Abstract
We previously demonstrated that a deficiency of natural antibodies against CD25, Mucin 1 (MUC1), and vascular endothelial growth factor receptor 1 (VEGFR1) could contribute to high risk of non‐small cell lung cancer (NSCLC). This study was designed to investigate whether natural IgG antibodies against POU domain class 5 transcription factor 1 (POU5F1), tumor necrosis factor‐α (TNF‐α), and the combination of CD25, VEGFR1, and MUC1 could play an anti‐tumorigenic role against developing NSCLC. An ELISA was developed in‐house to examine plasma IgG against peptide antigens derived from POU5F1, TNF‐α, and a combination of peptide antigens derived from CD25, MUC1, and VEGFR1 in 211 patients with NSCLC and 200 healthy controls. Mann–Whitney U test demonstrated that plasma IgG levels for the combination of peptide antigens derived from CD25, MUC1, and VEGFR1 were significantly lower in NSCLC patients than control subjects (Z = −12.978, P < 0.001) although plasma levels of IgG antibodies for POU5F1 and TNFα were not significantly changed. The in‐house ELISA made with the CD25‐MUC1‐VEGFR1 combination had a sensitivity of 49.6% against a specificity of 95% to detect early‐stage NSCLC. In conclusion, natural antibodies against the combination of CD25, VEGFR1, and MUC1 may be an effective biomarker for early diagnosis of NSCLC.
Keywords: CD25, MUC1, natural antibody, non‐small cell lung cancer, VEGFR1
Group I as an early stage (stages 1 and 2A, n = 121), group II as stage 2B (n = 41), and group III as stages 3 and 4 (n = 49). ROC curve analysis showed that the CD25‐MUC1‐VEGFR1 combination‐based ELISA showed the largest AUC of 0.883 (95% CI 0.844–0.923) with a sensitivity of 49.6% against the specificity of 95.0% in patients with early‐stage NSCLC. Natural antibodies against the combination of CD25, VEGFR1, and MUC1 may be an effective biomarker for early diagnosis of NSCLC.
Abbreviations
- ADCC
antibody‐dependent cellular cytotoxicity
- CSLC
cancer stem‐like cell
- CV
coefficients of variation
- ELISA
enzyme‐linked immunosorbent assay
- MUC1
mucin 1
- NC
negative control
- NSCLC
non‐small cell lung cancer
- OD
optical density
- PC
positive control
- POU5F1
POU domain class 5 transcription factor 1
- QC
quality control
- SBR
specific binding ratio
- SD
standard deviation
- TAA
tumor‐associated antigen
- TNF‐α
tumor necrosis factor‐α
- Treg
regulatory T cells
- VEGF
vascular endothelial growth factor
- VEGFR1
VEGF receptors 1
According to the 2018 global cancer statistics report, lung cancer is the tumor with the highest incidence and mortality among men, while the third highest morbidity and the second leading cause of cancer death in women worldwide [1]. In 2011, the mortality rate of lung cancer patients in China was as high as 25% [2]. According to histopathological classification, lung cancer has been divided into small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC), in which NSCLC accounts for 85% with three subtypes, including adenocarcinoma, squamous cell carcinoma, and large‐cell carcinoma [3]. Although the diagnostic technology and treatment regimens of lung cancer have continuously been improving, the 5‐year survival rate of all combined tumor stages is still less than 20% [4]. Therapeutic efficacy and prognosis of lung cancer are related to pathological stage. Patients with early‐stage lung cancer can have a 5‐year survival rate of 50% [5].
Natural antibodies are a subset of immunoglobulins that are produced by innate B‐lymphocytes without external antigenic stimulation. Natural antibodies can serve as the first line of defense against invading pathogens, removing apoptotic cells and cell debris, and minimizing the inflammatory response. IgM is the main subtype of natural antibodies, but natural IgG and IgA also exist with a range of physiological functions [6, 7]. There is evidence that natural antibodies could destroy transformed cells in the body and contribute to immune surveillance against the development of malignancy [8].
