Highlights
-
•
Antifungal activities of safe natural components against Aspergillus flavus growth and aflatoxins production.
-
•
Silver nanoparticles enhanced the antifungal activities of J. procera extract.
-
•
No correlation between the inhibition of fungal growth and mycotoxins production.
Keywords: Aflatoxins, Mycotoxigenic fungi, Juniperus procera, Silver nanoparticles
Abstract
From ancient to currently, it has been hard to prevent the exposure to mycotoxigenic fungi, due to these fungi occurs naturally in the environment. This paper reports the antifungal activities of the Juniperus procera stem extract with silver nanoparticles (AgNPs) against Aspergillus flavus growth and aflatoxins production. Numerous constituents of J. procera extract were detected by GC/MS analysis. Methanolic extract at 30, 60 and 90 mg/mL inhibited the growth of A. flavus, where the inhibition reached to 50.86, 51.60 and 52.58 %. respectively while weak inhibition was observed using the aqueous extract. Growth of A. flavus was reduced using AgNPs, the highest inhibition 39.31 % was recorded at 100 ppm AgNPs. Synergistic activity was observed by applying 50 ppm of AgNPs with aqueous and methanolic extracts of J. procera . A reduction in aflatoxin B2 and G2 synthesis was observed using different concentrations of methanolic stems extract of J. procera particularly with AgNPs.
1. Introduction
Juniperus procera belong to Cupressaceae family, its widely investigated as a source of natural drugs with potential antimicrobial, anticancer, antioxidant and insecticidal activities [[1], [2], [3]]. From different literatures, more than 65 species associated to Juniperus and distributed throughout the world. Analysis of J. procera extracts confirmed the existence of various ingredients that may reflect its pharmacological properties [4]. For example, J. communis is traditionally used for healing urinary infections and J. oxycedrus is used as a remedy for dermatological infections [5]. According to Newall et al. [6], plants producing non-phenolic essential oils, like some Juniperus species, are also used in folk medicines as antiseptics.
Aspergillus flavus contaminates a wide range of cereals, fruits, vegetables and nuts; and produce aflatoxins which are carcinogenic and mutagenic [7]. As mentioned before, the natural antifungal agents from plants can be potential exploited in controlling the growth of fungi consequently inhibiting aflatoxin formation [[8], [9], [10], [11]]. Pankaj et al. [12] investigated the different fractions of J. communis leaves and bark, it inhibit the growth of aflatoxigenic A. flavus and A. niger. Extracts from the aerial parts of J. lucayana were assayed against phytopathogenic fungus Botrytis cinerea. The results obtained by Abd El-Ghany [9] indicated that the productivity percentage of different mycotoxins including aflatoxins B1, aflatoxin B2, sterigmatocystin, cyclopiazonic acid and fusaric acid was reduced as a result of treatment by J. procera extract. Recently, Nivalenol, gliotoxin and neosolaniol production was inhibited with using J. procera fruit extract [4]. Different types of nanomaterials like copper, zinc, titanium [13], magnesium, gold [14], alginate [15] and silver have come up but silver nanoparticles (AgNPs) have proved to be most effective and applied as it has good antimicrobial efficacy [[16], [17], [18], [19], [20]].
According to Kim et al. [21] the antifungal activity of AgNPs against the phytopathogen Raffaelea sp. was recorded through repress the fungal growth and development and damaged cell walls and therefore AgNPs may use to eradicate phytopathogens. Not only, phytopathogens but human pathogenic fungi and human pathogenic bacteria [22] were controlled by AgNPs, beside other applications such as cytotoxic activity using rat splenocytes [23,24] and human normal melanocytes [25].
According to Duran et al. [26] utilizing of AgNPs can be exploited in medicine for burn treatment, dental materials, coating stainless steel materials, textile fabrics, water treatment, sunscreen lotions, and posses low toxicity to human cells, high thermal stability and low volatility. Unfortunately, studies on the antimicrobial activity of AgNPs have been performed mostly on animal pathogens [27]. Indeed, several pieces of evidence support the hypothesis that AgNPs have enhanced antimicrobial activity [19]. It has also been shown that AgNPs efficiently penetrate microbial cells [27]. In a previous study, it was observed that AgNPs disrupt transport systems and degradation of deoxyribonucleic acid [28]. Over the years, there has been an increase in the need to identify and isolate the fungi associated with their spoilage. The present study was conducted to control of A. flavus and aflatoxins production by J. procera extracts and study their synergistic activity with silver nanoparticles.
2. Material and methods
2.1. Plant used as antifungal
J. procera was collected from Jazan region-south of Saudi Arabia and identified by Dr. Yehia masrahy Associate Professor of Plant Ecology, Biology department, Faculty of science, Jazan University, KSA according to Migahid [29] and Chaudhary [30].
2.2. Preparation of plant extracts (PE)
Fresh stems (600 g) of J. procera were air dried at room temperature (40 °C) for 5 days, ground into powder using an electric grinder model: 5620, made in Korea. Samples from shade dried powder of plant stems were extracted with water (5400 mL) and methanol (1500 mL) alone. All the extracted were concentrated separately using rotary flask evaporator and preserved at 5 °C in an air tight brown bottle until future use.
2.3. Source of fungal isolation
Spoilage fruit and vegetables including Tomatoes Apple and Figs were collected from different localities of Jazan region. Randomly selected spoilt fruits and vegetables were cut into small segments (2 mm in diameter) with a sterilized blade, surface sterilized in 1 % hypochlorite for 2 min, plated on PDA aseptically and then incubated at 28 ± 2 °C for 5 days. A pure fungal culture was obtained and maintained by sub-culturing each of the different colonies that emerged onto the PDA plates and incubating at 28 ± 2 °C for 5 days. Healthy fruits and vegetables used as a control for fungal isolation as mentioned in spoilt fruits and vegetables.
2.4. Characterization and Identification of mycotoxin producing fungus
Identification of mycotoxin producing fungus was depend on macroscopic and microscopic examination including color and shapes of colonies, shape and diameter of hyphea, conidiophores, conidia and phialide according to Raper and Fennell [31], Samson et al. [32]. The identification was achieved by placing a drop of cotton blue in lacto phenol stain on a clean slide with the aid of a mounting needle where a small portion of the fungal mycelium from the edge of culture was removed and placed in a drop and lacto-phenol, a cover slip was gently placed with little pressure to eliminate air bubbles the slides was then mounted and observed with the aid of objectives lens (10−40x).
2.5. Silver nanoparticles (AgNPs)
AgNPs (chemically synthesized < 100 nm) were obtained from Sigma-Aldrich used as antifungal agent
2.6. Poisoned food technique assay against mycotoxigenic fungi
For Antifungal activity of plant extract: Potato dextrose agar medium (PDA) with different concentrations of aqueous and methanolic stems of J. procera extract will prepared separately. About 25 mL of the medium growth will pour into each petri-dish and allow to solidify. Disc (5 mm) of 5-day old culture of the tested fungus under study will inoculate at the center of the petri dish, after incubation period (6 days) at 30 °C the growth was measured in millimeter.
