Abstract
Background
The human ether-a-go-go-related gene (hERG) potassium channel is the rapidly activating component of cardiac delayed rectifier potassium current (IKr), which is a crucial determinant of cardiac repolarization. The reduction of hERG current is commonly believed to cause Long QT Syndrome (LQTs). Probucol, a cholesterol-lowering drug, induces LQTs by inhibiting the expression of the hERG channel. Unfortunately, there is currently no effective therapeutic method to rescue probucol-induced LQTs.
Methods
Patch-clamp recording techniques were used to detect the action potential duration (APD) and current of hERG. Western blot was performed to measure the expression levels of proteins.
Results
In this study, we demonstrated that 1 μM matrine and oxymatrine could rescue the hERG current and hERG surface expression inhibited by probucol. In addition, matrine and oxymatrine significantly shortened the prolonged action potential duration induced by probucol in neonatal cardiac myocytes. We proposed a novel mechanism underlying the probucol induced decrease in the expression of transcription factor Specificity protein 1 (Sp1), which is an established transactivator of the hERG gene. We also demonstrated that matrine and oxymatrine were able to upregulate Sp1 expression which may be one of the possible mechanisms by which matrine and oxymatrine rescued probucol-induced hERG channel deficiency.
Conclusion
Our current results demonstrate that matrine and oxymatrine could rescue probucol-induced hERG deficiency in vitro, which may lead to potentially effective therapeutic drugs for treating acquired LQT2 by probucol in the future.
Keywords: Probucol, Human ether-a-go-go-related gene, matrine, oxymatrine, specificity protein 1, cardiac repolarization
1. INTRODUCTION
The cardiac potassium channel hERG is the rapidly activating component of the delayed rectifier potassium current (IKr), which plays an important role in terminal repolarization in human ventricular myocytes [1]. Long or short QT syndrome can be induced by a reduction or an increase in IKr, both of which increase the risk of fatal arrhythmias and sudden cardiac death [2, 3]. In addition, long QT syndrome 2 (LQT2) can be caused by dysfunction of hERG channels with drugs that interfere with channel gating [4]. Dual mechanisms were shown in the drug-induced reduction of the IKr/hERG currents, including the direct blockade of IKr/hERG potassium channels and elective disruption of hERG protein trafficking to the cell surface membrane [5, 6].
Probucol, a cholesterol-lowering drug, has been found to cause long QT syndrome and torsades de pointes arrhythmia in patients [7]. Probucol selectively reduces hERG/IKr currents via inhibition of hERG trafficking [8]. The mechanisms of probucol reducing
functional hERG expression involve altering the membrane stability and turnover of the hERG-interacting protein caveolin-1 (Cav1) [9]. The clinical usefulness of probucol is restricted by its undesirable cardiotoxicity. Therefore, reducing probucol-induced cardiotoxicity is important. In addition, the use of cardioprotective agents may be an alternative approach to clinical therapeutics in the future.
Matrine and oxymatrine are extracted from the dried root of Sophora flavescens Ait, called Ku Shen; both are active ingredients of Ku Shen. Recent research demonstrated that oxymatrine protected cardiomyocytes from apoptotic death during ischemic myocardial injuries in rats [10]. A wide range of pharmacological effects of matrine and oxymatrine, such as anti-arrhythmia and antitumor activities, have been demonstrated in various studies [11-13]. Furthermore, our recent studies showed that the hERG channel was activated by oxymatrine [14] and that the hERG channel surface expression was increased by both matrine and oxymatrine [15]. Therefore, the application of matrine and oxymatrine to rescue hERG channel deficiency seems to be a promising strategy. Interestingly, in our previous studies, we found that matrine and oxymatrine can rescue arsenic trioxide-induced hERG expression deficiency via elevating the transcription factor Sp1, which could upregulate hERG gene transcription [16]. Based on this, we hypothesized that probucol-induced hERG channel deficiency may be reversed by matrine and oxymatrine. The two main goals of this study were to determine (a) whether the probucol-induced hERG channel deficiency and prolonged action potential duration (APD) can be rescued by matrine and oxymatrine and (b) obtain mechanistic insight into matrine and oxymatrine rescue the decreased hERG expression by probucol.
In this study, we investigated the rescue effect of matrine and oxymatrine on the inhibition of hERG protein expression and the hERG current caused by probucol in vitro. We further detected the effect of matrine and oxymatrine on probucol-prolonged APD in neonatal rat ventricular myocytes. Finally, our results revealed the one of the possible mechanism underlying the matrine and oxymatrine rescue of hERG expression disrupted by probucol.