Mucins 1 (MUC1) mainly provide lubrication to the epithelial surfaces and protect the mucosal barriers resisting external physical invasion or biological invasion as a family of glycoproteins widely distributed in various normal mucosal surfaces of the body [9]. MUC1 overexpression and aberrant glycosylation could promote the oncogenesis and metastasis of various malignant tumors including NSCLC [10]. Vascular endothelial growth factor (VEGF) receptor 1 (VEGFR1) promoting angiogenic signaling pathways has been identified as important therapeutic targets in many tumors, including NSCLC [11]. Circulating natural autoantibodies derived from VEGFR1 peptide antigens have been proved to inhibit the proliferation of malignant cell lines derived from hepatocellular carcinoma and pancreatic cancer [8, 12].
POU domain class 5 transcription factor 1 (POU5F1) plays a key role in embryonic development, stem cell pluripotency, proliferative potential, and self‐renewal as the basis of maintaining pluripotent cells. Tumor necrosis factor‐α (TNF‐α) is often found in human tumor tissues and associated with cancer therapy or host response against tumor [13, 14]. However, there are few studies on the relationship between natural autoantibodies against POU5F1 or TNF‐α in NSCLC.
The CD25 is one of the phenotypic markers of CD4+ regulatory T (Treg) cells that play an indispensable role in maintaining immune tolerance and regulating immune response [15]. Circulating natural autoantibodies against CD25‐derived peptide antigens have been proved to be altered in several types of cancer including breast cancer and esophageal cancer [16, 17]. Our previous work demonstrated that natural anti‐CD25 antibody might play a significant role in maintaining the homeostasis of the immune system as the detection of circulating natural antibody against CD25 might affect the immune surveillance function and promote tumorigenesis [18].
In a recent study, we have confirmed that the reduction of circulating natural antibodies against MUC1 and VEGFR1 was associated with the carcinogenesis of NSCLC (unpublished data). In this study, accordingly, the combination of three peptide antigens derived from CD25, MUC1, and VEGFR1 was used to develop an enzyme‐linked immunosorbent assay (ELISA) in‐house to obtain additive signals for detection of natural IgG antibodies against these 3 target molecules in NSCLC; we also used the in‐house ELISA to detect natural IgG antibodies for cancer‐related molecules POU5F1 and TNF‐α in this malignancy.
Materials and methods
Subjects
A total of 211 patients were recruited with the diagnosis of NSCLC based on radiographic examination and histological confirmation at the Department of Thoracic Surgery, China‐Japan Union Hospital of Jilin University in the period between November 2012 and August 2016. All patients were diagnosed with NSCLC for the first time and did not receive anti‐tumor therapy such as surgery, radiotherapy, chemotherapy, and biotherapy before collection of their plasma samples. Patients with other tumors or autoimmune diseases and those with unqualified blood samples were excluded. These 211 patients included 131 males and 80 females with the mean age of 58.7 ± 8.7 years ranging from 18 to 75 years old. The types of NSCLC were restricted to adenocarcinoma and squamous cell carcinoma only in this study. These NSCLC patients were divided into three subgroups based on the TNM (tumor, node, and metastasis) staging system: group I as an early stage (stages 1 and 2A, n = 121), group II as stage 2B (n = 41), and group III as stages 3 and 4 (n = 49). Blood samples were taken prior to any anticancer treatment. In the same period, 200 healthy volunteers with gender (103 males and 97 females), aged 58.6 ± 9.3 years, and smoking history matched with NSCLC patients were collected in the community. The control subjects did not include those who had either any type of malignant tumors or autoimmune diseases such as autoimmune thyroid disease, pernicious anemia, type 1 diabetes, celiac disease, multiple sclerosis, systemic lupus erythematosus, and inflammatory bowel diseases.
This study was approved by the Ethics Committee of the Second Hospital of Jilin University and in line with the Declaration of Helsinki. All participants were of Chinese Han origin, and all signed written informed consent to participate in the study.
