Abstract
Background
Clostridioides difficile infection (CDI) is a hospital acquired disease associated with significant morbidity, hospitalisation and mortality. Almost 30% of treated patients experience at least one recurrence after treatment of their first episode. Treatment of recurrent CDI (rCDI) utilises vancomycin or fidaxomicin, however, a newer treatment option is faecal microbial transplantation (FMT) administered by nasogastric tube (NGT) or colonoscopy. It is associated with higher cure and lower recurrence rates than fidaxomicin or vancomycin. The aim of this analysis is to evaluate the cost effectiveness of FMT for rCDI using the latest and best evidence.
Method
A cost utility analysis was conducted using a decision model representing the cost per additional Quality Adjusted Life Year (QALY) from a National Health Service (NHS) perspective. A Markov model was constructed to compare FMT NGT and colonoscopy to antibiotic treatment (fidaxomicin or vancomycin). The model was informed by a literature review of clinical evidence, specifically focussing on hospitalised patients with rCDI over 65 years. Both deterministic and probabilistic sensitivity analyses were performed to assess uncertainties around the model inputs and assumptions.
Findings
The base case analysis showed that FMT is a less costly and more effective treatment than either fidaxomicin or vancomycin. FMT colonoscopy was slightly more effective than FMT NGT leading to an additional 0.012 QALYs but more expensive and the incremental cost effectiveness ratio (ICER) was £242,514/QALY. The Probabilistic sensitivity analysis based on 10,000 simulations suggested the probability of FMT NGT being cost effective was almost 78% at £20,000/QALY Willingness–To-Pay (WTP) threshold.
Interpretation
FMT is both more effective and less costly option than antimicrobial therapy. FMT NGT was the preferred route of administration and is likely to be considered the most cost-effective strategy by decision makers given current acceptable thresholds.
Keywords: Clostridioides difficile infection (previously asclostridium difficile infection), Recurrence, Economic evaluation, Cost-effectiveness analysis, Cost utility analysis, Faecal microbiota transplantation, Fidaxomicin, Vancomycin
1. Introduction
Clostridioides difficile infection (CDI) is the leading cause of antibiotic associated gastrointestinal disease causing significant morbidity and mortality [1,2]. The risk factors associated with developing CDI include the excessive use of antibiotics, advanced age, other comorbidities and prolonged hospitalisation [2,3].
Approximately 30% of individuals with CDI will experience either a recurrence or will fail to respond to initial treatment.[1] It is recurrent CDI (rCDI) that is both the most dangerous and costly [4], [5], [6] with the majority of the costs associated with the required hospital admission. [7,8] The available evidence suggests that rCDI is associated with a substantial risk of death within 6 months of the initial treatment [9].
Standard treatment for rCDI relies on using antibiotics such as fidaxomicin or vancomycin whilst Faecal Microbial Transplantation (FMT) is advocated as an effective alternative to antibiotic treatment for rCDI [2]. To perform FMT, healthy individuals who have been screened for a wide range of potentially transmittable conditions, according to national guidelines, provide faeces for processing [1] [10]. Faeces are then emulsified with a cryoprotectant, filtered and dispensed into aliquots and frozen at −80 °C. [1,10] A number of routes of administration are available: nasogastric tube (NGT), colonoscopy, enema or oral capsules, the first two being the most commonly used [1,11].
The National Institute for Health and Care Excellence (NICE) guidance supports the use of FMT for rCDI where patients fail to respond to antibiotics [12]. Furthermore, the current guidance suggests the use of vancomycin and fidaxomicin for the antibiotic treatment for rCDI [13,14]. Previous economic analyses comparing treatment with FMT and antibiotics have shown the potential cost-effectiveness of FMT in treating rCDI [15], [16], [17], [18].
However, the previous economic studies used evidence based on effectiveness studies conducted on patients with their first or second episode of CDI [15], [16], [17], [18]. The previous studies demonstrated that fidaxomicin is more efficacious with higher cure rates and less recurrences when compared with the other treatment options [5,19]. This is now in conflict with new evidence from a randomised controlled trial (RCT) and a network meta-analysis of RCTs, which shows that FMT has a higher cure rate and lower recurrence rate than either fidaxomicin or vancomycin for rCDI [20,21], and also suggests that fidaxomicin was not superior to vancomycin in treating rCDI [22]. Thus a new analysis using this latest evidence is required.
The objective of the current study is to explore the relative cost-effectiveness of FMT for rCDI compared to the existing alternative treatments with antibiotics using the latest and best available evidence. We compare the relative cost-effectiveness of delivering FMT to patients aged 65 years and above by the two most common routes of administration (NGT or colonoscopy) and the two most frequently used antibiotics (fidaxomicin and vancomycin).
2. Methods
A cost utility analysis was undertaken to compare the four treatment options for rCDI. A model-based approach was deemed to be an appropriate to provide a framework where data relating to treatments effects, unit costs, resource use and health related quality of life weights are synthesised and used to identify the optimal intervention under conditions of uncertainty [23,24]. The data used to inform the model are based on a pragmatic review and supplemented with expert opinion from within the study team. The details of the review are presented in the supplementary material, table S1 & 2. The analysis took the perspective of the UK National Health Service (NHS), meaning that only direct medical costs related to the treatment, cost of administration and CDI related hospitalisation were included.
