Abstract
Modular polyketide synthases (PKSs) are a multidomain megasynthase class of biosynthetic enzymes that have great promise for the development of new compounds, from new pharmaceuticals to high value commodity and specialty chemicals. Their colinear biosynthetic logic has been viewed as a promising platform for synthetic biology for decades. Due to this colinearity, domain swapping has long been used as a strategy to introduce molecular diversity. However, domain swapping often fails because it perturbs critical protein-protein interactions within the PKS. With our increased level of structural elucidation of PKSs, using judicious targeted mutations of individual residues is a more precise way to introduce molecular diversity with less potential for global disruption of the protein architecture. Here we review examples of targeted point mutagenesis to one or a few residues harbored within the PKS that alter domain specificity or selectivity, affect protein stability and interdomain communication, and promote more complex catalytic reactivity.
Keywords: Polyketide synthase, Site directed mutagenesis, Rational design, Saturation mutagenesis, Synthetic biology, Protein engineering
Abbreviations: PKS, polyketide synthase; KS, ketosynthase; AT, acyltransferase; ACP, acyl carrier protein; KR, ketoreductase; MT, methyltransferase; ER, enoylreductase; DH, dehydratase; PS, pyran synthase; EI, enoylisomerase; SNAC, N-acetyl cysteamine; LM, loading module; DEBS, 6-deoxyerthronolide B synthase; Mod, module
Introduction
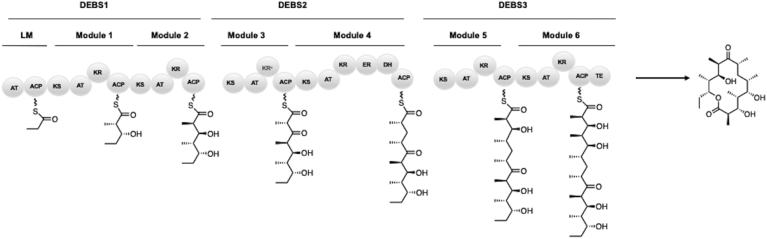
Bacterial modular type I polyketide synthases (PKSs) are responsible for the generation of a diverse range of natural product therapeutics including antibiotics (e.g. erythromycin), antifungals (e.g. amphotericin), immunosuppressants (e.g. rapamycin), and antiparasidics (e.g. avermectin). The incredible potential of PKSs as pharmaceutical generators has lent considerable interest in their biosynthesis. They possess a tantalizing, colinear biosynthetic logic, meaning they generate polyketides via megasynthase molecular assembly lines organized into modules. Each module performs one round of elongation followed by optional reductive processing with discrete enzymatic domains correlating to chemical transformations. A module, corresponding to one round of elongation and processing, is minimally composed of a ketosynthase (KS) domain which catalyzes decarboxylative Claisen condensation, an acyltransferase (AT) domain which is responsible for extender unit selection (malonyl-CoA or derivatives thereof), and an acyl carrier protein (ACP) which anchors the elongating carbon chain through a phosphopantetheinyl prosthetic group. Optional processing domains include a C-methyltransferase (MT) that methylates at the α position as well as β-carbon processing domains such as a ketoreductase domain (KR) which reduces the keto moiety to a hydroxy moiety, a dehydratase (DH) responsible for dehydration of the hydroxy group to form an olefin, and an enoylreductase (ER) which forms a saturated carbon backbone. A canonical example of this colinearity is shown below with the 6-deoxyerythronolide B (6-dEB) synthase (DEBS) (Fig. 1).
Fig. 1.
The 6-deoxyerythronolide B synthase (DEBS) which produces the aglycone precursor to erythromycin, 6-dEB through priming with a loading module (LM) and undergoing six successive decarboxylative Claisen condensations followed by reductive processing to the β-carbon.
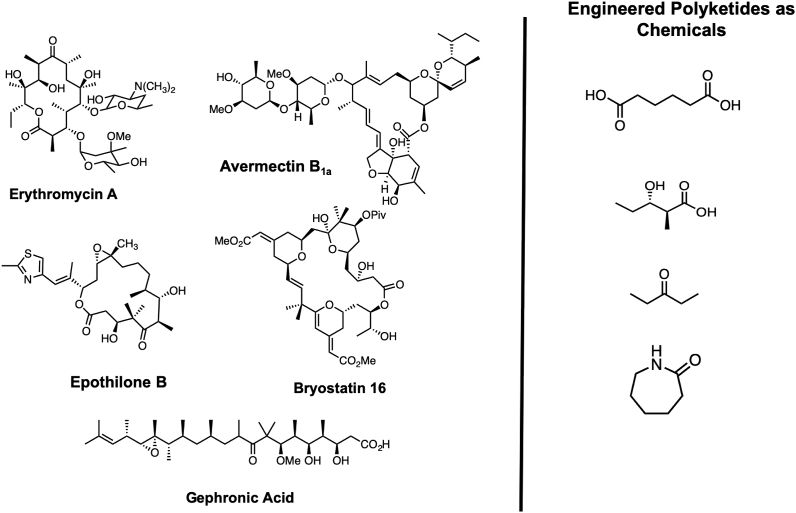
Because of issues with toxicity, analogs of natural products are often necessary for the development of a desired compound. However, due to the structural complexity of most natural product scaffolds, this can present some challenges when performed through traditional synthetic chemistry. Drawing inspiration from their colinear biosynthetic logic, the bioengineering community has had a longstanding interest in re-engineering polyketide synthases. More recent efforts have expanded their potential utility. For example, Keasling and coworkers have noted that sequential polymerizations of C2 and C3 synthons of varying degrees of oxygenation and reduction present the chemical transformations necessary for producing many petrochemical derivatives including commodity chemicals, specialty chemicals, and fuel additives (Fig. 2) [[1], [2], [3]].
Fig. 2.
Comparison of natural product scaffolds and petrochemically derived feedstocks such as adipic acid [3], 3-hydroxy acids [161,162], short chain ketones [97], and caprolactam [163] explored by Keasling and coworkers.
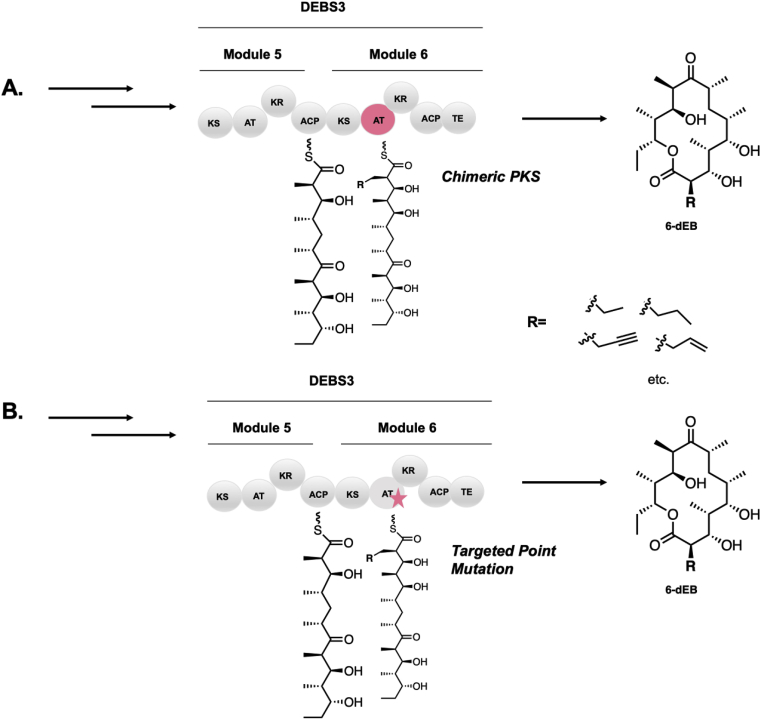
The “legoization” [4] of PKS domains has been envisioned as a conceptual strategy for engineering since the discovery of their colinear nature [5]. Because discrete domains catalyze transformations with specificity and selectivity, the idea of “domain swapping” to mix and match PKS biosynthetic machinery has been posited to realize the ramifications of colinearity [[6], [7], [8], [9], [10]] (Fig. 3A). A pioneering early example of the “molecular lego” approach that probed the modularity of domain swapping was performed by Menzella and coworkers. Restriction sites were introduced for the facile creation of both bimodular [11] and trimodular hybrid [4,12] polyketide synthases to generate model lactones with varying degrees of reduction and stereochemistry. While some success was observed, titers were low, often for reasons not easily rationalized. Further domain swapping experiments have been performed to alter selectivity of the AT [[13], [14], [15]], KR [16], and DH domains [3,17,18], of various PKSs, although typically with reduced or stalled activity. For example, more recently, Keasling and co-workers underwent an extensive study to engineer an enzyme from the borrelidin PKS that could produce adipic acid. Unfortunately, this study also produced trace amounts of active chimeric PKS with an accumulation of incompletely reduced product [3].
Fig. 3.
Comparison of A) a domain swapping approach and B) a site directed mutagenesis approach to alter the selectivity of EryAT6.
Over the past twenty years, significant advances have been made toward the structural characterization of PKS biosynthetic machinery [[19], [20], [21]]. These include the elucidation of PKS domain and didomain crystal structures [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]], the characterization of interdomain and intermodular linkers [[36], [37], [38], [39], [40], [41]], and the determination of a cryo-EM structure of a representative module [[42], [43], [44]]. The structural elucidation of PKSs has afforded us the ability to visualize the vast degree of evolutionary complexity and architecturally critical protein-protein interactions which ensure interdomain communication and stability of the dimer interface. When examining these characterized interactions, the rationale for why domain swapping has often failed to yield well folded or highly active protein [45,46] is apparent. However, when domain swapping is performed with critical consideration made to important interactions via judicious selection of domain boundaries, success can be observed. One of the most recent successful examples was performed by Yuzawa and coworkers who used a structure-based strategy to probe several AT domains and key associated linker regions to pinpoint boundaries for AT swaps. This study relied upon conserved motifs to install AT domains and suggested that the most successful boundaries preserved structurally distinct portions of the KS-AT linker and the post-AT linker, and were highly consistent between most ATs assayed. Indeed in one case, the kinetic data of the AT-swapped domain actually improved relative to wild type [47]. Nonetheless, examples of domain swapping with such success remain rare.
One alternative to domain swapping which minimizes global architectural disruption to the PKS involves engineering through individual point mutations (Fig. 3B). One or a few point mutations can drastically change the selectivity or specificity of domains harbored within the PKS, and in some circumstances, the communication between domains. Logically, making one or a few point mutations to a PKS is typically less globally destabilizing at the protein level than full domain swaps. One of the easiest transformations through site directed mutagenesis is to abolish activity through the mutation of a key active site residue. This has been performed numerous times for the purpose of mechanistic investigations, and loss-of-function mutations are frequently found in natural systems as a means of introducing molecular diversity. Furthermore, point mutations can be performed to alter the activity of a domain. This can be achieved through changing specificity (e.g. altering the extender unit choice in the AT domain), altering selectivity (shown in the KR and ER domains), or manipulating active site residues to perform new chemical transformations altogether (as is seen in the DH domain). Although not intended to be comprehensive, in this review we highlight examples of key amino acid residues identified through mutagenesis experiments which demonstrate the potential for precise manipulation of PKS biosynthesis. By capitalizing on our molecular-level structural understanding, this targeted “one at a time” approach is a powerful tool for creating chemical diversity.
Ketosynthase
Mutations to abolish activity: In nature, there are a few examples of KS domains lacking in catalytic function. Mostly commonly, these occur in trans-AT PKSs, which differ in that the AT domain is present on a separate polypeptide. These pathways have a number of other unique features with regard to their modular organization relative to the canonical PKSs (also called cis-AT PKSs) and are reviewed extensively by Piel [48,49]. One distinct feature is that catalytically inactive KS domains (KS0) that nonetheless appear to have a structural role [50] are more prevalent in trans-AT pathways than cis-AT pathways. Three catalytic residues are present in the KS domain, two histidines and a cysteine. In the KS0 domains of trans-AT PKSs, typically the histidine from the HGTG decarboxylation motif is missing [51]. Loading KS domains that lack condensing activity are termed KSQ domains and have a conserved glutamine residue replacing the catalytic cysteine. KSQ domains catalyze decarboxylation of a malonyl or methymalonyl unit to an acetyl or propionyl priming unit, respectively [52,53]. Thus, nature has evolved roles for the KS domain for performing its canonical Claisen condensation. To purposefully inactivate the KS, mutation of either the active site cysteine or histidine of the HGTG motif to alanine has been successful [54,55].
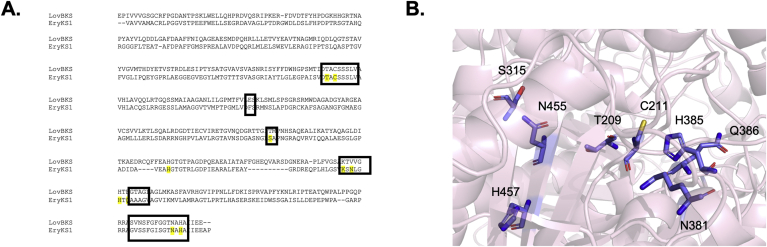
Mutations to alter activity: In a study performed by Khosla and coworkers, amino acid sequences of assembly line and iterative PKSs were compared, and a 90% consensus sequence was generated for each subgroup. Of those residues conserved universally or within each subgroup, those within 10 Å of the active site cysteine were selected for mutation in EryKS1. In most cases the residues were replaced with alanine including T209A, S315A, N381A, H457A. The two exceptions include N455L where an isosteric change was introduced and Q386E where the corresponding residue found in the subgroup of iterative KSs was introduced [54]. Three mutants of the Module 1 KS including C211A, H346A, and K379A resulted in dramatically reduced activity. The H346A mutant produced ~1% of product compared to the wild type, whereas the K379A mutant was added at 10-fold higher concentration and still produced only ~1% of product. These results suggest that both K379 and H346 are critical to catalyzing the formation of the C–C bond between the electrophilic and nucleophilic substrates, which differs from the mechanism of fatty acid synthesis regarding H346. H346 was also found to enhance decarboxylation of methylmalonyl-ACP by stabilizing the associated transition state. Similarly, K379 plays a role in C–C bond formation. Finally, the C211A mutant displayed no activity whatsoever, but was still a competent methylmalonyl-ACP carboxylase. Thus, the role of C211 is most likely to provide an extremely nucleophilic active site for anchoring the polyketide chain prior to decarboxylative Claisen condensation (Fig. 4) [54]. Considering that these residues appear to play an essential role in catalysis, homology-based methods may be used to uncover differences in the binding pocket affecting substrate selectivity.
Fig. 4.
A) Alignment of LovB and EryKS1 used in the mutagenesis experiment reported by Khosla and coworkers to identify conserved residues between modular and iterative PKSs essential for catalysis. Residues mutated in the report are indicated in yellow and residues found within 10 Å of the active site cysteine are in boxes. The alignment was performed with MUSCLE. B) Homology Model of EryKS1 with the residues mutated by Khosla and coworkers highlighted in blue. The homology model was made with Swiss PDB using EryKS3 as a template.
One of the most powerful reasons to alter KS activity is that the KS possesses “gatekeeping” behavior towards substrates. It was revealed that the KS domain is inactive or kinetically slow towards unnatural substrates [[56], [57], [58]], often resulting in a kinetic bottleneck when the structures resulting from proceeding modules have been altered. This sequential selectivity of the KS is one of the biggest hurdles to PKS engineering. One classic example is that EryKS2 within DEBS1 appears to be sensitive to alterations in stereochemistry [56,57], and is thus unable to condense unnatural stereoisomers.
