Abstract
Inflammatory bowel disease (IBD) refers to a group of disorders characterized by chronic inflammation of the gastrointestinal (GI) tract. The elevated levels of nitric oxide (NO) in serum and affected tissues; mainly synthesized by the inducible nitric oxide synthase (iNOS) enzyme; can exacerbate GI inflammation and is one of the major biomarkers of GI inflammation. Various natural and synthetic agents are able to ameliorate GI inflammation and decrease iNOS expression to the extent comparable with some IBD drugs. Thereby, the purpose of this study was to gather a list of natural or synthetic mediators capable of modulating IBD through the NO pathway. Electronic databases including Google Scholar and PubMed were searched from 1980 to May 2018. We found that polyphenols and particularly flavonoids are able to markedly attenuate NO production and iNOS expression through the nuclear factor κB (NF-κB) and JAK/STAT signaling pathways. Prebiotics and probiotics can also alter the GI microbiota and reduce NO expression in IBD models through a broad array of mechanisms. A number of synthetic molecules have been found to suppress NO expression either dependent on the NF-κB signaling pathway (i.e., dexamethasone, pioglitazone, tropisetron) or independent from this pathway (i.e., nicotine, prednisolone, celecoxib, β-adrenoceptor antagonists). Co-administration of natural and synthetic agents can affect the tissue level of NO and may improve IBD symptoms mainly by modulating the Toll like receptor-4 and NF-κB signaling pathways.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Nitric oxide, Nuclear factor-κB, Natural or synthetic mediators
Core tip: The present study aimed to investigate the correlation between the level of nitric oxide (NO), and inflammatory bowel disease (IBD). Collected data showed that elevated NO can induce gastrointestinal tract inflammation. Many natural and synthetic agents are able to decrease NO production through different pathways, thereby, improving inflammation and IBD symptoms. This study also determined the pharmacological effects of these agents in suppression of the NO pathway.
INTRODUCTION
Inflammatory bowel disease (IBD) is commonly comprised of Crohn's disease (CD) and ulcerative colitis (UC), and is a worldwide health issue, afflicting a continually increasing number of people[1]. Generally, IBD is characterized by chronic or relapsing inflammation of the gastrointestinal (GI) tract[2]. CD is associated with the transmural inflammation of potentially any part of the GI tract (mainly the terminal ileum and colon), usually without bleeding and concomitant complications including strictures, abscesses, and fistulas. However, UC only affects the mucosal layer of the colon (especially the rectum) and leads to rectal bleeding[3]. In spite of apparent differences in histological aspects and clinical manifestations of UC and CD, they share noticeable similarities from a pathophysiological point of view. In this regard, aberrant immune response to the microbial and environmental stimuli precipitated by genetic predispositions is assumed to result in IBD[4-6]. Notably, many factors involved in IBD converge within the intestinal inflammation, representing inflammatory cells infiltration and accelerating the inflammatory cytokines and nitric oxide (NO) release.
NO AND THE PATHOPHYSIOLOGY OF IBD
NO is an essential inorganic molecule with various physiological and pathological implications. NO is a free radical synthesized from the amino acid L-arginine (L-Arg) in a reaction catalyzed by the nitric oxide synthases (NOS) enzyme group. A number of NOS such as neuronal NOS (nNOS) and endothelial NOS (eNOS), also called constitutive NOS, are minimally expressed within the inflamed sites. However, inducible NOS (iNOS) is highly expressed in inflammatory cells in response to immunogenic stimuli, majorly in association with a different CD4+ helper T cell profile based on the Th2/Th1 paradigm and pro-inflammatory cytokines through the activation of mitogen-activated protein kinases (MAPK) and NF-κB[7-9]. Thus, CD was described as a Th1 type immune response promoted by signal transducer and activator of transcription (STAT)-4 and T-bet; which are able to produce interferon gamma (IFN-γ), interleukin (IL)-12, and tumor necrosis factor (TNF)-α. It has been shown that both IL-12 and IL-18 induce a high level of IFN-γ production, leading to reinforcement of the Th1 immune response. In contrast, UC is recognized as a Th2 type immune response prompted by the expression of transcription factor STAT-6 and GATA-3, as well as the secretion of IL-5, IL-4, and IL-13[9]. Obviously, an adequate amount of this immune response in the intestines is necessary for protection against infections, but excessive production of NO may exert pathologic effects[9-11].
iNOS is mainly activated through the activator protein-1 (AP-1) assembly; and activation of the extracellular signal-regulated kinase (ERK), leading to the phosphorylation and activation of IкB- of IкB-kinase-β (IKK-β) and cytosolic phospholipase A2, and upregulation of arachidonic acid (AA) release. An upsurge of NF-кB nuclear translocation, activates cyclooxygenase-2 (COX-2) by S-nitrosylation. COX-2 inevitably triggers the excessive production of prostaglandin E2 (PGE 2)[12].
The primary hypothesis of the involvement of NO in the pathogenesis of IBD was based on the presence of NO and its metabolites in the intestinal lumen and biological fluids (i.e., plasma and urine) in patients with IBD, more predominantly observed in their remission phase and in severe disease[11,13-15]. Rather than inflammation, many features of IBD are correlated with excessive NO; which can directly act on the endothelium and smooth muscle cells or may affect the epithelium, resulting in edema, vasodilatation, and increased mucosal permeability[16]. Overproduction of NO also stimulates chloride secretion in the colon, leading to diarrhea; one of the main features of IBD[17]. NO may play a pivotal role in the pathogenesis of some types of IBD such as toxic megacolon, through regulation of intestinal motility and mucosal blood supply[18].
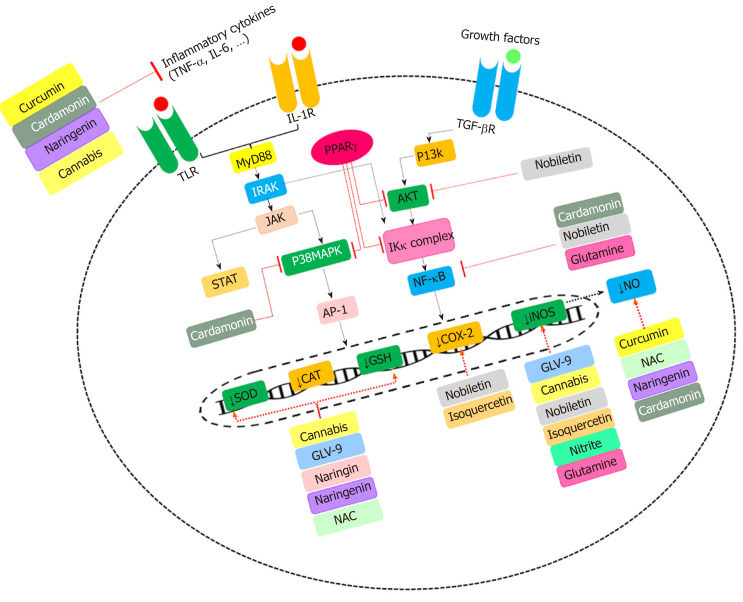
As mentioned earlier, iNOS is preliminarily activated by pro-inflammatory cytokines and immunogenic stimuli, acting via the JAK/STAT signaling pathways[9]. Activation of iNOS and irregular production of NO by pro-inflammatory (i.e., TNF-α, IFN-γ, IL-1b, IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, IL-17, IL-18 or IL-23) and inflammatory molecules (i.e., lipopolysaccharide (LPS)[9,10,19], results in further propagation of inflammatory responses by chemotaxis of neutrophils, natural killer (NK) cells and macrophages. In addition, NO indirectly induces reactive nitric oxygen species (RNOS) production. Then, it might react with superoxide, thus, generating peroxynitrite (OONO-). OONO- is an oxidant for several biological molecules, for example, it can activate the poly (-ADP-ribose) synthetase (PARS) enzyme, leading to depletion of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD) cellular supply, thereby, enhancing the epithelial permeability of the intestine[15,20,21]. Furthermore, NO can suppress mitochondrial function and DNA synthesis through OONO- production. It also enhances the intracellular release of iron, thus, acting as a cytotoxic agent and perpetuating the mucosal injury and inflammation[15]. Beyond OONO- production, NO induces RNOS species (i.e., NO2 and NO-) production[22]. It seems that there is a bidirectional interaction between NO production and inflammation in IBD, each one exacerbating the other[9]. Molecular pathways involved in IBD progression are shown in Figure 1.
Figure 1.
Main cellular mechanism of interventions in inflammatory bowel disease. COX-2: Cyclooxygenase-2; iNOS: Inducible nitric oxide synthase; TNF-α: Tumor necrosis factor alpha; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; IκB: Inhibitor of kappa B; IKκ complex: Inhibitor of kappa kinase complex; MyD88: Myeloid differentiation primary response 88; P38MAPK: P38Mitogen-activated protein kinase; GSH: Glutathione; P13k: Phosphatidylinositol 13-kinase; STAT: Signal transducer and activator of transcription proteins; IL-6: Interleukin-6; IL-1: Interleukin-1; PPARγ: Peroxisome proliferator-activated receptor gamma; TLR: Toll-like receptor; MCP-1: Monocyte chemoattractan-t protein-1; AP-1: Activator protein 1; IRAK: Interleukin-1 receptor-associated kinase; NAC: N-acetyl cysteine; NO: Nitric oxide; SOD: Superoxide dismutase; CAT: Catalase; TGFβR: Transforming growth factor beta receptor I.
Despite accumulating evidence supporting the role of excessive NO in the perpetuation of inflammatory responses, various research groups have shown that NO can exert immunomodulatory effects by inhibiting IL-12 secretion[23]. Additionally, some studies have shown that iNOS-deficient mice are more susceptible to colitis, as NO can suppress IL-17 secretion[24,25]. In line with previous findings, it has been shown that induction of iNOS alleviates mucosal injury and improves tissue repair[25]. In addition, nitrite supplementation and stimulation of NO release alleviated mucosal damage in a dextran sulfate sodium (DSS) model of colitis[26,27]. The pathophysiological significance of NO in the GI tract and possible efficacy of either natural or synthetic agents on NO have been reviewed by many studies, however, none of them extensively discussed both natural and synthetic interventions. This might allow opportunities for IBD drug development mediated by the NO pathway[2,28].
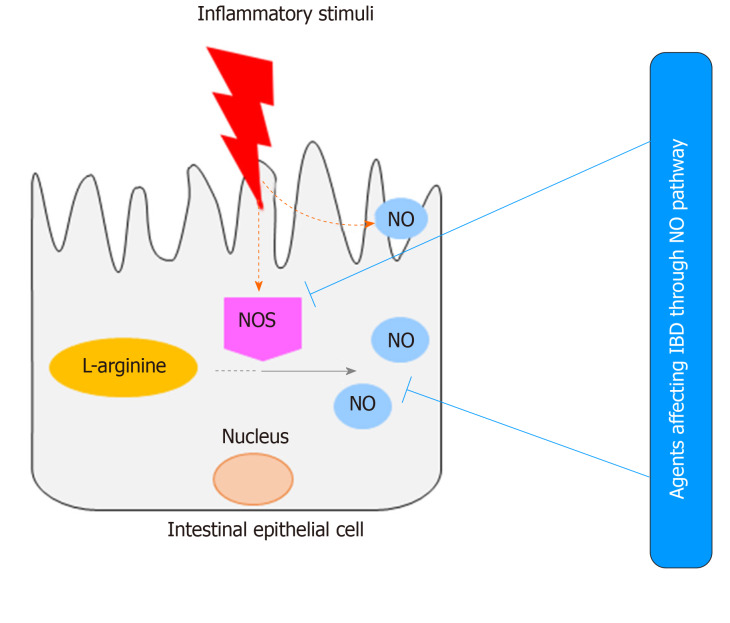
In spite of such controversies, suppression of NO-dependent pathological events by natural or synthetic agents was shown to attenuate IBD inflammation and symptoms in animal models (Figure 2). This paper reviews the pharmacological interventions of natural and synthetic agents that can affect IBD through the NO pathway.
Figure 2.
Inhibition of nitric oxide synthase suppresses inflammatory bowel disease. IBD: Inflammatory bowel disease; NOS: Nitric oxide synthase; NO: Nitric oxide.
SEARCH METHOD
We searched published literatures on studies that investigated the effects of natural and synthetic agents on IBD through modulation of the NO pathway. We used broad search terms: “Nitric oxide” or “NO” or “Nitric oxide pathways” and “Inflammatory bowel disease” or “IBD”. Electronic databases including Google Scholar and PubMed were searched from 1980 to May 2018, and the abstracts were screened for relevancy. The publications that investigated diseases other than IBD, or the interventions except that of the NO pathway, and studies that were not conducted on animal models, or did not include a synthetic or natural agent, were excluded.
PHARMACOLOGICAL INTERVENTIONS AFFECTING IBD THROUGH THE NO PATHWAY
Medicinal plants
The impressive roles of medicinal plants and their isolated chemical constituents (phytochemicals) in IBD management have been confirmed by numerous studies[29]. Therefore, medicinal plants seem to have a promising future in IBD treatment[30]. Following sections contain a number of plant species and their active ingredients that are able to inhibit NO, and were shown to have beneficial impacts on IBD (Table 1).
Table 1.