2.1. Model structure
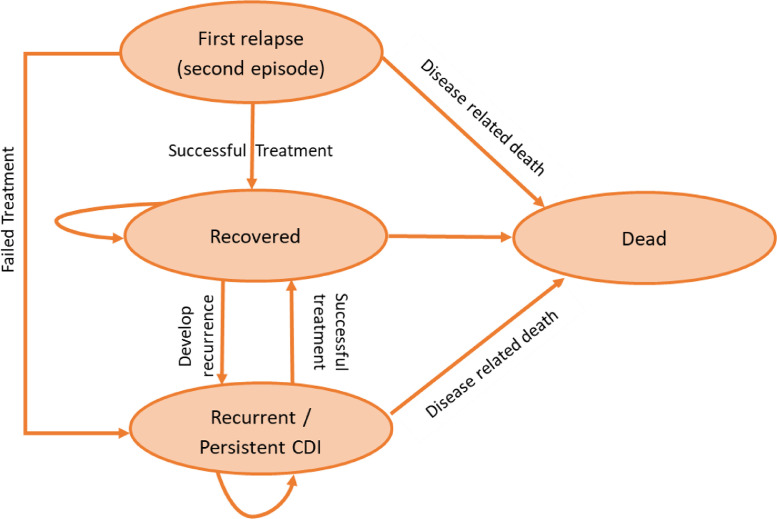
A Markov model was developed using TreeAge Pro 2019 R1.1 (TreeAge Software, Williamstown, Massachusetts, USA) to compare the treatment options on a hypothetical cohort of hospitalised patients over 65 years who had at least one CDI recurrence. People in this age group are more vulnerable for rCDI causing significant morbidity and mortality [3]. The model was based, in part, on previously published decision models assessing the cost effectiveness of FMT, fidaxomicin and vancomycin [16,18]. The model was constructed to ensure that it was consistent with the natural history of the disease, practical in a local context and capable of generating relevant outputs. In the model, patients are assumed to exist in one of four possible health states: relapsed, recovered, recurrent CDI and dead (Fig. 1). The cycle length is two months and reflects the duration of treatment and time to recurrence [2,4,6]. Response to treatment was defined as a resolution of CDI symptoms, whereas, treatment failure is the inability to resolve a CDI episode after the treatment has finished. Patients were simulated over a one year time horizon.
Fig. 1.
Markov transition state model for recurrent Clostridioides difficile infection.
In the model, following NICE guidance, patients with rCDI are assumed to be treated with one of the following treatment strategies, FMT via NGT, FMT via colonoscopy, oral fidaxomicin or oral vancomycin [12,13]. According to NICE, FMT administration via NGT and colonoscopy are the most commonly used procedures and this is supported by the British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) recommendations [10]. Those who respond to treatment move within the model to the recovered health state. However, after recovery there is still a chance of recurrence up to 2 months later. According to local practice in Birmingham, if the patient shows no response or develops recurrence after apparent recovery, the patient is assumed to receive treatment with vancomycin Taper Pulsed (TP) therapy. At any point, death as a result of CDI symptoms may occur if the patient shows no sign of response to treatment. For each treatment arm, patients who do not improve after completing the treatment will move to the recurrent health state where they remain on vancomycin TP until the model terminates. Figure S1 in the suppelemtary material summarises the patient pathway.
2.2. Model parameters / variables
All input parameters including transitions probabilities, costs and utilities with the corresponding distributions and sources are listed in Table 1a and 1b.
Table 1a.
Model input parameters – Probabilities and Health utilities.
| Base case* | Range | Distributionβ | Sources | |
|---|---|---|---|---|
| Effectiveness data | ||||
| FMT-NGT | ||||
| Response rate – single infusion | 0.81 | 0.73–0.88 | Beta (422, 331) | [6,20,25,33] |
| Response rate- multiple infusion | 0.88 | 0.82–0.94 | Beta (4,8) | [6,25,33] |
| Recurrence rate | 0.12 | 0.06–0.30 | Beta (4, 32) | [15,16,20] |
| FMT - colonoscopy | ||||
| Response rate - single infusion | 0.87 | 0.79–0.94 | Beta (597, 432) | [20,25,33,50] |
| Response rate- multiple infusion | 0.95 | 0.92–0.97 | Beta (25,30) | [25,33,50] |
| Recurrence rate | 0.09 | 0.06–0.30 | Beta (3, 26) | [15,16,51] |
| Fidaxomicin | ||||
| Response rate | 0.42 | 0.22–0.88 | Beta (7,14) | [20,22] |
| Recurrence rate | 0.15 | 0.12–0.50 | Beta (3,18) | [5,19,22] |
| Vancomycin | ||||
| Response rate | 0.31 | 0.19–0.75 | Beta (6,16) | [6,19,20,50] |
| Recurrence rate | 0.31 | 0.21–0.70 | Beta (12,23) | [6,19,22,52] |
| Vancomycin TP | ||||
| Response rate | 0.58 | 0.40–0.81 | Beta (25,30) | [49,53] |
| Recurrence rate | 0.31 | 0.10–0.40 | Beta (25,30) | [52,53] |
| Mortality | ||||
| CDI related mortality | 0.18 | 0.15–0.30 | Beta (9, 87) | [9,27] |
| Other cause mortality – 65 years | 0.002 | - | Fixed | [26] |
| Utilities- QALYs | ||||
| Cured - 65 years | 0.78 | 0.70–1.00 | Beta (21,6) | [28] |
| Relapsed CDI | 0.42 | 0.35–0.81 | Beta (77,107) | [8] |
| rCDI | 0.42 | 0.35–0.81 | Beta (77,107) | [8] |
| Dead | 0 | 0 | 0 |
Abbreviations: FMT, Feacal Microbiota Transplant; NGT, Nasogastric Tube; TP, Taper Pulsed; QALYs, Quality Adjusted Life Years; CDI, Clostridioids Difficile infection; rCDI, recurrent Clostridioids Difficile infection.
β Probabilistic sensitivity analysis, alpha and beta values for Beta distribution.
Table 1b.
Model input parameters – Hospital stay and Costs (£, UK 2018).
| Base case | Range* | Distributionγ | Sources | |
|---|---|---|---|---|
| Cost of FMT - NGT | £740.16 | £592-£888 | Gamma (100,0.13) | Table 2 |
| Cost of FMT – Colposcopy | £3006.17 | £2405-£3607 | Gamma (100,0.03) | Table 2 |
| Cost of oral Vancomycin 250 mga | £200.11 | £160-£240 | Gamma (100,0.48) | [29] |
| Cost of oral Vancomycin 500 mgb | £400.23 | £320-£480 | Gamma (100,0.24) | [29] |
| Cost of oral Vancomycin TPc | £297.60 | £238-£358 | Gamma (100,0.34) | [29] |
| Cost of Fidaxomicin d | £1350.00 | £1080-£1620 | Gamma (100,0.07) | [29] |
| Cost of hospital stay per day e | £404 | £323-£485 | Gamma (100,0.25) | [32] |
| Hospital stay – FMT (days) | 5 | 2–20 | Fixed | [10] |
| Hospital stay – Antibiotics (days) | 10 | 6–27 | Fixed | [10] |
Abbreviations: FMT, Feacal Microbiota Transplant; NGT, Nasogastric Tube, TP, Taper Pulsed.