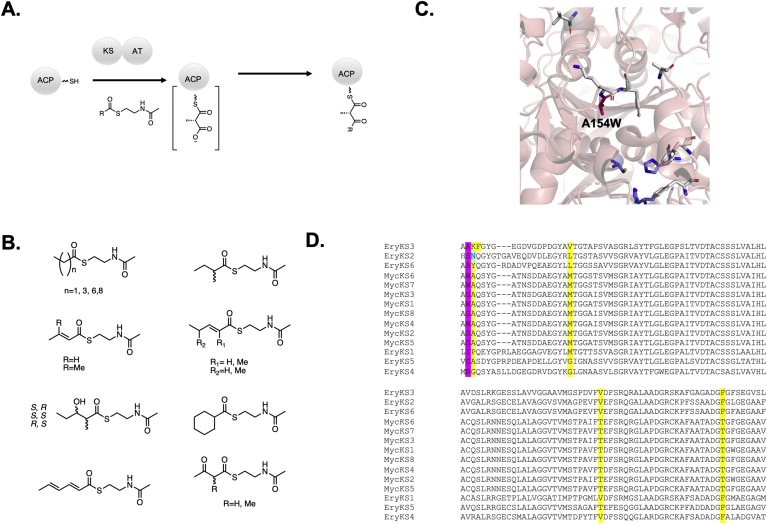
One of the most successful recent examples of altering the gatekeeping behavior of the KS domain was an investigation of EryKS3. Leadlay and coworkers performed a sequence alignment of EryKS3 with the mycolactone KS domains and discovered an exceptional degree of sequence similarity (>97%) despite condensing units of differing lengths and stereochemistry [59]. As EryKS3 has been shown to have gatekeeping activity and a crystal structure is available [32], residues that deviated between the mycolactone KS domains and EryKS3 were targeted for mutagenesis. Indeed this approach proved successful; a simple mutation of alanine 154 in EryKS3 to tryptophan greatly enhanced the steric flexibility of the KS while allowing for the condensation of bulkier substrates (Fig. 5) [58]. The success of this simple mutation indicates that relatively conservative changes can be employed to address gatekeeping and lends credence to this homology-based approach. In addition to using pathways with high homology between modules, KSs from terminal modules (such as EryKS6) also tend to have looser substrate promiscuity [60,61]. Thus, targeting mutations to either KSs that accept large substrates or KSs that retain a great degree of homology from within their biosynthetic pathways may be the key to enabling the retention of structural changes including altered stereochemistry, degrees of reduction, and successful processing of unnatural extender units.
Fig. 5.
A) Overview of the ability of KS1 to extend unnatural N-acetyl cysteamine substrates. B) Panel of substrates tested. C) Structure of EryKS3 (pdb code 2QO3) with the residues mutated to explore the sequence space divergence in the mycolactone KSs. D) Sequence alignment of the KSs within DEBS and the those within the mycolactone PKS. The residue found by our laboratory in a common assembly of DEBS1-TE is highlighted (mutated to histidine to introduce a restriction site). The alignment was performed with MUSCLE.
The KS domain is also responsible for whether one round of elongation per module is performed, as is typical of type I PKSs, or iteration occurs via back-transfer to the ACP. Programmed iteration appears in a number of modular type I PKSs including the aureothin, borrelidin, and stigmatellin PKSs [62]. Therefore, altering residues that influence the kinetics of the KS towards back transfer of the substrate from the ACP may be a means to change the PKS structure for expansion of the carbon skeleton. Khosla and coworkers explored transforming EryMod3 from a modular enzyme to an iterative one, however such changes focused on altering regions of the ACP in contact with the KS [63]. To date, targeted mutation of the KS for reprogramming iteration is an underexplored area.
Acyltransferase
Mutations to abolish activity: The AT domain selects malonyl-CoA derivative extender units, most commonly methylmalonyl and malonyl-CoA. It is responsible for introducing functionality including heteroatoms, halogens, and alterations to the carbon chain length that can drastically affect the scaffold of the PKS carbon skeleton [64]. Mechanistically, the AT domain selects an extender unit through a serine-histidine catalytic dyad and either transfers the acyl enzyme intermediate through transthioesterification to the next ACP or hydrolyzes the extender unit from the acyl enzyme intermediate [13,65,66]. Inactivation of the AT can be achieved directly through mutation of the active site serine to alanine in the GHSxG motif (Fig. 6). One example of the utility of inactivating the AT domain to create molecular diversity was demonstrated via trans-AT complementation, a strategy wherein a trans-AT is introduced to deliver an extender unit to the module with the inactivated AT domain. This approach was originally employed by Khosla and coworkers using the disorazole pathway. It was then employed by Khosla and coworkers to introduce ethymalonyl-CoA through KirCII, a trans-AT of the kirromycin pathway, to Mod6 of DEBS [67], and later by Chang and co-workers [68,69] as a means of delivering fluoridated extender units.
Fig. 6.
A) Example of a monomethylating MT domain harbored within in a PKS module. Frequently, other β-carbon processing domains are found, for example, a full reductive loop shown above. B) Example of a gem-dimethylating MT domain. C) Active site of CurMT3 (pdb code: 5THZ) from the curacin A pathway. Residues targeted for mutation are shown in orange and the SAH cofactor is shown in blue.
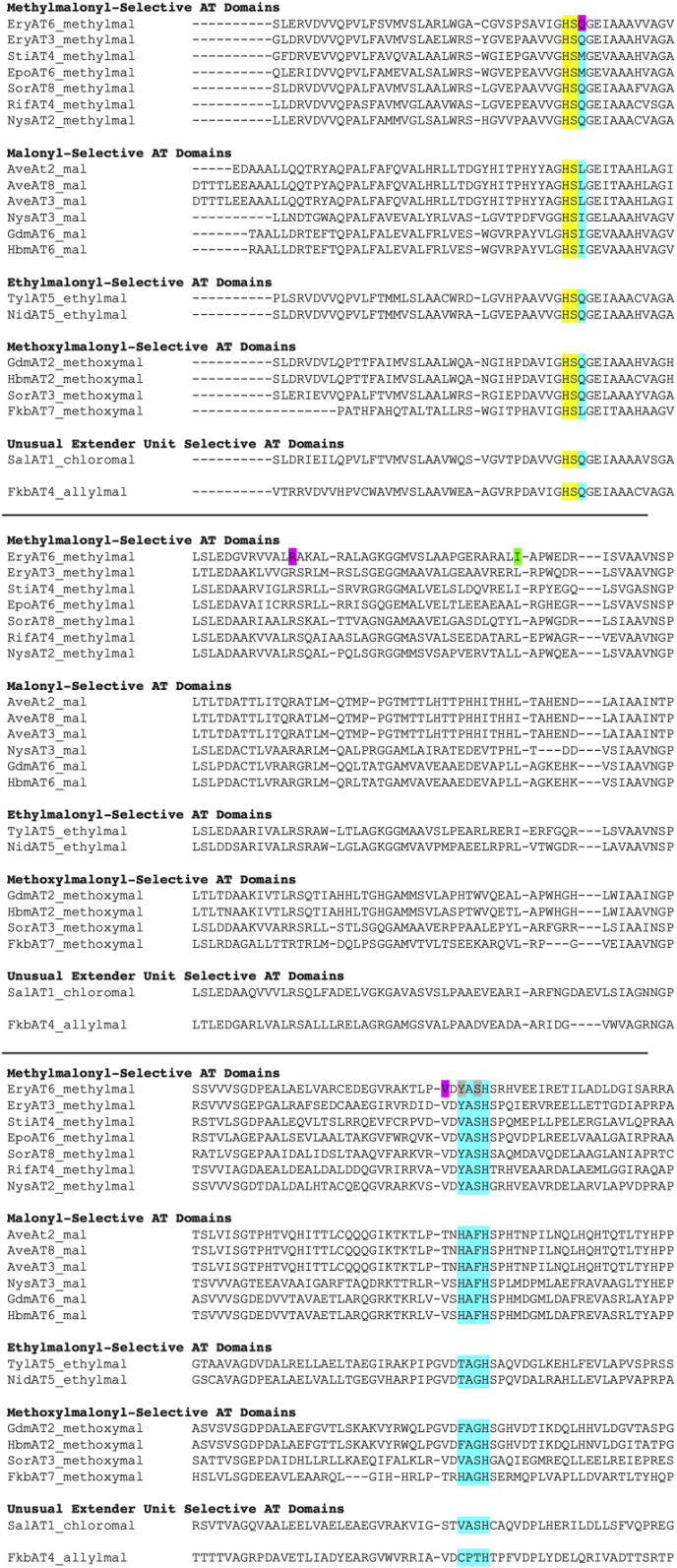
Mutations to alter activity: Altering the selectivity of the acyltransferase domain was one of the earliest examples of targeted point mutations in PKS domains. The two most commonly utilized extender units, malonyl-CoA and methylmalonyl-CoA, are readily identifiable by diagnostic selectivity motifs, HAFH and YASH for malonyl-CoA and methylmalonyl-CoA, respectively, although occasionally VASH is observed instead of YASH for methylmalonyl-CoA. Early attempts to alter selectivity focusing on these motifs were performed in EryAT1 [70], EryAT4 [70], and EryAT6 [71]. In each of these domains, the mutations increased promiscuity, although did not entirely reverse selectivity, indicating that mutations beyond this diagnostic “fingerprint” region are likely necessary to entirely reverse selectivity. This was later verified by Williams and coworkers vide infra [72]. Mixed selectivity motifs exist, including the HASH motif observed in epothilone AT3 which natively possesses a relaxed specificity and accepts a mixture of malonyl- and methylmalony-CoA [73]. Other motifs appear for rarer extender units including RPGH for SalAT1, a chloromalonyl-selective AT. Additionally, VASH is associated with a number of AT selectivities including methylmalonyl-, ethymalonyl-, and methoxymalonyl-CoA. Methoxymalonyl-CoA-selective ATs have a range of additional motifs including FAGH and HAGH. Finally, CPTH is observed in an allylmalonyl-selective AT in the FK506 pathway [74]. Thus, motifs correlating to ATs that accept rarer extender units tend to be much less conserved (Fig. 7).
Fig. 7.
Sequence alignment of AT domains. Signature motifs including YASH for methylmalonyl-CoA, HAFH for malonyl-CoA, TAGH for ethylmalonyl-CoA, and less conserved motifs for rarer extender units as well as the diagnostic Q versus I/L motif indicating methylmalonyl versus malonyl-CoA are highlighted in cyan. The active site histidine/serine dyad is highlighted in yellow. Mutations introduced by Schulz and coworkers in EryAT6 are indicated in magenta [76,77,115], those introduced by Williams and coworkers [72] are indicated in green, and those explored by both the Williams and Schulz groups are indicated in grey. The alignment was performed with MUSCLE.
A targeted approach was taken to expand the selectivity of the terminal AT domain of DEBS to accept varying extender units. Schulz and coworkers suggested that there are only subtle differences in the AT that afford discrimination between methylmalonyl-CoA and malonyl-CoA. In addition, they hypothesized that non-natural substrate exclusion is not strict but rather evolutionarily unimportant. This hypothesis is consistent with the observation that in pathways where a rare extender unit is employed, distributions of metabolites are often observed. For example, the salinosporamide PKS, SalAT1, predominantly accepts chloromethylmalonyl-CoA (salinosporamide A) but also accepts ethylmalonyl-CoA (salinosporamide B) and methylmalonyl-CoA (salinosporamide D) to a lesser degree, resulting in a distribution of metabolites [64]. An analogous phenomenon is observed in the FK506 pathway where allylmalonyl-CoA is used in addition to propylmalonyl-CoA, ethylmalonyl-CoA, and methylmalonyl-CoA. As additional precedence, Williams and coworkers discovered that EryMod6 is more intrinsically promiscuous than anticipated toward a range of unnatural substrates including ethylmalonyl-CoA, propargylmalonyl-CoA, isopropylmalonyl-CoA, butylmalonyl-CoA, methoxymalonyl-CoA, 2-azodomalonyl-CoA, and phenylethylmalonyl-CoA in vitro [75].
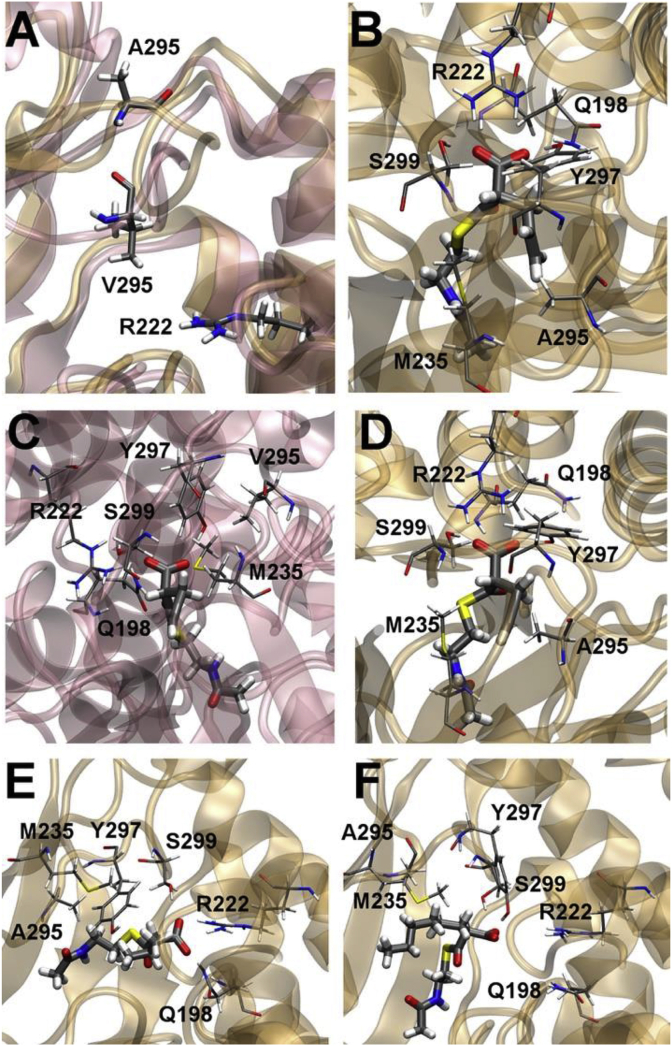
To test their hypothesis, Shulz and coworkers used a directed mutagenesis approach to mutate EryAT6. Initial studies used a truncated substrate of the phosphopanetheinyl arm, 2-propargyl-malonyl-N-Acetylcysteamine (SNAC) to identify promising residues [71] and a follow-up study used 2-propargyl-malonyl-CoA as an extender unit [76,77]. These experiments identified a new mutation that expanded the promiscuity of EryAT6, V295A. Molecular dynamic (MD) simulations of wild type EryAT6 suggested that the carboxylate of malonyl-CoA and methylmalonyl-SNAC interact with a few key residues in the active site such as Q198, M235, and V295 (Fig. 6, Fig. 8). Q198 was found to have contact with the thioester of methylmalonyl-SNAC while the latter two residues appeared to be responsible for stereochemical control [76]. Among the mutations most successful for introducing new extender units, MD simulations revealed that an EryAT6 V295A mutation widens the active site to allow additional substrates to bind (Fig. 8). The V295A EryAT6 mutant was found to relax, but not switch, substrate specificity (Fig. 8) [76].
Fig. 8.
