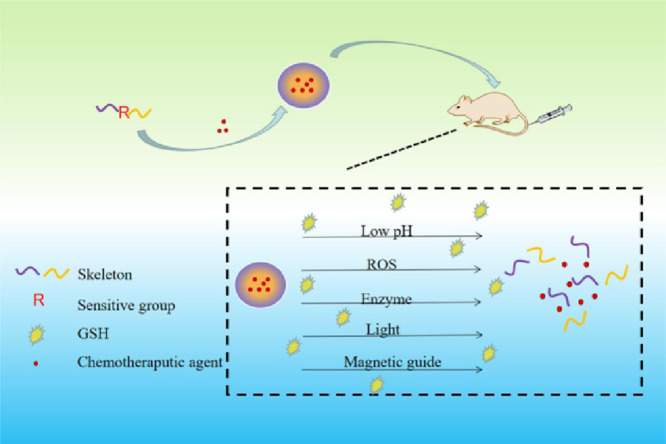
Abstract
Cancer is a big challenge that has plagued the human beings for ages and one of the most effective treatments is chemotherapy. However, the low tumor-targeting ability limits the wide clinical application of chemotherapy. The microenvironment plays a critical role in many aspects of tumor genesis. It generates the tumor vasculature and it is highly implicated in the progression to metastasis. To maintain a suitable environment for tumor progression, there are special microenvironment in tumor cell, such as low pH, high level of glutathione (GSH) and reactive oxygen species (ROS), and more special enzymes, which is different to normal cell. Microenvironment-targeted therapy strategy could create new opportunities for therapeutic targeting. Compared to other targeting strategies, microenvironment-targeted therapy strategy will control the drug release into tumor cells more accurately. Redox responsive drug delivery systems (DDSs) are developed based on the high level of GSH in tumor cells. However, there are also GSH in normal cell though its level is lower. In order to control the release of drugs more accurately and reduce side effects, other drug release stimuli have been introduced to redox responsive DDSs. Under the synergistic reaction of two stimuli, redox dual-stimuli responsive DDSs will control the release of drugs more accurately and quickly and even increase the accumulation. This review summarizes strategies of redox dual-stimuli responsive DDSs such as pH, light, enzyme, ROS, and magnetic guide to delivery chemotherapeutic agents more accurately, aiming at providing new ideas for further promoting the drug release, enhancing tumor-targeting and improving anticancer effects. To better illustrate the redox dual-stimuli responsive DDS, preparations of carriers are also briefly described in the review.
Keywords: Chemotherapy, Drug delivery system, Redox responsive, Dual-stimuli responsive, Tumor-targeting
Graphical abstract
Encapsulated drugs were accurately released at tumor cells based on sensitive groups or the characteristic of carrierself under redox dual stimulus including low pH, ROS, enzyme, light and magnetic guide.
1. Introduction
Cancer, one of the fatal diseases, remains a major challenge for human. And it is known that chemotherapy is one of the most common and effective methods in clinical therapy among many treatments of cancer [1]. However, chemotherapy still needs for further improvement in some fields, such as poor tumor-targeting, severe side effects and multidrug resistance. In order to solve issues mentioned above, types of drug delivery systems (DDSs) have been designed and fabricated under the efforts of researchers. The ideal DDS should have the following favorable features, e.g. (1) no leakage in blood circulation; (2) high accumulation in tumor tissues; (3) appropriate drug release site when give release stimuli. In addition, nanoscale DDSs can not only protect drug from avoiding leakage and eliminating in blood, but also be easily internalized into cancer cells through the enhanced permeability and retention (EPR) effect with the small size [2]. Despite many researchers have exhibited their design of nanocarriers for passive release after cell uptake, it is more preferable to well apply microenvironments of tumor tissues to achieve stimuli-responsive cargo release [3].
There are many differences in endogenous stimuli between tumor cells and normal tissues in microenvironment. Thus, stimuli-responsive nanomaterials have been applied to design and fabricate nanocarrier to enhance the drug delivery at targeted sites and minimize the leakage in unexpected tissues by endowing the nanocarrier with redox, pH, enzyme and reactive oxygen species (ROS) responsiveness [4], [5]. Currently, pH-sensitive DDSs are relatively mature, but redox-sensitive DDSs exhibit great potential among those internal microenvironment stimuli-responsive DDSs. Researchers have developed target-specific DDSs by exploiting unbalanced levels of glutathione (GSH) which is overexpressed in cancer cells cytoplasm [6], [7], [8]. The precise delivery and powerful effects of this strategy endow the redox-sensitive DDSs a series of significant advantages. Under the absence of another endogenous or exogenous signal stimulus, the strategy of this delivery could even exceed desired results such as no leakage in the blood circulation, tumor-targeting delivery and fast drug release.
The aim of this article is to supply an overview of current research activities that focus on redox responsive dual-stimuli DDSs. We discuss redox responsive dual-stimuli DDSs comprehensively including pH, light, enzyme, ROS and magnetic guide, which is to control the drug release more accurately and reduce the side effects. To perfect the whole article background, the description of redox responsive DDSs is included. Moreover, key examples of DDSs are presented.
2. Redox responsive DDSs
2.1. The mechanism of redox responsive DDSs
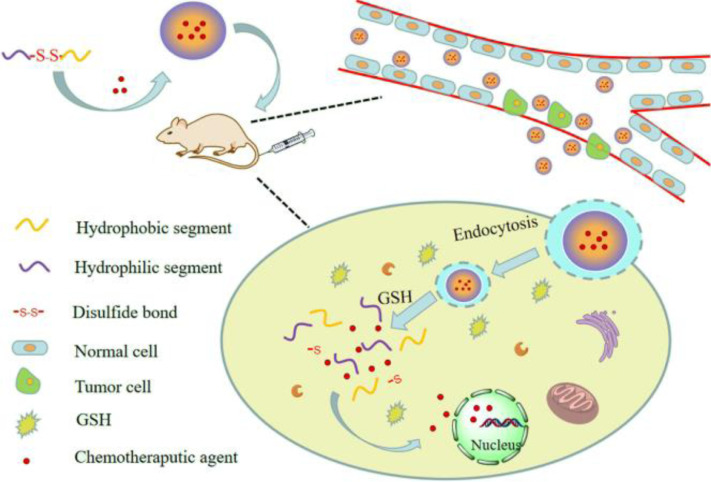
As known that, GSH plays a key role in human body, which can be oxidized to glutathione disulfide (GSSG) in normal tissue microenvironment, and GSSG can be concentrated back to GSH under the existence of the NADPH-dependent glutathione reductase to maintain cellular redox homeostasis necessary for cell growth and function [9]. However, there is a large quantity of GSH in cancer cells (approximately 2–20 mM) owing to the special environment and nutrients required for the tumor growth and multiple genetic alterations [10], [11]. The release mechanism of redox sensitive DDS in vivo is clearly noticed in Fig. 1. After administration, the nanocarrier could accumulate in tumor tissues through the EPR effect in the process of long blood circulation, and be uptaken into tumor cell via endocytosis. After that, the redox responsive linker broke down under the presence of high redox potential. And the structure of nanocarrier will be disassembled with the incorporated drugs released fast. Nevertheless, the nanocarrier still kept relatively stable in healthy tissue because the low redox potential could not destroy the structure of nanocarrier. Precise delivery, fast drug release and low side effects can be achieved according to the reduction potential gradient between cancer cells and healthy ones.
Fig. 1.
The release mechanism of redox sensitive DDS.
2.2. The application of redox responsive DDSs
Types of redox responsive groups have been used to design redox responsive DDSs. Disulfide bond, as one of the redox responsive bonds, is broadly involved in redox-responsive linkages. When subject to redox stimuli, DDSs can be directly cleaved to release the cargo. For example, Sun et al. [12] prepared a redox responsive micelle to deliver doxorubicin (DOX) to tumor sites. The amphiphilic conjugates coupled heparosan with deoxycholic acid via disulfide bond self-assembled into stable micelles in aqueous solution with much higher entrapment efficiency. In order to examine the drug release behavior, PBS with or without GSH (10 mM) was used to imitate the microenvironment of cancer or normal cells, respectively. Research results showed that the quantity of DOX release in PBS containing GSH (10 mM) was nearly two-fold compared with PBS without GSH. Except that, the release rate of DOX was distinctly accelerated under the existence of GSH. These phenomena were resulted from disulfide bond breakage under the GSH-triggered induction. This study showed that the micelles can deliver the DOX into cancer cells successfully and showed a GSH-mediated drug release behavior. Apart from this, Lv et al. [13] used cystamine as cross-linker between folate-conjugated poly (ethylene glycol)-b-copolycarbonates (the hydrophilic segment) and methoxy poly (ethylene glycol)-b-copolycarbonates (the hydrophobic block). The amphiphilic block copolymers could self-assemble into nanoscale micelles. Compared with uncrosslinked copolymer, these micelles exhibited higher drug loading capacity and displayed longer sustained drug release behavior. All the results of this study suggested that amphiphilic block copolymers micelles were applicable drug carriers for efficient tumor-targeting redox-responsive delivery of chemotherapeutic agents. Except for micelles, other preparations can be also applied to redox responsive DDSs such as liposomes [4], mesoporous silica nanoparticles (MSN) [14] and hyperbranched polymers [15].
3. Redox dual-stimuli responsive DDSs
Despite great potential for enhancing tumor-targeting, redox-responsive DDSs are still facing some challenges. Unfortunately, it should be noted that undesirable drug release behavior from redox responsive DDSs would be visible at non-targeted tissues, bringing about unpopular toxicities [16]. Moreover, the amount of chemotherapeutic agents still have a long way to reach the satisfying result in spite of the nanoscale size of DDSs. Adding another external or intracellular sensitive groups or materials to redox responsive DDSs has been a novel tactic to overcome the challenges mentioned above. External or intracellular sensitive groups or materials are shown in Table 1. As one of the representative instances, the rate of drug release could increase from redox and pH dual responsive DDSs with the synergistic actions of both low pH and high redox potential [17]. Moreover, redox and enzyme responsive DDSs could reduce the size before reaching cancer cells, leading high amount of anticancer drugs to penetrate into tumor cells [18].
