Abstract
Dermatological disorders have a huge psychosocial impact, causing significant impairment of patient's life. Topical therapy plays a pivotal role in management of such disorders. Conventional topical delivery systems result in overmedication/undermedication, leading to adverse effects and reduction in therapeutic efficacy. Consequently, researchers have been striving towards the development of alternative delivery systems for dermatological applications. In the last decade, microsponges emerged as an attractive option for topical delivery. Their characteristic particle size offers enhanced benefits, making them superior to the contemporary microcarriers. The present review furnishes a comprehensive account of state of the art, important factors affecting the performance and mechanism of drug release from topically applied microsponges, along with characterization techniques. Further, a list of marketed products and their applications for common dermatological disorders has been presented. All in all, this paper is an attempt to lay a bibliographic foundation for researchers working in this field and foster further investigations in this arena.
Keywords: Skin, Polymers, Drug release, Dermatology, Safety aspects, Stability
Graphical abstract
1. Introduction
Human skin is an important target site for drug application in dermatological problems. For the treatment of skin disorders, topical drug delivery is an appropriate approach to limit the therapeutic effect on the affected region and to reduce systemic side effects. The outermost layer of skin, stratum corneum (SC), presents an efficient barrier to external agents, including drugs. This innate feature of stratum corneum also tenders a significant challenge to the formulation scientists, and in order to deliver therapeutic effective drug concentrations in various skin layers, this barrier has to be overcome.
In contemporary times, lipid-based colloidal carriers have been widely explored and have proven their merit in facilitating drug delivery. This is supported by the fact that the lipids used in such formulations are similar to physiological lipids. In light of this, lipid based colloidal systems enable improved dermal penetration, offering a rewarding option for delivering dermatological actives. Additional features which make them particularly suitable for dermal applications include their ability to adhere to the skin surface, improved hydration, replenishment of epidermal lipids by allowing the exchange of lipids between the colloidal carriers and the outermost layer of epidermis [1]. With the advent of micro-colloidal drug delivery systems, it is possible to achieve drug targeting, in addition to enhanced percutaneous absorption. In this context, numerous studies have been undertaken as well as patents granted, wherein lipid based colloidal carriers have been exploited to provide therapeutic benefits in treating various dermatological disorders [2]. Most widely investigated micro-colloidal drug delivery systems encompass micelles, microemulsions, liposomes and niosomes.
Micelles may be defined as optically isotropic thermodynamically stable liquid systems comprising of water and amphiphile. Due to their limited solubilization capacity for oils, micelles become unstable in the presence of large quantity of apolar solvents (e.g. alkanes). The toxic potential of surfactants is another disadvantage associated with their application. On the other hand, emulsions constitute large quantities of oil droplets dispersed in aqueous phase and are stabilized by oil saturated micelles. The droplet size of these metastable colloids is usually more than 1 µm. Emulsions have capacity to allow greater amount of hydrophobic moieties to be incorporated in dispersed phase, employing minimum concentration of surfactant (1%) [3]. However, these highly viscous dispersions pose stability problems due to aggregation of oil droplets, thereby reducing the shelf-life of the product. Contrary to these, microemulsions represent thermodynamically stable systems, produced with the addition of a co-surfactant to the system consisting of an aqueous phase, lipophillic phase and a surfactant [3]. The resulting dispersion is a homogenous system having the property to diffuse light [4]. The major disadvantages pertaining to this system include high surfactant concentration and their potential toxicity. The effect of environmental factors on drug solubility is also a point of concern [3].
Liposomes are the most extensively studied particulate carrier systems which revolutionized the dermatological drug delivery by virtue of their controlled release property and potential for site specific delivery. They are vesicular systems constituted by one or more phospholipid bilayers, separated by an aqueous compartment [5]. Besides drug molecules, this system is well established for its ability to encapsulate proteins and enzymes. This can be attained by varying their composition, dimensions, surface and structural characteristics. The chief advantage of liposomes is the low toxicity and biocompatibility of their ingredients. Ease of preparation and alteration in their composition to yield better formulations are further advantageous. Still the scale-up issues, high viscosity and propensity to disintegrate upon administration (leading to in vivo instability and uncontrolled drug release) are the main drawbacks and the probable reasons for fewer marketed liposomal formulations [6].
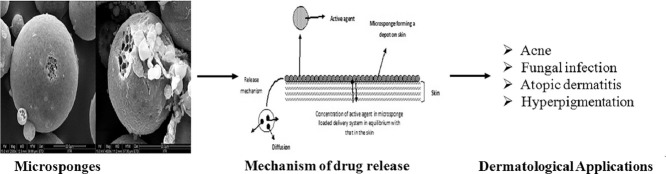
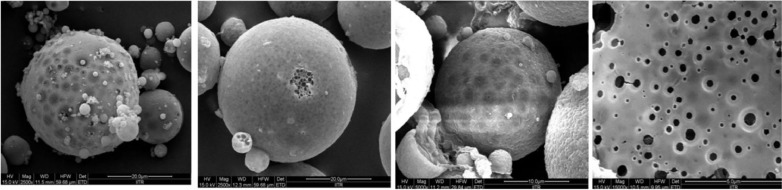
The emergence of microsponge colloidal carriers has presented an attractive alternative to the aforementioned delivery systems. This is apparently due to the fact that microsponges not only amalgamate the advantages of the former systems, but also circumvent their limitations and drawbacks. These colloidal carriers may be defined as tiny, highly cross-linked porous and spherical particles (Fig. 1). These are generally prepared by solvent removal from microemulsion templates. These sponge-like carriers are chiefly composed of polymers, dispersed in an aqueous system, with the aid of a stabilizing agent. Removal of solvent from microemulsion droplets leads to porosity of resultant carriers. Their inherent features of enhanced drug payload and stability, alongwith potential for reduced irritation, mutagenicity and allergenicity, contribute to their superiority over the contemporary colloidal carriers [7]. Thus, understanding of the structural and physiological characteristics of the human skin assumes utmost importance.
Fig. 1.
Microsponges under field emission scanning electron microscopy
Human skin is chiefly composed of epidermis resting upon dermis. Stratum corneum, as topmost layer of epidermis, has been thoroughly characterized by microscopic, bioengineering and biophysical techniques [8]. Briefly, it consists of keratinized, dead corneocytes which are embedded in an extracellular network of multilamellar lipid bilayers. The visualization studies in this regard have shown that barrier properties of SC are due to the presence of extracellular lipids, as percutaneous penetration is mainly attributed to intracellular pathway [9] and compromised SC barrier function brought about by lipid extraction or tape stripping of the skin [10], [11].
In view of the structure and physiology of human skin, microsponges offer enhanced efficacy of dermatological agents, as well as reduced local adverse effects. The characteristic size of microsponges (5–300 µm) [12], [13] is the most crucial feature for topical application, because it hinders their passage through stratum corneum [14]. Hence, these carriers are especially advantageous as delivery systems for dermal applications, as these allow the drug to be present on the skin surface/epidermis for a prolonged period. At the same time, the transdermal penetration of the active agent is minimized. This attribute is of paramount importance in the context of topical delivery of drugs [15]. Additionally, owing to their porous nature, microsponges permit controlled release of the entrapped drug, resulting in minimum deposition of the active moiety in the epidermis and dermis [16]. However, the particulate nature of microsponges makes them less appropriate for direct topical application. Therefore, they are incorporated in topical base, such as gel, emulgel, ointment, or cream for better efficacy. Literature reports suggest that gel base has been most commonly exploited for the loading of microsponges meant for dermatological use [7], [12], [17], [18], [19], [20]. Besides gel, creams have also found use in microsponge based drug delivery. Paenol and miconazole loaded microsponges have been incorporated into cream base [15], [21]. In addition to these, literature search revealed a paper for emulgel based delivery of mupirocin loaded microsponges [22] and another for benzoyl peroxide loaded microsponges incorporated in a lotion [23]. The selection of topical base is influenced by skin type and clinical symptoms associated with the disorder e.g. condition such as acne vulgaris, wherein the sebaceous secretions increase the oily character of the skin, a gel base or an o/w type cream base would be more appropriate [24]. Likewise, excessively dry nature of skin, such as in psoriatic patients, would be more benefited when the carrier is enthused in an oil-enriched cream, lotion or emulgel base. Further, a base with good occlusivity prevents the loss of moisture from the skin keeping it well hydrated [15], [21], [22], [23].
The nature of drug (hydrophilic or lipophilic) is also one of the deciding factors, when it comes to base selection. Upon application, the diffusion of the drug from the delivery system involves its passage through the base matrix [15]. The inherent nature of the latter, hydrophilic or lipophilic, and its interactions with the drug molecules, thereof, influences its release. This particularly concerns the o/w partition co-efficient of the drug. Another aspect of utmost importance is the excipients, composing the base. Especially, formulations intended for the treatment of allergic conditions, characterized by skin irritation, the ingredients of the base, viz. surfactants and cosolvents, should be chosen, as to avoid cutaneous irritation [25].
Foremost, the extent of drug release from microsponge as well as its pattern is affected by the surrounding base. In this regard, the rheological properties and the texture of the base are significant. The release of the drug from delivery system may be modified by altering the type and composition of the base. In view of this, gel-based formulations should be designed keeping in mind the fact that its microstructure undergoes a breakdown upon rubbing (due to increase in shear rate), thereby diminishing its apparent viscosity [17]. In the same breath, it may be said that the spreadability of the formulation also depends upon the viscosity and textural propreties of the base. Ideally, the base should permit the microsponge to be spread with the application of minimum shear [12]. From the viewpoint of stability of drug entrapped in the microsponges, the base is expected to impart protection from environmental factors. Hence, it would be noteworthy to mention here the relevance of stability studies of microsponge loaded in the base, in addition to microsponge alone. It is so because the ingredients present in the base are likely to interact with the drug and microsponge components. The selection of a topical base is influenced by various characteristics of skin. The development of a microsponge loaded delivery system for dermatological agents could further enhance drug efficacy and reduce the occurrence of local adverse effects.
To the best of our knowledge, there is no review till date, focusing on the role of microsponges in dermatological applications. In the present article, we have reviewed the use of microsponges in cosmetics and dermatological products, with the view to improve their characteristics and performance. Further, we have attempted to provide an update on the current status, trends of research in this area and the direction in which the field is progressing.
2. Merits of microsponges as carrier system for topically active agents
During the last decade, the interest in microsponges has increased profoundly, as is evident from the publications in this area. Advantages associated with microsponges compared with other microparticulate systems are: easy fabrication (in terms of composition), better drug loading and controlled release of drug. A number of articles suggest additional advantages of microsponge based delivery systems (MDS), enumerated in Table 1.
Table 1.
Advantages of microsponge based delivery systems
| Sr. No. | Advantages | Ref. |
|---|---|---|
| 1. | Shelf-life and product stability can be prolonged without using preservatives, since bacteria are too large to enter into the microsponge | [26] |
| 2. | Highly compartmentalized nature of microsponges results in very high internal surface area hence, they exhibit high pay loading capacity | [27] |
| 3. | Undesirable properties like oiliness and tackiness, or undesirable feel or odor of ingredients can be considerably reduced which makes them suitable for topical delivery to skin | [28] |
| 4. | Liquids can be transformed into free flowing powder, providing material handling benefits | [28] |
| 5. | Microsponges help in improving elegance of the formulation | [29] |
| 6. | MDS augment the efficacy of topically active agents and enables their sustained release | [30] |
| 7. | Microsponges consist of interconnecting voids within a non-collapsible structure, with large porous surface | [31] |
| 8. | Stable over a wide pH range of 1–11 and up to a temperature of 130 °C | [32] |
3. Composition of microsponges
Various polymers used in fabrication of microsponges for topical application result in formation of a microsponge ‘cage’. As per published literature, polymers explored so far include polymethacrylates or Eudragit© polymers (Eudragit RS100, Eudragit RSPO, Eudragit S100), polylactide-co-glycolic acid, polylactic acid, polydivinyl benzene, polyhydroxy butyrate and ethyl cellulose. Table 2 gives an account of such polymers employed in fabrication of microsponge formulations. Among these, Eudragit RS100 is the most widely studied polymer, owing to its versatile nature [37]. The wide range of Eudragit polymers, differing in charge, solubility and water permeability, allows for custom-tailored release characteristics in this system, facilitating a wide range of alternatives to achieve the desired performance [38]. Polymers belonging to polymethacrylate category are Food and Drug Administration (FDA) approved, safe, non-toxic and economic excipients, widely used in the pharmaceutical industry. The flexibility to combine different polymethacrylate polymers offers a better control on drug release behavior, especially due to drug–methacrylate–polymer interaction [39]. Being a foundation material for microsponges, ethyl cellulose is also used for engineering of microsponges due to its nonirritating, nontoxic and nonallergenic nature [40]. Another polymer, polydivinyl benzene, has been reported for the crafting of porous microspheres by liquid–liquid suspension polymerization technique [41]. Although, several polymers have been explored of late, but only few studies have been reported with biodegradable polymers. They can be potential excipients for the development of microsponge carriers for drug targeting. Hence, there is a strong need to explore biodegradable polymers for this delivery system. Beside this, the choice of polymer should take into the account skin irritant and dermal toxicity potential. This being a major concern in dermatological formulations, has been studied by some group of researchers working in the domain of microsponge based delivery systems. Recently, Kumar and Ghosh have addressed this aspect by conducting cell line toxicity studies and in vivo skin irritation study for silver sulfadiazine loaded microsponge gel [20].
