Abstract
Since the discovery of vasopressin (VP) and oxytocin (OT) in 1953, considerable knowledge has been gathered about their roles in cardiovascular homeostasis. Unraveling VP vasoconstrictor properties and V1a receptors in blood vessels generated powerful hemostatic drugs and drugs effective in the treatment of certain forms of circulatory collapse (shock). Recognition of the key role of VP in water balance via renal V2 receptors gave birth to aquaretic drugs found to be useful in advanced stages of congestive heart failure. There are still unexplored actions of VP and OT on the cardiovascular system, both at the periphery and in the brain that may open new venues in treatment of cardiovascular diseases. After a brief overview on VP, OT and their peripheral action on the cardiovascular system, this review focuses on newly discovered hypothalamic mechanisms involved in neurogenic control of the circulation in stress and disease.
Keywords: Vasopressin, oxytocin, blood pressure, heart rate, short-term variability, stress, hypertension, heart failure
1. INTRODUCTION
The golden era of peptide research gathered considerable knowledge about the biological functions of the neurohypophysial peptides vasopressin (VP) and oxytocin (OT) on the cardiovascular system, discovered in 1953 [1-3]. Understanding of the peripheral actions of VP on vascular resistance was crucial for the development of effective vasoconstrictor drugs, analogues of VP (felipressin, terlipressin) targeting V1a receptors (V1aR) in blood vessels, used today as first line treatment in some forms of upper gastrointestinal tract (esophageal) bleeding. Also, the vasoconstrictor properties of VP turned out to be life saving in late stages of shock (hemorrhagic, septic) when blood vessels become irresponsive to circulating catecholamines. Appreciating the role of VP in the pathogenesis of heart failure (HF) gave birth to new class of aquaretic drugs, nonselective and selective V2 receptor (V2R) antagonists for advanced stages of congestive HF associated with dilutional hyponatremia. The efficacy of these drugs is being critically reviewed in professional and medical journals [4-6].
VP and OT, both peripherally and centrally, have actions on the cardiovascular system still not well understood. It has been discovered that OT synthesized in the heart acts as an important factor in cardiac cell differentiation and cardiac healing. Furthermore, a cardio-protective potential of OT during cardiac ischemia and diabetes has been suggested. These exciting new data are comprehensively reviewed by the main contributors in the field [7, 8].
It is believed that shedding light on the central action of VP and OT on the neurogenic control of the circulation may open new venues in the treatment of cardiovascular diseases associated with hyperadrenergic states such as primary hypertension and HF. Autocrine and paracrine factors involved in the local control of VP and OT release have been discovered clarifying the functioning of magnocellular neurons (MCN) located in the supraoptic nucleus (SON) and the paraventricular (PVN) nucleus of the hypothalamus. Because peripheral and central actions of VP and OT on the cardiovascular system are tightly interconnected, in the following sections we will first give a brief and updated overview on VP, OT and of their peripheral actions on the cardiovascular system [9]. Then, we will focus this review on newly discovered hypothalamic mechanisms modulating the excitability of MCNs and of the pre-sympathetic VP and OT neurons implicated in neurogenic control of the circulation in stress and disease.
2. VP & OT: ORIGIN, STRUCTURE, RECEPTORS AND FUNCTIONS
VP and OT are synthetized in the brain mainly by the MCNs in the hypothalamic PVN and SON. They are small cyclic peptides consisting of 9 amino-acids transcribed from genes located adjacent to each other at chromosome 20, encoding large pre-pro-peptides [10, 11]. Although similar in structure (differing only in 2 amino-acids at position 3 and 9) VP an OT exert diverse, often opposing biological actions, and participate in cardiovascular homeostasis at the periphery and in the brain. VP and OT containing MCNs in SON and in the magnocellular part of the PVN project to neurohypohysis where they are released directly into systemic blood and hypophyseal portal circulation. Neurons from parvocellular part of the PVN project to extrahypothalamic structures performing classical “wire” neurotransmission [12]. In addition, VP and OT are released intranuclearly from somata and dendrites of MCNs where they act in autocrine/paracrine fashion, diffuse into extracellular fluid and exert “volume” communication by reaching distant receptors [13].