2. MATERIALS AND METHODS
2.1. Cell Culture
We used a stably transfected human embryonic kidney (HEK) cell line expressing high levels of functional hERG to study the effects of different compounds on the hERG channel. The hERG-HEK cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; TBD, Tianjin, China) at 37 °C in a humidified atmosphere of 5% CO2. Stably transfected cells were then selected with 400 mg/ml geneticin (G-418, Invitrogen, Carlsbad, CA, USA). For electrophysiological studies, the cells were harvested from the culture dish by trypsinization, washed twice with standard DMEM, and stored in this medium at room temperature for later use. The cells were studied within 8 h of harvest.
2.2. Reagents
Matrine and oxymatrine (purity of ≥98%, Shanxi Huike Botanical Development Co., Ltd., Shanxi, China) were dissolved in PBS as a stock solution. The probucol was dissolved in dehydrated alcohol to obtain a 10 mM stock solution, which was stored at -20 °C. For cellular experiments, hERG-HEK cells were incubated with different concentrations of the compounds diluted in the cultured medium.
2.3. Neonatal Rat Ventricular Myocyte Isolation
Single ventricular myocytes were isolated from 1- to 2-day-old Sprague-Dawley rats of either sex by enzymatic dissociation as described previously [8]. Neonatal rat ventricular myocytes were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal bovine serum. Cardiomyocytes were grown on glass coverslips for the electrophysiology studies. All animal procedures were approved by the Institutional Animal Care and Use Committee at Harbin Medical University (No. HMUIRB-2008-06) and the Institute of Laboratory Animal Science of China (A5655-01). All of the procedures conformed to the Directive 2010/63/EU of the European Parliament.
2.4. Patch-clamp Recording Techniques
The drug solutions were freshly prepared daily with an extracellular solution. For electrophysiological recordings, submaximally confluent hERG-HEK cells were used after culturing for 1-3 days at a seeding density of 1-4 × 105 cells per flask (NEST, 25cm2). The cells were harvested from the flask using 0.25% trypsin and 0.02% EDTA and transferred to a small cell bath mounted on the stage of an inverted microscope (IX-70; Olympus, Tokyo), where they were allowed to attach to the glass bottom for approximately 10 min. The cells were then superfused continuously at a rate of 1.5 ml/min.
The hERG currents were measured using the whole-cell configuration of the patch-clamp technique. The whole-cell configuration was formed using a glass pipette with a tip resistance of 2-5 mOhm when filled with a pipette solution: 130 mM KCl, 1 mM MgCl2•6H2O, 10 mM HEPES, 5 mM Mg-ATP, 5 mM EGTA, and 0.1 mM GTP (pH 7.3 with KOH). The extracellular solution contained 136 mM NaCl, 5.4 mM KCl, 5 mM HEPES, 1 mM MgCl2•6H2O, 1 mM CaCl2, and 10 mM glucose (adjusted to pH 7.4 with NaOH). An Axopatch-200B patch-clamp amplifier was used to record the membrane current and APD. Computer software (Clampex 9.2; Axon Instruments, USA) was used to generate voltage-clamp protocols and acquire data. Capacitance and series resistance compensation were optimized. The data were recorded on a computer via Digidata 1322A and analyzed using Clampfit 9.2 (Axon Instruments) and Prism (GraphPad Inc., San Diego, CA, USA) software. Graphical fits of the data were made using previously described standard equations [17, 18].
The APDs were recorded using the whole-cell configuration with a pipette solution containing 20 mM KCl, 110 mM KOH, 1 mM MgCl2•6H2O, 10 mM HEPES, 5 mM Na2-ATP, 5 mM EGTA, and 110 mM K-aspartate (adjusted to pH 7.3 with KOH). The extracellular solution was the same as the Ca2+-containing Tyrode’s solution. The neonatal rat ventricular myocytes were transferred to a small cell bath to attach to the glass bottom for approximately 10 min.
2.5. Western Blot Analysis
The expression of high levels of hERG protein was monitored by Western blot experiments. The 1 µM matrine/oxymatrine and 100 μM probucol were diluted and added to hERG-HEK cells for 48 h at 37 °C before analysis by Western blots. The cells were placed on ice and washed 3 times with 3 ml ice-cold PBS. Then, 60 µl RIPA (Bi Yun Tian, Jiangsu, China) and 0.6 µl PMSF (Shenneng Bocai, Shanghai, China) were added to the plates, and the cells were scraped from the plates and transferred into tubes. Protein (150 μg per sample) was separated using SDS-PAGE, transferred onto nitrocellulose membranes (Stratagene, La Jolla, CA), and incubated with a primary antibody against GAPDH (Affinity Reagents) and a specific polyclonal rabbit anti-hERG antibody (Santa Cruz Biotechnology, CA) at a 1:100 dilution. Goat anti-rabbit Alexa Fluor 700 (dilution 1:2000, Molecular Probes, Eugene, OR) was used as a secondary antibody. The Odyssey infrared fluorescent scanning system (LI-COR, Lincoln, NE) was used to detect membrane proteins. The band densities were quantified by densitometry using Scion Image software (Scion, Frederick, MD). The data were normalized to GAPDH.