Table 2.
Active moieties and polymers employed in microsponges formulations
| Active moieties | Polymer | Drug-polymer ratio | Ref. |
|---|---|---|---|
| Benzoyl peroxide | Ethyl cellulose | 1:1, 1:3,1:5, 1:7, 1:9, 1:11, 1:13 | [33] |
| Dicyclomine | Eudragit S100 | 1:3, 1:6, 1:9, 1:12 | [34] |
| Hydroxyzine HCl | Eudragit RS100 | 1:1, 1:2, 1:3, 1:4 | [1] |
| Mometasone furoate | Eudragit RS100 | 1:3, 1:5, 1:7, 1:9, 1:11, 1:13 | [35] |
| Acyclovir sodium | Ethyl cellulose | 1:2, 1:3 | [36] |
| Diclofenac diethylamine | Eudragit RS100 | 1:1, 1:2, 1:3, 1:4, 1:5, 1:6 | [12] |
| Terbinafine | Eudragit RSPO | 1:1.8, 1:4, 1:1 | [6] |
In addition to the polymers and active ingredients, some other excipients are needed for preparation of stable and effective microsponge formulations. For instance, triethyl citrate is used as a plasticizer to stabilize the buoyant microsponges, and addition of a porogen like hydrogen peroxide or sodium bicarbonate, results in the formation of evenly distributed and interconnected pores, which provide larger surface area for drug loading in these systems. The pores also increase the entrapment efficiency of this micro-colloidal drug delivery system [42]. In some reports, sucrose and pre-gelatinized starch have also been used as pore inducers to improve drug release rate [1]. Polyvinyl alcohol (PVA) and cellulose ethers have been reported as emulsifiers to maintain the viscosity of aqueous phase in quasi-emulsion solvent diffusion method [23], [43].
4. Engineering of microsponges
Apart from microsponge composition, preparation techniques play an important role in regulating the performance of this delivery system. However, the methods of microsponge preparation are limited due to their complexity and cost. Microsponge production can be achieved by techniques such as liquid-liquid suspension and quasi-emulsion solvent diffusion method, with or without modification. Grochowicz et al. reported a method for microsponges preparation based on free radical suspension polymerization technique. It is a one-step process in which monomers are dissolved with non-polar drug in a suitable solvent and the resulting solution is dispersed in aqueous phase containing suitable surfactant and suspending agent. Polymerization is initiated either by irradiation, addition of catalyst or by increasing the temperature. Although, it is a convenient method, it results in non-uniform structures with poor reproducibility. Further, it requires a long time for reaction of the monomers. Another limitation is the entrapment of unreacted monomer residues. This limitation can be overcome by using quasi-emulsion solvent diffusion method [41] .
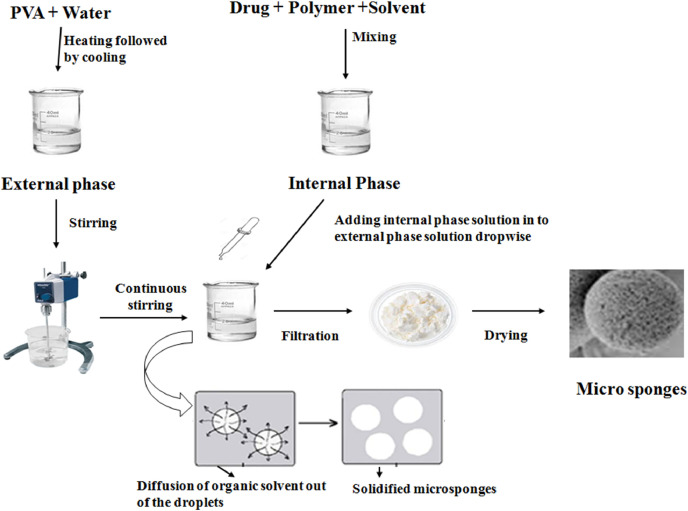
Quasi-emulsion solvent diffusion method represents the most commonly used technique to design microsponges (Fig. 2). It is a two-step process, in which internal phase consisting of a suitable polymer is dissolved in solvents such as dichloromethane (DCM), acetone or ethanol, in the presence of a plasticizer and a diffusible substance (porogen). This internal phase is, then, dispersed into an external aqueous phase, comprising of polyvinyl alcohol, which acts as a stabilizer. After emulsification, the system is continuously stirred for a suitable time interval and maintained at a high temperature, if needed. Porogen diffuses into the external medium, resulting in a highly porous scaffold structure called ‘microsponge’. The final product is subsequently washed and dried in vacuum oven at 60 °C for 24 h. This process might prove a better option when the active molecule is sensitive to polymerization conditions. Further, the process has the advantage of avoiding solvent toxicity. Importantly, some factors such as drug solubility, nature of solvent, temperature and speed of emulsification, nature of polymer cross-linking, diffusibility of porogen, type and concentration of plasticizer also affect the formation of microsponges. This process, besides being rapid, is simple and reproducible. Furthermore, this technique yields uniform microsponges with narrow size distribution [41].
Fig. 2.
Fabrication of microsponges through Quasi-emulsion solvent diffusion method
5. Characterization of microsponges
Physicochemical characterization of microsponges is a vital step in the successful design and fabrication of these versatile microcarriers. In addition to UV–visible spectroscopy and high pressure liquid chromatography (HPLC), this delivery system requires various complementary techniques like Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM) and porosity studies for investigating their structural and morphological characteristics. The functional properties of microsponges are investigated based on these characteristics. Various methods widely used by scientists for the purpose of characterization of microsponges for the relevant functional parameters are discussed in the following sections:
5.1. Fourier transform infrared (FTIR) spectroscopy
Fourier transform infrared spectroscopy is a primary tool to check the purity and chemical interaction of drug and excipients. To check the stability of the drug in microsponges, FTIR of blank and drug loaded microsystems can be performed. On encapsulation of drugs into microsponges, the FTIR spectra shows shifting or broadening of drug peak, on account of molecular interactions between the active moiety and the microsponge. In this context, FTIR study concerning purity and drug-excipient interaction of diclofenac diethylamine in microsponges was performed by Osmani et al. FTIR spectroscopy results revealed no new peak appearance or disappearance of existing peaks upon encapsulation, confirming compatibility of drug with the selected polymer and excipients. The study also confirmed good stability of diclofenac diethylamine in all microsponge formulations [12]. FTIR yields more information when coupled with DSC technique [44].
5.2. Differential scanning calorimetry
DSC is a relatively cheaper and easier technique of analysis in comparison to X-ray diffraction and solid state NMR [45], [46]. It provides information regarding purity of drug, interaction between drug and polymer, and confirmation of drug loading in microsponges.
The thermograms of drug, polymer, physical mixture of drug and excipients used, and drug loaded microsponges can be obtained using DSC. Exothermic and endothermic peaks are obtained due to melting, decomposition or moisture loss of samples. Broadening of peaks implies loss of crystallinity of drug during microsponge formation. Such results have been reported by Amrutiya et al., for mupirocin microsponges [22]. Arya and Pathak used DSC to ascertain the physical nature of curcumin loaded in microsponges and to check its compatibility with the excipients used (ethyl cellulose and Eudragit S100). This group reported that DSC of physical mixture represented additive spectra of the peaks of ethyl cellulose, Eudragit S100 and curcumin, advocating no interaction between the polymers and the drug. The thermogram of curcumin loaded microsponge was found similar to blank microsponge formulation, showing detectable loss of prominent peak for pure drug moiety, signifying its uniform molecular dispersion in microsponges [47].
5.3. Powder X-ray diffraction
Powder X-ray diffraction is a valuable analysis method to study physicochemical features of crafted microsponges. XRD pattern of the microsponge can be determined as a function of scattered angles due to dispersion of atoms in their lattice planes [48]. Powder X-ray diffraction has been used for assessing the changes in crystallinity of drug and chemical interaction between the ingredients in microsponges. The diffraction peaks of physical mixture of meloxicam and Eudragit L100 (1:1) showed distinctive crystalline diffraction peaks of drug, indicating the absence of interaction. A reduction in the intensity of peak indicated decrease in crystallinity of meloxicam in microsponges [49]. PXRD analysis also gives information about thermal stability of drug in microsponge formulation [12].
5.4. Scanning electron microscopy
Scanning electron microscopy is employed to study the particle size and morphology. Orlu et al. carried out SEM for microsponge formulations which revealed the fine, spherical and uniform nature of microsponges. The cross-sectional view was also taken, which showed the rigid shell of microsponge along with internal cavity enclosed. SEM photographs also showed the absence of entire drug crystals in microsponges [27]. In order to study the topographical features of the prepared microsponges, SEM technique has been invariably employed by all the researchers. Therefore, characterisation of microsponges without SEM could be considered to be incomplete.
5.5. Porosity studies
Porosity study can be performed to check the size of nanocavities formed in microsponges. Pore structure, diameter and pore volume can be determined in these studies. Porosity can be measured using mercury intrusion porosimetry [1]. In this test, a sample of microsponges is placed in a vacuum chamber and submerged under a mercury pool, in a volume-calibrated cell. When the pressure is gradually increased in the cell, mercury is forced into pores of microsponges. This leads to reduction in the apparent volume of mercury within the calibrated cell [1]. The formula for computing percent porosity is given below:
Rizkalla et al. investigated pore volume and pore size of microsponges using mercury intrusion porosimetry. High percentage porosity (60.9%–71.8%) in prepared microsponges was observed [1]. Pore size and volume directly affect drug encapsulation and release, which are important parameters for any delivery system. However, review of literature suggests that porosity studies, although vital for microsponges, have received meagre attention from the researchers.
6. Characterization of microsponges loaded in topical delivery systems
Besides regular physicochemical characterization of microsponges, more specific evaluation related to topical treatment has to be adopted for microsponges incorporated in topical base. This includes conducting in vitro permeation experiments using dialysis membrane or animal skin, transepidermal water loss measurements, assessment of rheology, spreadability, texture analysis, occlusive behavior and tube extrudability.
7. Effects of formulation parameters and process variables on microsponge characteristics
Microsponges are characterized for particle size, shape, encapsulation efficiency, production yield, drug loading, porosity, surface morphology and drug release [1]. The following sections provide an insight into some of the key parameters which have profound impact upon the efficacy of microsponges.
7.1. Size
The most important variable which needs to be monitored during preparation of microsponges for their satisfactory performance is their size. For effective topical application, the selected preparation method should result in optimum size range, with uniform distribution of microsponges [7]. Various parameters that affect the size of these porous microstructures include, drug: polymer ratio, volume of internal phase, amount of emulsifying agent and stirring speed. Among these, drug: polymer ratio has more pronounced effect on the size of prepared microsponges. It can be varied in two ways. Firstly, the effect of increase in polymer amount on size of microsponges can be investigated. With increase in drug-polymer ratio, increase in particle size of microporous structures has been reported by scientists. This might be due to the fact that the polymer available at high drug-polymer ratio is in considerable amount per microsponge, thereby increasing the polymer surrounding the drug. This, consequently, results in production of larger microsponges [25]. Secondly, drug-polymer ratio can also be varied by varying the amount of drug. When drug concentration is high, ethanol bound with the drug might be separated from the aqueous phase, resulting in viscous coacervate emulsion droplet, in which slow co-crystallization of the drug and polymer takes place, forming a spherical microsponge. At lower concentration of the drug, quick intermixing of free ethanol with the aqueous phase occurs, inducing a large reduction in the size of ethanol droplets and a rapid crystallization of drug. This leads to production of very small range of spongy micro structures. It has been observed that particle size of microsponges is directly proportional to apparent viscosity of the internal phase [22]. The larger the difference in viscosities between the dispersed phase and the dispersion medium, lower the resultant particle size and vice-versa. This might be credited to the fact that on mixing the dispersed phase with higher viscosity dispersion medium, the emulsion formed is hardly broken into small globules, and results in bigger droplets. In addition, with increase in the amount of emulsifying agent, particle size has been found to increase. It is probably due to the rise in apparent viscosity at augmented stabilizer concentration [25]. The volume of internal phase is another significant factor which has important role in microsponge preparation. On increasing the volume of internal phase, viscosity is found to decrease, resulting in finely dispersed emulsion droplets, which adhere together and coalesce. As a result, no microsponge is obtained. Hence, it is suggested that volume of the internal phase should be controlled carefully for successful microsponge formation [34].