For antifungal activity of AgNPs: Different levels of AgNPs will be added into appropriate volum of the sterile media perior it solidified. The medium containing AgNPs was poured into the sterile Petri plate and inoculated. Medium without AgNPs will use as a negative controls. Diameter of colony will measure after 6 days and inhibition % of the growth of fungi in connection to control treatment was calculated according to the given equation:
Where I = Percentage of inhibition, C = Radial growth at control, T = Radial growth at treatment.
2.7. Effect the plant extract and AgNPs on mycotoxins production
Potatoes dextrose broth media (100 mL) supplemented with different concentrations of plant extract and AgNPs in 250-mL Erlenmeyer conical flasks, followed by inoculation with 6-mm diameter discs of the A. flavus, then incubated at 28 ± 2 °C for 12 days in the dark. The filtrates of the culture media were obtained and assayed for the presence of mycotoxins with using GC/MS. Growth medium without plant extract and AgNPs was used as control.
2.8. Mycotoxins detection
The tested mycotoxins were determined by Gas chromatograph with mass selective (GC/MS) and the procedure based on analytical methods described elsewhere [33]. GC/MS detector 6890/5975B (Agilent Technologies) was combined with the column HP-5MS, 30 m, 0.25 mm and 0.25 μm. The program of ChemStation was from Agilent Technologies for the system control and data processing. The carrier gas was helium with the column flow rate of 1 mL/min. The split less injection mode was used and injection volume was 1 μL. The inlet temperature was 270 °C, MSD ion source temperature 170 °C, mass filter temperature 150 °C and GC-MSD interface temperature 280 °C. The column temperature program was: 60 °C held for 2 min, 25 °C/min to 240 °C and 5 °C/min to 300 °C. Electron ionization (EI) was carried out at 70 eV and spectra were monitored in selected ion monitoring (SIM) mode.
A certified combined standard of different mycotoxins was purchased from sigma Aldersh After reconstitution in acetonitrile, the concentration of each toxin in the solution was 100 μg/mL. Working standard solutions with the concentrations of each was 0.2 and 2.0 μg/mL were prepared diluting the stock standard solution with acetonitrile. The extraction solvent, the mixture of acetonitrile and deionized water (84 + 16) was used. Prior to use, glass vials for the derivatisation were deactivated with 5 % dichlorodimethylsilane solution (25 mL of dichlorodimethylsilane diluted to 500 mL with hexane).
3. Results and discussion
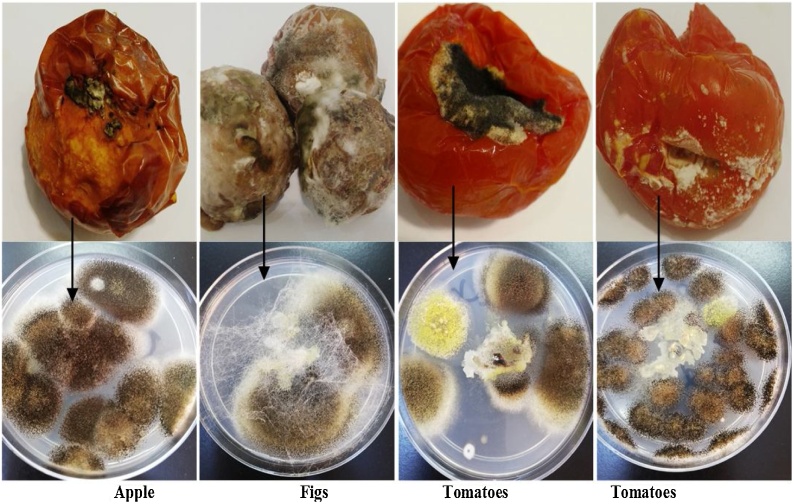
The identified mycotoxin producing fungus associated with spoilt fruits and vegetables (Fig. 1) in the study area include A. flavus suggesting that fungus could be responsible for the fruit spoilage. The isolated fungus is known to produce secondary metabolites known as mycotoxins to human which is associated with liver cancer in animal and human. A. flavus (green colony) was isolated from tomato (Fig. 1) Similar findings on the fungal isolation from fruits and vegetables stored in the market have been reported by earlier researchers [34].
Fig. 1.
Fungal isolates and their sources. Arrow direction from spoiled fruits or vegetables to fungal isolates.
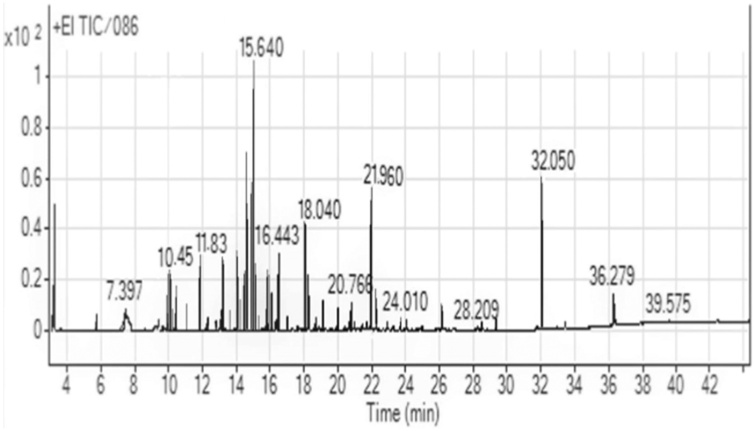
GC/MS analysis of J. procera extract reflect the presence of 46 constituents related to different secondary metabolites (Table 1 and Fig. 2). The obtained results revealed the presence of monoterpenes known as thymol with RC 3.13 % in J. procera extract. Previous results revealed that thymol had the highest antibacterial activity [35,36] and antifungal activity against plant pathogenic fungi [37,38] and against grey molds in horticultural products caused by Botrytis cinerea [39], through the damage to the enzymatic cell system [40] that responsible for spore germination or interference with the amino acid involved in germination [41].
Table 1.