Probabilistic sensitivity analysis, alpha and lambda values for Gamma distribution.
Cost of vancomycin 250 mg, 4 times a day for 10 days.
Cost of Vancomycin 500 mg, 4 times a day for 10 days.
Cost of Vancomycin pulse taper for 6 weeks starting with 250 mg.
Cost of Fidaxomicin 200 mg for 10 days.
Inpatient, specialist palliative care (adults only), average cost per bed day.
Cost range was based on assumption - varying the base value by +/−20%.
2.3. Effectiveness data
The rates of response to treatment and recurrence were informed by the available evidence from the pragmatic review of the literature which identified studies specifically focused on patients with rCDI (Available as supplementary data). The FMT response rates for colonoscopy and NGT were obtained from a recent systematic review and meta-analysis study which included 7 randomised controlled trials and thirty case series [25]. Data on recurrence rate for FMT NGT and colonoscopy were extracted from previous economic evaluations. [15,16] Both the response and recurrence rates for fidaxomicin and vancomycin were based on the results of a recently published RCT and observational study [20,22]. All rates were converted into 2 months probabilities using the rate – probability conversion equation that can be found in the supplementary material.
The probability of other cause mortality, excluding CDI related mortality, was obtained from a lifetable published by the office for national statistics [26]. An estimate for the rCDI associated mortality was derived from two studies specific on hospitalised patients with rCDI [9,27].
Utility values for patients with rCDI were derived from a non-interventional study conducted in the UK for inpatients with rCDI [8]. In that study, eligible patients completed the EuroQol 5 Dimensions, 3 levels (EQ-5D-3 L) questionnaire within 5 days of developing symptoms and the mean EQ-5D index score was reported. Utility values for patients in the cured state were derived from the EQ-5D index value population norms for 65–74 years adults [28]. All values were converted to QALY weights for each health state.
2.4. Resource use and cost
The British National Formulary and National Health System reference costs were used as references for drug acquisition and procedure prices respectively [29,30]. An average length of stay of 5 days was obtained from the BSG and HIS guidelines using the duration of treatment to achieve CDI resolution as a proxy. [10] For fidaxomycin and vancomycin, 10 days was used to represent the number of days required to be hospitalised [10,31]. The cost of the hospital stay assumes that all patients are treated as inpatient, specialist palliative care in the UK without critical care [32].
A micro-costing approach was taken to estimate the cost of the FMT administration route per patient by valuing the resources required for preparing FMT material, its administration and follows up, multiplied by respective unit costs obtained from routine sources. Following the joint BSG and HIS guidelines [10], 3 units of 50 ml FMT are required for the colonoscopy route and 1 unit for the NGT administration. The colonoscopy procedure is performed by a gastroenterologist, while a trained nurse can insert the NG tube. Details on cost components and the estimated cost of administering FMT colonoscopy and NGT are presented in Table 2. All costs were reported in 2018 GBP and values obtained from previous years were inflated to 2018 prices [32].
Table 2.
Cost of FMT according to the route of administration (UK £, 2018).
| Procedure | Resource use | Unit costγ | Total cost | Sources | ||||
|---|---|---|---|---|---|---|---|---|
| FMT- colonoscopy | ||||||||
| FMT material ± | 3 unit (150 ml)§ | £650.00 | £1950.00 | BSG & HIS guidelines [10] | ||||
| FMT administration | ||||||||
| Colonoscopy | 1 unit | £947.00 | £947.00 | NHS reference costs [30] | ||||
| Loperamide 2 mg for FMT retention | 1 tablet | £0.10 | £0.10 | BNF [29] | ||||
| Staff cost (gastroenterologist) | 1/2 h | £108.00 | £54.00 | PSSRU 2018 [32] | ||||
| Recovery time | 2 hrs nurse | £27.53 | £55.07 | Expert opinion | ||||
| Total cost | £3006.17 | |||||||
| FMT nasogastric tube (NGT) | ||||||||
| FMT material± | 1 unit (50 ml)§ | £650.00 | £650.00 | BSG & HIS guidelines [10] | ||||
| FMT administration | ||||||||
| Omeprazole 20 mg / 2 h prior procedure | 1 tablet¥ | £0.03 | £0.03 | BNF [29] | ||||
| Domperidone/ 2 h prior procedure | 1 tablet¥ | £0.03 | £0.03 | BNF [29] | ||||
| NG tube | 1 unit | £7.86* | £7.86 | ASGE [54] | ||||
| Staff cost (HCP) to place the tube | 12 min¥ | £27.53 | £5.51 | PSSRU 2018 [32] | ||||
| X ray | 1 unit | £55.67 | £57.06 | NHS reference cost [30] | ||||
| Recovery time | 1 hrs nurse | £27.53 | £27.53 | Expert opinion | ||||
| Total cost | £740.16 | |||||||
† FMT, Faecal Microbiota Transplant; BNF, British National formulary; PSSRU, Personal Social Service Research Unit; ASGE, American Society for Gastrointestinal Endoscopy; HCP, Health Care Professional.
- Costs of additional tests and follow up were not considered as they are the same for both.
Each unit price (obtained from Queen Elizabeth Hospital - Birmingham) represents the cost of donor selection and testing, preparation and storage prior administration.
Inflated to 2018 costs using the UK Hospital and Community Health Services pay and prices index.
Number of FMT units required for each procedure were based on expert opinion.
Data on resource use were obtained from a published study.
An informal price was provided from expert opinion at University Of Birmingham which was within the same range as the price presented by the ASGE Technology Committee when appropriately converted to UK currency and inflated to 2018 price year.