2.6. Statistical Analyses
The data are presented as the mean ± S.E.M. Statistical analysis was performed using SPSS 16.0 software to evaluate significant differences. Student’s t-test was used for comparisons between two groups, and one-way or two-way analysis of variance (ANOVA) was used to analyze significant differences between groups under different conditions. Two groups were considered significantly different when P-values were < 0.05 (two-tailed). All of the graphs were drawn by GraphPad Prism 5.0.
3. RESULTS
3.1. Matrine and Oxymatrine Rescue hERG Protein Expression after Probucol Treatment
Probucol reduces hERG expression levels in the plasma membrane [8]. In our study, we detected the effect of long-term treatment with 100 μM probucol for 48 h by detecting the hERG proteins located in the membrane. As illustrated in Fig. ((1), two forms of hERG channels were recognized by the anti-hERG antibody, a fully glycosylated mature protein with a molecular mass of 155 kDa and an immature core-glycosylated protein of 135 kDa in HEK-293 cells stably transfected with hERG. Fig. (1A and 1B) demonstrated that the expression of mature hERG protein decreased after treatment with 100 μM probucol (p < 0.05, n=6).
Fig. (1).
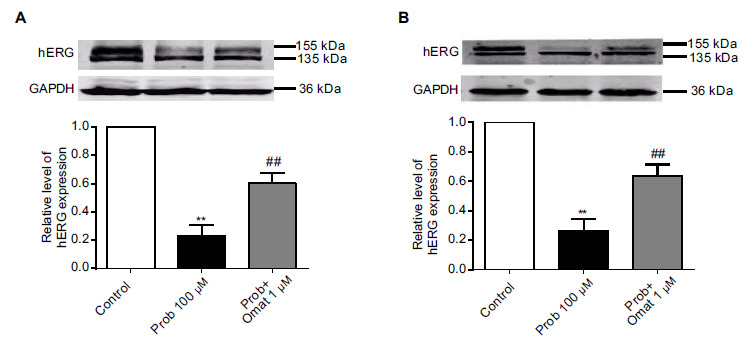
Matrine and oxymatrine rescue the decreased hERG expression after probucol treatment in hERG-HEK cells. (A) Matrine upregulates hERG channel surface expression after probucol treatment. (B) Oxymatrine upregulates hERG channel surface expression after probucol treatment. n=6, ** p <0.01 vs the control and ## p <0.01 vs probucol.
To investigate whether matrine and oxymatrine could recover the hERG deficiency induced by probucol, we coincubated the hERG-HEK cells with 1 μM matrine/oxymatrine and 100 μM probucol containing media at 37 °C for 48 h. Afterwards, we used western blot experiments to identify hERG proteins in the hERG proteins in the control groups, probucol groups, matrine/oxymatrine and probucol groups. As shown in Fig. (1A and 1B), the application of 1 μM matrine and 1 μM oxymatrine partially recovered the mature hERG protein in the presence of 100 μM probucol (p < 0.01, n=6).
3.2. Matrine and Oxymatrine Rescue hERG Currents After Probucol Treatment
To further determine the functionality of the hERG protein rescued by matrine and oxymatrine, we recorded and analyzed the currents from the hERG-HEK cells that were incubated with matrine or oxymatrine and probucol. The hERG current was elicited by a series of 4-s depolarization pulses ranging from -60 to +40 mV from a holding potential of -80 mV. A repolarization to -50 mV was used to elicit the tail current (Fig. 2A). Fig. (2B-D) shows that the tail current of the hERG-HEK cells was significantly inhibited after incubation with probucol for 48 h, whereas the application of 1 μM matrine/oxymatrine could attenuate this effect of probucol (p < 0.05, n=10).
Fig. (2).
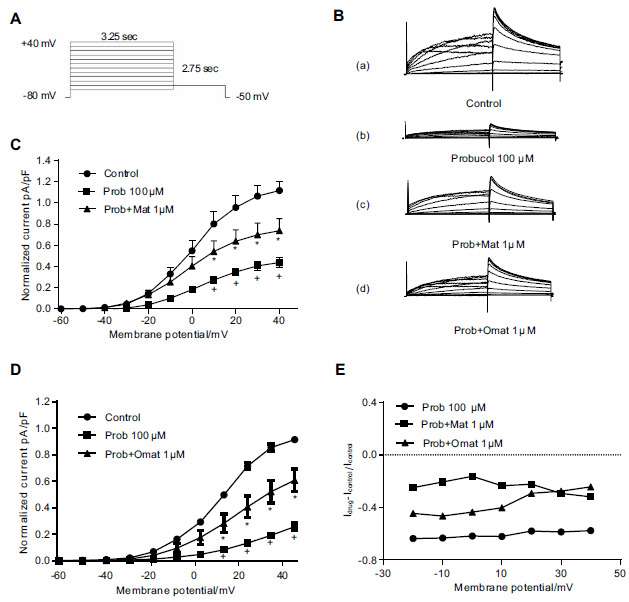
Matrine and oxymatrine reverse the inhibition of the hERG current after probucol treatment in hERG-HEK cells. (A) Voltage clamp protocol. (B) Representative hERG current traces recorded from a stably expressed hERG cell line in control conditions (a), in the presence of 100 μM probucol (b), in the presence of 100 μM probucol and 1 μM matrine (c), and in the presence of 100 μM probucol and 1 μM oxymatrine (d). (C-D) Normalized I-V relationships for the tail current before and after probucol treatment and rescue with 1 μM matrine and 1 μM oxymatrine. n=10, * p < 0.05 vs the control and + p <0.05 vs probucol. (E) The ratio of probucol, matrine, and oxymatrine voltage dependence peak tail current calculated using the function (I Drug-I Control)/I Control.