Detection of plasma IgG levels
An in‐house ELISA was developed to detect plasma natural IgG antibodies against linear peptide antigens derived from POU5F1, TNF‐α, and the combination of CD25, MUC1, and VEGFR1 (the mixture of all 3 peptides in equal concentrations). These peptide antigens were designed by a computational epitope prediction software (http://www.iedb.org) and then synthesized by solid‐phase chemistry with a purity of >95%. Detailed sequence information is given in Table 1. Each peptide antigen was dissolved in 67% acetic acid to obtain a stock solution of 5 mg·mL−1 that was diluted in coating buffer (0.1 m phosphate buffer containing 0.15 m NaCl and 10 mm EDTA, pH 7.2) to a working solution of 10 μg·mL−1 just before use. Maleimide‐activated 96‐well microplates (Thermo Fisher Scientific, Shanghai, China) were coated with 100 µL of antigen working solution according to manufacturer’s instruction. Plasma IgG antibodies were tested with the antigen‐coated 96‐well microplate, the sample well containing 50 µL plasma diluted 1: 100 in assay buffer in the sample wells, the negative control (NC) well containing 50 µL assay buffer, and the positive control (PC) well containing 50 µL purified IgG solution (50 µg·mL−1), which was purified from pooled plasma donated by >1000 healthy subjects [19, 20, 21]. All samples were tested in duplicate, and the variation between the two tests was evaluated by dispersion that is calculated as follows:
Table 1.
The sequence information for peptide antigens used for the development of ELISA for antibody test.
| Antigen | Sequence (N → C) | NCBI accession | Position (aa) |
|---|---|---|---|
| MUC1 | H‐crynltisdvsvsdvpfpfsaqsgah‐OH | NP_002447 | 149–173 |
| VEGFR1 | H‐dlklsctvnkflyrdvtwillrtvnnrtmhysi‐OH | NP_001153502 | 572–600 |
| CD25 | H‐iyhfvvgqmvyyqcvqgyralhrgpaesve‐OH | NP_000408 | 116–144 |
| POU5F1 | keleqfakllkqkritlgytqadvgltc | NP_001272916.1 | 49–75 |
| TNF‐α | cqlqwlnrranallangvelrdnqlv | NP_000585.2 | 101–125 |
If the dispersion was less than 10%, the result was valid; while the dispersion was ≥ 10%, the result was invalid and the test should be repeated. The specific binding ratio (SBR) was used to represent a relative level of plasma IgG, and the SBR was calculated using the optical density (OD) as follows:
To assess the reproducibility of the in‐house ELISA, pooled plasma collected from >30 unrelated healthy donors, called quality control (QC) sample, was tested on every plate. The inter‐assay deviation was estimated with SBR from QC sample test. The coefficient of variation (CV) was used to represent the reproducibility (Table 2).
Table 2.
The reproducibility of the in‐house ELISA in all IgG tests.
| Antigen | Mean ± SD (n) a | CV (%) |
|---|---|---|
| POU5F1 | 2.020 ± 0.206 (20) | 10.21 |
| TNF‐α | 1.365 ± 0.173 (20) | 12.60 |
| CD25‐MUC1‐VEGFR1 | 0.473 ± 0.043 (20) | 9.03 |
(n) represents the number of plates tested.
Statistical analysis
All antibody test data were expressed as means ± standard deviation (SD). Mann–Whitney U test was used to examine the differences in plasma IgG levels between patients with NSCLC and healthy controls due to the skewed distribution of plasma antigen‐specific IgG levels based on Kolmogorov–Smirnov one‐sample test (Table 3). Receiver operating characteristic (ROC) curve analysis was performed to work out the area under the ROC curve (AUC) with 95% confidence interval (CI) and the sensitivity of anti‐CD25 IgG assay against a specificity of >95%.
Table 3.