Active ingredients of J. procera stems extract detected by GC/MS.
| NO. | Compound Name | RT | RC (%) |
|---|---|---|---|
| 1 | β-Pinene | 9.91 | 0.687 |
| 2 | β-Myrcene | 9.96 | 1.687 |
| 3 | Camphene | 10.05 | 2.312 |
| 4 | α-Pinene | 10.14 | 2.062 |
| 5 | D,L-Limonene | 10.39 | 0.650 |
| 6 | L-Limonene | 10.45 | 2.687 |
| 7 | γ-Terpinene | 10.68 | 0.275 |
| 8 | α-Terpinolene | 10.95 | 0.450 |
| 9 | Linalool | 11.07 | 0.225 |
| 10 | Allo-Ocimene | 11.38 | 1.050 |
| 11 | Pinocarveol | 11.53 | 0.100 |
| 12 | Carveol | 11.63 | 0.750 |
| 13 | Camphor | 11.7 | 0.150 |
| 14 | Thymol | 11.83 | 3.125 |
| 15 | Verbenone | 12.35 | 0.112 |
| 16 | Hexyl isovalerate | 12.48 | 0.175 |
| 17 | Benzen, 1,3-bis-dimethylethyl | 12.57 | 0.262 |
| 18 | Cyclohexene, 2-ethenyl-1, 3, 3-trimethyl- | 13.2 | 0.150 |
| 19 | α-Terpinene | 13.47 | 3.225 |
| 20 | α-Cubebene | 13.59 | 1.150 |
| 21 | β-Elemene | 14.03 | 3.337 |
| 22 | Trans-Caryophyllene | 14.38 | 0.475 |
| 23 | β-Caryophyllene | 14.41 | 1.387 |
| 24 | γ-Elemene | 14.49 | 7.074 |
| 25 | Germacrene | 14.66 | 2.387 |
| 26 | α-Humulene | 14.79 | 0.637 |
| 27 | Copaene | 14.95 | 1.137 |
| 28 | Cedrene | 14.99 | 0.162 |
| 29 | β-Selinene | 15.51 | 1.012 |
| 30 | δ-Cadinene | 15.4 | 1.900 |
| 31 | Epoxy caryophyllene | 15.42 | 5.962 |
| 32 | γ-Cadinene | 15.54 | 3.200 |
| 33 | α-Muurolene | 15.59 | 0.287 |
| 34 | γ-Selinene | 15.62 | 0.437 |
| 35 | Germacrene B | 15.64 | 9.536 |
| 36 | Elemol | 15.73 | 2.750 |
| 37 | Hexadecane | 15.97 | 2.212 |
| 37 | α-Cadinol | 15.99 | 0.787 |
| 38 | Isospathulenol | 16.21 | 0.175 |
| 39 | α-Amorphene | 17.13 | 0.800 |
| 40 | Ledol | 17.35 | 0.162 |
| 41 | Cedrol | 19.98 | 4.249 |
| 42 | Eicosane | 21.96 | 0.400 |
| 43 | Docosane | 26.96 | 5.612 |
| 44 | Heptacosane | 29.06 | 0.162 |
| 45 | Nonacosane | 32.05 | 0.262 |
| 46 | Stenol | 18.04 | 6.049 |
Fig. 2.
GC–MS chromatogram of J. procera stem extract.
Trans-Caryophyllene, β-Caryophyllene and Epoxy caryophyllene were detected in stems of J. procera with RC 0.475, 1.387 and 5.962 %, respectively. Emami et al. [42] reported the presence of caryophyllene in J. excels. According to GC/MS of J. virginiana and J. communis aerial parts, caryophyllene was also detected [43] particularly in the branches. The antibacterial and antifungal activities of caryophyllene and caryophyllene oxide were exhibited in numerous studies [44,45].
The obtained results of GC/MS showed the existence of pinene derivatives including β-Pinene, α-Pinene and γ-Terpinene in J. procera extract (Table 1). α-pinene was identified as a component of active ingredients of J. communis, and exhibited fungistatic effect [46]. β-Elemene, γ-Elemene, Camphene, β-Pinene and α-cubebene were identified with highest concentration compared with other components (Table 1), these components with caryophyllene and pinene displayed great potential of antifungal activity as a mycelial growth inhibitor against the tested phytopathogenic fungi such as Rhizoctonia solani, Botrytis cinerea, Fusarium solani, Phytophthora capsici and Colletotricum capsici [47]. Antifungal and antibacterial activity against Aspergillus niger, Staphylococcus aureus and Bacillus cereus were attributed to the presence of active ingredient in the current tested plant J. procera extract such as α-Humulene (Table 1) [48], α-cadinol as a constituent of J. procera extract has shown activity against B. cereus and S. aureus according to Su and Ho [49]. The antibacterial and antifungal activity can be attributed to the relatively high concentrations of (E)-caryophyllene, α-humulene, δ-cadinene, and α-cadinol in the oil of Ocimum forskolei and Teucrium yemense (Lamiaceae) [50].
d,l-Limonene, l-Limonene and other active ingredients of J. procera plays an important role for antimicrobial activities as mentioned in numerous studies, where Several reports have proved that limonene interacts with the cytoplasmic membranes of bacteria, resulting in a loss of membrane integrity, the dissipation of the proton-motive forces, and the inhibition of the respiratory enzymes [51,52].
According to Sieniawska et al. [53], some terpenes comounds such as d,l-Limonene, l-Limonene, β-Myrcene, α-Pinene and β-Elemene enhanced the activity of tuberculostatic antibiotics. The oil isolated from the calyx of Salvia brachyodon contained β-pinene, α-pinene, camphene, borneol, and camphor as components also of J. procera, possessed the best antifungal activity against Trichophyton mentagrophytes, Aspergillus niger, and Candida albicans [54]. GC/MS analysis identified sesquiterpene alcohol (Cedrol) in the extract of J. procera (Table 1), those compound was identified in J. virginiana, and showed highest inhibitory effects against brown-rot fungus Gloeophyllum trabeum [54]. Also, Tumen et al. [2] reported the antifungal activity of three species of J. virginiana, J. ashei, J. occidentalis that attributed to cedrol.
Results of antifungal activity of aqueous and methanolic J. procera stems extract and AgNPs are presented in Table 2. The obtained observations suggested that methanolic extract had strong, while aqueous extract had negligible antifungal activity. Unfortunately low concentration of aqueous J. procera (30 mg/mL) extract stimulated the growth of A. flavus where the growth increased up to 4.4 mm compared with control (4.07 mm) without any treatment. Similar phenomenon was observed by Mughal et al. [55], who found that some allelochemicals can enhance the fungal growth at different concentrations. The differences in the toxicity of different extracts could be attributed to the presence of the active compounds that are extracted by different solvents, which may be influenced by numerous factors including extraction methods, type of extracting solvent and time of harvesting plant materials [56]. Heartwood samples from three species of J. virginiana, J. occidentalis, and J. ashei were extracted with hexane, methanol and ethanol; and tested for antifungal activity against Irpex lacteus, Gloeophyllum trabeum, Postia placenta, Trametes versicolor which known as wood-rot fungi. The ethanol extracts had higher antifungal activity than the hexane extracts [2].
Table 2.
