Abstract
The therapeutic potential of saquinavir, a specific inhibitor of human immunodeficiency virus (HIV)-1 and HIV-2 protease enzymes, has been largely limited because of a low solubility and consequnt low bioavailability. Thus, we aimed to design a supersaturated self-microemulsifying drug delivery system (S-SMEDDS) that can maintain a high concentration of saquinavir in gastro-intestinal fluid thorugh inhibiting the drug precipitation to enhance the lymphatic transport of saquinavir and to increase the bioavailability of saquinavir considerably. Solubilizing capacity of different oils, surfactants, and cosurfactants for saquinavir was evaluated to select optimal ingredients for preparation of SMEDDS. Through the construction of pseudo-ternary phase diagram, SMEDDS formulations were established. A polymer as a precipitation inhibitor was selected based on its viscosity and drug precipitation inhibiting capacity. The S-SMEDDS and SMEDDS designed were administered at an equal dose to rats. At predetermined time points, levels of saquinavir in lymph collected from the rats were assessed. SMEDDS prepared presented a proper self-microemulsification efficiency and dispersion stability. The S-SMEDDS fabricated using the SMEDDS and hydroxypropyl methyl cellulose 2910 as a precipitation inhibitor exhibited a signficantly enhanced solubilizing capacity for saquinavir. The drug concentration in a simulated intestinal fluid evaluated with the S-SMEDDS was also maintained at higher levels for prolonged time than that examined with the SMEDDS. The S-SMEDDS showed a considerably enhanced lymphatic absoprtion of saquinavir in rats compared to the SMEDDS. Therefore, the S-SMEDDS would be usefully exploited to enhance the lymphatic absorption of hydrophobic drugs that need to be targeted to the lymphatic system.
Keywords: Lymphatic drug delivery, Self-microemulsifying drug delivery system, Saquinavir, Precipitation inhibitor, Supersaturation, Lipid-based formulation
Graphical abstract
Supersaturated self-microemulsifying drug delivery systems designed in this study were demonstrated to enhance the intestinal lymphatic absorption of saquinavir in vivo through inhibiting the drug precipitation in gastrointestinal fluid.
1. Introduction
Saquinavir is an antiretroviral drug that specifically inhibits human immunodeficiency virus (HIV)-1 and HIV-2 protease enzymes. The oral bioavailability of saquinavir has been known to be very low because of its hydrophobic property and consequent low aqueous solubility and dissolution rate. In addition, saquinavir undergoes the extensive first pass metabolism when absorbed into the systemic circulation via the gastrointestinal (GI) tract [1]. For these reasons, the therapeutic potential of saquinavir has been considerably limited.
Intestinal lymphatic drug transport can be useful to enhance the bioavailability of saquinavir because drugs absorbed through the intestinal lymphatic pathway avoid the first pass metabolism [2]. In addition, the lymphatic transport of saquinavir would be beneficial for treatment of acute infection of HIV as the viruses actively replicate in the lymphatic system and colonize lymphoid organs from the early infection to the latent stage [3] The highly lipophilic property of saquinavir is also advantageous to be associated with chylomicrons composed of triglyceride, phospholipids, cholesterol and proteins in intestinal enterocytes, which is essential for the intestinal lymphatic absorption of drugs [2].
To promote the intestinal lymphatic uptake of saquinavir, lipid-based pharmaceutical formulations could be exploited. The lipid ingredients of the formulations are used for the production of chylomicrons when absorbed into the enterocytes, and in this process drugs dissolved or dispersed in the lipids would be integrated into chylomicrons [4]. Among diverse lipid-based pharmaceutical formulations, self-microemulsifying drug delivery systems (SMEDDS), an isotropic mixture of oil, surfactant, and possibly co-surfactant, are considered to be promising to enhance the lymphatic drug absorption [5]. After oral administration of SMEDDS, the oil ingredients dissolving drugs are spontaneously dispersed in the GI tract, consequently forming fine oil droplets [6]. Owing to high solubilizing capacity and large surface area of the oil droplets, the dissolution and absorption rates of hydrophilic drugs can be considerably increased. Furthermore, the oil ingredients may promote the association of lipophilic drugs with chylomicrons, thereby promoting the lymphatic drug absorption [4].
However, a metastable saturated state of saquinavir in SMEDDS formulations possibly causes the precipitation of the drug due to an insufficient solubilizing capacity of SMEDDS [7]. In addition, saquinavir could be precipitated in the GI tract after being exposed to the GI fluid, leading to the decreased absorption of the drugs into the lymphatic system [8]. To prevent the precipitation of hydrophobic drugs in the GI fluid, SMEDDS have been formulated with polymers capable of inhibiting the drug precipitation, which are generally called supersaturated SMEDDS (S-SMEDDS). Many studies have reported that the systemic absorption of hydrophobic drugs was considerably increased by S-SMEDDS because drug concentrations in the GI fluid were able to be maintained at high levels by the polymers contained in S-SMEDDS [7], [9], [10]. However, to the best of our knowledge, S-SMEDDS have not been investigated yet for the purpose of enhancing the lymphatic drug absorption. We hypothesized that the intestinal lymphatic absorption of saquinavir could be greatly increased by S-SMEDDS because the elevated drug levels in the GI fluid would lead to increase in the drug absorption to the enterocytes and the lymphatic system.
In the present study, therefore, we aimed to design supersaturated SMEDDS (S-SMEDDS) for efficient lymphatic transport of saquinavir. Based on the solubilizing capacity and the self-emulsification efficiency, we selected components for preparation of SMEDDS of saquinavir such as suitable oils, surfactants, and co-surfactants. Polymeric materials were then employed as a precipitation inhibitor to enhance the stability of the supersaturated state of saquinavir in SMEDDS. The lymphatic absorption of saquinavir through the S-SMEDDS was evaluated using a rat model.
2. Material and methods
2.1. Materials
Saquinavir was purchased from Vivagen Co., Ltd. (Seongnam, Korea). Capryol™ 90, Labrasol®, propylene glycol, Tween® 80, Labrafac® CM 10, and urethane (ethyl carbamate) were obtained from Daejung Chemicals & Metal Co., Ltd. (Siheung, Korea). Cremophor® ELP, polyvinylpyrrolidone (PVP) K90, PVP K30, hydroxypropyl methylcellulose (HPMC) 2910, poloxamer 407, poloxamer 188, copovidone, and polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®) were purchased from BASF (Cleveland, OH, USA). Polyethylene glycol (PEG) 400 was provided by Samchun Pure Chemical (Pyeongtaek, Korea). Transcutol® HP was obtained from Gattefosse (Lyon, France). Male specific pathogen-free/vial antibody-free outbred Sprague-Dawley (SD) rats (275–300 g) were purchased from Orient Bio Inc. (Seongnam, Korea). All solvents used in the experiments were of high-performance liquid chromatography (HPLC) grade.