According to Zaki Rizkalla et al. during preparation of hydroxyzine hydrochloride microsponges, addition of Span 80 to liquid paraffin causes a drastic decrease in microparticle size to nano range. Such nano size particles obtained after the addition of Span 80 may be due to the higher stabilizing effect imparted to the small emulsion droplets, which prevents them from collision with the larger ones. In this formulation, the addition of magnesium stearate in a specified concentration resulted in successful microencapsulation of hydroxyzine hydrochloride. Such concentration of magnesium stearate depends on the solvent volume and the amount of polymer. As reported, 3% (w/v) magnesium stearate with 1:5 polymer/solvent ratios yielded free flowing microsponges [1]. According to Jelvehgari et al. the dispersion of the drug and polymer into the aqueous phase has been found to be dependent on the stirring speed of agitator. As the agitation speed is increased, the size of microsponges is reduced [23]. Hence, optimum drug-polymer ratio, amount of emulsifying agent, volume of internal phase and stirring speed have been found to play crucial role in obtaining customize size of microsponges.
7.2. Production yield
Production yield has been found to be affected by drug-polymer ratio, amount and nature of emulsifying agent used, and stirring speed. Increase in the drug-polymer ratio may result in higher production yield. In dicyclomine microsponges reported by Jain and Singh, when drug-polymer ratio is 1:1, the production yield is very low, while with drug-polymer ratio 1:5, it is observed to be remarkably high. The abridged dichloromethane diffusion rate from concentrated solutions to aqueous phase at increased drug: polymer concentration provides more time for droplet formation, leading to an improvement in yield. However, an increase in emulsifying agent leads to reduction in production yield. Due to nonionic nature of the emulsifier (PVA), it forms some hydrophobic region and some of the drug and polymer gets dissolved. Stirring speed is also known to influence production yield. When the speed of stirrer is increased, a decrease in production yield is observed, possibly due to the reason that at higher stirring rates, the polymer adheres to the paddle because of creation of turbulence within the external phase [34].
7.3. Entrapment efficiency
Entrapment efficiency is the content of core material effectively entrapped in a formulation. It can be measured by an indirect method in which microsponge suspension can be centrifuged at 2000 rpm for 10 min. The supernatant obtained can be suitably diluted with suitable solvent and the amount of free drug present in supernatant can be quantified using UV–Visible spectroscopy or HPLC. The drug entrapment efficiency (EE) can be calculated using the following formula:
Entrapment efficiency depends on several parameters discussed here. As per literature reports, drug polymer ratio and amount of pore inducers affect. Increase in the drug-polymer ratio may lead to increase in the EE. The reason for increase in EE is the reduced diffusion rate of drug solution from concentrated polymeric solutions into the external phase. This allows more time for droplet formation, resulting in improvement of microsponge yield and entrapment efficiency. This may be attributed to the fact that the amount of drug per unit of polymer is more. The reduced diffusion rate of DCM from the concentrated solution takes more time for droplet formation, with higher precipitation of the drug molecules in the microsponge. This is responsible for improved EE [7]. In contrast to this, Osmani et al. reported that superior drug loading efficiencies were obtained at lower drug: polymer ratios. The reason ascribed to this observation is the availability of considerable polymer amount to each drug unit as opposed to the rest of the microsponges. Further, it has been reported that with increase in the amount of PVA, production yield and encapsulation efficiency are increased [21]. Pore inducers have also been reported to affect entrapment efficiency of microsponges. They increase surface area for absorption of drug onto their porous particles. In the given context, an increase in the entrapment efficiency with increase in pore inducer concentration has been reported by some researchers [1].
7.4. Drug release
Drug release is another important variable which needs to be monitored during microsponge fabrication for its best performance. Literature suggests that variables like drug: polymer ratio, DCM concentration, amount of PVA and pore inducers affect drug release from microporous structures. Increase in drug: polymer ratio has been found to result in decreased percent drug release. This could be explained as follows: with increase in drug: polymer ratio, the polymer amount available for each microsponge for drug encapsulation is higher, thus, leading to more pronounced polymer matrix wall thickness. It results in extended diffusion path, and ultimately, to lesser drug release. Consequently, the drug diffused and flux also decrease at higher drug: polymer ratio [21]. Similar results were reported by Osmani and his research group. This group of researchers also reported that the drug release went on decreasing with increasing polymer amount. It might be attributed to the fact that the polymer matrix releases drug after complete swelling and the time required for swelling is directly proportional to the concentration of stabilizer [13]. DCM concentration was also found to have a positive impact on the drug release. This is attributed to the fact that with increase in DCM concentration, more porous and spongy microstructures are obtained. Higher amount of DCM also results in the precipitation of the drug at the periphery of the microsponge, leading to extended drug release. With increase in amount of PVA, slight decrease in drug release has been noticed [13]. Pore inducers, hydrophilic powders that are generally added during the microsponge formulation with the view to obtain an optimum release of active ingredients, are reported to drain water from the dissolution medium, either by osmotic effect (like sucrose) or by adsorption and disintegrant effect (like pregelatinized starch (PGS)) [50]. According to Zaki Rizkalla et al., the rate of drug released from PGS containing hydroxyzine microsponges was found to be better than those containing sucrose. Better disintegrant effect of PGS in the microsponge matrix might be responsible for such results [1].
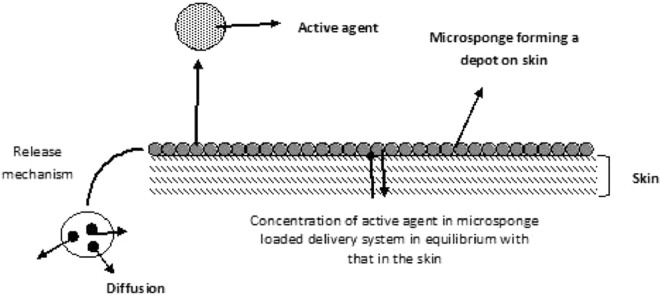
8. Mechanism of drug release from topical microsponges
For controlling the release of a drug from microsponges, a number of variables can be altered, taking into consideration the physicochemical features of the active agent and the cutaneous environment. As discussed above, the vehicle used for dissolving the polymer plays an important role in the release of the active agent from the system. Initially, an equilibrium exists between the concentration of active agent in the polymer and the vehicle. As the concentration of the active agent from the vehicle depletes in the skin, the MDS releases more active agent as per the demand created by the equilibrium shift. Such a system results in a continuous and steady release of the active agent onto the skin. In addition, the MDS can act as a depot, which continuously releases the active agent to the skin, even after the absorption or drying of vehicle by the skin (Fig. 3).
Fig. 3.
Mechanism of drug release from topical microsponges
In the reported microsponge based intra-dermal drug-delivery systems, researchers have used mupirocin, paenol, hydroxyzine hydrochloride, glabridin and benzoyl peroxide as the model drugs to evaluate the characteristics of this system, and revealed that the major drug release mechanism was diffusion and that the microsponge preparation could enhance the rate of drug release [15]. Drug release was expected to be based on interaction between microsponges and dermal secretions. Further, it may also be associated with its porous nature, as this enables penetration of the release media and accessibility to the encapsulated drug moiety [37]. According to Jelvehgari et al. upon penetration of release media into the porous microsponges, the drug gets dissolved into it gets released. As the release media first comes in contact with the surface of the microsponge, and then gradually into the internal region, the drug release measured over the initial few hours may be due to non-encapsulated drug on the surface of the micro carriers, followed by release of the drug entrapped in the pores, giving rise to sustained drug release [15], [23]. Hence, size of pores also plays significant role in drug release. Porosity of microsponges can be controlled by altering the drug and polymer concentration in the preparation, while keeping their content ratio constant [51].
For treating skin disorders, it is imperative that the drug stays in the treated areas and there is no absorption to other areas. Li et al., suggested that preparing a microsponge based formulation for paeonol may be a promising approach for skin targeting in the treatment of dermatological disorders. The greater amount of drug deposition in the skin from paeonol microsponge cream indicated better drug bioavailability topically [15]. Due to occlusive effect, higher drug retention by the microsponges produced a film on the skin surface, reducing transepidermal water loss. This increased the hydration of the stratum corneum, thereby, causing an increased drug penetration into the skin [52], [53]. Moreover, the high lipophilicity of the microsponge formulation prevented drug diffusion from the skin into the receiver fluid, maintaining effective local drug concentration for a long period of time [54].
In this study, microdialysis results represented the total amount of drug that penetrated through the stratum corneum, into the epidermis and finally, into the dermis. It was reported that microsponge formulations could deliver significant amounts of paeonol to the dermis, thus, leading to higher drug bioavailability, but with high inter-individual variability. Further, it has been confirmed that the insignificant amount of drug absorbed into the plasma, results in decline of side-effects [54].
In order to comprehend the drug permeation kinetics from microsponge-loaded formulations, the in vitro release data can be fitted to various drug release kinetic models, namely, zero order (cumulative percentage drug permeated vs time), first order (log cumulative percentage drug remaining to be permeated vs time), Higuchi (cumulative percentage drug permeated vs square root of time), Peppas and Korsmeyer-Peppas, and by assessing the highest r2 value, the best fit model may be decided. An empirical parameter, n value is used to characterize the release mechanism [22]. Based on the diffusion exponent, ‘n’ equal to 0.5 indicates that the drug release mechanism approaches to a Fickian diffusion controlled release, whereas n equal to 1.0 indicates zero-order release. The n value from 0.5 to 1 represents a drug release mechanism for non-Fickian diffusion. The in vitro drug release from diclofenac diethylamine microsponge formulation showed highest regression value for the Peppas model (0.998 for optimized batch). The n value for Korsmeyer-Peppas model was found to be in the range 0.5–1, which represented non-Fickian diffusion [12]. The data from in vitro release profile of hydroxyzine hydrochloride microsponges (in phosphate buffer at pH 5.5) were analyzed, and a diffusion controlled mechanism, according to simplified Higuchi model, was revealed [1]. In vitro drug permeation from diclofenac sodium microsponge-loaded gel formulations was best described by Higuchi's diffusion kinetics, as the plot was linear, with r2 values ranging from 0.9797 to 0.9889. Similar release kinetics was observed for mupirocin from the microsponge-based emulgel system [22]. Different kinetic models were employed to fit the data relating to the release of glabridin from microsponges incorporated in the gel. The highest value of r2 for Higuchi model indicated that drug release from glabridin microsponges followed diffusion kinetics. To investigate the release mechanism, further, the data was fitted in Korsmeyer-Peppas model. It was concluded that drug release from the glabridin microsponge-loaded gel followed non-fickian diffusion [55]. On application to skin, drying of aqueous gel was observed which left behind a layer of microsponges, adhering to the hydrophilic dermal layer, with the help of thin films of Carbopol. The drug was believed to get squeezed out of microsponges during application, thus, some amount of glabridin remained in the microsponge matrix and some in the gel base [17]. The benzoyl peroxide (BPO) release mechanism from conventional lotion was reported as non-Fickian diffusion, whereas from lotions containing microsponges, Fickian diffusion was observed, which was controlled by the porosity of the microsponges [56]. For benzoyl peroxide, the main site of pharmacological action is the pilosebaceous canal [24]. It exerts its antimicrobial activity by penetrating through the follicular opening, probably by dissolving into sebaceous lipids [57]. Skin irritation is the most common side effect of this drug and, a correlation exists between its efficacy and irritation [58]. This correlation could be altered by controlling release of BPO from delivery system to the skin, by maintaining intrafollicular penetration while reducing percutaneous absorption. Because of larger size, the microsponges are unable to pass through the stratum corneum, and hence, they remain on the skin surface forming a depot, gradually releasing their contents over time. This release pattern enhances the safety of locally applied drugs through prevention of excessive accumulation of active moiety in the skin. Microsponge entrapped BPO enabled reduction in skin irritation when compared to the formulations containing unencapsulated BPO powders [5]. It was ascertained that a controlled-release topical delivery system might reduce the percutaneous absorption of BPO without affecting its intrafollicular penetration, leading to alleviation of drug irritancy, without affecting efficacy.