Effect of different concentrations of J. procera aqueous and methanolic stems extract and AgNPs on A. flavus growth.
| Treatment | Aqueous |
Methanolic |
||
|---|---|---|---|---|
| Growth(mm) | Inhibition (%) | Growth(mm) | Inhibition (%) | |
| Control | 4.07 ± 0.12 | 0.00 | 4.07 ± 0.12 | 0.00 |
| 30 mg/mL PE | 4.40 ± 0.17 | 0.00 | 2.00 ± 0.01 | 50.86 |
| 60 mg/mL PE | 3.93 ± 0.12 | 3.44 | 1.97 ± 0.02 | 51.60 |
| 90 mg/mL PE | 3.93 ± 0.21 | 3.44 | 1.93 ± 0.06 | 52.58 |
| 25 ppm AgNPs | 3.00 ± 0.01 | 26.29 | 3.00 ± 0.01 | 26.29 |
| 50 ppm AgNPs | 3.00 ± 0.02 | 26.29 | 3.00 ± 0.02 | 26.29 |
| 100 ppm AgNPs | 2.47 ± 0.06 | 39.31 | 2.47 ± 0.06 | 39.31 |
| 30 mg/mL PE + 50 ppm AgNPs | 4.43 ± 0.12 | 0.00 | 1.97 ± 0.06 | 52.00 |
| 60 mg/mL PE + 50 ppm AgNPs | 3.90 ± 0.17 | 4.17 | 1.47 ± 0.06 | 63.88 |
| 90 mg/mL PE + 50 ppm AgNPs | 3.90 ± 0.26 | 4.17 | 1.47 ± 0.15 | 63.88 |
±, Standard Deviation.
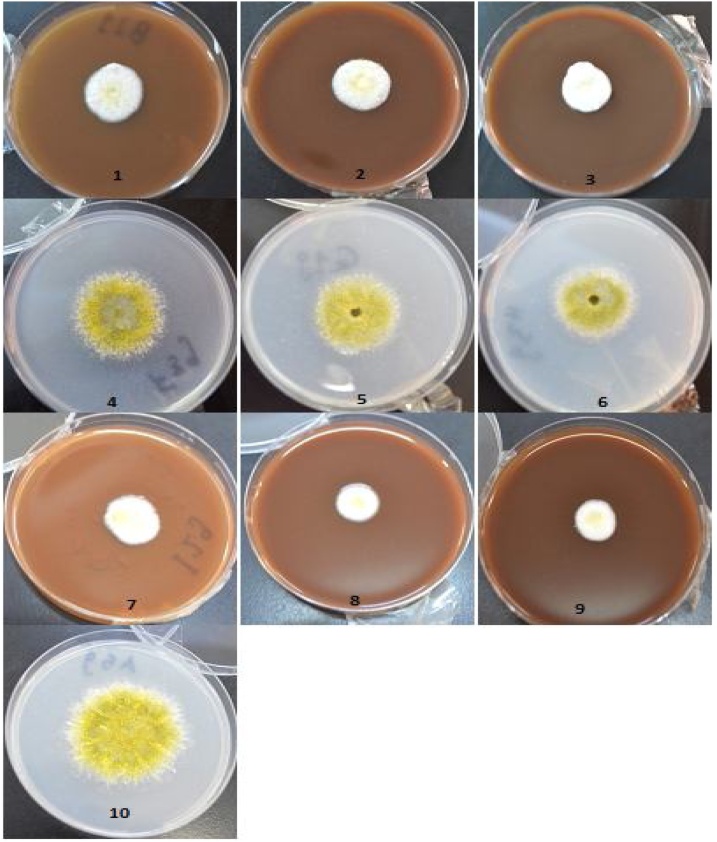
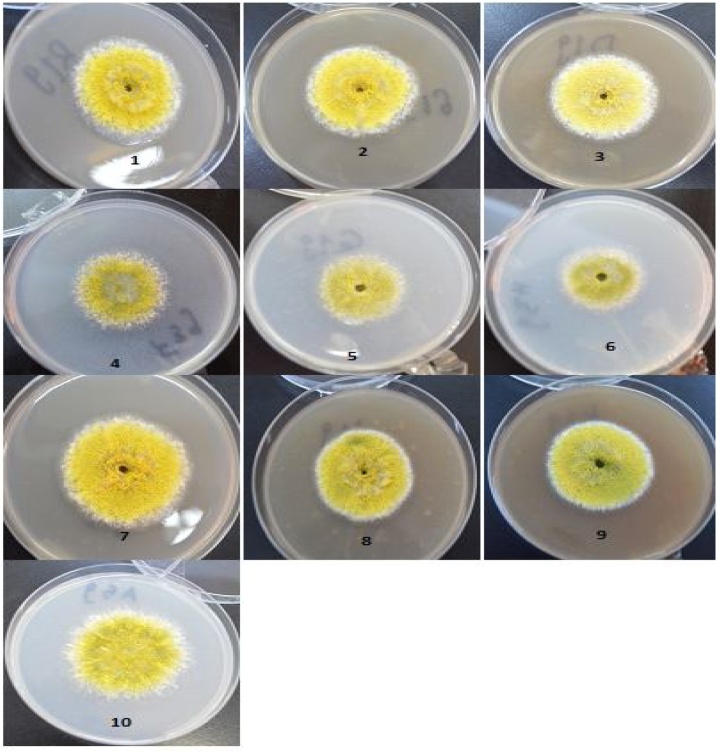
Various concentrations of methanolic J. procera extract (30, 60 and 90 mg/mL) showed antifungal activity on the growth of A. flavus, where the growth inhibition was 50.86, 51.60 and 52.58 % respectively. From microscopically examination of A. flavus at J. procera treatments showed that sporogenisis was inhibited. This observation was clear also from the appearance of white color of coloy compared with the coloy color at control or at AgNPs treatments (Fig. 3) unlike aqueous extract (Fig. 4), the colony color was similar to color of colony at control treatment. These results may explained on the bases of the presence of active ingredients in J. procera that recorded by GC/MS analysis. The previous studies on J. procera showed inhibitory effect on the growth of fungi [9,57]. Similarly, the essential oil from these plants was also found effective against fungal contamination of food products [58]. Antifungal activity of Juniperus essential oils of different species of Juniperus including J. communis ssp. alpina, J. oxycedrus ssp. oxycedrus and J. turbinata was reported against Aspergillus and dermatophytes [59].
Fig. 3.
Effect of different concentrations of J. procera methanolic stem extract and AgNPs on A. flavus growth. 1, 30 mg/mL extract; 2, 60 mg/mL extract;3, 90 mg/mL extract; 4, 25 ppm AgNPs; 5, 50 ppm AgNPs; 6, 100 ppm AgNPs; 7, 30 mg/mL extract +50 ppm AgNPs;8, 60 mg/mL extract+50 ppm AgNPs;9, 90 mg/mL extract+50 ppm AgNPs;10, Control without treatment.
Fig. 4.
Effect of different concentrations of J. procera aqueous stem extract and AgNPs on A. flavus growth. 1, 30 mg/mL extract; 2, 60 mg/mL extract;3, 90 mg/mL extract; 4, 25 ppm AgNPs; 5, 50 ppm AgNPs; 6, 100 ppm AgNPs; 7, 30 mg/mL extract +50 ppm AgNPs;8, 60 mg/mL extract+50 ppm AgNPs;9, 90 mg/mL extract+50 ppm AgNPs;10, Control without treatment.