Kolmogorov–Smirnov test for a normal distribution of plasma IgG level.
| IgG | Skewness | Kurtosis | P * , a |
|---|---|---|---|
| POU5F1 | |||
| Patient | 3.307 | 14.705 | <0.001 |
| Control | 3.625 | 18.398 | <0.001 |
| TNF‐α | |||
| Patient | 0.968 | 2.121 | <0.001 |
| Control | 0.180 | 2.551 | <0.001 |
| CD25‐MUC1‐VEGFR1 | |||
| Patient | 0.754 | 0.690 | 0.903 |
| Control | −0.083 | 0.013 | 0.001 |
P > 0.05 was considered to be normally distributed.
Results
The demographic information and clinical characteristics of all subjects recruited have been described in our previous publication [19].
The in‐house ELISA made with the CD25‐MUC1‐VEGFR1 combination showed significantly lower IgG levels in NSCLC patients than control subjects (Z = −12.978, P < 0.001), and both male and female patients contributed to the decrease in plasma IgG levels, while plasma anti‐POU5F1 IgG levels were significantly lower in female patients with NSCLC than female control subjects (Z = −2.477, P = 0.013) (Table 4). In addition, decreased IgG against POU5F1 and the CD25‐MUC1‐VEGFR1 combination levels mainly occurred in patients with early‐stage NSCLC (Table 5). There was a significant decrease in plasma IgG levels for CD25‐MUC1‐VEGFR1 combinations in patients with either adenocarcinoma or squamous cell carcinoma while a significant decrease in plasma IgG levels for POU5F1 in patients with adenocarcinoma (Table 6).
Table 4.
The levels of plasma IgG against peptide antigens derived from specific target molecules in patients with NSCLC and control subjects.
| IgG | Gender | Patients | Controls | Z * , a | P b |
|---|---|---|---|---|---|
| Pou5F1 | Male | 0.760 ± 0.556 (131) | 0.802 ± 0.623 (103) | −0.803 | 0.422 |
| Female | 0.692 ± 0.581 (80) | 0.811 ± 0.562 (97) | −2.477 | 0.013 | |
| Both | 0.734 ± 0.565 (211) | 0.806 ± 0.593 (200) | −2.249 | 0.025 | |
| TNF‐α | Male | 1.084 ± 0.325 (131) | 1.037 ± 0.318 (103) | −0.366 | 0.715 |
| Female | 1.066 ± 0.371 (80) | 1.123 ± 0.331 (97) | −1.270 | 0.024 | |
| Both | 1.077 ± 0.342 (211) | 1.079 ± 0.327 (200) | −0.713 | 0.476 | |
| CD25‐MUC1‐VEGFR1 | Male | 0.370 ± 0.101 (131) | 0.520 ± 0.121 (103) | −8.635 | <0.001 |
| Female | 0.346 ± 0.100 (80) | 0.555 ± 0.113 (97) | −9.453 | <0.001 | |
| Both | 0.361 ± 0.101 (211) | 0.537 ± 0.118 (200) | −12.978 | <0.001 |
Plasma IgG levels are expressed as mean ± SD in SBI, with n = 211 in the patient group (131 males and 80 females) and n = 200 in the control group (103 males and 97 females).
Mann–Whitney U test (two‐tailed).
P < 0.017 was considered statistically significant based on the Bonferroni correction.
Table 5.
The levels of plasma IgG against peptide antigens derived from specific target molecules in subgroups of NSCLC.
| IgG | Group a | Patient(n) | Control(n) | Z b | P c |
|---|---|---|---|---|---|
| POU5F1 | I | 0.735 ± 0.640 (121) | 0.806 ± 0.593 (200) | −2.463 | 0.014 |
| II | 0.678 ± 0.369 (41) | 0.806 ± 0.593 (200) | −1.340 | 0.180 | |
| III | 0.778 ± 0.503 (49) | 0.806 ± 0.593 (200) | −0.392 | 0.695 | |
| TNF‐α | I | 1.031 ± 0.338 (121) | 1.079 ± 0.327 (200) | −1.928 | 0.054 |
| II | 1.137 ± 0.354 (41) | 1.079 ± 0.327 (200) | −0.738 | 0.461 | |
| III | 1.142 ± 0.330 (49) | 1.079 ± 0.327 (200) | −0.876 | 0.381 | |
| CD25‐MUC1‐VEGFR1 | I | 0.347 ± 0.107 (121) | 0.537 ± 0.118 (200) | −11.504 | <0.001 |
| II | 0.376 ± 0.087 (41) | 0.537 ± 0.118 (200) | −7.279 | <0.001 | |
| III | 0.384 ± 0.093 (49) | 0.537 ± 0.118 (200) | −7.507 | <0.001 |
Plasma IgG levels were expressed as mean ± SD in SBI.