Table 1.
pH-/ROS-sensitive groups and enzyme materials commonly used in redox dual responsive DDSs.
| pH-sensitive group | |||
|---|---|---|---|
| Denomination | Acylamino | Schiff base | Calcium phosphate |
| Structure | 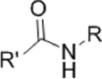 |
 |
Ca3(PO4)2 |
| Reference | [36] | [42] | [83] |
| ROS-sensitive group | Ferrocene | Boronic ester | Thioether |
| Denomination | |||
| Structure | 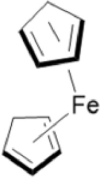 |
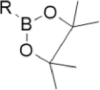 |
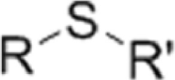 |
| Reference | [66] | [61] | [67] |
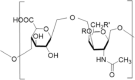
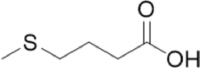
| Enzyme material | Hydroxyethyl starch | Chondroitin sulfate | Methionine |
| Denomination | |||
| Structure | 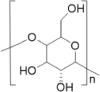 |
 |
 |
| Reference | [18] | [55] | [59] |
3.1. Redox and light dual responsive DDSs
Photodynamic therapy (PDT) based on the photodynamic effect has been recognized as a valid method [19]. When photosensitizers were delivered into body, tumor tissue was irradiated by light with a specific wavelength with singlet oxygen (1O2) generated by photosensitizers in the same instant, which would induce necrosis of tumor cells and activate an immune response against tumors [20], [21]. To be exact, that is photosensitizing reaction accompanying biological effects involving aerobic molecules. Besides, PDT could also clearly observe the sites of the anticancer drug action through fluorescence imaging [22]. However, its targeting ability still needs to further study. Despite that, photosensitizer delivery systems are facing the following two major matters. One issue is the potential trapping of the produced singlet oxygen inside cells due to the existence of the matrix of nanocarriers, which slows down even completely impedes the out-diffusion [23]. In addition, the photosensitizer encapsulated inside the nanosystem keeps the state of self-quenching to reduce PDT effect and NIR imaging efficiency by π-π stacking and hydrophobic interactions [24], [25], which is identified to be good for decreasing the side effects in the blood circulation [26], [27]. But that's why the limitation of PDT effect in tumor sites. The ideal result is to keep the fluorescence self-extinguishing in the blood circulation and be activated after reaching the target sites [28].
Combining the redox responsive drug release stimuli, photosensitizer-loaded DDSs are only disassembled in high redox potential, leading the photosensitizer fast release. Meanwhile, the photosensitizers could be activated into the excited state upon the cleavage of disulfide bond under the irradiation of NIR light, and generate singlet oxygen exerting the PDT effect, which might enhance the anticancer effect [29]. Application samples of redox and light dual responsive DDSs are shown in Table 2.
Table 2.
Application samples of redox and light dual responsive DDSs.
| Ref. | [27] | [28] | [29] | [33] |
|---|---|---|---|---|
| Carrier composition | MMP2; PEG | Dextran | LMWH; Alpha-TOS | Alginate |
| Preparation | Nanoparticles | Nanoparticles | Nanoparticles | Nanoparticles |
| Drug(s) | Chlorin e6 | Chlorin e6 | Chlorin e6; Paclitaxel |
Pheophorbide A; Doxorubicin |
| Drug loading method | Covalent conjugation | Covalent conjugation | Covalent conjugation | Covalent conjugation; Encapsulation |
| Sensitive groups/material | Photosensitizer; Disulfide linker |
Photosensitizer; Disulfide linker |
Photosensitizer; Cystamine |
Photosensitizer Disulfide linker |
3.1.1. Delivering photosensitizer
With the advantages of relatively noninvasive, selective and repeatable, PDT has been emerged as a novel strategy recently [30]. However, the concentration of singlet oxygen generated by photosensitizer could not reach the ideal level owing to the self-quenching of the photosensitizer at the core of nanoparticles in the blood circulation and the poor penetration of NIR light.
To solve the drawbacks above, the fabrication of novel redox responsive DDSs has been widely explored to control drug release under the high level of GSH in cancer cytoplasm. Hou et al. [27] synthesized polyethylene glycol-SS-chlorin e6- matrix metalloproteinase 2 (PEG-SS-Ce6-MMP2) self-assembly nanoparticles in aqueous solution. In addition, disulfide bond, as a cross-linker, connected PEG and Ce6. The release and toxicity experiments revealed that the PEG-SS-Ce6-MMP2 nanoparticles kept intact under the absence of DTT and displayed low toxicity without NIR light irradiation. Additionally, the Ce6 kept self-quenching state before it reached the appreciate site, which indicated that this strategy could minimize the side effects. Moreover, fluorescence intensity of Ce6 was evidently remarkable under the NIR irradiation due to the excitation stimulated by the cleavage of the disulfide linker. In other words, the cleavage of disulfide linker as the switch controlled the release of Ce6, which improved the poor penetration of NIR drastically. This research certified that PEG-SS-Ce6-MMP2 nanoparticles containing redox responsive linker might act as a novel anticancer strategy of both tumor-targeting imaging and PDT.
3.1.2. Delivering photosensitizer and chemotherapeutic agent simultaneously
The side effects of PDT are generally dose-dependent, so are chemotherapy, which seriously limits the treatment effect. Hence, the drug combination has been explored to broaden the application of PDT to reduce the dosage of drugs, increase the therapeutic effects, and abate side effects [31].
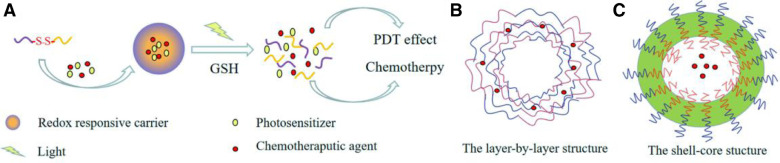
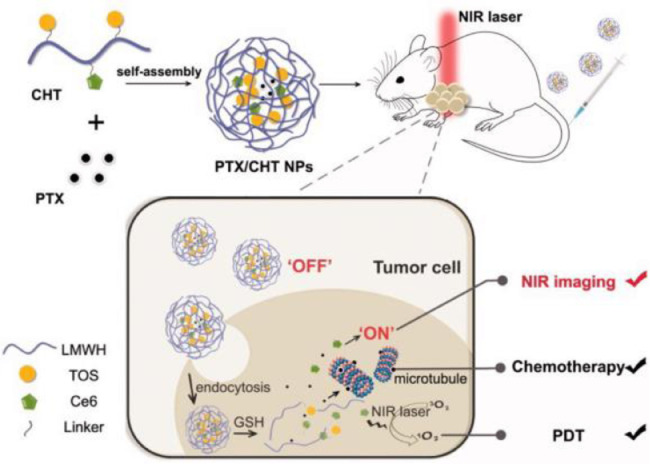
To utilize the superiorities of drug combination, DDSs delivering photosensitizer and chemotherapeutic agent simultaneously have been developed recently [32]. The process was simply illustrated in Fig. 2A. In the work of Yang et al. [29], Ce6 and alpha-tocopherol succinate (TOS) was conjugated to low molecular weight heparin (LWMH) through cystamine, forming amphiphilic Ce6-LMWH-TOS (CHT) polymer and then self-assembling into nanoparticles. Paclitaxel (PTX) was encapsulated inside the inner core of nanoparticles. Thanks to the redox responsive characteristic of cystamine, the enhanced NIR fluorescence intensity and the amount of singlet oxygen generated by Ce6 were observed in reductive environment as shown in Fig. 3. In addition, synergistic effects of drug combination were certified by experiments of MTT, in vitro apoptosis assays and NIR fluorescence capacity in tumor-bearing nude mice. Similarly, pheophorbide A (PheoA, a hydrophobic photosensitizer) was conjugated to alginate (PheoA-ALG) via a redox sensitive disulfide linkage; then PheoA-ALG was assembled nanoparticles via hydrophilic and hydrophobic interactions in the work of Zhang et al. [33]. This nanocarrier delivered photosensitizer and chemotherapeutic agent into B16 tumor cells (murine melanoma) successfully. Studies in vivo further certified these nanoparticles preferentially accumulated in tumor tissues, leading to substantial inhibition of B16 tumor growth under the simultaneous treatments of both chemotherapy and PDT.
Fig. 2.
(A) Delivering photosensitizer and chemotherapeutic agent simultaneously. (B) Layer-by-layer structure. (C) Shell-core structure.
Fig. 3.
Application of PTX/CHT nanoparticles in tumor NIR fluorescence imaging and synergistic photodynamic/chemotherapy. (Adapted with permission from [29].Copyright 2018 The Authors).
3.2. Redox and pH dual responsive DDSs
The pH responsive DDSs achieved by the difference of acidity is the relatively mature system among the intracellular drug release stimuli DDSs. Tumor tissues need more energy to maintain their growth. Hence, their energies mainly come from glycolysis instead of normal oxidative phosphorylation accompanying with more generation of H+ as a byproduct of glycolysis. Furthermore, the Warburg effect also leads to a lower pH value in the tumor microenvironment [34]. The pH levels of cancerous endosomes (pH 5.0–6.5) and lysosomes (pH 4.5–5.0) are both lower than those (pH 7.4) in blood and normal cells [35]. It has revealed that positively charged nanoparticles are more likely to enter cells through the endocytosis of proteoglycan adsorption in cell membranes than negatively charged nanoparticles [36]. However, positively charged nanoparticles are easily cleared in the blood circulation [37]. To overcome this issue, charge reversal achieved by the acid gradient between blood and cancer cells and pH responsive DDSs have been designed as a better strategy [38]. The interaction between drug and nanocarrier is generally through the following two ways: the weak interaction achieved by hydrogen bonds or electrostatic interactions and chemical cross-linking. Due to the poor stability, the former would cause the drug leakage in the blood circulation [39]. Whereas the latter have higher stability than the former, its responsive degree and selectivity to tumor cells are limited in some degree [40].