The inhibitory effect of AgNPs at different concentrations (25, 50 and 100 ppm) was recorded against A. flavus (Table 2). The lowest level of inhibition was observed againstA. flavus at 25 ppm concentration of AgNPs, while the highest level of inhibition was observed at 100 ppm concentration of AgNPs (39.31 %). The results clearly demonstrated that AgNPs are hopeful antifungal agents against fungi. The use of AgNPs as antifungal agents has become more common in the current time. AgNPs display numerous mechanisms of inhibitory action to microorganisms; they may be applied for repress different phytopathogens in a relatively safe way compared to synthetic fungicides [21]. The antifungal mechanism of AgNPs may be due to the fact that the formation of free radicles produced from the nanoparticles could disturb the membrane lipids and then finally spoil the functions of membrane [60,61]. Recent studies have indicated that the AgNPs is able to cause DNA and proteins to leak outside fungal cells [58], beside distortions and damage of fungal mycelia [24]
Combined activity of the AgNPs with aqueous and methanolic J. procera stems extract was studied also. When tested together the combination of J. procera stems extract with AgNPs showed synergistic effect against A. flavus growth. Enhancement of antifungal activities of J. procera stems extract was observed by calculating of growth inhibition. Extracts at 60 mg/mL and 90 mg/mL with 50 ppm AgNPs showed inhibition 63.88 % compared with treatments without AgNPs. The overall result is shown in Table 2. The current finding were agree with recent results of Bakri et al. [4]. The synergistic action of AgNPs with J. procera stems extract may open the door for a future combination treatment against mycotoxigenic fungi. Recently, AgNPs promoted the effect of numerous antibiotics against Escherichia coli [62] and epoxiconazole against Setosphaeria turcica [63].
Aflatoxins are a potent toxic created as secondary metabolites by the fungus A. flavus and other species. Antifungal agents extracted from plants could be exploited in repress the fungi growth consequently inhibiting aflatoxin synthesis [4,12]. A clearly complete inhibition in aflatoxin B2 synthesis was observed, when A. flavus treated with methanolic extract of J. procera, where the aflatoxin B2 production was zero in all concentrations compared with control was 10.43 μg/mL. The same effect showed when added the 50 ppm of AgNPs with 30, 60 and 90 mg/mL of methanolic J. procera extract. On the other hand, reduction in aflatoxin B2 production was observed with aqueous extract treatment, where the aflatoxin B2 was 8.23, 6.36 and 6.66 ppm at 30, 60 and 90 mg/mL plant extract (Table 3). The previous study by Abd El-Ghany [9] was agreement with current results, where the extract of J. procera demonstrated good inhibitory effect on mycotoxins of A. flavus, where the production of aflatoxins B1 was reduced, while aflatoxins B2 was completely inhibited with the treatment by J. procera extract. According to numerous studies, fungitoxic effects indicate that J. procera extract block the metabolic pathway of aflatoxins biosynthesis [64,65].
Table 3.
Effect of different concentrations of J. procera aqueous and methanolic stems extract with AgNPs on aflatoxins productions.
| Treatment | Aflatoxin concentration (μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aqueous extract |
Methanolic extract |
|||||||
| B1 | B2 | G1 | G2 | B1 | B2 | G1 | G2 | |
| Control | 0.00 | 10.43 | 0.00 | 0.29 | 0.00 | 10.43 | 0.00 | 0.29 |
| 30 mg/mL PE | 0.00 | 8.23 | 0.00 | 8.23 | 0.00 | 0.00 | 0.00 | 0.00 |
| 60 mg/mL PE | 0.00 | 6.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.43 |
| 90 mg/mL PE | 0.00 | 6.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.56 |
| 25 ppm AgNPs | 0.00 | 7.98 | 0.00 | 0.00 | 0.00 | 7.98 | 0.00 | 0.00 |
| 50 ppm AgNPs | 0.00 | 8.47 | 0.00 | 0.00 | 0.00 | 8.47 | 0.00 | 0.00 |
| 100 ppm AgNPs | 0.00 | 8.15 | 0.00 | 0.00 | 0.00 | 8.15 | 0.00 | 0.00 |
| 30 mg/mL PE + 50 ppm AgNPs | 0.00 | 8.88 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 60 mg/mL PE + 50 ppm AgNPs | 0.00 | 6.15 | 0.00 | 0.62 | 0.00 | 0.00 | 0.00 | 0.77 |
| 90 mg/mL PE + 50 ppm AgNPs | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.04 |
From the current results aflatoxins B1 and G1 not detected in the culture filtrate of untreated or treated growth medium (Table 3), therefore stimulation of its production by plant extracts not observed. The results showed the increasingly production of aflatoxin G2, when tested with aqueous stems extract at 30 mg/mL, but reduced when treated with methanolic stems extract at the same concentration. The production of aflatoxin G2 at 30 mg/mL was 8.23 μg/mL, compared with control where the aflatoxin G2 was 0.29 μg/mL. Antifungal index and aflatoxin production by Aspergillus parasiticus using antifungal, juniper EO, increased in parallel, while applying sub-lethal concentrations of EOs might induce stress response in A. parasiticus leading to increased aflatoxin production [66]. Gömöri et al. [67] tested the effect of essential oils (EOs) of cinnamon, clary sage, juniper, lemon and marjoram for inhibition of growth and aflatoxin production by Aspergillus parasiticus, and found decrease the amount of aflatoxin B1 and G2. Inhibition of growth of aflatoxigenic A. flavus and A. niger growth was recorded by Pankaj et al. [12] through different fractions of Juniperus leaves and bark extracts.
A great deal of scientific papers reported the antibacterial [68] and antifungal [17,19] activity of AgNPs. However, few studies reported the activity of AgNPs against mycotoxins production. The obtained results showed the inhibitory action of AgNPs against aflatoxin B2 production, at the same time aflatoxin G2 production was completely inhibited. In agreement with the current result, AgNPs have been found to be eff ;ective in thwarting the synthesizing of the mycotoxins of A. ochraceus [25],Fusarium graminearum [69], A. flavus and A. parasiticus [70].
The synergistic action of 90 mg/mL plant extract with 50 ppm of AgNPs was observed in case aflatoxin B2 production (Table 3), where its completely inhibited compared with using plant extract or AgNPs alone. The results are consistent with recent study reported the activity of AgNPs with J. procera extract toward mycotoxins production by Aspergillus fumigatus and Fusarium chlamydosporum [4]. Also, this observation parallels findings in a study carried out by Hafez et al. [71], who stated that AgNPs used as nanofungicides to inhibit the fungal growth and subsequent aflatoxins production in cereal grains during storage. Ayatollahi [72] demonstrated that a minimum inhibition concentration (MIC) equal to 180 μg/mL was determined for AgNPs against A. parasiticus, at the same time AgNPs effectively inhibited aflatoxin B1 production at a concentration of 90 μg/mL. Generally from the obtained results s of fungal growth of A. flavus and its mycotoxins production, there was no correlation between the inhibition of growth and mycotoxins production. However these notes were agreement with previous studies [73,74], but this phenomenon needs more studies. In study carried out by Neveen [75] found that the complete inhibition of A. flavus growth was observed at 1000 ppm oil concentration of Ocimum basilicum, while marked inhibition of aflatoxin B1 production was observed at 500, 750 and 1000 ppm oil concentrations tested.