Snapshots of molecular dynamics simulations of the wild type EryAT6 (rose) and EryAT6 V295A (ochre). A) Depiction of an alanine mutation which allows for greater flexibility of the binding pocket compared to valine. B) Allylmalonyl-SNAC placed in A295A EryAT6 which can be accommodated by the increased binding pocket of the alanine mutation. C) Propargylmalonyl-SNAC does not readily fit into the active site of wild type EryAT6. D) Propargylmalonyl-SNAC fits better in the V295A mutant than the wild type. E) V295A with isopropylmalonyl-SNAC. F) Hexanoylmalonyl-SNAC. Reproduced with permission from ref. [76].
Williams and coworkers capitalized on the previously uncovered intrinsic promiscuity of ATs through a different approach. Rather than directed mutagenesis, they utilized saturation mutagenesis. Residues likely to form the binding pocket were identified via sequence alignment and homology modeling as L118, Y198, and S191 (Fig. 6). While Y198 and S191 correlate to the YASH motif, L118 does not correlate to any previously described specificity-conferring motif. However, it was chosen due to its likeliness to form interactions with the extender unit determined via homology modeling. Partially degenerate mutagenic oligonucleotides were used to introduce an average of 12 different amino acids at each targeted position, and the variants were incubated with a diketide-SNAC thioester and methymalonyl-CoA for an initial screen to determine their activity towards propargylmalonyl-CoA. Following this initial screen, the mutants were subjected to a more complex system utilizing a substrate mimic developed by Sherman and coworkers to determine if DEBS3 would extend. The most successful mutation discovered through this study was Y189R, a completely novel mutation which afforded enhanced propargylmalonyl-CoA selectivity. When a double mutant of Y189R in concert with the V187A mutation identified by the Schulz group was assayed, EryAT6's extender unit specificity was completely inverted. Rather than merely becoming more promiscuous, Y189R/V187A EryAT6 preferentially accepted unnatural extender units over methylmalonyl-CoA, particularly allylmalonyl-CoA, ethylmalonyl-CoA, propylmalonyl-CoA, and 2-azidomalonyl-CoA. Such an inversion of specificity was unprecedented in an engineered AT domain [72].
AT promiscuity was also uncovered by taking advantage of the S. cinnamonensis malonyl-CoA ligase (MatB) enzyme which ligates a range of malonate derivatives to CoA to then be used as extender units by AT domains. Such substrates include propanenitrile, phenyl, and thiophene substituted malonyl-CoAs [78]. MatB enzymes have been extensively engineered for enhanced AT promiscuity toward a range of malonate derivatives by the group of Gavin Williams [79,80].
Uniquely among PKS domains, mutations to the AT domain have the most potential for metabolic engineering to alter metabolite scaffolds. Both in engineered and natural contexts, AT promiscuity is common and metabolite structure is dictated by precursor availability. One common biosynthetic pathway for uncommon malonyl-CoA derivatives is through a crotonyl-CoA carboxylase/reductase (CCR) enzyme reviewed by Moore [81,82]. An example of successful metabolic engineering of an extender unit was performed using the salinosporamide PKS. In this study, a strategy to engineer fluoromethylmalonyl-CoA production was implemented in Salinospora tropica. SalAT1 was able to accept this unnatural substrate and an analog of salinosporamide A, flourosalinosporamide, was successfully generated by replacing the chlorine atom with fluorine [83]. An example of utilizing engineered MatB in vivo to supply unnatural precursors was demonstrated by the groups of Weber, Lee, and Williams to generate “bioderivativized” kirromycin analogs [84]. Although trans-AT domains typically utilize malonyl-CoA, KirCII is a trans-AT that natively accepts ethylmalonyl-CoA and has promiscuity towards a broad range of other analogs including azidomalonyl-CoA, allylmalonyl-CoA, propargylmalonyl-CoA, and others [80,84,85]. Thus, manipulation of the available precursor flux of extender units in concert with targeted changes to the AT domain ultimately determines the final metabolite structure [72,84,85].
Methyltransferase
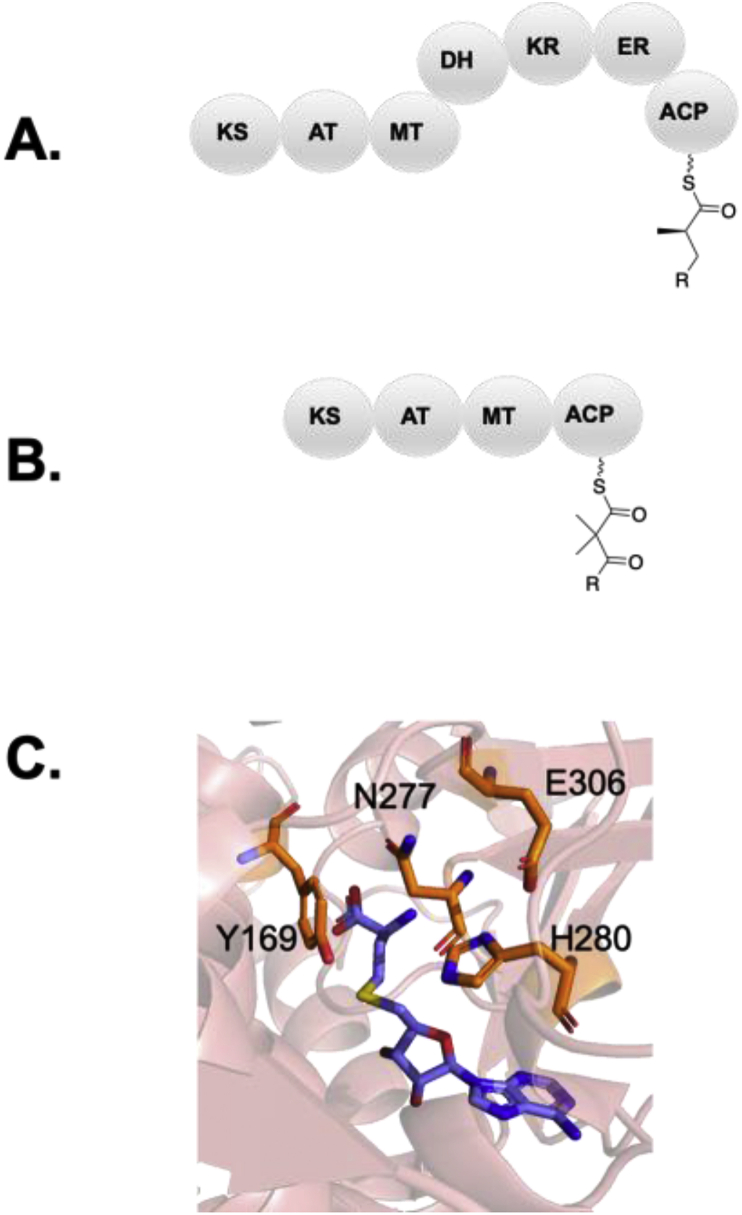
Mutations to abolish activity: The MT is the most recently characterized domain harbored within modular bacterial PKSs and is somewhat uncommon in cis-AT PKSs, although common in trans-AT PKSs [[86], [87], [88], [89], [90]]. The MT introduces methyl branches to the α-carbon via S-adensoyl-methionine (SAM) as opposed to methylmalonyl-CoA. Notably, iterative fungal type I PKSs exclusively use MT domains to introduce methyl branching, however bacterial modular PKSs have evolved two distinct means of doing so. Both monomethylating MT domains and gem-dimethylating MT domains exist (Fig. 6A and B). The recent structural characterization of a monomethylating cis-AT PKS from the curacin biosynthetic pathway, CurMT3, has lent greater insight into the active site environment [86]. To understand gem-dimethylation, an MT from the disorazole PKS, DisMT3, was recently crystalized [87]. An invariant glutamine/histidine catalytic dyad is present in all MTs both from bacterial and fungal PKS pathways, and it is hypothesized that the imidazole of the histidine residue may act as a catalytic base to deprotonate the substrate α-carbon. Consistent with this hypothesis, alanine mutations to the active site histidine/glutamine dyad abolished activity in CurMT3. The melting temperatures of these mutants were comparable to the wild type, suggesting that the elimination of activity was not due to compromised stability of the enzyme, but rather the removal of active site residues [86]. Likewise, analogous mutations were made in DisMT3 to H288 (H288N and H288A) which resulted in inactivation of the MT. Thus to inactivate the MT, the conserved active site histidine appears to be a reliable route (Fig. 6C) [87].
Mutations to alter activity: In CurMT3, other mutations to conserved residues in the active site including Y169F and N277A decreased activity, suggesting that they play a role in substrate positioning (Fig. 6C). Although chemoenzymatic methods to create analogs of SAM have been developed [91], no research has been put forth involving the possible transfer of other unnatural analogs with varying sidechains in bacterial PKSs. However, such an approach has been demonstrated to allow for the incorporation of a propargyl moiety through a propargyl SAM analog in a fungal PKS. Thus, tuning the kinetics to incorporate unnatural SAM analogs may be a highly valuable future area of exploration to increase molecular diversity [92].
To determine the residues that impact catalysis for gem-dimethylating MTs, mutations were made to Y170F, E341A, N285A, and E341Q. None of these mutants substantially affected the rate of gem-dimethylation. Other potentially relevant residues forming a hydrophobic tunnel were suggested including Y132, M166, L169, and Y170, although mutations to these residues were not performed [93]. For gem-dimethylating MTs, an intriguing application would be the generation of quaternary carbons possessing different functional groups, for example, methylation to a domain extended with ethylmalonyl-CoA. Only one example of such a substitution through domain swapping has been explored by Abe and coworkers, and the experiment was unsuccessful [94]. In the future, targeting mutations that expand the hydrophobic tunnel for the accommodation of bulkier groups may be highly desirable to alter metabolite conformation [95].
Ketoreductase
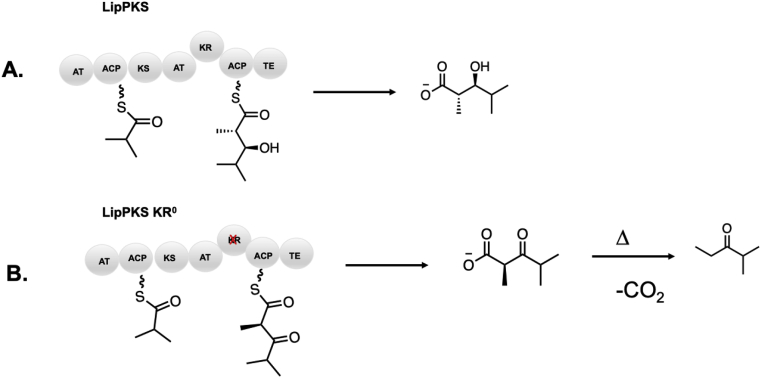
Mutations to abolish activity: The active site of the KR is comprised of three residues including a serine, tyrosine (sometimes replaced by histidine), and asparagine [21]. Alteration of either the active site serine (typically to an alanine) or tyrosine (typically to a phenylalanine) can abolish activity [96]. One example of this strategy was used by Keasling and coworkers to manipulate the lipomycin PKS. The inactivated KR yielded a terminal β-keto acid which was readily converted to the corresponding ketone upon thermal decarboxylation. This method was utilized for the production of small branched chain ketones that could be used as gasoline additives (Fig. 9) [47,97].
Fig. 9.
Example of strategic inactivation of KR residues to generate keto moieties. A) LipPKS, composed of the Lip1 gene (LM and Mod1) fused to EryTE. B) Inactivation of the KR to strategically generate short chain ketones [97].
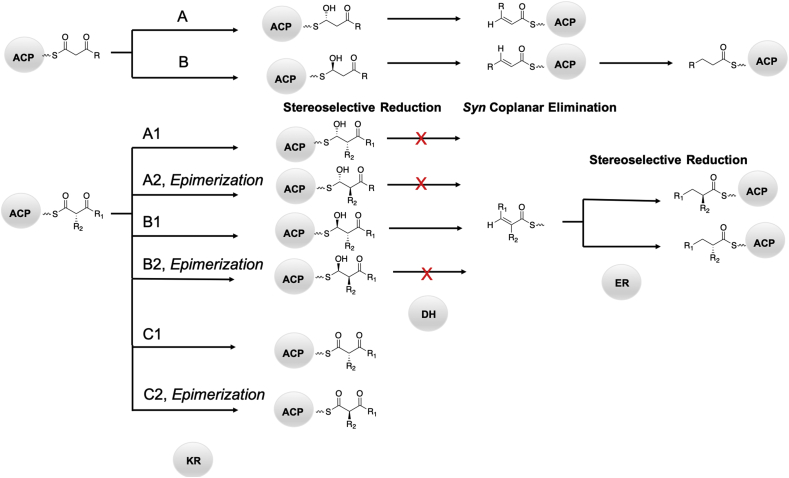
KR domains are categorized twofold: first by the stereoselectivity conferred to the hydroxy group during reduction (A-type KRs generate l-configured hydroxy groups whereas B-type KRs generate d-configured hydroxy groups) and second by whether they are non-epimerizing or epimerizing (denoted by a 1 for non-epimerizing and a 2 for epimerizing). Additionally, reductase-incompetent KRs are termed C-type KRs. These are common in PKSs and lack the conserved active site asparagine, however some retain epimerizing function (Fig. 9) [26]. Epimerizing C2-type KRs harbor the catalytic tyrosine and serine, and it was determined that these residues are indeed responsible for catalyzing epimerization [96]. Examples of C2-type KRs are present in several common macrolide scaffolds including erythromycin, picromycin, tylosin, niddamycin, and meglomyicin [26]. Entirely inactive KRs (classified as C1-type) lacking both the active site serine and tyrosine are far rarer, but are occasionally found, such as oligiomycin KR4 [27].
Mutations to alter activity: Mutations to alter the stereoselectivity of the KR domain have been extensively studied, particularly by the groups of Leadlay and Keatinge-Clay (reviewed by Keatinge-Clay [98]). During biosynthesis, when α substitution is present, the AT domain typically only selects 2S configured units (e.g. 2S-methylmalonyl-CA). The condensation reaction catalyzed by the KS domain inverts the configuration, resulting in 2R stereochemistry (Fig. 9). While the exact mechanistic path for epimerization is unknown, one potential hypothesis is that differences in conformation allow varying access to water in the active site [99]. Congruent with that hypothesis, non-epimerizing KRs can be converted to epimerizing KRs with only a single point mutation, which need not be an active site base [22,100]. Diagnostic fingerprint motifs have been identified for all KR types (Fig. 10) [27,101,102].
Fig. 10.
Schematic of chemical fates of the elongating polyketide by β-carbon processing domains.