2.2. Solubility testing of saquinavir in oils, surfactants, and co-surfactants
Solubility of saquinavir in various oils, surfactants, and co-surfactants shown in Table 1 was evaluated to select optimal components for preparation of saquinavir SMEDDS. Excessive amount of saquinavir was added to each ingredient, and the mixtures were agitated at 20 °C for 24 h on a magnetic stirrer. The mixtures were then centrifuged at 2000 × g for 1 h. The level of saquinavir in the supernatants was determined using Breeze™ 2 HPLC system, (Waters, Milford, MA, USA), Hypersil GOLD™ C18 column (250 mm × 4.6 mm, 5 µm particle size, Thermo Fisher Scientific Inc., Waltham, MA, USA), and the mobile phase consisting of 0.1% phosphoric acid: acetonitrile (4: 6) at a flow rate of 1 ml/min. The retention time of saquinavir was 3.8 min, and the detection wavelength was 240 nm.
Table 1.
Solubility of saquinavir evaluated in different oils, surfactants, and cosurfactants.
| Materials | Classification | Solubility of saquinavir (mg/g) |
|---|---|---|
| Water | Aqueous phase | 2.2 ± 0.3 |
| Triglyceride | Oil | 1.9 ± 0.4 |
| Labrafac® CM10 | Oil | 57 ± 4.2 |
| Capryol 90 | Oil | 145 ± 12.6 |
| Tween® 80 | Surfactant | 37 ± 4.3 |
| Cremophor® EL | Surfactant | 55 ± 5.0 |
| Labrasol® | Surfactant | 105 ± 5.0 |
| PEG 400 | Co-surfactant | 40 ± 3.4 |
| Transcutol® HP | Co-surfactant | 158 ± 17.8 |
| Propylene glycol | Co-surfactant | 228 ± 19.5 |
2.3. Construction of pseudo-ternary phase diagram
Based on the results of the saquinavir solubility test, Capryol™ 90, Labrasol® and propylene glycol were chosen as an oil, a surfactant, and a cosurfactant, respectively, to design SMEDDS formulation for saquinavir. To establish the compositions consisting of the oil, surfactant, and cosurfactant that can spontaneously form a microemulsion, the self-microemulsification region was evaluated by constructing pseudo-ternary phase diagrams with varying the ratio of each ingredient used [11]. The surfactant and cosurfactant were mixed at five different ratios of 3:1, 2:1, 1:1, 1:2, and 1:3. The mixtures of surfactant and cosurfactant at ratios of 3:1, 2:1, and 1:1 were then added to the oil at various weight ratios from 1:9 to 9:1 and homogeneously mixed. Distilled water was then added to each mixture under gentle stirring. The appearance of the mixtures was then observed, and the turbid and clear dispersions were considered as a coarse emulsion and a microemulsion, respectively [12]. The compositions of the mixtures that formed the transparent microemulsion were noted and pseudo-ternary diagrams were plotted using SigmaPlot 10 (Systat Software, Inc., San Jose, CA).
2.4. Preparation and in vitro characterization of SMEDDS formulations
Based on the pseudo-ternary phase diagrams obtained, SMEDDS formulations with three different compositions were prepared as shown in Table 2. The oil was used at a constant percentage of 20%, and the surfactant and cosurfactant were used at different ratios of 1:1, 2:1, and 3:1. The surfactant mixtures were prepared first, and the oil was added to the mixtures and homogeneously mixed. The in vitro characteristics of the SMEDDS formulations were evaluated as described below.
Table 2.
Compositions of self-microemulsifying drug delivery system formulations prepared.
| Formulation code | Ingredients (weight%) |
||
|---|---|---|---|
| Capryol™ 90 |
Labrasol® |
Propylene glycol | |
| A | 20 | 40 | 40 |
| B | 20 | 53 | 27 |
| C | 20 | 60 | 20 |
2.4.1. Assessment of self-microemulsification efficiency
The self-microemulficiation efficiency of SMEDDS formulations prepared was assessed by evaluating the time taken for the emulsification of the formulations. Each formulation (200 mg) was added in a drop-wise manner to a volumetric flask containing 25 ml distilled water, and the emulsification process was observed for 1 h [13]. When the equilibrium of the emulsification was attained, the evaluation of self-emulsification was performed according to the grading system of the self-microemulsification efficiency as previously reported (Table 3) [14].
Table 3.
Grading system for evaluation of self-emulsification efficiency of self-microemulsifying drug delivery systems in vitro[14].
| Grade | Features | Time of self-emulsification |
|---|---|---|
| I | Rapid forming microemulsion which is clear or slightly bluish in appearance | <1 min |
| II | Rapid forming, slightly less clear emulsion which has a bluish white appearance | <2 min |
| III | Bright white emulsion (similar to milk in appearance) | <3 min |
| IV | Dull, greyish white emulsion with a slightly oily appearance that is slow to emulsify | >3 min |
| V | Exhibits poor or minimal emulsification with large oil droplets present on the surface | >3 min |
2.4.2. Evaluation of dispersion stability of SMEDDS
Each SMEDDS formulation (200 mg) was diluted with 25 ml distilled water, and 1 ml of the diluted formulations was used for the analysis of the oil droplet size and zeta potential value. The droplet size and zeta potential of the diluted formulations were measured using Zetasizer Nano ZS (Malvern Instruments, Ltd., Malvern, UK) at 0.5, 1, 10, and 24 h after the dilution procedure.
2.4.3. Assessment of solubilization capacity of saquinavir SMEDDS formulations
An excess amount of saquinavir was added to formulations A, B, and C and then mixed at 20 °C for 24 h using a magnetic stirrer. The mixtures were then centrifuged at 2000 × g for 1 h, and the supernatants were appropriately diluted with acetonitrile and the level of saquinavir in the diluted supernatants was determined using the HPLC as described above.
2.5. Evaluation of viscosity of polymer solutions
Viscosity of aqueous solutions containing seven different polymers was assessed to select a suitable polymer as a precipitation inhibitor for preparation of the S-SMEDDS (Table 4). The polymers were dissolved in distilled water to prepare 2% (w/v) polymer solutions, and their viscosity was measured using a rotational rheometer (HAAKE™ Rheostress™ 1, Thermo Fisher Scientific Inc., MA, USA) fitted with a 2° × 6 cm titanium cone (C60/2° Ti L, Thermo Fisher Scientific Inc., MA, USA) at 20 °C. Each polymer solution was loaded on the rheometer plate and allowed to equilibrate for 10 min before the viscosity evaluation. The gap between the cone and plate was adjusted at 1.00 mm. The viscosity of the solutions was measured at a shear rate of 10/s.
Table 4.
Viscosity of different polymer aqueous solutions measured at a shear rate of 10 /sec. The concentration of polymers in the solutions was set at 2%.
| Ingredients | Viscosity (mPa·s) |
|---|---|
| PVP K90 | 10.5 |
| HPMC 2910 | 9.4 |
| PVP K30 | 6.3 |
| Poloxamer 407 | 3.4 |
| Poloxamer 188 | 3.0 |
| Copovidone | 2.0 |
| Polycaprolactam-polyvinyl acetate-polyethylene glycol graft copolymer | 1.7 |
2.6. Preparation and in vitro characterization of S-SMEDDS for saquinavir
To prepare the S-SMEDDS of saquinavir, different amounts of PVP K90 and HPMC 2910 were added to formulation A as presented in Table 5. The characteristics of each S-SMEDDS formulation were then evaluated, such as viscosity, droplet size, zeta potential, and solubilizing capacity for saquinavir as aforementioned.