4. Conclusion
The results obtained from the current study showed that plant extracts of the metanolic stems extract of J. procera exhibit antifungal effects against A. flavus growth and its mycotoxins. The study demonstrated the enhanced antifungal effect by combination of J. procera extract with AgNPs against A. flavus. The present study also helped in identifying phytoconstituents present in the extract which are responsible for various biological and antifungal activities.
Author contribution statement
Abdelghany T. M: Conceived and designed the experiments;Analyzed and interpreted the data; Wrote the paper.
Maryam M. Hasan : Performed the experiments and Wrote the paper.
Medhat A. El-Naggar: Contributed reagents,materials, and mycotoxins analysis Wrote the paper.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00496.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Ortiz Y., Spengler I., Rodríguez Y., Collado I.G., Hernandez-Galan R. Screening study of potential lead compounds for natural products based fungicides from Juniperus lucayana. Nat. Prod. Commun. 2008;3:469–473. [Google Scholar]
- 2.Tumen I., Fred J.E., Carol A.C., Jeffery A.T. Antifungal activity of heartwood extracts from three Juniperus species. BioResource. 2013;8:12–20. [Google Scholar]
- 3.Abd El-Ghany T.M., Hakamy M.O. Juniperus procera as food safe additive, their antioxidant, anticancer and antimicrobial activity against some food-borne Bacteria. J. Biol. Chem. Res. 2014;31:668–677. [Google Scholar]
- 4.Bakri M.M., El-Naggar Medhat A., Helmy E.A., Ashoor Mona S., Abdel Ghany T.M. Efficacy of Juniperus procera constituents with silver nanoparticles against Aspergillus fumigatus and Fusarium chlamydosporum. BioNanoScience. 2020;10:62–72. doi: 10.1007/s12668-019-00716-x. [DOI] [Google Scholar]
- 5.Cosentino S., Barra A., Pisano B., Cabizza M., Pirisi F.M., Palmas F. Composition and antimicrobial properties of Sardinian Juniperus essential oils against foodborne pathogens and spoilage microorganisms. J. Food Prot. 2003;66:1288–1291. doi: 10.4315/0362-028x-66.7.1288. [DOI] [PubMed] [Google Scholar]
- 6.Newall C., Anderson L., Phillipson J. A Guide for Health Care Professionals. The Pharmaceutical Press; London: 1996. Herbal medicines. [Google Scholar]
- 7.Kiswii T.M., Monda E.O., Okemo P.O., Bii C., Alakonya A.E. Efficacy of selected medicinal plants from eastern kenya against Aspergillus flavus. J. Plant Sci. 2014;2:226–231. [Google Scholar]
- 8.Grayer R.J., Harborne J.B. A survey of antifungal compounds from higher plants. Phytochemistry. 1994;37:19–42. doi: 10.1016/s0031-9422(00)00450-7. [DOI] [PubMed] [Google Scholar]
- 9.Abd El-Ghany T.M. Eco-friendly and safe role of Juniperus procera in controlling of fungal growth and secondary metabolites. J. Plant Pathol. Microbiol. 2014;5:231. doi: 10.4172/2157-7471.1000231. [DOI] [Google Scholar]
- 10.Abd El-Ghany T.M., Ganash M.A., Bakri M.M., Al-Rajhi A.M.H., Al Abboud M.A. Evaluation of natural sources for repress cytotoxic Trichothecenes and Zearalenone production with using Enzyme-linked immunosorbent assay. Life Sci. J. 2016;13:74–86. doi: 10.7537/marslsj130816.13. [DOI] [Google Scholar]
- 11.Abd El-Ghany T.M., El-Naggar M.A., Ganash M.A., Al Abboud M.A. PCR identification of Aspergillus niger with using natural additives for controlling and detection of malformins and maltoryzine production by HPLC. BioNanoSci. 2017;7:588–596. doi: 10.1007/s12668-017-0455-6. [DOI] [Google Scholar]
- 12.Pankaj K., Bhatt R.P., Sati O.P., Vinod K.D., Lokendra S. In-vitro antifungal activity of different fraction of Juniperus communis leaves and bark against Aspergillus niger and Aflatoxigenic Aspergillus flavus. Int. J. Pharma Bio Sci. 2010;1:1–7. [Google Scholar]
- 13.Retchkiman-Schabes P.S., Canizal G., Becerra-Herrera R., Zorrilla C., Liu H.B., Ascencio J.A. Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Opt. Mater. 2006;29:95–99. [Google Scholar]
- 14.Gu H., Ho P.L., Tong E., Wang L., Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Lett. 2003;3:1261–1263. [Google Scholar]
- 15.Ahmad Z., Pandey R., Sharma S., Khuller G.K. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Ind. J. Chest Dis. Allied Sci. 2005;48:171–176. [PubMed] [Google Scholar]
- 16.Gong P., Li H., He X., Wang K., Hu J., Tan W. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 2007;18:604–611. [Google Scholar]
- 17.Abd El-Ghany T.M. Stachybotrys chartarum: a novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indon. J. Biotechnol. 2013;18:75–82. [Google Scholar]
- 18.Abd El-Ghany T.M., Shater A.M., A.Abboud M., Alawlaqi M.M. Silver nanoparticles biosynthesis by Fusarium moniliforme and their antimicrobial activity against some food-borne bacteria. Mycopath. 2013;11:1–7. [Google Scholar]
- 19.Abd El-Ghany T.M., Al-Rajhi A.M., Al Abboud M.A., Alawlaqi M.M., Magdah G., Helmy E.A., Mabrouk A.S. Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A review. BioNanoSci. 2018;8:5–16. doi: 10.1007/s12668-017-0413-3. [DOI] [Google Scholar]
- 20.Abd El-Ghany T.M., Ganash M., Bakri M.M., Al-Rajhi A.M. Molecular characterization of Trichoderma asperellum and lignocellulolytic activity on barley straw treated with silver nanoparticles. BioResources. 2018;13:1729–1744. doi: 10.15376/biores.13.1.1729-1744. [DOI] [Google Scholar]
- 21.Kim K., Sung W.S., Moon S., Choi J., Kim G.J., Lee D.G. An in vitro study of the antifungal effect of silver nanoparticles on Oak Wilt Pathogen Raffaelea sp. J. Microbiol. Biotechnol. 2009;19:760–764. [PubMed] [Google Scholar]
- 22.Mythili R., Selvankumar T., Kamala-Kannan S., Sudhakar C., Ameen F., Al-Sabri A., Selvam K., Govarthanan M., Kim H. Utilization of market vegetable waste for silver nanoparticle synthesis and its antibacterial activity. Mater. Lett. 2018;225:101–104. doi: 10.1016/j.matlet.2018.04.111. [DOI] [Google Scholar]