Medicinal plants affecting inflammatory bowel disease via modulating nitric oxide pathways
| Ref. | Plant | Plant part/ ingredients | Type of animal/cells | Model of IBD | Route of administra-tion | Duration of treatment | Numbers of animals in intervention group and control group | Outcomes |
| [199] | Terminalia catappa | Stem bark | Rat | 2,4,6-trinitrobenzene sulfonic acid (TNBS) | Oral | Two days before colitis induction | n = 6 | Disease activity index (DAI) ↓, myeloperoxida-se (MPO) ↓, glutathione content ↑, tumor necrosis factor (TNF)-α ↓, interleukin (IL)-6 ↓, IL-23 ↓, cytokine-induced neutrophil chemoattractan-t 1 ↓, mucin (MUC)-2 ↑, MUC-3 ↑, villin ↑ |
| [200] | Veronica polita | Whole plant | Mice | Dextran sulfate sodium (DSS) | Oral | 7 d | n = 10 | DAI ↓, malondialdehy-de (MDA) ↓, nitric acid (NO) ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, inducible nitric oxide synthase (iNOS) ↓, cyclooxygenase (COX-2) ↓, nuclear factor (NF)-κB ↓, phosphoryla-tion of Junas kinase/signal transducer and activator of transcription (JAK2/STAT-3) ↓ |
| [32] | Lavandula stoechas/ Lavandula dentate | Aerial parts | Rat | TNBS | Intrarectal | 7 d | n =10 | DAI ↓, MPO ↓, reduced glutathione (GSH) content ↓, iNOS ↓, IL-1β ↓, IL-6 ↓, monocyte chemoattrac-tant protein 1 (MCP-1) ↓, intercellular adhesion molecule 1 (ICAM1) ↓, IL-17 ↓, MUC-3 ↑, trefoil factor 3 gene (TFF)-3 ↑ |
| [201] | Ziziphora clinopoides | Aerial parts | Mice | DSS | Oral | 7 d before colitis induction | n = 6 | Colon lipid peroxidation (LPO) ↓, total thiol molecules (TTM) ↑, total antioxidant capacity (TAC) ↑, NO ↑, TNF-α ↓, superoxide dismutase (SOD) ↑, catalase (CAT) ↑ |
| [202] | Lavandula intermedia (cultivar Okanagan) | Essential oil | Mice | Citrobacter rodentium | Oral | 5-10 d | - | Mortality and morbidity ↓, cecal damage ↓, damage of distal colon↔, iNOS ↓, IFN-γ ↓, IL-22 ↓, macrophage inflammatory protein (MIP)-2α ↓ |
| [203] | Pisum sativum (Green Pea) | Powdered fruit | Mice | DSS | Oral | 9 wk | n = 7 | DAI ↓, MCP-1 ↓, COX-2 ↓, IL-6 ↓, IFN-γ ↓, IL-17 ↓, iNOS ↓, MUC-2 secretion ↑, TFF-3 ↑, kruppel-like factor 4 ↑, sam-pointed domain Ets transcription factor-1 ↑, activating transcription factor 6 (ATF)-6 ↓ |
| [33] | Cannabis sativa | Cannabidiol | Mice | Dinitrobenzene sulfonic acid (DNBS) | Intracolonic | 6 d | - | DAI ↓, iNOS ↓, nitrite production ↓, IL-1β↓, IL-10 ↑, anandamide & 2-arachydonylgly-cerol ↓, reactive oxygen species (ROS) formation ↓ |
| [204] | Cannabis sativa | Cannabigerol | Mice | DNBS | Intracolonic | Preventive protocol: 6 d/ curative protocol: 2 d | - | Pre-treatment→DAI ↓, Fluorescein isothiocyanate (FITC)-conjugated dextran in the serum ↓, nitrite ↓ treatment→ MPO ↓, SOD ↑, iNOS ↓, IL-1β ↓, interferon (INF)-γ ↓, IL-10 ↑ |
| [34] | Olea europaea (olive) | Leaves | Rat | Acetic acid (AA) | Oral | 3 d | n = 6 | DAI ↓, TNF-α ↓, NO ↓, IL-1β ↓, IL-6 ↓, iNOS ↓, IL-2 ↓ |
| [83] | Crude canola (rapeseed) oil | Phenolic compound 4-vinyl-2,6-dimethoxyphen-ol (canolol) | Mice | DSS | Oral | 7 d | - | DAI ↓, COX-2 ↓, free 8-hydroxy-2' deoxyguanosin-e (OHdG) in the plasma ↓, IL-12 ↓, TNF-α ↓, NO ↓ |
| [37] | Retama monosperma | Aerial parts/ flavonoids | Rat | TNBS | Oral | 48, 24 and 1 h prior to the induction of colitis & 24 h later | n = 10-11 | DAI ↓, COX-2 ↓, iNOS ↓, p38 mitogen-activated protein kinase (MAPK) ↓ |
| [38] | Hibiscus rosa sinensis | Leaves/ alkaloid, flavonoids, steroid and phenols | Mice and rat | AA | Oral | 7 d before colitis induction | n = 6 | DAI ↓, spleen enlargement ↓, white blood cell (WBC) count ↑, red blood cell (RBC) count ↑, hemoglobin (Hb) ↑, hematocrit ↑, platelet count ↑, SOD ↑, GSH ↑, LPO ↓, MPO ↓, nitrite/nitrate levels ↓, TNF-α ↓ |
| [205] | Changtai granule | Traditional Chinese empirical formula comprised of Phellodendro Chinense, Sanguisorba officinalis , Euphorbia humifusa and polygonum hydropiper | Rat | TNBS | Oral | 7 d | - | DAI ↓, MPO ↓, COX-2 ↓, iNOS ↓, Th1 cytokine response ↓, translocation of NF-κB in lamina propria mononuclear cells ↓ |
| [206] | Syringa vulgaris | Verbascoside/ phenylpropano-id glycosides | Rat | DNBS | Oral | 3 d | n = 10 | DAI ↓, TNF-α ↓, IL-1β ↓, iNOS ↓, NO ↓, poly(ADP ribose) ↓, IκB-α levels in colon ↑, pro-matrix metalloproteina-se (MMP)-2 ↑, MMP-9 ↑ |
| [39] | Curcuma longa | Curcumin | Rat | TNBS | Oral | 3 d before induction of IBD & was continued for 5 d after | n = 8 | DAI ↓, TNF-α ↓, MPO ↓, NO ↓, colonic hydroxyproline & ceruloplasmin levels ↓, expression of MMP-1, MMP-3 and tissue inhibitors of metalloproteina-ses 1 (TIMP)-1 ↓ |
| [39] | Ginkgo biloba | Root | Rat | TNBS | Oral | 3 d before induction of IBD & was continued for 5 d after | n = 8 | DAI ↓, TNF-α ↓, MPO ↓, NO ↓, colonic hydroxyproline and ceruloplasmin levels ↓, expression of MMP-1, MMP-3 and TIMP-1 ↓ |
| [207] | Cordyceps militaris Grown on Germinated Soybeans (GSC) | Mycelia | Mice | DSS | Oral | 2 or 9 d before colitis induction | n ≥ 15 | DAI ↓, MMP-3 ↓, MMP-9 ↓, TNF-α ↓, iNOS ↓, p53 ↓ |
| [208] | Ficus bengalensis | Stem bark | Rat | TNBS | Oral | 21 d | n = 6 | DAI ↓, colon mucosal damage index ↓, MPO ↓, MDA ↓, NO ↓, SOD ↑ |
| [46] | Vaccinium corymbosum (blueberry) | Fruits/phenolic acids and flavonoids | Mice | DSS | Oral | 14 d | n = 6 | DAI ↓, COX-2 ↓, IL-1β ↓, p65 NF-κB ↓, IFN-γ ↓, iNOS ↓, MDA ↓, CAT ↑, SOD ↑, prostaglandin E2 (PGE2) ↑ |
| [209] | Hericium erinaceus (Lion's Mane Medicinal Mushroom) | Mycelia | Mice | DSS | Oral | 7 d | - | DAI ↓, MPO ↓, TNF-α ↓, IL-1β ↓, IL-6 ↓, NO ↓, MDA ↓, SOD ↓ in serum |
| [40] | Panax notoginseng | Root/saponin | Mice | AOM and DSS | Oral | 15 d | n = 3 | DAI ↓, COX-2 ↓, iNOS ↓ |
| [75] | Citrus nobiletin | Nobiletin | Rat | TNBS | Intragastric | 7 d (1 d after colitis induction) | - | MPO ↓, iNOS ↓, COX-2 ↓, myosin light-chain kinase (MLCK) ↓, NF-kB ↓, protein kinase B (Akt) phosphoryla-tion ↓, trans epithelial electrical resistance ↓, inhibition of the Akt–NF‐κB–MLCK pathway |
| [66] | Vaccinium corymbosum (Portuguese blueberries) | Anthocyanin-rich fraction | Rat | TNBS | Intragastric | 8 d | n = 8 | DAI ↓, iNOS ↓, COX-2 ↓ |
| [41] | Malus sylvestris (apple) | Fruit | Rat | AA | Oral | 6 d | n = 5-6 | iNOS expression ↓, COX-2 expression ↓, Copper Zinc (CuZn) SOD expression ↑ protein expression of iNOS ↓ in ulcerated area, COX-2↔ & 8-OHdG ↔ |
| [210] | Scutellariae baicalensis | Oroxyloside | Mice | DSS | Intragastrically | 10 d | n = 8 | DAI ↓, MPO ↓, iNOS ↓, pro-inflammatory, cytokines in serum & colon ↓, peroxisome proliferator-activated receptor (PPAR)c ↑ →NF-κB ↓ |
| [211] | Rheum tanguticum | Polysaccharide | Rat | TNBS | Intrarectal | 5 d | n = 12 | DAI ↓, NF-κB p65 ↓, TNF-α ↓, COX-1↔, COX-2 ↓, PGE2 ↑, iNOS ↓ |
| [212] | Hypericum perforatum (St. John's Wort) | Hypericum perforatum extract | Rat | TNBS | Intraperitoneal | 3 and 7 d treatments | - | DAI ↓, CAT ↓, GSH ↑, tissue NO ↓, MPO, glutathione reductase (GR), MDA, GSH-Px ↔ |
| [42] | Allium sativum (garlic) | Diallyl sulfide (DAS) and diallyl disulfide (DADS) | Mice | DNBS + DAS DADS | Oral | 2 d after first day of treatment | n = 10 | In vivo→ DAI ↓ In vitro→ DADS→ IL-6 ↓, hydrogen sulfide ↑ DAS→ nitrite ↓, STAT-1 ↓, hydrogen sulfide ↑ |
| [213] | Avena sativa (oat) | β-glucan | Mice | DSS | Intragastric | 14 d | n = 20 | DAI, TNF-α, IL-1β, IL-6, iNOS, NO, MDA, MPO ↓ |
| [31] | Eryngium duriaei subsp. Juresianum; Laserpitium eliasii subsp. Thalictrifolium; Lavandula luisieri, Thapsia villosa | Essential oils (EO) | Primary human chondrocyte & C2BBe1 | IL-1β or a cytokine mixture (IFN-γ, IL-1β TNF-α) | - | EO added 30 min before cytokine stimulation | - | EO of L. luisieri→ iNOS ↓, p-IκB-α ↓ in both cell models EO of E. duriaei subsp. juresianum→ iNOS ↓, p-IκB-α ↓ in human chondrocytes EO of L. eliasii subsp. thalictrifolium & O. maritimus → iNOS ↓ in C2BBe1 cells EO of T. villosa → inactive in both cell types |
| [214] | Rhizophora apiculate | Whole plant | Mice | AA | Intraperitoneal | 7 d | n = 6 | SOD ↑, GSH ↑, LPO ↓, NO ↓, MPO ↓, lactate dehydrogenase (LDH) ↓, iNOS ↓, COX-2 ↓, translocation of NF-κBp65 & p50 subunits ↓ |
| [215] | Polygonum multiflorum | 2,3,5,4'-tetrahydroxystilbene-2-O-beta-d-glucoside (THSG) | Mice | AA+ mitomycin C | Oral | 7 d and 24 d | n = 12 | THSG (60 mg/kg) →DAI ↓, MPO ↓, MDA ↓, NO level ↓, iNOS ↓, SOD ↑ THSG (120 mg/kg) & 24th day of mitomycin C→ > positive control, 5-aminosalicylic acid (5-ASA) |
| [94] | Oryza sativa L. (black rice ) | Anthocyanin and rosmarinic acid | Mice | DSS | Oral | 8 d | n = 8 | Macroscopic damage ↓, microscopic damage ↓, body weight loss ↓, iNOS ↓- COX-2 ↓, IL-6 ↓, IL-1β ↓, TNF-α ↓ |
| [67] | Dioscorea alata | Anthocyanidin-es | Mice | TNBS | Rectal | 7 d | n = 10 | Macroscopic damage ↓, microscopic damage↓, body weight loss ↓, tight junction proteins ↑, MPO ↓, iNOS ↓, TNF-α ↓, IFN-γ ↓ |
| [216] | Patrinia scabiosaefolia | Root | Mice | DSS | Oral | 7 d | n = 8 | Macroscopic damage ↓, microscopic damage↓, IL-6 ↓, IL-1β ↓, TNF-α ↓, iNOS ↓ |
| [217] | Morinda citrifolia (noni) | Fruit juice/ flavonoids | Mice | DSS | Oral | 9 d | n = 8 | Microscopic damage ↓, IL-6 ↓, INF-γ ↓, NO ↓, MPO ↓ |
| [201] | Ziziphora clinopoides (kahlioti) | Aerial parts | Mice | DSS | Oral | 7 d | n = 6 | TNF-α ↓, TAC ↑, TTM ↑, LPO ↓, NO ↓ |
| [44] [43] | Camellia sinensis (Black tea) | Theaflavin-3,30-digallate | Mice | TNBS | Oral | 18 d | - | Macroscopic damage ↓, microscopic damage ↓, iNOS ↓, MPO ↓, TNF-α↓, IFN-γ ↓, IL-12 p40 ↓, NFκB ↓ |
| Thearubigin | Mice | TNBS | Oral | 18 d | - | Macroscopic damage ↓, microscopic damage ↓, iNOS ↓, O2- ↓, MPO ↓, IFN-γ ↓, IL-12 ↓, IL-4 ↓, NFκB ↓ | ||
| [218] | Picrasma quassiodes | Dried branches/ alkaloids | Mice | TNBS | Gastric lavage | 7 d | n = 10 | Macroscopic damage, microscopic damage, body weight loss, MPO, TNF-α, IL-8, COX-2, iNOS↓ |
| [95] | Aronia berries | Berry extract/ polyphenols | Mice | DSS | Oral | 10 d | - | Macroscopic damage, microscopic damage, body weight loss, TNF-α, IL-6, PGE2, NO, MAPK ↓ |
| [199] | Terminalia catappa | Stem/phenolic compound | Rat | TNBS | Oral | 9 d | - | Macroscopic damage ↓, microscopic damage ↓, NO ↓, IL-1β ↓, MUC2 ↑, MUC3 ↑ |
| [45] | Glycyrrhiza glabra (liquorice) | Glabridin | Mice | DSS | Oral | 7 d | - | Macroscopic damage, microscopic damage, body weight loss, iNOS, MPO, COX-2, TNF-α, IL-6↓ |
| [219] | Punica granatum (pomegranate) | Extract/ phenolic compounds | Rat | DSS | Oral | 30 d | n = 8 | Macroscopic damage ↓, Bifidobacteria and Lactobacilli ↑, lipid peroxidation ↓, iNOS ↓- COX-2 ↓, P53 ↑, cluster of differentiation molecule (CD) 40 ↓, IL-1β ↓, IL-4 ↓ |
| [220] | Physalis peruviana | Calyx/flavonoi-ds, terpenoids & glycosides | Rat | TNBS | Intraperitoneal | 3 d in protective protocol and 15 d in therapeutic protocol | - | Macroscopic damage ↓, microscopic damage ↓, COX-2 ↓, iNOS ↓, MPO ↓, NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) ↓, IL-1β ↓, IL-6 ↓, IL-10 ↓, MUC2 ↑ |
| [221] | Perilla frutecens | Whole plant | Mice | DSS | Oral | 14 d (starting from 7 d before induction of colitis) | n = 6 | NF-кB ↓, COX-2 ↓, iNOS ↓, cyclin D1 ↓, STAT-3 activation ↓, nuclear factor erythroid 2–related factor (Nrf)2 ↑, heme oxygenase-1 (HO-1) ↑, interferon regulatory factor 3 (IRF3) ↓ PE (10 mg/mL) in CCD841CoN human normal colon epithelial cells→ TNF-α ↓, iNOS ↓, P-IкB α ↓, P-STAT-3 ↓, C-X-C Motif Chemokine Receptor 2 (CXCR)2 ↓ |
IBD: Inflammatory bowel disease.
Lavandula spica L. or L. angustifolia Mill.: Members of the Lavandula genus, Lamiaceae, have been associated with a reduction of chronic inflammation in various settings, including IBD. Interestingly, in vitro studies suggested that more than one species of Lavandula works through the NO pathway to reduce inflammation in various cell lines. For instance, the essential oil of L. eliasii subsp. thalictrifolium specifically reduced iNOS expression in intestinal cell lines, indicating that these subspecies may be more appropriate for IBD settings. As expected, the anti-inflammatory effect of Lavandula species was exerted through the NF-κB signaling pathway[31,32].
Cannabis sativa L.: The marijuana plant C. sativa and its derivatives, Cannabinoids, are known to be a potential therapy for IBD. Cannabigerol and Cannabidiol were found to regulate a set of functions in the body and play a key role in curing IBD, particularly by reducing the production of nitric compounds, with therapeutic effect in colitis mice[33].
Olea europaea L.: Extracts from olive (Olea europaea) leaves are used in different traditional medicines as anti-inflammatory agents. The extract could be a great choice for the treatment of oxidative stress-induced inflammatory conditions, including IBD, mainly due to its antioxidant phenolic content, i.e., Oleuropeoside. This extract inhibited IBD progression, most probably by reducing the production of chemokines and nitrite compounds such as NO[34].
Retama monosperma (L.) Boiss.: Retama spp. is an indigenous component of traditional medicine of Mediterranean regions, and its anti-inflammatory and antioxidant effects have been well accepted. The hypoglycemic effects of this plant have been attributed to the high concentration of Pinitol, a cyclic polyol, present in the aerial parts of R. monosperma[35]. However, the antioxidant activities of this plant have been directly correlated with the titrated concentrations of its flavonoid content[36]. Oral administration of an aqueous extract of the aerial parts of R. monosperma led to a decrease in iNOS expression mediated through the NF-κB and p38 MAPK signaling pathways[37].
Hibiscus rosa-sinensis L.: Hibiscus rosa-sinensis, also known as rose mallow or China rose, is a species of flowering plant belonging to the family Malvaceae. H. rosa-sinensis was shown to have potential therapeutic value in ameliorating experimental colitis in laboratory animals, by inhibiting pro-inflammatory mediators such as NO and TNF-α. The hydroalcoholic extract of the leaves of H. rosa-sinensis; containing alkaloids, flavonoids, steroids, and phenols; significantly reduced severity of acetic acid-induced colitis symptoms as assessed by the clinical disease activity score[38].
Curcuma longa L.: Curcumin is the main constituent of the rhizome of Curcuma longa. Inhibition of COX-1, COX-2, TNF-α, iNOS, and NF-κB, and the potent anti-oxidant and anti-inflammatory effects of curcumin, makes this compound a great pharmacological candidate for patients with IBD[39].
Vaccinium corymbosum L.: An aqueous extract of highbush blueberry fruit (Vaccinium corymbosum), rich in phenolic acids especially flavonoids, was shown to inhibit iNOS overexpression through the NF-κB signaling pathway.
Panax notoginseng (Burkill) F.H.Chen: Panax notoginseng (P. notoginseng) is a well-known Chinese herbal medicine. An interesting character of P. notoginseng is its potential therapeutic effects on chronic diseases. The root extract of P. notoginseng, containing saponin, was found to exert inhibitory effects on iNOS and inflammatory cytokines[40].
Malus pumila Mill.: Apples (Malus spp., Rosaceae) and their products, rich in polyphenols, have shown diverse biological activities and may contribute to a variety of beneficial health events such as protecting the intestine against inflammation initiated by IBD. The preventive effect of polyphenolic concentrated apple extract was illustrated in an acetic acid-induced IBD rat model, resulting in inhibition of iNOS overexpression through the NF-κB pathway[41].
Allium sativum L.: Plants of the genus Allium are known for their production of organosulfur compounds, which have marked biological and pharmacological properties. Garlic (Allium sativum) is one of the most widely used species, displaying a broad spectrum of beneficial anti-inflammatory effects. Diallyl sulfide and Diallyl disulfide are the main organosulfur compounds, with proved inhibitory effects on nitrite derivatives and pro-inflammatory elements involved in IBD pathogenesis[42].
Camellia sinensis (L.) Kuntze: Camellia sinensis (C. sinensis) leaves are rich in vitamins (B and C), minerals, polyphenols, caffeic acid, fertaric acid, tannins, and volatiles. In vivo studies showed that the polyphenol components of C. sinensis, theaflavin, and thearubigins, downregulated iNOS overexpression through the NF-κB pathway in oxidative-induced inflammatory complications such as IBD[43,44].
Glycyrrhiza glabra L.: Licorice, derived from the root of Glycyrrhiza glabra, is extensively used in traditional medicines for a variety of complications and ailments. Licorice possesses immune-modulatory and adaptogenic properties, required for the pathogenesis of IBD. The ethanolic extract of this plant, rich in glabridin, was found to be effective in IBD as it attenuated pro-inflammatory elements and iNOS production[45].
Phytochemicals
Table 2 depicts plant-derived compounds that affect IBD by modulating the NO pathway. Polyphenols are the most investigated plant-derived constituents with well-documented positive effects for IBD treatment via the NO pathway[46]. According to their chemical structures and phenolic content, polyphenols are classified into several subgroups. This section mostly focused on the effects of these subgroups on the NO pathways.
Table 2.