In recent years, redox and pH dual responsive DDSs have been introduced to relief issues mentioned above. Application samples of redox and pH dual responsive DDSs are shown in Table 3. The redox responsive structure is equivalent to the protective shell of pH responsive DDSs, reducing the leakage of cargo in the blood circulation. In addition, it could also enhance the tumor-targeting and the anticancer drug release abilities owing to the cleavage of redox chemical bond under the higher GSH in cancer cells cytoplasm. Apart from this, literature points out that the acidic group is almost completely protonated at pH 6.5, and the further reduction of the pH value has no more effect. Nevertheless, the addition of redox sensitive drug release stimuli will further accelerate the drug release and enhance the treatment effects [41]. Many groups have successfully developed redox and pH dual responsive DDSs and achieved very favorable results [42], [43], [44].
Table 3.
Application samples of redox and pH dual responsive DDSs.
| Ref. | Carrier composition | Drug | Preparation | Sensitive groups/material |
|---|---|---|---|---|
| [43] | BAC;4-AMPD; PEG | Doxorubicin | Micelles | Acylamino; Disulfide linkage |
| [47] | PCL; PDEA; Sulfobetaine | Doxorubicin | Micelles | Tertiary amines;Disulfide linkage |
| [73] | Reducible PHA | Doxorubicin | Micelles | Tertiary amine; Disulfide linkage |
| [74] | PCL-PDEA; mPEG-SS-PCL | Curcumin | Micelles | Tertiary amines; |
| [42] | Polysaccharide ADA derivative; Cystamine dihydrochloride | Docetaxel | Nanoparticles | Schiff base; Disulfide linkage |
| [36] | Poly(β-amino ester); D-α-tocopheryl poly ethylene glycol 1000 | Docetaxel | Nanoparticles | Tertiary amine; Disulfide linkage |
| [40] | 4-MBA; Carboxymethyl chitosan | Methotrexate | Nanoparticles | Acylamino; Disulfide bonds |
| [44] | TAT peptide; Mesoporous silica nanoparticles; PAH-Cit; GTC | Doxorubicin;siRNA | Nanoparticles | Acylamino; Disulfide linkage |
| [48] | rGO; Plu-SH | Doxorubicin | Nanoparticles | Tertiary amines;Disulfide linkage |
| [75] | Human serum albumin; Calcium phosphate | Cisplatin | Nanoparticles | Calcium phosphate; |
MBA = mercaptobenzoic acid; AMPD = (aminomethyl) piperidine; BAC = N,N-cystaminebis(acrylamide); PAH-Cit = poly(allylamine hydrochloride)-citraconic anhydride; GTC = galactose-modified trimethyl chitosan-cysteine.
3.2.1. Redox and pH dual responsive DDSs with the layer-by-layer structure
The layer-by-layer (LBL) structure means that the polymer chains are spirally wound into microcapsule, and the anticancer drugs are wrapped in the center as shown in Fig. 2B [45]. Redox and pH sensitive bonds alternately connected to form the polymer chains. Compared with the single pH responsive DDSs, the polymer chains are relatively difficult to be destroyed in the blood under the protection of redox responsive structure and to be disrupted quickly at the synergistic effects of acid and redox condition on the contrary. Gao et al. [42] designed pH and redox dual responsive polysaccharide-based microcapsules including Schiff base and disulfide bond via LBL techniques which have been extensively studied [46]. Owing to the hydrolysis of the Schiff base, the microcapsules became permeable at low pH. When adding 10 mM reduced DTT in the media at corresponding pH conditions, the permeability increased interestingly. This literature certified that microcapsules encapsulated docetaxel (DTX) could lead to pinpointed intracellular delivery and good therapeutic effect subsequently.
3.2.2. Redox and pH dual responsive DDSs with a shell-core structure
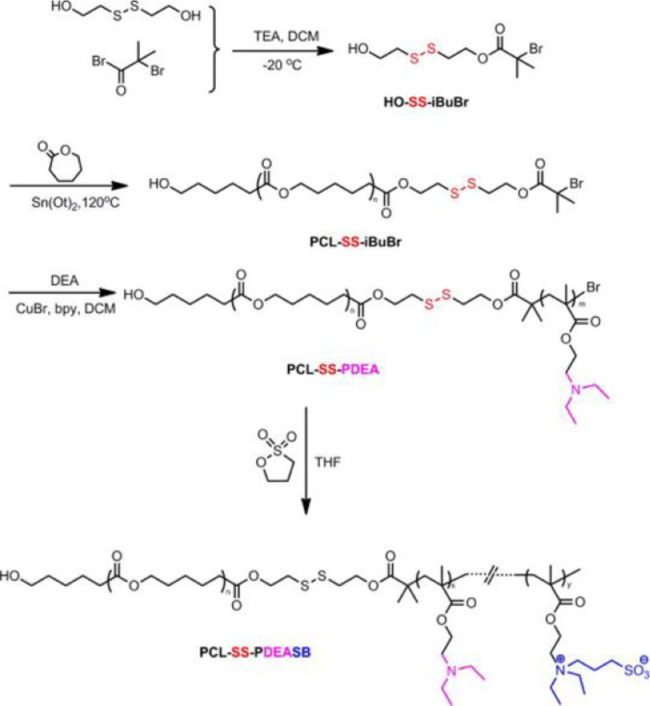
Generally, redox and pH dual responsive DDSs with shell-core structure consist of amphiphilic polymers as shown in Fig. 2C. The specificity of the core-shell structure with disulfide bond in the core and the hydrogen bond or the ester bond on the shell leads to the nanocarrier wrecked by low pH firstly, followed by the high level of GSH in cancer microenvironment. Hence, the combination of pH and redox stimuli enhanced the tumor-targeting enormously and reduced side effects significantly. According to the work of Cai's group [47], the pH and redox dual responsive micelles were designed and prepared successfully using amphiphilic poly(ε-caprolactone)-b-poly(N-(3-sulfopropyl)-N-methacrylate-N,N-diethylammoniu-m-betaine)(PCL-SS-PDEASB). And the synthetic line was shown in Fig. 4. DOX was encapsulated in micelles as a model drug to observe the behavior of drug release. After administration, the micelles carried drugs into the cancer cells via endocytosis. Tertiary amino groups of N, N-diethylaminoethyl methacrylate (DEA) were protonated as the pH decreasing from 7.4 to 5.0, and the micelles structure damaged preliminary, with the less release of DOX and the exposure of disulfide bonds to GSH. Subsequently, the structure of micelles was completely disrupted and the rate of DOX release had increased dramatically owing to the break of disulfide bond under the action of GSH. In addition, studies have shown that redox sensitivity plays a relatively important role in drug release and pH sensitivity may be an accelerate element which would benefit the penetration of external GSH and exposure of the disulfide bond in cores [40].
Fig. 4.
Synthetic line to PCL-SS-PDEASB. (Adapted with permission from [47]. Copyright 2015 Elsevier B.V.).
On the contrary, there was also core-shell structure in the pH and redox dual responsive DDSs with acid group in the core and redox group on the shell as reported by Abdullah Al-Nahaina's group [48]. Reduced graphene oxide (rGO) has been prepared as the matrix 1 to load DOX via hydrophobic interaction. Disulfide-containing pluronic (Plu-SH) as matrix 2 was wrapped in the outer layer of the rGO matrix formed a core-shell structure. Under the microenvironment of the cancer cells, oppositely, the delivery system was wrecked via the rupture of disulfide bond firstly, followed by acid group. More importantly, the high DOX loaded efficiency was achieved in this report due to the special structure of this carrier.
To sum up, compared with layer-by-layer and core-shell structure of DDSs, the former could release the drug faster and more with its special structure and the latter could enhance the tumor-targeting and reduce the side effects more remarkably.
3.3. Redox and enzyme dual responsive DDSs
Enzymes are widely present in various tissues of the human body and play an important role in maintaining the normal operation of the body. Likewise, dysregulation of enzymatic activity has been observed in a number of severe pathological conditions. For example, some enzymes are overexpressed in cancer tissues [49]. Hence, enzyme-sensitive materials have been widely utilized to design enzyme responsive DDSs [50]. The qualities of enzyme's high biocompatibility, specificity and efficiency endow the redox responsive DDSs higher tumor-targeting and drug release efficiency. Starches and peptides are usually the backbones of enzyme responsive DDSs, which will be destroyed by enzyme such as phospholipases, cancer-associated proteases, kinases, and acetyltransferases [51]. As known that starch is one of plant polysaccharides, α-amylase could hydrolyse the glycosidic bond of internal starch to dextrin or oligosaccharides. In addition, the degradation products of peptides are amino acids under the action of enzymes. Compared with other skeletons, these scaffolds are famous for high biocompatibility, easy degradation, and being removed quickly after delivery, reducing the adverse effects of exogenous substances. Although enzyme responsive DDSs have such powerful superiorities mentioned above, the biggest limitation in clinical application currently is low tumor-targeting ability, which could not distinguish cancerous and normal cells leading to serious toxic-side effects [52]. Hence, the strategy of redox sensitive drug release has been added to enzyme responsive DDSs as an auxiliary method to enhance drug release and some application samples are shown in Table 4. This combination could not only increase the accumulation of drugs in tumor cytoplasm under the high efficiency of the intracellular enzymes but also amplify the permeation by taking advantage of changes in particle size due to extracellular enzyme cleavage.