Table 5.
Composition and viscosity of S-SMEDDS formulations. For preparation of S-SMEDDS formulations formulation A was used as a SMEDDS and precipitation inhibitors were added to the SMEDDS and homogeneously mixed.
| Formulation code |
Weight fraction of precipitation inhibitor in S-SMEDDS formulations (%) |
|
|---|---|---|
| PVP K90 | HPMC 2910 | |
| Sa1 | 1 | |
| Sa2 | 2 | |
| Sa3 | 3 | |
| Sa4 | 4 | |
| Sa5 | 5 | |
| Sb1 | 4 | |
| Sb2 | 5 | |
| Sb3 | 6 | |
| Sb4 | 7 | |
| Sb5 | 8 | |
2.7. In vitro drug release from SMEDDS and S-SMEDDS of saquinavir
The in vitro release behavior of saquinavir from SMEDDS and S-SMEDDS was assessed according to the United States Pharmacopeia (USP) dissolution apparatus II method [15]. The formulations equivalent to 200 mg saquinavir and the same amount of saquinavir bulk powders were added to distilled water (500 ml, pH 6.5) in 900 ml vessels and agitated at 50 rpm and 37 °C. The aliquots (2 ml) were withdrawn at 5, 10, 15, 30, 60, and 120 min and filtered through a 0.45 µm polyvinylidene difluoride membrane filter. The dissolution media were replaced with fresh media after taking the aliquots. The levels of saquinavir in the samples were then evaluated using the HPLC.
2.8. Evaluation of drug precipitation inhibiting ability of S-SMEDDS
The drug precipitation inhibiting ability of S-SMEDDS was assessed in a simulated intestinal fluid to expect changes in the solubility of saquinavir in the intestinal fluid in vivo. Saquinavir SMEDDS (formulation A), saquinavir S-SMEDDS (formulation Sa3 and Sb4), and saquinavir bulk powders equivalent to 200 mg saquinavir were added to 10 ml of the simulated intestinal fluid containing 5 mM porcine bile extract and 1.25 mM phosphatidyl choline (pH 6.5) placed in a round-bottomed flask [16], [17]. The samples were kept in a shaking incubator at 50 rpm and 37 °C. Aliquots (2 ml) were withdrawn at 5, 15, 30, 60 and 90 min, and the levels of saquinavir in the aliquots were analyzed using the HPLC.
2.9. In vivo assessment of lymphatic absorption of saquinavir
The assessment of lymphatic absorption of saquinavir in rats and its protocol were approved by Chung-Ang University Support Center for Animal experiments. All the procedures were conducted according to the guidelines and regulations. The animals were sacrificed using carbon dioxide gas after finishing the experiments, and all efforts were made to minimize suffering.
Male SD rats (275–300 g) were housed under constant temperature condition (20 °C) in the animal facility center of College of Pharmacy, Chung-Ang University and fed a commercial diet, with tap water provided ad libitum. The rats were acclimatized for 10 days and fasted for 24 h prior to the experiments. The SMEDDS, S-SMEDDS formulations, and a saquinavir aqueous suspension were administered to the rats via oral gavage at a dose of 50 mg saquinavir/g. One hour after the administration, the rats were anesthetized with a urethane solution in pyrogen-free water through intraperitoneal injection at a dose of 1300 mg/kg. Urethane was used as an anesthetic because of its long duration of action and excellent depth of analgesia and anesthesia [18], [19]. Mesenteric lymphatic duct cannulation on the unconscious rats was conducted as reported [20], [21], and lymph samples were collected at 2 h intervals for 8 h. During the lymph collection procedure, the body temperature and hydration state of the rats were maintained.
Acetonitrile (1 ml) was added to each lymph sample (200 µl) and vortexed for 10 min to extract saquinavir from the samples. The samples were then centrifuged at 2000 × g for 10 min to remove insoluble ingredients in the samples. The supernatants were separated and evaporated using a stream of nitrogen gas. The dried residues were reconstituted with 1 ml HPLC mobile phase and the level of saquinavir in the solutions was analyzed using the HPLC system according to the procedure aforementioned.
2.10. Statistical analysis
Data are presented as means ± standard deviation (means ± standard error of mean for the animal experiment results). Student's t-test was performed to determine the statistical significance (P < 0.05, unless otherwise indicated) of result values between experimental groups.
3. Results and discussion
3.1. Solubility of saquinavir in oils, surfactants, and co-surfactants
Solubility of saquinavir in different oils, surfactants, and co-surfactants was examined to choose optimal ingredients for design of SMEDDS with a maximized solubilization capacity for saquinavir. The HPLC analysis method used for quantification of saquinavir was validated and showed appropriate specificity, linearity, precision, and accuracy. Thus, the HPLC analysis method was considered to be suitable for the intended purpose. As presented in Table 1, among oils the solubility of saquinavir was the greatest in Capryol™ 90. The reason for this might be attributed to the amphiphilic structure of Capryol™ 90 with a hydrophilic-lipophilic balance value of 5.0, which was more appropriate to enhance the solubility of saquinavir than the other oils [22]. In case of surfactants tested, the squinavir solubility was the greatest in Labrasol®, which consists of polyethylene glycol (PEG) esters, a small glyceride fraction, and free PEG and has been frequently used as a surfactant for preparation of SMEDDS formulations [23]. Among co-surfactants tested, propylene glycol exhibited the highest solubility of saquinavir. Therefore, Capryol™ 90, Labrasol®, and propylene glycol were selected to formulate the composition of SMEDDS for saquinavir.
3.2. Pseudo-ternary phase diagram
Pseudo-ternary phase diagram provides the information on phase behaviors under various compositions of lipid-based formulations, and its construction is thus essential for evaluation of the self-microemulsification ability of SMEDDS formulations and establishment of the optimal ratio of the components of the formulations.
Pseudo-ternary phase diagrams were obtained with varying the volume ratio of water, oil (Capryol™ 90), and mixture of surfactant (Labrasol®) and co-surfactant (propylene glycol) as shown in Fig. 1. The formulations were considered to possess a high self-emulsification efficiency when they exhibited a large area of the self-microemulsifying region [22]. The self-emulsifying region was generally increased with changing the ratio of surfactant and cosurfactant from 3:1 to 1:1. However, considerable phase separations were observed when the proportion of cosurfactant in the formulations was higher than that of surfactant, implying that the amount of surfactant was not sufficient to form microemulsions. The formulation with the surfactant/cosurfactant ratio of 1:1 was thus considered to have the best self-emulsifying potential among the tested formulations.
Fig. 1.
Pseudo-ternary phase diagrams of formulations composed of Capryol™ 90 (oil), Labrasol® (surfactant) and propylene glycol (cosurfactant) dispersed in distilled water. The surfactant mixtures (Smix) used consisted of surfactant and cosurfactant at three different ratios of (A) 1:1, (B) 2:1, and (C) 3:1. The gray area indicates that the formulations formed oil-in-water microemulsions.
