Highlights
-
•
This is a novel case of a 53 years old, nulliparous lady, who had been treated over a 10-year period for recurrent and symptomatic polypoid endometriosis of the pelvis. During this time, she underwent four surgical resections, the final one being a total pelvic exenteration, with histology demonstrating the presence of a rare myxoid low grade Endometrial Stromal Sarcoma (ESS) that had arisen in Polypoid Endometriosis (PE).
-
•
Polypoid endometriosis is a rare and uncommon variant of endometriosis that may present as a polypoid mass that simulates a malignant neoplasm. Typically, PE develops locally as a large benign pelvic mass. However, it may sometimes conceal a malignant disease in its context.
-
•
PE may involve different pelvic organs and can mimic a malignant mass in the pelvis. It mostly affects women in their peri- or post-menopausal age and it is not always related to hormonal therapy. Malignances, such as Endometrial Stromal Sarcoma in this case, can arise in the context of PE and their diagnosis can be challenging. Surgical excision may constitute radical multi-organ resection, particularly for recurrent and symptomatic cases. However, the recurrence rates after surgery can be significant.
-
•
Histopathologic findings for Polypoid Endometriosis and Endometrial Stromal Sarcoma are well described in this article (in literature there is great lack of this information).
Abbreviations: PE, polypoid endometriosis; ESS, endometrial stromal sarcoma; MRI, magnetic resonance imaging; MDT, multidisciplinary team
Keywords: Polypoid endometriosis, Endometrial stromal sarcoma, Pelvic mass, Total pelvic exenteration, Endometriosis, Surgery
Abstract
Background
Polypoid endometriosis (PE) is a rare and uncommon variant of endometriosis that may present as a polypoid mass that simulates a malignant neoplasm. Typically, PE develops locally as a large benign pelvic mass. However, it may sometimes conceal a malignant disease in its context.
Case presentation
A 53 years old, nulliparous lady, had been treated over a 10-year period for recurrent and symptomatic polypoid endometriosis of the pelvis. During this time, she underwent four surgical resections, the final one being a total pelvic exenteration, with histology demonstrating the presence of a rare myxoid low grade Endometrial Stromal Sarcoma (ESS) that had arisen in PE.
Conclusion
PE is a rare variant growth pattern of endometriosis which may involve different pelvic organs and can mimic a malignant mass in the pelvis. It mostly affects women in their peri- or post-menopausal age and it is not always related to hormonal therapy. Malignances, such as Endometrial Stromal Sarcoma in this case, can arise in the context of PE and their diagnosis can be challenging. Surgical excision may constitute radical multi-organ resection, particularly for recurrent and symptomatic cases. However, the recurrence rates after surgery can be significant.
1. Background
Polypoid endometriosis (PE) is a rare and uncommon variant of endometriosis that may present as a polypoid mass that simulates a malignant neoplasm. Usually PE develops locally as a large benign pelvic mass. However, it may sometimes conceal a malignant disease in its context.
We present the case of a 53-year old lady with a 10-year history of recurrent PE of the pelvis, from which eventually the presence of an Endometrial Stromal Sarcoma (ESS) was found to have arisen in polypoid endometriosis. ESS is a rare type of mesenchymal tumor of gynecological nature, generally starting from the uterus and rarely from other anatomical sites. The characterization of these lesions is often challanging in the preoperative setting, leading to a state of unclarity about the management.
A few cases of PE have been described in the literature. However, it still remains uncertain whether this entity can constitute a background for the onset of malignancies. We herein report this rare case with a brief review of the literature on this rare gynecological condition. The work has been reported in line with the SCARE criteria [1].
2. Case presentation
A 53 years old, nulliparous lady, had been treated at our institute over a 10-year period for recurrent polypoid endometriosis of the pelvis. During this time, she underwent four surgical resections, with the final one demonstrating the presence of a rare myxoid low grade Endometrial Stromal Sarcoma (ESS) that had arisen in polypoid endometriosis.