Group I for stages 1and 2A, group II for stage 2B, and group III for stages 3 and 4.
Mann–Whitney U test (two‐tailed).
P < 0.017 was considered statistically significant based on the Bonferroni correction.
Table 6.
The levels of plasma IgG against peptide antigens derived from specific target molecules in two histological types of NSCLC. AC, adenocarcinoma; SCC, squamous cell cancer.
| IgG | Type | Patient (n) | Control (n) | Z a | P b |
|---|---|---|---|---|---|
| POU5F1 | SCC | 0.775 ± 0.637 (87) | 0.806 ± 0.593 (200) | −1.015 | 0.310 |
| AC | 0.705 ± 0.509 (124) | 0.806 ± 0.593 (200) | −2.503 | 0.012 | |
| TNF‐α | SCC | 1.105 ± 0.347 (87) | 1.079 ± 0.327 (200) | −0.058 | 0.954 |
| AC | 1.058 ± 0.339 (124) | 1.079 ± 0.327 (200) | −1.093 | 0.275 | |
| CD25‐MUC1‐VEGFR1 | SCC | 0.375 ± 0.106 (87) | 0.537 ± 0.118 (200) | −9.413 | <0.001 |
| AC | 0.351 ± 0.097 (124) | 0.537 ± 0.118 (200) | −11.639 | <0.001 |
Plasma IgG levels are expressed as mean ± SD in SBI.
Mann–Whitney U test (two‐tailed).
P < 0.017 was considered statistically significant based on the Bonferroni correction.
Receiver operating characteristic curve analysis showed that the CD25‐MUC1‐VEGFR1 combination‐based ELISA showed the largest AUC of 0.883 (95% CI 0.844–0.923) with a sensitivity of 49.6% against the specificity of 95.0% in patients with early‐stage NSCLC (Fig. 1).
Fig. 1.
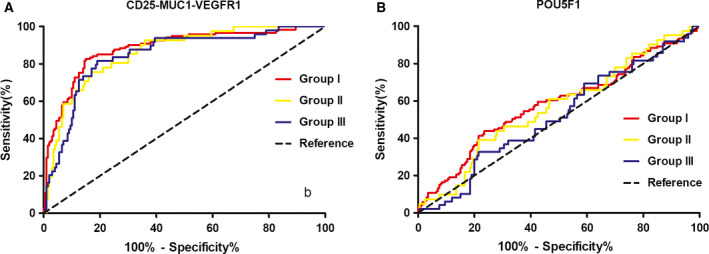
Receiver operating characteristic curve analysis of circulating IgG levels in three subgroups of NSCLC. Group I for stages 1 and 2A, group II for stage 2B, and group III for stages 3 and 4. (A) The CD25‐MUC1‐VEGFR1 IgG assay with a specificity of 95% showed an AUC of 0.883 (95% CI 0.844–0.923) with a sensitivity of 49.6% in group I, an AUC of 0.861 (95% CI 0.804–0.918) with a sensitivity of 39.0% in group II, and an AUC of 0.846 (95% CI 0.785–0.907) with a sensitivity of 26.5% in group III. (B) The POU5F1 IgG assay with a specificity of 95% showed an AUC of 0.582 (95% CI 0.516–0.648) with a sensitivity of 10.7% in group I, an AUC of 0.566 (95% CI 0.470–0.663) with a sensitivity of 7.3% in group II, and an AUC of 0.518 (95% CI 0.429–0.607) with a sensitivity of 2.0% in group III.