Table 4.
Application samples of redox and enzyme dual responsive DDSs.
| Reference | [18] | [52] | [54] | [55] |
|---|---|---|---|---|
| Carrier composition | Hydroxyethyl starch | Polycaprolactone; PBA; PEG; mPEG | Starch | CSCD conjugates |
| Preparation | Nanoparticles | Nanoparticles | Hydrogels | Nanoparticles |
| Drug | Paclitaxel | Camptothecin | Rhodamine B | Docetaxel |
| Drug loading method | Covalent conjugation | Chemical conjugation |
Encapsulation | Encapsulation |
| Sensitive groups/material | Hydroxyethyl starch; Disulfide linker |
Azo bonds; Disulfide linker |
Starch; Diselenide crosslinking part | Chondroitin sulfate; Disulfide linker |
CSCD = chondroitin sulfate-ss-deoxycholic acid.
3.3.1. Enhancing the drug release by synergistic effects
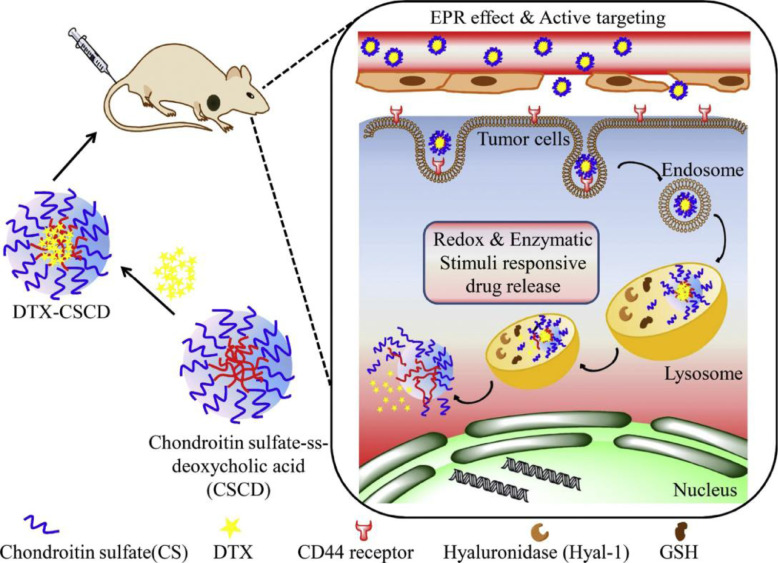
Introducing redox bond to enzyme DDSs endows the nanocarrier exhibiting a redox responsive character. The drug could accurately release into the appropriate site based on the high level of GSH in cancer cells cytoplasm [53]. Thereby, drugs which are encapsulated or chemically linked release faster under the two drug release signals simultaneously, which might increase the tumor-targeting and the cumulative release amounts of drug as well as inhibit multidrug resistance to some extent. With the combination of the high efficiency of the enzyme and the tumor-targeting of redox responsive endow this nanocarrier with powerful superiorities. Sun et al. [54] synthesized redox and enzyme responsive hydrogels with a biodegradable nanocarrier consisting of starch chain cross-linked via diselenide, and Rhodamine B (RB) as model drug was loaded to describe the drug release behavior from hydrogels more clearly. The release behavior of RB from starch-based hydrogels in different conditions showed that both enzyme and redox agent could promote the release of RB for the starch chains and diselenide linker. It should be pointed out that after adding α-amylase, the RB could release from hydrogels more quickly, which was attributed to the unique efficiency of enzymatic hydrolysis. Moreover, the release effect of RB was more pronounced in the presence of both α-amylase and redox agent, owing to the synergistic effects of enzymatic hydrolysis and redox responsive cleavage. Liu et al. [55] also successfully designed enzyme and redox dual responsive nanoparticles with the enhanced drug release pattern under the presence of GSH and hyaluronidase-1 (Hyal-1). The introduction of the formation, tumor-targeted accumulation and redox/enzyme-responsive drug release of self-assembled nanoparticles was shown in Fig. 5. The biodistribution study certified that there were high tumor and lung accumulation of nanoparticles compared with that of the free drugs, which further exhibited the high efficiency, specificity of the enzyme and redox dual responsive DDSs.
Fig. 5.
Schematic illustration of the formation, tumor-targeted accumulation and redox/enzyme-responsive drug release of self-assembled nanoparticles. (Adapted with permission from [55]. Copyright 2018 Elsevier B.V.).
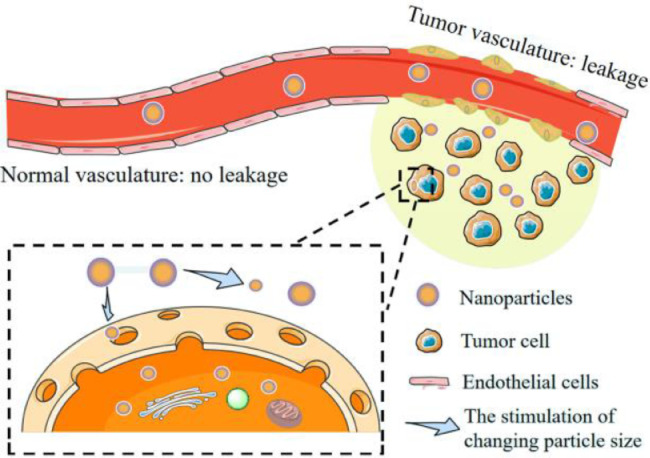
3.3.2. Enhancing internalization by changing particle size
Generally, the chemotherapeutic agent is primarily internalized into cancer cells by the EPR effect [56] or endocytosis [57]. It is known that the EPR effect results from the extravasation of macromolecules or nanoparticles through tumor blood vessels. Drugs loaded in nanocarrier can preciously accumulate in tumor tissues by the EPR effect in the blood circulation. This process is easier to implement according to the leakage characteristic of tumor vessel. But the nanoagents’ transport is limited by the low tumor permeability which is related to the nanoagents’ size. In addition, the size of nanoagents has a drastic effect on half-life in the blood circulation. Reducing the nanoagents’ size to enhance the permeability of tumor may be not a good choice for that it will sacrifice circulation time and lead to less accumulation of nanoagents in tumor tissues. The key to overcome this contradiction is to change particle size. The mechanism of enhancing internalization by changing particle size is shown in Fig. 6. The nanoagents firstly accumulate in tumor tissues by the EPR effect in the blood circulation, followed by being cut to smaller size under some kinds of stimulations, such as enzyme. Nanoagents will enter tumor cells more easily with the superiority of smaller size, improving the tumor permeability and resulting in more accumulation. Li et al. [18] changed the particle size via cutting nanocarrier directly under the condition of α-amylase. The hydroxyethyl starch (HES) nanoparticles were prepared and PTX was conjugated to HES via disulfide bond which exhibited redox responsive characteristic. The nanocarrier was decomposed into small particle under the degradation effect of α-amylase in the blood circulation, which improved the permeability of nanoparticles in tumor tissues. Fortunately, there was no drug leakage after cutting nanoparticle by the action of α-amylase. Thanks to the strategy of changing particle size and redox responsive characteristic, the HES nanoparticles can preciously reach to tumor sites and achieve better anticancer efficacy.
Fig. 6.
The mechanism of enhancing internalization by changing particle size.
3.4. Redox and ROS dual responsive DDSs
ROS such as hydroxyl radicals (OH.), hydrogen peroxides (H2O2), peroxynitrites (ONOO−), and superoxides (O2−) are byproducts generated from mitochondrial electron transport in normal cells [58]. Malignant cells can generate higher level of ROS than normal cells mainly due to oncogene stimulation, mitochondrial malfunction and chronic inflammation [59]. For example, the H2O2 concentration in cancer cells is about 2–5 times as that of normal cells. Hence, ROS have been utilized to design tumor-targeting DDSs, which has been a novel study in the last few years. Plenty of ROS responsive groups have been prosperously developed for cancer therapy such as thioketal [60], boronic ester [61] and thioether [62]. In Deepagan et al. [63] and Wang et al. [64] studies, ROS responsive DDSs have been successfully synthesized to achieve higher therapeutic effects and minimize undesirable toxicities. However, there was literature pointing out that the efficiency and selectivity of ROS responsive release from DDSs were insufficient to reach high tumor-targeting and therapeutic effect because of the heterogeneity of tumors [65]. Although this strategy has some weak points, applying it to redox responsive DDSs will produce powerful synergistic effects. And the application samples of redox and ROS dual responsive DDSs are shown in Table 5. That's a novel study hotpot nowadays.
Table 5.
Application samples of redox and ROS dual responsive DDSs.
| Reference | Carrier composition | Preparation | Drug | Drug loading method | Sensitive groups/material |
|---|---|---|---|---|---|
| [66] | Ferrocene; β-CD; mPEG | Micelles | Camptothecin | Non-covalent supramolecular inclusion interactions | The interation between β-CD and Ferrocene; Disulfide linker |
| [67] | mPEG-b-P(Desalt-Cys) | Micelles | Camptothecin | Encapsulation | Diethyl sulfide; Disulfide-containing cystamine |
Des = diethyl sulfide; Cys = disulfide-containing cystamine.