Moreover, we calculated the ratio of the hERG currents between Control groups and drug groups to observe the effect of matrine/oxymatrine on inhibition hERG currents by probucol. The inhibitory ratio of 100 μM probucol was computed from the tail current from -20 mV to 40 mV with the following function: (I Probucol –I Control)/I Control. As shown in Fig. (2E), the ratio was -0.61 at 0 mV (-0.57 at 40 mV). The rescue ratio of matrine or oxymatrine was -0.16 and -0.43 at 0 mV (-0.31 and -0.24 at 40 mV), respectively, as calculated by the functions (I Matrine -I Control)/I Control and (I Oxymatrine -I Control)/I Control.
3.3. Matrine and Oxymatrine Recover the APD Prolongation Induced by Probucol in Neonatal Cardiac Myocytes
The next series of experiments were performed to confirm whether our analysis might be extended to other expression systems such as ventricular cardiomyocytes. We examined the effects of matrine or oxymatrine and probucol on action potentials recorded in neonatal rat ventricular myocytes. Fifty percent and ninety percent
repolarization of APD (APD50 and APD90) was used to describe the action potential changes. When 100 μM probucol was added to the ventricular cardiomyocyte culture medium for 48 h, the APD90 was significantly prolonged (Fig. 3A and 3B). To investigate the recovery from prolongation of cardiac action potentials induced by probucol administration, we added matrine or oxymatrine to myocytes incubated with 100 μM probucol. The prolonged APD90 was significantly shortened from 145.4±16.8 ms to 51.3±4.5 ms by 1 μM matrine (Fig. 3A and 3B), and 1 μM oxymatrine shortened the prolonged APD90 from 167.7±27.8 ms to 75.9±5.4 ms in neonatal cardiac myocytes (Fig. 3C and 3D).
Fig. (3).
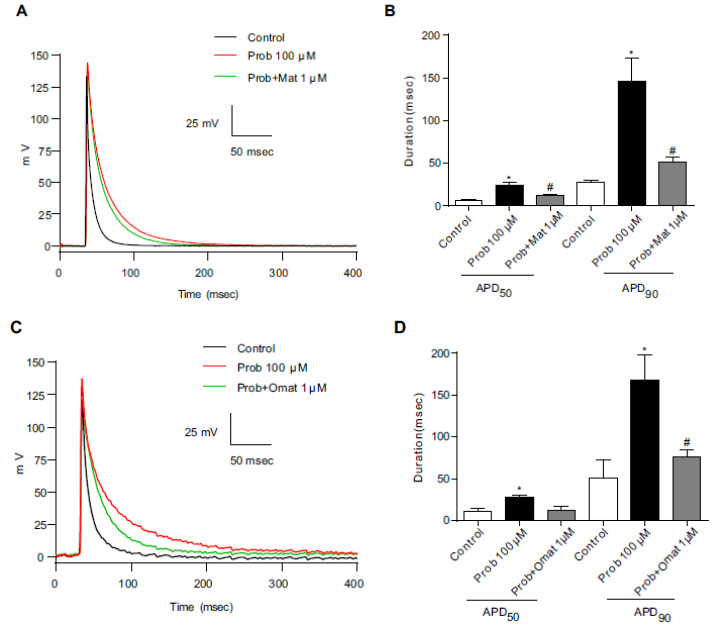
The effects of matrine and oxymatrine on the prolongation of APD by probucol in neonatal cardiac myocytes. (A) and (C) Representative action potential traces from control (black line), probucol (red line) and probucol/1 μM matrine or 1 μM oxymatrine groups (green line) in neonatal cardiac myocytes. (B) and (D) The APD50 and APD90 were prolonged by probucol and recovered by 1 μM matrine or 1 μM oxymatrine. n=5, * p <0.05 vs the control and # p <0.05 vs probucol. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4. Matrine and Oxymatrine Rescue hERG Expression Deficiency by Probucol Via Upregulation of Sp1 Expression
Specificity protein 1 (Sp1), an extensively expressed transcription factor, is a member of the Sp/KLF family. In our previous study, we demonstrated that Sp1 was essential in driving hERG promoter transcription, and both matrine and oxymatrine increased the expression of Sp1 in hERG-HEK cells. Therefore, in our present study, to determine the possible mechanisms of matrine and oxymatrine rescued probucol-induced hERG channel deficiency, Sp1 expression was examined using western blot analysis.