To conduct the analysis, pragmatic conservative assumptions were required and were informed by the literature and expert opinion. Those assumptions are listed in table 3.
Table 3.
Model Assumptions relating to model structure, effectiveness and resource use.
| • Patients failed to respond to treatment options will move to the recurrent health state. |
| • Patients across all treatment arms, except for fidaxomicin, who fail the initial treatment, will receive an additional dose of the same initial treatment. |
| • Vancomycin TP was given as a treatment if the patient develops a recurrence or failed to respond to the second dose of treatment. This assumption is based on local practice and because there is some evidence to suggest the response rate for Vancomycin TP is higher [49]. |
| • A recurrent episode is assumed to be instigated by the same bacterial strain and not reinfection by a different strain. |
| • Constant response and recurrence rates for the same treatment option throughout the model regardless of the number of previous relapses. |
| • Utility values for single first recurrence and multiple recurrences are the same. |
| • Patients on either of the FMT treatments are assumed to spend 5 days in the hospital and 10 days if treated with antibiotics, reflecting the time needed to finish the treatment [10]. |
| • Patients will remain hospitalised for a defined number of days for each treatment option even if they recovered before the end of the defined period. |
| • Patients receiving vancomycin TP were discharged to continue treatment at home after a 10 day hospitalization period, as expert opinion believes that 10 days is enough to evaluate the patient's response to treatment. |
| • Cost of tests and follow up were not included in the final cost as it is considered to be the same for all treatment options. |
2.5. Model analysis
The outcome of the base case analysis is reported in terms of an incremental cost effectiveness ratio (ICER) presented in terms of cost per QALY. This is based on the mean difference in costs and QALYs for FMT NGT compared to the other treatment options. Discounting was not carried out as the model does not exceed 1 year. A half-cycle correction was used for initial values of health related outcomes. One-time costs incurred at the beginning of the cycle, such as the costs of antibiotic treatments, FMT treatment options and hospital stay, were not subject to half-cycle correction. An additional and separately reported base case analysis was conducted using evidence from an alternative meta-analysis [33].
To assess the uncertainty of the model variables, assumptions, and their impact on the model results, deterministic and probabilistic sensitivity analyses (PSA) were performed [24]. In the deterministic analysis, key parameters were varied individually to observe the impact on the model outcome such as cost of treatments, hospital stay, antibiotics response rates and CDI related mortality. Different scenarios were explored to include (i) the response to treatment and recurrence rates for FMT colonoscopy and NGT were a assumed to be the same, (ii) length of hospital stay for FMT options were changed from 5 to 10 days (iii) similar recurrence rates for all treatment options (iv) constant efficacy for the first and second dose of FMT (v) the cost of all treatment options was varied using the parameter range presented in Table 1. Further sensitivity analysis was carried out to explore other potential assumption relating to the lower CDI related mortality associated with FMT [34,35].
Wherever possible, 95% confidence or credible intervals should be used to inform the ranges used in sensitivity analysis. However, such intervals are not available for the cost parameters in this model because they are based on secondary data which did not contain any measure of uncertainty. Thus we assumed plus or minus 20% to accommodate this.
For the PSA, distributions were assigned to the model parameters as presented in Table 1. About 10,000 Monte Carlo simulations were generated from fitted distributions to determine mean cost and effectiveness values to estimate the probability of the intervention being cost-effective using the NICE threshold of 20,000/QALY and plotted as a Cost Effectiveness Acceptability Curve (CEAC) [36]. Beta distributions were used to characterize the uncertainty of the probabilities and utility values, whereas gamma distributions were applied to total costs [24].
3. Results
3.1. Base case analysis
The results of the base case analysis are presented in Table 4. The model results show that FMT NGT strategy is the least costly strategy of all those compared in the model and has a mean cost of almost £8877 per patient with a total QALY of 0.645 per patient. It is followed by FMT colonoscopy which is the next least costly option at £11,716 per patient. However, FMT colonoscopy was also slightly more effective with an additional 0.012 QALY compared to FMT NGT. Thus, the ICER for FMT colonoscopy compared to FMT NGT is estimated at approximately £242,514 per QALY gained.
Table 4.
Base case results for treatment options relative to FMT NGT for rCDI.
| Treatment option | Expected cost per patient (UK £ 2018) | Difference in Costs (UK £ 2018) | QALYs | Difference in QALYs | ICER b |
|---|---|---|---|---|---|
| FMT – NGT a | 8877 | - | 0.645 | - | - |
| FMT – colonoscopy | 11,716 | +2839 | 0.657 | +0.012 | 242,514 |
| Fidaxomicin | 14,399 | +5521 | 0.577 | −0.068 | Dominated |
| Vancomycin | 17,279 | +8402 | 0.513 | −0.132 | Dominated |
Abbreviations: FMT, Faecal Microbiota Transplantation; NGT, Nasogastric Tube; ICER, Incremental Cost Effectiveness Ratio; QALYs, Quality Adjusted Life Years.
This represents the least costly option and the baseline with which subsequent options are compared.
Incremental cost effectiveness ratio expressed as the additional cost per additional QALY.
The strategy of treatment with vancomycin is the most costly with an expected cost of £17,279 per patient. It is also the least effective with the lowest expected effect of 0.513 QALY. Thus, the strategy of vancomycin is said to be dominated by other treatment options as it is more costly and less effective than all other strategies. Fidaxomicin is also dominated by both FMT NGT and FMT colonoscopy as it is more costly and less effective with a mean QALY of 0.577 at the end of the time horizon. The results obtained from the analysis based on an alternative review that was identified made no material difference to the overall results. These data are presented in supplementary file table S3.
3.2. Sensitivity analysis
The deterministic sensitivity analysis revealed that the model was not sensitive to the cost of different treatments, cost of hospital stay, the response rates for the antibiotics and mortality associated with CDI. However, the analysis showed that FMT NGT was more effective and less costly than colonoscopy if the efficacy of FMT NGT was similar to FMT colonoscopy. The length of hospital stay for FMT interventions was tested using the same duration for antibiotic treatment and the results suggested that FMT NGT and colonoscopy are still less costly than antibiotic treatment. The results of the deterministic sensitivity analysis using different values for selected model parameters are presented in Table 5.