Plant-derived compounds affecting inflammatory bowel disease by modulating nitric oxide pathways
| Ref. | Phytochemical | type of animal | Model of IBD | Route of administration | Duration of treatment | Numbers of animals in intervention group and control group | Outcomes |
| [71] | Naringenin (25, 50, 100 mg/kg per day) | Rat | Acetic acid (AA) | Transrectal | 7 d before colitis induction | n = 6 | Disease activity index (DAI) ↓, total glutathione sulphadryls (T-GSH) ↑, non-protein sulphadryls ↑, DNA, RNA and total protein content ↑, nitrit oxide (NO) ↓, catalase (CAT) ↑, superoxide dismutase (SOD) ↑, tumor necrosis factor (TNF)-α ↓, interleukin (IL)-1β ↓, IL-6 ↓ |
| [82] | Eupatilin (ethanol extract of aerial parts of Artemisiae herba, EIE) and quercetin-3-β-D-glucuronopyranoside from Rumex aquaticus (EIQ) (EIE, 100 mg/kg & EIQ, 30 mg/kg) | Rat | 2,4,6-trinitrobenzene sulfonic acid (TNBS) | Oral | 48, 24 and 1 h prior to the TNBS instillation and again 24 h later | n = 6 | DAI ↓, myeloperoxidase (MPO) ↓, NO ↓, TNF-α ↓, total glutathione sulphadryls (GSH) ↑, malondialdehyde (MDA) ↓ |
| [74] | Naringin (20, 40, or 80 mg/kg) | Rat | AA | Oral | 12 d (8 d before colitis induction and 4 d after) | n = 6 | DAI ↓, spleen weight ↓, white blood cell (WBC) ↑, red blood cell (RBC) ↑, hemoglobin (Hb) ↑ & platelet ↑, lactate dehydrogenase (LDH) ↓, alkaline phosphatase (ALP) ↓, SOD ↑, GSH ↑, Colon lipid peroxidation (LPO) ↓, NO ↓, MPO ↓, xanthine oxidase activity ↓, protein carbonyl content ↓, the number of unwinded double strand DNA ↓ |
| [222] | Oligonol (0.5 and 5 mg/kg/d) | Mice | Dextran sulfate sodium (DSS) | Oral | 7 d before colitis induction | n = 5 | DAI ↓, IkBα phosphorylation & degradation ↓, p65 phosphorylation & nuclear translocation ↓, cyclooxygenase (COX-2) ↓, iNOS ↓, expression of antioxidant enzymes ↑, colon carcinogenesis ↓, the incidence and the multiplicity of colonic adenoma ↓ |
| [223] | Algal meroterpene11hydroxy11Omethylamentadione (1, 10 & 20 mg/ kg) | Mice | DSS | Oral | 7 d after colitis induction | DAI ↓, MPO ↓, TNF-α ↓, IL-1β ↓, IL-10 ↓, COX-2 ↓, iNOS ↓ | |
| [224] | Fraxinellone | Mice | DSS and lipopolysacchari-de (LPS) | intraperitoneal | 9 d | n = 10 | DAI ↓, MPO ↓, alkaline phosphatase (ALP) ↓, glutathione ↑, IL-1β ↓, IL-6 ↓, IL-18 ↓, TNF-α ↓, inhibition of cluster of differentiation molecule 11B (CD11B).+ macrophage infiltration, ICAM1 ↓, vascular cell adhesion molecule 1 ↓, iNOS ↓, COX-2 ↓, NO ↓, NF-κB signaling ↓ & NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome ↑ |
| [79] | Isoquercitrin (1 and 10 mg/kg/d) | Rat | DSS | Oral | 14 d (DSS induced the second half) | n = 8 | Colon shortening ↓, COX-2 ↓, iNOS ↓, tissue healing did not encompass rectum |
| [225] | Oridonin (5.0 and 7.5 mg/kg) | Mice | TNBS | ntraperitoneal | 7 d | n = 3-9 | DAI ↓, TNF-α ↓, interferon (INF) ↓, IL-17A ↓, iNOS ↓, COX-2 ↓, in vitro lymphocyte proliferation ↓, NF-κB p65 expression & activity ↓ |
| [68] | Cardamonin (20, 50, and 100 mg/kg) | Mice | DSS | Oral | 2 d before DSS treatment & 7 d after | n = 10 | NO ↓, TNF-α ↓, IL-6 ↓, toll like receptor (TLR)-4 ↓, myeloid differentiation factor (MDF) 88 ↓, IL-1 receptor-associated kinase-1 ↓, IκBα ↓, IκBK-α/β ↓, mitogen-activated protein kinase (MAPK) ↓, c-Jun NH2-terminal kinase ↓, nuclear translocation of NF-κB p65 ↓ |
IBD: Inflammatory bowel disease.
Flavonoids: Flavonoids are natural products present in a wide range of vascular plant species. They function in several categories including protection against UV-light and phytopathogens (i.e., phytoalexins in legumes), attraction of pollinators (i.e., anthocyanins in berries), and reduction of reactive oxygen species (ROS) in conditions of oxidative stress (i.e., quercetin and other flavonols)[47]. Previous studies rigorously support the effect of flavonoids on suppressing various elements involved in innate immunity in a variety of hypersensitivity and inflammatory conditions including osteoarthritis, nephrotoxicity, photodamaged skin, diabetes mellitus, and IBD[48-53]. As mentioned, iNOS is an inflammatory marker, creating a situation of oxidative stress by producing high amounts of NO, resulting in a slippery slope of ONOO− formation, oxidative damage, nitration, and S-nitrosylation of certain biomolecules such as proteins, lipids, and DNA[54].
During inflammation, flavonoids regulate/reduce NO production and iNOS expression via 2 main pathways; the NF-κB and JAK/STAT signaling pathways[55-57]. These pathways are stimulated during the inflammatory process, then macrophages are infiltrated to affected tissues. Thereafter, TLR-4 and INF-γ receptors are activated and induce the mentioned molecular cascades. Consequently, expression of iNOS is up-regulated. Flavonoids also downregulate the NO pathway, due to their ability to suppress elements of the above-mentioned pathways[47].
The peroxisome proliferator-activated receptor γ (PPARγ) was found to be a key participant in downregulation of iNOS[58]. PPARγ is a major transcription factor of lipid oxidation and metabolism genes in macrophages, and has anti-inflammatory activities. Apigenin, chrysin, and kaempferol were found to especially reduce iNOS expression via PPARγ activation in vitro[59]. Park et al[60] suggested that beyond NF-κB inhibition, the anti-oxidant activities of flavonoids must be mediated by other mechanisms. It is reported that trimeric flavonoids exhibit stronger anti-inflammatory and anti-oxidant properties than monomeric flavonoids. Trimeric flavonoids activate the NF-κB pathway, while monomeric flavonoids were shown to have a suppressive effect.
Several protein docking simulation analyses have proposed that flavonoids such as silibinin and deguelin, can directly bind iNOS and are able to suppress iNOS activity. Therefore, a number of flavonoids might directly bind iNOS and inhibit its functions, in a way other than modulating its expression at gene level[61,62]. Overall, flavonoids significantly diminish pathologic lesions in colonic tissue, attributed to their anti-inflammatory and antioxidant effects, resulting in NO reduction.
Anthocyanins: Anthocyanins are an important subcategory of flavonoids, available in many fruits and vegetables with a prominent red, blue, or purple hue. Anthocyanins were shown to effectively suppress inflammation and tumorigenesis processes in different cell lines[63-65]. A significant decrease was reported in the expression levels of TNF-α and iNOS, when anthocyanin-rich fractions (ARF) of purple yam (Dioscorea alata L.) and Portuguese blueberries (Vaccinium corymbosum L.) were orally administered to animals. The ARF prepared from the latter fruit reduced iNOS expression as effectively as 5-aminosalicylic acid (5-ASA) at a molar anthocyanin concentration approximately 30 times lower than that of 5-ASA[66,67].
Cardamonin: Oral administration of Cardamonin, a chalcanoid produced in members of the Alpinia genus, reversed the upregulation of TLR-4 and cytokine receptors along with the cascade of proteins downstream to these receptors (i.e., myeloid differentiation factor 88, IL-1 receptor-associated kinase-1, inhibitor κBα, and inhibitor κB kinase-α/β, as well as MAPK and c-Jun NH2-terminal kinase), resulting in inhibition of the nuclear localization of NF-κB p65 and inactivation of the MAPK pathway. Cardamonin also suppressed the expression of their target genes, i.e., iNOS[68].
Luteolin: Luteolin, a yellow, naturally occurring flavone, interferes with iNOS expression in IBD models by inhibiting elements of the NF-κB cascade as mentioned above[69]. Luteolin significantly inhibited IL-8 production, COX-2 and iNOS expression, and cytokines-induced NO overproduction; indicating that luteolin negatively modulates the key inflammatory signaling cascades underlying intestinal inflammation. Mechanistically, inhibition of the JAK/STAT pathway was identified as a critical mechanism by which luteolin exerts its intestinal anti-inflammatory action[70].
Naringenin and naringin: Naringenin and naringin, the glycosidic form of naringenin, belong to the flavanones and are frequently found in grapefruit. These compounds were shown to decrease the colonic level of NO and inflammatory cytokines[71,72]. Moreover, naringin reduces colonic xanthine oxidase level, which catalyzes the conversion of nitrite, both the physiological storage pool of NO and its metabolites[73]. Concerning NO reduction, pre-treatment with 50 mg/kg/d of naringenin showed efficiency comparable to Mesalazine (300 mg/kg/d)[71].
Nobiletin: Nobiletin is a widely distributed O-methylated flavone found in citrus peels, which has recently attracted attentions due to its anti-insulin resistance, anti-inflammatory, and anti-cancer characteristics[74]. In addition to decreasing the nuclear localization of NF-κB, nobiletin regulated the tissue production of NO through an Akt-dependent manner. Oral treatment of nobiletin (40 mg/kg) was shown to be as potent as 100 mg/kg of Sulfasalazine, a first-line drug in IBD treatment[75].
Quercetin and isoquercetin: Quercetin, the most widely distributed flavonoid, has been shown to suppress LPS-induced IKK, NF-κB and AP-1 activation; and the IFN-γ-induced NF-κB, STAT1, and interferon regulatory factor-1 (IRF-1) activation in vitro, almost all of which are upstream of the NF-κB and JAK/STAT signaling pathways. Quercetin also induces heme oxygenase-1 (HO-1) expression via activation of tyrosine kinase and MAPK. Quercetin was shown to partly downregulate iNOS expression through this pathway[76]. Suppression of PI3K/AKT might reduce the nuclear translocation of NF-κB and the subsequent increase in iNOS expression[77,78].
Isoquercetin, the 3-O-glucoside of quercetin is present in fruits such as mango, and has been shown to repress iNOS expression in IBD models, in a dose-dependent manner[79]. However, isoquercetin displayed little to no effect on histological damage and the iNOS level in lower segments of the colon, where the damage was considerably severe, suggesting that the isoquercetin effect might be correlated with severity and histology of target tissues[79].
Wogonoside: Wogonoside is a bioactive flavonoid derived from the root of Scutellaria baicalensis Georgi. This glucuronide metabolite of wogonin has been shown to possess anti-inflammatory and anticancer effects. Recent studies revealed that NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome are implicated in IBD, mainly by inducing IL-1β production. To date, only limited agents have been nominated to target both NF-κB and NLRP3 inflammasome in IBD[80]. In DSS-induced colitis mice, wogonoside alleviated body weight loss, colon length shortening, colonic pathological damage, inflammatory cells infiltration, myeloperoxidase (MPO) and pro-inflammatory mediators levels, and iNOS activity. Furthermore, this compound reduced IL-1β, TNF-α, and IL-6 production, and downregulated the mRNA expression of pro-IL-1β and NLRP3 in phorbol myristate acetate (PMA)-differentiated monocytic THP-1 cells, through suppression of NF-κB and NLRP3 inflammasomes. Wogonoside also repressed iNOS expression, twice more potent than 5-ASA, by suppressing both NF-κB and NLRP-3 inflammasomes, which were not targeted by 5-ASA[81].
Other flavonoids: A number of flavonoids have also been reported to reduce iNOS expression and inflammatory cytokines in IBD models such as (1) Rutin (the glycoside combining the flavonol quercetin and the disaccharide rutinose found in citrus fruits but not quercetin itself)[81]; (2) Glabridin (an isoflavone of the root extract of licorice)[45]; (3) Eupatilin (an O-methylated flavone from the aerial parts of Artemisiae herba); and (4) Quercetin-3-β-D-glucuronopyranoside (isolated from Rumex aquaticus)[82].
Other polyphenols: In this section, we discuss other polyphenolic compounds rather than flavonoids.
Canolol
A major component of crude canola oil called canolol, has been put in the spotlight due to its anti-mutagenesis roles. Canolol was shown to affect IBD and NO production in the manner discussed above[83]. It was reported that following canolol treatment, IL-12, TNF-α, COX-2, iNOS, and the oxidative responding molecules (i.e., HO-1) were suppressed[83].
Curcumin
Hydroxycinnamic acids are a subcategory of polyphenols. Among these compounds, curcumin a bright yellow curcumoid found in turmeric, is the main spice in curry, and is considered as an antioxidant, anti-inflammatory, and anti-tumorigenesis substance. In addition to suppressing NO production and iNOS expression during reducing inflammation in IBD models[39], curcumin plays an anti-epileptic role in the central nervous system by downregulating neuronal NOS, improving endothelial dysfunction and vascular remodeling through upregulation of eNOS[84-86]. It has also been reported that the anti-inflammatory effect of curcumin has been implicated in suppression of the protein components of the NF-κB and JAK/STAT pathways[51,86,87].
Gallic acid, thea-3,3'-bigallate, and thearubigin
Among the polyphenols, hydrobenzoic acids have received considerable attentions. Gallic acid itself and its derivatives such as thea-3,3'-bigallate and thearubigin (a polymer of epigallocatechin and epigallocatechin gallate found in black tea), were shown to modulate iNOS expression and NF-κB suppression[45,46,88]. In addition, gallic acid acts through the IL-6/STAT-3 signaling pathway, not via iNOS expression[88,89].
Oligonol
Oligonol is a lychee-fruit-derived low molecular weight polyphenol containing catechin-type monomers and oligomers with beneficial effects on memory in amyloid β-induced Alzheimer's disease models, and reduces tissue injury in various organs by inhibiting the expression of NF-κB p65, COX-2, and iNOS[90,91]. It was shown that oligonol improved inflammation through downregulation of iNOS expression and NO production, although the compound enhanced cardiac health by increasing NO production and vasodilation, which was mediated by eNOS upregulation[91,92]. Oligonol can mediate IBD symptoms mainly via iNOS suppression[93].
Rosmarinic acid
Rosmarinic acid is abundantly present in phenolic acid-rich species such as black rice and Aronia berry. Oral administration of rosmarinic acid ameliorated colonic inflammation and downregulated iNOS expression[94,95]. Moreover, rosmarinic acid reduced IL-6, IL-1β, and IL-22 expression, and suppressed the protein levels of COX-2 and iNOS in IBD by inhibiting the NF-κB and STAT-3 signaling pathways[96].
Dairy products
Therapeutic dietary products have long been of interest in mitigating inflammation. For instance, goat cheese whey; a by-product of the cheese-making process, is rich in amino acids threonine and cysteine, and oligosaccharides such as sialic acid; which can act as an antioxidant with considerable immunomodulatory function[97,98]. In addition to increasing mucin synthesis by threonine and cysteine[99,100], it can modulate the colonic flora in a murine model of colitis[100]. In acetic acid-induced colitis rats, the anti-oxidative and anti-inflammatory properties of goat cheese whey have been shown to reduce iNOS expression, comparable to Sulfasalazine[101]. Whether specific component/s of goat cheese whey is responsible for such effects requires clarification[101].
It has been demonstrated that glycomacropeptide, a product of the enzymatic hydrolysis of casein, can have immunomodulatory effects by altering the colonic bacterial population, i.e., accommodating host-friendly microorganisms and confronting pathogens[102-104]. Pre-treatment of colitis rats with glycomacropeptide, inhibited pro-inflammatory cytokines production, iNOS expression, and improved anorexia[105].
Probiotics and prebiotics
Although various data suggest that intestinal microorganisms trigger inflammation, and the efficacy of antibiotics is being clarified in IBD subjects; it seems the aberrant immune response to intestinal antigens plays a major role in the pathogenesis of IBD. In this regard, extensive research on commensal flora has revealed noticeable variations in the composition of gut microbiota in IBD patients compared with healthy controls[106-108], active phases of IBD compared with inactive phases, and between the parts of the intestine affected by IBD[109-111]. During past decades, ingestion of live microorganisms available as probiotics[112] or non-digestible substrates for selective microorganisms known as prebiotics[113], has been introduced as a method of altering the intestinal microbiome and to potentially prevent or reduce inflammation in IBD[114]. Herein, we review evidence on the efficiency of pro- and pre-biotics in IBD treatment, specifically through modulation of NO production. Overall, it seems that pro- and pre-biotics attenuate NO production by suppression of the IκB/NF-κB pathway[115], i.e., via IκB-α degradation and ubiquitination in epithelial cells[116,117]. The anti-inflammatory properties of probiotics may be related to the suppression of proteasome function and inhibition of the transmission of complexes shaped by NF-κB and PPAR-γ from the nucleus[118]. In addition, IL-10 secretion and the suppression of dendritic cells-induced IL-12 secretion attenuated the immunomodulatory effects and iNOS activity[118].
Lactobacillus farciminis: L. farciminis has been shown to release NO in vitro. In 2004, Lamine et al[119] showed that oral administration of L. farciminis in a 2,4,6-trinitrobenzene sulfonic acid (TNBS) model of rat colitis, similar to sodium nitroprusside, led to intraluminal NO release, alleviated the macroscopic evidence of colitis, and attenuated iNOS and MPO activities. In 2006, Peran et al[120] designed a study to investigate the potency of L. fermentum in the TNBS model of rat colitis. Treatment with L. fermentum promoted Lactobacillus species growth and short-chain fatty acids (SCFAs) production, reduced microscopic colitis, extenuated oxidative stress, and ameliorated TNF-α secretion and iNOS expression. They subsequently performed a comparative analysis of the effectiveness of probiotics in the same model, in which treatment with Bifidobacterium lactis, also known as L. acidophilus, reduced iNOS expression, and prevented intestinal inflammation and diarrhea[121]. However, L. casei seemed to have no significant effect on iNOS expression[121].
B. lactis was shown to transiently activate NF-κB expression and p38 MAPK, the bacterium also inhibited the expression of iNOS and COX-2, and TNF-α production. This was achieved by facilitating a cross-talk between the intestine epithelial cells and immune cells[122], indicating the anti-inflammatory effect of B. lactis. On the other hand, L. acidophilus and L. fermentum, secondary to a decrease in inflammatory cytokines release and neutrophil activation, reduced iNOS expression and oxidative stress[120]. In contrast, L. farciminis, seems to exert its anti-inflammatory effect mainly by generating NO in the intestine, and partly through reducing the release of pro-inflammatory cytokines, and by enhancing the barrier integrity and modification of intestinal flora[123].
In DSS-induced colitis mice, administration of L. plantarum AN1 strain cells, which were derived from the fermented fish aji-narezushi, enhanced endogenous Lactobacillus growth, especially L. reuteri, and protected against colitis with a significant effect on mucosal damage[124]. On the other hand, in vitro administration of both live and heated L. plantarum AN1 strains to murine macrophage RAW264.7 cells, improved the anti-inflammatory effect by reducing NO secretion[124], supporting the outcomes of previous studies.
