Highlights
-
•
PLGA microspheres based on exenatide encapsulated in nanoparticles were developed.
-
•
Ex-NPs-PLGA-Ms was prepared by modified S/O/W method with a low initial burst.
-
•
Ex-NPs-PLGA-Ms achieved zero-order controlled release.
-
•
S/O/W method could preserve the bioactivity of exenatide without cytotoxicity.
Keywords: Microspheres, PLGA, Peptides, Lipid nanoparticles, Sustained drug release
Abstract
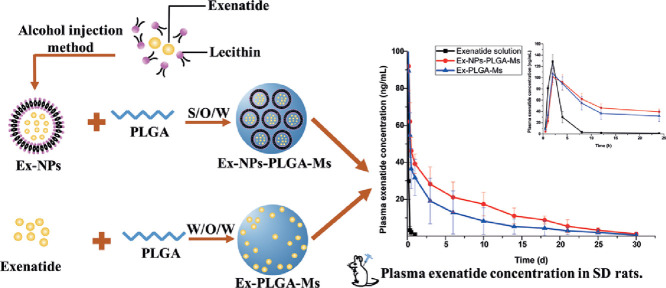
This study aimed to prepare poly (D, L-lactic-co-glycolic acid) microspheres (PLGA-Ms) by a modified solid-in-oil-in-water (S/O/W) multi-emulsion technique in order to achieve sustained release with reduced initial burst and maintain efficient drug concentration for a prolonged period of time. Composite PLGA microspheres containing exenatide-encapsulated lecithin nanoparticles (Ex-NPs-PLGA-Ms) were obtained by initial fabrication of exenatide-loaded lecithin nanoparticles (Ex-NPs) via the alcohol injection method, followed by encapsulation of Ex-NPs into PLGA microspheres. Compared to Ms prepared by the conventional water-in-oil-in-water (W/O/W) technique (Ex-PLGA-Ms), Ex-NPs-PLGA-Ms showed a more uniform particle size distribution, reduced initial burst release, and sustained release for over 60 d in vitro. Cytotoxicity studies showed that Ms prepared by both techniques had superior biocompatibility without causing any detectable cytotoxicity. In pharmacokinetic studies, the effective drug concentration was maintained for over 30 d following a single subcutaneous injection of two types of Ms formulation in rats, potentially prolonging the therapeutic action of Ex. In addition, administration of Ex-NPs-PLGA-Ms resulted in a more smooth plasma concentration-time profile with a higher area under the curve (AUC) compared to that of Ex-PLGA-Ms. Overall, Ex-NPs-PLGA-Ms prepared by the novel S/O/W method could be a promising sustained drug release system with reduced initial burst release and prolonged therapeutic efficacy.
Graphical abstract
1. Introduction
Type 2 diabetes is a chronic and progressive condition with the number of diabetic patients increasing in the recent years. The pathogenesis of type 2 diabetes is insulin resistance or hyposensitivity, leading to diminished insulin secretion [1], [2]. Exenatide (Ex), consisting of 39 amino acids and sharing 53% of sequence homology with the mammalian glucagon-like peptide-1 (GLP-1) [3], is the first incretin mimetics approved by FDA and European Medicines Agency for the treatment of type 2 diabetes. Commercial Ex formulations are currently injected via the subcutaneous route either twice daily for an immediate-release formulation (ByettaⓇ, Eli Lily/Amylin, 2005) or once weekly for extended-release suspensions (Bydureon™, AstraZeneca, 2012) [4]. In order to achieve enhanced therapeutic efficacy for a prolonged period of time and reduce the need for frequent injections, recent investigations have focused on development of long-acting drug delivery systems, such as poly (D, L-lactic-co-glycolic acid) microspheres (PLGA-Ms), in order to achieve enhanced drug bioavailability, sustained drug release, and reduced frequency of injections.
PLGA, one of the polymers approved by FDA for human uses, has gained tremendous attention due to its great biocompatibility and has been used as carrier matrix to encapsulate proteins/peptides as well as sustain their release [5]. Water-in-oil-in-water (W/O/W) double emulsion is a commonly used method to prepare protein/peptide loaded PLGA-Ms due to its simple preparation procedure [6]. However, the high initial burst and low encapsulation efficiency (EE) limit its application, as the encapsulated drugs could easily migrate to the external water phase during the fabrication process with eventually deposited on the surface of Ms. In addition, the structure and functionality of proteins/peptides may be changed due to exposure to organic solvents. Indeed, the initial burst may result in unwanted side effects due to the dramatically elevated drug concentration especially for the drugs with a narrow therapeutic interval and short half-lives [7]. The low EE and inactivation of drugs may also increase the costs as well as decrease the therapeutic efficacy.
In this study, a modified solid-in-oil-in-water (S/O/W) multi-emulsion technique was developed to prepare composite PLGA-Ms containing exenatide-loaded lecithin nanoparticles (Ex-NPs) and their physicochemical properties, including morphology, particle size, EE, and in vitro drug release, were compared to that of PLGA-Ms prepared by the conventional W/O/W method. The modified S/O/W technique was based on a combination of exenatide-loaded lecithin nanoparticles and PLGA-Ms, where Ex-NPs were fabricated by the alcohol injection method and were further encapsulated into PLGA-Ms. Egg yolk lecithin (PC-98T), a natural phospholipid extracted from egg yolk and approved by CFDA for injections [8], was used as the matrix of NPs [9], [10], [11]. The structural integrity of Ex extracted from two types of Ms was also analyzed by circular dichroism (CD) spectrum and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Moreover, cytotoxicity of Ms was evaluated in a human keratinocyte (HaCaT) cell line and in vivo pharmacokinetics of Ms after subcutaneous administration was assessed in Sprague-Dawley (SD) rats.