After exposure of hERG-HEK cells to 100 μM probucol for 48 h, the expression level of Sp1 was significantly reduced (p < 0.05, n = 5, Fig. 4A and 4B). Meanwhile, 1 μM matrine or oxymatrine reversed the Sp1 reduction in the presence of 100 μM probucol (p < 0.05, n = 5, Fig. 4A and 4B).
Fig. (4).
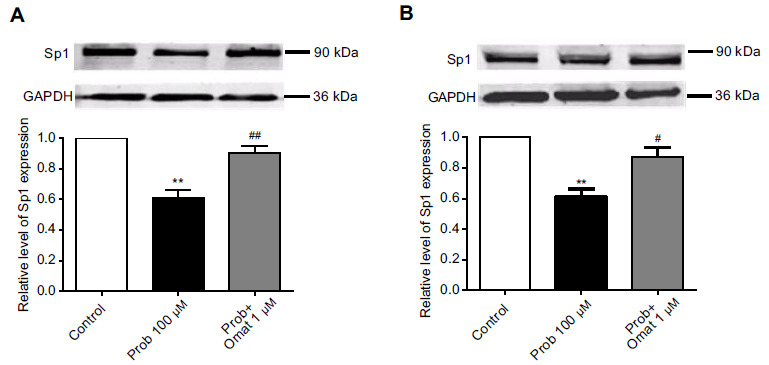
The effect of probucol, matrine, and oxymatrine on the expression of the transcription factor Sp1 after incubation for 48 h. (A) Matrine upregulated Sp1 expression to normal levels after probucol treatment. (B) Oxymatrine upregulates Sp1 expression to normal after probucol treatment. n=6, ** p <0.01 vs the control, # p <0.05 vs probucol and ## p < 0.01 vs probucol.
4. DISCUSSION
Probucol is a cholesterol-lowering drug that can induce cardiovascular system abnormalities. In patients, long QT syndrome and torsades de pointes arrhythmia were caused by probucol [19]. Dysfunction of hERG channels can induce LQTs.
In a previous study, the hERG current was decreased in hERG-HEK cells after treatment with probucol for 48 h with an IC50 of 10.6 μM [9]. In rat neonatal cardiomyocytes, probucol reduced the native IKr with an IC50 of 20.6 μM for 48 h [8]. In patients who received 1 g probucol daily for periods of 1 to 12 months, mean plasma levels ranged from 18.2 to 39.2 g/ml (35.2-75.9 μM) [20].
Therefore, the reduced expression of hERG channels and the prolonged QT interval could be induced by clinically relevant concentrations of probucol. In this study, we chose a concentration of 100 μM probucol to form the hERG-deficiency pattern because the maximum inhibitory effect of probucol can be achieved under this concentration. The turnover of the hERG channel occurs at a rather slow rate (approximately 11 h) [21], so we incubated the hERG-HEK cells with probucol for 48 h. The results illustrate the inhibitory effect of probucol on the hERG channel. Fig. (1A)and (B) show that the expression of mature hERG protein was reduced, and (Fig. 2B-D) show that the tail current of hERG-HEK cells was inhibited when the cells were cultured for 48 h in the presence of probucol.
The inhibition of hERG expression induced by the hERG channel mutations has been shown to be rescued by low temperature and E4031 [22]. Astemizole was shown to rescue the pentamidine-induced hERG trafficking inhibition [23]. However, an effective therapeutic method that can rescue the inhibition of hERG channel expression caused by probucol has not been confirmed. Previously, in our studies, 1 μM matrine and 1 μM oxymatrine were reported to enhance the hERG tail current. Furthermore, we confirmed that hERG expression could be increased by matrine and oxymatrine via upregulating the expression of Sp1 [14, 16]. Therefore, we investigated whether these two drugs could recover the hERG expression deficiency caused by probucol in vitro.