In addition to the beneficial effects of Lactobacillus in reducing NO production in animal models of colitis, the protective effects of L. rhamnosus GG on normal human colon epithelial cells and murine macrophages, by increasing iNOS expression, has been reported[125]. Thus, it appears that Lactobacillus may be beneficial in alleviating colitis by modulating iNOS expression. In the DSS model of colitis[126], oral administration of Rhodobacter sphaeroides extract (LycogenTM), reduced pro-inflammatory cytokines and NO production, decreased colonic bacteria, and prevented weight loss and colon shortening, while improving survival[126].
More recently, combination therapy with probiotics has been examined in animal models of colitis. A combination of four live bacterial strains (L. acidophilus, L. plantarum, B. lactis and B. breve) form a probiotic cocktail named "Ultrabletique". It was shown that oral administration of Ultrabiotique in DSS-induced colitis, attenuated microscopic evidence of inflammation, reduced NO production, as measured in the supernatants of peritoneal macrophages (PMQ) cultures[127]. Later, a study by Toumi et al[128] demonstrated that Ultrabletique treatment ameliorated plasma NO and IFN-γ production in association with reduced expression of colonic TLR-4, iNOS and NF-κB in DSS-induced colitis. LPS-induced TLR-4 stimulation, activated the NF-κB pathway, thereby, inducing iNOS expression. On the other hand, LPS-induced production of IFN-γ leads to the activation of IRF-1 and NF-κB. In vivo, co-localization and interaction of IRF-1 and NF-κB in the macrophage nucleus, physically bends the iNOS promoter DNA and leads to NO production[129]. In this study, Ultrabiotique significantly reversed these mechanisms.
Kefir, a natural beverage consisting of a fermented dairy product obtained by exposing milk to yeast and bacteria; composed of Lactobacillus, Acetobacteria, Streptococcus and yeasts; attenuated colitis in DSS mouse model by extenuating pro-inflammatory cytokines and NO production[128]. In a study by Soufli et al[130] the laminated layer of Echinococcus granulosus cyst increased IL-10 expression, and decreased the expression of TNF-α, IFN-γ, NF-κB, and iNOS, thus, improving colitis. Helminth antigens from organisms such as Trichuris suis have previously been used in IBD subjects; however, the underlying mechanisms are undetermined[131-133], investigations into their potential to relieve inflammation in IBD are warranted.
In addition to direct anti-inflammatory effects of bacterial antigens, probiotic products may have such effects. Fermentation of water-soluble fibers by anaerobic bacteria leads to SCFA production. For instance, hydrolyzation and fermentation of inulin, a natural beta-fructan extracted from many types of plants, by microbiota and Lactobacilli in the intestinal lumen results in SCFAs formation (i.e., butyric acid). It was shown that inulin suppressed inflammation in DSS-induced colitis rats, at least partly mediated by reducing NO production[127]. Previous studies have shown that SCFAs significantly suppressed pro-inflammatory cytokines expression in intestinal cells via downregulation of NF-κB[134,135]. Arribas et al[136] developed a caramel with a high content of difructose dianhydrides and their glycosylated derivatives, cyclic fructans that were shown to provide an appropriate environment for Lactobacillus and Bifidobacterium species. In addition, the intestinal microbiota is able to produce SCFAs by fermentation of DFAs, and can further promote their anti-inflammatory effects by reducing iNOS expression.
Animal oils
The advantage of polyunsaturated fatty acids (PUFAs) intake in IBD was elucidated by epidemiologic studies in Eskimos[137] and by lower levels of PUFAs in patients' sera[138]. Although, data are conflicting, most of the studies support the efficiency of PUFAs in IBD[137]. PUFAs are able to attenuate inflammation, as in IBD, by altering the production of eicosanoids and COX-2, and by modulating PPAR-γ and NF-κB[139-143]. The inefficacy of omega-3 PUFAs has been reported in a study on protection against IBD, which was in contrast to conjugated linoleic acid (CLA)[143].
Omega-6 PUFAs such as (17S)-hydroxy-docosapentaenoic acid, but not (10, 17S)-dihydroxy-docosapentaenoic acid, exhibited inhibitory effects on iNOS expression in a DSS model of colitis, as well as predominating M2 macrophages with anti-inflammatory properties in vitro[144]. Additionally, (5E,7Z,10Z,13Z,16Z,19Z)-4-Hydroxy-5,7,10,13,16,19-docosahexaenoic acid, a potential agonist of PPAR-γ with antidiabetic property[145], has been shown to modulate colitis through suppression of iNOS. Although, these effects are independent of PPAR-γ inhibition[146].
Amino acids
Glutamine: Glutamine at concentrations above 0.5 mmol/L reduced NF-κB nuclear transportation, iNOS expression, and attenuated colitis severity[147]. Indeed, the intrarectal administration of glutamine has been associated with less intestinal damage and reduced the expression of STAT-1, STAT-5, and NF-κB, decreased pro-inflammatory cytokines (i.e., IL-8 and IL-6) production, and enhanced IL-10 in an IBD model[148].
L-Arg: Administration of L-Arg enhanced the survival time of colitis animals, while reducing the expression of iNOS and NF-κB. However, different studies have reported various results on the beneficial effect of L-Arg on the colon[149,150].
Synthetic molecules
Previous studies showed that NF-κB inhibitors directly suppresse the expression of NF-κB dependent pro-inflammatory mediators, including iNOS. Therefore, blockade of the NF-κB signal transduction pathway may be one of the major mechanisms underlying several synthetic molecules in management of IBD. Hence, the following sections will discuss studies in which NF-κB/NO signaling helped to improve IBD complications (Table 3).
Table 3.
Synthetic compounds affecting inflammatory bowel disease by modulation of nitric oxide pathways
| Ref. | Type of animal | Model of IBD | Intervention | Duration of treatment | Numbers of animals in intervention group and control group | Outcomes |
| [226] | Rat | Dextran sulfate sodium (DSS) | Dinitrate-barbiturate (rectally twice daily) | 5 d | n = 12 | Matrix metalloproteinase (MMP)-9 activity ↓, disease activity ↓, colonic injury ↓ |
| [227] | Rats | Lipopolysaccharide (LPS) | Ursodeoxycholate (gavage) | 4 d | n = 4 | Circulating nitrite/nitrate ↓, intestinal epithelial inducible nitric oxide synthase (iNOS) activity ↓, colonic injury ↓ |
| [177] | Rat | Acetic acid (AA) | N-Acetylcysteine (NAC) (100 mg/kg for 7 d, 20 mg/kg for 2 d) (intraperitoneal, intracolonic) | 2 d, 7 d | - | 100 mg/kg NAC → tissue myeloperoxidase ↓, glutathione ↓, NO ↓, colonic injury ↓ 20 mg/kg NAC→ no protective effects |
| [229] | Rat | 2,4,6-trinitrobenzene sulfonic acid (TNBS), AA | NG-nitro-L arginine methyl ester (L-NAME) | accompanied by TNBS or 7 d before AA | n = 55 | TNBS-treated rats→ tissue injury ↓, lesion area ↓, colonic weight ↓, myeloperoxidase activity ↓, nitric oxide synthase (NOS) activity ↓ 24 hours after AA+ capsaicin pretreated rats→ tissue injury ↓, lesion area ↓, colonic weight ↓, NOS activity ↓ TNBS + L-NAME treated rats→ mean arterial blood pressure was higher than in TNB treated rats |
| [228] | Mice | Dinitrobenzene sulfonic acid (DNBS) | Trichinella spiralis antigens (helminth ags), (rectal submucosal) | 5 d before DNBS induction | n = 6-8 | Macroscopically and histologically colitis ↓, mortality rate↓, MPO activity↓, IL-1β production↓, inducible nitric oxide synthase (iNOS) expression ↓, IL-13 ↑, transforming growth factor beta (TGF)-β ↑, TH2 dominancy |
| [170] | Human mixed mono- nuclear cells (MMCs) co-cultured with HT-29 cells from UC patients | IFN-γ and LPS | Budesonide or prednisolone (corticosteroids) | Incubation or Pre-treatment | - | Nitrite content ↓, iNOS expression ↓ |
| [191] | Rat | TNBS | Arginine-Glycine-Aspartic acid (RGD)-functionalized silk fibroin nanoparticles (SFN) (intrarectal, 1 mg/rat) | 7 d | n = 10 | Adhesion of integrins of the cell surface to the extra- cellular matrix of connective tissue ↓→ leukocyte recruitment to the inflamed intestinal tissue ↓→ iNOS expression ↓ |
| [179] | Mice | DSS | Pravastatin, an 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitor, (intraperitoneal, 1 mg/kg) | 10 d | n = 4-9 | Cachexia ↓, hematochezia ↓, intestinal epithelial permeability ↓ with no effect on serum cholesterol, colonic injury ↓, expression of mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1) ↓, mucosal endothelial nitric-oxide synthase (eNOS) mRNA degradation ↓, eNOS expression ↑, protective effects of pravastatin in DSS-induced colitis were not found in eNOS-deficient mice |
| [153] | Mice | Pathogen Citrobacter rodentium as an infection model and DSS as an injury model | Antennapedia-linked Apolipoprotein E-mimetic peptide COG112, (intraperitoneal) | Concurrent with induction of colitis, during induction plus recovery, or only during the recovery phase of disease | n = 4 | C. rodentium treated mice→ improving the clinical parameters of survival, body weight loss, colon weight, histologic injury, expression of iNOS & the CXC chemokine keratinocytes (KC) & macrophage inflammatory protein (MIP)-2, more effective in iNOS-deficient mice, DSS treated mice→ body weight loss ↓, colon length ↓, histologic injury ↓, iNOS ↓, KC ↓, TNF-α ↓, IFN-γ ↓, IL-17 mRNA expression↓, nuclear translocation of NF-κB ↓, IKB kinase (IKK) activity ↓ |
| [192] | Mice | DSS | 3,3-Diindolylmethane (oral) | 7 d, commencing at the same time DSS exposure began | n = 5 | Number of iNOS- & COX-2-producing cells ↓ |
| [147] | Rat | DSS | Glutamine (oral) | 7 d with DSS | n = 13 | Cytokine-induced iNOS expression↓, nuclear translocation of NF-κB p65 subunit ↓, cellular heat shock proteins (HSP)25 & HSP70 in a dose-dependent manner ↑ |
| [180] | Rat | TNBS | Lactulose (oral) | 2 wk before TNBS adminstration | n = 10 | MPO activity ↓, colonic TNF-α ↓, leukotriene B4↓, iNOS expression ↓, levels of Lactobacilli and Bifidobacteria species in colonic contents ↑ |
| [229] | Rat | Iodoacetamide | NG-nitro - L-NAME (oral) | 21 d | n = 9-16 | lesion area ↓, NOS activity ↓ |
| [200] | Mice | DSS | GL-V9, a synthesized flavonoid derivative (intragastric) | From day 1 to day 10 | - | Inflammatory cells infiltration↓, myeloperoxida-se (MPO) activity ↓, iNOS activity ↓, ROS & MDA generation ↓, SOD ↓, GSH reservoir ↑, pro-inflammatory cytokines production in serum & colon ↓; pro-inflammatory cytokines ↓, ROS production ↓, antioxidant defenses ↑ in mouse macrophage RAW264.7 cells by promoting thioredoxin-1 expression |
| [159] | Mice | DSS | Glucosamine oligomers (Chitooligosaccharid-es) (oral) | 5 d | n = 8 | Number of positive areas of iNOS & nuclear factor (NF)-κB staining in the colonic epithelium ↓ |
| [27] | Mice | DSS | Nitrite (1 mmol/L) or nitrate (10 mmol/L) (oral) | 7 d | n = 27-29 | Nitrite (1 mM) → DAI score↓, colon length ↑, iNOS expression ↓, histopathology ↓, DSS-induced decrease in colonic mucus thickness↓, goblet cell abundance ↑. Nitrate (10 mM)→ DAI-score ↓ |
| [176] | Rats, mice | TNBS and DSS | Doxycycline (oral) | 5 d after the first DSS colitis induction | n = 10-21 | Macrophages → IL-8 production ↓ by intestinal epithelial cells & NO production ↓. TNBS-colitic rats (5, 10 and 25 mg/kg) → DAI score ↓, colonic tissue damage ↓, mRNA expression of IL-6, TNF, IL-17, intercellular adhesion molecule 1 (ICAM1), iNOS & MCP-1 ↓, partial restoration of the mRNA levels of markers of intestinal barrier function (ZO-1, occludin & mucin (MUC)-3 |
| [173] | Ulcerative Colitis (UC) and Crohn's Disease (CD) patients | - | Corticosteroids (Sulfasalazine and Azathioprine) (intravenous) | - | (1) UC treated with high dose corticosteroids (6 patients, 10 blocks); (2) UC patients who had never received corticosteroids (10 patients, 16 blocks); (3) CD treated with high dose corticosteroids (12 patients, 24 blocks); (4) Non-inflammatory, non-neoplastic controls (4 patients, 6 blocks) | Immunostaining with an antibody raised against the C terminal end of iNOS for NOS→ diffuse in UC & patchy in CD in epithelial cells & was most intense near areas of inflammation, Non-inflamed epithelium showed no immunoreactivity, treatment with corticosteroids made no difference to the amount of NOS |
| [162] | Rat | TNBS | Telmisartan (angiotensin II receptor antagonist) (oral, 10 mg/kg) | 10 d before TNBS and until day 4 of TNBS | n = 8 | DAI score ↓, colon weight/length ratio ↓, macroscopic damage ↓, histopathological findings ↓. Inflammation ↓, leukocyte migration ↓, TNF-α ↓, prostaglandin E2 (PGE2) ↓, MPO activity ↓, restoration of IL-10 mRNA and protein expression of NF-κB p65↓, mRNA expression of COX-2 and iNOS ↓, peroxisome proliferator-activated receptor (PPAR)-γ ↑, oxidative stress ↓ [lipid peroxidation (LPO) ↓, NO ↓, GSH ↑, TAC ↑, SOD ↑, glutathione peroxidase activity↑], apoptosis ↓ (mRNA, protein expression and activity of caspase-3 ↓, cytochrome C and Bax mRNA ↓, Bcl-2 ↓) |
IBD: Inflammatory bowel disease.
NF-κB-NO dependent synthetic molecules
To date, numerous “NF-κB and NO inhibitors” have been introduced with potentially beneficial effects in colitis treatment[151,152]. Notably, the NF-κB inhibitory effects of these molecules are mostly due to the blockade of NF-κB translocation to the nucleus, either by inhibiting P65 or IκB degradation.
COG112: It is known that Apolipoprotein E has immunomodulatory effects and synthetically derived Apolipoprotein E-mimetic peptides were found to be useful in models of sepsis and neuroinflammation. For example, one of these peptides, COG112, was shown to cause a significant reduction of iNOS expression in a colitis model[153]. COG112 also improved the clinical parameters of survival, body weight, colon weight, and histologic injury in these animals. Moreover, COG112 inhibited colon tissue iNOS, keratinocytes, TNF-α, IFN-γ, and IL-17 mRNA expression, and reduced the nuclear translocation of NF-κB. The IKK activity was also reduced, a necessary factor for activation of the canonical NF-κB pathway[153].
Dexamethasone: Dexamethasone is a corticosteroid, and exhibited a predictable positive effect on colitis-induced colon damage, which was correlated with reduced iNOS expression and NF-κB activity. This effect was even stronger when dexamethasone was administered in particle or microsphere form[151,152].
Pioglitazone: Pioglitazone is a PPARγ ligand, approved for diabetes treatment. Pioglitazone was also found to be an NF-κB-DNA binding inhibitor, allowing a reduction in iNOS expression and inflammation. Moreover, PPARγ itself can bind NF-κB and block its normal function, hence, reducing iNOS expression and other inflammatory end products of the NF-κB pathway[154,155].
Tropisetron: Tropisetron is a serotonin 5-hydroxytryptamine 3 receptor antagonist and is mainly used as an antiemetic agent. Tropisetron was shown to have an anti-inflammatory effect via PPARγ upregulation, resulting in NF-κB blockade and diminishing NO production[156]. This drug reduced the expression of β-catenin and COX-2 in a colitis-associated cancer experimental group, while the levels of IL-1β, TNF-α, TLR-4, and Myd88 were significantly decreased with tropisetron treatment[157].
Cyclopentenone prostaglandin 15-deoxy-Δ 12,14-PGJ 2: Treatment of experimental colitis model rats with 15-deoxy-Δ 12,14-PGJ 2 (15d-PGJ2) resulted in significant attenuation of severity of colon damage, which was attributed to reduced iNOS and suppressed NF-κB-DNA binding in 15d-PGJ2 treated group[158].
Glucosamine oligomers: Colitis animals treated with glucosamine oligomers have shown a significant reduction in the expression of iNOS and NF-κB, and prolongation of survival time[159]. Oral administration of glucosamine oligomers inhibited inflammation in colonic mucosa by suppressing MPO activity in inflammatory cells, and by reducing NF-κB, COX-2, iNOS, and the serum levels of TNF-α and IL-6[159].
8-hydroxydeoxy guanosine: 8-hydroxydeoxy guanosine (8-OHdG) is a product of ROS attacking guanine bases in DNA, which can bind thymidine rather than cytosine. Therefore, 8-OHdG is a biomarker of mutagenesis consequent to oxidative stress. It was shown that the administration of exogenous 8-OHdG can repress NF-κB signaling, pro-inflammatory cytokines, COX-2 and iNOS expression in stress-induced and inflammation-based GI tract conditions. Such outcomes might show a potential beneficial therapeutic effect[160].
Macrophage migration inhibitory factor inhibitor: In RAW 264.7 cells, migration inhibitory factor (MIF) inhibitor significantly reduced inflammation by inhibiting MIF-induced NF-κB nuclear translocation and NO production, proposing a new strategy for colitis management[161].
Telmisartan: Telmisartan administration diminished NF-κB p65 protein expression, and reduced the expression of iNOS and COX-2 in a colitis rat model[162].
Amitriptyline: Amitriptyline is an antidepressant that is used to control the psychosomatic symptoms of GI disorders. It was reported that amitriptyline inhibited the degradation of IκB, and the production of NO and TNF-α[163], suggesting a possible role for amitriptyline in colitis treatment.