2. Materials and methods
2.1. Materials
Exenatide (Ex, purity 98.5%) was purchased from Jill Biochemical co., Ltd. (China). Poly D, L-lactic-co-glycolic acid (PLGA, Mw = 40,000; lactic: glycolic acid = 50:50) was obtained from Ji'nan Daigang Biology (China). Egg yolk lecithin (PC-98T) was gained from A.V.T. Pharmaceutical Co., Ltd. (China). Tert-butanol was purchased from Tianjin Zhiyuan Chemical Reagent Co., Ltd. (China). Dichloromethane (DCM), D- (+) -trehalose dihydrate, and poly vinyl alcohol (PVA 1788, Mw 74.9 kDa) were obtained from Aladdin Reagent co., Ltd. (China). Micro-BCA protein assay kit was purchased from CoWin Biotech Co., Ltd. (China). Sprague-Dawley (SD) rats were supplied by Guangdong Medical Laboratory Animal Center (China). All other reagents were analytical grade.
2.2. Preparation of Ex-NPs
Ex-NPs were prepared via the alcohol injection-lyophilization method. Briefly, Ex (2 mg) was firstly dissolved in 1.0 ml of trehalose solution (1 mg/ml) to obtain an aqueous phase, while egg yolk lecithin was dissolved in tert-butanol to form an organic phase (30 mg/ml). The organic phase was slowly dropped into the aqueous phase under stirring at 1200 rpm at 37 °C for 20 min. Afterwards, the mixture was snap frozen into liquid nitrogen and lyophilized to form freeze dried Ex-NPs (S). The particle size of Ex-NPs dispersed in deionized water was measured via dynamic light scattering (Malvern Master Sizer 2000, Malvern Instruments, UK). The morphology of Ex-NPs dispersed in DCM-acetone mixture was observed by transmission electron microscopy (TEM) (JEM-2100F, JEOL Ltd., Japan).
2.3. Preparation of Ex-NPS-loaded PLGA-Ms (Ex-NPs-PLGA-Ms) and Ex-loaded PLGA-Ms (Ex-PLGA-Ms)
Ex-NPs (10 mg, S) were resuspended uniformly in a mixture of DCM and acetone (2 ml, 1:1, v/v) containing PLGA (10%, w/v, O) and were immediately emulsified by adding into 20 ml PVA solution (5%, w/v, W). Afterwards, the resultant mixture was sonicated at a power of 300 W in ice bath for 30 s to obtain the S/O/W multi-emulsion. The emulsion was instantly transferred into a large flask containing 500 ml sodium chloride solution (5%, w/v) with constant agitation for 3 h to volatilize organic solvents and formulate Ms. Subsequently, Ms were collected by centrifugation and washing with deionized water for three times. Finally, Ex-NPs-PLGA-Ms were pre-frozen at −80 °C overnight and lyophilized.
Ex-PLGA-Ms prepared by conventional water-in-oil-in-water (W/O/W) multi-emulsion method were used as a comparision. Ex (2 mg) and trehalose were dissolved in deionized water to form an internal aqueous phase (W), which was then suspended in a mixture of DCM and acetone containing PLGA (10%, w/v, O) through vortex mixing to obtain a primary emulsion (W/O). Afterwards, the primary emulsion was rapidly dispersed in PVA solution (W) by ultra-sonication to form a W/O/W emulsion. Ex-PLGA-Ms were obtained and collected as mentioned above in the preparation of Ex-NPs-PLGA-Ms.
2.4. Morphology and particle size distribution
The morphology of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms was observed using scanning electron microscopy (SEM) (JSM-6330F, JEOL Ltd., Japan). The particle size and size distribution were measured via dynamic light scattering using Malvern Master Sizer 2000 (Malvern Instruments, UK). Polydispersity was determined by the span value expressed in
| (1) |
where DV,90%, DV,50%, and DV,10% are volume size diameters at 90%, 50%, and 10% of the cumulative volume, respectively. The smaller span value represents the narrower particle size distribution.
2.5. Drug loading content (DLC) and EE
To determine the DLC and EE of Ms, Ex-NPs-PLGA-Ms and Ex-PLGA-Ms (∼5 mg) were precisely weighed and dissolved in 2 ml 0.1 M NaOH solution containing 0.5% (w/v) of sodium dodecyl sulfate (SDS) in a centrifuge tube [12], [13], [14]. Afterwards, the mixture was vibrated slowly using an orbital shaker (HZS-H, Dongming medical instrument factory, China) at 37 °C for 24 h and centrifuged at 12 000 rpm for 10 min. Finally, the supernatant was collected and the concentration of Ex was measured by the micro-BCA assay (CWBIO, China). DLC and EE of Ms were determined using the following Eq. 2 and Eq. 3, respectively.
| (2) |
| (3) |
2.6. Fourier-transform infrared (FTIR) and circular dichroism (CD)
FTIR was performed to detect the interaction between proteins and polymer matrix. Ex, blank PLGA-Ms, Ex-NPs-PLGA-Ms, Ex-PLGA-Ms, and a physical mixture of Ex and PLGA (according to the ratio in Ms) were determined using a FTIR spectroscope (Bruker Tensor 37 spectrophotometer Bruker, Germany). Each sample was initially mixed with potassium bromide at a ratio of 1:30–1:100 and compressed into flakes for FTIR assay. The detection wavelength was in the range of 400–4000 cm−1 and the number of scans was 64 times with a resolution of 4 cm−1.
The influence of preparation process on the secondary structure of Ex was investigated by CD spectroscopy (Chirascan, Applied Photophysics Ltd., UK). Ex-NPs-PLGA-Ms and Ex-PLGA-Ms were weighed, dissolved in DCM, and centrifuged at 12,000 rpm for 5 min. The sediment was subsequently collected and dissolved in deionized water to obtain the sample. The secondary structure of extracted Ex was determined by CD assay and compared to that of the original Ex solution. The samples were measured at the detection wavelength of 180–260 nm under a nitrogen atmosphere using a quartz cell with a path length of 1.0 mm. Each sample was tested in triplicate.