3.3. Self-microemulsification efficiency of SMEDDS
Based on pseudo-ternary phase diagrams obtained, three different SMEDDS formulations (formulation A, B, and C) were prepared as shown in Table 2. The SMEDDS formulations were composed of 20% oil and 80% surfactant mixture, which consisted of surfactant and cosurfactant at three different ratios of 1:1, 2:1, and 3:1. The self-emulsification efficiency of the formulations was then assessed using a grade system of self-emulsification efficiency as presented in Table 3 [14].
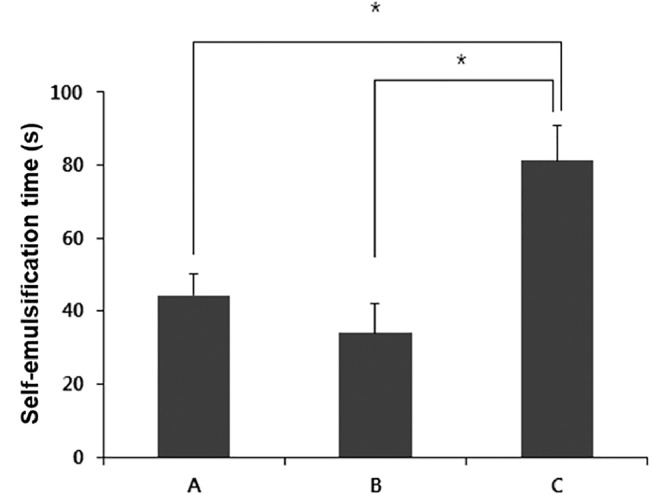
Formulation A and B exhibited the self-emulsification time less than 1 min, while formulation C was self-emulsified within 2 min (Fig. 2). The microemulsions formed from formulation A and B were clear and transparent, while the emulsion obtained from formulation C was slightly turbid. According to the grade system, the self-emulsification efficiency of formulation A and B was graded I, and that of formulation C was classified as II. Self-emulsification times of formulation A and B were not significantly different (P > 0.05). Thus, formulation A and B showed better self-emulsification efficiency than formulation C.
Fig. 2.
The time taken for self-emulsification of self-microemulsifying drug delivery system formulations of A, B, and C, which were composed of Capryol™ 90, Labrasol®, and propylene glycol at three different weight ratios of 20:40:40, 20:53:27, and 20:60:20, respectively. Asterisk (*) indicates a statistical significance of P < 0.05.
3.4. Dispersion stability of SMEDDS
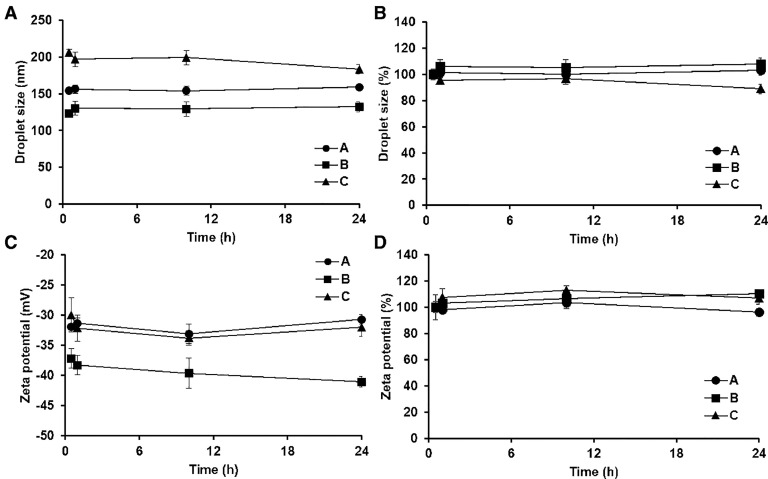
The droplet size and zeta potential of oil phases of the three different SMEDDS were assessed for 24 h to evaluate the dispersion stability of the SMEDDS formulations (Fig. 3). The mean diameters of oil droplets of formulation A, B, and C at 1 h were 154 ± 4.7 nm, 123 ± 2.8 nm, and 206 ± 5.4 nm, respectively. In general, the droplet sizes of the formulations were not significantly varied for the tested period, exhibiting the percentile changes less than 10%. Therefore, the three different formulations showed proper dispersion stabilities. The oil droplet sizes ranging from 100 nm to 150 nm have been known to be advantageous for the absorption in the GI tract [24]. Thus, formulation A and B with a mean oil droplet size of approximately 100–150 nm were considered to be more suitable self-emulsification ability to promote the intestinal lymphatic transport of saquinavir.
Fig. 3.
Changes in droplet size (A and B) and zeta potential (C and D) of oil phase of self-microemulsifying drug delivery systems (200 mg) evaluated for 24 h after dilution of the formulations in distilled water (25 mL). Fig. 3(a) and (c) present changes in original values of the droplet size and zeta potential, and Fig. 3(b) and (d) show percentile changes in the droplet size and zeta potential. A, B, and C indicate the self-microemulsifying drug delivery system formulations consisting of Capryol™ 90, Labrasol®, and propylene glycol at three different weight ratios of 20:40:40, 20:53:27, and 20:60:20, respectively. Asterisk (*) indicates a statistical significance of P < 0.05.
Zeta potential values of a dispersed phase indicate the potential stability of a colloidal system [25], and those of ± 30 mV are widely regarded as a criterion to define stable and unstable colloids [26]. The zeta potentials of formulations A, B, and C were −32 ± 0.5 mV, −37 ± 1.6 mV, and −30 ± 2.8 mV, respectively, 1 h after beginning the dispersion stability testing. The zeta potentials of the formulations were maintained to be less than −30 mV during the whole tested period, indicating that the dispersion stability of the SMEDDS formulations was well retained over 24 h.