When studying drug release mechanism of topically applied microsponge formulation, confocal laser scanning microscopy could prove a valuable tool. With aid of this technique, the extent of drug release, its penetration and targeting to the desired cutaneous tissue /site can be featured out, although, this technique has not been so utilised for studying the release behaviour of MDS.
9. Applications of microsponges in cosmetics and dermatology
The original patents for microsponge technology developed by Won in 1987 were assigned to Advanced Polymer Systems, Inc. This company applied the microsponge technology for cosmetic, over-the-counter (OTC) and prescription pharmaceutical products [59]. MDS gives assurance regarding drug localization on skin surface and within the epidermis, abridging systemic and local cutaneous side effects. Microsponges for the dermatological and cosmetic products differ only in the technological aspects. Due to minor regulatory conditions, production, marketing and introduction time of cosmetic products is much shorter as compared to dermatological products [60]. Some examples of the microsponge based cosmetic products currently available in the market are enlisted in Table 3 [61], [62].
Table 3.
Some examples of MDS currently marketed as cosmetic products
| Name of product | Active moiety | Indications | Manufactures |
|---|---|---|---|
| Retin-A-Micro | Tretinoin | Acne vulgaris | Ortho-MCNeil Pharmaceutical, Inc. USA. |
| Carac Cream | Fluorouracil | Actinic keratoses | Dermik Laboratories, Inc. USA |
| Line Eliminator Dual Retinol Facial Treatment | Retinol | Antiwrinkle | Avon Products, Inc. UK |
| Retinol Night Cream | Retinol | Antiwrinkle | Biomedical IMPORIUM, South Africa |
| Lactrex™ 12% Moisturizing Cream | Lactic acid | Moisturizer | SDR Pharmaceuticals Pvt. Ltd, India |
| EpiQuin Micro | Hydroquinone and retinol | Hyperpigmentation | SkinMedica, Inc. USA |
| Oil free Matte Block SPF20 | Zinc Gluconate | Sunscreen | Dermalogica, LLC, USA |
| Ultra Guard | Dimethicone | Protective for babies | Scott Paper Company, USA |
| Aramis Fragrances | Antiperspirant | Aramis, Inc. USA | |
| Micro Peel Plus/Acne Peel | Salicylic acid | Acne vulgaris | Biomedical IMPORIUM, South Africa |
| Retinol Cream | Retinol | Skin supplement | Biomedical IMPORIUM, South Africa |
| Glycolic Acid Moisturizer w/SPF 15 | Glycolic acid | Antiwrinkle and soothing agent | AMCOL Health and Beauty Solutions, Inc. USA |
| Salicylic Peel 20 and 30 | Salicylic acid | Excellent exfoliation | Biophora Medical Skin Care, Ontario, Canada |
One of the most important features of microsponge is their ability to absorb skin secretions, i.e., oil and sweat [6]. Due to their highly absorbent nature, many microsponge loaded deodorants, antiperspirants and sunscreens are commercially available. Further, microsponge drug delivery systems can be used for skin targeting, avoiding excessive absorption of drug into the percutaneous blood circulation. This feature may prove a boon in skin disorders, like skin cancer, wounds, acne, alopecia, sunburn, hyperhidrosis and wrinkles. Table 4 summarizes microsponge based formulations developed with various drugs for dermatological applications. The use of active compounds with microsponges provides an overview of the breadth of MDS platform, which serve as carrier for the cosmetic and dermatological products.
Table 4.
Drug candidates explored using microsponge delivery systems for dermatological and cosmetic applications
| Sr. No | Drug Candidates | Formulation Type | Cosmetic and Dermatological Applications | References |
|---|---|---|---|---|
| 1 | Benzoyl peroxide | Cream, gel, lotion | Antiacne Treatment (Keratolytic agent) | [23] |
| 2 | Hydroquinone and retinol | – | Depigmentation | [63] |
| 3 | Flucinolone acetonide | Gel | Skin inflammation | [64] |
| 4 | Mupirocin | Emulgel | Primary and secondary skin infections | [22] |
| 5 | Mometasone furoate | – | Skin inflammatory diseases | [35] |
| 6 | Hydroxyzine HCl | – | Atopic dermatitis | [1] |
| 7 | Fluconazole | Gel | Antifungal | [18] |
| 8 | Voriconazole | Gel | Antifungal | [65] |
| 9 | Ketoconazole | Gel | Antifungal | [66] |
| 10 | Eberconazole nitrate | Gel | Antifungal | [19] |
| 11 | Acyclovir sodium | Gel | Herpes simplex virus infections | [36] |
| 12 | Glabridin | – | Depigmenting agent | [17] |
| 13 | Paeonol | Cream | Antiinflammatory and antimelanin activity | [15] |
| 14 | Erythromycin | – | Antibacterial | [67] |
| 15 | Miconazole | Gel | Antifungal | [21] |
| 16 | Itraconazole | Gel | Antifungal | [68] |
| 17 | Terbinafine | Gel | Antifungal (hair and skin infection) | [6] |
| 18 | Oxybenzone | Gel | Sun screen agent | [7] |
| 19 | Sertaconazole nitrate | Gel | Antifungal | [69] |
| 20 | Fluconazole | Gel | Antifungal | [70] |
| 21 | Silver sulfadiazine | Gel | For partial thickness burn wounds | [20] |
| 22 | Babchi oil | Gel | Antibacterial | [71] |
| 23 | Miconazole | Gel | Antifungal | [72] |
| 24 | Tea tree oil | Gel | Antibacterial | [73] |
9.1. Microsponges for anti-acne drugs
Acne is a common skin disorder in young adults [74]. Although, topical therapy is the main strategy for its treatment, associated side effects with various topical antiacne bioactive molecules affect their utility and patient compliance. Some potential novel carriers and delivery systems like liposomes, microemulsions, solid lipid nanoparticles, and nanolipid carriers have been explored to enhance topical anti acne therapy. Recently, microsponges have been proposed as an advanced drug delivery system, able to optimize drug activity profile for anti acne agents. Benzoyl peroxide (BPO) is first line topical agent employed for management of acne, due to its bactericidal activity against Propionibacterium acnes [75]. It is superior to other antibiotics, as the bacteria do not develop resistance to it. However, a drawback of the treatment of BPO containing products is that they generally cause skin irritation to the individuals [76]. Degree of skin irritation depends on the concentration of BPO present in the skin [58]. Irritation is generally influenced by the delivery system, formulation and nature of skin. Encapsulation of BPO reduces skin irritation, due to reduction in the release rate of the drug from the formulation [5].
Jelvehgari et al. selected microsponge delivery system to facilitate topical delivery of BPO. In order to optimize the microporous formulation, parameters affecting the physical properties of the system were also investigated. Emulsion-solvent diffusion method was used for preparation of ethyl cellulose based microsponges. BPO was added to the organic internal phase. Drug content, particle size and loading capacity were determined in the prepared formulation. BPO microsponges were, then, incorporated into creams and evaluated for drug release. Scanning electron microscopy was used to study the morphology of the microsponges. The micrograph of microsponges showed that they were spherical in shape, with porous structure. It was observed that the drug-polymer ratio, stirring rate and volume of dispersed phase, influenced the particle size and drug release behavior, and that the presence of emulsifier was an essential requirement for microsponge formation. The results showed that, generally, an increase in drug: polymer ratio results in reduction in the release rate of BPO from the microsponges, which may be attributed to their decreased internal porosity [23].
Wester et al., explored factors affecting the morphology of BPO microsponges. Absorption of this anti acne agent from a topical lotion containing freely dispersed drug was compared with the same lotion in which the drug was entrapped in a controlled release styrene-divinylbenzene microsponge. In an in vitro diffusion system, statistically significant differences were obtained for BPO in excised human skin and in percutaneous absorption. Significantly less drug was absorbed through Rhesus monkey skin from the prepared polymeric system, as reported from in vivo research investigation. This controlled release of BPO to skin can modify the dose relation between efficacy and skin irritation. Corresponding studies showed reduced irritation in rabbits and human beings, whereas in vivo human antimicrobial efficacy studies depicted that application of the formulations loaded with BPO significantly reduced counts of Propionibacterium acnes (P < 0.001) and aerobic bacteria (P < 0.001) and the free fatty acid/triglyceride ratio in skin lipids. These findings supported the hypothesis that, at least for this drug, controlled topical delivery can enhance safety without sacrificing its efficacy [5]. In another study of BPO, microporous particles were prepared using a quasi-emulsion solvent diffusion method. Various concentrations of BPO microsponges were then incorporated into various formulations (creams, gels and lotions) for release studies. It was shown that the drug-polymer ratio, stirring rate and volume of the dispersed phase have considerable impact on the particle size and drug release. Various ratios of formulation were prepared with Eudragit RS100 and evaluated for loading efficiency, drug entrapment and surface morphology. Further, topography and in vivo release kinetics of particles were studied. The reports of this research work showed that by careful control of the process variables, desirable features in this system can be obtained. Drug release mechanism of microsponge loaded lotion is Fickain diffusion and is controlled by the porosity of the microsponges [33].
Erythromycin is another drug of choice used to treat acne by reducing the amount of Propionebacterium acnes on the skin. However, the drug causes gastric irritation, nausea, vomiting, abdominal pain and is easily inactivated in the gastric environment. Ravi and Senthil prepared erythromycin loaded microsponge based gel, which exhibited lesser skin irritation, greater skin tolerance and slow release of drug [67]. Osmani et al. formulated microsponge loaded miconazole nitrate cream as a promising delivery system for acne and skin infection. Besides characterization of drug entrapped microsponges and microsponge loaded cream, in vitro release was also carried out. Results of in vitro release have shown that microsponge loaded cream with 1:2 drug-polymer ratio were found to be more efficient. The system provided controlled release for about 8 h as compared to conventional gel, which lasted nearly for 4 h [21].
9.2. Microsponges for anti-fungal drugs
Fungal infection of the skin is one of the most widely experienced dermatological diseases worldwide [77]. According to recent reports, more than 25% of world's population is affected by this disorder [78]. The progression of fungal infection can be rapid and serious due to compromising immune function [79]. Oral administration of an antifungal drug increases the chances of gastric irritation and systemic side effects. Therefore, topical therapy is an attractive choice for the treatment of cutaneous infections. This therapy also provides advantages like drug targeting at the site of infection and reduction in systemic side effects [80]. The most common and effective topical antifungals are polyenes, azoles, allylamines, and their derivatives.
Bhimavarapu et al. investigated the topical delivery of itraconazole loaded microsponges which were developed with the objective of enhancement of bioavailability of drug. The porous microsponges were prepared using quasi-emulsion solvent diffusion technique. Microsponges with 1:6 ratios were found to possess better controlled release characteristics and followed Higuchi model of release [68].
Terbinafine is a BCS (Biological Classification System) class-II drug, used for treating various skin and hair infections. It possesses low bioavailability, as it can be easily inactivated by acidic medium in gastrointestinal tract. Gastric irritation, abdominal pain, nausea, vomiting are the common side effects of this drug, when administered orally [66]. Recently, Barde and Basarkar developed microsponges loaded with terbinafine, as an alternative to conventional topical and oral formulations. Microsponges loaded with terbinafine were prepared, optimized and incorporated in a Carbopol gel. Microsponges were prepared using Eudragit RSPO polymer, by quasi-emulsion solvent diffusion method. In order to optimize the formula for microsponges, factors affecting the physical properties of this system were evaluated. It was observed that the polymer and PVA concentration influenced the particle size and drug content of microsponges. Parameters namely production yield, loading efficiency, surface morphology and particle size were checked. In vitro permeation studies of microsponge loaded gel formulations were performed using Franz diffusion cell. Also, drug release was compared with that of the marketed formulation [6].
In some studies, attention has been paid to the triazole derivatives, having broad-spectrum antifungal activity and low toxicity [81]. Fluconazole, a triazole derivative widely used for mycoses (especially superficial fungal infection) acts by inhibition of cytochrome P450 system and prevents the ergosterol synthesis, a main component of fungal membrane [82]. It is used orally in the treatment of dermatophytosis and topically for cutaneous leishmaniasis [83], [84]. Oral administration of fluconazole (FLZ) results in gastric irritation, heart burn, vomiting and even ulceration. Microsponge-loaded hydrogel containing FLZ was prepared using ethyl cellulose (EC) and Eudragit RS100 based microsponges by quasi-emulsion solvent diffusion method. The effect of process variables such as drug: polymer ratio, polymer type, PVA concentration and type of internal phase on the physical properties of the microsponges were investigated using 24 factorial design. Results demonstrated that FLZ loading and particle size of microsponges were increased with increase in the polymer amount. Moreover, EC significantly improved the drug EE and the mean particle size. There was a reverse proportionality observed between the PVA concentration and, both, the EE and the mean particle size. In comparison to methylene chloride, ethanol significantly increased the EE and the particle size. Optimized formulation of FLZ was selected, as the FLZ microsponge (consisting of FLZ and EC in 1:1 ratio and prepared using 0.75% PVA and methylene chloride) was loaded in Carbopol gel and explored for its in vitro release characteristics. The developed microsponges were found to be spherical and porous. The drug release using cellulose dialysis membrane exhibited Fickian release pattern. The research group further recommended antifungal activity and in vivo animal activity for future studies [18]. Recently, Moin et al. fabricated, characterized and evaluated fluconazole microsponges for topical fungal therapy. This group reported fluconazole microsponges as an alternative to conventional therapy for safe efficient and facilitated eradication of fungal infection topically [85].