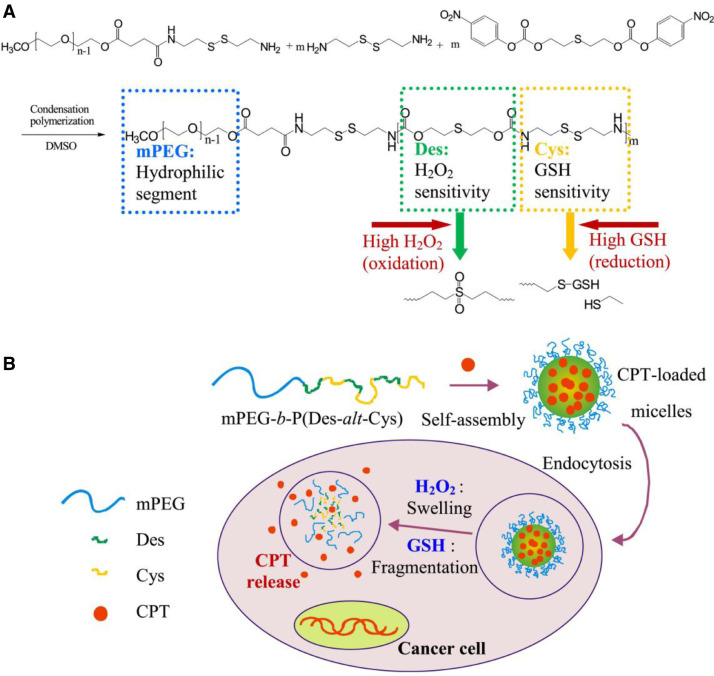
Compared with ROS or redox single triggered DDSs, dual responsive DDSs exhibit great potential for tumor-targeting and fast release. Kang et al. [66] successfully developed self-assembled redox and ROS dual sensitive mPEG-β-CD/Fc-CPT micelle. After endocytosis, the disulfide bonds or the interaction between β-cyclodextrin (β-CD) and ferrocene (Fc) would be rapidly destroyed under high GSH or H2O2 in cytoplasm, respectively, followed by high-fast camptothecin (CPT) release at tumor sites. Chiang et al. [67] utilized diethyl sulfide and disulfide-containing cystamine for designing redox and ROS dual responsive micelles. The chemical structure of copolymer, dual redox-responsive micelles and CPT release triggered by ROS and GSH were illustrated in Fig. 7. Micelles did not produce therapeutic effect in blood circulation only as a shelter for CPT. When micelles had been deposited in tumor tissues and induced endocytosis, the diethyl sulfide was oxidized and micelles were swollen under the high levels of ROS. Meanwhile, the disulfide structure will be cleaved at the presence of GSH-rich environment, which led to CPT release at tumor cells. It should be pointed out that compared with H2O2 condition, the particle size changed greatly under GSH condition. All of those literatures certificated that the new study of redox and ROS dual responsive DDSs might serve as novel promising chemotherapeutic agent nanocarriers in tumor treatment.
Fig. 7.
Schematic illustration of (A) chemical structure of mPEG-b-P(Des-alt-Cys) copolymer and (B) dual redox-responsive micelles and CPT release triggered by ROS and GSH. (Adapted with permission from [67]. Copyright 2015 Elsevier B.V.).
3.5. Redox responsive and magnetic guide DDSs
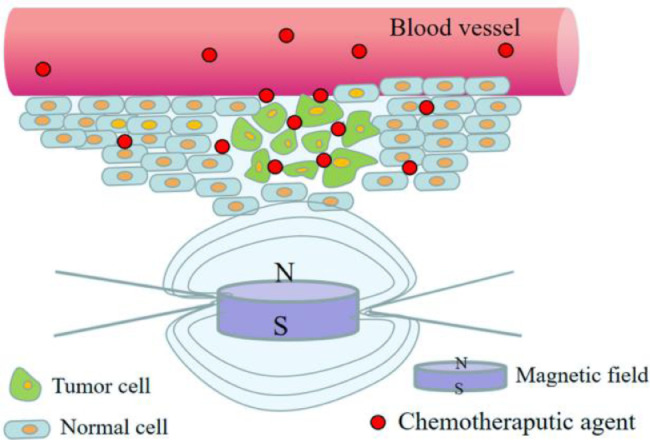
Except the dual sensitive DDSs mentioned above, magnetic guide has become hotpot in the studies of redox dual-stimuli responsive DDSs recently, which aimed at further delivering drugs to targeted location and protecting the normal tissues from harming. Magnetic guide means that after administration, the vehicle containing magnetic nanoparticles (MNPs), reaches the tumor tissues exactly under the action of an external magnetic field, endowing anticancer drugs to be accumulated around the tumor tissues under the EPR effect and the magnetic targeting. The drug distribution of magnetic and redox responsive DDS is shown in Fig. 8 [68]. The key point for the smart magnetic guide drug delivery is to accurately control the release of drugs into tumor cell cytoplasm.
Fig. 8.
The drug distribution of magnetic and redox responsive DDS.
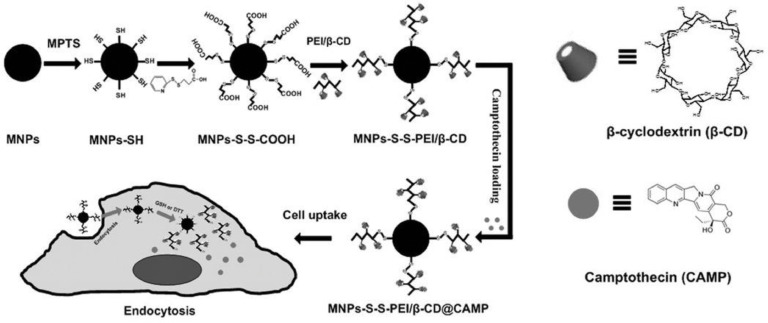
Additionally, redox responsive DDSs could control drug release at the tumor sites, which may not alter the primitive distribution in vivo. Introducing magnetic guide into redox responsive DDSs compensates for this deficiency and solves the magnetic guide-self issue of drug release to a certain extent. The magnetic guide and redox responsive DDS has been fabricated through combining the function both magnetic guide and redox sensitiveness. Application samples of redox responsive and magnetic guide DDSs are shown in Table 6. Among those, at the work of Luo et al. [69], disulfide bonds as coupling linkers immobilized β-CD grafting poly ethylenimine (PEI) molecules onto MNPs. Schematic illustration of the nanaoparticles’ fabrication of was shown in Fig. 9. CPT as a model drug was easily encapsulated into β-CD molecules through hydrophobic interactions for that β-CD contains a relatively hydrophobic central cavity and hydrophilic outer surface [70]. This DDSs can respond to redox agents and external magnetic fields for site-specific intracellular delivery of chemotherapeutic agent, which had potential biomedical applications such as clinical chemotherapy of cancer and magnetic resonance imaging. Moreover, Tang et al. [71] designed and fabricated star-shaped self-assembled micelles constituted by four-arm PEG-PCL copolymers with disulfide bonds as intermediate linkers, and PBA ligands were chemically conjugated to the end of the hydrophilic PEG segments. DOX and magnetic iron oxide nanoparticles (Fe3O4) were simultaneously encapsulated into the hydrophobic cores. The experiments of antitumor efficacy in vivo exhibited that the redox responsive and magnetic guide dual-targeted micelles obviously increased the drug accumulation in tumor tissues and minimized the side effects towards normal sites.
Table 6.
Application samples of redox responsive and magnetic guide DDSs.
| Reference | [69] | [71] | [78] |
|---|---|---|---|
| Carrier composition | Magnetic nanoparticles; PEI/β-CD | Four-arm PEG-PCL copolymers; Fe3O4 | Hen egg white proteins; Fe3O4 magnetic nanoparticles |
| Preparation | Nanoparticles | Micelles | Microcapsules |
| Drug | Camptothecin | Doxorubicin | Coumarin 6 |
| Drug loading method | Encapsulation | Encapsulation | Encapsulation |
| Sensitive groups/material | Magnetic nanoparticles; Disulfide linker |
Fe3O4; Disulfide linker |
Fe3O4 magnetic nanoparticles; Disulfide bond |
PEI/β-CD = β-cyclodextrin grafting polyethylenimine.
Fig. 9.
Schematic illustration of the nanaoparticles’ fabrication. (Adapted with permission from [69]. Copyright 2012 Wiley Online Library.).
4. Double sensitive preparations
Double sensitive preparations were typically modified on the materials or linkers to endow them targeted drug release according to a stimulus. Compared with conventional DDSs, the greatest advantage of dual-sensitive drug delivery systems is to control the drug release, thereby increasing drug targeting and antitumor effects.
4.1. Polymer micelles
Polymer micelles generally consist of a hydrophobic core and a hydrophilic shell. As a drug carrier, the typical core-shell structure gives it unique advantages, which can improve the narrow clinical application of chemotherapeutic agents caused by their hydrophobicity. However, polymer micelles can increase the solubility of poorly soluble drugs, improve drug stability, and increase drug loading due to the hydrophobic core [72]. Furthermore, polymer micelles can also improve the tumor-targeting ability and have excellent clinical application with the superiority of their skeleton consists.
In polymer micelles, methods of drug entrapment are generally divided into two ways, including chemical binding and physical entrapment. The method of chemical binding is to attach drug to polymer to prepare prodrug and self-assemble into micelles in an aqueous solution through hydrophobic and hydrophilic interactions. In the work of Zhang et al. [52], CPT was linked to mPEG via a disulfide bond and self-assembled into micelles with the polymer phenylboronic acid-poly (ethylene glycol)−4,4′-(diazene-1,2-diyl) benzoyl-poly(ε-caprolactone) (PBA-PEG-Azo-PCL) in an aqueous solution. The chemical combination of drugs and micelles can greatly reduce the leakage of drugs and the drugs can be released under the high level of GSH, which can reduce non-targeting toxicity and increase antitumor effects. In the work of Li et al. [73], the cationic polymer poly (β-hydroxyl amine) (PHA) containing tertiary amino group and disulfide bond were both pH and redox-sensitive and self-assembled into polymer micelles in aqueous solution. In addition, polymers PCL-PDEA and PEG-SS-PCL were prepared into double-sensitive mixed micelles by solvent evaporation [74]. Micelles could achieve above 90% of drug release under low pH and reducing environment within 10 h, which enhanced intracellular drug release. Interestingly, the micelles can contain magnetic particles while encapsulating the chemotherapeutic agent, which can achieve magnetic guidance and increase the tumor-targeting of chemotherapeutic agents. Star-type four-arm polymer micelles self-assembled from PEG-SS-PCL copolymers containing both DOX and Fe3O4 in the work of Tang et al. [71], they also achieved active-targeted and magnetic-guided functions.