Recombinant human IL-11: In vitro treatment of macrophages with recombinant human IL-11 (rhIL-11) caused a meaningful decrease in NF-κB-DNA binding and the production of pro-inflammatory cytokines and NO. Co-administration of normal intestinal cells with rhIL-11 reduced cellular proliferation, representative of a possible function of rhIL-11 in treating colitis patients with IL-11[164].
Non-NF-κB dependent synthetic molecules
Herein, we address studies that evaluated the effect of synthetic compounds on NO production and colitis severity, regardless of the NF-κB pathway.
NO intervention: Although the whole context of this review has been concentrated on the detrimental effect of the overproduction of NO in colitis progression, some studies indicated that a basal amount of NO, mainly produced by eNOS, is necessary for normal colon physiology. In animal models of colitis, the administration of nitrite or nitrate results in attenuation of colitis activity and damage, covering both preventive and therapeutic approaches[28]. Considering the key role of NO in colon damage induced by colitis, one would think it is reasonable to develop a set of direct iNOS inhibitors to manage the condition. Despite such a straightforward idea, the iNOS inhibitors raised a contentious scientific arguement, as a number of investigations reported no improvement, while some others have found promising results[165,166]. For instance, Methylene blue which is a well-known NOS inhibitor was shown to reduce mucosal inflammation damage and decrease the expression of iNOS and MPO in colitis experimental models[167]. The difference in eNOS antagonism affinity of antagonists used in each study was assumed to be the underlying reason for this inconsistency.
Nicotine: The positive effect of nicotine on UC, but not CD, has been known for quite a long time. However, data are not congruent regarding the correlation between NO and nicotine; some have noted a significant decrease in iNOS expression and NO production, while in some cases these effects were not observed. However, it has been accepted that nicotine is able to reduce the severity of colon inflammation in some settings[168,169].
Anti-inflammatory drugs: As noted above, dexamethasone had positive effects on colitis mainly by reducing NF-κB activity. Budesonide and Prednisolone, which are corticosteroids, attenuated inflammation and iNOS expression in UC cases[170]. Celecoxib, Rofecoxib, and Nimesulide, which are non-steroidal COX-2 inhibitor anti-inflammatory drugs, have also significantly repressed iNOS activity and inflammatory damage in the colon[171,172]. Sulfasalazine and Azathioprine did not affect NO production in colitis[173].
Drugs with collateral anti-inflammatory effects: A broad list of chemical compounds and drugs has been investigated as NO inhibitors. Of note, Trimetazidine, Minocycline, N-Acetylcysteine, Pravastatin, Glutamine, Lactulose, Carvedilol, and Melatonin are all among the approved drugs for other medical conditions rather than inflammation, nonetheless, they have also been shown to possess anti-inflammatory and iNOS inhibitory effects, at least to some extent.
Trimetazidine: Trimetazidine is mainly used for angina pectoris and is considered to be a favorable drug for colitis treatment that reduces colon damage severity and NO production. The effect of Trimetazidine was correlated with the antioxidant properties of this drug[174].
Minocycline: Apart from Minocycline's antibiotic properties, it has also been shown to regulate inflammation in some medical conditions. In colitis, Minocycline inhibited iNOS expression and reduced colon inflammation, damage and mortality[175]. Although activation of the NF-κB pathway by Minocycline is greatly supported by the literature, enhancement of NF-κB in colonic tissue is still in doubt[175].
Doxycycline: Another antibiotic, Doxycycline, has shown promising immuno-modulatory properties. Doxycycline can ameliorate colitis and colon damage by decreasing the pro-inflammatory cytokine, IL-8 and NO generation, an effect which is even enhanced when it is concurrently administered with Saccharomyces boulardii[176].
N-acetylcysteine: N-acetylcysteine (NAC) is a known antioxidant agent and has been shown to substantially reduce colon damage and NO production, particularly in relatively higher doses[177]. In a DSS-induced colitis model, NAC attenuated macroscopic and histopathologic colonic damage similar to 5-ASA treated mice. In addition, NAC reduced colonic MPO activity, ROS, TNF-α, and IL-1β levels, while elevating paraoxonase/arylesterase 1 (PON1) activity, and GSH concentration. Overexpression of PON1 and scavenging of oxygen-derived free radicals might be the mechanisms underlying the protective effect of NAC in colitis treatment[178].
Pravastatin: Pravastatin is a β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase inhibitor, able to ameliorate colitis severity and reduce colitis symptoms. Colitis model mice lacking eNOS have not shown this effect, suggesting that Pravastatin exerts its anti-inflammatory effects by upregulating eNOS expression rather than inhibiting iNOS[179].
Lactulose: Lactulose is a well-known osmotic anti-constipation agent, but also reduces TNF-α and leukotriene B4 production, as well as iNOS inhibition, mostly due to its probiotic properties, leading to Lactobacilli and Bifidobacteria overgrowth in the colon[180].
Carvedilol: Carvedilol, a nonselective β-adrenoceptor antagonist with α1-adrenoceptor antagonist activity, is also an antioxidant and anti-inflammatory agent. Carvedilol attenuated colon histopathological damage and iNOS expression, and reduced TNF-α, IL-1 β, IL-6 and PGE2 levels[181].
Melatonin: Melatonin administration in colitis models improved the disease symptoms, and decreased the expression of iNOS, COX-2 and MPO[182,183]. There have been some efforts to develop colon-specific sodium alginate gels containing melatonin, to confirm melatonin associated anti-inflammatory effects[182]. The fact that melatonin is a sleep-related neurohormone might propose a relationship between sleep patterns and colitis pathogenesis, which can be a topic of future studies.
Miscellaneous compounds
Soluble guanylate cyclase inhibitors: Colitis is associated with a reduced soluble guanylate cyclase (SGc) sensitivity, hence, a reduced colonic response to nitrergic stimuli. This finding puts forward the idea that inhibition of SGc might enhance colon protection in colitis. In contrast, several studies claimed that the pharmacological inhibition of SGc does not exert any protective effects in colitis[184].
Calpain inhibitor I: Calpain is a family of calcium-dependent proteases, and although their physiologic functions are poorly understood, they are thought to be of importance in cellular functions such as cell mobility and apoptosis[185]. Calpain inhibition has been found to cause a significant reduction in iNOS expression in colitis animals[186].
IL-4: Transfection of colon with IL-4-gene-carrying adenovirus vectors has successfully increased IL-4 concentration, and significantly reduced the expression of INF-γ, MPO and iNOS[187].
TNF-α Convertase (TACE/ADAM17) inhibition: BB1101, a TACE/ADAM17 inhibitor has shown a promising suppressive effect on iNOS expression and TNF-α release in the colon of IBD animals, leading to a significant anti-inflammatory effect[188].
α-Melanocyte-stimulating hormone: In colitis settings, administration of α-Melanocyte-stimulating hormone, a melanogenesis stimulator, can significantly affect NO production, modulating inflammation and providing protective benefits in the colon[189].
Ursodeoxycholate: Ursodeoxycholate, a bile acid, has successfully protected the colon against LPS-induced colitis in rats, mainly by hampering iNOS activity[190].
(Arginine-glycine-aspartic acid) RGD motif: It has been proposed that integrins have great importance in colitis pathogenesis and their dysfunction can cause an inflamed colon due to leukocyte recruitment. As an integrin motif, administration of RGD to the colon, using silk functionalized particles, resulted in a significant decrease in iNOS expression[191].
3,3′-diindolylmethane: 3,3’-diindolylmethane, a cruciferous family of vegetables derived molecule has been shown to inhibit iNOS and COX-2 expression[192]. The underlying mechanism is not fully understood.
GL-V9: This compound is a synthetic flavonoid, capable of inhibiting inflammatory cells infiltration and decreasing MPO and iNOS activities[193]. Furthermore, GL-V9 decreased pro-inflammatory cytokines and ROS production, increased antioxidant defenses in mouse macrophage RAW264.7 cells; mainly by promoting Trx-1 expression. It has been demonstrated that GL-V9 decreased oxidative stress by up-regulating Trx-1 via activation of the AMPK/FOXO3a pathway, suggesting that GL-V9 might be a potential choice for IBD[193].
Propionyl-L-carnitine: Propionyl-L-carnitine, an antioxidant molecule, inhibited oxidative stress-induced CAM expression, thus, reducing leukocyte infiltration in the colon, which then led to a significant reduction in iNOS expression[194].
Tetrahydrobiopterin: The enzyme cofactor tetrahydrobiopterin is a fundamental part of biogenic amine synthesis, lipid metabolism and redox coupling of NOS. An oral suspension of tetrahydrobiopterin was shown to reduce iNOS activity and increase regulatory T cells in the colon environment, leading to reduced colon inflammation, shrinkage and swelling[195].
CONCLUSION
It has been shown that NO has a significant role in the pathogenesis and treatment of IBD. In this paper, pharmacological interventions that affect IBD, especially those modulating the NO pathways, were reviewed in two distinct categories: natural agents and synthetic agents. The outcomes of this study demonstrated that herbal agents i.e., flavonoids can decrease NO production and iNOS expression, leading to therapeutic effects comparable with Mesalazine in IBD. NF-κB and JAK/STAT are the major pathways involved in this process. Activation of PPARγ, inhibition of iNOS, and suppression of TLR-4 upregulation; in addition to the secretion of inflammatory cytokines are considered to be the main therapeutic mechanisms of flavonoids in this regard. This review indicates that other polyphenol compounds could also be beneficial, mainly due to iNOS modulation and NF-κB suppression. Interestingly, a number of these compounds could even upregulate eNOS, which is necessary for normal colon physiology. Probiotics and prebiotics can also alter the intestinal microbiome and have the ability to prevent or reduce inflammation in IBD. Previous studies indicated that pro- and pre-biotics reduce NO production and suppress iNOS activity through different mechanisms such as suppressing the IκB/NF-κB pathway, IL-10 secretion, dendritic cells-induced IL-12 secretion, and modulating the expression of iNOS and TLR 4. It has been suggested that dairy products have similar properties, seemingly by alterating the colonic bacterial population. Fatty acids such as PUFAs can also improve IBD by modulating the production of eicosanoids, COX-2, PPAR-γ, and NF-κB, and exhibiting inhibitory effects on iNOS.
Sulfasalazine and Azathioprine are fundamental anti-inflammatory and immunomodulatory drugs for IBD treatment, but have no effect on NO production in colitis. Synthetic compounds could also be effective treatments for IBD, of which some (i.e., Dexamethasone, Pioglitazone, Tropisetron) can act through the NO and NF-κB signaling pathways. Other therapeutic compounds could regulate NO, independent of NF-κB. For instance, nicotine; anti-inflammatory agents (i.e., Prednisolone and Celecoxib); β-adrenoceptor antagonist with α1-adrenoceptor antagonist activity (i.e., Carvedilol); antioxidants (i.e., N-Acetylcysteine); and specific iNOS inhibitors (i.e., Methylene blue) are able to attenuate iNOS expression and NO production, directly or by mechanisms other than the NF-κB signaling pathway.
It is important to mention that the IBD inhibitory effects of phytochemicals or their combination with conventional therapeutics are principally implicated due to their ability to modulate the main pathophysiological factors of IBD development; inflammatory cytokines (i.e., IL-6, IL-12) production. Although, such treatments are affordable, they can alleviate the pain and inhibit inflammation in IBD patients, however, the major drawback of natural compounds is their mode of action, and their limited bioavailability at the site of inflammation. In comparison, the antibody based treatments such as anti-TNF biologics (i.e., Infliximab, Adalimumab or Etanercept) have more specificity, can lower cytokine production, inhibit inflammatory cell recruitment, and induce cell death of inflammatory cells, while representing less efficacy, as a significant percentage of patients do not respond to anti-TNF treatment. Furthermore, such therapeutics are expensive and showed some adverse effects such as increased risk of infection and malignancy or may cause de novo autoimmune diseases[196-198]. Overall, this review highlights the importance of NO in the pathogenesis and treatment of IBD. It seems the (co)administration of natural or chemical NO-regulating compounds might be favorable for patients suffering from IBD. Further investigations and well-designed trials are worth consideration.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to disclose.
Manuscript source: Invited manuscript
Peer-review started: February 24, 2020
First decision: April 22, 2020
Article in press: June 4, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farhat S, Gazouli M, Vradelis S S-Editor: Gong ZM L-Editor: Webster JR E-Editor: Ma YJ
Contributor Information
Aida Kamalian, Department of Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Masoud Sohrabi Asl, Department of Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Mahsa Dolatshahi, Department of Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran; Students' Scientific Research Center, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Khashayar Afshari, Department of Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Shiva Shamshiri, Department of Traditional Pharmacy, School of Persian Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Nazanin Momeni Roudsari, Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran 1941933111, Iran.
Saeideh Momtaz, Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Tehran 1417614411, Iran; Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), and Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran; Gastrointestinal Pharmacology Interest Group, Universal Scientific Education and Research Network, Tehran 1417614411, Iran.
Roja Rahimi, Department of Traditional Pharmacy, School of Persian Medicine, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Mohammad Abdollahi, Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), and Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran.
Amir Hossein Abdolghaffari, Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran Medical Sciences, Islamic Azad University, Tehran 1941933111, Iran; Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Tehran 1417614411, Iran; Toxicology and Diseases Group (TDG), Pharmaceutical Sciences Research Center (PSRC), The Institute of Pharmaceutical Sciences (TIPS), and Department of Toxicology and Pharmacology, School of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran; Gastrointestinal Pharmacology Interest Group, Universal Scientific Education and Research Network, Tehran 1417614411, Iran; Department of Toxicology and Pharmacology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran. amirhosein172@hotmail.com.