Srilakshami and Prathima encapsulated voriconazole in microsponges with varying proportions of Eudragit RS100 and Eudragit L100 using quasi-emulsion diffusion method. These microsponges were further loaded in gels. Characterization studies showed high encapsulation efficiency in these carriers. In vivo activity was performed in guinea pigs by inducing fungal infection using Candida albicans. Voriconazole showed a significant antimicrobial and antifungal activity as compared to the conventional fluconazole gel, which was used as a reference. The antimicrobial study revealed greater zone of inhibition with microsponge loaded voriconazole gel in comparison to the marketed fluconazole gel, used as a control. Hence, voriconazole microsponge loaded gel may be considered as a potential option for treatment of fungal infections [65].
Recently, Dinesh Mohan and Gupta formulated and evaluated fluconazole loaded microsponge gel for topical sustained delivery. However, the study was restricted to preparation and characterization of drug loaded microsponges and microsponge loaded gel [70]. Bothiraja et al. prepared ethyl cellulose based microsponges of eberconazole and incorporated it into gel for topical delivery. The characterization of microsponges and skin irritation studies were conducted to demonstrate controlled release and non-irritancy. Further, antifungal activity was carried out on the microsponge gel. Results of in vivo skin deposition study demonstrated four times higher drug retention in the stratum corneum when compared to commercial cream. Results signified that the prepared eberconazole microsponge gel may be a potential topical delivery system for antifungal therapy [19]. Recently, Pande et al. fabricated and characterized sertaconazole nitrate microsponges for topical drug delivery. This group prepared drug loaded microsponges and characterized them for various parameters like particle size, production yield, entrapment efficiency and drug content. Microsponges were further loaded into 1% Carbopol gel and evaluated appropriately. Further, in vivo studies were not carried out [69].
9.3. Microsponges for atopic dermatitis
Hydroxyzine hydrochloride is an antihistaminic drug used for the treatment of urticaria and atopic dermatitis. Blurred vision, dizziness, and anticholinergic responses are the most common side effects of this drug, when administered orally. The MDS is a unique technology reported for the controlled delivery of the topically active agent. Therefore, these were studied as vehicle for topical administration of hydroxyzine hydrochloride with an attempt to reduce the side effects and to target the drug to the site of action. It has been shown by Zaki Rizkalla et al. that controlled release of hydroxyzine hydrochloride from the delivery system could reduce the side effects while reducing percutaneous absorption. Eudragit RS100, based microsponges of the drug were fabricated by the oil in oil emulsion solvent diffusion method, with acetone as dispersing solvent and liquid paraffin as the continuous medium. Magnesium stearate was added to the dispersed phase in order to prevent flocculation and to obtain free flowing microsponges. Pore inducers, such as sucrose and PGS, were used to enhance the release rate of drug due to their water absorption and disintegrant properties. Microsponges with nearly 98% encapsulation efficiency and 60–70% porosity were obtained. The pharmacodynamic effect of the chosen preparation was investigated using histamine-sensitized rabbits. Histopathological studies were also carried for the detection of the healing of inflamed tissues [1].
The percutaneous absorption of drug increases the risk associated with systemic absorption of topically applied formulation. D'souza and Harinath, prepared fluocinolone acetonide entrapped microporous particles, which limit the percutaneous absorption by controlling release of the drug [64]. Mometasone furoate is a medium potency, synthetic, non-fluorinated topical corticosteroid, used for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses, including psoriasis. Controlled release topical formulation of the drug is expected to reduce the side effects while decreasing percutaneous absorption as the same time. Therefore, Rekha and Manjula aimed to produce mometasone furoate entrapped microporous particles to control its release in skin. Mometasone furoate microsponges were fabricated using emulsion solvent diffusion method. For optimization of the microsponges, factors affecting the physical parameters of formulation were determined. Excipients and drug compatibility interaction was studied by FTIR spectroscopic analysis. Production yield, loading efficiency and surface morphology of microsponges were also investigated. It was observed that the drug-polymer ratio, stirring rate, volume of external and internal phase, affected the particle size and drug release behaviour of microsponges. The results indicated that, generally an increase in the drug-polymer ratio results in a reduction in the release rate of mometasone furoate from microsponges [35].
Paeonol is one of the major active components from the root bark of Paeonia suffruticosa. It has the potential to treat neurodegenerative diseases by alleviating morphological damage [86] increasing neuron viability [87] and reducing cerebral infraction [88]. It also possesses anti-arrhythmic [89] anti-atherogenic [90] anti-tumor [91] and anti-inflammatory activity [92]. Further, it is widely used in cardiovascular diseases [93], [94] and eczema due to its anti-anaphylactic activity [95]. However, it is unable to penetrate the stratum corneum, as it has a low aqueous solubility, with an oil/water partition coefficient of 2.21 [96], [97]. Paeonol microsponges were prepared by using the quasi-emulsion solvent-diffusion method and incorporated in a cream base. In vitro release studies were carried out using Franz diffusion cells. Results depicted that the drug delivered via microsponges possessed increased permeation rate. Ex vivo drug deposition studies demonstrated that this novel formulation improved drug residence in the skin. In addition, in vivo microdialysis showed that the value for the area under the concentration versus time curve for the paeonol microsponge cream was much better than that of paeonol cream without microsponges. Results indicated that paeonol microsponge formulations could be better choice for treating skin disease, as the formulation improved drug bioavailability by increasing the drug residence time in the skin. Reduction in the amount of drug entering into the systemic circulation offers the added advantages of diminishing the adverse effects associated with the drug [15].
9.4. Microsponges for anti-hyperpigmenting agents
Hyperpigmentation disorders such as melasma and post inflammatory hyperpigmentation (PIH) are difficult to treat as well as can be distressing for patients. Many of the skin lightening products such as hydroquinone creams available in the market prove toxic to skin melanocytes and cause skin irritation as well. Some researchers explored microsponge based topical delivery system to overcome these problems. Grimes et al. reported the potential use of hydroquinone (HQ) 4% and retinol 0.15% entrapped in microsponge reservoirs for the treatment of melasma and PIH. Results reported minimum skin irritation as microsponges altered the release rate of the drug and prolonged the treatment exposure. The safety and efficacy studies were carried out as 12 weeks open-label study with 25 patients. The encapsulated 4% HQ/0.15% retinol formulation showed good tolerability and improvement at each visit, except in one patient who discontinued the treatment because of an allergic reaction. Overall improvement in disease and hyperpigmentation intensity was found statistically significant at 4, 8 and 12 weeks when discontinued because of allergic reaction [63].
Glabridin, another topical depigmenting agent, effective in the treatment of melasma, freckle and age spots has been reported. It is isolated from the plant Glycerrhiza glabra. This tyrosinase inhibitor prevents melanin synthesis. Glabridin microsponges were incorporated in gel for topical delivery. Drug loaded microsponges were characterized by scanning electron microscopy and FTIR spectroscopy. In vitro diffusion and drug loading studies were carried out. Highest correlation was found with Higuchi treatment as revealed by in vitro release studies. Glabridin loaded microsponge based gel showed better depigmenting activity [17].
9.5. Microsponges for anti-bacterial drugs
Infections, especially skin infections triggered by multiple bacteria constitute an extensive complication that threats the human health. This encourages the researchers to find an alternative for management of skin disorders by encapsulating the antibacterial drugs in novel carrier systems to enhance their efficacy [98]. A topical antibiotic used for skin infection is mupirocin. It is a drug of choice for the suppression of inflammation [99] produced by Pseudomonas fluorescens, bacteria that inhibits the growth of various dermatophytes and Pityrosporum [100]. It binds to the enzyme iso-leucyl of bacterial RNA synthetase, and inhibits bacterial protein synthesis [101], [102]. It is metabolized slowly in skin to the antimicrobially inactive metabolite monic acid.
Amrutiya et al. investigated emulgel bases loaded with microsponges for topical delivery. Briefly, mupirocin containing microsponges were prepared by emulsion solvent diffusion method. Formulation and process variables were optimized using 32 factorial design. Optimized microsponges were further incorporated into an emulgel base. In vitro drug release, ex vivo drug deposition, and in vivo antibacterial activity of formulations were studied. Drug release through cellulose dialysis membrane showed diffusion-controlled release. Drug deposition studies using rat abdominal skin exhibited significant retention of the drug in the skin from microsponge loaded gel, in 24 h. The optimized formulations were stable and non-irritant to skin as revealed by Draize patch test. Microsponge-based emulgels exhibited prolonged efficacy in mouse surgical wound model infected with Staphylococcus aureus. The results suggested that this topical delivery system could be a potential delivery system for the treatment of primary and secondary skin infections like impetigo, eczema, and atopic dermatitis [22].
Kumar et al., fabricated silver sulfadiazine microsponges through water in oil in water emulsion by solvent evaporation technique for burn wounds application. To optimize the formulation variables, 32 factorial design was employed. The optimized microsponges were incorporated in Carbopol gel. Various analytical tools, such as particle size analysis, FTIR, PXRD, DSC, mercury intrusion porosimetry and SEM analysis, were used to characterize the prepared microsponges. The dermal toxicity was assessed by MTT assay on mouse embryonic fibroblast (NIH-3T3) and epidermal keratinocyte (HaCaT) cell lines. To compare the antibacterial inhibitory efficacy of the optimized gel with commercial product, in vitro antibacterial studies were performed against the Pseudomonas aeruginosa and Staphylococcus aureus. Burn wound mouse model was employed to assess the potency of the optimized gel. Results indicated three fold enhancements in drug retention in skin layers and antibacterial inhibitory efficacy of the prepared formulation was found comparable to the commercial product. Silver sulfadiazine microsponge delivery system exhibited no skin irritation, low cytotoxicity on cell lines with enhanced wound healing efficacy [20].
Anti bacterial potential of babchi oil loaded microsponges was explored by Wadhwa et al. using dermal bacteria (Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli). In vitro cytotoxicity was evaluated to explore dermal safety of fabricated microsponges, on HaCaT cell lines (dermal cells) with respect to pure babchi oil. Further, improved photostability and stability of babchi oil loaded microsponges was demonstrated. This study advocated the micropsonges as potential carriers for enhancement of safety, stability and efficacy of babchi oil [71].
The therapeutic efficacy of tea tree (Melaleuca alternifolia) oil is diminished due to its poor aqueous solubility, low stability and high volatility. To circumvent these limitations, tea tree oil (TTO) loaded microsponges were fabricated using ethyl cellulose and PVA by Yadav and her research group. The optimized formulations were incorporated into Carbopol gel and subsequently, evaluated for drug release, photostability, antibacterial activity and skin irritation. MS-loaded gels were observed to be stable and non-irritant. Antibacterial potential of TTO microsponge gel displayed enhanced zones of inhibition with respect to TTO gel against Pseudomonas aeruginosa, Staphylococcus aureus and E. coli. The prepared microformulation can be a better alternative to commercial antibacterial formulations for dermal microbial disorders [73].
9.6. Miscellaneous applications of microsponges
Oxybenzone is one of the most widely used chemical filters found in commercial sunscreen products. This broad spectrum sunscreen agent reduces dermal penetration of radiations [103], [104]. Topically available conventional oxybenzone sunscreen products cause dermatitis, skin irritation and systemic absorption [105], [106]. Pawar and coworkers investigated the topical delivery of this molecule using microsponge loaded gel. Drug loaded microsponges were successfully formulated by quasi-emulsion solvent diffusion method using ethyl cellulose. 32 factorial designs were used for optimization of the formulation. The optimized microparticles were dispersed into hydrogel and further evaluated. Particle size measurements and drug entrapment efficiency were also checked. Results demonstrated that the controlled release of oxybenzone from the porous structures and barrier effect of gel resulted in prolonged retention of drug with reduced permeation, irritation or toxicity, and enhanced sun protection factor [7].