4.2. Nanoparticles
The size of the nanoparticles is usually between 1–100 nm and small size can enter the tumor cells based on the EPR effect more easily. The redox dual-sensitive nanoparticle carrier simultaneously combines the advantages of small size of nanoparticle and controlled drug release of redox dual sensitive carrier. Platinum (Pt) anticancer drugs particularly need for controlling drug delivery because of their severe side effects. Shi et al. [75] designed Pt prodrug of cisplatin, and pH and redox dual responsive delivery system was designed by using hybrid nanoparticles of human serum albumin (HSA) and calcium phosphate (CaP) for the Pt prodrug of cisplatin. Additionally, PTX was conjugated to HES via a redox sensitive disulfide bond to form HES-SS-PTX, and the conjugates assembled into stable and monodispersed nanoparticles in the work of Li et al. [18]. In redox and light-sensitive DDSs, photosensitizers were typically attached to the nanoparticle backbone through redox-sensitive groups and self-assembled into nanoparticles in an aqueous solution with drugs encapsulated [29], [33], [76]. Redox responsive and magnetic guide DDSs can be obtained by coating a surface of MNPs with a redox-sensitive polymer or by attaching other inclusion complexes via redox-sensitive groups [71], [77]. In addition to the above, MSN and upconversion nanoparticles were also commonly used nanoparticles.
4.3. Microcapsules
Microcapsules are drug-package microcapsules that use natural or synthetic polymeric materials such as polysaccharides, proteins, etc. as capsule wall shells to encapsulate solid or liquid drugs. The biomaterials such as proteins, lipids, and amino acids are good microcapsule materials and have prospective applications in the fields of drug or gene delivery, drug-controlled release, and magnetic resonance imaging. In the work of Gao et al. [42], pH and redox responsive microcapsules were successfully assembled by covalent cross-linking of polysaccharide alginate dialdehyde (ADA) derivative and cystamine dihydrochloride (CM) through a layer-by-layer (LBL) technique. It was Schiff base and disulfide bonds that played key roles in tumor-targeting ability based on the disparity of microenvironments. In addition, Zhong et al. [78] fabricated redox responsive magnetic protein microcapsules with Fe3O4 MNPs encapsulated inside, which were conveniently delivered to targeted site under the action of magnetic field. And microcapsules also had redox responsiveness with disulfide linkage. What's more, Li et al. [79] synthesized the redox responsive and magnetic guide dual targeted folic acid-cysteine-Fe3O4 microcapsules via the sonochemical technique.
4.4. Others
Hydrogels are network formed by a hydrophilic polymer and have good biocompatibility due to the large amount of moisture absorbed by the polymer [80]. It is more suitable as a controlled release material for macromolecular drugs due to the large amount of aqueous environment which adapts to the diffusion of polar protein molecule. Stimuli-responsive hydrogels have a unique property of undergoing volume changes from their collapsed and swollen states under different external triggers. In addition to the above, graphene is widely used in various fields as a two-dimensional carbon nanomaterial [48].
5. Conclusion
In recent years, a series of delivery vehicles have been developed to control the release of chemotherapeutic agents at tumor sites under the internal microenvironment stimuli based on the differences of tumor and normal cells, such as low pH [7], high redox potential [81] and high level of ROS [82]. Redox responsive DDSs can be disassembled in a specially controlled manner, further leading to the prompt release of encapsulated drugs. However, there are also some issues needed to be further improved. Although the primitive distribution in vivo may not be altered, other stimuli can be introduced to redox responsive DDSs to improve and enhance treatment effects under the common efforts of researchers.
Moreover, the synergistic effects of the redox responsive dual-stimuli responsive DDSs have a series of powerful advantages. The pH and ROS sensitiveness could increase the release of drugs at the tumor cells and the combined application of photosensitization and chemotherapy could achieve synergistic effects presenting higher effects of anticancer and attenuation of toxicity. Magnetic guidance might increase the cumulative release amount of drugs at the tumor sites. Furthermore, enzyme sensitivity could not only accelerate drug release, but also increase the accumulation in tumor. Hence, redox responsive dual-stimuli responsive DDSs could increase accumulation of drugs, enhance tumor-targeting, accelerate drug release and improve anticancer therapy. Strategies and preparations of redox dual-stimuli responsive DDSs such as pH, light, enzyme, ROS, and magnetic guide for chemotherapeutic agents have been summarized in this review, which aimed at providing new ideas for further expanding the redox-sensitive release of drugs, enhancing drug tumor-targeting and improving anticancer effects. Besides strategies of increasing drug release stimuli, the modification of targeted-ligands, such as hyaluronic acid [83], transferrin [31], phenylboronic acid [84], and arginine-glycine-aspartic acid [85], have been introduced to improve the delivery system of the chemotherapeutic agents. Furthermore, except tumor-targeting, cancer cells spread and metastasize need to be further studied eagerly.
Declaration of Competing Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81202480, 81302723) and Natural Science Foundation of Liaoning Province (2015020749).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2019.06.003.
Appendix. Supplementary materials
References
- 1.Perez-Herrero E., Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Oh N., Park J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9(Suppl 1):51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhuang Y.Y., Deng H.P., Su Y., He L., Wang R.B., Tong G.S. Aptamer-functionalized and backbone redox-responsive hyperbranched polymer for targeted drug delivery in cancer therapy. Biomacromolecules. 2016;17(6):2050–2062. doi: 10.1021/acs.biomac.6b00262. [DOI] [PubMed] [Google Scholar]
- 4.Hirata E., Sahai E. Tumor microenvironment and differential responses to therapy. Cold Spring Harb Perspect Med. 2017;7(7):1–14. doi: 10.1101/cshperspect.a026781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan J., Zhou Z.Q., Chen M.H., Li H.Y., Tong D.N., Yao J. Folate-conjugated and pH-responsive polymeric micelles for target-cell-specific anticancer drug delivery. Acta Biomater. 2017;60:244–255. doi: 10.1016/j.actbio.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Phillips D.J., Gibson M.I. Redox-sensitive materials fordrug delivery: targeting the correct intracellular environment, tuning release rates, and appropriate predictive systems. Antioxid Redox Signal. 2014;21(5):786–803. doi: 10.1089/ars.2013.5728. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier M.A. Redox-responsive drug delivery. Antioxid Redox Signal. 2014;21(5):705–706. doi: 10.1089/ars.2014.5980. [DOI] [PubMed] [Google Scholar]
- 8.McCarley R.L. Redox-responsive delivery systems. Annu. Rev Anal Chem. 2012;5:391–411. doi: 10.1146/annurev-anchem-062011-143157. [DOI] [PubMed] [Google Scholar]
- 9.Gulzar A., Xu J.X., Xu L.G., Gai S.L., Yang P.P., He F. Redox responsive UCNPS-DPA conjugated NGO-PEG-BPEI for cancer theranostic. Dalton Trans. 2013;47:3921–3930. doi: 10.1039/c7dt04093h. [DOI] [PubMed] [Google Scholar]
- 10.Kuppusamy P., Li H.Q., Ilangovan G., Cardounel A.J., Zweier J.L. Noninvasive imaging of tumor redox status and its modification by tissue glutathionelevels. Cancer Res. 2002;62(1):307–312. [PubMed] [Google Scholar]
- 11.Cheng R., Feng F., Meng F.H., Deng C., Jan F.J., Zhong Z.Y. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152(1):2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Sun C.L., Li X.Y., Du X.D., Wang T. Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy. Int J Biol Macromol. 2018;112:65–73. doi: 10.1016/j.ijbiomac.2018.01.136. [DOI] [PubMed] [Google Scholar]
- 13.Lv Y., Yang B., Li Y.M., He F., Zhuo R.X. Folate-conjugated amphiphilic block copolymer micelle for targeted and redox-responsive delivery of doxorubicin. J Biomater Sci Polym Ed. 2018;29(1):92–106. doi: 10.1080/09205063.2017.1400146. [DOI] [PubMed] [Google Scholar]
- 14.Chi Y.Y., Yin X.L., Sun K.X., Feng S.S., Liu J.H., Chen D.Q. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Rel. 2017;261:113–125. doi: 10.1016/j.jconrel.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 15.An N., Lin H.M., Yang C.Y., Zhang T., Tong R.H., Chen Y.H. Gated magnetic mesoporous silica nanoparticles for intracellular enzyme-triggered drug delivery. Mater Sci Eng C Mater Biol Appl. 2016;69:292–300. doi: 10.1016/j.msec.2016.06.086. [DOI] [PubMed] [Google Scholar]