Discussion
The present study demonstrated that patients with NSCLC, including both adenocarcinoma and squamous cell carcinoma, had a significant decrease in plasma IgG antibodies against the combination of three peptide antigens derived from CD25, MUC1, and VEGFR1, respectively. Patients with adenocarcinoma mainly contributed to the alteration of plasma anti‐POU5F1 IgG levels. Altered anti‐POU5F1 IgG antibody is likely to be involved in a female population, suggesting that deficiency of anti‐POU5F1 IgG is likely to be indicative of a subgroup of NSCLC. It is worth noting that circulating IgG for the CD25‐MUC1‐VEGFR1 combination had the highest sensitivity of 49.6% against a specificity of 95%, with an AUC of 0.883 (95% CI 0.844–0.923) in early‐stage NSCLC, suggesting that natural antibodies for this combination may be an effective biomarker for early diagnosis of NSCLC.
A decrease in natural antibody levels in the body may be involved in many health conditions, such as cancer, cardiovascular disease, and diabetes [22, 23]. In our research, we observed that deficiency of natural IgG antibodies against the CD25‐MUC1‐VEGFR1 peptide combination was strongly associated with lung cancer and that patients with early‐stage NSCLC were more likely to have low antigen‐specific IgG levels in the circulation (Table 5). The above findings raise the possibility that deficiency of natural anticancer antibodies could contribute to a high risk of developing malignancy as natural antibodies could destroy transformed cells and contribute to immune surveillance against carcinogenesis [8, 24, 25]. Therefore, detection of decreased natural antibody levels in the circulation may be a powerful tool for early diagnosis of malignant tumors including NSCLC.
CD25 is the interleukin‐2 receptor alpha chain (IL2RA) and is highly expressed in Treg cells. Increased number of Treg cells in the circulation and high infiltration in tumor microenvironment have been reported in several types of solid tumors [26]. Decreased anti‐CD25 IgG levels may enhance the activity of Treg cells and immune tolerance, and weaken immune surveillance, leading to immune escape of tumor cells [27, 28]. In a recent study, we found that circulating anti‐CD25 IgG antibody levels were significantly decreased in patients with NSCLC [18]. In combination of tumorigenic mechanism and the expression of MUC1 and VEGFR1 in lung cancer, the combined detection of three individual natural autoantibodies is more valuable in early diagnosis of NSCLC.
A series of studies have shown that a small number of cells in lung cancer have tumor initiation activity, which is called cancer stem‐like cell (CSLC) and may be related to the recurrence of lung cancer after surgery or other anti‐tumor treatments [29, 30]. Abnormal expression of POU5F1 contributes to tumorigenesis and metastasis [31]. Our results suggest that anti‐POU5F1 IgG is likely to be indicative of a subgroup of NSCLC, although the resulting ROC curve has failed to show a diagnostic value.
The previous studies have confirmed that peripheral blood antibodies against tumor‐associated antigens (TAAs) are useful biomarkers for early diagnosis of tumors including lung cancer [32, 33]. Chapman and co‐workers developed an in‐house ELISA made either from 6 TAAs (p53, Nyeso‐1, CAGE, GBU4‐5, Annexin 1, and SOX2) or from 7 TAAs (p53, NY‐ESO‐1, CAGE, GBU4‐5, SOX2, HUD, and MAGE A4) to detect plasma anti‐TAA autoantibodies, but they applied increased levels of plasma anti‐TAA antibodies for early diagnosis of lung cancer [33]. In fact, plasma TAA autoantibody levels could be increased with cancer progression, so that such ELISA tests are unlikely to be sensitive enough for early diagnosis. In function, natural autoantibodies can exert anti‐tumor effects by directly inducing antibody‐dependent cellular cytotoxicity and complement‐dependent cytotoxicity. Because of the effect of the immune amplification, even if the tumor antigen content in peripheral blood is lower than a detectable range, the natural autoantibody can still show a higher concentration, even before tumors are formed. Therefore, natural autoantibodies have more advantages than TAAs in early diagnosis of lung cancer.