Table 5.
Summary of sensitivity analysis results relative to FMT- NGT.
| Treatment option | Expected Value | Difference | ICER | ||
|---|---|---|---|---|---|
| Costs £ | QALYs | Cost £ | QALYs | £/ QALYs | |
| Scenario 1: Similar efficacy for FMT colonoscopy and NGT | |||||
| FMT – NGT | 8406 | 0.663 | – | – | – |
| FMT – Colonoscopy | 11,716 | 0.657 | 3311 | −0.007 | Dominated |
| Fidaxomicin | 14,399 | 0.577 | 5993 | −0.086 | Dominated |
| Vancomycin | 17,279 | 0.513 | 8873 | −0.151 | Dominated |
| Scenario 2: Recurrence rate=0.2 for all treatment options | |||||
| FMT – NGT | 10,706 | 0.615 | – | – | – |
| FMT – Colonoscopy | 14,779 | 0.600 | 4073 | −0.015 | Dominated |
| Fidaxomicin | 15,389 | 0.561 | 4683 | −0.053 | Dominated |
| Vancomycin | 16,635 | 0.520 | 5929 | −0.094 | Dominated |
| Scenario 3: constant efficacy for FMT options for the 1st and 2nd dose | |||||
| FMT – NGT | 8281 | 0.671 | – | – | – |
| FMT – Colonoscopy | 11,402 | 0.670 | 3121 | −0.001 | Dominated |
| Fidaxomicin | 14,395 | 0.577 | 6117 | −0.094 | Dominated |
| Vancomycin | 17,279 | 0.513 | 8998 | −0.159 | Dominated |
| Scenario 4: 10 days hospital stay for FMT | |||||
| FMT – NGT | 11,786 | 0.645 | – | – | – |
| Fidaxomicin | 14,399 | 0.577 | 2612 | −0.068 | Dominated |
| FMT – Colonoscopy | 14,585 | 0.657 | 2799 | +0.012 | 239,063 |
| Vancomycin | 17,279 | 0.513 | 5493 | −0.132 | Dominated |
| Scenario 5: 6 weeks hospital stay for vancomycin TP | |||||
| FMT – NGT | 23,498 | 0.645 | – | – | – |
| FMT – Colonoscopy | 25,404 | 0.657 | 1906 | +0.012 | 162,801 |
| Fidaxomicin | 41,325 | 0.577 | 17,827 | −0.068 | Dominated |
| Vancomycin | 46,564 | 0.513 | 23,066 | −0.132 | Dominated |
| Scenario 6: CDI related Mortality for FMT = 12% | |||||
| FMT – NGT | 9034 | 0.653 | – | ||
| FMT – colonoscopy | 11,843 | 0.662 | 2809 | +0.009 | 301,022 |
| Fidaxomicin | 14,399 | 0.577 | 5364 | −0.076 | Dominated |
| Vancomycin | 17,279 | 0.513 | 8245 | −0.140 | Dominated |
Abbreviations: ICER, Incremental cost effectiveness ratio; QALYs, Quality Adjusted Life Years; FMT, Faecal Microbiota Tube; NGT, Nasogastric Tube.
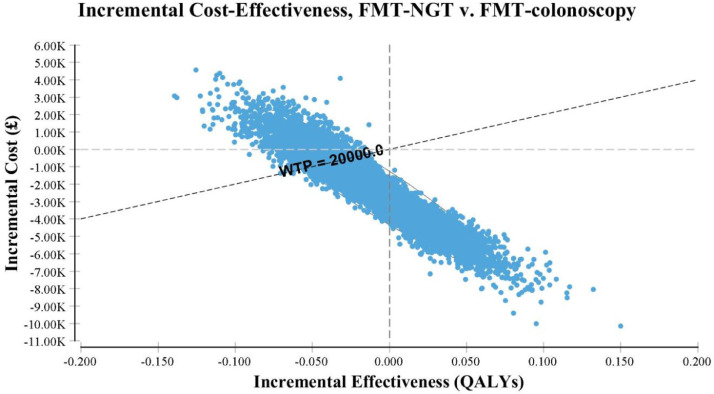
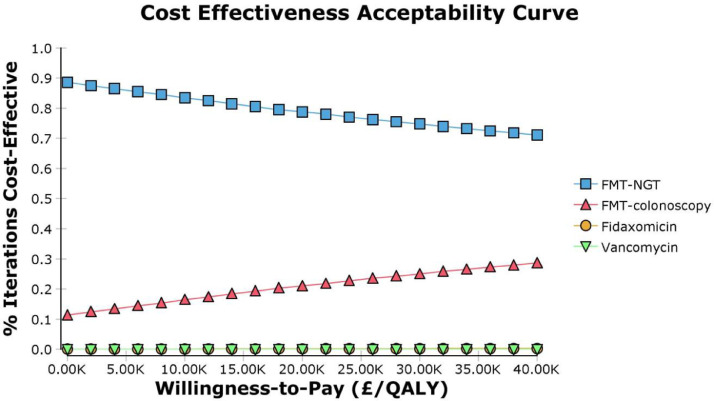
The results from the PSA in Fig. 2 showed that 34% of the 10,000 simulations were grouped in the south-east quadrant where FMT NGT would be considered as more effective and less costly than colonoscopy. The CEAC in Fig. 3 shows that FMT NGT has a 78% probability of being cost-effective compared to FMT colonoscopy at the NICE threshold of £20,000 [36] which is recommended by NICE as a threshold at which a treatment can be taken to be cost-effective.
Fig. 2.
Scatterplot of the incremental cost effectiveness of FMT NGT compared with FMT colonoscopy
†FMT refers to faecal microbiota transplant, NGT to nasogastric tube, QALY to Quality adjusted life year and WTP is willingness to pay.
Fig. 3.
Cost effectiveness acceptability curve
†FMT refers to faecal microbiota transplant, NGT to nasogastric tube, QALY to Quality adjusted life year and WTP is willingness to pay.