- 16.Liu N., Tan Y.N., Hu Y.W., Meng T.T., Wen L.J., Liu J.W. A54 peptide modified and redox-responsive glucolipid conjugate micelles for intracellular delivery of doxorubicin in hepatocarcinoma therapy. ACS Appl Mater Interfaces. 2016;8(48):33148–33156. doi: 10.1021/acsami.6b09333. [DOI] [PubMed] [Google Scholar]
- 17.Cheng W.R., Kumar J.N., Zhang Y., Liu Y. pH- and redox-responsive poly (ethylene glycol) and cholesterol-conjugated poly (amido amine)s based micelles for controlled drug delivery. Macromol Biosci. 2014;14(3):347–358. doi: 10.1002/mabi.201300339. [DOI] [PubMed] [Google Scholar]
- 18.Li Y.H., Hu H., Zhou Q., Ao Y.X., Xiao C., Wan J.L. α-Amylase- and redox-responsive nanoparticles for tumor-targeted drug delivery. ACS Appl Mater Interfaces. 2017;9(22):19215–19230. doi: 10.1021/acsami.7b04066. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y.Y., Chen Y.K., Hu C.S., Xiao L.Y., Huang W.L., Chi T.C. MAL-PDT inhibits oral precancerous cells and lesions via autophagic cell death. Oral Dis. 2019 doi: 10.1111/odi.13036. Jan 8. [DOI] [PubMed] [Google Scholar]
- 20.Avcil P., Erdem S.S., Hamblin M.R. Photodynamic therapy: one step ahead with self-assembled nanoparticles. J Biomed Nanotechnol. 2014;10(9):1937–1952. doi: 10.1166/jbn.2014.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovell J.F., Chen J., Jarvi M.T., Cao W.G., Allen A.D., Liu Y.Q. FRET quenching of photosensitizer singlet oxygen generation. J Phys Chem B. 2009;113(10):3203–3211. doi: 10.1021/jp810324v. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda T., Kaibori M., Hishikawa H., Nakatake R., Okumura T., Ozeki E. Near-infrared fluorescence imaging and photodynamic therapy with indocyanine green lactosome has antineoplastic effects for hepatocellular carcinoma. PLoS ONE. 2017;12(8):1–12. doi: 10.1371/journal.pone.0183527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega D.L., Lodge P., Vivero-Escoto J.L. Redox-responsive porphyrin-based polysilsesquioxane nanoparticles for photodynamic therapy of cancer cells. Int J Mol Sci. 2016;17(1):56. doi: 10.3390/ijms17010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren H., Liu J., Su F., Ge S., Yuan A., Dai W. Relighting photosensitizers by synergistic integration of albumin and perfluorocarbon for enhanced photodynamic therapy. ACS Appl Mater Interfaces. 2017;9(4):3463–3473. doi: 10.1021/acsami.6b14885. [DOI] [PubMed] [Google Scholar]
- 25.Amirshaghaghi A., Yan L., Miller J., Daniel Y., Stein J.M., Busch T.M. Chlorin e6-coated superparamagnetic iron oxide nanoparticle (SPION) nanoclusters as a theranostic agent for dual-mode imaging and photodynamic therapy. Sci Rep. 2019;9(1):2613. doi: 10.1038/s41598-019-39036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juarranz Á., Jaén P., Sanz-Rodríguez F., Cuevas J., González S. Photodynamic therapy of cancer. basic principles and applications. Clin Transl Oncol. 2008;10(3):148–154. doi: 10.1007/s12094-008-0172-2. [DOI] [PubMed] [Google Scholar]
- 27.Hou W.X., Xia F.F., Alves C.S., Qian X.Q., Yang Y.M., Cui D.X. MMP2-targeting and redox-responsive PEGylated chlorin e6 nanoparticles for cancer near-infrared imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2016;8(2):1447–1457. doi: 10.1021/acsami.5b10772. [DOI] [PubMed] [Google Scholar]
- 28.Liu P., Yue C.X., Sheng Z.H., Yue C.X., Sheng Z.H., Gao G.H. Photosensitizer-conjugated redox-responsive dextran theranostic nanoparticles for near-infrared cancer imaging and photodynamic therap. Ploym Chem. 2015;5(3):874–881. [Google Scholar]
- 29.Yang X.Y., Shi X.Q., Ji J.B., Zhai G.X. Development of redox-responsive theranostic nanoparticles for near-infrared fluorescence imaging-guided photodynamic/chemotherapy of tumor. Drug Deliv. 2018;25(1):780–796. doi: 10.1080/10717544.2018.1451571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan N.C., Cheng F.Y., Annie Ho J.A., Yeh C.S. Photocontrolled targeted drug delivery: photocaged biologically active folic acid as a light-responsive tumor-targeting molecule. Angew. Chem. Int Ed Engl. 2012;51(35):8806–8810. doi: 10.1002/anie.201203339. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y., Wang Z.H., Li T.L., McNally H., Park K., Sturek M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J Control Rel. 2014;176:76–85. doi: 10.1016/j.jconrel.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W., Zhu G., Wang S., Yu G., Yang Z., Lin L., et al. In situ dendritic cell vaccine for effective cancer immunotherapy. 2019;Mar 7. doi: 10.1021/acsnano.8b08346. [DOI] [PubMed]
- 33.Zhang C.N., Shi G.N., Zhang J., Niu J.F., Huang P.S., Wang Z.H. Redox- and light-responsive alginate nanoparticles as effective drug carriers for combinational anticancer therapy. Nanoscale. 2017;9(9):3304–3314. doi: 10.1039/c7nr00005g. [DOI] [PubMed] [Google Scholar]
- 34.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J.Y., Wan S.S., Zheng D.W., Lei Q., Zhuo R.X., Feng J. Propelled transnuclear gene transport achieved through intracellularly redox-responsive and acidity-accelerative decomposition of supramolecular florescence-quenchable vectors. ACS Appl Mater Interfaces. 2017;9(1):255–265. doi: 10.1021/acsami.6b14730. [DOI] [PubMed] [Google Scholar]
- 36.Chen F.Q., Zhang J.M., Wang L., Wang Y.T., Chen M.W. Tumor pHe-triggered charge-reversal and redoxresponsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale. 2015;7(38):15763–15779. doi: 10.1039/c5nr04612b. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y., Yang M., Park J.H., Singelyn J., Ma H.Q., Sailor M.J. A surface-charge study on cellular-uptake behavior of F3-peptide-conjugated iron oxide nanoparticles. Small. 2009;5(17):1990–1996. doi: 10.1002/smll.200900520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu C., Song R.J., Lu P., Chen J.C., Zhou Y.Q., Shen G. pH-triggered charge-reversal and redox-sensitive drug-release polymer micelles codeliver doxorubicin and triptolide for prostate tumor therapy. Int J Nanomedicine. 2018;13:7229–7249. doi: 10.2147/IJN.S182197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B.H., Li Y.P., Sun X.R., Meng X.L., Chen P., Liu N. A pH-responsive drug release system based on doxorubicin conjugated amphiphilic polymer coated quantum dots for tumor cell targeting and tracking. J Chem Technol Biotechnol. 2013;88(12):2169–2175. [Google Scholar]
- 40.Gao C., Liu T., Dang Y.H., Yu Z.Y., Zhang X.Q., He G.H. pH/redox responsive core cross-linked nanoparticles from thiolated carboxymethyl chitosan for in vitro release study of methotrexate. Carbohydr Polym. 2014;111:964–970. doi: 10.1016/j.carbpol.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Mavuso S., Choonara Y.E., Marimuthu T., Kumar P., du Toit L.C., Kondiah P.P.D. A dual pH/Redox responsive copper-ligand nanoliposome bioactive complex for the treatment of chronic inflammation. Int J Pharm. 2016;509(1–2):348–359. doi: 10.1016/j.ijpharm.2016.05.069. [DOI] [PubMed] [Google Scholar]
- 42.Gao L., Fei J.B., Zhao J., Cui W., Cui Y., Li J.B. pH- and redox-responsive polysaccharide-based microcapsules with autofluorescence for biomedical applications. Chemistry (Easton) 2012;18(11):3185–3192. doi: 10.1002/chem.201103584. [DOI] [PubMed] [Google Scholar]
- 43.Cheng W.R., Kumar J.N., Zhang Y., Zhang Y., Liu Y. pH- and redox-responsive self-assembly of amphiphilic hyperbranched poly (amido amine)s for controlled doxorubicin delivery. Biomater Sci. 2015;3(4):597–607. doi: 10.1039/c4bm00410h. [DOI] [PubMed] [Google Scholar]
- 44.Han L., Tang C., Yin C.H. Dual-targeting and pH/redox-responsive multi-layered nanocomplexes for smart co-delivery of doxorubicin and siRNA. Biomaterials. 2015;60:42–52. doi: 10.1016/j.biomaterials.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 45.del Mercato L.L., Rivera-Gil P., Abbasi A.Z., Ochs M., Ganas C., Zins I. LbL multilayer capsules: recent progress and future outlook for their use in life sciences. Nanoscale. 2010;2(4):458–467. doi: 10.1039/b9nr00341j. [DOI] [PubMed] [Google Scholar]
- 46.De Cock L.J., De Koker S., De Geest B.G., Grooten J., Vervaet C., Remon J.P. Polymeric multilayer capsules in drug delivery. Angew Chem Int Ed Engl. 2010;49(39):6954–6963. doi: 10.1002/anie.200906266. [DOI] [PubMed] [Google Scholar]
- 47.Cai M.T., Leng M.T., Lu A.J., He L., Xie X.X., Huang L. Synthesis of amphiphilic copolymers containing zwitterionic sulfobetaine as pH and redox responsive drug carriers. Colloids Surf B Biointerfaces. 2015;126:1–9. doi: 10.1016/j.colsurfb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Al-Nahain A., Lee S.Y., In I., Lee K.D., Park S.Y. Triggered pH/redox responsive release of doxorubicin from prepared highly stable graphene with thiol grafted pluronic. Int J Pharm. 2013;450(1–2):208–217. doi: 10.1016/j.ijpharm.2013.04.053. [DOI] [PubMed] [Google Scholar]