3.5. Solubility of saquinavir in SMEDDS
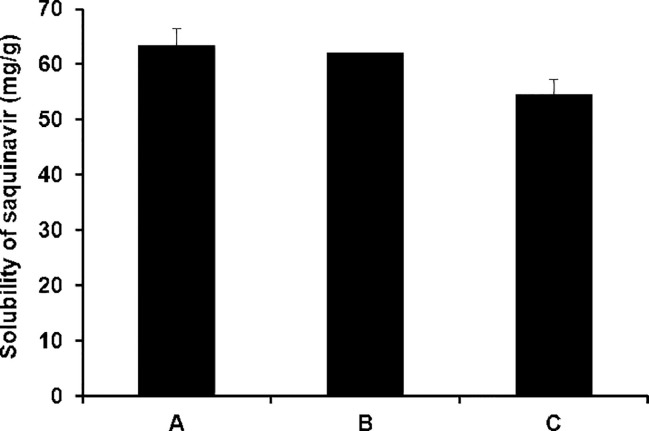
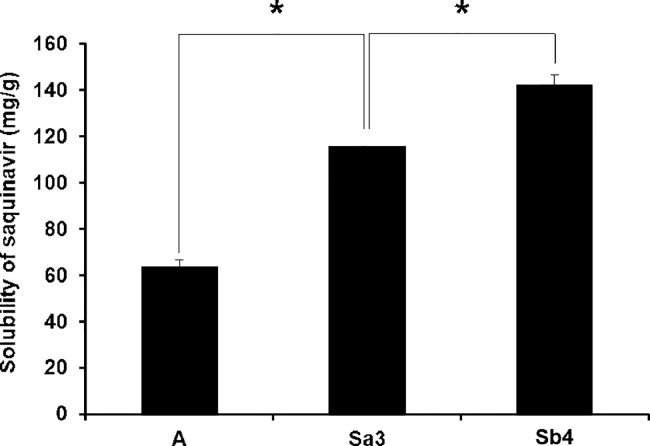
The solubilization capacity of SMEDDS for hydrophobic drugs is crucial to enhance the drug absorption in the GI tract because it is closely associated with the drug dissolution rate in the GI tract. The solubilization capacity of the three different SMEDDS formulations for saquinavir was therefore evaluated. As shown in Fig. 4, the saquinavir solubility in formulation A, B, and C was measured to be 63.2 ± 3.30 mg/g, 61.9 ± 0.04 mg/g, and 54.4 ± 2.82 mg/g, respectively. In general, a SMEDDS formulation containing a larger amount of cosurfactant, i.e. propylene glycol, exhibited a greater solubilization capacity for saquinavir. The reason for this might be ascribed to the highest solubilizing capacity of propylene glycol for saquinavir, which was demonstrated in the solubility testing of saquinavir as described above.
Fig. 4.
Solubility of saquinavir evaluated in self-microemulsifying drug delivery systems. A, B, and C indicate the self-microemulsifying drug delivery system formulations composed of Capryol™ 90, Labrasol®, and propylene glycol at three different weight ratios of 20:40:40, 20:53:27, and 20:60:20, respectively. Asterisk (*) indicates a statistical significance of P < 0.05.
3.6. Viscosity of polymer solutions
The precipitation of saquinavir possibly occurs in the GI tract when the saquinavir SMEDDS is orally administered because the drug would be released at a fast rate from the oil droplets formed from the SMEDDS and the drug concentration in the GI fluid might be rapidly increased up to the saturated state. The drug precipitation is not desirable to enhance the drug absorption in the GI tract. Polymers have been known to be able to inhibit the precipitation of hydrophobic drugs with low aqueous solubility [27]. They can stabilize the supersaturated state of the drugs through intermolecular interaction such as hydrogen bonding, van der Waals force, or steric hindrance [28] The precipitation inhibiting capacity of polymers depends on various factors, and among them viscosity of the polymer solutions is particularly important because the drug diffusion rate could be decreased by high viscosity, thereby preventing the crystallization of the drugs [27]. For this reason, we assessed the viscosity of aqueous solutions of seven different polymers that have been investigated as a drug precipitation inhibitor to select the most suitable polymer for preparation of S-SMEDDS of saquinavir.
As shown in Table 5, the aqueous solution of PVP K90 presented the highest viscosity, followed by HPMC 2910, PVP K30, poloxamer 407, polxamer 188, copovidone, and polycaprolactam-polyvinyl acetate-polyethylene glycol graft copolymer. PVP K90 and HPMC 2910 were chosen to prepare S-SMEDDS formulations of saquinavir owing to their comparatively high viscosity.
3.7. Viscosity and colloidal properties of S-SMEDDS
S-SMEDDS formulations were prepared using formulation A and precipitation inhibitors selected above as shown in Table 5 because formulation A exhibited a good self-emulsification efficiency and the best solubilization capacity for saquinavir. It has been known that high levels of precipitation inhibitors in SMEDDS may decrease the self-emulsification efficiency [29] and therefore, the colloidal properties of the S-SMEDDS formulations containing different levels of precipitation inhibitors were examined to determine an optimal level of the polymers in S-SMEDDS.
In all the S-SMEDDS formulations, the precipitation inhibitors could be homogeneously dispersed, and the viscosity of the formulations increased with increasing the amount of the polymers used (Table 5). In general, S-SMEDDS formulations with a viscosity value less than 100 mPa·s exhibited a relatively small droplet size of oil phase without significant changes over 24 h after dilution with distilled water as presented in Table 6, indicating good self-emulsification efficiencies of the formulations. The reason for this was likely because the oil phase could be dispersed well in the aqueous phase at comparatively low viscosities. However, S-SMEDDS formulations with viscosity values greater than 100 mPa·s showed considerably greater droplet sizes of the oil phase, implying that the self-emulsification of the S-SMEDDS formulations was inhibited by the high viscosity. Based on this result, S-SMEDDS formulation Sa3 and Sb4 were selected for further studies, which presented good self-emulsification behaviors with relatively high viscosities.
Table 6.
Results of evaluation of self-emulsification efficiency and dispersion stability of S-SMEDDS formulations. For assessment of the dispersion stability of S-SMEDDS formulations, each S-SMEDDS formulation (200 mg) was diluted with 25 ml distilled water, and the droplet size and zeta potential of the dispersed oil phase were measured at 1 h and 24 h after the dilution procedure.
| Formulation code | Grade of self- emulsification efficiency |
Viscosity (mPa·s) | Droplet size of oil phase (nm) |
Zeta potential (mV) |
||
|---|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | |||
| Sa1 | I | 34.3 ± 0.5 | 264 ± 8.4 | 271 ± 4.3 | −38 ± 0.8 | −36 ± 1.7 |
| Sa2 | I | 85.6 ± 1.2 | 280 ± 2.3 | 273 ± 3.3 | −30 ± 0.7 | −29 ± 0.8 |
| Sa3 | I | 101.2 ± 1.1 | 290 ± 4.0 | 285 ± 3.2 | −31 ± 0.5 | −28 ± 0.7 |
| Sa4 | III | 146.6 ± 2.7 | 428 ± 4.6 | 387 ± 5.6 | −31 ± 0.3 | −34 ± 0.5 |
| Sa5 | III | 234.4 ± 4.3 | 473 ± 13.0 | 418 ± 18.2 | −27 ± 0.4 | −23 ± 1.3 |
| Sb1 | I | 52.2 ± 0.7 | 162 ± 3.5 | 190 ± 5.4 | −37 ± 1.6 | −25 ± 1.7 |
| Sb2 | I | 76.1 ± 0.9 | 171 ± 2.6 | 191 ± 3.9 | −35 ± 1.4 | −31 ± 0.4 |
| Sb3 | I | 88.3 ± 0.5 | 125 ± 1.9 | 134 ± 2.3 | −28 ± 0.7 | −25 ± 0.8 |
| Sb4 | I | 101.3 ± 2.1 | 189 ± 2.1 | 201 ± 4.3 | −24 ± 1.9 | −27 ± 0.9 |
| Sb5 | III | 117.7 ± 1.8 | 273 ± 3.1 | 291 ± 5.2 | −29 ± 0.3 | −33 ± 1.2 |
3.8. Solubilizing capacity of S-SMEDDS for saquinavir
To evaluate the drug precipitation inhibiting capacity of S-SMEDDS formulation Sa3 and Sb4, the saquinavir solubility in the formulations was examined. As illustrated in Fig. 5, the solubility of saquinavir in S-SMEDDS formulation Sa3 and Sb4 was significantly greater than that in SMEDDS formulation A devoid of a precipitation inhibitor, meaning that the supersaturated state of saquinavir was well maintained by the presence of the precipitation inhibitors in the S-SMEDDS formulations. The solubility of saquinavir in formulation Sb4 was considerably higher than that in formulation Sa3. It has been known that HPMC 2910 contained in formulation Sb4 has much more functional groups such as hydroxyl group that can form hydrogen bonds than PVP K90 in formulation Sa3 [30]. Thus, it was supposed that HPMC 2910 was able to interact with saquinavir molecules more strongly than PVP K90 through forming hydrogen bonds with the drugs and thereby inhibited the drug precipitation at greater degrees than PVP K90, leading to the higher solubility of saquinavir in the S-SMEDDS formulation.