2.7. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis
SDS-PAGE analysis was performed to detect the structural integrality of Ex prepared by the two techniques. Ex-NPs-PLGA-Ms and Ex-PLGA-Ms were weighed, dissolved in DCM, and extract Ex by adding PBS (pH 7.4) and vortex. The mixture was then centrifuged at 12 000 rpm for 10 min and the supernatant was obtained for SDS-PAGE analysis. All protein samples were heated up in boiling water for 5 min and electrophoresed at a constant voltage of 120 V. After migration, Coomassie Bright Blue was added to the gel for staining and revealing protein [15]. Finally, the gel was de-stained repeatedly until the protein strips became obvious and detected using GelDoc-IT Imaging System.
2.8. In vitro drug release
The amount of 5 mg Ms was dispersed in 1.0 ml PBS (pH 7.4) and was placed in an orbital shaker shaking at 100 rpm at 37 °C. At predetermined time points, the supernatant was withdrawn after centrifugation at 10,000 rpm for 5 min and the entire release medium was replaced with 1.0 ml fresh PBS to maintain the sink conditions [16]. The quantification of released Ex was carried out using a Microplate Absorbance Reader (BioTek ELX800, BioTek Instruments, Inc., Highland Park, USA). All tests were performed in triplicate.
2.9. Cytotoxicity
Cytotoxicity of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms was investigated using an MTT assay in a human keratinocyte (HaCaT) cell line [17]. The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% (v/v) fetal bovine serum and 1% penicillin and streptomycin under an atmosphere of 5% CO2 at 37 °C. HaCaT cells were then seeded in 96-well microplates at a concentration of 5 × 103 cells per well with 100 µl medium and incubated at 37 °C for 24 h. Subsequently, Ms suspensions with concentrations ranged from 0.25 to 2.0 mg/ml were added and incubated at 37 °C for 24 and 48 h. Afterwards, the culture medium was substituted by MTT solutions (5 mg/ml) and the microplates were further incubated at 37 °C for 4 h. After removal of the culture medium and adding dimethyl sulfoxide (DMSO), optical densities were measured using a microplate absorbance reader at a wavelength of 490 nm.
2.10. In vivo pharmacokinetics
Pharmacokinetics of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms were investigated in male Sprague-Dawley (SD) rats weighed between 250–300 g. All animal experimental procedures were in conformity with National Institute of Health and Nutrition Guidelines for the Care and Use of Laboratory Animals and authorized by the Ethical Committee on Animal Experimentation at Sun Yat-sen University. The animals housing under controlled conditions of 12/12 h light/dark cycle received food and water ad libitum. All animals acclimated for 1 week prior to studies.
Fifteen rats were randomly divided into three groups (n = 5) and administered Ex-NPs-PLGA-Ms, Ex-PLGA-Ms, or native Ex solution at 100 µg/kg via subcutaneous injections. Ms were uniformly resuspended in an injectable medium containing 0.75% (w/v) sodium carboxymethyl cellulose and 0.1% (w/v) Tween 20, and all formulations were sterilized by radiation for 60 min before injections. Blood samples were collected from the orbit of rats using ice-cold heparinized polyethylene tubes at predetermined times and immediately treated with aprotinin solution (2 mg/ml, 1/40 volume of blood). All the samples were centrifuged at 3500 rpm for 15 min and the supernatant was stored at −80 °C for future experiments. Quantitative analysis of Ex was performed using commercial exenatide-4 EIA kits (EK-070-94 425D, Phoenix Pharmaceuticals, Inc. USA).
2.11. Histology
Rats were sacrificed after completion of pharmacokinetics studies and their skin tissues at the subcutaneous injection site were collected, fixed with 10% formalin solution, embedded with paraffin, stained with hematoxylin and eosin (H&E), and imaged using a light microscope.
2.12. Statistical analysis
All experimental data were obtained at least in triplicates and expressed as mean values ± standard deviation (mean ± SD). Statistical significance was analyzed using one-way ANOVA with differences considered statistically significant if P < 0.05.
3. Results and discussion
3.1. Physicochemical properties
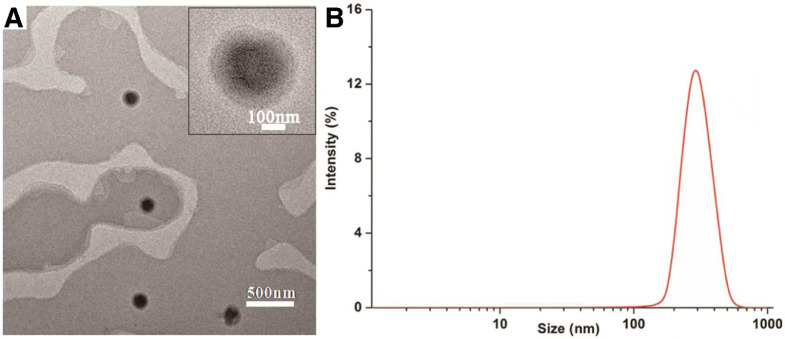
Ex-NPs appeared as dispersed solid spheres with an average particle size of 276.9 ± 4.6 nm (Fig. 1), suggesting that Ex-NPs have been successfully prepared by the alcohol injection-lyophilization method and their spherical morphology was well maintained after lyophilization.
Fig. 1.
(A) TEM image of lyophilized Ex-NPs dispersed in the mixture of DCM and acetone; A representative magnified image is displayed in the top right corner; scale bar = 500 nm. (B) Size distribution of Ex-NPs.
Physicochemical characteristics of Ms prepared by S/O/W and W/O/W methods, including particle size, Span values, and EE%, were evaluated and summarized in Table 1. Ms prepared by the S/O/W method showed a relatively large particle size (5.29 ± 0.98 µm) and a narrow size distribution with a Span value of 1.32 ± 0.01, indicating that the S/O/W method formulated Ms with a good uniformity of particle size.