- 23.Aravinthan A., Govarthanan M., Praburaman K.S., Selvankumar T., Balamurugan R., Kamala-Kannan S., Kim J.-H. Sunroot mediated synthesis and characterization of silver nanoparticles and evaluation of its antibacterial and Rat splenocyte Ccytotoxic effects. Int. J. Nanomed. 2015;11:1977–1983. doi: 10.2147/IJN.S79106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengottaiyan A., Mythili R., Selvankumar T., Aravinthan A., Kamala-Kannan S., Manoharan K., Thiyagarajan P., Govarthanan Muthusamy, Kim J.-H. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res. Chem. Intermed. 2016;42:3095–3103. doi: 10.1007/s11164-015-2199-7. [DOI] [Google Scholar]
- 25.Khalil N.M., Abd El-Ghany M.N., Rodríguez-Couto S. Antifungal and anti-mycotoxin efficacy of biogenic silver nanoparticles produced by fusarium chlamydosporum and Penicillium chrysogenum at non-cytotoxic doses. Chemosphere. 2019;218:477–486. doi: 10.1016/j.chemosphere.2018.11.129. [DOI] [PubMed] [Google Scholar]
- 26.Duran N., Marcarto P.D., De Souza G.I.H., Alves O.L., Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007;3:203–208. [Google Scholar]
- 27.Duran N., Duran M., de Jesus M.B., Seabra A.B., Favaro W.J., Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2015;12:789–799. doi: 10.1016/j.nano.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Shen T., Wang Q., Li C., Zhou B., Li Y., Liua Y. Transcriptome sequencing analysis reveals silver nanoparticles antifungal molecular mechanism of the soil fungi Fusarium solani species complex. J. Hazard. Mater. 2020;388 doi: 10.1016/j.jhazmat.2020.122063. [DOI] [PubMed] [Google Scholar]
- 29.Migahid A.M. 4th ed. Vol. 1. King Saud University Press; Riyadh, Saudi Arabia: 1974. Flora of Saudi Arabia. (Cryptogams and Dicotyledons Equisetaceae to Neuradaceae). [Google Scholar]
- 30.Chaudhary S.A. vol. 1. National Agriculture and Water Research Centre. Ministry of Agriculture; Saudi Arabia: 1997. (Flora of the Kingdom of Saudi Arabia). 691. [Google Scholar]
- 31.Raper K.B., Fennell D.I. Robert E Krieger Publishing Company; New York: 1973. The Genus Aspergillus. [Google Scholar]
- 32.Samson R.A., Hoekstra E.S., Van Oorschot C.A. Centraalbureau voor Schimmelcultures; 1981. Introduction to Food-Borne Fungi. [Google Scholar]
- 33.Binder E.M., Tan L.M., Chin L.J., Handl J., Richard J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007;137:265–282. [Google Scholar]
- 34.Mailafia S., Okoh R.G., Olabode H.O., Osanupin R. Isolation and identification of fungi associated with spoilt fruits vended in Gwagwalada market, Abuja, Nigeria. Vet. World. 2017;10:393–397. doi: 10.14202/vetworld.2017.393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawar V.C., Thaker V.S. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses. 2007;49:316–323. doi: 10.1111/j.1439-0507.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 36.Abdel Rasoul A.M., Marei G.I., Abdelgaleil S.A. Evaluation of antibacterial properties and biochemical effects of monoterpenes on plant pathogenic bacteria. Afr. J. Microbiol. Res. 2012;6:3667–3672. doi: 10.5897/AJMR12.118. [DOI] [Google Scholar]
- 37.Tsao R., Zhou T. Antifungal activity of monoterpenoids against postharvest pathogens Botrytis cinerea and Monilinia fructicola. J. Essent. Oil Res. 2000;12:113–121. [Google Scholar]
- 38.Sokovic M., Tzakou O., Pitarakoli D., Couladis M. Antifungal activities of selected aromatic plants growing wild in Greece. NahrungFood. 2002;46:317–320. doi: 10.1002/1521-3803(20020901)46:5<317::AID-FOOD317>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J., Ma S., Du S., S.Chen H. Sun. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019;56:2611–2620. doi: 10.1007/s13197-019-03747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conner D.E., Beuchat L.R. Effects of essential oils from plants on growth of food spoilage yeast. J. Food Sci. 1984;49:429–434. [Google Scholar]
- 41.Nychas G.J.E. Natural antimicrobial from plants. In: Gould G.W., editor. New Methods of Food Preservations. Blackie Academic and Professional; Glasgow, UK: 1995. pp. 58–89. [Google Scholar]
- 42.Emami S.A., Abedindo B.F., Hassanzadeh-Khayyat M. Antioxidant activity of the essential oils of different parts ofJuniperus excelsa M. Bieb. subsp. excelsa and J. excelsa M. Bieb. subsp. polycarpos (K. Koch) Takhtajan (Cupressaceae) Iran. J. Pharm. Res. 2010;10:799–810. [PMC free article] [PubMed] [Google Scholar]
- 43.Hadaruga N.G., Branic A.G., Hadaruga D.I., Gruia A., Pleșa C., Costescu C., Ardelean A., Lupea A.X. Comparative study of Juniperus communis and Juniperus virginiana essential oils: TLC and GC analysis. J. Planar Chromatogr. 2011;24:130–135. doi: 10.1556/JPC.24.2011.2.9. [DOI] [Google Scholar]
- 44.Barrero A.F., Moral J.F.Q., José F Quílez del, Lara A., Herrador M.M. Herrador. Antimicrobial activity of sesquiterpenes from the essential oil of Juniperus thurifera wood. Planta Med. 2005;71:67–71. doi: 10.1055/s-2005-837753. [DOI] [PubMed] [Google Scholar]
- 45.Keskes H., Belhadj S., Jlail L., El Feki A., Damak M., Sayadi S., Allouche N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm. Biol. 2017;55:88–95. doi: 10.1080/13880209.2016.1230139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glisic S.B., Milojevic S.Z., Dimitrijevic-Brankovic S.I., Orlovic A.M., Skala D.U. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007;72(4):311–320. [Google Scholar]
- 47.Hossain M.A., Zhari I., Atiqur R., Sun C. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind. Crops Prod. 2008;27:328–334. doi: 10.1016/j.indcrop.2007.11.008. [DOI] [Google Scholar]
- 48.Schmidt J.M., Noletto J.A., Vogler B., Setzer W.N. Abaco bush medicine: chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J. Herbs Spices Med. Plants. 2006;12:43–65. doi: 10.1300/J044v12n03_04. [DOI] [Google Scholar]