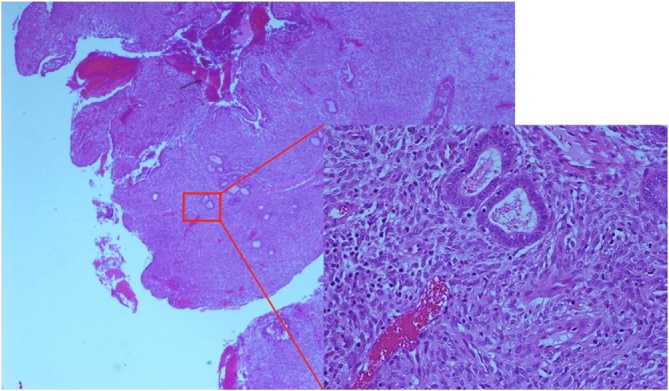
In April 2009, she was first diagnosed with a 10 × 5 cm sized soft tissue mass involving the left ovary, with the presence of ascites. CA125 was 476 U/mL. The other tumour markers CA19-9, CA15-3 and CEA were 209 U/mL, 34 U/mL and 1 UG/L, respectively. She was not using any hormonal therapy. As there was a strong suspicion for primary ovarian malignancy, she underwent laparotomy for excision of the mass. Intraoperatively, the friable mass was densely adherent to the presacral fascia and the posterior aspect of uterus, with smaller satellite lesions found on the pelvic peritoneum, base of the bladder, bowel serosa, mesentery and omentum. En bloc resection of the mass was undertaken, with total hysterectomy and bilateral salpingoophorectomy and omentectomy. The nodules were individually excised. Histopathological analysis revealed extensive PE (Fig. 1) with foci of simple and complex hyperplasia involving the surface of the uterus, ovaries, fallopian tubes, omentum and the mesentery of ileum, caecum and transverse colon. There was no malignancy present.
Fig. 1.
Polypoid Endometriosis, histopathologic sample.
Eight years later, a pelvic magnetic resonance imaging (MRI) was carried out due to recurrent symptoms of abdomino-pelvic pain. It revealed a large heterogeneous mass of 9.4 × 8.6 × 8.3 cm in the pelvis, causing left-sided hydroureteronephrosis due to ureteric compression at the pelvic inlet. The mass had a thickened wall with multiple irregular solid components showing significant enhancement. CA125 was 71 U/mL. At laparotomy, the recurrent mass was inseparable from the rectosigmoid junction. Hence en bloc resection of the mass was performed with anterior resection of the rectum and end colostomy. Histopathology showed endometriosis with a predominance of PE, with involvement of large bowel serosa and muscularis propria. No features of atypical endometriosis or malignancy was present.
A year later in 2018, surveillance MRI demonstrated a de novo cystic lesion of 4.7 × 2.4 cm, abutting the rectosigmoid stump, extending to left internal iliac vessels and in close proximity to the sciatic foramen. This appeared suspicious for malignant disease. Histological diagnosis was attempted by executing a trucut biopsy vaginally, which again revealed endometriosis without any signs of cellular atypia. Resection of the pelvic mass from the left pelvic side wall was performed, along with reversal of the end colostomy to re-establish bowel continuity and formation of a covering loop ileostomy. The histology of the mass was again found to be PE, and seven months later, her ileostomy was reversed as well.
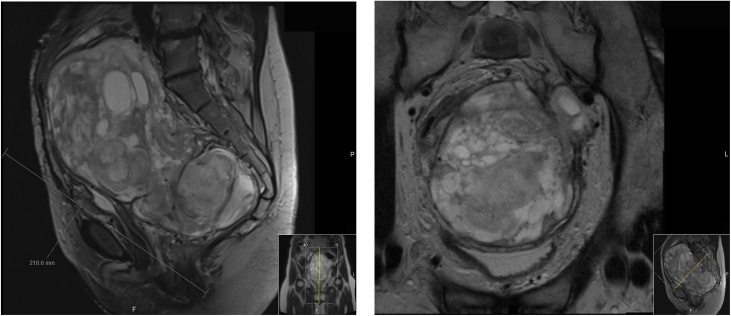
After having requested second opinions from other specialists in the field of complex endometriosis, the decision of the multidisciplinary team (MDT) was to continue a regular, close follow-up for which she also underwent a 3–6 monthly pelvic MRI between September 2018 and July 2019. Her symptoms of abdominal suprapubic discomfort and lower back pain recurred in June 2019, and MRI demonstrated regrowth of a soft tissue mass in the left pelvic side wall from 2.3 cm maximum diameter to 13.9 × 10.9 × 21.9 cm (Fig. 2). The left ureter was encased and occluded, with associated left hydroureteronephrosis. No distant metastasis was evident.
Fig. 2.