A well known topical antiviral agent for the treatment of herpes simplex infections of skin is acyclovir. Chandramouli et al., developed acyclovir loaded microsponges employing quasi-emulsion solvent diffusion technique, in order to eliminate problems associated with topical formulations. Two grades of Eudragit polymers (Eudragit RSPO and RLPO) and methyl cellulose were used in the formulation as rate controlling polymers. In addition to characterization, the microsponges were evaluated for particle size, production yield, loading efficiency, entrapment efficiency and dissolution behavior. The selected microsponges were entrapped in Carbopol gels. In vitro release was determined using egg membrane which depicted prolonged release of the drug from microsponges. On the basis of results, it was suggested that acyclovir microsponge loaded topical gel showed sustained release and enhanced retention of drug in skin [36].
Recently, sertaconazole nitrate, a 14α-demethylase inhibitor used in various skin disorders, was encapsulated in Eudragit microsponges. The microsponges were fabricated employing quasi emulsion solvent diffusion method. The fabricated microsponges were investigated for production yield, particle size, drug content and entrapment efficiency. Topography and pore structure analysis were carried out through SEM and mercury intrusion porosimetry, showing spherical and porous nature of the formulation, respectively. Further, microsponges incorporated into Carbopol gel (1%) were analyzed for drug content, pH, in vitro release and gel texture. From the results obtained, the Carbopol gel loaded with sertaconazole nitrate microsponges was found promising for topical delivery of its antifungal agent [69].
Nebivolol was formulated in microsponges using oil in oil solvent diffusion method. The prepared microsponges were further incorporated in Carbopol 934 gel (2%) for management of moist wound environment. The prepared formulations were evaluated for physicochemical parameters. In vitro diffusion study of the optimized formulation displayed slow release behavior with 80% drug release in 8 h. The gel properties like pH, drug content and viscosity were also explored. Excision wound and streptozotocin-induced diabetic rat model were used for in vivo studies, which exhibited wound closure activity and wound healing within ten days. Histopathological results like, neovascularization and inflammatory cell infiltrations in granulation tissues signified wound healing potential of prepared microformulation. The obtained results demonstrated that nebivolol-loaded microsponge gel may act as a potential carrier system for diabetic wound healing [107].
10. Safety aspects
The true worth of a delivery system is judged on the basis of its ability to deliver effective concentration of the active agent without compromising on the safety aspects. In other words, the drug should be released from the delivery system in such a manner that it does not induce any irritation, cytotoxicity, genotoxicity or immunogenicity. With particular reference to dermatological application, the system should also prevent any dermal sensitization reactions, if reported with the active agent incorporated.
In context of dermal topical preparations, skin irritation is a commonly encountered adverse effect, however, skin irritation can be directly correlated with the concentration of the active agent. In this concern, the delivery system loaded with the active agent can play a key role by modulating its release and, further, may even modify this correlation by facilitating intrafollicular penetration and decreasing its percutaneous uptake [58]. Some groups of researchers have checked skin irritation potential of microsponge based topical formulations, whose results are evaluated in terms of erythma, oedema and irritation, using rats or rabbits as animal model. Active agents such as eberconazole nitrate [19], oxybenzone [7] and silver sulfadiazine [20] have been, thus, investigated. Their findings have proven the potential of these delivery systems to subdue or eliminate the skin irritancy.
Cytotoxicity evaluation of the drug loaded microporous carriers constitutes an important aspect of formulation development. The cytotoxic effects of both, the active agent as well as the ingredients comprising the delivery system, should be taken into account. The percent cell viability and IC50 values should be found out for the drug alone, drug loaded microsponges and blank microsponges. Such a comparative evaluation will present a clear picture pertaining to cytotoxic effects of the prepared formulations. Howbeit, evaluation of microsponges from this perspective seems to have been overlooked. Recently, Kumar and Ghosh, conducted dermal cytotoxicity studies as a part of evaluation of silver sulfadiazine loaded microsponge gel [20].
11. Stability issues
Microsponges have been found to remain stable in the range of physiological pH and, therefore, can be used as a versatile carrier system. Due to their property of thermostability, they can withstand temperatures upto 130 °C. As their average pore size is 0.25 µm, microsponge formulations are self-sterilizing, limiting bacterial penetration [108]. Although, microbial entry to the bulk is prevented, they can grow on the surface of microsponges [109].
For stability testing of the microsponge formulations, generally ICH guidelines are followed. Osmani et al. filled optimized diclofenac diethylamine microsponge loaded gel in clean, lacquered, collapsible aluminum tubes, and various replicates were kept at 40 °C and 75% RH in a humidity chamber. Gel was assessed for change in its appearance, pH or in vitro release profile for 90 d. During stability studies, no change in physical appearance and no significant deviation in pH were observed. Results indicated no considerable change in drug content as well as percent drug release. Therefore, no evidence of drug degradation was seen in this study. After comparing the optimized formulation, drug release profiles prior to and after 3 months were determined, and similarity factor was also calculated. f2 value was found to be 82.38 (>50). As similarity factor was greater than 50, it was concluded that the product possessed good stability over a period of 3 months [12]. For curcumin microsponges, researchers also performed Fourier transform infrared spectroscopy (FTIR) studies for the analysis of stability of the product, in addition to the above mentioned parameters. The FTIR spectra revealed no sign of instability of the drug, suggesting good shelf life of microsponge delivery system [110].
12. Recent advances in porous drug delivery systems
Although merits of microporous systems in dermatological preparations are well proven, in the current times when nanotechnology is dominating all the spheres of scientific endeavours, nanosized porous systems are being approached as a further advancement to their microsized counterparts. Nanosponges are hyper cross-linked polymer based colloidal structures, consisting of countless interconnecting voids within a collapsible structure with porous surface [111]. These offer passive targeting of dermal agents to skin leading to dosage form retention on skin, total dose reduction and systemic absorption avoidance. Very few research groups have attempted to investigate these nanoporous carriers for encapsulating dermally relevant moieties. Swaminanthan et al. formulated cyclodextrin nanosponges for solubility enhancement of itraconazole, a poorly water soluble drug [112]. The babchi oil loaded cyclodextrin nanosponges were also fabricated by our research group for solubility and photostability enhancement of entrapped essential oil [113]. Sharma and Pathak fabricated ethyl cellulose nanosponges as an alternative system for targeting econazole nitrate to the skin through hydrogel formulation [114]. Hence, nanosponges can be looked upon as an emerging alternative for dermatological disorders. In view of the safety concerns associated with nanoscale particles, exploration of these nano porous systems as carriers for dermatological agents demands a great deal of attention and in depth investigation. Veritably, this domain offers tremendous scope and scientists looking to enter this field should give due consideration to the issues stated above.
13. Expert opinion
Given the drawback of conventional dermatological formulations yielding relatively greater drug concentrations in short duration of time or vice-versa, many serious adverse effects ensue occasionally. By virtue of their structure, size, composition and drug release pattern, microsponges represent a promising strategy for dermatologists. A critical analysis of published research work in this field drives us to certain lacunas present in the research carried out so far. Further, based on an in depth analysis of the investigations undertaken, and results thereof, certain key points may be deduced, which pave way for the upcoming research in this area. These keypoints are listed as under:
(1) As far as fabrication technique is concerned, suspension polymerization technique is the commercially used method which suffers from a significant drawback that majority of active compounds undergo decomposition at polymerization temperature. As an alternative to this, quasi-emulsion solvent diffusion has been extensively utilized for preparing microsponges. The higher yield obtained with this technique is a valuable advantage.
(2) As it is, the formulation and process variables have a profound impact on the quality and efficiency of the microsponges, the relationship of such factors affecting product performance can be investigated more effectively by implementing quality by design (QbD) fundamentals in the development of microsponges. Recently, Pandit et al., and Kumar and Ghosh applied multivariate statistical analysis and Design of Experiments to optimise the critical factors in microsponge development [20], [107].
(3) In vitro drug release studies for microsponges till date, have been restricted to determination of drug release profile and extent of release, as well as application of release kinetics models. These studies seem incomplete without measuring the drug deposition in skin. Although, it is common knowledge that topical delivery improves the residence time of topically applied drugs, which is an important aspect of a controlled release formulation, at the same time avoiding increase in drug permeation. From this point of view, skin deposition studies, are valuable tools to validate this.
(4) In vivo investigations, with the view to quantify the drug reaching different strata of the skin, provides a clear view of cutaneous drug distribution as well as targeting, making the evaluation of topical microsponge based formulations more meaningful. Li et al. employed microdialysis technique for quantitative determination of paeonol in skin layers [15].
(5) Microsponges are best applied in a suitable base, which may be chosen from cream, lotions, gel or emulgel. However, the choice of suitable base should be made on the basis of well-planned studies with the objective of ensuring product performance.
(6) The safety testing of any drug product cannot be overemphasized. In respect of microsponge based dermal products, the major point of merit is that these are devoid of any surfactant. On the other hand, most of the topical drug delivery systems employ one or more surfactant(s), which are known for their ability to enhance permeation, cause irritation and induce toxic reactions. The safety studies for microsponges may be further supplemented by carrying out histopathological examination to confirm absence of any adverse skin reactions with advantage of reduced systemic side effects. Further, the cytotoxicity testing of these products should be conducted on cell lines specific to the dermatological route of drug delivery, such as human keratinocytes, and dermal fibroblasts.
14. Conclusions
The aim of dermatologists and cosmetic chemists is to develop new technology and tailor made products. Innovative cosmetic and dermatological formulations can be designed by an improved understanding of skin physiology and understanding its structure and function. In addition, the pharmaceutical formulator must possess a good knowledge of the physicochemical properties of drugs and polymers used, to be able to apply this new technology successfully, in drug delivery. Without further optimisation, addition of polymers to the existing formulations will seldom result in acceptable outcome.
Microsponge based delivery systems have been one of the key technologies investigated in the last few years. These systems display a strong rationale for cosmetic and dermal applications, in order to enhance the therapeutic performance of active molecules, with improved patient compliance. As Li et al. reported that the microsponge delivery system could not only enhance paeonol permeation but also decline transdermal penetration of the active molecule, which should lead to its augmented bioavailability in skin, with reduction in side effects while treatment of skin problems. Additionally, microsponges have the capacity to extend the drug residence in skin and allow prolonged drug release for up to 12 h, resulting in a long active time for the drug in topical treatment. These properties ascertain that MDS could be a promising platform for a new generation of dermatological and cosmetic treatments [15]. However, the level of drug distribution in the stratum corneum, epidermis, and dermis layers of skin and the mechanism of drug penetration are still at infancy, thus further studies to explore these aspects are needed.
Based on the scientific literature, it may be concluded that microsponge technology is a crucial tool, not only for scientific research based on this system, but also for the industrial development of new cosmetic products. In commercial formulations, microsponges are already being used or being proposed for the future use, as carrier of cosmetic and dermatological actives. Researchers, regulators and manufacturers should come together to adopt a scientific approach so as to produce safe topical products, using new technologies for both medicinal and cosmetic purposes and help in passing on these benefits to the consumers in a cost effective manner. Although, topical application for cosmetic and dermal benefits is associated with the advantage of reduced systemic side effects, certain drugs have the potential to induce problems like local irritation. Microsponges for topical delivery have overcome this obstacle to a great extent. Furthermore, these facilitate controlled release of potentially irritating molecules with better therapeutic delivery, resulting in improved adherence of patients to topical treatment. Additionally, the microsponge particles are too large to be absorbed through skin, which contributes to the safety of these microcarriers. However, there are still some unexplored, possible grey areas regarding the biocompatibility and toxicity of these porous microparticles, which makes an appeal for more exhaustive studies on their safety profile for the success of this system. Fascinatingly, a rapid increase in microsponge based cosmetic and dermatologic products can be expected in near future.
Acknowledgments
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgment
The co-author Mr. Sunil Kumar, is thankful to Indian Council of Medical Research, New Delhi for providing Senior Research Fellowship (Letter No: 45/44/2018-Nan/BMS).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2019.05.004.