Fig. 5.
Solubility of saquinavir in self-microemulsifying drug delivery system (formulation A) and supersaturated self-microemulsifying drug delivery systems (formulation Sa3 and Sb4). Asterisk (*) indicates a statistical difference of P < 0.05.
3.9. In vitro drug release study
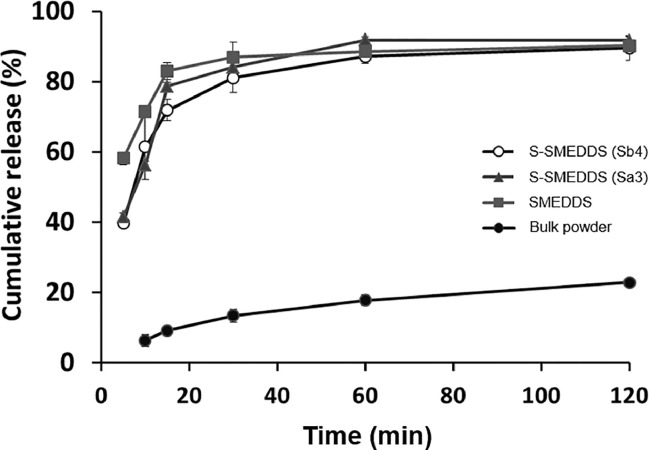
In vitro release behaviors of saquinavir from S-SMEDDS (Sa3 and Sb4), SMEDDS (formulation A), and saquinavir bulk powders were evaluated. As shown in Fig. 6, the drug dissolution rate from saquinavir bulk powders was gradually increased as a function of time, but it was only ∼20% at the end of the experiment, which might be due to the low aqueous solubility of saquinavir. As for the SMEDDS and S-SMEEDS formulations, the drug release rate from the SMEDDS formulation was considerably higher than those from the S-SMEDDS formulations (Sa3 and Sb4) at early stage, which might be because the high viscosity of the S-SMEDDS formulations caused by the precipitation inhibitors delayed the diffusion of saquinavir from the formulations. However, thereafter the drug dissolution rates from the SMEDDS and S-SMEDDS became similar, implying that the oil phase of the formulations was self-microemulsifed in the media and saquinavir was successfully released from the dispersed oil droplets.
Fig. 6.
In vitro release behavior of saquinavir from S-SMEDDS (Sa3 and Sb4), S-SMEDDS (formulation A) and saquinavir bulk powder.
3.10. Drug precipitation inhibiting ability of S-SMEDDS evaluated in simulated intestinal fluid
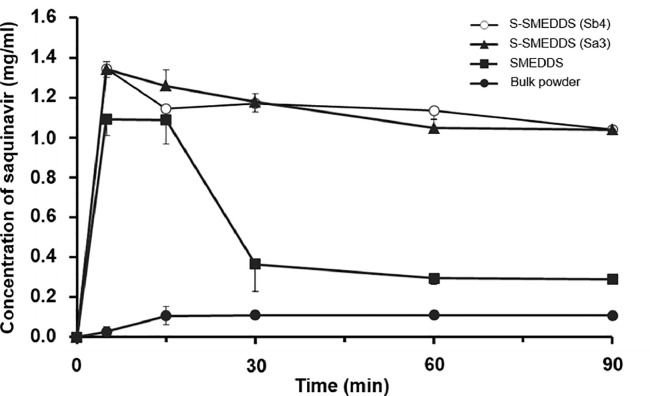
Changes in the concentration of saquinavir in an artificial intestinal fluid were evaluated to simulate the drug precipitation inhibition by the S-SMEDDS formulation in the GI tract in vivo. As illustrated in Fig. 7, the concentration of saquinavir released from S-SMEDDS formulations (Sa3 and Sb4) was rapidly increased at early stage and it was maintained for the whole tested period without significant changes. Thus, it was demonstrated that the precipitation inhibitors contained in the S-SMEDDS formulations successfully prevented the drug precipitation. Between formulation Sa3 and Sb4, no considerable changes in the saquinavir concentration were observed, which might be attributed to the similar viscosity of the S-SMEDDS formulations and consequent comparable precipitation inhibiting ability. However, the concentration of saquinavir examined with SMEDDS formulation was considerably decreased from 30 min, indicating that saquinavir was precipitated. In case of saquinaivr bulk powders, the drug concentration was very low compared to those evaluated from the S-SMEDDS and SMEDDS formulations. The results, therefore, implied that the precipitation inhibitors should be used to prevent the drug precipitation in the GI tract and to enhance the intestinal lymphatic delivery of saquinavir because the precipitated drug would not be efficiently absorbed via the enterocytes.
Fig. 7.
Result of the drug precipitation inhibiting ability of S-SMEDDS (formulation Sa3 and Sb4) evaluated in a simulated intestinal fluid in comparison with SMEDDS (formulation A) and saquinavir bulk powders. Changes in concentration of saquinavir in the simulated intestinal fluid were monitored for 90 min after adding the samples equivalent to 200 mg of saquinavir to 10 ml of the simulated intestinal fluid.
3.11. In vivo intestinal lymphatic drug absorption study of S-SMEDDS and SMEDDS
S-SMEDDS, SMEDDS and saquinavir bulk powders dispersed in distilled water were orally administered to rats at an equivalent dose of saquinavir, and the intestinal lymphatic absorption of the drug was assessed. As of now, two types of pharmaceutical formulations for oral administration of saquinavir have been marketed by F. Hoffmann-La Roche Ltd. (Basel, Switzerland) such as hard gel capsules and film-coated tablets, which are all conventional dosage forms and not formulated to deliver the drug efficiently to the lymphatic circulation. The saquinavir suspension was composed of pure saquinavir powders dispersed in distilled water and did not contain any pharmaceutical excipients that were expected to enhance the lymphatic drug uptake. We thus considered that the saquinavir suspension would show lymphatic drug absorption behaviors comparable to those of the marketed dosage forms and used the suspension in the lymphatic drug absorption study.