References
- 1.Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:942–951. doi: 10.3109/00365521.2015.1014407. [DOI] [PubMed] [Google Scholar]
- 2.Wadwa M, Klopfleisch R, Adamczyk A, Frede A, Pastille E, Mahnke K, Hansen W, Geffers R, Lang KS, Buer J, Büning J, Westendorf AM. IL-10 downregulates CXCR3 expression on Th1 cells and interferes with their migration to intestinal inflammatory sites. Mucosal Immunol. 2016;9:1263–1277. doi: 10.1038/mi.2015.132. [DOI] [PubMed] [Google Scholar]
- 3.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Fiocchi C. Etiopathogenesis of inflammatory bowel diseases. World J Gastroenterol. 2006;12:4807–4812. doi: 10.3748/wjg.v12.i30.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soufli I, Toumi R, Rafa H, Touil-Boukoffa C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J Gastrointest Pharmacol Ther. 2016;7:353–360. doi: 10.4292/wjgpt.v7.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427–437. doi: 10.1111/j.1365-2567.2004.01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross RK, Wilson KT. Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:179–189. doi: 10.1097/00054725-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Slomiany BL, Slomiany A. Role of LPS-elicited signaling in triggering gastric mucosal inflammatory responses to H. pylori: modulatory effect of ghrelin. Inflammopharmacology. 2017;25:415–429. doi: 10.1007/s10787-017-0360-1. [DOI] [PubMed] [Google Scholar]
- 13.Avdagić N, Zaćiragić A, Babić N, Hukić M, Seremet M, Lepara O, Nakaš-Ićindić E. Nitric oxide as a potential biomarker in inflammatory bowel disease. Bosn J Basic Med Sci. 2013;13:5–9. doi: 10.17305/bjbms.2013.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachmilewitz D, Eliakim R, Ackerman Z, Karmeli F. Direct determination of colonic nitric oxide level--a sensitive marker of disease activity in ulcerative colitis. Am J Gastroenterol. 1998;93:409–412. doi: 10.1111/j.1572-0241.1998.00409.x. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, Serizawa H, Ishii H. Increased expression of an inducible isoform of nitric oxide synthase and the formation of peroxynitrite in colonic mucosa of patients with active ulcerative colitis. Gut. 1998;42:180–187. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirst DG, Robson T. Nitric oxide physiology and pathology. Methods Mol Biol. 2011;704:1–13. doi: 10.1007/978-1-61737-964-2_1. [DOI] [PubMed] [Google Scholar]
- 17.Tamai H, Gaginella TS. Direct evidence for nitric oxide stimulation of electrolyte secretion in the rat colon. Free Radic Res Commun. 1993;19:229–239. doi: 10.3109/10715769309056511. [DOI] [PubMed] [Google Scholar]
- 18.Guslandi M. Nitric oxide: an ubiquitous actor in the gastrointestinal tract. Dig Dis. 1994;12:28–36. doi: 10.1159/000171434. [DOI] [PubMed] [Google Scholar]
- 19.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy M, Denenberg AG, Szabó C, Salzman AL. Poly(ADP-ribose) synthetase activation mediates increased permeability induced by peroxynitrite in Caco-2BBe cells. Gastroenterology. 1998;114:510–518. doi: 10.1016/s0016-5085(98)70534-7. [DOI] [PubMed] [Google Scholar]
- 21.Colgan SP. Nitric oxide and intestinal epithelia: just say NO. Gastroenterology. 1998;114:601–603. doi: 10.1016/s0016-5085(98)70545-1. [DOI] [PubMed] [Google Scholar]
- 22.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 23.Xiong H, Zhu C, Li F, Hegazi R, He K, Babyatsky M, Bauer AJ, Plevy SE. Inhibition of interleukin-12 p40 transcription and NF-kappaB activation by nitric oxide in murine macrophages and dendritic cells. J Biol Chem. 2004;279:10776–10783. doi: 10.1074/jbc.M313416200. [DOI] [PubMed] [Google Scholar]
- 24.Jianjun Yang, Zhang R, Lu G, Shen Y, Peng L, Zhu C, Cui M, Wang W, Arnaboldi P, Tang M, Gupta M, Qi CF, Jayaraman P, Zhu H, Jiang B, Chen SH, He JC, Ting AT, Zhou MM, Kuchroo VK, Morse HC 3rd, Ozato K, Sikora AG, Xiong H. T cell–derived inducible nitric oxide synthase switches off Th17 cell differentiation. J Exp Med. 2013;210:1447–1462. doi: 10.1084/jem.20122494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–1027. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- 26.Tun X, Yasukawa K, Yamada K. Involvement of nitric oxide with activation of Toll-like receptor 4 signaling in mice with dextran sodium sulfate-induced colitis. Free Radic Biol Med. 2014;74:108–117. doi: 10.1016/j.freeradbiomed.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Jädert C, Phillipson M, Holm L, Lundberg JO, Borniquel S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol. 2014;2:73–81. doi: 10.1016/j.redox.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toumi R, Abdelouhab K, Rafa H, Soufli I, Raissi-Kerboua D, Djeraba Z, Touil-Boukoffa C. Beneficial role of the probiotic mixture Ultrabiotique on maintaining the integrity of intestinal mucosal barrier in DSS-induced experimental colitis. Immunopharmacol Immunotoxicol. 2013;35:403–409. doi: 10.3109/08923973.2013.790413. [DOI] [PubMed] [Google Scholar]
- 29.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16:4504–4514. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahimi R, Nikfar S, Abdollahi M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: a meta-analysis. World J Gastroenterol. 2013;19:5738–5749. doi: 10.3748/wjg.v19.i34.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rufino AT, Ferreira I, Judas F, Salgueiro L, Lopes MC, Cavaleiro C, Mendes AF. Differential effects of the essential oils of Lavandula luisieri and Eryngium duriaei subsp. juresianum in cell models of two chronic inflammatory diseases. Pharm Biol. 2015;53:1220–1230. doi: 10.3109/13880209.2014.970701. [DOI] [PubMed] [Google Scholar]
- 32.Algieri F, Rodriguez-Nogales A, Vezza T, Garrido-Mesa J, Garrido-Mesa N, Utrilla MP, González-Tejero MR, Casares-Porcel M, Molero-Mesa J, Del Mar Contreras M, Segura-Carretero A, Pérez-Palacio J, Diaz C, Vergara N, Vicente F, Rodriguez-Cabezas ME, Galvez J. Anti-inflammatory activity of hydroalcoholic extracts of Lavandula dentata L. and Lavandula stoechas L. J Ethnopharmacol. 2016;190:142–158. doi: 10.1016/j.jep.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V, Izzo AA. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 2009;87:1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- 34.Fakhraei N, Abdolghaffari AH, Delfan B, Abbasi A, Rahimi N, Khansari A, Rahimian R, Dehpour AR. Protective effect of hydroalcoholic olive leaf extract on experimental model of colitis in rat: involvement of nitrergic and opioidergic systems. Phytother Res. 2014;28:1367–1373. doi: 10.1002/ptr.5139. [DOI] [PubMed] [Google Scholar]
- 35.González-Mauraza NH, León-González AJ, Espartero JL, Gallego-Fernández JB, Sánchez-Hidalgo M, Martin-Cordero C. Isolation and Quantification of Pinitol, a Bioactive Cyclitol, in Retama spp. Nat Prod Commun. 2016;11:405–406. [PubMed] [Google Scholar]
- 36.Belmokhtar Z, Harche MK. In vitro antioxidant activity of Retama monosperma (L.) Boiss. Nat Prod Res. 2014;28:2324–2329. doi: 10.1080/14786419.2014.934237. [DOI] [PubMed] [Google Scholar]
- 37.González-Mauraza H, Martín-Cordero C, Alarcón-de-la-Lastra C, Rosillo MA, León-González AJ, Sánchez-Hidalgo M. Anti-inflammatory effects of Retama monosperma in acute ulcerative colitis in rats. J Physiol Biochem. 2014;70:163–172. doi: 10.1007/s13105-013-0290-3. [DOI] [PubMed] [Google Scholar]
- 38.Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Gosavi TP, Badole SL, Bodhankar SL. Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pac J Trop Biomed. 2012;2:337–344. doi: 10.1016/S2221-1691(12)60053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motawi TK, Rizk SM, Shehata AH. Effects of curcumin and Ginkgo biloba on matrix metalloproteinases gene expression and other biomarkers of inflammatory bowel disease. J Physiol Biochem. 2012;68:529–539. doi: 10.1007/s13105-012-0168-9. [DOI] [PubMed] [Google Scholar]
- 40.Wen XD, Wang CZ, Yu C, Zhao L, Zhang Z, Matin A, Wang Y, Li P, Xiao SY, Du W, He TC, Yuan CS. Panax notoginseng attenuates experimental colitis in the azoxymethane/dextran sulfate sodium mouse model. Phytother Res. 2014;28:892–898. doi: 10.1002/ptr.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pastrelo MM, Dias Ribeiro CC, Duarte JW, Bioago Gollücke AP, Artigiani-Neto R, Ribeiro DA, Miszputen SJ, Fujiyama Oshima CT, Ribeiro Paiotti AP. Effect of Concentrated Apple Extract on Experimental Colitis Induced by Acetic Acid. Int J Mol Cell Med. 2017;6:38–49. [PMC free article] [PubMed] [Google Scholar]
- 42.Fasolino I, Izzo AA, Clavel T, Romano B, Haller D, Borrelli F. Orally administered allyl sulfides from garlic ameliorate murine colitis. Mol Nutr Food Res. 2015;59:434–442. doi: 10.1002/mnfr.201400347. [DOI] [PubMed] [Google Scholar]
- 43.Maity S, Ukil A, Karmakar S, Datta N, Chaudhuri T, Vedasiromoni JR, Ganguly DK, Das PK. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur J Pharmacol. 2003;470:103–112. doi: 10.1016/s0014-2999(03)01760-6. [DOI] [PubMed] [Google Scholar]
- 44.Ukil A, Maity S, Das PK. Protection from experimental colitis by theaflavin-3,3'-digallate correlates with inhibition of IKK and NF-kappaB activation. Br J Pharmacol. 2006;149:121–131. doi: 10.1038/sj.bjp.0706847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon HS, Oh SM, Kim JK. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2008;151:165–173. doi: 10.1111/j.1365-2249.2007.03539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pervin M, Hasnat MA, Lim JH, Lee YM, Kim EO, Um BH, Lim BO. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J Nutr Biochem. 2016;28:103–113. doi: 10.1016/j.jnutbio.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farid R, Rezaieyazdi Z, Mirfeizi Z, Hatef MR, Mirheidari M, Mansouri H, Esmaelli H, Bentley G, Lu Y, Foo Y, Watson RR. Oral intake of purple passion fruit peel extract reduces pain and stiffness and improves physical function in adult patients with knee osteoarthritis. Nutr Res. 2010;30:601–606. doi: 10.1016/j.nutres.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 49.Farris P, Yatskayer M, Chen N, Krol Y, Oresajo C. Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. Journal of Drugs Dermatology. 2014;13(12):1467 [PMID 25607790]. [PubMed] [Google Scholar]
- 50.Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010;87:240–245. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Nones K, Dommels YE, Martell S, Butts C, McNabb WC, Park ZA, Zhu S, Hedderley D, Barnett MP, Roy NC. The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases. Br J Nutr. 2009;101:169–181. doi: 10.1017/S0007114508009847. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Zhu L, Tan J, Zhou X, Xiao L, Yang X, Wang B. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia Pilosa Ledeb. BMC Complement Altern Med. 2014;14:12. doi: 10.1186/1472-6882-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta R, Mathur M, Bajaj VK, Katariya P, Yadav S, Kamal R, Gupta RS. Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes. J Diabetes. 2012;4:164–171. doi: 10.1111/j.1753-0407.2011.00173.x. [DOI] [PubMed] [Google Scholar]
- 54.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837, 837a-837d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terra X, Valls J, Vitrac X, Mérrillon JM, Arola L, Ardèvol A, Bladé C, Fernandez-Larrea J, Pujadas G, Salvadó J, Blay M. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J Agric Food Chem. 2007;55:4357–4365. doi: 10.1021/jf0633185. [DOI] [PubMed] [Google Scholar]
- 56.Limtrakul P, Yodkeeree S, Pitchakarn P, Punfa W. Suppression of Inflammatory Responses by Black Rice Extract in RAW 264.7 Macrophage Cells via Downregulation of NF-kB and AP-1 Signaling Pathways. Asian Pac J Cancer Prev. 2015;16:4277–4283. doi: 10.7314/apjcp.2015.16.10.4277. [DOI] [PubMed] [Google Scholar]
- 57.Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28:841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 58.Feng A, Zhou G, Yuan X, Huang X, Zhang Z, Zhang T. Inhibitory effect of baicalin on iNOS and NO expression in intestinal mucosa of rats with acute endotoxemia. PLoS One. 2013;8:e80997. doi: 10.1371/journal.pone.0080997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang YC, Tsai SH, Tsai DC, Lin-Shiau SY, Lin JK. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 60.Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000;465:93–97. doi: 10.1016/s0014-5793(99)01735-4. [DOI] [PubMed] [Google Scholar]
- 61.Maldonado-Rojas W, Olivero-Verbel J. Food-related compounds that modulate expression of inducible nitric oxide synthase may act as its inhibitors. Molecules. 2012;17:8118–8135. doi: 10.3390/molecules17078118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye H, Xie C, Wu W, Xiang M, Liu Z, Li Y, Tang M, Li S, Yang J, Tang H, Chen K, Long C, Peng A, Chen L. Millettia pachycarpa exhibits anti-inflammatory activity through the suppression of LPS-induced NO/iNOS expression. Am J Chin Med. 2014;42:949–965. doi: 10.1142/S0192415X14500608. [DOI] [PubMed] [Google Scholar]
- 63.Peiffer DS, Zimmerman NP, Wang LS, Ransom BW, Carmella SG, Kuo CT, Siddiqui J, Chen JH, Oshima K, Huang YW, Hecht SS, Stoner GD. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev Res (Phila) 2014;7:574–584. doi: 10.1158/1940-6207.CAPR-14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ha US, Bae WJ, Kim SJ, Yoon BI, Hong SH, Lee JY, Hwang TK, Hwang SY, Wang Z, Kim SW. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med J. 2015;56:16–23. doi: 10.3349/ymj.2015.56.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, Vanamala JK. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J Nutr Biochem. 2015;26:1641–1649. doi: 10.1016/j.jnutbio.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Pereira SR, Pereira R, Figueiredo I, Freitas V, Dinis TC, Almeida LM. Comparison of anti-inflammatory activities of an anthocyanin-rich fraction from Portuguese blueberries (Vaccinium corymbosum L.) and 5-aminosalicylic acid in a TNBS-induced colitis rat model. PLoS One. 2017;12:e0174116. doi: 10.1371/journal.pone.0174116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen T, Hu S, Zhang H, Guan Q, Yang Y, Wang X. Anti-inflammatory effects of Dioscorea alata L. anthocyanins in a TNBS-induced colitis model. Food Funct. 2017;8:659–669. doi: 10.1039/c6fo01273f. [DOI] [PubMed] [Google Scholar]
- 68.Ren G, Sun A, Deng C, Zhang J, Wu X, Wei X, Mani S, Dou W, Wang Z. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G517–G527. doi: 10.1152/ajpgi.00133.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Shen L, Luo H. Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway. Int Immunopharmacol. 2016;40:24–31. doi: 10.1016/j.intimp.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 70.Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017;8:387–396. doi: 10.1039/c6fo01529h. [DOI] [PubMed] [Google Scholar]
- 71.Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633–5644. doi: 10.3748/wjg.v19.i34.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132–145. doi: 10.7555/JBR.27.20120082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shiva S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013;1:40–44. doi: 10.1016/j.redox.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harbrecht BG, Nweze I, Smith JW, Zhang B. Insulin inhibits hepatocyte iNOS expression induced by cytokines by an Akt-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G116–G122. doi: 10.1152/ajpgi.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong Y, Chen D, Yu C, Lv B, Peng J, Wang J, Lin Y. Citrus nobiletin ameliorates experimental colitis by reducing inflammation and restoring impaired intestinal barrier function. Mol Nutr Food Res. 2015;59:829–842. doi: 10.1002/mnfr.201400614. [DOI] [PubMed] [Google Scholar]
- 76.Chen JC, Ho FM, Pei-Dawn Lee Chao, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung Wu, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Pisonero-Vaquero S, Martínez-Ferreras Á, García-Mediavilla MV, Martínez-Flórez S, Fernández A, Benet M, Olcoz JL, Jover R, González-Gallego J, Sánchez-Campos S. Quercetin ameliorates dysregulation of lipid metabolism genes via the PI3K/AKT pathway in a diet-induced mouse model of nonalcoholic fatty liver disease. Mol Nutr Food Res. 2015;59:879–893. doi: 10.1002/mnfr.201400913. [DOI] [PubMed] [Google Scholar]
- 78.Xiang T, Fang Y, Wang SX. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J Huazhong Univ Sci Technolog Med Sci. 2014;34:740–744. doi: 10.1007/s11596-014-1345-6. [DOI] [PubMed] [Google Scholar]
- 79.Cibiček N, Roubalová L, Vrba J, Zatloukalová M, Ehrmann J, Zapletalová J, Večeřa R, Křen V, Ulrichová J. Protective effect of isoquercitrin against acute dextran sulfate sodium-induced rat colitis depends on the severity of tissue damage. Pharmacol Rep. 2016;68:1197–1204. doi: 10.1016/j.pharep.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 80.Sun Y, Zhao Y, Yao J, Zhao L, Wu Z, Wang Y, Pan D, Miao H, Guo Q, Lu N. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem Pharmacol. 2015;94:142–154. doi: 10.1016/j.bcp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Kwon KH, Murakami A, Tanaka T, Ohigashi H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Joo M, Kim HS, Kwon TH, Palikhe A, Zaw TS, Jeong JH, Sohn UD. Anti-inflammatory Effects of Flavonoids on TNBS-induced Colitis of Rats. Korean J Physiol Pharmacol. 2015;19:43–50. doi: 10.4196/kjpp.2015.19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang J, Seki T, Tsukamoto T, Qin H, Yin H, Liao L, Nakamura H, Maeda H. Protection from inflammatory bowel disease and colitis-associated carcinogenesis with 4-vinyl-2,6-dimethoxyphen-ol (canolol) involves suppression of oxidative stress and inflammatory cytokines. Carcinogenesis. 2013;34:2833–2841. doi: 10.1093/carcin/bgt309. [DOI] [PubMed] [Google Scholar]
- 84.Zhu W, Su J, Liu J, Jiang C. The involvement of neuronal nitric oxide synthase in the anti-epileptic action of curcumin on pentylenetetrazol-kindled rats. Biomed Mater Eng. 2015;26 Suppl 1:S841–S850. doi: 10.3233/BME-151376. [DOI] [PubMed] [Google Scholar]