Table 1.
Physicochemical characteristics of Ms prepared by S/O/W and W/O/W methods (mean ± SD, n = 3).
| Preparation method | DV,50 (µm) | Span value | DLC (%) | EE (%) |
|---|---|---|---|---|
| S/O/W | 5.29 ± 0.98 | 1.32 ± 0.01 | 0.66 ± 0.04 | 66.33 ± 3.75 |
| W/O/W | 4.82 ± 1.27 | 1.66 ± 0.03 | 0.62 ± 0.05 | 61.82 ± 4.85 |
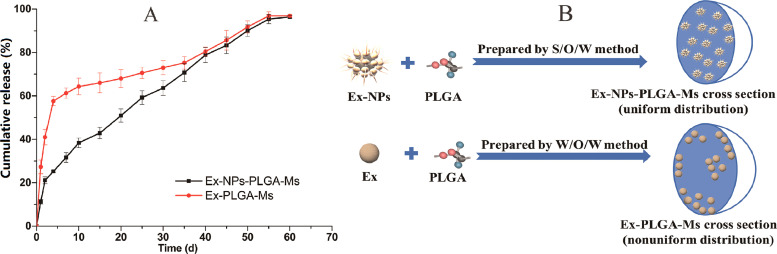
The morphology of Ms prepared by both S/O/W and W/O/W methods was investigated using SEM with images shown in Fig. 2. Ex-NPs-PLGA-Ms showed a more uniform size distribution and more even spherical shapes (Fig. 2A) compared to Ex-PLGA-Ms (Fig. 2A). This might be attributed to the superior surfactant and amphoteric properties of lecithin originating from hydrophilic phosphates and hydrophobic fatty acid groups, which could reduce the interfacial tension between oil and water, allowing a quick formation of uniform multi-emulsion [18]. On the contrary, the hydrophilic internal water phase of double emulsions was not well dispersed in the PLGA solution during the preparation process of MS via the W/O/W method, which could hinder the formation of uniform particles [19]. Thus, Ex-NPs-PLGA-Ms showed a more uniform particle size distribution compared to Ex-PLGA-Ms.
Fig. 2.
SEM images of Ms prepared by S/O/W (A) and W/O/W (B) methods.
The EE of Ex-NPs-PLGA-Ms was 66.33% ± 3.75%, which was higher than that of Ex-PLGA-Ms (61.82% ± 4.85%). It was speculated that water-soluble Ex was easily diffused to the external water phase during the process of preparation via the W/O/W method, leading to drug absorption on the surface of Ms [20]. For Ms prepared by the modified S/O/W method, on the other hand, Ex was initially encapsulated and stabilized into nanoparticles, resulting in an even drug distribution in Ms and increased EE%.
3.2. FTIR and CD analysis
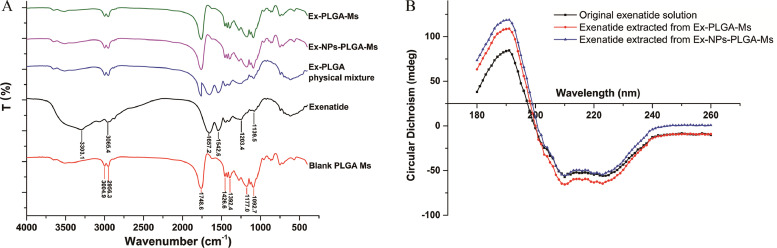
FTIR spectroscopy was used to investigate interactions between drug molecules and PLGA polymers. As shown in Fig. 3A, Ex was a macromolecular peptide, exhibiting hydrogen-bonded stretching vibrations υN-H (3303.1 cm−1) and υC-H (3065.4 cm−1) as well as characteristic absorption peaks of peptides (υC=O stretching vibration at 1657.2 cm−1, δN-H in-plane bending vibration and υC-N stretching vibration at 1542.6 cm−1, υC-C=O stretching vibration peak at 1203.4 cm−1, and δC—C skeleton stretching vibration at 1139.5 cm−1) [21]. Blank PLGA Ms showed characteristic hydrocarbon absorption peaks at 3004.9–2956.3 cm−1 and 1748.6 cm−1 originating from υC-N stretching bands and ester functional groups of υC=O stretching bands, respectively [22]. All the typical bands of Ex and PLGA were observed in the spectrum of their physical mixture, demonstrating that no interactions were formed between Ex and PLGA [23]. On the contrary, no characteristic absorption peaks of Ex were shown in the spectra of Ms prepared by the two methods, while all the PLGA characteristic absorption peaks were found without obvious chemical shifts. These results indicated that Ex was completely encapsulated into PLGA matrix with both modified S/O/W and W/O/W methods showing efficient drug loading. Therefore, Ex was well surrounded by a protective PLGA layer that prevented Ex from rapid degradation in the preparation process of Ms.
Fig. 3.
(A) FTIR spectra of Ex, PLGA, physical mixture of Ex and PLGA, Ex-NPs-PLGA-Ms, and Ex-PLGA-Ms. (B) Far-UV CD spectrum of original Ex solution and Ex extracted from Ex-NPs-PLGA-Ms as well as Ex-PLGA-Ms.
It is well known that CD could determine the α-helix (double negative peaks at 208 and 223 nm) and β-sheet (positive peak at 192 nm) structures of peptides [24]. In this study, Ex extracted from both Ex-NPs-PLGA-Ms and Ex-PLGA-Ms was analyzed by CD spectroscopy to identify if its unique secondary/tertiary structures were changed during the preparation process of Ms, which may affect biological activities of Ex. As shown in Fig. 3B, two minima at 208 and 223 nm with double negative peaks were observed in the spectrum of original Ex solutions, indicating a typical α-helix structure [25]. Compared to the original Ex solution, there were no remarkable changes in the secondary structure of Ex extracted from Ms prepared by two methods, suggesting that both S/O/W and W/O/W preparation techniques did not affect the chemical construction of Ex and thereby preserving the biological functionality of encapsulated Ex.