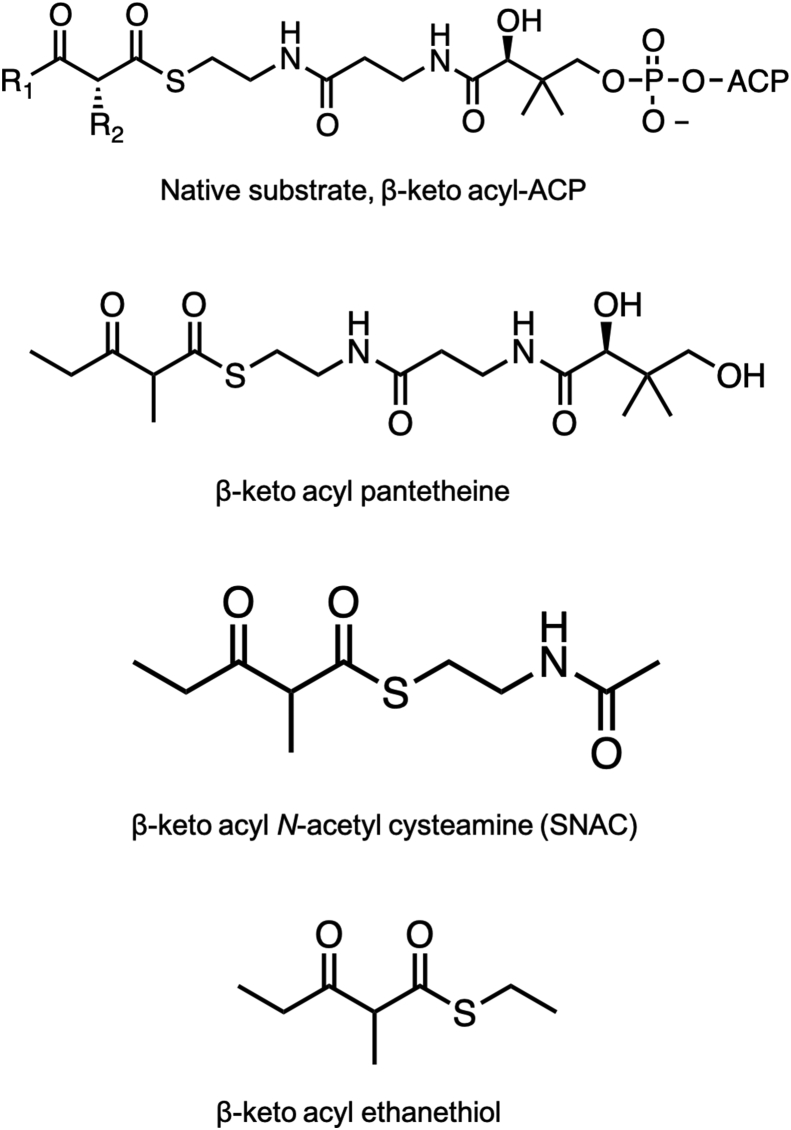
The KR domain is one of the most thoroughly studied PKS domains. Biochemically, it is extremely stable when excised as a standalone domain and numerous studies have been performed on KRs with model substrates such as N-acetyl cysteamine (SNAC)-linked β-keto thioesters (Fig. 13). The residue numbering of standalone KRs (especially EryKR1) vary from report to report. The KR domain is composed of two Rossman folds: a catalytic subdomain and a structural subdomain that does not bind NADPH but is nonetheless part of the complete structure [24,98]. In some reports, only the catalytic subdomain is numbered, while in others the entire intact KR domain is numbered, and sometimes the gene numbering (e.g. EryKR1's position in DEBS1) is used. This inconsistency can cause confusion while comparing and analyzing mutations from different studies. For the sake of clarity, we refer the readers to Fig. 12 where we have used the standalone domain numbering for EryKR1 (including both catalytic and structural subdomains) to display the active site.
Fig. 13.
Native β-keto acyl-ACP and truncated small molecule substrate mimics for in vitro studies of decreasing complexity used by standalone KR domains.
Fig. 12.
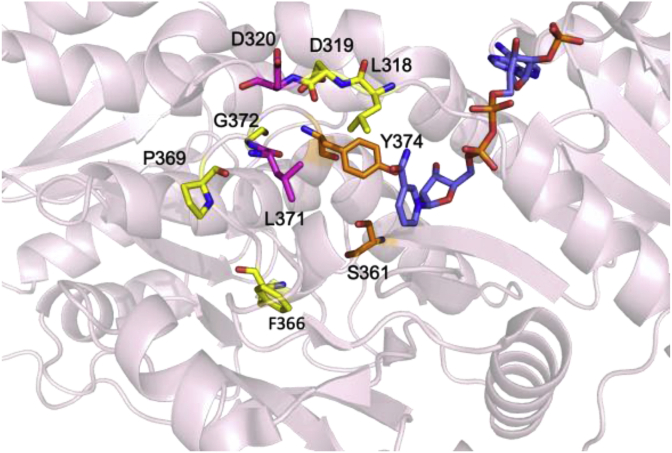
Active site of EryKR1 (pdb code 2FR1). The active site tyrosine and serine are indicated in orange. The NADP+ cofactor is indicated in blue. Residues mutated by the Keatinge-Clay group are indicated in magenta [100]. Residues mutated by the Leadlay group are indicated in yellow [104,105]. D320 was mutated by both Keatinge-Clay and coworkers and Leadlay and coworkers.
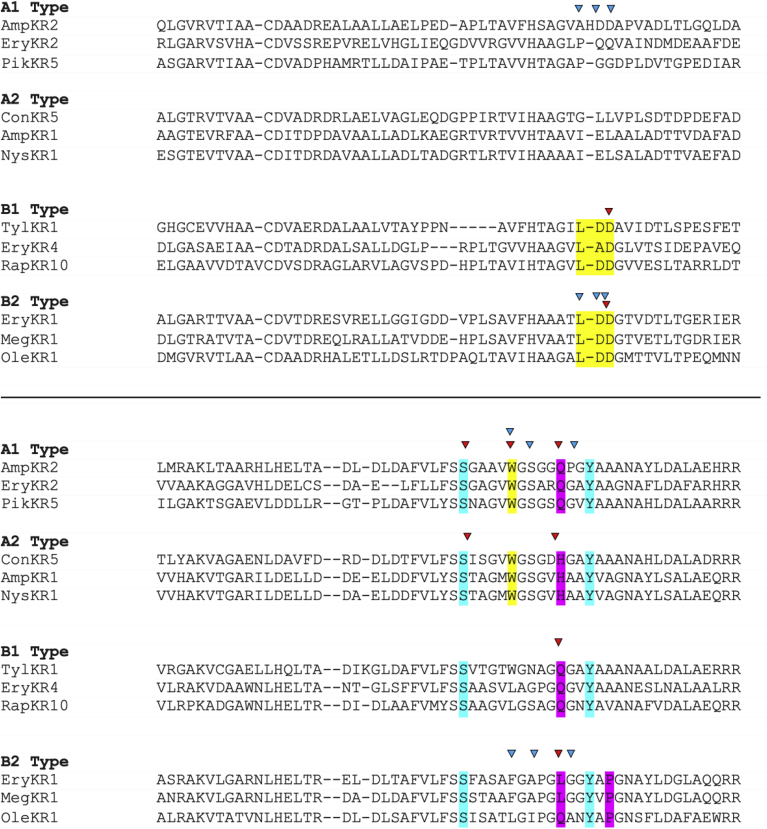
Seminal early investigations using excised KRs were performed by Leadlay and coworkers who examined EryKR1 and EryKR2. These were cloned as standalone domains and assayed for the reduction of truncated β-ketoacyl SNAC substrate mimics (Fig. 13) [[103], [104], [105]]. First, Leadlay and coworkers implemented a rationally designed directed mutagenesis approach based on apparent sequence differences between different KR types [104]. These motifs targeted two regions of the KR domain, first, the LDD motif diagnostic of B-type KRs, and second, the residues in a loop containing conserved tryptophan diagnostic of A type KRs. In EryKR1 a phenylalanine is present in this position (Fig. 11). At the time, only homology models were available for designing residue exchanges, however these predicted a reasonable structure for the Rossman fold and remain essentially in agreement with more recent structural characterization by X-ray crystallography [[22], [23], [24],26,27,98,106,107]. In EryKR1, phenylalanine, proline, and glycine residues were determined to be part of a loop directly adjacent to the catalytic tyrosine, whereas the LDD motif was present in a loop adjacent to the active site. In EryKR2, the residues corresponding to the LDD motif were PQS. Therefore, up to six residues were mutated including the residues corresponding to the LDD motif (EryKR1 PQS and EryKR2 LDD) along with residues corresponding to tryptophan, proline, and glycine. For this motif, in EryKR1 and EryKR2, WGG and LPN triple mutants were generated, respectively. Finally, all six mutations were implemented (Fig. 12). All mutants probed in this study had comparable activity to the wild type on an SNAC β-keto thioester substrate, however a complete reversal of stereoselectivity to form the epimeric A2 product was observed with the EryKR1 WGG triple mutant. The EryKR2 PQS mutant also resulted in mixed stereoselectivity, with a close to equal mixture of the native A1 and epimeric A2 products. Upon the introduction of all six mutations, however, complete reversal to A2 stereoselectivity occurred in EryKR2 [104]. A complementary experiment involved a saturation mutagenesis approach on these motifs [105]. The libraries were screened against decalone, which had been shown previously to be an effective model substrate for screening activity [103,108,109]. Selected mutants with high activity towards decalone were then assayed with a β-keto thioester model substrate. Like the directed mutagenesis study, a handful of mutations affected both the B2-type EryKR1 and the A1-type EryKR2 through alterations in stereoselectivity, typically requiring at least 3 mutations for a complete reversal, but activity was reduced compared to wild type. Most frequently, the A2 isomer was observed, although for some mutations, trace amounts of other stereochemical alterations were also observed [105].
Fig. 11.
Sequence alignment indicating diagnostic motifs in the KR domain. Active site residues are shown in cyan. Residues that are fingerprints for hydroxy stereochemistry are indicated in yellow (for A type KRs a W and for B type KRs an LDD motif). Residues associated with α stereochemistry are indicated in magenta. Residues mutated by Leadlay and coworkers are indicated by blue arrows [104,105]. Residues mutated by Keatinge-Clay and coworkers are indicated by red arrows [22,25,100]. The alignment was performed with MUSCLE.
Keatinge-Clay and coworkers demonstrated that individual point mutations could affect the stereoselectivity of the second KR from the amphotericin PKS, AmpKR2, an A1-type [22,25]. Intriguingly, two point mutations, Q364H and G355T, entirely resulted in the epimeric A2 product as well as a fourfold increase in catalytic activity [22]. Additionally, a single mutation from a glutamine to a histidine three residues away from the catalytic tyrosine changed the behavior of AmpKR2 from an A1-type to a nonspecific A-type KR [25]. Keatinge-Clay and coworkers later discovered that extremely truncated mimics of the phosphopantetheinyl arm, including ethanethiol thioesters could retain native stereoselectivity in a panel of representative KRs, which was somewhat corroborated by studies of TylKR1 by Lüdeke and coworkers [110], [111]. With the goal of understanding why such poor mimics of the phosphopantethienyl arm retained near natural stereocontrol, Keatinge-Clay and coworkers examined motifs correlated with controlling α stereochemistry. They also sought to determine if subtle non-covalent interactions in the active site would dictate stereochemical outcome. To this end, a residue that correlates with α stereochemical outcome was identified three residues away from the catalytic tyrosine, typically leucine in B2-type, glutamine in A1-and B1-type, and histidine in A2-type KRs (Fig. 11). In EryKR1, alteration of leucine to glutamine or histidine only resulted in a catalytically deficient enzyme. However, a leucine to alanine mutation in EryKR1 resulted in formation of the epimeric A2 product with increased catalytic activity [100]. Indeed, when a analogous mutations were made to the other KR types including TylKR1, AmpKR2, and RifKR7, a similar switch of selectivity to form the A2 product was observed in all but RifKR7, which is naturally an A2-type KR. From this, Keatinge-Clay and coworkers rationalized that the observed anti selectivity follows Felkin-Anh stereoselectivity, suggesting that when the active site is sterically flexible, an enzyme will default to the thermodynamically favored product [100]. Both this result and the results from directed mutagenesis of AmpKR2 [22,25] were consistent with Leadlay and coworkers’ findings that the A2 isomer was most frequently observed in both directed mutagenesis [104] and saturation mutagenesis [108] experiments. However, the directed mutagenesis experiments performed by Keatinge-Clay and coworkers [22,25,100] achieved the same selectivity with fewer mutations compared to those by Leadlay and coworkers [104,105] (1–2 as compared to 3–6) and resulted in an enzyme with increased catalytic activity compared to the wild type rather than decreased.
As noted by Keatinge-Clay and coworkers, the difference required to generate an enantiomeric excess of 99% is only ~3 kcal/mol in ΔΔG‡ at room temperature [100]. This suggests that effecting stereochemical changes through extremely subtle alterations of the active site should be achievable. It is currently unknown whether or not binding interactions of the phosphopantetheinyl arm play a large role in orienting the substrate, yet it is energetically possible to achieve total stereocontrol with a small energetic barrier. Thus, it is not implausible that minor changes to the active site from a single residue substitution, as was observed in model β-keto thioester substrates, could be retained in the context of a module fused to an ACP-linked substrate. The fact that kinetic parameters of β-keto SNAC thioester substrates and the more natural pantetheinyl thioester substrates remain comparable further supports this hypothesis [112]. The thermodynamic values for binding to the ACP compared to those of binding the KR to various mimics of the phosphopantethienyl arm have been probed by isothermal titration calorimetry. These Kd values are on the same order of magnitude for KRs binding to the ACP compared to increasingly truncated phosphopantetheinyl arm mimics, suggesting that any binding contributions from the phosphopantetheinyl arm of the ACP to the KR likely only account for less than 1 kcal/mol in ΔΔG‡ at room temperature [113].
Translation of standalone mutant KR activity towards β-keto thioester SNAC substrates in the context of a module has been tested in an investigation by Leadlay and coworkers. When the most effective mutations from their studies on EryKR1 and EryKR2 were introduced to DEBS1-TE and grown in vivo in Streptomyces coelicolor, the result was either no metabolite formation or trace amounts of incompletely reduced ketolactone. These results suggest that the mutations were not retained in the more complex environment of the module [112] despite the kinetic arguments outlined above. However, from these experiments it is unclear whether the EryKR1 and EryKR2 mutants have not retained the altered selectivity possessed as standalone domains toward model small molecule substrates in the context of a full module on a covalently attached ACP-linked substrate, or if this is due to downstream effects such as gatekeeping from downstream domains. More work needs to be done to discriminate between these two possibilities. Nonetheless, the fact that small energetic differences are sufficient to alter stereoselectivity at a chemical level is extremely promising. These results suggest that point mutations can be used as a means to subtly alter the KR active site environment and reliably alter molecular stereochemistry through enzymatic asymmetric induction.
Dehydratase
Mutations to abolish activity: The active site of the DH domain contains of a conserved catalytic histidine which acts as a general acid and general base [114] along with a conserved aspartate residue [30]. Mutation of the histidine residue to another abolishes the activity of the DH in nature. Examples of inactive DHs exist in antilid module 2, ansamitocin module 1, PMM110117 module 5, and nanchanomycin module 1. Schulz and coworkers successfully inactivated several DH domains in the monensin PKS via an active site histidine to phenylalanine mutation. Perplexingly, this approach appeared to be effective in only four out of six cases [115]. DH domains that retain the active site histidine but have substitutions to the active site aspartate tend to have new catalytic activities, a phenomenon which offers a potentially intriguing expansion of chemical space (vide infra).
Mutations to alter activity: The DH domain undergoes a syn coplanar elimination [[28], [29], [30],[116], [117], [118], [119], [120]], thus the stereochemistry of the KR domain proceeding the DH domain enforces the stereochemical outcome of this reaction (Fig. 10). DH domains proceeding KR domains conferring l stereochemistry result in cis double bonds whereas DH domains proceeding KR domains that confer d stereochemistry result in trans double bonds [57]. The relationship between E and Z stereochemistry with α substitution is slightly more complex [29,118,121], but nonetheless appears to be dependent on KR stereochemistry. Thus, facile alterations to alter olefin geometry through DH domain engineering alone would likely be unsuccessful. However, DH domains do appear to be sensitive to the stereoelectronic geometry of the substrate they dehydrate [3,119,122,123]. Structural studies of the active sites of fluviricin DH1 and borrelidin DH3 suggest that this sensitivity is influenced by a diagnostic region of the substrate binding cavity [122]. Tuning this binding pocket with minor substitutions may be a feasible way to expand the substrates accommodated by DH domains. Similar to the KS domain, the DH domain may be a gatekeeper that prevents structural changes introduced in upstream modules. As the DH domain catalyzes a reversible, thermoneutral reaction that is further shifted towards the right by being in 55 M water, a poor or loose steric fit between substrate and binding pocket is likely to shift the equilibrium towards the hydroxy as opposed to the olefin, which poses a particular challenge for engineered systems [3,119,123].