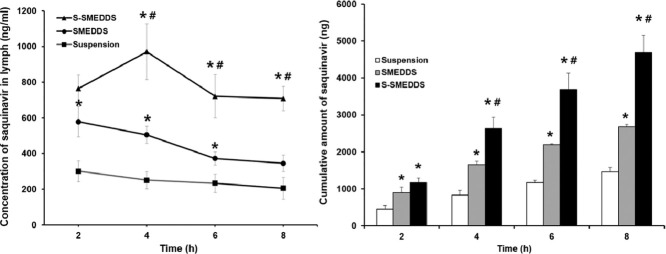
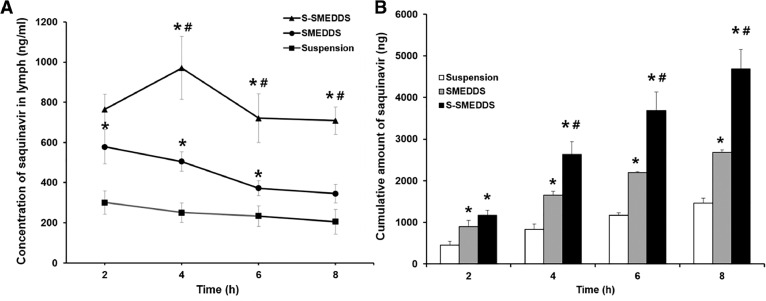
As an S-SMEDDS formulation used for this study, Sb4 was selected because it had a greater solubilization capacity for saquinavir than that of Sa3. For measurement of levels of saquinavir in rat lymph, the validated HPLC method used for in vitro studies was employed. We used an external calibration method for quantification of saquinavir because the drug in the lymph samples was completely dissolved in acetonitrile. Fig. 8A shows the concentration of saquinavir in lymph evaluated at pre-determined times. In case of the S-SMEDDS formulation, the concentration of saquinavir in lymph was the highest at 4 h after oral administration. This result was in good agreement with a previous study in which the saquinavir concentration in plasma was measured to be the highest at 2–4 h after oral administration of the drug to HIV-infected patients [31]. However, the time at which the maximum drug concentration is observed (Tmax) was not found in case of the SMEDDS formulation and suspension. Based on the data obtained, it was supposed that Tmax of the SMEDDS and suspension was earlier than 2 h. However, we were not able to assess the concentrations of saquinavir in lymph at earlier than 2 h because we could not obtain a sufficient amount of lymph necessary for the quantification of saquinavir through the HPLC analysis due to low lymph flow rates of rats. The reason for the delayed Tmax of the S-SMEDDS formulation compared to that of the SMEDDS formulation and suspension was might be because the precipitation inhibitor contained in the S-SMEDDS sustained the drug release in the GI tract of rats, leading to the later time of maximum drug concentration in lymph.
Fig. 8.
(A) Concentration and (B) cumulative amount of saquinavir in lymph obtained from rats evaluated during in vivo lymphatic drug absorption study. For the experiment, S-SMEDDS (formulation Sb4), SMEDDS (formulation A), and saquinavir suspension were administered to rats at an equal dose. Data are presented as mean ± standard error of mean (n = 5). Asterisk (*) and sharp (#) represents P < 0.05 versus saquinavir suspension and SMEDDS, respectively.
The concentrations of saquinavir in the lymph examined with the S-SMEDDS and SMEDDS were significantly higher than that evaluated with the saquinavir suspension for the whole tested period, implying that the S-SMEDDS and SMEDDS were self-microemulsified in the GI tract of the rats and the absorption of saquinavir via enterocytes could be promoted. In addition, the oil ingredients of the S-SMEDDS and SMEDDS were supposed to be able to facilitate the association of saquinavir with chylomicron, leading to enhanced lymphatic absorption of the drug. Between the S-SMEDDS and SMEDDS, the S-SMEDDS showed considerably higher concentrations of saquinavir in lymph than the SMEDDS. The reason for this might be because the precipitation inhibitor contained in the S-SMEDDS prevented the drug precipitation, thereby maintaining high levels of saquinavir in the GI fluid and enhancing the drug absorption in the GI tract [32].
When the cumulative amounts of saquinavir contained in the lymph samples obtained over 8 h at 2 h intervals were calculated, the same trend to results described above was observed as shown in Fig. 8B. The cumulative amount of saquinavir evaluated from the lymph was found to be the largest when the drug was administered to the rats as a form of the S-SMEDDS, followed by the SMEDDS and the saquinavir suspension. Thus, the lymphatic absorption of saquinavir was able to be maximized through the SMEDDS combined with the precipitation inhibitor that could retain high drug concentrations in the GI fluid.
4. Conclusion
For efficient lymphatic transport of saquinavir, SMEDDS were designed using Capryol™ 90, Labrasol®, and propylene glycol as an oil, surfactant, and cosurfactant, respectively, which showed better solubilizing capacity for saquinavir than other tested materials. The SMEDDS prepared showed a good self-emulsification efficiency and dispersion stability. To maximize the drug absorption in the GI tract and into the lymphatic system, HPMC 2910 was chosen based on its miscible property with the SMEDDS, appropriate viscosity and drug precipitation inhibiting ability. Owing to the precipitation inhibitor, the S-SMEDDS exhibited the higher concentrations of saquinavir in a simulated intestinal fluid than the SMEDDS and saquinavir bulk powders. When examining the lymphatic transport of saquinavir using rats in vivo, significantly enhanced lymphatic absorption of saquinavir was demonstrated from rats administered the S-SMEDDS. Therefore, the S-SMEDDS designed in this study would be usefully exploited to enhance the lymphatic absorption of hydrophobic drugs that need to be targeted to the lymphatic system.
Conflict of interest
The author reports no conflicts of interest in this work.