All actions, including the cardiovascular actions of VP and OT, are mediated via specific, very similar, membrane G protein linked receptors, widely distributed in the body [14-34]. The majority of central nervous system actions [35-40] and of the peripheral actions of VP including vasoconstriction [41, 42], glycogenolysis [43, 44] adrenal angiotensin II secretion [45] and platelet adhesion [46] are mediated via type 1 receptors (V1aR), coupled to a Gq/11 protein, phospholipases C, D and A2 as well as intracellular calcium increase [22, 23]. In the kidneys, VP binds to type 2 receptors (V2R) coupled to cAMP production, and control aqaporin-2 (Aqp-2) gene expression and synthesis, affecting water reabsorption and urine concentration [47, 48]. V1bR discovered later, were found to modulate the effect of corticotrophin releasing factor (CRF) on adrenocorticotrophic hormone (ACTH) synthesis and release in the stress response [49]. V1bR were also found in the limbic system where they exert anxiogenic effects [50-54]. OT, popularly called the “love hormone” for its role in the regulation of reproductive and social behaviors, binds to specific OT receptors (OTR) coupled to Gq/11 protein, and also to V1aR [23, 24]. OT synthetized in the heart was reported to support cardiac function, development and regeneration [7, 8], and to reduce cardiovascular reactivity to stress [55-60].
It is important to mention that changes of the structure of VP and OT and of their receptors are common, and, are due to naturally occurring genetic polymorphisms and mutations. These structural changes have been shown to affect VP and OT release, receptor distribution and predilection to disease [61]. In addition, the OT and VP pathways of the brain show sexual dimorphism that impact reproductive and social behaviors [62, 63]. But we still do not know how these phenomena affect cardiovascular homeostasis and susceptibility to cardiovascular diseases.
3. VP & OT IN PERIPHERAL CONTROL OF THE CIRCULATION
Blood osmolality is the best fine-tuned bodily variable required for normal functioning of excitable tissues primarily the brain and the heart. Changes in blood osmolality as small as 1-2% are detected by osmo-sensitive receptors located in the circumventricular organs and conveyed to the PVN to modulate the synthesis and the release of VP [64]. An increase in blood osmolality stimulates VP release into the blood. VP then binds to V2R in the membrane of epithelial cells of the collecting duct of the kidneys, triggering fusion of Aqp-2 (structural component of water channels) containing vesicles to the apical plasma membrane that enhances water re-absorption with consequent normalization of blood osmolality and urine concentration. Long-lasting osmotic stimuli increase Aqp-2 gene expression and the number of water pores in the apical membrane of the collecting ducts [47, 48].
Loss of circulating blood volume during dehydration and hemorrhage, is another strong stimulus to VP release into the circulation [65-67]. Increased blood concentration of VP will then stimulate V1aRs that are less sensitive than V2R, located in the peripheral arteries, constrict them and increase peripheral resistance to maintain the circulation. At a molar basis VP has been shown to be the most potent vasoconstrictor, even more powerful than angiotensin II and noradrenalin [41]. However, in some vascular beds, primarily the kidneys, lungs and basilaries, VP produces vasodilatation involving V2Rs or V1aRs and calcium dependent NO release [68-72].
OT also binds to V1aR in peripheral arteries and produces weak vasoconstriction except in the umbilical artery at term [73, 74]. In some vascular beds with increased vascular tone, OT produces vasodilation similarly to VP via V1aR and calcium dependent NO liberation [75, 76]. Intriguingly, the effect of OT on vascular tone is unaffected by pregnancy [77].
VP and OT when applied peripherally and centrally in very high, non-physiological (pharmacological) doses change the mean level of arterial blood pressure (BP) in opposing manner [39, 78, 79]. However, under basal physiological conditions, they do not contribute to BP maintenance [39, 78, 79]. In rats with naturally occurring VP gene deletion [80] and V1aR knock-out (KO) mice [45] basal values of BP and heart rate (HR) were reported to be slightly lower than those of the wild type controls. The lack of V1aRs-mediated enhancement of BP baro-receptor reflex sensitivity (BRS) in area postrema and the loss of V1aR-mediated facilitation of renin release in macula densa seem to be the cause [81]. In contrast, OT KO mice exhibit slightly higher values of BP and HR [82].