3.3. SDS-PAGE analysis
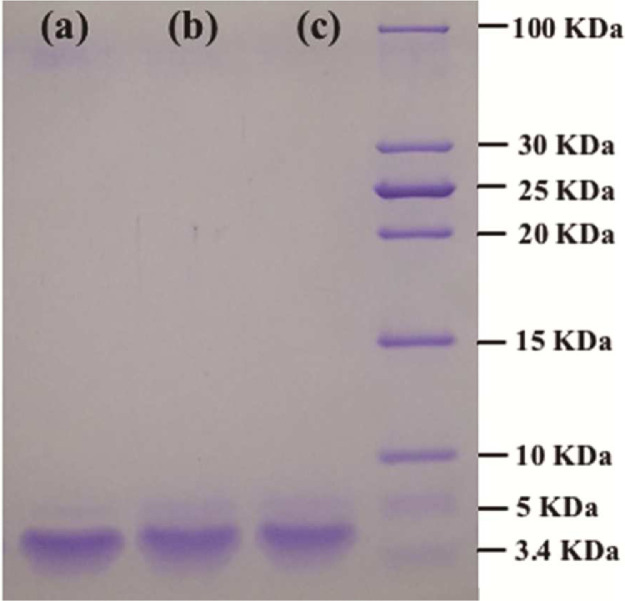
As shown in Fig. 4, the characteristic bands of Ex were presented in all the groups with their positions remaining consistent, suggesting that the molecular weight and structural integrity of Ex were not changed during the preparation of Ms via both S/O/W and W/O/W methods. This could be due to the present of cryoprotectant trehalose, which formed hydrogen bonding with polar head groups of amino acids to protect encapsulated Ex during the process of lyophilization [16], [29], [30].
Fig. 4.
SDS-PAGE analysis of original Ex (a) and Ex extracted from Ex-NPs-PLGA-Ms (b) as well as Ex-PLGA-Ms (c).
3.4. In vitro drug release
In vitro drug release of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms in PBS (pH 7.4) medium was investigated. As shown in Fig. 5A, Ex was slowly released from Ms prepared by both methods for over 60 d The drug release profile of Ex-PLGA-Ms showed a tri-phase pattern with an obvious initial burst release (27.32% ± 3.21%) observed within 24 h. In the first 4 d, drugs were rapidly released from Ex-PLGA-Ms with the pattern closely matching the Higuchi model. Afterwards, a lag phase was subsequently observed between day 4 and 35 due to the slow diffusion of encapsulated drugs from Ms. PLGA carrier matrix was continuously hydrolyzed, resulting in a direct contact between internal pores and the release medium. In the following phase, the rate of drug release gradually accelerated until encapsulated Ex was completely released from Ms, with the pattern fitting the first-order model. The obvious initial burst of Ex-PLGA-Ms was presumably attributed to the rapid escape of drugs adsorbed on the surface or leakage through the water channels of Ms [26]. Drugs could easily migrate to the external water phase during the fabrication process of MS via the W/O/W method due to the high hydrophilicity of Ex, resulting in an uneven drug distribution in Ex-PLGA-Ms with drugs adsorbed on the surface. In addition, as shown in Fig. 2 and Table 1, Ex-PLGA-Ms prepared via the conventional W/O/W technique possessed a relatively small particle size with a large surface area and irregular aggregates, which could lead to remarkable initial burst and insufficient therapeutic concentrations of drugs released from microspheres in later stages.
Fig. 5.
(A) In vitro drug release profiles of Ms prepared by S/O/W and W/O/W methods (n = 3). (B) A schematic diagram of the mechanism resulting in different structure of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms.
In contrast, drugs were constantly and slowly released from Ex-NPs-PLGA-Ms with a reduced initial burst release (less than 11.90% ± 1.73%) within 24 h. The overall drug release profile closely matched to the Ritger–Peppas model with the fitting equation showing as follows:
| (4) |
The fitting results indicated that the mechanism of drugs released from Ex-NPs-PLGA-Ms was a combination of Fick's diffusion and erosion of skeleton structures. Specifically, the mechanism of Ex diffusion accelerated erosion of the PLGA skeleton, which in turn slowed down the migration of Ex to the external surface of PLGA skeletons [27]. The initial burst of Ex-NPs-PLGA-Ms was thus greatly reduced compared to that of Ex-PLGA-Ms. The immobility of Ex and its uniform distribution in Ex-NPs-PLGA-Ms could also contribute to the constantly slow drug release (Fig. 5B) and thereby avoid potential risks of excess drugs at the initial stage and insufficient therapeutic concentrations at later stages as well as peak-valley fluctuations [19]. Also, lecithin in Ex-NPs might affect the properties of Ms polymer matrix, slowing down the hydrolysis process of Ms and prolonging the duration of sustained drug release [28]. Overall, Ms prepared by both techniques achieved sustained drug release over 60 d and Ex-NPs-PLGA-Ms exhibited a more constant drug release profile with reduced initial burst compared to Ex-PLGA-Ms.
3.5. Cytotoxicity
Cytotoxicity of HaCaT cells after incubation with Ex-NPs-PLGA-Ms and Ex-PLGA-Ms was investigated. As shown in Fig. 6, cell viability of HaCaT cells was more than 90% in both groups treated with Ms at all the concentrations between 0.25–2.0 mg/ml at 24 and 48 h post treatment, suggesting negligible cytotoxicity and desirable biocompatibility of Ex-NPs-PLGA-Ms and Ex-PLGA-Ms. It is commonly believed that PLGA polymers with superior biocompatibility and safety can be used as an injectable carrier material [31], [32]. Cytotoxicity results in this work further support the safety of PLGA based Ms.
Fig. 6.
Cell viability of HaCaT cells after 24 (A) and 48 h (B) of incubation with Ex-NPs-PLGA-Ms and Ex-PLGA-Ms (mean ± SD, n = 6).