Appendix. Supplementary materials
References
- 1.Zaki Rizkalla C.M., Latif Aziz R., Soliman I.I. In vitro and in vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS PharmSciTech. 2011;12:989–1001. doi: 10.1208/s12249-011-9663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone C., Leonardi A., Cupri S., Puglisi G., Pignatello R. Pharmaceutical and biomedical applications of lipid-based nanocarriers. Pharm Pat Anal. 2014;3(2):199–215. doi: 10.4155/ppa.13.79. [DOI] [PubMed] [Google Scholar]
- 3.Bagwe R.P., Kanicky J.R., Palla B.J., Patanjali P.K., Shah D.O. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 2001;18(1):77–140. [PubMed] [Google Scholar]
- 4.Bothorel P. Les microemulsions. L'Act Chim. 1985;(5):23–27. [Google Scholar]
- 5.Wester R.C., Patel R., Nacht S., Leyden J., Melendres J., Maibach H. Controlled release of benzoyl peroxide from a porous microsphere polymeric system can reduce topical irritancy. J Am Acad Dermatol. 1991;24(5):720–726. doi: 10.1016/0190-9622(91)70109-f. [DOI] [PubMed] [Google Scholar]
- 6.Barde P.M., Basarkar G.D. Formulation, development and in vitro evaluation of terbinafine HCl microsponge gel. Int J Pharm Sci Rev Res. 2015;32:310–314. [Google Scholar]
- 7.Pawar A.P., Gholap A.P., Kuchekar A.B., Bothiraja C., Mali A.J. Formulation and evaluation of optimized oxybenzone microsponge gel for topical delivery. J Drug Deliv. 2015;2015:1–9. doi: 10.1155/2015/261068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Euromoniter International. Market research on beauty and personal care, 2016. Available fromhttp://www.euromonitor.com/beauty-and-personal-care.
- 9.Sena M. Beauty industry analysis 2016-cost & trends, 2016. Available fromhttps://www.franchisehelp.com/industry-reports/beauty-industry-report/.
- 10.Jadoul A., Bouwstra J., Preat V. Effects of iontophoresis and electroporation on the stratum corneum: review of the biophysical studies. Adv Drug Deliv Rev. 1999;35(1):89–105. doi: 10.1016/s0169-409x(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?) 2016. Available from http://www.fda.gov/Cosmetics/GuidanceRegulation/LawsRegulations/ucm074201.htm#Intended_use.
- 12.Osmani R.A., Aloorkar N.H., Ingale D.J., Kulkarni P.K., Hani U., Bhosale R.R. Microsponges based novel drug delivery system for augmented arthritis therapy. Saudi Pharm J. 2015;23:562–572. doi: 10.1016/j.jsps.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmani R.A., Aloorkar N.H., Thaware B.U., Kulkarni P.K., Moin A., Hani U. Microsponge based drug delivery system for augmented gastroparesis therapy: formulation development and evaluation. Asian J Pharm Sci. 2015;10:442–451. [Google Scholar]
- 14.Maiti S., Kaity S., Ray S., Sa B. Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm. 2011;61(3):257–270. doi: 10.2478/v10007-011-0022-6. [DOI] [PubMed] [Google Scholar]
- 15.Li S.S., Li G.F., Liu L., Jiang X., Zhang B., Liu Z.G. Evaluation of paeonol skin-target delivery from its microsponge formulation: in vitro skin permeation and in vivo microdialysis. PLoS ONE. 2013;8(11):e79881. doi: 10.1371/journal.pone.0079881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain V., Singh R. Design and characterization of colon-specific drug delivery system containing paracetamol microsponges. Arch Pharm Res. 2011;34:733–740. doi: 10.1007/s12272-011-0506-4. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh K., Poddar S.S. Tyrosinase inhibitor-loaded microsponge drug delivery system: new approach for hyperpigmentation disorders. J Microencapsul. 2012;29(6):559–568. doi: 10.3109/02652048.2012.668955. [DOI] [PubMed] [Google Scholar]
- 18.Shah C.N., Shah D.P. Design and optimization of fluconazole microsponges containing ethyl cellulose for topical delivery system using quality by design approach. Pharma Science Monitor. 2014;5:95–133. [Google Scholar]
- 19.Bothiraja C., Gholap A.D., Shaikh K.S., Pawar A.P. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv. 2014;5(7):781–794. doi: 10.4155/tde.14.43. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P.M., Ghosh A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur J Pharm Sci. 2017;96:243–254. doi: 10.1016/j.ejps.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Osmani R.A., Aloorkar N.H., Kulkarni A.S., Kulkarni P.K., Hani U., Tharumaleshwar S., Bhosale R.R. Novel cream containing microsponges of anti-acne agent: formulation development and evaluation. Curr Drug Deliv. 2015;12:504–516. doi: 10.2174/1567201812666150212122421. [DOI] [PubMed] [Google Scholar]
- 22.Amrutiya N., Bajaj A., Madan M. Development of microsponges for topical delivery of mupirocin. AAPS PharmSciTech. 2009;10:402–409. doi: 10.1208/s12249-009-9220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelvehgari M., Siahi-Shadbad M.R., Azarmi S., Martin G.P., Nokhodchi A. The microsponge delivery system of benzoyl peroxide: preparation, characterization and release studies. Int J Pharm. 2006;308:124–132. doi: 10.1016/j.ijpharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Nacht S. Proceedings of the 1980th research and scientific developments conference. The Proprietry Association (Ed.); New York City: 1981. Methods to assess the transepidermal and intrafolicular penetration of anti-acne agents; pp. 88–91. [Google Scholar]
- 25.Horita K., Horita D., Tomita H., Yasoshima M., Yagami A., Matsunaga K. Effects of different base agents on prediction of skin irritation by sodium lauryl sulfate using patch testing and repeated application test. Toxicology. 2017;382:10–15. doi: 10.1016/j.tox.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Patel S.B., Patel H.J., Seth A.K. Microsponge drug delivery system: an overview. J Global Pharma Technol. 2010;2(8):1–9. [Google Scholar]
- 27.Orlu M., Cevher E., Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm. 2006;318(1):103–117. doi: 10.1016/j.ijpharm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Patravale V.B., Mandawgade S.D. Novel cosmetic delivery systems: an application update. Int J Cosmet Sci. 2008;30(1):19–33. doi: 10.1111/j.1468-2494.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 29.Osmani R.A., Aloorkar N.H., Kulkarni A.S., Harkare B.R., Bhosale R.R. A new cornucopia in topical drug delivery: microsponge technology. Asian J Pharm Sci Technol. 2014;4:48–60. [Google Scholar]
- 30.Chanchal D., Swarnlata S. Novel approaches in herbal cosmetics. J Cosmet Dermatol. 2008;7(2):89–95. doi: 10.1111/j.1473-2165.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 31.Chadawar V., Shaji J. Microsponge delivery system. Curr Drug Deliv. 2007;4(2):123–129. doi: 10.2174/156720107780362320. [DOI] [PubMed] [Google Scholar]
- 32.Embil K., Nacht S. The microsponge® delivery system (MDS): a topical delivery system with reduced irritancy incorporating multiple triggering mechanisms for the release of actives. J Microencapsul. 1996;13(5):575–588. doi: 10.3109/02652049609026042. [DOI] [PubMed] [Google Scholar]
- 33.Nokhodchi A., Jelvehgari M., Siahi M.R., Mozafari M.R. Factors affecting the morphology of benzoyl peroxide microsponges. Micron. 2007;38(8):834–840. doi: 10.1016/j.micron.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Jain V., Singh R. Dicyclomine-loaded Eudragit®-based microsponge with potential for colonic delivery: preparation and characterization. Trop J Pharm Res. 2010;9:67–72. [Google Scholar]
- 35.Rekha U., Manjula B.P. Formulation and evaluation of microsponges for topical drug delivery of mometasone furoate. Int J Pharm Pharm Sci. 2011;3(4):133–137. [Google Scholar]
- 36.Chandramouli Y., Firoz S., Rajalakshmi R., Vikram A., Yasmeen B.R., Chakravarthi R.N. Preparation and evaluation of microsponge loaded controlled release topical gel of acyclovir sodium. Int J Biopharm. 2012;3(2):96–102. [Google Scholar]
- 37.Srivastava R., Pathak K. Microsponges: a futuristic approach for oral drug delivery. Expert Opin Drug Deliv. 2012;9:863–878. doi: 10.1517/17425247.2012.693072. [DOI] [PubMed] [Google Scholar]
- 38.Kadian S.S., Harikumar S.L.Eudragit and its pharmaceutical significance. (2009). Available from: https://www.researchgate.net/publication/228097715_Eudragit_and_its_Pharmaceutical_Significance.
- 39.Yuksel N., Tinçer T., Baykara T. Interaction between nicardipine hydrochloride and polymeric microspheres for a controlled release system. Int J Pharm. 1996;140:145–154. [Google Scholar]
- 40.Kathe K., Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J Pharm Sci. 2017;12(6):487–497. doi: 10.1016/j.ajps.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grochowicz M., Bartnicki A., Gawdzik B. Preparation and characterization of porous polymeric microspheres obtained from multifunctional methacrylate monomers. J Polym Sci A Polym Chem. 2008;46:6165–6174. [Google Scholar]
- 42.Bae S.E., Son J.S., Park K., Han D.K. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J Control Release. 2009;133:37–43. doi: 10.1016/j.jconrel.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Sangnim T., Limmatvapirat S., Nunthanid J., Sriamornsak P., Sittikijyothin W., Wannachaiyasit S. Design and characterization of clindamycin-loaded nanofiber patches composed of polyvinyl alcohol and tamarind seed gum and fabricated by electrohydrodynamic atomization. Asian J Pharm Sci. 2018;13:450–458. doi: 10.1016/j.ajps.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandes L., Oliveira W., Sztatisz J., Szilágyi I., Novák C.S. Solid state studies on molecular inclusions of Lippia sidoides essential oil obtained by spray drying. J Therm Anal Calorim. 2008;95(3):855–863. [Google Scholar]
- 45.Giordano F., Novak C., Moyano J.R. Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim Acta. 2001;380(2):123–151. [Google Scholar]
- 46.Fernandes L.P., Éhen Z., Moura T.F., Novak C., Sztatisz J. Characterization of Lippia sidoides oil extract-b-cyclodextrin complexes using combined thermoanalytical techniques. J Therm Anal Calorim. 2004;78(2):557–573. [Google Scholar]
- 47.Arya P., Pathak K. Assessing the viability of microsponges as gastro retentive drug delivery system of curcumin: optimization and pharmacokinetics. Int J Pharm. 2014;460(1):1–2. doi: 10.1016/j.ijpharm.2013.10.045. [DOI] [PubMed] [Google Scholar]
- 48.Suryanarayanan R. Physical characterization of pharmaceutical solids. CRC Press; 1995. X-ray powder diffractometry; pp. 187–221. [Google Scholar]
- 49.Nief R.A., Hussein A.A. Preparation and evaluation of meloxicam microsponges as transdermal delivery system. Iraqi J Pharm Sci. 2017;23(2):62–74. [Google Scholar]
- 50.Song M., Li N., Sun S., Tiedt L.R., Liebenberg W., de Villiers M.M. Effect of viscosity and concentration of wall former, emulsifier and pore-inducer on the properties of amoxicillin microcapsules prepared by emulsion solvent evaporation. П Farmaco. 2005;60:261–267. doi: 10.1016/j.farmac.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Kawashima Y., Takeuchi H., Yoji I.T. Control of prolonged drug release and compression properties of ibuprofen microsponges with acrylic polymer, eudragit RS, by changing their intraparticle porosity. Chem Pharm Bull. 1992;40:196–201. doi: 10.1248/cpb.40.196. [DOI] [PubMed] [Google Scholar]
- 52.de Jalón E.G., Blanco-Príeto M.J., Ygartua P., Santoyo S. Topical application of acyclovir-loaded microparticles: quantification of the drug in porcine skin layers. J Control Release. 2001;75:191–197. doi: 10.1016/s0168-3659(01)00395-9. [DOI] [PubMed] [Google Scholar]
- 53.Sakai S., Kikuchi K., Satoh J., Tagami H., Inoue S. Functional properties of the stratum corneum in patients with diabetes mellitus: similarities to senile xerosis. Br J Dermatol. 2005;153:319–323. doi: 10.1111/j.1365-2133.2005.06756.x. [DOI] [PubMed] [Google Scholar]
- 54.Desai P.R., Shah P.P., Patlolla R.R., Singh M. Dermal microdialysis technique to evaluate the trafficking of surface-modified lipid nanoparticles upon topical application. Pharm Res. 2012;29(9):2587–2600. doi: 10.1007/s11095-012-0789-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoaib M.H., Tazeen J., Merchant H.A., Yousuf R.I. Evaluation of drug release kinetics from ibuprofen matrix tablets using HPMC. Pak J Pharm Sci. 2006;19(2):119–124. [PubMed] [Google Scholar]
- 56.Kligman A.M., Mills O.H., McGinley K.J., Leyden J.J. Acne therapy with tretinoin in combination with antibiotics. Acta Derm Venereol Suppl (Stockh) 1975;74:111–115. [PubMed] [Google Scholar]
- 57.Leyden J.J., McGinley K., Mills O.H., Kligman A.M. Topical antibiotics and topical antimicrobial agents in acne therapy. Acta Derm Venereol Suppl (Stockh) 1980;(Suppl 89):75–82. [PubMed] [Google Scholar]
- 58.Fulton J.E., Farzad-Bakshandeh A., Bradley S. Studies on the mechanism of action of topical benzoyl peroxide in acne vulgaris. J Cutan Pathol. 1974;1:191–200. doi: 10.1111/j.1600-0560.1974.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 59.Won R. Method for delivering an active ingredient by controlled time release utilizing a novel delivery vehicle which can be prepared by a process utilizing the active ingredient as a porogen. USA:4690825, 1987-9-1.