Matrine and oxymatrine are two major alkaloid components extracted from the Sophora root [24]. Our studies demonstrated that the hERG protein expression increased significantly and the hERG current was enhanced when hERG-HEK cells were exposed to 1 μM matrine or oxymatrine for 24 h [16]. Acute application of probucol had no effect on the hERG current, but hERG protein trafficking could be disrupted by treatment with probucol for 48 h [8]. Thus, we study whether matrine or oxymatrine had an effect on probucol-induced hERG deficiency after incubation matrine/oxymatrine and probucol at 37 °C for 48 h in hERG-HEK cells. In our previous research, we demonstrated that 1 μM matrine or oxymatrine promoted hERG expression, whereas high concentrations (100 μM) of the compounds could decrease the current of the hERG channel[15]. For this reason, 1 μM matrine or oxymatrine was chosen for our study. As shown in Fig. ((1), 1 μM matrine or oxymatrine could partially recover mature hERG protein after treatment with probucol. In hERG-HEK cells incubated with 1 μM matrine or oxymatrine, the reduction of the hERG tail current in the presence of probucol could be reversed. Meanwhile, the acute application of matrine and oxymatrine could increase the current of the hERG channel by regulating the kinetics of channel gating [14, 16]. Nevertheless, our study found that the acute application of matrine or oxymatrine had no effect on probucol-induced hERG deficiency (data not shown). These results demonstrated that the rescue effect of matrine and oxymatrine on probucol-induced hERG deficiency most likely depended on the upregulation of hERG protein expression but not the alteration of channel kinetics. Furthermore, probucol-induced prolongation of action potential duration could be shortened by matrine or oxymatrine in neonatal rat ventricular myocytes. Both matrine and oxymatrine could shorten the APD90 prolonged by probucol. Thus, matrine or oxymatrine could rescue LQT2 induced by probucol in our study.
In the preceding study, hERG inhibition induced by probucol affected the cell plasma membrane, but whether probucol affects the transcription of hERG remains unknown. Sp1 is a transcriptional activator or a repressor of target genes [25]. Sp1 can regulate certain ion channels, such as the potassium channel subunits Kv7.2, Kv7.3 [26] and Kv4.3 [27], the CLC-2 lung epithelial chloride channel [28], and a nonselective cation channel (the NCCa-ATP channel) [29]. Our prior study identified an extensively expressed transcription factor, Sp1, which was demonstrated to regulate the expression of hERG, and matrine or oxymatrine could increase hERG expression through the upregulation of Sp1 [16]. Thus, Sp1 may be the target for probucol to inhibit the expression of the hERG channel. Our data indicated that matrine or oxymatrine upregulated the decreased expression of Sp1 by probucol. These results revealed that matrine and oxymatrine recovered the probucol-induced expression deficiency of the hERG protein via the upregulation of Sp1. However, as shown in Fig. (1) and Fig. (4), the effects of probucol on the surface expression of the hERG channel and hERG current appear to be more pronounced compared to its effect on the expression of Sp1. This finding indicates that probucol may have Sp1-independent effects on the maturation process of the hERG channel. Many factors are involved in hERG protein expression, including mRNA transcription, protein synthesis, maturation, intracellular trafficking, and incorporation into the cell membrane. All of these steps are possible candidates for the site of action of probucol and matrine. Nevertheless, more mechanisms and in vivo evidence need to be evaluated in further studies.
CONCLUSION
In conclusion, our study confirmed the protective effect of matrine and oxymatrine against the probucol-induced reduction of hERG channels in both the heterologous hERG-HEK system and neonatal cardiac myocytes. The protective effect is possibly mediated by upregulated Sp1 expression. Therefore, matrine or oxymatrine may have potential therapeutic value for acquired LQT2 by probucol, which has an adverse effect on hERG channel deficiency.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS' CONTRIBUTIONS
BXL planned the study. YQS, PF, and XT wrote the manuscript. GCZ and YHZ performed the experiments, including cell culture and western blotting. MZL and FW participated in the patch-clamp experiments. YQS and PF conducted the statistical analysis. All authors read and approved the manuscript and agreed to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work was appropriately investigated and resolved.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All of the animal procedures were approved by the Institutional Animal Care and Use Committee at Harbin Medical University (No. HMUIRB-2008-06) and the Institute of Laboratory Animal Science of Harbin China (A5655-01). All of the procedures conformed to the Directive 2010/63/EU of the European Parliament.