MRI abdomen and pelvis in July 2019 showing the large solid-cystic pelvic tumour (13,9 × 10,9 × 21,9 cm). The tumour occupied most of the pelvis: caudally from the ano-rectal junction with components extending to the left greater sciatic notch and intimately related to the left sciatic nerve, whereas cranially reaching up to the level of L4/L5, and hereby displacing the surrounding anatomy. It bordered around the following structures: anterior abdominal wall musculature, multiple loops of large- and small bowel, left iliacus muscle, common iliac vessels bilaterally, left external iliac vessels, left pelvic side wall fascia, presacral fascia, displaced urinary bladder, vaginal vault, left piriformis muscle, left sacro-spinous and sacro-tuberous ligaments and at least the left levator sling.
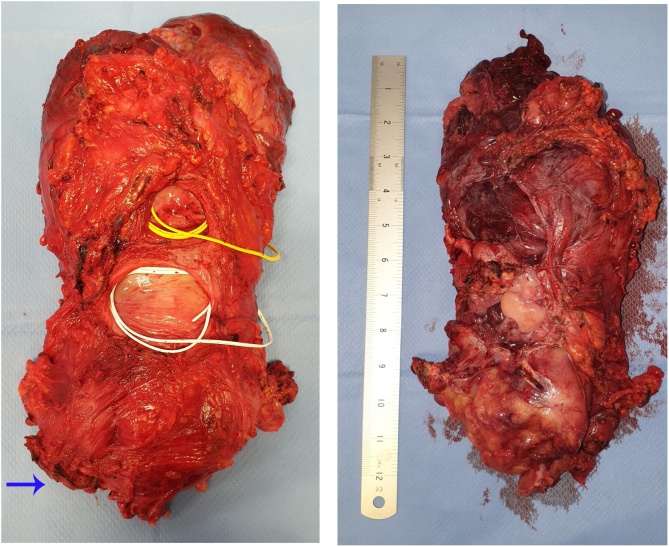
Following a new MDT discussion, a surgical removal of this mass was planned. The strong adherence of the mass to the surrounding structures necessitated a total pelvic exenteration with ligation of the left internal iliac artery, fashioning of a permanent end-colostomy and formation of an ileal conduit. An en bloc removal of the specimen was achieved (Fig. 3), which included the rectal stump, sigmoid, descending colon, top of vagina, bladder, distal ureters bilaterally, left pelvic side-wall with its fascia, left internal iliac artery, left pelvic wall fascia, part of obturator internus muscle, part of the left piriformis muscle and the top of the anal canal.
Fig. 3.
Specimen removed during the Total Pelvic Exenteration. On the right: upper face of the pelvic mass. On the left: lower face of the pelvic mass; it can be noted the rectum (blue arrow), the superior dome of the vagina after previous total histerectomy (white loop) and the bladder (yellow loop).
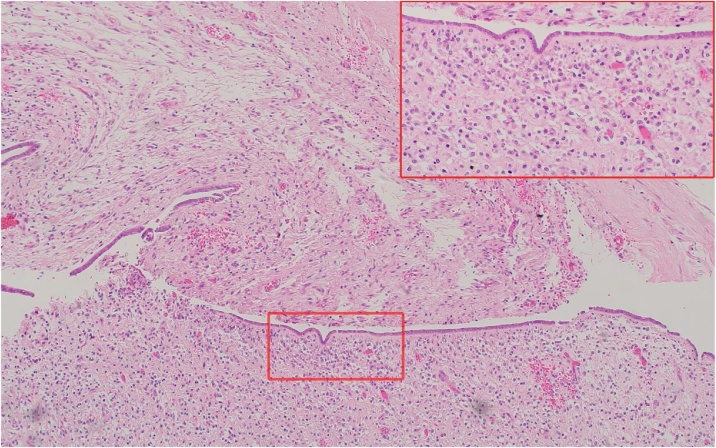
The histopathological analysis revealed this time a low-grade sarcoma that had arisen in endometriosis with myxoid mesenchymal proliferation (Fig. 4). Any subclassification was considered difficult as the differential diagnoses included a rare myxoid low grade Endometrial Stromal Sarcoma (ESS) and a Müllerian adenosarcoma. Despite some unusual features - given the resemblance to endometrial stromal cells and the positive immunoprofile for CD10, WT1, ER and PR - the findings were suggestive of a rare myxoid low grade ESS arising from PE. The positive expression for CD34 in this lesion was however, unusual as it is typically negative in low grade ESS.