3.6. In vivo pharmacokinetics
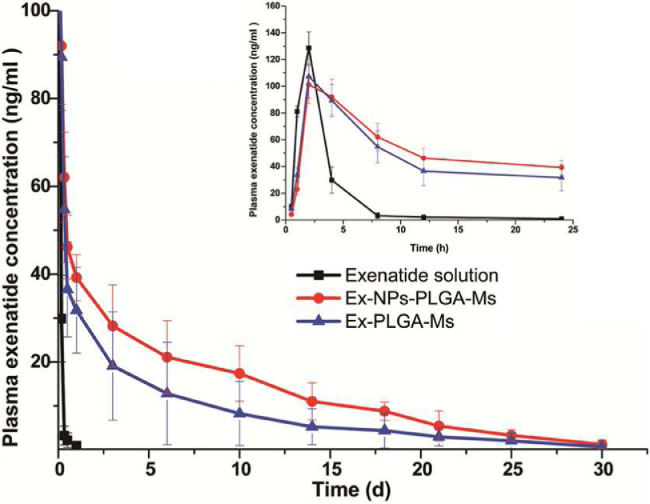
In vivo studies were performed in SD rats and pharmacokinetic parameters of formulations were summarized in Table 2. Both Ex-NPs-PLGA-Ms and Ex-PLGA-Ms achieved sustained drug release over 30 d in vivo after a single injection (Fig. 7). According to the instruction of ByettaⓇ, the minimal dose of Ex to achieve effective glycemic control was approximately 1.04 ng/ml. The Ex concentration in plasma reached an extremely high Cmax of 128.72 ± 12.31 ng/ml with a short T1/2 of 11.33 ± 10.39 h after injection of Ex solution due to the rapid absorption and elimination, which may lead to inefficient treatment. Two types of Ms, on the other hand, exhibited sustained drug release behavior for up to 30 d in vivo. The Cmax in the group treated with Ex solution was slightly higher than that in the other groups received Ms (106.14 ± 10.31 ng/ml for Ex-NPs-PLGA-Ms and 109.08 ± 14.16 ng/ml for Ex-PLGA-Ms), however, the T1/2 was much shorter than that in the Ms formulation groups (108.41 ± 16.22 h for Ex-NPs-PLGA-Ms and 109.20 ± 17.50 h for Ex-PLGA-Ms). Therefore, both Ex-NPs-PLGA-Ms and Ex-PLGA-Ms could potentially provide long-term therapeutic efficacy with reduced frequency of injection. It is worth mentioning here that the plasma drug concentration in the Ex-NPs-PLGA-Ms group was higher than that in Ex-PLGA-Ms group at 4 h post injection, which could be due to the initial burst release and rapid drug clearance within the first 2 h. Moreover, the AUC0-∞ in the group treated with Ex-NPs-PLGA-Ms was 10,298.89 ng·h/ml, which was significantly higher than that in the group received Ex-PLGA-Ms (6408.19 ng·h/ml) (P < 0.01). This could be due to the uniform distribution of Ex-NPs in the PLGA matrix and constantly slow Ex release from Ex-NPs-PLGA-Ms for 4 weeks.
Table 2.
Pharmacokinetic parameters of Ex solution and Ex-NPs-PLGA-Ms as well as Ex-PLGA-Ms after subcutaneous administration in SD rats (mean ± SD, n = 5).
| Pharmacokinetic parameters | Ex solution | Ex-NPs-PLGA-Ms | Ex-PLGA-Ms |
|---|---|---|---|
| Cmax (ng/ml) | 128.72 ± 12.31 | 106.14 ± 10.31 | 109.08 ± 14.16 |
| T1/2 (h) | 11.33 ± 10.39 | 108.41 ± 16.22 | 109.20 ± 17.50 h |
| AUC0-∞ (ng·h/ml) | 393.65 ± 45.33 | 10,298.89 ± 1027.10 | 6408.19 ± 2775.31 |
| MRT(h) | 3.22 ± 0.43 | 196.88 ± 8.88 | 155.22 ± 43.17 |
Fig. 7.
Ex concentration-time curve in plasma after subcutaneous administration in SD rats; the insert presents Ex concentration-time curve in plasma within the first 24 h post injection (mean ± SD, n = 5).
Compared to in vitro data, Ms prepared by both techniques degraded faster in vivo. The predominant mechanism of drug release in vitro was degradation of PLGA carrier matrix and Ex diffusion; however, the complex in vivo environment with non-specific enzymatic catalysis and fluid infiltration in the surroundings could further accelerate the process [33], [34]. In comparison with Ex-NPs-PLGA-Ms, the contact surface area of Ex-PLGA-Ms with enzymes in vivo could be greater due to their smaller particle size. Therefore, the burst initial release of Ex-PLGA-Ms might be affected by enzymatic catalysis in vivo more obviously, particularly within the first 2 h. On the contrary, the rate of drug release from Ex-NPs-PLGA-Ms was constantly slow, which could avoid unwanted side effects, such as hypoglycemia [35], induced by peaks and valleys in blood glucose levels. Overall, Ex-NPs-PLGA-Ms with great biocompatibility and low toxicity could be a promising drug delivery system for the long-term treatment of diabetes.
3.7. Histology
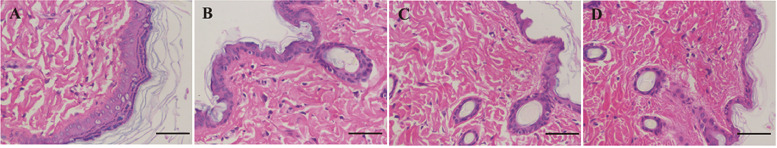
Histological examination was performed to evaluate the biocompatibility of Ms. As shown in Fig. 8, the tissue at the injection site has recovered with no pathological changes including plasma cell infiltration, local lymphocytic infiltration, and capillary hyperplasia observed in all the groups. Although PLGA polymer may cause damage to the tissue due to their slow degradation and generated acidic oligomers, it was expected that PLGA based formulations with low doses would not result in detectable damage [35], [36]. Therefore, Ms prepared by both methods were biocompatible and safe for subcutaneous injection.