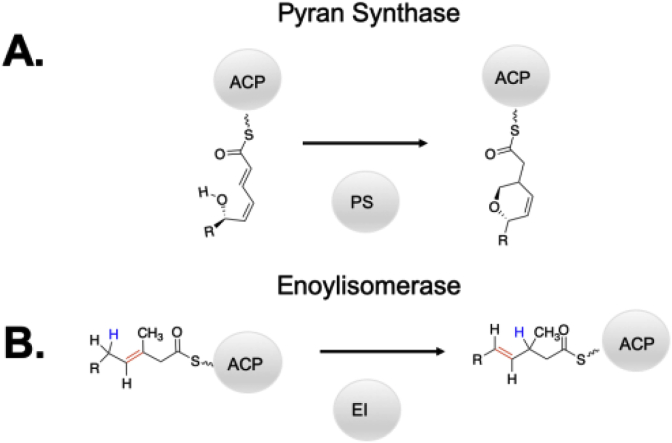
Perhaps one of the most exciting prospects for DH engineering involves domains possessing the DH's characteristic double hot dog fold, but lacking activity as a dehydratase. These domains have been shown to perform multiple types of isomerization reactions when dehydration activity is absent, as the DH is the only non-reductive β-carbon processing domain harbored within PKSs. The general acid-general base chemistry catalyzed by the DH can be co-opted for other transformations. Specifically, this includes enoyl isomerization and the isomerization of a linear polyketide to form a pyran (Fig. 14). Enoyl isomerases possess an asparagine in place of the catalytic aspartate [124,125]. Pyran synthases typically have either histidine or asparagine at this location [126]. An unusual case where both the catalytic histidine and aspartate are present is found in the third dehydratase in the ambrucitin pathway [127,128]. AmbDH3 performs both dehydration and cyclization in dual steps. The typical H51 and D215 residues are essential for dehydration, but through examination and structural characterization, V173 was determined to be unique to the cyclizing activity of AmbDH3. In most dehydrating DHs, the residue homologous to V173 is a tyrosine. Homology models suggests that putting a hydrophobic branched residue in that location creates enough space in the active site to facilitate cyclization [128]. Hence, these studies provide both insight into the evolutionary path from a dehydratase to an isomerizing enzyme and clues for how subtle alterations to DH active sites enable alternative chemistry from the catalytic histidine.
Fig. 14.
Types of reactions catalyzed by domains with DH double hot dog folds including A) cyclization to form a pyran and B) isomerization of the olefin.
Enoylreductase
Mutations to abolish activity: The ER domain stereospecifically reduces an enzyme-bound 2-enoyl intermediate. It is the least structurally and biochemically characterized β-carbon processing domain. To date, the exact active site residues are not entirely established, although they appear to be largely composed of an active site lysine, aspartate, and tyrosine [129]. As a member of the medium chain dehydrogenase (MDR) super family, it was assumed that, like the mammalian fatty acid synthase (FAS), the tyrosine would be a critical residue. Mutation of any one of the proposed active site residues does not entirely abolish catalytic activity, however in the spinosad enoylreductase, SpinER2, a lysine to alanine mutation resulted in the largest loss of activity [31]. For this reason, it is difficult to inactivate an ER domain through a catalytic residue. The most effective mutations of the ER domain appear to be in the NADPH binding pocket, as shown by Schulz and coworkers, who mutated the first two glycines in the TGGVG motif to S and P in the monensin PKS [115]. One example of an inactive ER domain in nature is fostreicin ER1 [130]. Fostreicin ER1 has a proline in place of the usual tyrosine or valine, however the NADPH binding site appears to be uninterrupted.
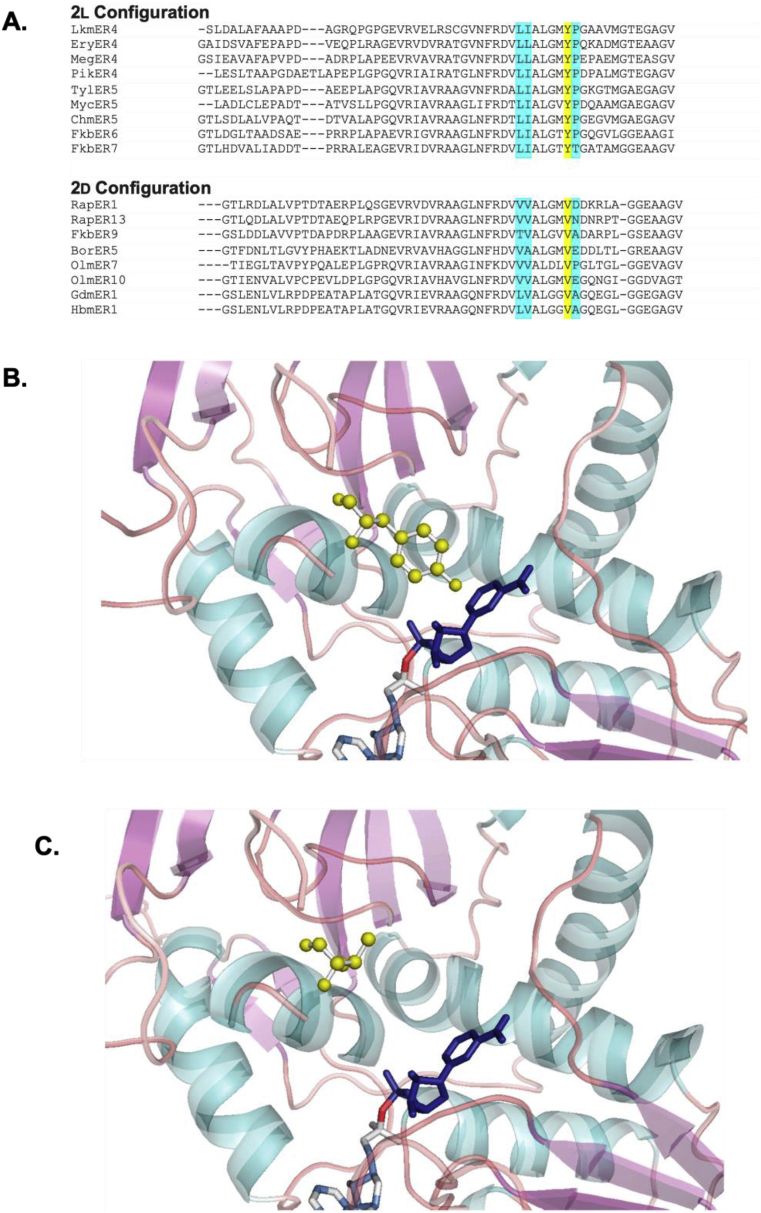
Mutations to alter activity: A study performed by Kwan and Leadley revealed key active site residues influencing the stereospecificity of enoylreduction in the rapamycin PKS. Additionally, several residues previously identified as important in the medium-chain dehydrogenase/reductase family of enzymes were found to be unimportant for enoylreduction in rapamycin biosynthesis [129]. For modular PKS ER domains in which the residue at position 52 is tyrosine, the stereochemical outcome consistently favors the formation of a (2l)-alkyl-branched product, whereas for ER domains in which the residue at position 52 is valine, alanine, or phenylalanine, the (2r) product is almost always formed instead (Fig. 15) [131]. Following this discovery, amino acid mutations were performed in two different ER domains to determine whether stereochemistry of the product would be influenced. Mutagenesis of tyrosine to valine in EryER4 resulted in switching the stereochemistry of enoylreduction while mutatagenesis of valine to tyrosine in position 52 of RapER13 resulted in unchanged stereochemistry [129].
Fig. 15.
A) Sequence alignment of ER types by stereochemical outcome. The diagnostic tyrosine and valine are indicated in yellow. Other residues that correlate with stereochemical outcome but do not appear to have any impact in mutagenesis studies are indicated in yellow. The alignment was performed with MUSCLE. B) Homology model of EryER4 indicating a hypothesis for the role of the diagnostic tyrosine, which provides steric crowding and prevents approach from the re face. C) Homology model of RapER13 indicating a hypothesis for the role of the diagnostic valine, which sterically affords space for reduction from the re face. Panels B–C reproduced with permission from ref. [129], [131].
Further analysis of multiple sequence alignments of ER domains led to the investigation of additional residues, several of which were surprisingly found to have little to no effect on the stereochemistry of enoylreduction. These include V52, N53, V46, and V47. Additionally, different active site residues were mutated in a model triketide lactone synthase termed TKS Rap13 to test the effect on catalysis. TKS-Rap13 is a variant of DEBS1-TE where EryKR2 is swapped with the full set of reductive domains from rapamycin module 13. From sequence alignment data, several residues were chosen for mutagenesis studies including N41, D43, Thr122, Y124, Y125, and K236. Most of the strains of S. erythraea containing the amino acid mutations to TKS-Rap13 produced significantly less triketide lactone than the parent strain, the fully reduced triketide lactone, or in the case of the K236A mutant, both diastereomers. Each of the mutants also produced a portion of unreduced 3-keto triketide lactone in similar amounts. Although the amount of unreduced 3-keto triketide lactone was comparable between mutant strains, there were vast differences in the production of the fully reduced triketide lactone between different mutants. Surprisingly, mutagenesis of K236 in TKS-Rap13, which corresponds to the Lys1771 proposed as a catalytic residue in the mammalian FAS, had no effect on the production of fully reduced triketide lactone. However, the mutation resulted in a shift in enzyme stereospecificity with two different diastereomers produced in a ratio of 1:6. Therefore, K236 might act as a proton donor to C-2 [129]. This work serves as a valuable starting point for exploration into ER mutagenesis and its effect on stereoselectivity as well as catalytic activity.
Acyl carrier protein
Mutations to alter activity: While the ACP domain does not catalyze any chemical reactions, recognition between the ACP and the catalytic domains has been demonstrated as important for catalysis [34,56,85,[132], [133], [134], [135]]. Thus, disrupting key interfaces along this region influences the kinetics of catalysis. In a study performed by Khosla and coworkers, the effect of protein-protein interactions on PKS activity was explored using chimeric bimodular and trimodular systems from DEBS. It was predicted that chain translocation from DEBS Mod1 to Mod3 in a bimodular PKS would be improved by applying an E23K mutation to EryACP1. This mutation was selected because a cationic residue also exists at the corresponding position of EryACP2. As a result, the turnover rate of the E23K mutant of DEBS Mod1+Mod3+TE was more than two-fold over the wild type bimodular PKS. This result shows the importance of ACP-KS interactions during PKS chain translocation [136]. Although a type II PKS rather than a type I modular PKS, studies of the actinorhodin PKS revealed critical insights regarding the apo and holo forms of the ACP's conformation. Conformational differences exist in the helix II residues including L45, M46, G47, and G53. The rearrangement of helix III is responsible for the observed apo-to-holo chemical shift differences for D62. The whole of D62 swings toward helix II and folds across the cleft. Additionally, the transition from the apo to holo form results in a subtle switch from Leu43 being solvent exposed to forming intramolecular interactions with the newly added phosphopantetheine side chain. Tryptophan fluorescence and FAS holo-synthase (ACPS) assays revealed that apo-ACP has a twofold higher affinity than holo-ACP. Site-directed mutations to L43 and D62 were performed to examine the influence on binding and ACPS activity. Four point mutations were compared including L43A, L43R, D62A, and D62N. The mutation L43A produced a binding profile like that of the holo-ACP with twofold weaker binding. Conversely, the mutant L43R had tenfold weaker binding and was completely inactive in the phosphopantetheine transfer assay. Furthermore, the mutations D62A and D62N resulted in ~40% and 20% reductions in binding affinity, respectively. However, both mutants were completely activated after a period; the D62A mutant was completely activated after 2 h while the D62N mutant was activated after 1 h. Therefore, point mutations to L43 had a much greater effect on binding and activity than point mutations to A62 [132]. Both examples demonstrate the ability of the ACP to dramatically impact the efficiency of catalytic domains through protein-protein interactions.
Thioesterase
Mutations to abolish activity: The TE domain consists of a typical α/β hydrolase fold with a serine, histidine, aspartate catalytic triad. TE domains can either be hydrolyzing or lactonizing, where the acyl enzyme intermediate is attacked by water or a hydroxy group on the polyketide chain, respectively [[137], [138], [139]]. There is little reason to inactivate the TE domain intentionally for synthetic biology engineering endeavors, as inactivating chain termination results in no catalytic turnover. One potential application for intentional TE inactivation would be for a few PKSs where the TE domain is present in trans rather than at the C-terminus, such as the monensin and nanchangomycin PKSs. Inactivation of the TE and complementation with one in trans possessing distinct substrate selectivity, or changing a TE from lactonizing to hydrolyzing may be approaches to consider [[140], [141], [142]]. As one would anticipate, mutation to the catalytic serine abolishes activity, and mutations to the other residues within the catalytic triad (e.g. an aspartate to asparagine mutation) compromise activity severely [143]. The most reliable inactivation to the TE domain is an active site serine to alanine mutation, which has been performed in mechanistic studies [143,144].
Mutations to alter activity: Although TE domains can be either hydrolyzing or lactonizing, many that are natively lactonizing such as EryTE or the pikromycin TE, PikTE, perform hydrolysis when a nucleophilic hydroxy group is absent [3,60,61,145]. Structural studies of the tautomycetin TE, a hydrolyzing TE, compared to lactonizing TEs indicate the presence of a constrained substrate tunnel that prevents macrocyclization [138,[146], [147], [148]]. Therefore, using mutagenesis to alter residues in the active site may be a means to toggle between chain termination via lactonization or hydrolysis. Other modes of chain termination exist including reductase (R) domains that reduce the thioester to a terminal alcohol [[149], [150], [151]] and sulfotransferase (SULT) domains which result in a terminal olefin [152,153]. However, introducing such chemistry would require domain swapping and is thus out of the scope of this review.