HUMAN AND ANIMAL RIGHTS
No human subjects were used in the study. The reported experiments on animal were in accordance with the Standards set forth in the 8th Edition of Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-thecare-and-use-of-laboratory-animals.pdf) published by the National Academy of Sciences, The National Academies Press, Washington DC, United States of America.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
The present study was supported by grants from the National Natural Science Foundation of China (No. 81673636 to Dr. Baoxin Li and No. 81700303 to Dr. Yuanqi Shi), the China Postdoctoral Science Foundation (No. 2018M631958) and the Fundamental Research Funds for the Provincial Universities (No. 2017LCZX17).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sanguinetti M.C., Jiang C., Curran M.E., Keating M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [http://dx.doi.org/10.1016/0092-8674(95)90340-2]. [PMID: 7736582]. [DOI] [PubMed] [Google Scholar]
- 2.Keating M.T., Sanguinetti M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–580. doi: 10.1016/s0092-8674(01)00243-4. [http://dx.doi.org/10.1016/S0092-8674(01)00243-4]. [PMID: 11239413]. [DOI] [PubMed] [Google Scholar]
- 3.Patel C., Yan G.X., Antzelevitch C. Short QT syndrome: from bench to bedside. Circ Arrhythm Electrophysiol. 2010;3(4):401–408. doi: 10.1161/CIRCEP.109.921056. [http://dx.doi.org/10.1161/CIRCEP.109.921056]. [PMID: 20716721]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raschi E., Vasina V., Poluzzi E., De Ponti F. The hERG K+ channel: Target and antitarget strategies in drug development. Pharmacol. Res. 2008;57(3):181–195. doi: 10.1016/j.phrs.2008.01.009. [http://dx.doi.org/10.1016/j.phrs.2008.01.009]. [PMID: 18329284]. [DOI] [PubMed] [Google Scholar]
- 5.Dennis A., Wang L., Wan X., Ficker E. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem. Soc. Trans. 2007;35(Pt. 5):1060–1063. doi: 10.1042/BST0351060. [http://dx.doi.org/10.1042/BST0351060]. [PMID: 17956279]. [DOI] [PubMed] [Google Scholar]
- 6.van der Heyden M.A., Smits M.E., Vos M.A. Drugs and trafficking of ion channels: a new pro-arrhythmic threat on the horizon? Br. J. Pharmacol. 2008;153(3):406–409. doi: 10.1038/sj.bjp.0707618. [http://dx.doi.org/10.1038/sj.bjp.0707618]. [PMID: 18059314]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamura M., Ueki Y., Ohtsuka E., et al. Probucol-induced QT prolongation and syncope. Jpn. Circ. J. 1994;58(5):374–377. doi: 10.1253/jcj.58.374. [http://dx.doi.org/10.1253/jcj.58.374]. [PMID: 8022053]. [DOI] [PubMed] [Google Scholar]
- 8.Guo J., Massaeli H., Li W., et al. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J. Pharmacol. Exp. Ther. 2007;321(3):911–920. doi: 10.1124/jpet.107.120931. [http://dx.doi.org/10.1124/jpet.107.120931]. [PMID: 17377062]. [DOI] [PubMed] [Google Scholar]
- 9.Guo J., Li X., Shallow H., et al. Involvement of caveolin in probucol-induced reduction in hERG plasma-membrane expression. Mol. Pharmacol. 2011;79(5):806–813. doi: 10.1124/mol.110.069419. [http://dx.doi.org/10.1124/mol.110.069419]. [PMID: 21278233]. [DOI] [PubMed] [Google Scholar]
- 10.Hong-Li S., Lei L., Lei S., et al. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother. Res. 2008;22(7):985–989. doi: 10.1002/ptr.2452. [http://dx.doi.org/10.1002/ptr.2452]. [PMID: 18389484]. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Wang T., Wen X., et al. Effect of matrine on HeLa cell adhesion and migration. Eur. J. Pharmacol. 2007;563(1-3):69–76. doi: 10.1016/j.ejphar.2007.01.073. [http://dx.doi.org/10.1016/j.ejphar.2007.01.073]. [PMID: 17343841]. [DOI] [PubMed] [Google Scholar]
- 12.Ho J.W., Ngan Hon P.L., Chim W.O. Effects of oxymatrine from Ku Shen on cancer cells. Anticancer. Agents Med. Chem. 2009;9(8):823–826. doi: 10.2174/187152009789124673. [http://dx.doi.org/10.2174/187152009789124673]. [PMID: 19538168]. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Zhu M., Shi R., Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am. J. Chin. Med. 2003;31(3):337–354. doi: 10.1142/S0192415X03001107. [http://dx.doi.org/10.1142/S0192415X03001107]. [PMID: 12943166]. [DOI] [PubMed] [Google Scholar]
- 14.Hu M.Q., Dong Z.X., Zhao W.X., et al. The novel mechanism of oxymatrine affecting HERG currents at different temperatures. Cell. Physiol. Biochem. 2010;26(4-5):513–522. doi: 10.1159/000322319. [http://dx.doi.org/10.1159/000322319]. [PMID: 21063089]. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Du J., Zhang Y., et al. Yao Xue Xue Bao. 2007;42(2):139–144. [Effects of matrine, oxymatrine and resveratrol on HERG channel expression]. [PMID: 17518040]. [PubMed] [Google Scholar]