- 85.Boonla O, Kukongviriyapan U, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Prachaney P, Greenwald SE. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Skidmore FM, Spetsieris PG, Anthony T, Cutter GR, von Deneen KM, Liu Y, White KD, Heilman KM, Myers J, Standaert DG, Lahti AC, Eidelberg D, Ulug AM. A full-brain, bootstrapped analysis of diffusion tensor imaging robustly differentiates Parkinson disease from healthy controls. Neuroinformatics. 2015;13:7–18. doi: 10.1007/s12021-014-9222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, Kawachi H, Watanabe K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol Nutr Food Res. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 88.Pandurangan AK, Mohebali N, Esa NM, Looi CY, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int Immunopharmacol. 2015;28:1034–1043. doi: 10.1016/j.intimp.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets. 2008;9:369–374. doi: 10.2174/138945008784221116. [DOI] [PubMed] [Google Scholar]
- 90.Ahn JH, Choi JW, Choi JM, Maeda T, Fujii H, Yokozawa T, Cho EJ. Protective role of oligonol from oxidative stress-induced inflammation in C6 glial cell. Nutr Res Pract. 2015;9:123–128. doi: 10.4162/nrp.2015.9.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thirunavukkarasu M, Zhan L, Wakame K, Fujii H, Moriyama H, Bagchi M. Safety of oligonol, a highly bioavailable lychee-derived polyphenolic antioxidant, on liver, kidney and heart function in rats. Toxicol Mech Methods. 2012;22:555–559. doi: 10.3109/15376516.2012.702795. [DOI] [PubMed] [Google Scholar]
- 92.Zhang XH, Yokoo H, Nishioka H, Fujii H, Matsuda N, Hayashi T, Hattori Y. Beneficial effect of the oligomerized polyphenol oligonol on high glucose-induced changes in eNOS phosphorylation and dephosphorylation in endothelial cells. Br J Pharmacol. 2010;159:928–938. doi: 10.1111/j.1476-5381.2009.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yum HW, Zhong X, Park J, Na HK, Kim N, Lee HS, Surh YJ. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid Redox Signal. 2013;19:102–114. doi: 10.1089/ars.2012.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao L, Zhang Y, Liu G, Hao S, Wang C, Wang Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018;9:2796–2808. doi: 10.1039/c7fo01490b. [DOI] [PubMed] [Google Scholar]
- 95.Kang SH, Jeon YD, Moon KH, Lee JH, Kim DG, Kim W, Myung H, Kim JS, Kim HJ, Bang KS, Jin JS. Aronia Berry Extract Ameliorates the Severity of Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. J Med Food. 2017;20:667–675. doi: 10.1089/jmf.2016.3822. [DOI] [PubMed] [Google Scholar]
- 96.Jin BR, Chung KS, Cheon SY, Lee M, Hwang S, Noh Hwang S, Rhee KJ, An HJ. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci Rep. 2017;7:46252. doi: 10.1038/srep46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hernández-Ledesma B, Ramos M, Gómez-Ruiz JÁ. Bioactive components of ovine and caprine cheese whey. Small Ruminant Res. 2011;101:196–204. [Google Scholar]
- 98.Thum C, Cookson A, McNabb WC, Roy NC, Otter D. Composition and enrichment of caprine milk oligosaccharides from New Zealand Saanen goat cheese whey. J Food Compos Anal. 2015;42:30–37. [Google Scholar]
- 99.Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuillé D, Obled C, Corthésy-Theulaz I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr. 2006;136:1558–1564. doi: 10.1093/jn/136.6.1558. [DOI] [PubMed] [Google Scholar]
- 100.Sprong RC, Schonewille AJ, van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium-induced colitis: role of mucin and microbiota. J Dairy Sci. 2010;93:1364–1371. doi: 10.3168/jds.2009-2397. [DOI] [PubMed] [Google Scholar]
- 101.Araújo DFS, Guerra GCB, Júnior RFA, Antunes de Araújo A, Antonino de Assis PO, Nunes de Medeiros A, Formiga de Sousa YR, Pintado MME, Gálvez J, Queiroga RCRDE. Goat whey ameliorates intestinal inflammation on acetic acid-induced colitis in rats. J Dairy Sci. 2016;99:9383–9394. doi: 10.3168/jds.2016-10930. [DOI] [PubMed] [Google Scholar]
- 102.Brück WM, Graverholt G, Gibson GR. A two-stage continuous culture system to study the effect of supplemental alpha-lactalbumin and glycomacropeptide on mixed cultures of human gut bacteria challenged with enteropathogenic Escherichia coli and Salmonella serotype Typhimurium. J Appl Microbiol. 2003;95:44–53. doi: 10.1046/j.1365-2672.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- 103.Brück WM, Kelleher SL, Gibson GR, Nielsen KE, Chatterton DE, Lönnerdal B. rRNA probes used to quantify the effects of glycomacropeptide and alpha-lactalbumin supplementation on the predominant groups of intestinal bacteria of infant rhesus monkeys challenged with enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 2003;37:273–280. doi: 10.1097/00005176-200309000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Brody EP. Biological activities of bovine glycomacropeptide. Br J Nutr. 2000;84 Suppl 1:S39–S46. doi: 10.1017/s0007114500002233. [DOI] [PubMed] [Google Scholar]
- 105.Daddaoua A, Puerta V, Zarzuelo A, Suárez MD, Sánchez de Medina F, Martínez-Augustin O. Bovine glycomacropeptide is anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2005;135:1164–1170. doi: 10.1093/jn/135.5.1164. [DOI] [PubMed] [Google Scholar]
- 106.Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106–111. doi: 10.1097/01.MIB.0000200323.38139.c6. [DOI] [PubMed] [Google Scholar]
- 107.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, Inoue N, Ogata H, Iwao Y, Nomoto K, Tanaka R, Hibi T. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 108.Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14:147–161. doi: 10.1002/ibd.20330. [DOI] [PubMed] [Google Scholar]
- 109.Sepehri S, Kotlowski R, Bernstein CN, Krause DO. Microbial diversity of inflamed and noninflamed gut biopsy tissues in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:675–683. doi: 10.1002/ibd.20101. [DOI] [PubMed] [Google Scholar]
- 110.Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N, Brostoff J, Parkhill J, Dougan G, Petrovska L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. doi: 10.1186/1471-2180-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, Zhu W, Sartor RB, Boedeker EC, Harpaz N, Pace NR, Li E. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:179–184. doi: 10.1002/ibd.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mack DR. Probiotics in inflammatory bowel diseases and associated conditions. Nutrients. 2011;3:245–264. doi: 10.3390/nu3020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009;15:454–462. doi: 10.1002/ibd.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orel R, Kamhi Trop T. Intestinal microbiota, probiotics and prebiotics in inflammatory bowel disease. World Journal of Gastroenterology. 2014;20(33):11505–11524. doi: 10.3748/wjg.v20.i33.11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Korhonen R, Korpela R, Saxelin M, Mäki M, Kankaanranta H, Moilanen E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001;25:223–232. doi: 10.1023/a:1010971703271. [DOI] [PubMed] [Google Scholar]
- 116.Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 117.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, Karmali V, Rao AS, Madara JL. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science. 2000;289:1560–1563. doi: 10.1126/science.289.5484.1560. [DOI] [PubMed] [Google Scholar]
- 118.Rachmilewitz D, Stamler JS, Bachwich D, Karmeli F, Ackerman Z, Podolsky DK. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn's disease. Gut. 1995;36:718–723. doi: 10.1136/gut.36.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lamine F, Fioramonti J, Bueno L, Nepveu F, Cauquil E, Lobysheva I, Eutamène H, Théodorou V. Nitric oxide released by Lactobacillus farciminis improves TNBS-induced colitis in rats. Scand J Gastroenterol. 2004;39:37–45. doi: 10.1080/00365520310007152. [DOI] [PubMed] [Google Scholar]
- 120.Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, Olivares M, Xaus J, Zarzuelo A, Galvez J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Colorectal Dis. 2006;21:737–746. doi: 10.1007/s00384-005-0773-y. [DOI] [PubMed] [Google Scholar]
- 121.Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836–844. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- 122.Ruiz PA, Hoffmann M, Szcesny S, Blaut M, Haller D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology. 2005;115:441–450. doi: 10.1111/j.1365-2567.2005.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamine F, Eutamène H, Fioramonti J, Buéno L, Théodorou V. Colonic responses to Lactobacillus farciminis treatment in trinitrobenzene sulphonic acid-induced colitis in rats. Scand J Gastroenterol. 2004;39:1250–1258. doi: 10.1080/00365520410007953. [DOI] [PubMed] [Google Scholar]
- 124.Yokota Y, Shikano A, Kuda T, Takei M, Takahashi H, Kimura B. Lactobacillus plantarum AN1 cells increase caecal L. reuteri in an ICR mouse model of dextran sodium sulphate-induced inflammatory bowel disease. Int Immunopharmacol. 2018;56:119–127. doi: 10.1016/j.intimp.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 125.Senol A, Isler M, Sutcu R, Akin M, Cakir E, Ceyhan BM, Kockar MC. Kefir treatment ameliorates dextran sulfate sodium-induced colitis in rats. World J Gastroenterol. 2015;21:13020–13029. doi: 10.3748/wjg.v21.i46.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu WS, Chen MC, Chiu KH, Wen ZH, Lee CH. Amelioration of dextran sodium sulfate-induced colitis in mice by Rhodobacter sphaeroides extract. Molecules. 2012;17:13622–13630. doi: 10.3390/molecules171113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdelouhab K, Rafa H, Toumi R, Bouaziz S, Medjeber O, Touil-Boukoffa C. Mucosal intestinal alteration in experimental colitis correlates with nitric oxide production by peritoneal macrophages: effect of probiotics and prebiotics. Immunopharmacol Immunotoxicol. 2012;34:590–597. doi: 10.3109/08923973.2011.641971. [DOI] [PubMed] [Google Scholar]
- 128.Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C. Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol. 2014;27:615–627. doi: 10.1177/039463201402700418. [DOI] [PubMed] [Google Scholar]
- 129.Saura M, Zaragoza C, Bao C, McMillan A, Lowenstein CJ. Interaction of interferon regulatory factor-1 and nuclear factor kappaB during activation of inducible nitric oxide synthase transcription. J Mol Biol. 1999;289:459–471. doi: 10.1006/jmbi.1999.2752. [DOI] [PubMed] [Google Scholar]
- 130.Soufli I, Toumi R, Rafa H, Amri M, Labsi M, Khelifi L, Nicoletti F, Touil-Boukoffa C. Crude extract of hydatid laminated layer from Echinococcus granulosus cyst attenuates mucosal intestinal damage and inflammatory responses in Dextran Sulfate Sodium induced colitis in mice. J Inflamm (Lond) 2015;12:19. doi: 10.1186/s12950-015-0063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 132.Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garg SK, Croft AM, Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2014:CD009400. doi: 10.1002/14651858.CD009400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn's mucosa through modulation of antioxidant defense machinery. PLoS One. 2012;7:e32841. doi: 10.1371/journal.pone.0032841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Cavaglieri CR, Nishiyama A, Fernandes LC, Curi R, Miles EA, Calder PC. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 136.Arribas B, Suárez-Pereira E, Ortiz Mellet C, García Fernández JM, Buttersack C, Rodríguez-Cabezas ME, Garrido-Mesa N, Bailon E, Guerra-Hernández E, Zarzuelo A, Gálvez J. Di-D-fructose dianhydride-enriched caramels: effect on colon microbiota, inflammation, and tissue damage in trinitrobenzenesulfonic acid-induced colitic rats. J Agric Food Chem. 2010;58:6476–6484. doi: 10.1021/jf100513j. [DOI] [PubMed] [Google Scholar]
- 137.Belluzzi A, Boschi S, Brignola C, Munarini A, Cariani G, Miglio F. Polyunsaturated fatty acids and inflammatory bowel disease. Am J Clin Nutr. 2000;71:339S–342S. doi: 10.1093/ajcn/71.1.339s. [DOI] [PubMed] [Google Scholar]
- 138.Siguel EN, Lerman RH. Prevalence of essential fatty acid deficiency in patients with chronic gastrointestinal disorders. Metabolism. 1996;45:12–23. doi: 10.1016/s0026-0495(96)90194-8. [DOI] [PubMed] [Google Scholar]
- 139.Gil A. Polyunsaturated fatty acids and inflammatory diseases. Biomed Pharmacother. 2002;56:388–396. doi: 10.1016/s0753-3322(02)00256-1. [DOI] [PubMed] [Google Scholar]
- 140.Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777–791. doi: 10.1053/j.gastro.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 141.Huang CH, Hou YC, Yeh CL, Yeh SL. A soybean and fish oil mixture with different n-6/n-3 PUFA ratios modulates the inflammatory reaction in mice with dextran sulfate sodium-induced acute colitis. Clin Nutr. 2015;34:1018–1024. doi: 10.1016/j.clnu.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 142.Mbodji K, Charpentier C, Guérin C, Querec C, Bole-Feysot C, Aziz M, Savoye G, Déchelotte P, Marion-Letellier R. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-κB in rats with TNBS-induced colitis. J Nutr Biochem. 2013;24:700–705. doi: 10.1016/j.jnutbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 143.Bassaganya-Riera J, Hontecillas R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin Nutr. 2006;25:454–465. doi: 10.1016/j.clnu.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 144.Chiu CY, Gomolka B, Dierkes C, Huang NR, Schroeder M, Purschke M, Manstein D, Dangi B, Weylandt KH. Omega-6 docosapentaenoic acid-derived resolvins and 17-hydroxydocosahexaenoic acid modulate macrophage function and alleviate experimental colitis. Inflamm Res. 2012;61:967–976. doi: 10.1007/s00011-012-0489-8. [DOI] [PubMed] [Google Scholar]
- 145.Yamamoto K, Itoh T, Abe D, Shimizu M, Kanda T, Koyama T, Nishikawa M, Tamai T, Ooizumi H, Yamada S. Identification of putative metabolites of docosahexaenoic acid as potent PPARgamma agonists and antidiabetic agents. Bioorg Med Chem Lett. 2005;15:517–522. doi: 10.1016/j.bmcl.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto K, Ninomiya Y, Iseki M, Nakachi Y, Kanesaki-Yatsuka Y, Yamanoue Y, Itoh T, Nishii Y, Petrovsky N, Okazaki Y. 4-Hydroxydocosahexaenoic acid, a potent peroxisome proliferator-activated receptor gamma agonist alleviates the symptoms of DSS-induced colitis. Biochem Biophys Res Commun. 2008;367:566–572. doi: 10.1016/j.bbrc.2007.12.188. [DOI] [PubMed] [Google Scholar]
- 147.Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr. 2011;35:188–197. doi: 10.1177/0148607110381407. [DOI] [PubMed] [Google Scholar]
- 148.Coëffier M, Marion-Letellier R, Déchelotte P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:518–524. doi: 10.1002/ibd.21017. [DOI] [PubMed] [Google Scholar]
- 149.Al-Drees A, Khalil MS. Histological and immunohistochemical effects of L-arginine and silymarin on TNBS-induced inflammatory bowel disease in rats. Histol Histopathol. 2016;31:1259–1270. doi: 10.14670/HH-11-757. [DOI] [PubMed] [Google Scholar]
- 150.Coburn LA, Gong X, Singh K, Asim M, Scull BP, Allaman MM, Williams CS, Rosen MJ, Washington MK, Barry DP, Piazuelo MB, Casero RA Jr, Chaturvedi R, Zhao Z, Wilson KT. L-arginine supplementation improves responses to injury and inflammation in dextran sulfate sodium colitis. PLoS One. 2012;7:e33546. doi: 10.1371/journal.pone.0033546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakase H, Okazaki K, Tabata Y, Uose S, Ohana M, Uchida K, Nishi T, Debreceni A, Itoh T, Kawanami C, Iwano M, Ikada Y, Chiba T. An oral drug delivery system targeting immune-regulating cells ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. J Pharmacol Exp Ther. 2001;297:1122–1128. [PubMed] [Google Scholar]
- 152.Nakase H, Okazaki K, Tabata Y, Chiba T. Biodegradable microspheres targeting mucosal immune-regulating cells: new approach for treatment of inflammatory bowel disease. J Gastroenterol. 2003;38 Suppl 15:59–62. [PubMed] [Google Scholar]
- 153.Singh K, Chaturvedi R, Barry DP, Coburn LA, Asim M, Lewis ND, Piazuelo MB, Washington MK, Vitek MP, Wilson KT. The apolipoprotein E-mimetic peptide COG112 inhibits NF-kappaB signaling, proinflammatory cytokine expression, and disease activity in murine models of colitis. J Biol Chem. 2011;286:3839–3850. doi: 10.1074/jbc.M110.176719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alleva DG, Johnson EB, Lio FM, Boehme SA, Conlon PJ, Crowe PD. Regulation of murine macrophage proinflammatory and anti-inflammatory cytokines by ligands for peroxisome proliferator-activated receptor-gamma: counter-regulatory activity by IFN-gamma. J Leukoc Biol. 2002;71:677–685. [PubMed] [Google Scholar]
- 155.Takagi T, Naito Y, Tomatsuri N, Handa O, Ichikawa H, Yoshida N, Yoshikawa T. Pioglitazone, a PPAR-gamma ligand, provides protection from dextran sulfate sodium-induced colitis in mice in association with inhibition of the NF-kappaB-cytokine cascade. Redox Rep. 2002;7:283–289. doi: 10.1179/135100002125000802. [DOI] [PubMed] [Google Scholar]
- 156.Rahimian R, Zirak MR, Keshavarz M, Fakhraei N, Mohammadi-Farani A, Hamdi H, Mousavizadeh K. Involvement of PPARγ in the protective action of tropisetron in an experimental model of ulcerative colitis. Immunopharmacol Immunotoxicol. 2016;38:432–440. doi: 10.1080/08923973.2016.1231202. [DOI] [PubMed] [Google Scholar]
- 157.Amini-Khoei H, Momeny M, Abdollahi A, Dehpour AR, Amiri S, Haj-Mirzaian A, Tavangar SM, Ghaffari SH, Rahimian R, Mehr SE. Tropisetron suppresses colitis-associated cancer in a mouse model in the remission stage. Int Immunopharmacol. 2016;36:9–16. doi: 10.1016/j.intimp.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 158.Cuzzocrea S, Ianaro A, Wayman NS, Mazzon E, Pisano B, Dugo L, Serraino I, Di Paola R, Chatterjee PK, Di Rosa M, Caputi AP, Thiemermann C. The cyclopentenone prostaglandin 15-deoxy-delta(12,14)- PGJ2 attenuates the development of colon injury caused by dinitrobenzene sulphonic acid in the rat. Br J Pharmacol. 2003;138:678–688. doi: 10.1038/sj.bjp.0705077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Azuma K, Osaki T, Kurozumi S, Kiyose M, Tsuka T, Murahata Y, Imagawa T, Itoh N, Minami S, Sato K, Okamoto Y. Anti-inflammatory effects of orally administered glucosamine oligomer in an experimental model of inflammatory bowel disease. Carbohydr Polym. 2015;115:448–456. doi: 10.1016/j.carbpol.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 160.Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-Hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol. 2012;18:302–308. doi: 10.3748/wjg.v18.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alam A, Pal C, Goyal M, Kundu MK, Kumar R, Iqbal MS, Dey S, Bindu S, Sarkar S, Pal U, Maiti NC, Adhikari S, Bandyopadhyay U. Synthesis and bio-evaluation of human macrophage migration inhibitory factor inhibitor to develop anti-inflammatory agent. Bioorg Med Chem. 2011;19:7365–7373. doi: 10.1016/j.bmc.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 162.Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One. 2014;9:e97193. doi: 10.1371/journal.pone.0097193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rahimi HR, Shiri M, Razmi A. Antidepressants can treat inflammatory bowel disease through regulation of the nuclear factor-κB/nitric oxide pathway and inhibition of cytokine production: A hypothesis. World J Gastrointest Pharmacol Ther. 2012;3:83–85. doi: 10.4292/wjgpt.v3.i6.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trepicchio WL, Dorner AJ. The therapeutic utility of Interleukin-11 in the treatment of inflammatory disease. Expert Opin Investig Drugs. 1998;7:1501–1504. doi: 10.1517/13543784.7.9.1501. [DOI] [PubMed] [Google Scholar]