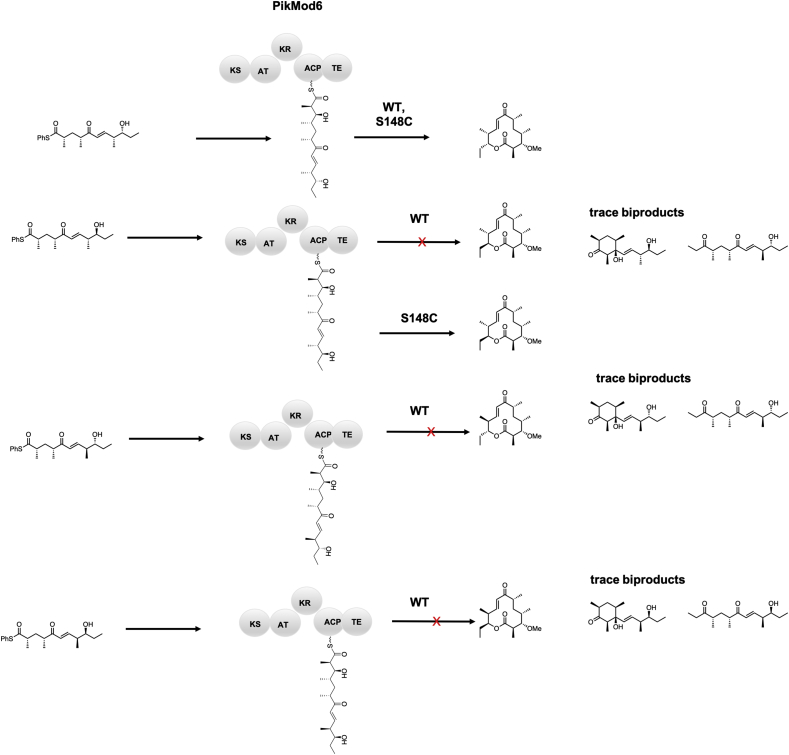
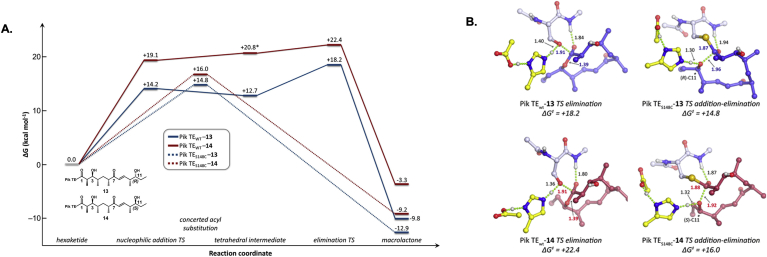
The TE domain is stereospecific for the configuration of the hydroxy group acting as a nucleophile for macrolactonization in both DEBS and the pikromycin PKS [60,145,154,155]. Because of steric sensitivity, the ability to alter stereochemical scaffolds is greatly hindered by the inability of the TE to cyclize varying stereochemical backbones. Inspired by proteases, Sherman and coworkers mutated the active site serine in PikTE to a cysteine which surprisingly resulted in the cyclization of an unnatural epimer (Fig. 16A). Additionally, the catalytic activity of the S148C mutant improved by approximately 4-6-fold in kcat/Km. The computationally verified mechanistic rationale suggested that the acyl enzyme intermediate was less kinetically stable, thus decreasing the barrier for the macrocylization reaction and affording a kinetically feasible path for the less sterically favorable macrolactonization (Fig. 17) [155,156]. Although not translated to another system, this method could be broadly applicable for the expansion of the stereochemical scaffolds of macrolides, as high stereospecificity is not unique to the pikromycin PKS.
Fig. 16.
Stereoisomers biocatalytically generated by the wild type and S148C mutant of PikTE through a biocatalytic extension of model substrates via methylmalonyl-SNAC [155,156].
Fig. 17.
A) Energy diagram of and B) transition state calculations of wild type and S148C with the two epimeric substrates. Reproduced with permission from Ref. [156].
Interdomain linkers
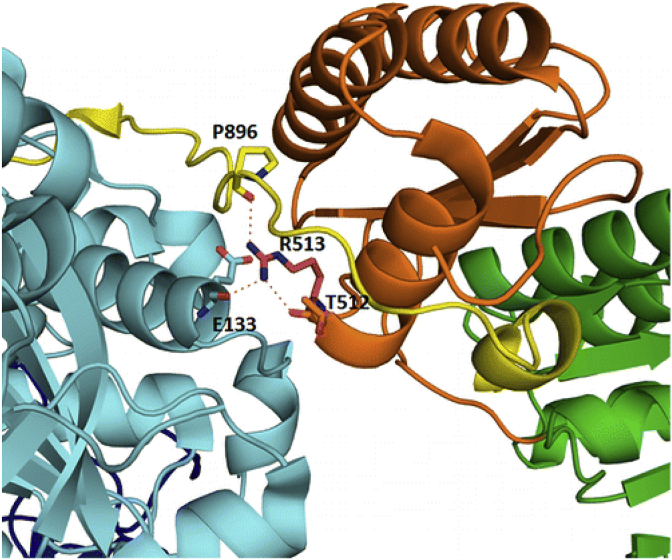
Mutations to alter activity: A striking example of the ability to alter catalytic efficiency through individual point mutations to interdomain linkers is revealed in a study by Khosla and coworkers involving DEBS Mod3. A crystal structure of the KS-AT didomain was available to visualize its interface [32]. It was assumed that mechanistically important residues in the KS-AT linker of DEBS are evolutionarily conserved. Thus, the amino acid sequence of the DEBS KS-AT linker was compared with other PKSs. The most conserved residues including V475, V476, S477, R479, L484, E487, I491, L509, R513, H516, H518, R519, L534, and I537 were individually replaced with alanine. The corresponding mutants were assayed, and the rate of triketide lactone formation was measured. Most mutants showed initial rates comparable to that of the wild type enzyme, however the R513A mutant had a 7-fold decrease in activity at 23 °C and a 20-fold decrease in activity at 30 °C. The rates for individual KS- and AT-catalyzed self-acylation of the R513A mutant were comparable to those of the wild type enzyme, suggesting a lack of significant structural changes. Proteolysis experiments revealed that the R513A mutant has a more flexible structure than the wild type enzyme and two prototypically susceptible sites emerged as a result of the R513A mutation. As such, the significantly reduced turnover rate of the R513A mutant is not due to a direct role of R513 during interactions with the ACP domain, but is rather likely due to the role of the residue in stabilizing the KS-AT didomain quaternary structure. Thus, while not targeting an active site residue directly, the impact that key residues have on the stability of the overall fold can have ramifications on catalytic activity. The location of R513 in the KS-AT linker is shown in Fig. 18 [157].
Fig. 18.
Critical arginine salt bridge mediating contact between the KS-AT linker. Reproduced with permission from Ref. [157].
For protein-protein interactions among sequential modules that are not present on the same gene, N-terminal and C-terminal docking domains, termed NDD and CDD domains respectively, allow for the proteins between PKSs to associate. Charge complementarity has been observed between matching NDD/CDD pairings [37,38,40,41,158,159], and these linkers have also been used to promote binding in non-natural contexts [40,93,158]. Such critical linkers are key regions to exclude when performing random mutagenesis studies in order to retain critical protein-protein interactions.
Domain skipping and modular elasticity
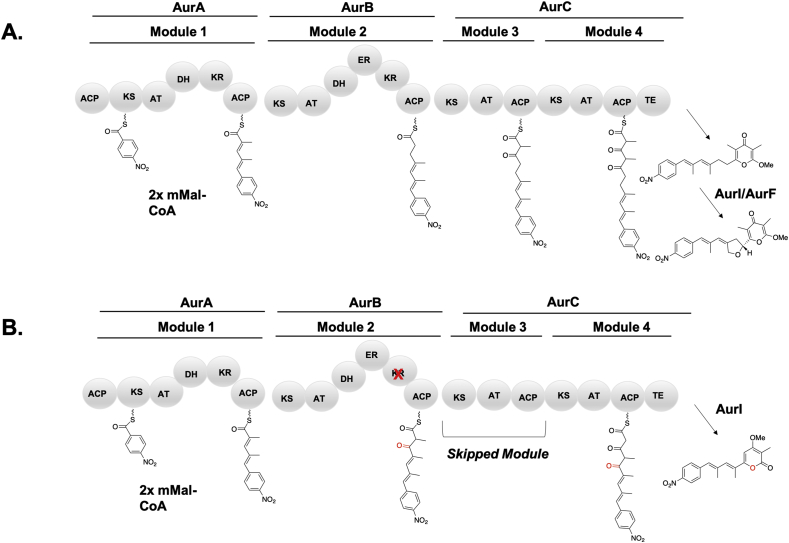
More uncommonly, a single mutation can result in domain skipping which then alters the size and shape of the resulting metabolite. A key example of this phenomenon in the aureothin pathway was recently reported by Hertweck and coworkers. They noticed that the native Streptomyces thioluteus strain produces a minor truncated side product, luteoretculin (Fig. 19). Intriguingly, upon mutation of the AurKR2 active site tyrosine to phenylalanine, a keto moiety was produced instead of a fully reduced carbon. Therefore, AurKS3 skipped condensation, which was likely due to KS gatekeeping. Interestingly, mutations to the other β-carbon processing domains had different effects on product distributions. In AurDH2, mutation of the catalytic histidine to phenylalanine resulted in a combination of stalled but extended products, as one would expect from a typical domain inactivation. A null mutant of AurER2 was made by mutating the conserved NADPH binding motif of GGVGMA to SPVGMA. This also resulted in the expected stalled products. Only inactivation of the KR domain resulted in this reliable module skipping. Because the KS is often sensitive to the steric shape of its substrate, removal of functionality through a single point mutation can result in more dramatic alterations to the carbon-carbon backbone if condensation becomes kinetically stalled. The precedent for the formation of luteoretculin from the aureothin PKS indicates the need for caution, as a single point mutation can have more drastic effects on molecular structure than initially anticipated [160]. This kinetic phenomenon of module skipping at AurKS3 demonstrates the interplay between simple domain inactivation and more complex issues regarding interacting selectivities of sequential domains.
Fig. 19.
Example of domain skipping by a single KR inactivation in the aureothin pathway [160].
Synthetic biology ramifications
Due to the high GC content of many genes derived from actinobacteria and myxobacteria which average 75% and 70% GC, respectively, as well as the very large sizes of these genes, the assembly of PKS constructs has typically presented a challenge. Prior to the invention of scar-free strategies such as Gibson and Golden Gate cloning, typically, restriction sites were added to PKS genes to enable their assembly. To date, the secondary structure arising from high GC content presents a significant challenge for reliable assembly using modern scar-free strategies like Gibson cloning which are reliant on regions of homology. It is not always possible to introduce restriction sites resulting in silent mutations, thus historically, researchers have tolerated conservative mutations to the DNA sequence. Recently, our laboratory has discovered that a mutation commonly introduced into DEBS1 to add a BsJ1 site for assembly is in a site analogous to one that affects the substrate selectivity in DEBS KS3 [58]. This residue is homologous with K154 in DEBS3 which was mutated to alanine in a homology directed experiment guided by the mycolactone PKS (vide supra, Fig. 5D). Although the native DEBS1 sequence contains an asparagine, to introduce the restriction site it was altered to a histidine. While typically, mutations to introduce a restriction site are believed to be innocuous, as they are performed with the intention of avoiding key active site residues, much structural and biochemical elucidation has occurred since many of these classic constructs were first assembled. Many mutations introduced in early papers detailing domain swaps and functional assays of DEBS remain unannotated. Therefore, the degree to which these “conservative” mutations affect stability or enzymatic function compared to the wild type is currently unknown. Some mutations may appear in residues near the active site, as the active site residues are not always close together in primary sequence. Prior to structural elucidation it was impossible to determine the proximity of distal residues to the active site. It is worth highlighting the need to rigorously sequence all plasmids commonly utilized in the PKS literature received from previous groups, as such restriction sites often remain unannotated.
Summary
Due to the wealth of available structural information on PKS domains, our ability to target mutations for specific purposes is fairly sophisticated. Both rational and random mutagenesis approaches can be utilized to effect desired changes in activity, selectivity, and kinetics among these complex multidomain systems. Targeted mutation has enormous potential to influence the catalytic activity of PKSs while being much more precise and less inherently globally disruptive than domain swapping, which often results in poor expression and low titers. So far, much mechanistic information on the catalytic efficiency, selectivity, and stereospecificity of PKS domains has been gleaned from point mutation experiments. Indeed, key active site residues, substrate binding motifs, and residues facilitating key didomain interactions have been identified. Additionally, the phenomenon of domain skipping has been explored, and is expected to be the focus of many future studies. Going forward, one of the biggest challenges will be using targeted point mutations that do not affect selectivity, but instead affect gatekeeping behavior due to stalled kinetics of downstream domains upon the introduction of unnatural substrates. Furthermore, recently characterized domains such as the MT and underexplored domains such as the DH and ER should be the subjects of future mutagenesis experiments. It is also important to note any “conservative” mutations used to insert restriction sites for genetic engineering, as these could influence enzyme structure and function more severely than expected. Taken together, point mutagenesis approaches are a powerful way to unleash the potential of PKSs for synthetic biology applications. This foundational work has provided the basis for expanding the scope from PKS mechanistic exploration to engineering application.