- 49.Su Y.C., Ho C.L. Composition of the leaf essential oil of Phoebe formosana from Taiwan and its in vitro cytotoxic, antibacterial, and antifungal activities. Nat. Prod. Commun. 2016;11:845–848. [PubMed] [Google Scholar]
- 50.Nasser A.A., Bhuwan K.C., Noura S.D., Khola S., Ahmed J.A., Ludger W., William N.S. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines (Basel) 2017;4:17. doi: 10.3390/medicines4020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J. D-limonene: safety and clinical applications. Altern. Med. Rev. 2007;12:259–264. [PubMed] [Google Scholar]
- 52.Sieniawska E., Swatko-Ossor M., Sawicki R., Skalicka-Woźniak K., Ginalska G. Natural terpenes influence the activity of antibiotics against isolated Mycobacterium tuberculosis. Med. Princ. Pract. 2017;26:108–112. doi: 10.1159/000454680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marina S., Mihailo R. Chemical composition and antifungal activities of essential oils from leaves, calyx and corolla of Salvia brachyodon Vandas. J. Essent. Oil Res. 2011;17:227–229. doi: 10.1080/10412905.2005.9698884. [DOI] [Google Scholar]
- 54.Mun S.P., Prewitt L. Antifungal activity of organic extracts from Juniperus virginiana heartwood against wood decay Fungi. For. Prod. J. 2011;61:443–449. [Google Scholar]
- 55.Mughal M.A., Khan T.Z., Nasir M.A. Antifungal activity of some plant extracts. Pak. J. Phytopath. 1996;8:46–48. [Google Scholar]
- 56.Qasem J.R. Fungitoxicity of weed extracts to tomato wilt pathogen (Fusarium oxysporum f. sp. lycopersici) Emir. J. Agric. Sci. 1996;8:103–112. [Google Scholar]
- 57.Muhammad I., Mossa J.S., El-Feraly F.S. Additional antibacterial diterpenes from the bark of Juniperus procera. Phytother. Res. 1996;10:604–607. [Google Scholar]
- 58.El Jemli M., Naima K., Khadija L., Driss T., Yousra E., Ilias M., El Mahdi W., Yahia C., Katim A. Antifungal and insecticidal properties of Juniperus thurifera leaves. Nat. Prod. Commun. 2018;13:1047–1049. [Google Scholar]
- 59.Cavaleiro C., Pinto E., Gonçalves M.J., Salgueiro L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J. Appl. Microbiol. 2006;100:1333–1338. doi: 10.1111/j.1365-2672.2006.02862.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim J.S., Kuk E., Yu K.N. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Ganash M., Abdel Ghany T.M., Omar A.M. Morphological and biomolecules dynamics of phytopathogenic fungi under stress of silver nanoparticles. BioNanoScience. 2018;8:566–573. doi: 10.1007/s12668-018-0510-y. [DOI] [Google Scholar]
- 62.Abo-Shama U.H., El-Gendy H., Mousa W.S., Hamouda R.A., Yousuf W.E., Hetta H.F., Abdeen E.E. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect. Drug Resist. 2020;13:351–362. doi: 10.2147/IDR.S234425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang W., Yan M., Duan H., Bi Y., Cheng X., Yu H. Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against setosphaeria turcica. J. Nanomater. 2020 doi: 10.1155/2020/9535432. 7. [DOI] [Google Scholar]
- 64.Mahmoud A.L.E. Inhibition of growth and aflatoxin biosynthesis of Aspergillus flavus by extracts of some Egyptian plants. Lett. Appl. Microbiol. 1999;29:334–336. doi: 10.1046/j.1472-1765X.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 65.Sánchez E., Heredia N., García S. Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. Int. J. Food Microbiol. 2005;98:271–279. doi: 10.1016/j.ijfoodmicro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Gömöri C., Nacsa-Farkas E., Kerekes E.B., Vidács A., Bencsik O., Kocsubé S., Khaled J.M., Alharbi N.S., Vágvölgyi C., Krisch J. Effect of essential oil vapours on aflatoxin production of Aspergillus parasiticus. World Mycotoxin J. 2018;11:579–588. doi: 10.3920/WMJ2017.2260. [DOI] [Google Scholar]
- 67.Gömöri C., Nacsa-Farkas E., Kerekes E.B., Kocsubé S., Vágvölgyi C., Krisch J. Evaluation of five essential oils for the control of food spoilage and mycotoxin producing fungi. Acta Biol. Szeged. 2013;57:113–116. [Google Scholar]
- 68.Ameen F., Srinivasan P., Selvankumar T., Kamala-Kannan S., Al Nadhari S., Almansob A., Dawoud T., Govarthanan M. Phytosynthesis of silver nanoparticles using Mangifera indica flower extract as bioreductant and their broad-spectrum antibacterial activity. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102970. [DOI] [PubMed] [Google Scholar]
- 69.Ibrahim E., Zhang M., Zhang Y., Hossain A., Qiu W., Chen Y., Wang Y., Wu W., Sun G., Li B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials. 2020;10:219. doi: 10.3390/nano10020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Zaban M.I., Abd El-Aziz A.R.M., Abdelazim N.S.H. Antifungal and anti-aflatoxin efficacy of mycosynthesis nanosilver particles produced by fusarium species: a physicocultural and molecular study. Dig. J. Nanomater. Biostruct. 2019;14:943–961. [Google Scholar]
- 71.Hafez R.A., Abdel-Wahhab M.A., Sehab A.F., Al-Zahraa A.A.K. Green synthesis of silver nanoparticles using Morus nigra leave extract and evaluation their antifungal potency on phytopathogenic fungi. J. Appl. Pharm. Sci. 2017;7:041–048. doi: 10.7324/JAPS.2017.70206. [DOI] [Google Scholar]
- 72.M.S. Ayatollahi Inhibitory effects of silver nanoparticles on growth and aflatoxin B1 production by Aspergillus parasiticus. Iran. J. Med. Sci. 2015;40:501–506. [PMC free article] [PubMed] [Google Scholar]
- 73.Masood A., Ranjan K.S. The effect of aqueous plant extracts on growth and aflatoxin production by Aspergillus flavus. Lett. Appl. Microbiol. 1991;13:32–34. [Google Scholar]
- 74.Abd El-Aziz A.R.M., Al-Othman M.R., Al-Sohaibani S.A., Mahmoud M.A., Kasi M. Prevention of aflatoxin contamination of maize by Aspergillus flavus through aqueous plant extracts in Saudi Arabia. Afr. J. Microbiol. Res. 2012;6:6931–6935. [Google Scholar]
- 75.Neveen H.A. Chemical composition and antifungal activity of Ocimum basilicum L. Essent. Oil. 2015;3(3):374–379. doi: 10.3889/oamjms.2015.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.