Acknowledgments
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2015R1A5A1008958). This work was also supported by the Industry Technology Development Program (10077593) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
References
- 1.Mouly S.J., Paine M.F., Watkins P.B. Contributions of CYP3A4, P-glycoprotein, and serum protein binding to the intestinal first-pass extraction of saquinavir. J Pharmacol Exp Ther. 2004;308(3):941–948. doi: 10.1124/jpet.103.056390. [DOI] [PubMed] [Google Scholar]
- 2.Griffin B.T., O'Driscoll C.M. A comparison of intestinal lymphatic transport and systemic bioavailability of saquinavir from three lipid-based formulations in the anaesthetised rat model. J Pharm Pharmacol. 2006;58(7):917–925. doi: 10.1211/jpp.58.7.0006. [DOI] [PubMed] [Google Scholar]
- 3.Van Gyseghem E., Baert L., Van Remoortere P., van't Klooster G., Rouan M.C., Voorspoels J. Co-administration of darunavir and a new pharmacokinetic booster: formulation strategies and evaluation in dogs. Eur J Pharm Sci. 2010;41(2):193–200. doi: 10.1016/j.ejps.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Kim H., Seong I., Ro J., Hwang S.H., Yun G., Lee J. Enhanced association of probucol with chylomicron by pharmaceutical excipients: an in vitro study. Drug Dev Ind Pharm. 2015;41(7):1073–1079. doi: 10.3109/03639045.2014.927479. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian N., Ray S., Ghosal S.K., Bhadra R., Moulik S.P. Formulation design of self-microemulsifying drug delivery systems for improved oral bioavailability of celecoxib. Biol Pharm Bull. 2004;27(12):1993–1999. doi: 10.1248/bpb.27.1993. [DOI] [PubMed] [Google Scholar]
- 6.Kyatanwar A.U., Jadhav K.R., Kadam V.J. Self micro-emulsifying drug delivery system (SMEDDS) Rev J Pharm Res. 2010;3:75–83. [Google Scholar]
- 7.Mukherjee T., Plakogiannis F.M. Development and oral bioavailability assessment of a supersaturated self-microemulsifying drug delivery system (SMEDDS) of albendazole. J Pharm Pharmacol. 2010;62(9):1112–1120. doi: 10.1111/j.2042-7158.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 8.Nan Z., Lijun G., Tao W., Donggin Q. Evaluation of carbamazepine (CBZ) supersaturatable self-microemulsifying (S-SMEDDS) formulation in vitro and in vivo. Iran J Pharm Res. 2012;11(1):257–264. [PMC free article] [PubMed] [Google Scholar]
- 9.Jaisamut P., Wiwattanawongsa K., Graidist P., Sangsen Y., Wiwattanapatapee R. Enhanced oral bioavailability of curcumin using a supersaturatable self-microemulsifying system incorporating a hydrophilic polymer; in vitro and in vivo investigations. Aaps PharmSciTech. 2018;19(2):730–740. doi: 10.1208/s12249-017-0857-3. [DOI] [PubMed] [Google Scholar]
- 10.Kim M.S., Ha E.S., Choo G.H., Baek I.H. Preparation and in vivo evaluation of a Dutasteride-loaded solid-supersaturatable self-microemulsifying drug delivery system. Int J Mol Sci. 2015;16(5):10821–10833. doi: 10.3390/ijms160510821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixit A.R., Rajput S.J., Patel S.G. Preparation and bioavailability assessment of SMEDDS containing valsartan. AAPS PharmSciTech. 2010;11(1):314–321. doi: 10.1208/s12249-010-9385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deokate U., Shinde N., Bhingare U. Novel approaches for development and characterization of SMEDDS. Int J Curr Pharm Res. 2013;5(4):5–12. [Google Scholar]
- 13.Bachynsky M.O., Shah N.H., Patel C.I., Malick A.W. Factors affecting the efficiency of a self-emulsifying oral delivery system. Drug Dev Ind Pharm. 1997;23(8):809–816. [Google Scholar]
- 14.Pouton C. Effects of the inclusion of a model drug on the performance of self emulsifying formulations. J Pharm Pharmacol. 1985;37(S12):1P. P. [Google Scholar]
- 15.The United States Pharmacopoeial Convention. USP41-NF36, General Chapter <711>Dissolution, 2018.
- 16.Fatouros D.G., Nielsen F.S., Douroumis D., Hadjileontiadis L.J., Mullertz A. In vitro-in vivo correlations of self-emulsifying drug delivery systems combining the dynamic lipolysis model and neuro-fuzzy networks. Eur J Pharm Biopharm. 2008;69(3):887–898. doi: 10.1016/j.ejpb.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Gao P., Morozowich W. Development of supersaturatable self-emulsifying drug delivery system formulations for improving the oral absorption of poorly soluble drugs. Expert Opin Drug Deliv. 2006;3(1):97–110. doi: 10.1517/17425247.3.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Abbott S.B., Stornetta R.L., Fortuna M.G., Depuy S.D., West G.H., Harris T.E. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29(18):5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field K.J., White W.J., Lang C.M. Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim. 1993;27(3):258–269. doi: 10.1258/002367793780745471. [DOI] [PubMed] [Google Scholar]
- 20.Boyd M., Risovic V., Jull P., Choo E., Wasan K.M. A stepwise surgical procedure to investigate the lymphatic transport of lipid-based oral drug formulations: cannulation of the mesenteric and thoracic lymph ducts within the rat. J Pharmacol Toxicol Methods. 2004;49(2):115–120. doi: 10.1016/j.vascn.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Edwards G.A., Porter C.J.H., Caliph S.M., Khoo S.M., Charman W.N. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliver Rev. 2001;50(1–2):45–60. doi: 10.1016/s0169-409x(01)00148-x. [DOI] [PubMed] [Google Scholar]
- 22.Tung N.T., Tran C.S., Pham T.M.H., Nguyen H.A., Nguyen T.L., Chi S.C. Development of solidified self-microemulsifying drug delivery systems containing l-tetrahydropalmatine: design of experiment approach and bioavailability comparison. Int J Pharmaceut. 2018;537(1–2):9–21. doi: 10.1016/j.ijpharm.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Yi T., Lam C.W. Effects of spray-drying and choice of solid carriers on concentrations of labrasol (R) and transcutol (R) in solid self-microemulsifying drug delivery systems (SMEDDS) Molecules. 2013;18(1):545–560. doi: 10.3390/molecules18010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trevaskis N.L., Charman W.N., Porter C.J.H. Lipid-based delivery systems and intestinal lymphatic drug transport: a mechanistic update. Adv Drug Deliver Rev. 2008;60(6):702–716. doi: 10.1016/j.addr.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H., Zhong M. Preparation and evaluation of self‐microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J Pharm Pharmacol. 2006;58(9):1183–1191. doi: 10.1211/jpp.58.9.0004. [DOI] [PubMed] [Google Scholar]
- 26.Kovalenko M.V., Bodnarchuk M.I., Zaumseil J., Lee J.S., Talapin D.V. Expanding the chemical versatility of colloidal nanocrystals capped with molecular metal chalcogenide ligands. J Am Chem Soc. 2010;132(29):10085–10092. doi: 10.1021/ja1024832. [DOI] [PubMed] [Google Scholar]
- 27.Warren D.B., Benameur H., Porter C.J.H., Pouton C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: a mechanistic basis for utility. J Drug Target. 2010;18(10):704–731. doi: 10.3109/1061186X.2010.525652. [DOI] [PubMed] [Google Scholar]
- 28.DiNunzio J.C., Miller D.A., Yang W., McGinity J.W., Williams R.O. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol Pharmaceut. 2008;5(6):968–980. doi: 10.1021/mp800042d. [DOI] [PubMed] [Google Scholar]
- 29.Charman W.N.A., Stella V.J. Estimating the maximal potential for intestinal lymphatic transport of lipophilic drug molecules. Int J Pharmaceut. 1986;34(1–2):175–178. [Google Scholar]
- 30.Chen Z.Q., Liu Y., Zhao J.H., Wang L., Feng N.P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int J Nanomed. 2012;7:1115–1125. doi: 10.2147/IJN.S28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merry C., Barry M.G., Mulcahy F., Halifax K.L., Back D.J. Saquinavir pharmacokinetics alone and in combination with nelfinavir in HIV-infected patients. AIDS. 1997;11(15):F117–F120. doi: 10.1097/00002030-199715000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Brouwers J., Brewster M.E., Augustijns P. Supersaturating drug delivery systems: the answer to solubility-limited oral bioavailability? J Pharm Sci US. 2009;98(8):2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]