Acknowledgements
This work was supported by funding provided by the University of Tennessee, Knoxville.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Barajas J.F. Engineered polyketides: synergy between protein and host level engineering. Synth Syst Biotechnol. 2017;2:147–166. doi: 10.1016/j.synbio.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuzawa S. Commodity chemicals from engineered modular type I polyketide synthases. 2018;608 doi: 10.1016/bs.mie.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Hagen A. Engineering a polyketide synthase for in vitro production of adipic acid. ACS Synth Biol. 2016;5:21–27. doi: 10.1021/acssynbio.5b00153. [DOI] [PubMed] [Google Scholar]
- 4.Menzella H.G. Rational design and assembly of synthetic trimodular polyketide synthases. Chem Biol. 2007;14:143–151. doi: 10.1016/j.chembiol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Donadio S. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan A. Knowledge-based design of bimodular and trimodular polyketide synthases based on domain and module swaps: a route to simple statin analogues. Chem Biol. 1999;6:731–741. doi: 10.1016/s1074-5521(00)80020-4. [DOI] [PubMed] [Google Scholar]
- 7.Maervoet V.E.T., Briers Y. Synthetic biology of modular proteins. Bioengineered. 2017;8:196–202. doi: 10.1080/21655979.2016.1222993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hans M. Mechanistic analysis of acyl transferase domain exchange in polyketide synthase modules. J Am Chem Soc. 2003;125:5366–5374. doi: 10.1021/ja029539i. [DOI] [PubMed] [Google Scholar]
- 9.Kellenberger L. A polylinker approach to reductive loop swaps in modular polyketide synthases. Chembiochem. 2008;9:2740–2749. doi: 10.1002/cbic.200800332. [DOI] [PubMed] [Google Scholar]
- 10.Eng C.H. ClusterCAD: a computational platform for type I modular polyketide synthase design. Nucleic Acids Res. 2018;46:D509–D515. doi: 10.1093/nar/gkx893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzella H.G. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat Biotechnol. 2005;23:1171–1176. doi: 10.1038/nbt1128. [DOI] [PubMed] [Google Scholar]
- 12.Chandran S.S. Activating hybrid modular interfaces in synthetic polyketide synthases by cassette replacement of ketosynthase domains. Chem Biol. 2006;13:469–474. doi: 10.1016/j.chembiol.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Dunn B.J., Khosla C. Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. J R Soc Interface. 2013;10:20130297. doi: 10.1098/rsif.2013.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan X. Acyltransferase domain substitutions in erythromycin polyketide synthase yield novel erythromycin derivatives. J Bacteriol. 1997;179:6416–6425. doi: 10.1128/jb.179.20.6416-6425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petkovic H. A novel erythromycin, 6-desmethyl erythromycin D, made by substituting an acyltransferase domain of the erythromycin polyketide synthase. J Antibiot. 2003;56:543–551. doi: 10.7164/antibiotics.56.543. [DOI] [PubMed] [Google Scholar]
- 16.Annaval T. Evaluating ketoreductase exchanges as a means of rationally altering polyketide stereochemistry. Chembiochem. 2015;16:1357–1364. doi: 10.1002/cbic.201500113. [DOI] [PubMed] [Google Scholar]
- 17.Yong J.-H., Byeon W.-H. Alternative production of avermectin components in Streptomyces avermitilis by gene replacement. J Microbiol. 2005;43:277–284. [PubMed] [Google Scholar]
- 18.Luhavaya H. Site-specific modification of the anticancer and antituberculosis polyether salinomycin by biosynthetic engineering. Chembiochem. 2014;15:2081–2085. doi: 10.1002/cbic.201402300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissman K.J. The structural biology of biosynthetic megaenzymes. Nat Chem Biol. 2015;11:660–670. doi: 10.1038/nchembio.1883. [DOI] [PubMed] [Google Scholar]
- 20.Robbins T. Structure and mechanism of assembly line polyketide synthases. Curr Opin Struct Biol. 2016;41:10–18. doi: 10.1016/j.sbi.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keatinge-Clay A.T. The structures of type I polyketide synthases. Nat Prod Rep. 2012;29:1050–1073. doi: 10.1039/c2np20019h. [DOI] [PubMed] [Google Scholar]
- 22.Zheng J. Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling α-substituent stereochemistry. ACS Chem Biol. 2013;8:1964–1971. doi: 10.1021/cb400161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu C. Substrate-bound structures of a ketoreductase from amphotericin modular polyketide synthase. J Struct Biol. 2018;203:135–141. doi: 10.1016/j.jsb.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keatinge-Clay A.T., Stroud R.M. The structure of a ketoreductase determines the organization of the beta-carbon processing enzymes of modular polyketide synthases. Structure. 2006;14:737–748. doi: 10.1016/j.str.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J. Structural and functional analysis of A-type ketoreductases from the amphotericin modular polyketide synthase. Structure. 2010;18:913–922. doi: 10.1016/j.str.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J., Keatinge-Clay A.T. Structural and functional analysis of C2-type ketoreductases from modular polyketide synthases. J Mol Biol. 2011;410:105–117. doi: 10.1016/j.jmb.2011.04.065. [DOI] [PubMed] [Google Scholar]
- 27.Keatinge-Clay A.T. A tylosin ketoreductase reveals how chirality is determined in polyketides. Chem Biol. 2007;14:898–908. doi: 10.1016/j.chembiol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Akey D.L. Crystal structures of dehydratase domains from the curacin polyketide biosynthetic pathway. Structure. 2010;18:94–105. doi: 10.1016/j.str.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gay D. Structure and stereospecificity of the dehydratase domain from the terminal module of the rifamycin polyketide synthase. Biochemistry. 2013;52:8916–8928. doi: 10.1021/bi400988t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keatinge-Clay A. Crystal structure of the erythromycin polyketide synthase dehydratase. J Mol Biol. 2008;384:941–953. doi: 10.1016/j.jmb.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng J. Divergence of multimodular polyketide synthases revealed by a didomain structure. Nat Chem Biol. 2012;8:615–621. doi: 10.1038/nchembio.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang Y. The 2.7-Angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci USA. 2006;103:11124–11129. doi: 10.1073/pnas.0601924103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gay D.C. A close look at a ketosynthase from a trans-acyltransferase modular polyketide synthase. Structure. 2014;22:444–451. doi: 10.1016/j.str.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alekseyev V.Y. Solution structure and proposed domain domain recognition interface of an acyl carrier protein domain from a modular polyketide synthase. Protein Sci. 2007;16:2093–2107. doi: 10.1110/ps.073011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai S.-C. Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases. Biochemistry. 2002;41:12598–12606. doi: 10.1021/bi0260177. [DOI] [PubMed] [Google Scholar]
- 36.Weissman K.J., Müller R. Protein-protein interactions in multienzyme megasynthetases. Chembiochem. 2008;9:826–848. doi: 10.1002/cbic.200700751. [DOI] [PubMed] [Google Scholar]
- 37.Broadhurst R.W. The structure of docking domains in modular polyketide synthases. Chem Biol. 2003;10:723–731. doi: 10.1016/s1074-5521(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 38.Weissman K.J. The structural basis for docking in modular polyketide biosynthesis. Chembiochem. 2006;7:485–494. doi: 10.1002/cbic.200500435. [DOI] [PubMed] [Google Scholar]
- 39.Dorival J. Characterization of intersubunit communication in the virginiamycin trans-acyl transferase polyketide synthase. J Am Chem Soc. 2016;138:4155–4167. doi: 10.1021/jacs.5b13372. [DOI] [PubMed] [Google Scholar]
- 40.Whicher J.R. Cyanobacterial polyketide synthase docking domains: a tool for engineering natural product biosynthesis. Chem Biol. 2013;20:1340–1351. doi: 10.1016/j.chembiol.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchholz T.J. Structural basis for binding specificity between subclasses of modular polyketide synthase docking domains. ACS Chem Biol. 2009;4:41–52. doi: 10.1021/cb8002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith J.L. Architecture of the polyketide synthase module: surprises from electron cryo-microscopy. Curr Opin Struct Biol. 2015;31:9–19. doi: 10.1016/j.sbi.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whicher J.R. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature. 2014;510:560–564. doi: 10.1038/nature13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dutta S. Structure of a modular polyketide synthase. Nature. 2014;510:512–517. doi: 10.1038/nature13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poust S. Narrowing the gap between the promise and reality of polyketide synthases as a synthetic biology platform. Curr Opin Biotechnol. 2014;30:32–39. doi: 10.1016/j.copbio.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Klaus M., Grininger M. Engineering strategies for rational polyketide synthase design. Nat Prod Rep. 2018;35:1070–1081. doi: 10.1039/c8np00030a. [DOI] [PubMed] [Google Scholar]
- 47.Yuzawa S. Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production. ACS Synth Biol. 2017;6:139–147. doi: 10.1021/acssynbio.6b00176. [DOI] [PubMed] [Google Scholar]
- 48.Helfrich E.J.N., Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat Prod Rep. 2016;33:231–316. doi: 10.1039/c5np00125k. [DOI] [PubMed] [Google Scholar]
- 49.Piel J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat Prod Rep. 2010;27:996–1047. doi: 10.1039/b816430b. [DOI] [PubMed] [Google Scholar]
- 50.Jenner M. Mechanism of intersubunit ketosynthase-dehydratase interaction in polyketide synthases. Nat Chem Biol. 2018;14:270–275. doi: 10.1038/nchembio.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen T. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat Biotechnol. 2008;26:225–233. doi: 10.1038/nbt1379. [DOI] [PubMed] [Google Scholar]
- 52.Long P.F. Engineering specificity of starter unit selection by the erythromycin-producing polyketide synthase. Mol Microbiol. 2002;43:1215–1225. doi: 10.1046/j.1365-2958.2002.02815.x. [DOI] [PubMed] [Google Scholar]
- 53.Brautaset T. Site-specific mutagenesis and domain substitutions in the loading module of the nystatin polyketide synthase, and their effects on nystatin biosynthesis in Streptomyces noursei. J Biol Chem. 2003;278:14913–14919. doi: 10.1074/jbc.M212611200. [DOI] [PubMed] [Google Scholar]
- 54.Robbins T. Roles of conserved active site residues in the ketosynthase domain of an assembly line polyketide synthase. Biochemistry. 2016;55:4476–4484. doi: 10.1021/acs.biochem.6b00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y. Characterization of the ketosynthase and acyl carrier protein domains at the LnmI nonribosomal peptide synthetase-polyketide synthase interface for leinamycin biosynthesis. Org Lett. 2016;18:4288–4291. doi: 10.1021/acs.orglett.6b02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu N. Quantitative analysis of the relative contributions of donor acyl carrier proteins, acceptor ketosynthases, and linker regions to intermodular transfer of intermediates in hybrid polyketide synthases. Biochemistry. 2002;41:5056–5066. doi: 10.1021/bi012086u. [DOI] [PubMed] [Google Scholar]
- 57.Keatinge-Clay A.T. Stereocontrol within polyketide assembly lines. Nat Prod Rep. 2016;33:141–149. doi: 10.1039/c5np00092k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy A.C. Broadening substrate specificity of a chain-extending ketosynthase through a single active-site mutation. Chem Commun. 2016;52:8373–8376. doi: 10.1039/c6cc03501a. [DOI] [PubMed] [Google Scholar]
- 59.Pidot S.J. Deciphering the genetic basis for polyketide variation among mycobacteria producing mycolactones. BMC Genom. 2008;9:462. doi: 10.1186/1471-2164-9-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harper A.D. Preparative, in vitro biocatalysis of triketide lactone chiral building blocks. Chembiochem. 2012;13:2200–2203. doi: 10.1002/cbic.201200378. [DOI] [PubMed] [Google Scholar]
- 61.Hughes A.J. Employing a polyketide synthase module and thioesterase in the semipreparative biocatalysis of diverse triketide pyrones. Medchemcomm. 2012;3:956. [Google Scholar]
- 62.Chen H., Du L. Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl Microbiol Biotechnol. 2016;100:541–557. doi: 10.1007/s00253-015-7093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kapur S. Reprogramming a module of the 6-deoxyerythronolide B synthase for iterative chain elongation. Proc Natl Acad Sci USA. 2012;109:4110–4115. doi: 10.1073/pnas.1118734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eustáquio A.S. Biosynthesis of the salinosporamide A polyketide synthase substrate chloroethylmalonyl-coenzyme A from S-adenosyl-L-methionine. Proc Natl Acad Sci USA. 2009;106:12295–12300. doi: 10.1073/pnas.0901237106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lau J. Dissecting the role of acyltransferase domains of modular polyketide synthases in the choice and stereochemical fate of extender units. Biochemistry. 1999;38:1643–1651. doi: 10.1021/bi9820311. [DOI] [PubMed] [Google Scholar]
- 66.Haydock S.F. Divergent sequence motifs correlated with the substrate specificity of (methyl)malonyl-CoA:acyl carrier protein transacylase domains in modular polyketide synthases. FEBS Lett. 1995;374:246–248. doi: 10.1016/0014-5793(95)01119-y. [DOI] [PubMed] [Google Scholar]
- 67.Dunn B.J. Comparative analysis of the substrate specificity of trans- versus cis-acyltransferases of assembly line polyketide synthases. Biochemistry. 2014;53:3796–3806. doi: 10.1021/bi5004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker M.C. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science. 2013;341:1089–1094. doi: 10.1126/science.1242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ad O. Elucidating the mechanism of fluorinated extender unit loading for improved production of fluorine-containing polyketides. Proc Natl Acad Sci USA. 2017;114:E660–E668. doi: 10.1073/pnas.1614196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeves C.D. Alteration of the substrate specificity of a modular polyketide synthase acyltransferase domain through site-specific mutations. Biochemistry. 2001;40:15464–15470. doi: 10.1021/bi015864r. [DOI] [PubMed] [Google Scholar]
- 71.Sundermann U. Enzyme-directed mutasynthesis: a combined experimental and theoretical approach to substrate recognition of a polyketide synthase. ACS Chem Biol. 2013;8:443–450. doi: 10.1021/cb300505w. [DOI] [PubMed] [Google Scholar]
- 72.Koryakina I. Inversion of extender unit selectivity in the erythromycin polyketide synthase by acyltransferase domain engineering. ACS Chem Biol. 2017;12:114–123. doi: 10.1021/acschembio.6b00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petković H. Substrate specificity of the acyl transferase domains of EpoC from the epothilone polyketide synthase. Org Biomol Chem. 2008;6:500–506. doi: 10.1039/b714804f. [DOI] [PubMed] [Google Scholar]
- 74.Mo S. Biosynthesis of the allylmalonyl-CoA extender unit for the FK506 polyketide synthase proceeds through a dedicated polyketide synthase and facilitates the mutasynthesis of analogues. J Am Chem Soc. 2011;133:976–985. doi: 10.1021/ja108399b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koryakina I. Promiscuity of a modular polyketide synthase towards natural and non-natural extender units. Org Biomol Chem. 2013;11:4449–4458. doi: 10.1039/c3ob40633d. [DOI] [PubMed] [Google Scholar]
- 76.Bravo-Rodriguez K. Substrate flexibility of a mutated acyltransferase domain and implications for polyketide biosynthesis. Chem Biol. 2015;22:1425–1430. doi: 10.1016/j.chembiol.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 77.Klopries S. Data in support of substrate flexibility of a mutated acyltransferase domain and implications for polyketide biosynthesis. Data Brief. 2015;5:528–536. doi: 10.1016/j.dib.2015.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grote M., Schulz F. Exploring the promiscuous enzymatic activation of unnatural polyketide extender units in vitro and in vivo for monensin biosynthesis. Chembiochem. 2019;20:1183–1189. doi: 10.1002/cbic.201800734. [DOI] [PubMed] [Google Scholar]
- 79.Koryakina I., Williams G.J. Mutant malonyl-CoA synthetases with altered specificity for polyketide synthase extender unit generation. Chembiochem. 2011;12:2289–2293. doi: 10.1002/cbic.201100383. [DOI] [PubMed] [Google Scholar]
- 80.Koryakina I. Poly specific trans-acyltransferase machinery revealed via engineered acyl-CoA synthetases. ACS Chem Biol. 2013;8:200–208. doi: 10.1021/cb3003489. [DOI] [PubMed] [Google Scholar]
- 81.Ray L., Moore B.S. Recent advances in the biosynthesis of unusual polyketide synthase substrates. Nat Prod Rep. 2016;33:150–161. doi: 10.1039/c5np00112a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson M.C., Moore B.S. Beyond ethylmalonyl-CoA: the functional role of crotonyl-CoA carboxylase/reductase homologs in expanding polyketide diversity. Nat Prod Rep. 2012;29:72–86. doi: 10.1039/c1np00082a. [DOI] [PubMed] [Google Scholar]
- 83.Eustáquio A.S., Moore B.S. Mutasynthesis of fluorosalinosporamide, a potent and reversible inhibitor of the proteasome. Angew Chem Int Ed Engl. 2008;47:3936–3938. doi: 10.1002/anie.200800177. [DOI] [PubMed] [Google Scholar]