- 60.Puglia C., Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin Drug Deliv. 2012;9(4):429–441. doi: 10.1517/17425247.2012.666967. [DOI] [PubMed] [Google Scholar]
- 61.Aloorkar N.H., Kulkarni A.S., Ingale D.J., Patil R.A. Microsponges as innovative drug delivery systems. Int J Pharm Sci Nanotechnol. 2012;5:1597–1606. [Google Scholar]
- 62.Patel A., Upadhyay P., Trivedi J., Shah S., Patel J. Microsponges as the versatile tool for topical route: a review. Int J Pharm Sci Res. 2012;3(9):2926–2937. [Google Scholar]
- 63.Grimes P.E. A microsponge formulation of hydroquinone 4% and retinol 0.15% in the treatment of melasma and postinflammatory hyperpigmentation. Cutis. 2004;74(6):362–368. [PubMed] [Google Scholar]
- 64.D'souza J.I., More H.N. Topical anti-inflammatory gels of fluocinolone acetonide entrapped in eudragit based microsponge delivery system. Res J Pharm Technol. 2008;1(4):502–506. [Google Scholar]
- 65.Srilakshmi P., Srinivas M.P. Development and evaluation of voriconazole microsponges for topical delivery. Invent Rapid: Pharm Technol. 2011;2011:75–81. [Google Scholar]
- 66.Saboji J.K., Manvi F.V., Gadad A.P., Patel B.D. Formulation and evaluation of ketoconazole microsponge gel by quassi emulsion solvent diffusion. J Cell Tissue Res. 2011;11(1):2691–2696. [Google Scholar]
- 67.Ravi R., Senthi K.S.K. Standarization of process parameters involved erythormycin microsponges by Quassi emulsion solvent diffusion method. Int J Pharm Dev Technol. 2013;3:28–34. [Google Scholar]
- 68.Bhimavarapu R., Chitra K.P., Karunkiran P., Raviteja G., Meharagavendra Y., Sundaramma S., Chaitanya D. Itraconazole loaded microsponges – a novel carrier system. Int J Inv Pharm Sci. 2011;1:67–76. [Google Scholar]
- 69.Pande V.V., Kadnor N.A., Kadam R.N., Upadhye S.A. Fabrication and characterization of sertaconazole nitrate microsponge as a topical drug delivery system. Ind J Pharm Sci. 2015;77(6):675–680. doi: 10.4103/0250-474x.174986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dineshmohan S., Gupta V.R. Formulation and in vitro evaluation of fluconazole loaded microsponge gel for topical sustained delivery. IOSR-JPBS. 2015;10:15–20. [Google Scholar]
- 71.Wadhwa G., Kumar S., Mittal V., Rao R. Encapsulation of babchi essential oil into microsponges: physicochemical properties, cytotoxic evaluation and anti-microbial activity. J Food Drug Anal. 2018 doi: 10.1016/j.jfda.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salah S., Awad G.E., Makhlouf A.I. Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: formulation development and in vivo therapeutic efficacy in rats. Eur J Pharm Sci. 2018;114:255–266. doi: 10.1016/j.ejps.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 73.Yadav E., Rao R., Kumar S., Mahant S., Vohra P. Microsponge based gel of tea tree oil for dermatological microbial infections. Nat Prod J. 2018;8:1. doi: 10.2174/2210315508666180605080426. [DOI] [Google Scholar]
- 74.Date A.A., Naik B., Nagarsenker M.S. Novel drug delivery systems: potential in improving topical delivery of antiacne agents. Skin Pharmacol Physiol. 2006;19(1):2–16. doi: 10.1159/000089138. [DOI] [PubMed] [Google Scholar]
- 75.Billow J.A. Handbook of nonprescription drugs. 8th ed. APhA; Washington: DC: 1986. Acne products. [Google Scholar]
- 76.Lorenzetti O.J., Wernet T., Mcdonald Alcon T. Some comparisons of benzoyl peroxide formulations. J Soc Cosmet Chem. 1977;28:533–549. [Google Scholar]
- 77.Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010;28(2):197–201. doi: 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Hay R. Superficial fungal infections. Medicine (Baltimore) 2009;37(11):610–612. [Google Scholar]
- 79.Ramos-e-Silva M., Lima C.M., Schechtman R.C., Trope B.M., Carneiro S. Superficial mycoses in immunodepressed patients (AIDS) Clin Dermatol. 2010;28(2):217–225. doi: 10.1016/j.clindermatol.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Lee C.M., Maibach H.I. Deep percutaneous penetration into muscles and joints. J Pharm Sci. 2006;95(7):1405–1413. doi: 10.1002/jps.20666. [DOI] [PubMed] [Google Scholar]
- 81.Toraskar M.P., Kadam V.J., Kulkarni V.M. Synthesis and antimicrobial activity of functional analogues of fluconazole. IJPPS. 2010;2:132–133. [Google Scholar]
- 82.Gennaro A.R. Lippincott Williams & Wilkins; Baltimore, M.D.: 2000. Remington: the science and practice of pharmacy. [Google Scholar]
- 83.Lesher J.L., Jr. Oral therapy of common superficial fungal infections of the skin. J Am Acad Dermatol. 1999;40:S31–S34. doi: 10.1016/s0190-9622(99)70395-6. [DOI] [PubMed] [Google Scholar]
- 84.Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 85.Moin A., Deb T.K., Osmani R.A., Bhosale R.R., Hani U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J Basic Clin Pharm. 2016;7(2):39. doi: 10.4103/0976-0105.177705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J.B., Song N.N., Wei X.B., Guan H.S., Zhang X.M. Protective effects of paeonol on cultured rat hippocampal neurons against oxygen–glucose deprivation-induced injury. J Neurol Sci. 2008;264(1):50–55. doi: 10.1016/j.jns.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 87.Zhong S.Z., Ge Q.H., Qu R., Li Q., Ma S.P. Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by D-galactose in ICR mice. J Neurol Sci. 2009;277(1):58–64. doi: 10.1016/j.jns.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 88.Hsieh C.L., Cheng C.Y., Tsai T.H., Lin I.H., Liu C.H., Chiang S.Y. Paeonol reduced cerebral infarction involving the superoxide anion and microglia activation in ischemia-reperfusion injured rats. J Ethnopharmacol. 2006;106(2):208–215. doi: 10.1016/j.jep.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 89.Nizamutdinova I.T., Oh H.M., Min Y.N., Park S.H., Lee M.J., Kim J.S. Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-κB signaling pathways. Int Immunopharmacol. 2007;7(3):343–350. doi: 10.1016/j.intimp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Ma Y.L., Bates S., Gurney A.M. The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur J Pharmacol. 2006;545(2):87–92. doi: 10.1016/j.ejphar.2006.06.064. [DOI] [PubMed] [Google Scholar]
- 91.Sun G.P., Wang H., Xu S.P., Shen Y.X., Wu Q., Chen Z.D. Anti-tumor effects of paeonol in a hepa-hepatoma bearing mouse model via induction of tumor cell apoptosis and stimulation of IL-2 and TNF-α production. Eur J Pharmacol. 2008;584(2):246–252. doi: 10.1016/j.ejphar.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 92.Himaya S.W., Ryu B., Qian Z.J., Kim S.K. Paeonol from hippocampus kuda bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicol in Vitro. 2012;26(6):878–887. doi: 10.1016/j.tiv.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 93.Pan L.L., Dai M. Paeonol from paeonia suffruticosa prevents TNF-α-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine. 2009;16(11):1027–1032. doi: 10.1016/j.phymed.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 94.Li Y.J., Bao J.X., Xu J.W., Murad F., Bian K. Vascular dilation by paeonol—a mechanism study. Curr Vasc Pharmacol. 2010;53(3):169–176. doi: 10.1016/j.vph.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 95.Kim S.H., Kim S.A., Park M.K., Kim S.H., Park Y.D., Na H.J. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-α. Int Immunopharmacol. 2004;4(2):279–287. doi: 10.1016/j.intimp.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 96.Tsao J.Y., Tsai H.H., Wu C.P., Lin P.Y., Su S.Y., Chen L.D. Release of paeonol-β-CD complex from thermo-sensitive poly (N-isopropylacrylamide) hydrogels. Int J Pharm. 2010;402(1):123–128. doi: 10.1016/j.ijpharm.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 97.Tsai Y., Tsai H.H., Wu C.P., Tsai F.J. Preparation, characterisation and activity of the inclusion complex of paeonol with β-cyclodextrin. Food Chem. 2010;120(3):837–841. [Google Scholar]
- 98.Barzic A.I., Ioan S. Antibacterial drugs - From basic concepts to complex therapeutic mechanisms of polymer systems. In: Bobbarala V., editor. Concepts, compounds and the alternatives of antibacterials. InTechOpen; London: 2015. doi: [DOI] [Google Scholar]
- 99.Pappa K.A. The clinical development of mupirocin. J Am Acad Dermatol. 1990;22(5):873–979. doi: 10.1016/0190-9622(90)70116-y. [DOI] [PubMed] [Google Scholar]
- 100.Ward A., Campoli-Richards D.M. Mupirocin. Drugs. 1986;32(5):425–444. doi: 10.2165/00003495-198632050-00002. [DOI] [PubMed] [Google Scholar]
- 101.Conly J.M., Johnston B.L. Mupirocin – are we in danger of losing it? Can J Infect Dis Med Microbiol. 2002;13(3):157–159. doi: 10.1155/2002/692581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parenti M.A., Hatfield S.M., Leyden J.J. Mupirocin: a topical antibiotic with a unique structure and mechanism of action. Clin Pharm. 1987;6(10):761–770. [PubMed] [Google Scholar]
- 103.Cross S.E., Innes B., Roberts M.S., Tsuzuki T., Robertson T.A., McCormick P. Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol Physiol. 2007;20(3):148–154. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- 104.Kale S., Sonawane A., Ansari A.M., Ghoge P., Waje A.S. Formulation and in-vitro determination of sun protection factor of ocimum basilicum, Linn. leaf oils sunscreen cream. Int J Pharm Pharm Sci. 2010;2(4):147–149. [Google Scholar]
- 105.Burnett M.E., Wang S.Q. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27(2):58–67. doi: 10.1111/j.1600-0781.2011.00557.x. [DOI] [PubMed] [Google Scholar]
- 106.European Commission. Health and Consumer Protection Directorate-General. Opinion on benzophenone-3. 2008. Available fromhttp://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_159.pdf.
- 107.Pandit A.P., Patel S.A., Bhanushali V.P., Kulkarni V.S., Kakad V.D. Nebivolol-loaded microsponge gel for healing of diabetic wound. AAPS PharmSciTech. 2016:1–9. doi: 10.1208/s12249-016-0574-3. [DOI] [PubMed] [Google Scholar]
- 108.Aritomi H., Yamasaki Y., Yamada K., Honda H., Koshi M. Development of sustained release formulation of chlorpheniramine maleate using powder coated microsponges prepared by dry impact blending method. J Pharm Sci Technol. 1996;56:49–56. [Google Scholar]
- 109.Love FS, Taylor TS, Meeks RG, Alexander JL, Stavrakas KH. Nonwoven towel with microsponges. USA, 12/214,456[P].2008-6-19.
- 110.Sareen R., Nath K., Jain N., Dhar K.L. Curcumin loaded microsponges for colon targeting in inflammatory bowel disease: fabrication, optimization, and in vitro and pharmacodynamic evaluation. BioMed Res Int. 2014;2014:1–7. doi: 10.1155/2014/340701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tejashri G., Amrita B., Darshana J. Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm. 2013;63(3):335–358. doi: 10.2478/acph-2013-0021. [DOI] [PubMed] [Google Scholar]
- 112.Swaminathan S., Vavia P.R., Trotta F., Torne S. Formulation of betacyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem. 2007;57(1–4):89–94. [Google Scholar]
- 113.Kumar S., Pooja, Trotta F., Rao R. Encapsulation of babchi oil in cyclodextrin-based nanosponges: physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics. 2018;10(4):169. doi: 10.3390/pharmaceutics10040169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharma R., Pathak K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm Dev Technol. 2011;16(4):367–376. doi: 10.3109/10837451003739289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.