This study has a couple of limitations. First, sample size used in this study is rather small, so the sample power is not enough to analyze the results from stage 1A patients. Further investigation should focus on stage 1A of NSCLC with large sample size that will lead to a firm conclusion. Second, a prospective study should be carried out to confirm if people with a low level of circulating IgG for the CD25‐MUC1‐VEGFR1 combination are more likely to develop NSCLC than those with a normal level of such autoantibodies.
Conclusions
The in‐house ELISA made by the combination of three peptide antigens derived from CD25, MUC1, and VEGFR1 could enhance the sensitivity for detection of natural anticancer antibodies that have a diagnostic value in screening of early‐stage NSCLC.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
SL carried out laboratory work, data analysis, and drafting the manuscript. XZ conceived this study and corrected the manuscript. QJ was responsible for identification of patients and healthy controls, and collection of samples and clinical information. TL supervised laboratory work and data analysis.
Acknowledgements
We sincerely thank the Tissue Specimen Bank of China‐Japan Union Hospital of Jilin University for providing plasma samples collected from patients with lung cancer. We thank the patients and healthy volunteers for their support and participation.
Contributor Information
Xuan Zhang, Email: zhangxuankj@163.com.
Tingting Liang, Email: liangting_2006@163.com.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. She J, Yang P, Hong Q and Bai C (2013) Lung cancer in China: challenges and interventions. Chest 143, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 3. Sher T, Dy GK and Adjei AA (2008) Small cell lung cancer. Mayo Clin Proc 83, 355–367. [DOI] [PubMed] [Google Scholar]
- 4. Spira A and Ettinger DS (2004) Multidisciplinary management of lung cancer. N Engl J Med 350, 379–392. [DOI] [PubMed] [Google Scholar]
- 5. Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, Wiewrodt R, Barnes AC and Robertson JF (2008) Autoantibodies in lung cancer: possibilities for early detection and subsequent cure. Thorax 63, 228–233. [DOI] [PubMed] [Google Scholar]
- 6. Holodick NE, Rodriguez‐Zhurbenko N and Hernandez AM (2017) Defining natural antibodies. Front Immunol 8, 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palma J, Tokarz‐Deptula B, Deptula J and Deptula W (2018) Natural antibodies – facts known and unknown. Central‐Europ J Immunol 43, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Y, Yan Z, Huang Y, Qiu C, Chen X, Hu Y, Meng Q and Wei J (2017) Study of natural IgG antibodies against vascular endothelial growth factor receptor 1 in hepatocellular carcinoma. Am J Cancer Res 7, 603–609. [PMC free article] [PubMed] [Google Scholar]
- 9. Kaur S, Kumar S, Momi N, Sasson AR and Batra SK (2013) Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol 10, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen PL, Niehans GA, Cherwitz DL, Kim YS and Ho SB (1996) Membrane‐bound (MUC1) and secretory (MUC2, MUC3, and MUC4) mucin gene expression in human lung cancer. Tumour Biol 17, 176–192. [DOI] [PubMed] [Google Scholar]
- 11. Hicklin DJ and Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23, 1011–1027. [DOI] [PubMed] [Google Scholar]
- 12. Wu L, He Z, Huang Y, Cai W, Meng Q and Wei J (2017) Inhibitory role of natural IgG antibodies against VEGFR1 in the proliferation of pancreatic cancer. J Pancreas 18, 133–137. [Google Scholar]
- 13. Frankenberg S and Renfree MB (2013) On the origin of POU5F1. BMC Biol 11, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9, 361–371. [DOI] [PubMed] [Google Scholar]
- 15. Sakaguchi S, Sakaguchi N, Asano M, Itoh M and Toda M. (2011) Pillars article: immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J. Immunol. 1995. J Immunol (Baltimore, Md : 1950) 186, 3808–3821. [PubMed] [Google Scholar]