Fig. 8.
Histological examination of tissues collected at the injection site in SD rats. (A) Saline; (B) Ex solution; (C) Ex-NPs-PLGA-Ms; (D) Ex-PLGA-Ms; scale bar = 200 nm.
4. Conclusion
In this study, a modified S/O/W multi-emulsion method was developed to prepare composite PLGA microspheres containing exenatide loaded nanoparticles (Ex-NPs-PLGA-Ms). Compared to Ms prepared by the conventional (W/O/W) multi-emulsion method, Ex-NPs-PLGA-Ms showed a uniform particle size distribution and in vitro sustained drug release for 60 d with reduced initial burst. Moreover, Ex was constantly and slowly released from Ex-NPs-PLGA-Ms for 4 weeks in vivo after a single injection via the subcutaneous route. In conclusion, Ex-NPs-PLGA-Ms prepared by the newly developed S/O/W method could be a promising formulation for treatment of type 2 diabetes.
Acknowledgments
Conflict of interest
There is no conflict of interest for this article.
Acknowledgments
This work was funded by the China Postdoctoral Science Foundation (Grant No. 2016M602442), the Science and Technology Plan Projects of Guangdong Province (Grant No. 2015B020232010), the 111 project (Grant No. B16047), and the Natural Science Fund Project of Guangdong Province (Grant No. 2018A030310555, Grant No. 2016A030312013).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2019.01.002.
Contributor Information
Chune Zhu, Email: chunezhu1108@gdut.edu.cn.
Xin Pan, Email: panxin2@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Noguchi H. Stem cell applications in diabetes. J Stem Cells. 2012;7:229–244. [PubMed] [Google Scholar]
- 2.Hinton P.S. Role of reduced insulin-stimulated bone blood flow in the pathogenesis of metabolic insulin resistance and diabetic bone fragility. Med Hypotheses. 2016;93:81–86. doi: 10.1016/j.mehy.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Tonneijck L., Smits M.M., Muskiet M.H.A., Hoekstra T., Kramer M.H.H., Danser A.H.J. Acute renal effects of the GLP-1 receptor agonist exenatide in overweight type 2 diabetes patients: a randomised, double-blind, placebo-controlled trial. Diabetologia. 2016;59:1412–1421. doi: 10.1007/s00125-016-3938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott L.J. Exenatide extended-release: a review of its use in type 2 diabetes mellitus. Drugs. 2012;72:1679–1707. doi: 10.2165/11209750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Kang J., Wu F., Cai Y., Xu M., He M., Yuan W. Development of recombinant human growth hormone (rhGH) sustained-release microspheres by a low temperature aqueous phase/aqueous phase emulsion method. Eur J Pharm Sci. 2014;62:141–147. doi: 10.1016/j.ejps.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Lin Q., Cai Y., Yuan M., Ma L., Qiu M., Su J. Development of a 5-fluorouracil-loaded PLGA microsphere delivery system by a solid-in-oil-in-hydrophilic oil (S/O/hO) novel method for the treatment of tumors. Oncol Rep. 2014;32:2405–2410. doi: 10.3892/or.2014.3480. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A.S., Sapin A., Damgé C., Leroy P., Socha M., Maincent P. Reduction of the in vivo burst release of insulin-loaded microparticles. J Drug Deliv Sci Technol. 2015;30:486–493. [Google Scholar]
- 8.Gładkowski W., Chojnacka A., Kiełbowicz G., Trziszka T., Wawrzeńczyk C. Isolation of pure phospholipid fraction from egg yolk. J Am Oil Chem Soc. 2012;89:179–182. [Google Scholar]
- 9.Li J., Wang X., Zhang T., Wang C., Huang Z., Luo X. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10:81–98. [Google Scholar]
- 10.Singh R.P., Gangadharappa H.V., Mruthunjaya K. Phospholipids: unique carriers for drug delivery systems. J Drug Deliv Sci Tec. 2017;39:166–179. [Google Scholar]
- 11.Wałęsa R., Man D., Engel G., Siodłak D., Kupka T., Ptak T. The impact of model peptides on structural and dynamic properties of egg yolk lecithin liposomes-experimental and DFT studies. Chem Biodivers. 2015;12:1007–1024. doi: 10.1002/cbdv.201400179. [DOI] [PubMed] [Google Scholar]
- 12.Samadi N., Abbadessa A., Di Stefano A., van Nostrum C.F., Vermonden T., Rahimian S. The effect of lauryl capping group on protein release and degradation of poly(D,L-lactic-co-glycolic acid) particles. J Control Release. 2013;172:436–443. doi: 10.1016/j.jconrel.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Cho K., Choi S., Kim C., Nam Y., Park T., Park J. Protein release microparticles based on the blend of poly(D,L-lactic-co-glycolic acid) and oligo-ethylene glycol grafted poly(L-lactide) J Control Release. 2001;76:275–284. doi: 10.1016/s0168-3659(01)00442-4. [DOI] [PubMed] [Google Scholar]
- 14.Genta I., Perugini P., Pavanetto F., Maculotti K., Modena T., Casado B. Enzyme loaded biodegradable microspheres in vitro-ex vivo evaluation. J Control Release. 2001;77:287–295. doi: 10.1016/s0168-3659(01)00511-9. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.Y., Lee H., Oh K.S., Kweon S., Jeon O.C., Byun Y. Multilayer nanoparticles for sustained delivery of exenatide to treat type 2 diabetes mellitus. Biomaterials. 2013;34:8444–8449. doi: 10.1016/j.biomaterials.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Li L., Wang X., Ren Y., Zhou T., Lu W. Application of model-based methods to characterize exenatide-loaded double-walled microspheres: in vivo release, pharmacokinetic/pharmacodynamic model, and in vitro and in vivo correlation. J Pharm Sci. 2012;101:3946–3961. doi: 10.1002/jps.23236. [DOI] [PubMed] [Google Scholar]