- 84.Musiol-Kroll E.M. Polyketide bioderivatization using the promiscuous acyltransferase kircii. ACS Synth Biol. 2017;6:421–427. doi: 10.1021/acssynbio.6b00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye Z. Reprogramming acyl carrier protein interactions of an Acyl-CoA promiscuous trans-acyltransferase. Chem Biol. 2014;21:636–646. doi: 10.1016/j.chembiol.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skiba M.A. Domain organization and active site architecture of a polyketide synthase C-methyltransferase. ACS Chem Biol. 2016;11:3319–3327. doi: 10.1021/acschembio.6b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meinke J.L. Structural and functional studies of a gem-dimethylating methyltransferase from a trans-acyltransferase assembly line. ACS Chem Biol. 2018;13:3306–3314. doi: 10.1021/acschembio.8b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stevens D.C. Methyltransferases excised from trans-AT polyketide synthases operate on N-acetylcysteamine-bound substrates. J Antibiot. 2016;69:567–570. doi: 10.1038/ja.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagner D.T. α-Methylation follows condensation in the gephyronic acid modular polyketide synthase. Chem Commun. 2016;52:8822–8825. doi: 10.1039/c6cc04418b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie X. Elucidation of the stereospecificity of C-methyltransferases from trans-AT polyketide synthases. J Am Chem Soc. 2017;139:6102–6105. doi: 10.1021/jacs.7b02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J., Zheng Y.G. SAM/SAH analogs as versatile tools for SAM-dependent methyltransferases. ACS Chem Biol. 2016;11:583–597. doi: 10.1021/acschembio.5b00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Winter J.M. Expanding the structural diversity of polyketides by exploring the cofactor tolerance of an inline methyltransferase domain. Org Lett. 2013;15:3774–3777. doi: 10.1021/ol401723h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meinke J.L. Employing 25-residue docking motifs from modular polyketide synthases as orthogonal protein connectors. ACS Synth Biol. 2019 doi: 10.1021/acssynbio.9b00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Awakawa T. Reprogramming of the antimycin NRPS-PKS assembly lines inspired by gene evolution. Nat Commun. 2018;9:3534. doi: 10.1038/s41467-018-05877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsen E.M. Conformation-activity relationships of polyketide natural products. Nat Prod Rep. 2015;32:1183–1206. doi: 10.1039/c5np00014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie X. Epimerase and reductase activities of polyketide synthase ketoreductase domains utilize the same conserved tyrosine and serine residues. Biochemistry. 2016;55:1179–1186. doi: 10.1021/acs.biochem.6b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuzawa S. Short-chain ketone production by engineered polyketide synthases in Streptomyces albus. Nat Commun. 2018;9:4569. doi: 10.1038/s41467-018-07040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng J., Keatinge-Clay A.T. The status of type I polyketide synthase ketoreductases. Medchemcomm. 2013;4:34–40. [Google Scholar]
- 99.Weissman K.J. Genetic engineering of modular PKSs: from combinatorial biosynthesis to synthetic biology. Nat Prod Rep. 2016;33:203–230. doi: 10.1039/c5np00109a. [DOI] [PubMed] [Google Scholar]
- 100.Bailey C.B. Substrate structure-activity relationships guide rational engineering of modular polyketide synthase ketoreductases. Chem Commun. 2016;52:792–795. doi: 10.1039/c5cc07315d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reid R. A model of structure and catalysis for ketoreductase domains in modular polyketide synthases. Biochemistry. 2003;42:72–79. doi: 10.1021/bi0268706. [DOI] [PubMed] [Google Scholar]
- 102.Khan N. A labile point in mutant amphotericin polyketide synthases. Biotechnol Lett. 2011;33:1121–1126. doi: 10.1007/s10529-011-0538-3. [DOI] [PubMed] [Google Scholar]
- 103.Siskos A.P. Molecular basis of Celmer's rules: stereochemistry of catalysis by isolated ketoreductase domains from modular polyketide synthases. Chem Biol. 2005;12:1145–1153. doi: 10.1016/j.chembiol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Baerga-Ortiz A. Directed mutagenesis alters the stereochemistry of catalysis by isolated ketoreductase domains from the erythromycin polyketide synthase. Chem Biol. 2006;13:277–285. doi: 10.1016/j.chembiol.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 105.O'Hare H.M. High-throughput mutagenesis to evaluate models of stereochemical control in ketoreductase domains from the erythromycin polyketide synthase. Chem Biol. 2006;13:287–296. doi: 10.1016/j.chembiol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Lohman J.R. Structural and evolutionary relationships of “AT-less” type I polyketide synthase ketosynthases. Proc Natl Acad Sci USA. 2015;112:12693–12698. doi: 10.1073/pnas.1515460112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonnett S.A. Structural and stereochemical analysis of a modular polyketide synthase ketoreductase domain required for the generation of a cis-alkene. Chem Biol. 2013;20:772–783. doi: 10.1016/j.chembiol.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bali S. Broad substrate specificity of ketoreductases derived from modular polyketide synthases. Chembiochem. 2006;7:478–484. doi: 10.1002/cbic.200500430. [DOI] [PubMed] [Google Scholar]
- 109.Bali S., Weissman K.J. Ketoreduction in mycolactone biosynthesis: insight into substrate specificity and stereocontrol from studies of discrete ketoreductase domains in vitro. Chembiochem. 2006;7:1935–1942. doi: 10.1002/cbic.200600285. [DOI] [PubMed] [Google Scholar]
- 110.Häckh M. Substrate-dependent stereospecificity of Tyl-KR1: an isolated polyketide synthase ketoreductase domain from Streptomyces fradiae. Chem Eur J. 2013;19:8922–8928. doi: 10.1002/chem.201300554. [DOI] [PubMed] [Google Scholar]
- 111.Häckh M. Hidden specificities in enzyme catalysis: structural basis of substrate structure-selectivity relationship of a ketoreductase. Chembiochem. 2019;20:1150–1154. doi: 10.1002/cbic.201800799. [DOI] [PubMed] [Google Scholar]
- 112.Kwan D.H. Insights into the stereospecificity of ketoreduction in a modular polyketide synthase. Org Biomol Chem. 2011;9:2053–2056. doi: 10.1039/c1ob00022e. [DOI] [PubMed] [Google Scholar]
- 113.Moretto L. Dissecting how modular polyketide synthase ketoreductases interact with acyl carrier protein-attached substrates. Chem Commun. 2017;53:11457–11460. doi: 10.1039/c7cc04625a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie X., Cane D.E. pH-Rate profiles establish that polyketide synthase dehydratase domains utilize a single-base mechanism. Org Biomol Chem. 2018;16:9165–9170. doi: 10.1039/c8ob02637h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kushnir S. Minimally invasive mutagenesis gives rise to a biosynthetic polyketide library. Angew Chem Int Ed Engl. 2012;51:10664–10669. doi: 10.1002/anie.201202438. [DOI] [PubMed] [Google Scholar]
- 116.Guo X. Mechanism and stereospecificity of a fully saturating polyketide synthase module: nanchangmycin synthase module 2 and its dehydratase domain. J Am Chem Soc. 2010;132:14694–14696. doi: 10.1021/ja1073432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y. Functional characterization of a dehydratase domain from the pikromycin polyketide synthase. J Am Chem Soc. 2015;137:7003–7006. doi: 10.1021/jacs.5b02325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Valenzano C.R. Stereospecificity of the dehydratase domain of the erythromycin polyketide synthase. J Am Chem Soc. 2010;132:14697–14699. doi: 10.1021/ja107344h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Faille A. Insights into substrate modification by dehydratases from type I polyketide synthases. J Mol Biol. 2017;429:1554–1569. doi: 10.1016/j.jmb.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 120.Fiers W.D. Tylosin polyketide synthase module 3: stereospecificity, stereoselectivity and steady-state kinetic analysis of β-processing domains via diffusible, synthetic substrates. Chem Sci. 2015;6:5027–5033. doi: 10.1039/c5sc01505g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu J. Polyketide double bond biosynthesis. Mechanistic analysis of the dehydratase-containing module 2 of the picromycin/methymycin polyketide synthase. J Am Chem Soc. 2005;127:17393–17404. doi: 10.1021/ja055672+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barajas J.F. Structural insights into dehydratase substrate selection for the borrelidin and fluvirucin polyketide synthases. J Ind Microbiol Biotechnol. 2019;46:1225–1235. doi: 10.1007/s10295-019-02189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zargar A. Chemoinformatic-guided engineering of polyketide synthases. BioRxiv. 2019 doi: 10.1101/805671. [DOI] [PubMed] [Google Scholar]
- 124.Gay D.C. A double-hotdog with a new trick: structure and mechanism of the trans-acyltransferase polyketide synthase enoyl-isomerase. ACS Chem Biol. 2014;9:2374–2381. doi: 10.1021/cb500459b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kusebauch B. Functionally distinct modules operate two consecutive alpha, beta-->beta,gamma double-bond shifts in the rhizoxin polyketide assembly line. Angew Chem Int Ed Engl. 2010;49:1460–1464. doi: 10.1002/anie.200905467. [DOI] [PubMed] [Google Scholar]
- 126.Wagner D.T. Structural and functional studies of a pyran synthase domain from a trans-acyltransferase assembly line. ACS Chem Biol. 2018;13:975–983. doi: 10.1021/acschembio.8b00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berkhan G., Hahn F. A dehydratase domain in ambruticin biosynthesis displays additional activity as a pyran-forming cyclase. Angew Chem Int Ed Engl. 2014;53:14240–14244. doi: 10.1002/anie.201407979. [DOI] [PubMed] [Google Scholar]
- 128.Sung K.H. Insights into the dual activity of a bifunctional dehydratase-cyclase domain. Angew Chem Int Ed Engl. 2018;57:343–347. doi: 10.1002/anie.201707774. [DOI] [PubMed] [Google Scholar]
- 129.Kwan D.H., Leadlay P.F. Mutagenesis of a modular polyketide synthase enoylreductase domain reveals insights into catalysis and stereospecificity. ACS Chem Biol. 2010;5:829–838. doi: 10.1021/cb100175a. [DOI] [PubMed] [Google Scholar]
- 130.Kong R. Elucidation of the biosynthetic gene cluster and the post-PKS modification mechanism for fostriecin in Streptomyces pulveraceus. Chem Biol. 2013;20:45–54. doi: 10.1016/j.chembiol.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 131.Kwan D.H. Prediction and manipulation of the stereochemistry of enoylreduction in modular polyketide synthases. Chem Biol. 2008;15:1231–1240. doi: 10.1016/j.chembiol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Evans S.E. An ACP structural switch: conformational differences between the apo and holo forms of the actinorhodin polyketide synthase acyl carrier protein. Chembiochem. 2008;9:2424–2432. doi: 10.1002/cbic.200800180. [DOI] [PubMed] [Google Scholar]
- 133.Wong F.T. Protein-protein recognition between acyltransferases and acyl carrier proteins in multimodular polyketide synthases. Biochemistry. 2010;49:95–102. doi: 10.1021/bi901826g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kapur S. Molecular recognition between ketosynthase and acyl carrier protein domains of the 6-deoxyerythronolide B synthase. Proc Natl Acad Sci USA. 2010;107:22066–22071. doi: 10.1073/pnas.1014081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Klaus M. Protein-protein interactions, not substrate recognition, dominate the turnover of chimeric assembly line polyketide synthases. J Biol Chem. 2016;291:16404–16415. doi: 10.1074/jbc.M116.730531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar P. Enhancing the modularity of the modular polyketide synthases: transacylation in modular polyketide synthases catalyzed by malonyl-CoA: ACP transacylase. J Am Chem Soc. 2003;125:14307–14312. doi: 10.1021/ja037429l. [DOI] [PubMed] [Google Scholar]
- 137.Horsman M.E. Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: logic gate or a victim of fate? Nat Prod Rep. 2016;33:183–202. doi: 10.1039/c4np00148f. [DOI] [PubMed] [Google Scholar]
- 138.Tsai S.C. Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: versatility from a unique substrate channel. Proc Natl Acad Sci USA. 2001;98:14808–14813. doi: 10.1073/pnas.011399198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tsai S.-C. Insights into channel architecture and substrate specificity from crystal structures of two macrocycle-forming thioesterases of modular polyketide synthases†,‡. Biochemistry. 2002;41:12598–12606. doi: 10.1021/bi0260177. [DOI] [PubMed] [Google Scholar]
- 140.Harvey B.M. Evidence that a novel thioesterase is responsible for polyketide chain release during biosynthesis of the polyether ionophore monensin. Chembiochem. 2006;7:1435–1442. doi: 10.1002/cbic.200500474. [DOI] [PubMed] [Google Scholar]
- 141.Liu T. Identification of NanE as the thioesterase for polyether chain release in nanchangmycin biosynthesis. Chem Biol. 2006;13:945–955. doi: 10.1016/j.chembiol.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 142.Grote M. Identification of crucial bottlenecks in engineered polyketide biosynthesis. Org Biomol Chem. 2019;17:6374–6385. doi: 10.1039/c9ob00831d. [DOI] [PubMed] [Google Scholar]
- 143.Lu H. Expression, site-directed mutagenesis, and steady state kinetic analysis of the terminal thioesterase domain of the methymycin/picromycin polyketide synthase†. Biochemistry. 2002;41:12590–12597. doi: 10.1021/bi026006d. [DOI] [PubMed] [Google Scholar]
- 144.Hughes A.J. Investigating the reactivities of a polyketide synthase module through fluorescent click chemistry. Chem Commun. 2014;50:5276–5278. doi: 10.1039/c3cc47513a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma K.K., Boddy C.N. The thioesterase domain from the pimaricin and erythromycin biosynthetic pathways can catalyze hydrolysis of simple thioester substrates. Bioorg Med Chem Lett. 2007;17:3034–3037. doi: 10.1016/j.bmcl.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 146.Tripathi A. Thioesterase domain swapping of a linear polyketide tautomycetin with a macrocyclic polyketide pikromycin in Streptomyces sp. CK4412. J Ind Microbiol Biotechnol. 2016;43:1189–1193. doi: 10.1007/s10295-016-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Akey D.L. Structural basis for macrolactonization by the pikromycin thioesterase. Nat Chem Biol. 2006;2:537–542. doi: 10.1038/nchembio824. [DOI] [PubMed] [Google Scholar]
- 148.Scaglione J.B. Biochemical and structural characterization of the tautomycetin thioesterase: analysis of a stereoselective polyketide hydrolase. Angew Chem Int Ed Engl. 2010;49:5726–5730. doi: 10.1002/anie.201000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barajas J.F. Comprehensive structural and biochemical analysis of the terminal myxalamid reductase domain for the engineered production of primary alcohols. Chem Biol. 2015;22:1018–1029. doi: 10.1016/j.chembiol.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 150.Zhu G. The reductase domain in a Type I fatty acid synthase from the apicomplexan Cryptosporidium parvum: restricted substrate preference towards very long chain fatty acyl thioesters. BMC Biochem. 2010;11:46. doi: 10.1186/1471-2091-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chhabra A. Nonprocessive [2 + 2]e- off-loading reductase domains from mycobacterial nonribosomal peptide synthetases. Proc Natl Acad Sci USA. 2012;109:5681–5686. doi: 10.1073/pnas.1118680109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gehret J.J. Terminal alkene formation by the thioesterase of curacin A biosynthesis: structure of a decarboxylating thioesterase. J Biol Chem. 2011;286:14445–14454. doi: 10.1074/jbc.M110.214635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gu L. Polyketide decarboxylative chain termination preceded by o-sulfonation in curacin a biosynthesis. J Am Chem Soc. 2009;131:16033–16035. doi: 10.1021/ja9071578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pinto A. 6-Deoxyerythronolide B synthase thioesterase-catalyzed macrocyclization is highly stereoselective. Org Lett. 2012;14:2278–2281. doi: 10.1021/ol300707j. [DOI] [PubMed] [Google Scholar]
- 155.Hansen D.A. Identification of a thioesterase bottleneck in the pikromycin pathway through full-module processing of unnatural pentaketides. J Am Chem Soc. 2017;139:13450–13455. doi: 10.1021/jacs.7b06432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Koch A.A. A single active site mutation in the pikromycin thioesterase generates a more effective macrocyclization catalyst. J Am Chem Soc. 2017;139:13456–13465. doi: 10.1021/jacs.7b06436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yuzawa S. Role of a conserved arginine residue in linkers between the ketosynthase and acyltransferase domains of multimodular polyketide synthases. Biochemistry. 2012;51:3708–3710. doi: 10.1021/bi300399u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yan J. Functional dissection of a multimodular polypeptide of the pikromycin polyketide synthase into monomodules by using a matched pair of heterologous docking domains. Chembiochem. 2009;10:1537–1543. doi: 10.1002/cbic.200900098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Starcevic A. A novel docking domain interface model predicting recombination between homoeologous modular biosynthetic gene clusters. J Ind Microbiol Biotechnol. 2011;38:1295–1304. doi: 10.1007/s10295-010-0909-0. [DOI] [PubMed] [Google Scholar]
- 160.Peng H. Loss of single-domain function in a modular assembly line alters the size and shape of a complex polyketide. Angew Chem Int Ed Engl. 2019;58:18252–18256. doi: 10.1002/anie.201911315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yuzawa S. Heterologous gene expression of N-terminally truncated variants of LipPks1 suggests a functionally critical structural motif in the N-terminus of modular polyketide synthase. ACS Chem Biol. 2017;12:2725–2729. doi: 10.1021/acschembio.7b00714. [DOI] [PubMed] [Google Scholar]
- 162.Yuzawa S. Broad substrate specificity of the loading didomain of the lipomycin polyketide synthase. Biochemistry. 2013;52:3791–3793. doi: 10.1021/bi400520t. [DOI] [PubMed] [Google Scholar]
- 163.Barajas J.F. Biochemical characterization of β-amino acid incorporation in fluvirucin B2 biosynthesis. Chembiochem. 2018;19:1391–1395. doi: 10.1002/cbic.201800169. [DOI] [PubMed] [Google Scholar]