- 16. Liu T, Song YN, Shi QY, Liu Y, Bai XN and Pang D (2014) Study of circulating antibodies against CD25 and FOXP3 in breast cancer. Tumour Biol 35, 3779–3383. [DOI] [PubMed] [Google Scholar]
- 17. Guan S, Liu B, Zhang C, Lee KH, Sun S and Wei J (2013) Circulating autoantibody to CD25 may be a potential biomarker for early diagnosis of esophageal squamous cell carcinoma. Clin Trans Oncol 15, 825–829. [DOI] [PubMed] [Google Scholar]
- 18. Zhao H, Zhang X, Han Z, Xie W, Yang W and Wei J (2018) Alteration of circulating natural autoantibodies to CD25‐derived peptide antigens and FOXP3 in non‐small cell lung cancer. Sci Rep 8, 9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao H, Zhang X, Han Z, Wang Z and Wang Y (2018) Plasma anti‐BIRC5 IgG may be a useful marker for evaluating the prognosis of nonsmall cell lung cancer. FEBS Open Bio 8, 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao H, Zhang X, Han Z and Wang Y (2018) Circulating anti‐p16a IgG autoantibodies as a potential prognostic biomarker for non‐small cell lung cancer. FEBS Open Bio 8, 1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu S, Zhang X, Wang J, Yang H, Jiang Y, Qiu C and Meng Q (2020) Analysis of plasma autoantibodies for inflammatory cytokines in patients with first‐episode schizophrenia among a Chinese population. J Neuroimmunol 341, 577165. [DOI] [PubMed] [Google Scholar]
- 22. Meier LA and Binstadt BA (2018) The contribution of autoantibodies to inflammatory cardiovascular pathology. Front Immunol 9, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holmes D (2016) Diabetes: Natural autoantibodies protect against T1DM. Nature Rev Endocrinol 12, 560. [DOI] [PubMed] [Google Scholar]
- 24. Ebrahimnezhad S, Jazayeri M, Hassanian SM and Avan A (2017) Current status and prospective regarding the therapeutic potential of natural autoantibodies in cancer therapy. J Cell Physiol 232, 2649–2652. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz‐Albiez R, Laban S, Eichmüller S and Kirschfink M (2008) Cytotoxic natural antibodies against human tumours: an option for anti‐cancer immunotherapy? Autoimmun Rev 7, 491–495. [DOI] [PubMed] [Google Scholar]
- 26. Shang B, Liu Y, Jiang SJ and Liu Y (2015) Prognostic value of tumor‐infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta‐analysis. Sci Rep 5, 15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T and Sakaguchi S (2005) Treatment of advanced tumors with agonistic anti‐GITR mAb and its effects on tumor‐infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med 202, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T and Nakayama E (1999) Tumor rejection by in vivo administration of anti‐CD25 (interleukin‐2 receptor alpha) monoclonal antibody. Can Res 59, 3128–3133. [PubMed] [Google Scholar]
- 29. Sullivan JP and Minna JD (2010) Tumor oncogenotypes and lung cancer stem cell identity. Cell Stem Cell 7, 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levina V, Marrangoni A, Wang T, Parikh S, Su Y, Herberman R, Lokshin A and Gorelik E (2010) Elimination of human lung cancer stem cells through targeting of the stem cell factor‐c‐kit autocrine signaling loop. Can Res 70, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS et al (2010) Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell‐like properties and epithelial‐mesenchymal transdifferentiation. Can Res 70, 10433–10444. [DOI] [PubMed] [Google Scholar]
- 32. Lam S, Boyle P, Healey GF, Maddison P, Peek L, Murray A, Chapman CJ, Allen J, Wood WC, Sewell HF et al (2011) EarlyCDT‐Lung: an immunobiomarker test as an aid to early detection of lung cancer. Cancer Prev Res (Phila) 4, 1126–1134. [DOI] [PubMed] [Google Scholar]
- 33. Chapman CJ, Healey GF, Murray A, Boyle P, Robertson C, Peek LJ, Allen J, Thorpe AJ, Hamilton‐Fairley G, Parsy‐Kowalska CB et al (2012) EarlyCDT(R)‐Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol 33, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]