4. Discussion
The model based economic evaluation carried out in this paper explores the relative cost effectiveness of four different treatment options recommended for hospitalised patient with rCDI. The results of the base case analysis show that FMT, regardless of route of delivery, is less costly and more effective as a treatment strategy than either fidaxomicin or vancomycin. Consequently, both antibiotic therapies are considered to be dominated by the two FMT strategies. The strategy of FMT NGT is the less costly of the two FMT strategies, but FMT colonoscopy is the most effective option. The incremental cost effectiveness ratio for FMT colonoscopy versus FMT NGT was estimated to be £242,514 per QALY gained and as such does not fall within the cost-effectiveness acceptability threshold used by NICE. In contrast, FMT NGT, which was shown to be the second most effective treatment option and the cheapest overall, is likely to be supported on cost-effectiveness grounds compared to the other three treatment strategies being compared in this analysis as it dominates both the antibiotic strategies. It is also supported by the results of the PSA shown in the cost effectiveness plane; furthermore the CEAC shows that FMT NGT has a 78% probability of being cost-effective at the £20,000 NICE cut off.
To our knowledge, this study is the first to estimate the cost-effectiveness of alternative options of FMT versus antibiotics for rCDI based on the new evidence that shows FMT has a higher cure rate and lower recurrence rate than either fidaxomicin or vancomycin for rCDI [20,21]. A strength of the analysis is that the majority of model parameters were derived from the most recent randomised controlled trials, meta-analysis and observational cohort studies on patients with rCDI. Moreover, the model used quality of life data from a primary study of patients who were experiencing rCDI [8]. Both the deterministic sensitivity analysis and the PSA explored the uncertainty in the model and the results were robust to the variations. The purpose of this economic evaluation was to capture the economic benefit of using FMT and it was assumed that there would be a reduction in the length of hospital stay. These assumptions have been supported by a novel prospective cohort study which recently reported that patients treated with FMT had fewer hospitalisations compared with antibiotics [35].
This model has several limitations relating to the limited evidences. The model inputs rely on studies with relatively short-term follow-up and so outcomes beyond the time horizon considered by the model are, as yet, unknown. The potential side effects associated with the different treatment options were not included in the model although these are considered to be minimal and likely similar for all treatment options [20] and may not affect the final outcome. A study by Wang et al. demonstrated that upper gastrointestinal route of FMT is associated with only 2% of serious side effects rate compared 6% for lower gastrointestinal route [38].
The severity of CDI and the number of previous recurrences were not considered in this analysis despite the fact that they are both associated with variation in the resource use and health outcome [8]. However, this omission is not anticipated to affect the relative cost-effectiveness of the different strategies although the importance of previous severity in recurrences and its impact on the effectiveness of treatment is not known. Additionally, CDI related morality was similar amongst all treatment options despite the recent studies showing that mortality rates with FMT are relatively lower than antibiotic treatment [34], [35]. The results of the sensitivity analysis have shown that this assumption has minimal effect on the base case result.
The model focussed on rCDI and did not include other comorbidities. Such comorbidities include cancer, dysphagia, renal diseases and irritable bowel diseases. Patients in the age group, of those considered in this model, are usually in receipt of other medication such as immunosuppressive drugs or other medications for chronic diseases that would impact on the chosen treatment option for rCDI [3].
In the model, a recurrent episode is assumed to be caused by the same bacterial strain and not reinfection by a different strain. This is a limiting assumption but required for the modelling because in reality, CDI recurring a year after the initial diagnosis or developing three consecutive recurrences is more likely to be due to new infection by a different C difficile strain [42]. This could have underestimated the actual effectiveness of the treatment options if CDI is caused by a different C. difficile strain. Furthermore, patients with C. difficile caused by ribotype 027 are less responsive to treatment with fidaxomicin or vancomycin [39]. However, FMT may be superior to antibiotics for both ribotype 027 and 002 [40], [41] thus the dominance of FMT will be further supported. The model did not consider those differences due to the lack of evidence from head to head comparisons for each type of ribotype and treatment options. Additionally the effectiveness of antibiotics in the model is constant regardless of the number of previous recurrences or failure to respond. However, in practice, antibiotic efficacy decreases after first exposure [6]. Similarly the risk of recurrence will increase depending on the number of previous recurrences [5]. Although this is less evident with FMT [25]. Additionally, the model assumes that vancomycin TP is more effective than vancomycin, however, this assumption is potentially questionable as evidence suggest that the vancomycin taper regimen was not superior to standard vancomycin treatment [43]. Although given the result of the model this assumption bears no impact on the results.
The method of producing and administering FMT is not yet standardized, although the Medicines and Healthcare products Regulatory Agency (MHRA) have licensed some UK centres for production as medical products [1,44]. Accordingly, the cost of an FMT unit and the process of administration may vary between different hospitals settings. This study attempted to estimate the cost of FMT using a micro-costing approach using a commercially costed product although overheads are not included. Mitigating this limitation is that the costs associated with antibiotic prescribing strategies typically do not include the cost of antimicrobial resistance and given the costs associated with antimicrobial resistance, the true cost of using fidaxomicin or vancomycin might be much higher compared to FMT than this study has suggested.
Despite the limitations, the results reported here are supported by other studies which report that treatment with FMT is a cost effective treatment for rCDI [[15], [16], [17], [18],[45], [46], [47]]. However, the cost of all treatments in this analysis was higher than others as a result of including the cost of hospitalisation, which was ignored in some studies. Economic analyses conducted in the USA, Australia and China, using health systems perspectives, concluded that irrespective of the mode of delivery, FMT is an effective and cost saving treatment for those with rCDI compared to antibiotic treatment [18,[45], [46], [47]]. Furthermore, two other analyses from France and the USA suggested FMT treatment for rCDI was more costly and more effective than vancomycin, and was still considered a cost effective intervention at the local willingness to pay threshold [15,17]. Regarding the route of FMT, a study in Canada found that FMT colonoscopy dominated the strategies that were analysed to treat rCDI [16]. Another study showed that the difference between FMT routes of administration was not significant [18].