- 16.Zhang Y., Dong Z., Jin L., et al. Arsenic trioxide-induced hERG K(+) channel deficiency can be rescued by matrine and oxymatrine through up-regulating transcription factor Sp1 expression. Biochem. Pharmacol. 2013;85(1):59–68. doi: 10.1016/j.bcp.2012.09.002. [http://dx.doi.org/10.1016/j.bcp.2012.09.002]. [PMID: 23103450]. [DOI] [PubMed] [Google Scholar]
- 17.Du X., Lu D., Daharsh E.D., Yao A., Dewoody R., Yao J.A. Dimethyl sulfoxide effects on hERG channels expressed in HEK293 cells. J. Pharmacol. Toxicol. Methods. 2006;54(2):164–172. doi: 10.1016/j.vascn.2006.03.002. [http://dx.doi.org/10.1016/j.vascn.2006.03.002]. [PMID: 16782359]. [DOI] [PubMed] [Google Scholar]
- 18.Gu D.F., Li X.L., Qi Z.P., et al. Blockade of HERG K+ channel by isoquinoline alkaloid neferine in the stable transfected HEK293 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009;380(2):143–151. doi: 10.1007/s00210-009-0419-7. [http://dx.doi.org/10.1007/s00210-009-0419-7]. [PMID: 19424681]. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K., Shimizu M., Ino H., et al. Probucol aggravates long QT syndrome associated with a novel missense mutation M124T in the N-terminus of HERG. Clin. Sci. (Lond.) 2004;107(2):175–182. doi: 10.1042/CS20030351. [http://dx.doi.org/10.1042/CS20030351]. [PMID: 15043509]. [DOI] [PubMed] [Google Scholar]
- 20.Heeg J.F., Tachizawa H. Plasma levels of probucol in man after single and repeated oral doses (author’s transl). Nouv. Presse Med. 1980;9(40):2990–2994. [PMID: 7443437]. [PubMed] [Google Scholar]
- 21.Ficker E., Dennis A.T., Wang L., Brown A.M. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 2003;92(12):e87–e100. doi: 10.1161/01.RES.0000079028.31393.15. [http://dx.doi.org/10.1161/01.RES.0000079028.31393.15]. [PMID: 12775586]. [DOI] [PubMed] [Google Scholar]
- 22.Delisle B.P., Anson B.D., Rajamani S., January C.T. Biology of cardiac arrhythmias: Ion channel protein trafficking. Circ. Res. 2004;94(11):1418–1428. doi: 10.1161/01.RES.0000128561.28701.ea. [http://dx.doi.org/10.1161/01.RES.0000128561.28701.ea]. [PMID: 15192037]. [DOI] [PubMed] [Google Scholar]
- 23.Dennis A.T., Wang L., Wan H., Nassal D., Deschenes I., Ficker E. Molecular determinants of pentamidine-induced hERG trafficking inhibition. Mol. Pharmacol. 2012;81(2):198–209. doi: 10.1124/mol.111.075135. [http://dx.doi.org/10.1124/mol.111.075135]. [PMID: 22046004]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai J.P., He X.W., Jiang Y., Chen F. Preparative separation and determination of matrine from the Chinese medicinal plant Sophora flavescens Ait by molecularly imprinted solid-phase extraction. Anal. Bioanal. Chem. 2003;375(2):264–269. doi: 10.1007/s00216-002-1675-2. [http://dx.doi.org/10.1007/s00216-002-1675-2]. [PMID: 12560971]. [DOI] [PubMed] [Google Scholar]
- 25.Kang J.E., Kim M.H., Lee J.A., et al. Histone deacetylase-1 represses transcription by interacting with zinc-fingers and interfering with the DNA binding activity of Sp1. Cell. Physiol. Biochem. 2005;16(1-3):23–30. doi: 10.1159/000087728. [http://dx.doi.org/10.1159/000087728]. [PMID: 16121030]. [DOI] [PubMed] [Google Scholar]
- 26.Mucha M., Ooi L., Linley J.E., et al. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J. Neurosci. 2010;30(40):13235–13245. doi: 10.1523/JNEUROSCI.1981-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.1981-10.2010]. [PMID: 20926649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q., Zhang Y., Sheng Y., et al. Large T-antigen up-regulates Kv4.3 K+ channels through Sp1, and Kv4.3 K+ channels contribute to cell apoptosis and necrosis through activation of calcium/calmodulin-dependent protein kinase II. Biochem. J. 2012;441(3):859–867. doi: 10.1042/BJ20111604. [http://dx.doi.org/10.1042/BJ20111604]. [PMID: 22023388]. [DOI] [PubMed] [Google Scholar]
- 28.Vij N., Zeitlin P.L. Regulation of the ClC-2 lung epithelial chloride channel by glycosylation of SP1. Am. J. Respir. Cell Mol. Biol. 2006;34(6):754–759. doi: 10.1165/rcmb.2005-0442OC. [http://dx.doi.org/10.1165/rcmb.2005-0442OC]. [PMID: 16456185]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simard J.M., Chen M., Tarasov K.V., et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006;12(4):433–440. doi: 10.1038/nm1390. [http://dx.doi.org/10.1038/nm1390]. [PMID: 16550187]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