Fig. 4.
myxoid low grade endometrial stromal sarcoma arosen from polypoid endometriosis.
Currently, the patient is asymptomatic and her 4th-month follow-up MRI and CT scan did not reveal any sign of recurrence.
3. Polypoid Endometriosis: a variant of endometriosis mimicking malignancy. Clinical and pathological aspects
PE is a distinct and rare variant growth pattern of endometriosis with histological features simulating those of an endometrial polyp that frequently forms a nodular polypoid mass, which sometimes can mimic a malignant tumour at presentation, with the possibility of recurrence after surgical excision [2]. Whilst usual endometriosis often affects women in their reproductive age, PE usually occurs in peri- or postmenopausal women and can affect different pelvic organs, such as rectosigmoid colon, adnexa, uterine serosa, cervix, vagina, ureters and omentum.
The etiology of PE is not entirely clear. Only one series has been reported [3] and although some case reports have suggested an association with estrogenic stimulation by unopposed exposure to hormonal treatment, such as Tamoxifen or (phyto)estrogen, this is not always the case [4], which was also reflected in the here described case as it continued to grow, even after the interruption of hormonal therapy. PE causes symptoms related to a pelvic mass with other non-specific symptoms such as dysmenorrhea, abdomino-pelvic pain and/or occasional vaginal bleeding. Some of the most common presentations consist of a pelvic mass, vaginal polypoid mass(es), vaginal spotting, abdominal pain, large bowel obstruction, haematuria with ureteric obstruction on imaging, adrenal mass, ovarian cyst and/or urinary urgency/frequency [3].
The management of PE can sometimes be a challenge as it can be mistaken for malignancy, both radiologically and histologically. Parker et al. [3] reported their clinicopathologic analysis of 24 cases of PE. 11/24 cases were referred due to problems in differential diagnosis of an adenosarcoma, low-grade adenocarcinoma and Sertoli cell tumour. The age of affected females in their series ranged from 23 years to 78 years, with 60 % of the patients being above 50 years age. The typical endometriosis was expressed in less than 5% of the post-menopausal females.
Imaging in the form of an MRI can help distinguish the diagnosis suggestive of PE by highlighting features such as of a T2 hyperintense polypoid mass with signal characteristics similar to endometrium, T2 hypointense peripheral rim, contrast enhancement pattern reflecting enhancement of the endometrium, and a lack of diffusion restriction [5].
The lesions can frequently involve multiple sites in the same patient, including serosal and mucosal surfaces. The rectosigmoid colon seems to be the most frequently involved [3]. Macroscopically, PE is very variable. However, its main feature is to grow as a polypoid mass with inconstant degrees of cystic and hemorrhagic aspects, growing on serosal surfaces, mucosal surfaces or both. Histopathologically, it consists of endometrial glands and inactive or proliferative stroma with a wide variety of architectural patterns of cytologic atypia. Metaplastic changes found can be tubal, mucinous, squamous or papillary syncitial [3]. Other histologic findings can consist of a stromal haemorrhage with haemosiderin laden macrophages, stromal decidualisation and/or focal periglandular cuffs with slightly increased cellularity. The two challanging differential diagnoses are of the Müllerian (mesodermal) adenosarcoma and the low-grade ESS with a glandular differentiation. Stromal papillae or broad polypoid fronds that project into glandular spaces and/or mild stromal atypia with periglandular cellular stromal cuffs are suspicious for adenosarcoma [6]. Mitoses can be low in adenosarcoma, but a high mitotic count would support malignancy [7]. The presence of glands, both in endometriosis and in PE, may mask the presence of ESS with glandular differentiation, even when the charachteristics of the latter are to have a tongue-like permeative pattern invasion and vascular invasion.
Synchronous endometriosis of usual type is often found in the PE samples. Malignant potential of PE is no different to typical endometriosis and hyperestrinism could cause the growth of malignancies on its background. Tumours that may arise are endometrioid carcinoma, clear cell carcinoma, seromucinous tumours and rarely a sarcoma [2]. However, PE as an individual entity is still typically associated with a benign course.
4. Conclusions
Polypoid Endometriosis (PE) is a rare variant growth pattern of endometriosis which may involve different pelvic organs and can mimic a malignant mass in the pelvis. It mostly affects women in their peri- or post-menopausal age and it is not always related to hormonal therapy. Malignances, such as Endometrial Stromal Sarcoma in this case, can arise in the context of PE and their diagnosis can be challanging. Surgical excision may constitute radical multi-organ resection, particularly for recurrent and symptomatic cases. However, the recurrence rates after surgery can be significant.