- 49.de la Rica R., Aili D., Stevens M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv Drug Deliv Rev. 2012;64(11):967–978. doi: 10.1016/j.addr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Han H., Wang J., Chen T., Yin L., Jin Q., Ji J. Enzyme-sensitive gemcitabine conjugated albumin nanoparticles as a versatile theranostic nanoplatform for pancreatic cancer treatment. J Colloid Interface Sci. 2017;507:217–224. doi: 10.1016/j.jcis.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X.X., Eden H.S., Chen X.Y. Peptides in cancer nanomedicine: drug carriers, targeting ligands and protease substrates. J Control Release. 2012;159(1):2–13. doi: 10.1016/j.jconrel.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L., Wang Y., Zhang X., Wei X., Xiong X., Zhou S. Enzyme and redox dual-triggered intracellular release from actively targeted polymeric micelles. ACS Appl Mater Interfaces. 2017;9(4):3388–3399. doi: 10.1021/acsami.6b14078. [DOI] [PubMed] [Google Scholar]
- 53.Anajafi T., Yu J., Sedigh A., Haldar M.K., Muhonen W.W., Oberlander S. Nuclear localizing peptide-conjugated, redox-sensitive polymersomes for delivering curcumin and doxorubicin to pancreatic cancer microtumors. Mol Pharm. 2017;14(6):1916–1928. doi: 10.1021/acs.molpharmaceut.7b00014. [DOI] [PubMed] [Google Scholar]
- 54.Sun T.B., Zhua C.Z., Xu J. Multiple stimuli-responsive selenium-functionalized biodegradable starch-based hydrogels. Soft Matter. 2018;14(6):921–926. doi: 10.1039/c7sm02137b. [DOI] [PubMed] [Google Scholar]
- 55.Liu M., Du H., Khan A.R., Ji J., Yu A., Zhai G. Redox/enzyme sensitive chondroitin sulfate-based self-assembled nanoparticles loading docetaxel for the inhibition of metastasis and growth of melanoma. Carbohydr Polym. 2018;184:82–93. doi: 10.1016/j.carbpol.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 56.Khawar I.A., Kim J.H., Kuh H.J. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Rel. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 57.Gao W., Ye G., Duan X., Yang X., Yang V.C. Transferrin receptor-targeted pH-sensitive micellar system for diminution of drug resistance and targetable delivery in multidrug-resistant breast cancer. Int J Nanomedicine. 2017;12:1047–1064. doi: 10.2147/IJN.S115215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tao W.H., He Z.G. ROS-responsive drug delivery systems for biomedical applications. Asian J Pharm Sci. 2018;13(2):101–112. doi: 10.1016/j.ajps.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S.H., Gupta M.K., Bang J.B., Bae H., Sung H.J. Current progress in reactive oxygen species (ROS)-responsive materials for biomedical applications. Adv Healthc Mater. 2013;2(6):908–915. doi: 10.1002/adhm.201200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y., Guo Q., An S., Lu Y., Li J., He X. ROS-switchable polymeric nanoplatform with stimuli-responsive release for active targeted drug delivery to breast cancer. ACS Appl Mater Interfaces. 2017;9(14):12227–12240. doi: 10.1021/acsami.6b16815. [DOI] [PubMed] [Google Scholar]
- 61.Zhang M., Song C.C., Su S., Du F.S., Li Z.C. ROS-activated ratiometric fluorescent polymeric nanoparticles for self-reporting drug delivery. ACS Appl Mater Interfaces. 2018;10(9):7798–7810. doi: 10.1021/acsami.7b18438. [DOI] [PubMed] [Google Scholar]
- 62.Tang M., Hu P., Zheng Q., Tirelli N., Yang X., Wang Z. Polymeric micelles with dual thermal and reactive oxygen species (ROS) responsiveness for inflammatory cancer cell delivery. J Nanobiotechnol. 2017;15(1):39. doi: 10.1186/s12951-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deepagan V.G., Kwon S., You D.G., Nguyen V.Q., Um W., Ko H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials. 2016;103:56–66. doi: 10.1016/j.biomaterials.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Meng G., Zhang S., Liu X. A reactive 1O2-responsive combined treatment system of photodynamic and chemotherapy for cancer. Sci Rep. 2016;6:29911. doi: 10.1038/srep29911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye M., Han Y., Tang J., Piao Y., Liu X., Zhou Z. A tumor-specific cascade amplification drug release nanoparticle for overcoming multidrug resistance in cancers. Adv Mater. 2017;29(38) doi: 10.1002/adma.201702342. [DOI] [PubMed] [Google Scholar]
- 66.Kang Y., Ju X., Ding L.S., Zhang S., Li B.J. Reactive oxygen species and glutathione dual redox-responsive supramolecular assemblies with controllable release capability. ACS Appl Mater Interfaces. 2017;9(38):4475–4484. doi: 10.1021/acsami.6b14640. [DOI] [PubMed] [Google Scholar]
- 67.Chiang Y.T., Yen Y.W., Lo C.L. Reactive oxygen species and glutathione dual redox-responsive micelles for selective cytotoxicity of cancer. Biomaterials. 2015;61:150–161. doi: 10.1016/j.biomaterials.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Pradhan P., Giri J., Rieken F., Koch C., Mykhaylyk O., Döblinger M. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Rel. 2010;142(1):108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Luo Z., Cai K., Hu Y., Li J., Ding X., Zhang B. Redox-responsive molecular nanoreservoirs for controlled intracellular anticancer drug delivery based on magnetic nanoparticles. Adv Mater. 2012;24(3):431–435. doi: 10.1002/adma.201103458. [DOI] [PubMed] [Google Scholar]
- 70.Thurein S.M., Lertsuphotvanit N., Phaechamud T. Physicochemical properties of β-cyclodextrin solutions and precipitates prepared from injectable vehicles. Asian J Pharm Sci. 2018;13(5):438–449. doi: 10.1016/j.ajps.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Z., Zhang L., Wang Y., Li D., Zhong Z., Zhou S. Redox-responsive star-shaped magnetic micelles with active-targeted and magnetic-guided functions for cancer therapy. Acta Biomater. 2016;42:232–246. doi: 10.1016/j.actbio.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 72.Pan Y., Wang X.H., Yin Z.N. Synthesis and evaluation of cationic polymeric micelles as carriers of lumbrokinase for targeted thrombolysis. Asian J Pharm Sci. 2018 doi: 10.1016/j.ajps.2018.03.004. May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D.W., Bu Y.Z., Zhang L.N., Wang X., Yang Y.Y., Zhuang Y.P. Facile construction of pH- and redox-responsive micelles from a biodegradable poly (β-hydroxyl amine) for drug delivery. Biomacromolecules. 2016;17(1):291–300. doi: 10.1021/acs.biomac.5b01394. [DOI] [PubMed] [Google Scholar]
- 74.Cai M., Zhu K., Qiu Y., Liu X., Chen Y., Luo X. pH and redox-responsive mixed micelles for enhance dintracellular drug release. Colloids Surf B Biointerfaces. 2014;116:424–431. doi: 10.1016/j.colsurfb.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 75.Shi H., Cheng Q., Yuan S., Ding X., Liu Y. Human serum albumin conjugated nanoparticles for pH and redox-responsive delivery of a prodrug of cisplatin. Chemistry (Easton) 2015;21(46):16547–16554. doi: 10.1002/chem.201502756. [DOI] [PubMed] [Google Scholar]
- 76.Molina A.M., Morales-Cruz M., Benítez M., Berríos K., Figueroa C.M., Griebenow K. Redox-sensitive cross-linking enhances albumin nanoparticle function as delivery system for photodynamic cancer therapy. J Nanomed Nanotechnol. 2012;6(3):294. doi: 10.4172/2157-7439.1000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stephen Z.R., Kievit F.M., Veiseh O., Chiarelli P.A., Fang C., Wang K. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors. ACS Nano. 2014;8(10):10383–10395. doi: 10.1021/nn503735w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong S.L., Cui X.J., Tian F.Y. Fabrication of redox-responsive magnetic protein microcapsules from hen egg white by the sonochemical method. J Microencapsul. 2015;32(7):705–710. doi: 10.3109/02652048.2015.1073389. [DOI] [PubMed] [Google Scholar]
- 79.Li Z., Zhang C., Wang B., Wang H., Chen X., Möhwald H. Sonochemical fabrication of dual-targeted redox-responsive smart microcarriers. ACS Appl Mater Interfaces. 2014;6(24):22166–22173. doi: 10.1021/am5057097. [DOI] [PubMed] [Google Scholar]
- 80.Rafael D., Andrade F., Martinez-Trucharte F., Basas J., Seras-Franzoso J., Palau M. Sterilization procedure for temperature-sensitive hydrogels loaded with silver nanoparticles for clinical applications. Nanomaterials (Basel) 2019;9(3):1–14. doi: 10.3390/nano9030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanamala M., Wilson W.R., Yang M., Palmer B.D., Wu Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials. 2016;85:152–167. doi: 10.1016/j.biomaterials.2016.01.061. [DOI] [PubMed] [Google Scholar]
- 82.Hu J.J., Lei Q., Peng M.Y., Zheng D.W., Chen Y.X., Zhang X.Z. A positive feedback strategy for enhanced chemotherapy based on ROS-triggered self-accelerating drug release nanosystem. Biomaterials. 2017;128:136–146. doi: 10.1016/j.biomaterials.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Chen D., Dong X., Qi M., Song X., Sun J. Dual pH/redox responsive and CD44 receptor targeting hybrid nano-chrysalis based on new oligosaccharides of hyaluronan conjugates. Carbohydr Polym. 2017;157:1272–1280. doi: 10.1016/j.carbpol.2016.10.089. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Zhang Z., Su X., Cai M., Zhuo R., Zhong Z. Phenylboronic acid-functionalized polymeric micelles with a hepg2 cell targetability. Biomaterials. 2013;34(38):10296–10304. doi: 10.1016/j.biomaterials.2013.09.042. [DOI] [PubMed] [Google Scholar]
- 85.Kunjachan S., Pola R., Gremse F., Theek B., Ehling J., Moeckel D. Passive versus active tumor targeting using RGD- and NGR-modified polymeric nanomedicines. Nano Lett. 2014;14(2):972–981. doi: 10.1021/nl404391r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.