The study reported here is likely to be generalizable to other high income country settings where FMT is available with resources and relative costs are similar to the UK. However, the results of this study may not be applicable to some Low Middle Income Countries (LMIC) settings.
In the UK, health centres have been using their own procedures and method for FMT administration based on their experience [11]. The FMT material is approved by the MHRA as a medicinal product under the Human Medicines Regulations 2012 [44]. Following a survey which shows that only 28% (36/130) of hospitals reported using FMT for rCDI [48], a service for the supply of FMT to centres throughout the UK has been established [1].
The long-term side effects associated with using FMT as a treatment for rCDI is not known. NICE recommends further research into faecal microbiota transplant for CDI to investigate optimal dosage, mode of administration and choice of donor [12]. These data could be captured through the establishment of a patient registry with long term follow up of outcomes and health resource utilisation.
In conclusion , FMT NGT is shown to be a cost-effective treatment for patients suffering from rCDI particularly when compared to fidaxomicin. Given the severity and life threatening nature of this condition, the provision of FMT should be expedited as this highly cost-effective treatment will save lives and use limited resources efficiently.
Role of funding
The authors received no specific funding for this work.
Contributors
TR and PH had the idea for the study and designed the study. ZA carried out the literature review and the model based analysis and interpreted the results under the supervision of TR. PH provided clinical data used in the model based analysis, patient pathway and clinical interpretation. PB provided advice on modelling techniques and used data. TR and ZA drafted the manuscript. All authors commented on all drafts of the study.
Declaration of Competing Interest
All authors have nothing to disclose
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100420.
Appendix. Supplementary materials
References
- 1.McCune VL M.S., Quraishi M.N. . The development of faecal transplant as a medical product for the treatment of Clostridium difficile infection. Eclin Med. 2019 (In press) [Google Scholar]
- 2.Mullish B.H., Williams H.R. Clostridium difficile infection and antibiotic-associated diarrhoea. Clin Med. 2018;18(3):237–241. doi: 10.7861/clinmedicine.18-3-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asempa T.E., Nicolau D.P. Clostridium difficile infection in the elderly: an update on management. Clin Interv Aging. 2017;12:1799–1809. doi: 10.2147/CIA.S149089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer M.P., Notermans D.W., van Benthem B.H. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 5.Cornely O.A., Crook D.W., Esposito R. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12(4):281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 6.van Nood E., Vrieze A., Nieuwdorp M. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. New England J Med. 2013;368(5):407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 7.Tresman R., Goldenberg S.D. Healthcare resource use and attributable cost of Clostridium difficile infection: a micro-costing analysis comparing first and recurrent episodes. J Antimicrob Chemother. 2018;73(10):2851–2855. doi: 10.1093/jac/dky250. [DOI] [PubMed] [Google Scholar]
- 8.Wilcox M.H., Ahir H., Coia J.E. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72(9):2647–2656. doi: 10.1093/jac/dkx174. [DOI] [PubMed] [Google Scholar]
- 9.Olsen M.A., Yan Y., Reske K.A., Zilberberg M.D., Dubberke E.R. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect. 2015;21(2):164–170. doi: 10.1016/j.cmi.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Mullish B.H., Quraishi M.N., Segal J.P. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British society of gastroenterology (BSG) and healthcare infection society (HIS) guidelines. Gut. 2018;67(11):1920–1941. doi: 10.1136/gutjnl-2018-316818. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg S.D., Batra R., Beales I. Comparison of different strategies for providing fecal microbiota transplantation to treat patients with recurrent clostridium difficile infection in two english hospitals: a review. Infect Dis Ther. 2018;7(1):71–86. doi: 10.1007/s40121-018-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of health and Care Excellence (NICE): Faecal microbiota transplant for recurrent Clostridium difficile infection 2014. https://www.nice.org.uk/guidance/ipg485/. [Accessed 25 July 2019].
- 13.Public health England updated quidance on the managment and treatment of Clostridium difficile. infection. 2013 [Google Scholar]
- 14.Debast S.B., Bauer M.P., Kuijper E.J. European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 15.Konijeti G.G., Sauk J., Shrime M.G., Gupta M., Ananthakrishnan A.N. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014;58(11):1507–1514. doi: 10.1093/cid/ciu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapointe-Shaw L., Tran K.L., Coyte P.C. Cost-effectiveness analysis of six strategies to treat recurrent clostridium difficile infection. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baro E., Galperine T., Denies F. Cost-effectiveness analysis of five competing strategies for the management of multiple recurrent community-onset Clostridium difficile infection in France. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merlo G., Graves N., Brain D., Connelly L.B. Economic evaluation of fecal microbiota transplantation for the treatment of recurrent Clostridium difficile infection in Australia. J Gastroenterol Hepatol (Australia) 2016;31(12):1927–1932. doi: 10.1111/jgh.13402. [DOI] [PubMed] [Google Scholar]
- 19.Louie T.J., Miller M.A., Mullane K.M. Fidaxomicin versus vancomycin for Clostridium difficile infection. New England J Med. 2011;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 20.Hvas C.L., Dahl Jørgensen S.M., Jørgensen S.P. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology. 2019;156(5):1324–1332. doi: 10.1053/j.gastro.2018.12.019. e3. [DOI] [PubMed] [Google Scholar]
- 21.Rokkas T., Gisbert J.P., Gasbarrini A. A network meta-analysis of randomized controlled trials exploring the role of fecal microbiota transplantation in recurrent Clostridium difficile infection. United Eur Gastroenterol J. 2019;7(8):1051–1063. doi: 10.1177/2050640619854587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tieu J.D., Williams R.J., 2nd, Skrepnek G.H., Gentry C.A. Clinical outcomes of fidaxomicin vs oral vancomycin in recurrent Clostridium difficile infection. J Clin Pharm Ther. 2019;44(2):220–228. doi: 10.1111/jcpt.12771. [DOI] [PubMed] [Google Scholar]
- 23.Drummond M.F., Sculpher M.J., Claxton K., Stoddart G.L., Torrance G.W. Oxford university press; 2015. Methods for the economic evaluation of health care programmes. [Google Scholar]
- 24.Briggs A., Sculpher M., Claxton K. OUP Oxford; 2006. Decision modelling for health economic evaluation. [Google Scholar]
- 25.Quraishi M.N., Widlak M., Bhala N. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479–493. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 26.Office for National Statistics. National life tables: UK September 2019. [accessed 30 September 2019]
- 27.Karas J.A., Bradshaw S., Mahmud W., Enoch D.A. Mortality in hospitalized older adults associated with Clostridium difficile infection at a district hospital. Infect Dis Rep. 2010;2(1):e8. doi: 10.4081/idr.2010.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szende A., Janssen B., Cabases J. Springer Netherlands; Dordrecht, The Netherlands: 2014. Self-reported population health: an international perspective based on eq-5d. [PubMed] [Google Scholar]
- 29.British National Formulary . BMJ Group and the Royal Pharmaceutical Society of Great Britain; 2018. Medicines complete.https://www.medicinescomplete.com/about (accessed 22/6/2019) [Google Scholar]
- 30.National Health Service. NHS reference cost 17/18. https://improvement.nhs.uk/resources/reference-costs/.[Accessed 10 June 2019].