- 17.Saïdi L., Vilela C., Oliveira H., Ajd S., Csr F. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohyd Polym. 2017;169:357–365. doi: 10.1016/j.carbpol.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Shah P.R., Gaitonde U.N., Ganesh A. Influence of soy-lecithin as bio-additiive with straight vegetable oil on CI engine characteristics. Renew Energ. 2018;115:685–696. [Google Scholar]
- 19.Chen L., Mei L., Feng D., Huang D., Tong X., Pan X. Anhydrous reverse micelle lecithin nanoparticles/PLGA composite microspheres for long-term protein delivery with reduced initial burst. Colloid Surf B. 2017;163:146–154. doi: 10.1016/j.colsurfb.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Duncanson W.J., Arriaga L.R., Ung W.L., Kopechek J.A., Porter T.M., Weitz D.A. Microfluidic fabrication of perfluorohexane-shelled double emulsions for controlled loading and acoustic-triggered release of hydrophilic agents. Langmuir. 2014;30:13765–13770. doi: 10.1021/la502473w. [DOI] [PubMed] [Google Scholar]
- 21.Wang M., Lu X., Yin X., Tong Y., Peng W., Wu L. Synchrotron radiation-based Fourier-transform infrared spectromicroscopy for characterization of the protein/peptide distribution in single microspheres. Acta Pharm Sin B. 2015;5:270–276. doi: 10.1016/j.apsb.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C., Huang Y., Zhang X., Mei L., Pan X., Li G. Comparative studies on exenatide-loaded poly (d, l -lactic-co-glycolic acid) microparticles prepared by a novel ultra-fine particle processing system and spray drying. Colloid Surf B. 2015;132:103–110. doi: 10.1016/j.colsurfb.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Sinha B., Mukherjee B., Pattnaik G. Poly-lactide-co-glycolide nanoparticles containing voriconazole for pulmonary delivery: in vitro and in vivo study. Nanomed-Nanotechnol. 2013;9:94–104. doi: 10.1016/j.nano.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Liu D., Wu P., Jiao P. Researching rumen degradation behaviour of protein by FTIR spectroscopy. Czech J Anim Sci. 2015;60:25–32. [Google Scholar]
- 25.Das N.K., Pawar L., Kumar N., Mukherjee S. Quenching interaction of BSA with DTAB is dynamic in nature: a spectroscopic insight. Chem Phys Lett. 2015;635:50–55. [Google Scholar]
- 26.Nijdam J., Trouillet V., Kachel S., Scharfer P., Schabel W., Kind M. Coat formation of surface-active proteins on aqueous surfaces during drying. Colloid Surf B. 2014;123:53–60. doi: 10.1016/j.colsurfb.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 27.Hirota K., Doty A.C., Ackermann R., Zhou J., Olsen K.F., Feng M.R. Characterizing release mechanisms of leuprolide acetate-loaded PLGA microspheres for IVIVC development I: in vitro evaluation. J Control Release. 2016;244:302–313. doi: 10.1016/j.jconrel.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Kastellorizios M., Tipnis N., Papadimitrakopoulos F., Burgess D.J. Drug distribution in microspheres enhances their anti-inflammatory properties in the gottingen minipig. Mol Pharmaceut. 2015;12:3332–3338. doi: 10.1021/acs.molpharmaceut.5b00326. [DOI] [PubMed] [Google Scholar]
- 29.Vilén E.M., Sandström C. NMR study on the interaction of trehalose with lactose and its effect on the hydrogen bond interaction in lactose. Molecules. 2013;18:9735–9754. doi: 10.3390/molecules18089735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreani T., Kiill C.P., Souza A.L.R.D., Fangueiro J.F., Doktorovová S., Garcia M.L. Effect of cryoprotectants on the reconstitution of silica nanoparticles produced by sol–gel technology. J Therm Anal Calorim. 2015;120:1001–1007. [Google Scholar]
- 31.Ding Y., Wang Y., Opokudamoah Y., Wang C., Shen L., Yin L., Zhou J. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi: 10.1016/j.biomaterials.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 32.He Z., Wang Q., Sun Y., Shen M., Zhu M., Gu M. The biocompatibility evaluation of mPEG-PLGA-PLL copolymer and different LA/GA ratio effects for biocompatibility. J Biomat Sci-Polym E. 2014;25:943–964. doi: 10.1080/09205063.2014.914705. [DOI] [PubMed] [Google Scholar]
- 33.Tomic I., Vidismillward A., Muellerzsigmondy M., Cardot J.M. Setting accelerated dissolution test for PLGA microspheres containing peptide, investigation of critical parameters affecting drug release rate and mechanism. Int J Pharm. 2016;505:42–51. doi: 10.1016/j.ijpharm.2016.03.048. [DOI] [PubMed] [Google Scholar]
- 34.Szlęk J., Pacławski A., Lau R., Jachowicz R., Mendyk A. Heuristic modeling of macromolecule release from PLGA microspheres. Int J Nanomedicine. 2013;8:4601–4611. doi: 10.2147/IJN.S53364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y., Sun T., Zhang Y., Chaurasiya B., Huang L., Liu X. Exenatide loaded PLGA microspheres for long-acting antidiabetic therapy: preparation, characterization, pharmacokinetics and pharmacodynamics. RSC Adv. 2016;6:37452–37462. [Google Scholar]
- 36.Wang M., Feng Q., Guo X., She Z., Tan R. A dual microsphere based on PLGA and chitosan for delivering the oligopeptide derived from BMP-2. Polym Degrad Stabil. 2011;96:107–113. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.