- 165.Armstrong AM, Campbell GR, Gannon C, Kirk SJ, Gardiner KR. Oral administration of inducible nitric oxide synthase inhibitors reduces nitric oxide synthesis but has no effect on the severity of experimental colitis. Scand J Gastroenterol. 2000;35:832–838. doi: 10.1080/003655200750023200. [DOI] [PubMed] [Google Scholar]
- 166.Pilichos CJ, Kouerinis IA, Zografos GC, Korkolis DP, Preza AA, Gazouli M, Menenakos EI, Loutsidis AE, Zagouri F, Gorgoulis VG, Fotiadis CI. The effect of nitric oxide synthases inhibitors on inflammatory bowel disease in a rat model. In Vivo. 2004;18:513–516. [PubMed] [Google Scholar]
- 167.Dinc S, Caydere M, Akgul G, Yenidogan E, Hücümenoglu S, Rajesh M. Methylene Blue inhibits the inflammatory process of the acetic acid-induced colitis in the rat colonic mucosa. Int Surg. 2015 doi: 10.9738/INTSURG-D-15-00118.1. [DOI] [PubMed] [Google Scholar]
- 168.Sykes AP, Brampton C, Klee S, Chander CL, Whelan C, Parsons ME. An investigation into the effect and mechanisms of action of nicotine in inflammatory bowel disease. Inflamm Res. 2000;49:311–319. doi: 10.1007/s000110050597. [DOI] [PubMed] [Google Scholar]
- 169.Eliakim R, Karmeli F, Rachmilewitz D, Cohen P, Fich A. Effect of chronic nicotine administration on trinitrobenzene sulphonic acid-induced colitis. Eur J Gastroenterol Hepatol. 1998;10:1013–1019. [PubMed] [Google Scholar]
- 170.Linehan JD, Kolios G, Valatas V, Robertson DA, Westwick J. Effect of corticosteroids on nitric oxide production in inflammatory bowel disease: are leukocytes the site of action? Am J Physiol Gastrointest Liver Physiol. 2005;288:G261–G267. doi: 10.1152/ajpgi.00336.2004. [DOI] [PubMed] [Google Scholar]
- 171.El-Medany A, Mahgoub A, Mustafa A, Arafa M, Morsi M. The effects of selective cyclooxygenase-2 inhibitors, celecoxib and rofecoxib, on experimental colitis induced by acetic acid in rats. Eur J Pharmacol. 2005;507:291–299. doi: 10.1016/j.ejphar.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 172.Karmeli F, Cohen P, Rachmilewitz D. Cyclo-oxygenase-2 inhibitors ameliorate the severity of experimental colitis in rats. Eur J Gastroenterol Hepatol. 2000;12:223–231. doi: 10.1097/00042737-200012020-00015. [DOI] [PubMed] [Google Scholar]
- 173.Leonard N, Bishop AE, Polak JM, Talbot IC. Expression of nitric oxide synthase in inflammatory bowel disease is not affected by corticosteroid treatment. J Clin Pathol. 1998;51:750–753. doi: 10.1136/jcp.51.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuralay F, Yildiz C, Ozutemiz O, Islekel H, Caliskan S, Bingol B, Ozkal S. Effects of trimetazidine on acetic acid-induced colitis in female Swiss rats. J Toxicol Environ Health A. 2003;66:169–179. doi: 10.1080/15287390306402. [DOI] [PubMed] [Google Scholar]
- 175.Huang TY, Chu HC, Lin YL, Lin CK, Hsieh TY, Chang WK, Chao YC, Liao CL. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicol Appl Pharmacol. 2009;237:69–82. doi: 10.1016/j.taap.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 176.Garrido-Mesa J, Algieri F, Rodriguez-Nogales A, Utrilla MP, Rodriguez-Cabezas ME, Zarzuelo A, Ocete MA, Garrido-Mesa N, Galvez J. A new therapeutic association to manage relapsing experimental colitis: Doxycycline plus Saccharomyces boulardii. Pharmacol Res. 2015;97:48–63. doi: 10.1016/j.phrs.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 177.Akgun E, Caliskan C, Celik HA, Ozutemiz AO, Tuncyurek M, Aydin HH. Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res. 2005;33:196–206. doi: 10.1177/147323000503300207. [DOI] [PubMed] [Google Scholar]
- 178.You Y, Fu JJ, Meng J, Huang GD, Liu YH. Effect of N-acetylcysteine on the murine model of colitis induced by dextran sodium sulfate through up-regulating PON1 activity. Dig Dis Sci. 2009;54:1643–1650. doi: 10.1007/s10620-008-0563-9. [DOI] [PubMed] [Google Scholar]
- 179.Sasaki M, Bharwani S, Jordan P, Joh T, Manas K, Warren A, Harada H, Carter P, Elrod JW, Wolcott M, Grisham MB, Alexander JS. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther. 2003;305:78–85. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- 180.Camuesco D, Peran L, Comalada M, Nieto A, Di Stasi LC, Rodriguez-Cabezas ME, Concha A, Zarzuelo A, Galvez J. Preventative effects of lactulose in the trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel Dis. 2005;11:265–271. doi: 10.1097/01.mib.0000160808.30988.d9. [DOI] [PubMed] [Google Scholar]
- 181.Fatani AJ, Al-Hosaini KA, Ahmed MM, Abuohashish HM, Parmar MY, Al-Rejaie SS. Carvedilol attenuates inflammatory biomarkers and oxidative stress in a rat model of ulcerative colitis. Drug Dev Res. 2015;76:204–214. doi: 10.1002/ddr.21256. [DOI] [PubMed] [Google Scholar]
- 182.Esiringü F, Tuğcu-Demiröz F, Acartürk F, Coşkun Cevher Ş, Bircan F, Sarı Kılıçaslan SM. Investigation of the effect of intracolonic melatonin gel formulation on acetic acid-induced colitis. Drug Deliv. 2016;23:2318–2326. doi: 10.3109/10717544.2014.982773. [DOI] [PubMed] [Google Scholar]
- 183.Cuzzocrea S, Mazzon E, Serraino I, Lepore V, Terranova ML, Ciccolo A, Caputi AP. Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J Pineal Res. 2001;30:1–12. doi: 10.1034/j.1600-079x.2001.300101.x. [DOI] [PubMed] [Google Scholar]
- 184.Van Crombruggen K, Van Nassauw L, Demetter P, Cuvelier C, Timmermans JP, Lefebvre RA. Influence of soluble guanylate cyclase inhibition on inflammation and motility disturbances in DSS-induced colitis. Eur J Pharmacol. 2008;579:337–349. doi: 10.1016/j.ejphar.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 185.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochem J. 1997;328(Pt 3):721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cuzzocrea S, McDonald MC, Mazzon E, Mota-Filipe H, Centorrino T, Terranova ML, Ciccolo A, Britti D, Caputi AP, Thiemermann C. Calpain inhibitor I reduces colon injury caused by dinitrobenzene sulphonic acid in the rat. Gut. 2001;48:478–488. doi: 10.1136/gut.48.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Macdonald TT. Viral vectors expressing immunoregulatory cytokines to treat inflammatory bowel disease. Gut. 1998;42:460–461. doi: 10.1136/gut.42.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Colón AL, Menchén LA, Hurtado O, De Cristóbal J, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine. 2001;16:220–226. doi: 10.1006/cyto.2001.0969. [DOI] [PubMed] [Google Scholar]
- 189.Rajora N, Boccoli G, Catania A, Lipton JM. alpha-MSH modulates experimental inflammatory bowel disease. Peptides. 1997;18:381–385. doi: 10.1016/s0196-9781(96)00345-2. [DOI] [PubMed] [Google Scholar]
- 190.De AK, Sana S, Datta S, Mukherjee A. Protective efficacy of ursodeoxycholic acid nanoparticles in animal model of inflammatory bowel disease. J Microencapsul. 2014;31:725–737. doi: 10.3109/02652048.2014.918666. [DOI] [PubMed] [Google Scholar]
- 191.Rodriguez-Nogales A, Algieri F, De Matteis L, Lozano-Perez AA, Garrido-Mesa J, Vezza T, de la Fuente JM, Cenis JL, Gálvez J, Rodriguez-Cabezas ME. Intestinal anti-inflammatory effects of RGD-functionalized silk fibroin nanoparticles in trinitrobenzenesulfonic acid-induced experimental colitis in rats. Int J Nanomedicine. 2016;11:5945–5958. doi: 10.2147/IJN.S116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim YH, Kwon HS, Kim DH, Shin EK, Kang YH, Park JH, Shin HK, Kim JK. 3,3'-diindolylmethane attenuates colonic inflammation and tumorigenesis in mice. Inflamm Bowel Dis. 2009;15:1164–1173. doi: 10.1002/ibd.20917. [DOI] [PubMed] [Google Scholar]
- 193.Zhao Y, Sun Y, Ding Y, Wang X, Zhou Y, Li W, Huang S, Li Z, Kong L, Guo Q, Lu N. GL-V9, a new synthetic flavonoid derivative, ameliorates DSS-induced colitis against oxidative stress by up-regulating Trx-1 expression via activation of AMPK/FOXO3a pathway. Oncotarget. 2015;6:26291–26307. doi: 10.18632/oncotarget.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scioli MG, Stasi MA, Passeri D, Doldo E, Costanza G, Camerini R, Fociani P, Arcuri G, Lombardo K, Pace S, Borsini F, Orlandi A. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis Through its Action on the Immune Function and Microvasculature. Clin Transl Gastroenterol. 2014;5:e55. doi: 10.1038/ctg.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zschiebsch K, Fischer C, Pickert G, Häussler A, Radeke H, Grösch S, Ferreirós N, Geisslinger G, Werner ER, Tegeder I. Tetrahydrobiopterin Attenuates DSS-evoked Colitis in Mice by Rebalancing Redox and Lipid Signalling. J Crohns Colitis. 2016;10:965–978. doi: 10.1093/ecco-jcc/jjw056. [DOI] [PubMed] [Google Scholar]
- 196.Sehgal P, Colombel JF, Narula N. Adverse Events During Anti-TNFα Therapies in IBD (Excluding Infections and Malignancies): When to Stop, Continue, or Switch Therapies. Inflamm Bowel Dis. 2016;22:1239–1245. doi: 10.1097/MIB.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 197.Bantel H, Schulze-Osthoff K. TNF Antagonists in IBD: Novel Antiinflammatory Mechanisms Beyond Cytokine Inhibition. Inflamm Bowel Dis. 2013;19:E51–E52. doi: 10.1002/ibd.22988. [DOI] [PubMed] [Google Scholar]
- 198.Li P, Zheng Y, Chen X. Drugs for Autoimmune Inflammatory Diseases: From Small Molecule Compounds to Anti-TNF Biologics. Front Pharmacol. 2017;8:460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Abiodun OO, Rodríguez-Nogales A, Algieri F, Gomez-Caravaca AM, Segura-Carretero A, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J Ethnopharmacol. 2016;192:309–319. doi: 10.1016/j.jep.2016.07.056. [DOI] [PubMed] [Google Scholar]
- 200.Akanda MR, Nam HH, Tian W, Islam A, Choo BK, Park BY. Regulation of JAK2/STAT3 and NF-κB signal transduction pathways; Veronica polita alleviates dextran sulfate sodium-induced murine colitis. Biomed Pharmacother. 2018;100:296–303. doi: 10.1016/j.biopha.2018.01.168. [DOI] [PubMed] [Google Scholar]
- 201.Amini-Shirazi N, Hoseini A, Ranjbar A, Mohammadirad A, Khoshakhlagh P, Yasa N, Abdollahi M. Inhibition of tumor necrosis factor and nitrosative/oxidative stresses by Ziziphora clinopoides (Kahlioti); a molecular mechanism of protection against dextran sodium sulfate-induced colitis in mice. Toxicol Mech Methods. 2009;19:183–189. doi: 10.1080/15376510701533996. [DOI] [PubMed] [Google Scholar]
- 202.Baker J, Brown K, Rajendiran E, Yip A, DeCoffe D, Dai C, Molcan E, Chittick SA, Ghosh S, Mahmoud S, Gibson DL. Medicinal lavender modulates the enteric microbiota to protect against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G825–G836. doi: 10.1152/ajpgi.00327.2011. [DOI] [PubMed] [Google Scholar]
- 203.Bibi S, de Sousa Moraes LF, Lebow N, Zhu MJ. Dietary Green Pea Protects against DSS-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients. 2017;9 doi: 10.3390/nu9050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Borrelli F, Fasolino I, Romano B, Capasso R, Maiello F, Coppola D, Orlando P, Battista G, Pagano E, Di Marzo V, Izzo AA. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem Pharmacol. 2013;85:1306–1316. doi: 10.1016/j.bcp.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 205.Liu BG, Jia XM, Cao YY, Chen SH, Gao PH, Wang Y, Jiang YY, Cao YB. Changtai granule, a traditional Chinese drug, protects hapten-induced colitis by attenuating inflammatory and immune dysfunctions. J Ethnopharmacol. 2008;115:1–8. doi: 10.1016/j.jep.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 206.Mazzon E, Esposito E, Di Paola R, Riccardi L, Caminiti R, Dal Toso R, Pressi G, Cuzzocrea S. Effects of verbascoside biotechnologically produced by Syringa vulgaris plant cell cultures in a rodent model of colitis. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:79–94. doi: 10.1007/s00210-009-0400-5. [DOI] [PubMed] [Google Scholar]
- 207.Park DK, Park HJ. Ethanol extract of Cordyceps militaris grown on germinated soybeans attenuates dextran-sodium-sulfate- (DSS-) induced colitis by suppressing the expression of matrix metalloproteinases and inflammatory mediators. Biomed Res Int. 2013;2013:102918. doi: 10.1155/2013/102918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Patel M, Patel P, Patel M. Aqueous Extract of Ficus bengalensis Linn. Bark for Inflammatory Bowel Disease. J Young Pharm. 2010;2:130–136. doi: 10.4103/0975-1483.63149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Qin M, Geng Y, Lu Z, Xu H, Shi JS, Xu X, Xu ZH. Anti-Inflammatory Effects of Ethanol Extract of Lion's Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), in Mice with Ulcerative Colitis. Int J Med Mushrooms. 2016;18:227–234. doi: 10.1615/IntJMedMushrooms.v18.i3.50. [DOI] [PubMed] [Google Scholar]
- 210.Wang X, Sun Y, Zhao Y, Ding Y, Zhang X, Kong L, Li Z, Guo Q, Zhao L. Oroxyloside prevents dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB pathway through PPARγ activation. Biochem Pharmacol. 2016;106:70–81. doi: 10.1016/j.bcp.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 211.Liu L, Shang Y, Li M, Han X, Wang J, Wang J. Curcumin ameliorates asthmatic airway inflammation by activating nuclear factor-E2-related factor 2/haem oxygenase (HO)-1 signalling pathway. Clin Exp Pharmacol Physiol. 2015;42:520–529. doi: 10.1111/1440-1681.12384. [DOI] [PubMed] [Google Scholar]
- 212.Dost T, Ozkayran H, Gokalp F, Yenisey C, Birincioglu M. The effect of Hypericum perforatum (St. John's Wort) on experimental colitis in rat. Dig Dis Sci. 2009;54:1214–1221. doi: 10.1007/s10620-008-0477-6. [DOI] [PubMed] [Google Scholar]
- 213.Liu B, Lin Q, Yang T, Zeng L, Shi L, Chen Y, Luo F. Oat β-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct. 2015;6:3454–3463. doi: 10.1039/c5fo00563a. [DOI] [PubMed] [Google Scholar]
- 214.V VP, C G. Protective effect of marine mangrove Rhizophora apiculata on acetic acid induced experimental colitis by regulating anti-oxidant enzymes, inflammatory mediators and nuclear factor-kappa B subunits. Int Immunopharmacol. 2014;18:124–134. doi: 10.1016/j.intimp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 215.Wang X, Zhao L, Han T, Chen S, Wang J. Protective effects of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur J Pharmacol. 2008;578:339–348. doi: 10.1016/j.ejphar.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 216.Cho EJ, Shin JS, Noh YS, Cho YW, Hong SJ, Park JH, Lee JY, Lee JY, Lee KT. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol. 2011;136:428–435. doi: 10.1016/j.jep.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 217.Coutinho de Sousa B, Reis Machado J, da Silva MV, da Costa TA, Lazo-Chica JE, Degasperi TD, Rodrigues Junior V, Sales-Campos H, Uber Bucek E, Freire Oliveira CJ. Morinda citrifolia (Noni) Fruit Juice Reduces Inflammatory Cytokines Expression and Contributes to the Maintenance of Intestinal Mucosal Integrity in DSS Experimental Colitis. Mediators Inflamm. 2017;2017:6567432. doi: 10.1155/2017/6567432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhao W, Sun C, He J, Chen L, Zhang Y, Sun W. The possible mechanisms of Picrasma quassiodes (D. Don) Benn. in the treatment of colitis induced by 2,4,6-trinitrobenzene sulfonic acid in mice. J Ethnopharmacol. 2013;145:424–430. doi: 10.1016/j.jep.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 219.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 220.Castro J, Ocampo Y, Franco L. Cape Gooseberry [Physalis peruviana L.] Calyces Ameliorate TNBS Acid-induced Colitis in Rats. J Crohns Colitis. 2015;9:1004–1015. doi: 10.1093/ecco-jcc/jjv132. [DOI] [PubMed] [Google Scholar]
- 221.Park DD, Yum HW, Zhong X, Kim SH, Kim SH, Kim DH, Kim SJ, Na HK, Sato A, Miura T, Surh YJ. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets. Front Pharmacol. 2017;8:482. doi: 10.3389/fphar.2017.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xu BL, Zhang GJ, Ji YB. Active components alignment of Gegenqinlian decoction protects ulcerative colitis by attenuating inflammatory and oxidative stress. J Ethnopharmacol. 2015;162:253–260. doi: 10.1016/j.jep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 223.Zbakh H, Talero E, Avila J, Alcaide A, de Los Reyes C, Zubía E, Motilva V. The Algal Meroterpene 11-Hydroxy-1'-O-Methylamentadione Ameloriates Dextran Sulfate Sodium-Induced Colitis in Mice. Mar Drugs. 2016;14 doi: 10.3390/md14080149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wu XF, Ouyang ZJ, Feng LL, Chen G, Guo WJ, Shen Y, Wu XD, Sun Y, Xu Q. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicol Appl Pharmacol. 2014;281:146–156. doi: 10.1016/j.taap.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 225.Liu QQ, Wang HL, Chen K, Wang SB, Xu Y, Ye Q, Sun YW. Oridonin derivative ameliorates experimental colitis by inhibiting activated T-cells and translocation of nuclear factor-kappa B. J Dig Dis. 2016;17:104–112. doi: 10.1111/1751-2980.12314. [DOI] [PubMed] [Google Scholar]
- 226.O'Sullivan S, Wang J, Pigott MT, Docherty N, Boyle N, Lis SK, Gilmer JF, Medina C. Inhibition of matrix metalloproteinase-9 by a barbiturate-nitrate hybrid ameliorates dextran sulphate sodium-induced colitis: effect on inflammation-related genes. Br J Pharmacol. 2017;174:512–524. doi: 10.1111/bph.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Invernizzi P, Salzman AL, Szabó C, Ueta I, O'Connor M, Setchell KD. Ursodeoxycholate inhibits induction of NOS in human intestinal epithelial cells and in vivo. Am J Physiol. 1997;273:G131–G138. doi: 10.1152/ajpgi.1997.273.1.G131. [DOI] [PubMed] [Google Scholar]
- 228.Motomura Y, Wang H, Deng Y, El-Sharkawy RT, Verdu EF, Khan WI. Helminth antigen-based strategy to ameliorate inflammation in an experimental model of colitis. Clin Exp Immunol. 2009;155:88–95. doi: 10.1111/j.1365-2249.2008.03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rachmilewitz D, Karmeli F, Okon E. Sulfhydryl blocker-induced rat colonic inflammation is ameliorated by inhibition of nitric oxide synthase. Gastroenterology. 1995;109:98–106. doi: 10.1016/0016-5085(95)90273-2. [DOI] [PubMed] [Google Scholar]