- 31.Zhang S., Palazuelos-Munoz S., Balsells E.M., Nair H., Chit A., Kyaw M.H. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. 2016;16(1):447. doi: 10.1186/s12879-016-1786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtis LAaB . University of Kent; 2018. Amanda. unit costs of health and social care 2018. [Google Scholar]
- 33.Ianiro G., Maida M., Burisch J. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Euro Gastroenterol J. 2018;6(8):1232–1244. doi: 10.1177/2050640618780762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y.W., Phelps E., Nemes S. Fecal Microbiota Transplant decreases mortality in patients with refractory severe or fulminant Clostridioides difficile infection. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2019.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Ianiro G., Murri R., Sciumè G.D. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann Intern Med. 2019;171(10):695–702. doi: 10.7326/M18-3635. [DOI] [PubMed] [Google Scholar]
- 36.National Institute of health and Care Excellence. Guide to the methods of technology appraisal. 2013https://wwwniceorguk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. [Accessed 1 July 2019]. [PubMed]
- 38.Wang S., Xu M., Wang W. Systematic review: adverse events of fecal microbiota transplantation. PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrella L.A., Sambol S.P., Cheknis A. Decreased cure and increased recurrence rates for Clostridium difficile infection caused by the epidemic C. difficile BI strain. Clin Infect Diseases: Off Public Infect Dis Soc Am. 2012;55(3):351–357. doi: 10.1093/cid/cis430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagier J.C., Delord M., Million M. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015;34(8):1597–1601. doi: 10.1007/s10096-015-2394-x. [DOI] [PubMed] [Google Scholar]
- 41.Jiang M., Leung N.H., Ip M., You J.H.S. Cost-effectiveness analysis of ribotype-guided fecal microbiota transplantation in Chinese patients with severe Clostridium difficile infection. PLoS ONE. 2018;13(7) doi: 10.1371/journal.pone.0201539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durovic A., Widmer A.F., Frei R., Tschudin-Sutter S. Distinguishing Clostridium difficile recurrence from reinfection: independent validation of current recommendations. Infect Control Hosp Epidemiol. 2017;38(8):891–896. doi: 10.1017/ice.2017.119. [DOI] [PubMed] [Google Scholar]
- 43.Gentry C.A., Giancola S.E., Thind S., Kurdgelashvili G., Skrepnek G.H., Williams R.J., 2nd A Propensity-matched analysis between standard versus tapered oral vancomycin courses for the management of recurrent Clostridium difficile infection. Open Forum Infect Dis. 2017;4(4) doi: 10.1093/ofid/ofx235. ofx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medicines and Healthcare products Regulatory Agency (MHRA). Faecal microbiota transplantation (FMT) MHRA's position. 2015.
- 45.Jiang M., Leung N.H., Ip M., You J.H.S. Cost-effectiveness analysis of ribotype-guided fecal microbiota transplantation in Chinese patients with severe Clostridium difficile infection. PLoS ONE. 2018;13(7) doi: 10.1371/journal.pone.0201539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varier R.U., Biltaji E., Smith K.J. Cost-effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015;36(4):438–444. doi: 10.1017/ice.2014.80. [DOI] [PubMed] [Google Scholar]
- 47.Zowall H., Brewer C., Deutsch A. Cost-effectiveness of fecal microbiota transplant in treating clostridium difficile infection in Canada. Value Health. 2014;17(7):A676. doi: 10.1016/j.jval.2014.08.2512. [DOI] [PubMed] [Google Scholar]
- 48.Quraishi M.N., Segal J., Mullish B. National survey of practice of faecal microbiota transplantation for Clostridium difficile infection in the UK. J Hosp Infect. 2017;95(4):444–445. doi: 10.1016/j.jhin.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Sirbu B.D., Soriano M.M., Manzo C., Lum J., Gerding D.N., Johnson S. Vancomycin taper and pulse regimen with careful follow-up for patients with recurrent Clostridium difficile infection. Clin Infect Diseases: Off Public Infect Dis Soc Am. 2017;65(8):1396–1399. doi: 10.1093/cid/cix529. [DOI] [PubMed] [Google Scholar]
- 50.Cammarota G., Masucci L., Ianiro G. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41(9):835–843. doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 51.Brandt L.J., Aroniadis O.C., Mellow M. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107(7):1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 52.McFarland L.V., Elmer G.W., Surawicz C.M. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 53.Murphy M.M., Patatanian E., Gales M.A. Extended duration vancomycin in recurrent Clostridium difficile infection: a systematic review. Ther Adv Infect Dis. 2018;5(6):111–119. doi: 10.1177/2049936118798276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwon R.S., Banerjee S., Desilets D. Enteral nutrition access devices. Gastrointest Endosc. 2010;72(2):236–248. doi: 10.1016/j.gie.2010.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.