Declaration of Competing Interest
None of the authors have conflicts of interest to declare.
Sources of funding
Nothing to declare.
Ethical approval
This research was conducted following good ethical and scientific principles. Authors have no conflict of interests. Ethical committee approval was not required. Written informed consent was obtained from the patient for collecting and using personal information and images for research intent in anonymous form. No patient and authors details are included in the figures and in the manuscript.
Consent
Written informed consent was obtained from the patient for collecting and using personal informations and images for research intent in anonymous form.
Author contribution
-
-
Fabio Carbone: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing;
-
-
Manou Manpreet Kaur: manuscript review, data analysis and interpretation;
-
-
Aik Yong Chok: manuscript review, data analysis and interpretation;
-
-
Christos Kontovounisios: data analysis and interpretation, administrative support, final approval of manuscript;
-
-
Thomas Ind: surgeon involved in the care of the patient, final approval of the manuscript;
-
-
Shahnawaz Rasheed: surgeon involved in the care of the case, final approval of the manuscript.
Registration of research studies
Not required.
Guarantor
-
-
Fabio Carbone.
-
-
Aik Yong Chok.
-
-
Christos Kontovounisios.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
Thank to the histopathology department of the Royal Marsden Hospital, for providing the histopathologic pictures, and to The Royal Marsden School, for supporting the literature searches.
Contributor Information
Fabio Carbone, Email: fa.carbone87@gmail.com.
Manou Manpreet Kaur, Email: manou.m.kaur@gmail.com.
Aik Yong Chok, Email: chokaikyong@gmail.com.
Christos Kontovounisios, Email: c.kontovounisios@imperial.ac.uk.
Thomas Ind, Email: thomasind@me.com.
Shahnawaz Rasheed, Email: shahnawaz.rasheed@rmh.nhs.uk.
References
- 1.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A.J., Orgill D.P. The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60(December):132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 2.M. Mostoufizadeh, and RS-C obstetrics, 1980 undefined. Malignant tumors arising in endometriosis. journals.lww.com [Internet]. [cited 2019 Nov 24]; Available from: https://journals.lww.com/clinicalobgyn/Citation/1980/09000/Malignant_Tumors_Arising_in_Endometriosis.24.aspx. [PubMed]
- 3.R. Parker, F. Dadmanesh, … RY-TA journal, 2004 undefined. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. journals.lww.com [Internet]. [cited 2019 Nov 24]; Available from: https://journals.lww.com/ajsp/Fulltext/2004/03000/Papillary_Tumor_of_the_Pineal_Region.1.aspx. [DOI] [PubMed]
- 4.Kaushal S., Dadhwal V., Mathur S.R., Ray R., Durgapal P., Deka D. Multifocal polypoid endometriosis in a young woman simulating vaginal and pelvic neoplasm. J. Clin. Pathol. [Internet] 2010;63(5):452–454. doi: 10.1136/jcp.2009.073312. http://www.ncbi.nlm.nih.gov/pubmed/20299388 May 1 [cited 2019 Nov 25], Available from: [DOI] [PubMed] [Google Scholar]
- 5.Ghafoor S., Lakhman Y., Park K.J., Petkovska I. Polypoid endometriosis: a mimic of malignancy. Abdom. Radiol. [Internet] 2019 doi: 10.1007/s00261-019-02143-8. http://www.ncbi.nlm.nih.gov/pubmed/31372776 Aug 1 [cited 2019 Nov 24]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eichhorn J.H., Young R.H., Clement P.B., Scully R.E. Mesodermal (müllerian) adenosarcoma of the ovary: a clinicopathologic analysis of 40 cases and a review of the literature. Am. J. Surg. Pathol. 2002;26(October (10)):1243–1258. doi: 10.1097/00000478-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 7.J. Eichhorn, R. Young, … PC-TA journal of, 2002 undefined. Mesodermal (müllerian) adenosarcoma of the ovary: a clinicopathologic analysis of 40 cases and a review of the literature. journals.lww.com [Internet]. [cited 2019 Nov 24]; Available from: https://journals.lww.com/ajsp/Fulltext/2002/10000/Pancreatic_Intraepithelial_Neoplasia__A_New.1.aspx. [DOI] [PubMed]