Both VP and OT have been found to produce direct actions on the heart. In large doses VP was found to exert negative inotropic effects via V1R, whereas in low doses it produces an opposite effect [83]. Furthermore, the heart synthesizes OT and expresses OTR, and under hypervolemic conditions cardiac OTRs were found to increase atrial natriuretic peptide release and enhance natriuresis [84-87]. OTRs were also found in the macula densa of the kidneys where they produce natriuresis by the modulation of tubuloglomerular feed-back and solute transport [84]. In addition, in the rat SON, every OT cell is osmo-sensitive that projects to the posterior pituitary [88]. Nonetheless, OT natriuretic properties have not been confirmed in humans [89, 90].
It is worth noting that heart-derived OT seems to have a role in the heart development and cardiac stem cell renewal and regeneration [7]. The highest activation of the OT system in the heart was observed in fetal and newborn rats [91]. It has been shown that OT stimulates cardiomyogenesis in several lines of embryonic stem cells isolated from rat hearts [92, 93]. Moreover, OT has been found to exert cardioprotective role under pathophysiological conditions such as cardiac ischemia. Several peripheral mechanisms were found to be implicated in the cardioprotective action of OT. Firstly, OT was found to reduce HR by direct vagomimetic and NO generating action, and by doing so, it increases diastole during which cardiac oxygenation occurs [94]. Secondly, OT acts as an antioxidant and prevents reactive oxygen species synthesis presumably by opening mitochondrial K+ATP channels and by inhibiting mitochondrial permeability transition pore [95, 96]. Finally, during sepsis [97] and myocardial infarction [98], OT has been found to exert anti-inflammatory action via down-regulation of cytokines gene expression, primarily tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β) and interleukin 6 (IL-6). OT has also been shown to alleviate diabetic cardiomyopathy in rats [8]. Surprisingly, chronic increase of OTR in the heart of transgenic mice has been reported to be detrimental, not beneficial [99].
4. VP & OT IN NEUROGENIC CONTROL OF THE CIRCULATION
4.1. Cardio-respiratory Reflexes
Receptors located in the large thoracic vessels and the heart that perceive changes in blood pressure - baro-receptors [100-103], blood volume - stretch-receptors [104-110] and blood gases - chemo-receptors [111-116] modulate VP synthesis and release [47, 48].
The key negative corrector of BP is the arterial baro-receptor reflex. It is involved in the short-term and the long-term BP control and its failure has been associated with cardiovascular morbidity and mortality [117]. Changes of BP are detected by baro-receptors that are located in the upper part of the body in the aortic arch and carotid sinuses to ensure, at all times, adequate blood supply to the brain. Afferent branches of the baro-receptor reflex, the vagus nerve (X) and glossopharingeal nerve (IX), transmit information about BP level directly to nucleus of the solitary tract (NTS) located beneath the fourth cerebral ventricle. From NTS information diverge to caudal (CVLM) and rostral ventrolateral medulla (RVLM), vagal nuclear complex, and the hypothalamus controlling VP release. Efferent branches of the baro-receptor reflex arise from vagal nuclear complex (dorsal nucleus and nucleus ambiguus) and RVLM where the parasympathetic and sympathetic outflow to the cardiovascular system is set [118].
In experimental settings it has been observed that VP acting at the periphery increases the sensitivity of the baro-receptor reflex (BRS). Infusion of small doses of VP to conscious dogs were found to increase total peripheral resistance and concomitantly to decrease cardiac output without affecting the level of BP [119]. Furthermore, it was reported that VP decreases HR and RSNA more than other vasoconstrictors for a given rise in BP, and that these effects could be abolished by sino-aortic deafferentation and electrolytic destruction of area postrema [100-103]. Thus, it was postulated that peripheral VP acts via area postrema, a structure easily accessible from the blood and abundantly connected to NTS [120, 121], to sensitize the baro-receptor reflex [65, 122]. Pharmacological evidence pointed to both V1aR and V2R receptors [123-129] as mediators of baro-receptor reflex desensitization in area postrema. The role of V1aRs in baro-receptor desensitization was confirmed in V1aR KO mice [130], but the role of V2Rs were neither confirmed nor excluded. Despite the effectiveness of selective V2R agonists and antagonists in the modulation of BRS, histological studies did not confirm the existence of V2R in area postrema [21].
Changes of blood volume, are sensed by specific receptors located at the junction of great thoracic veins and the left atrium and initiate a stretch-receptor reflex in order to maintain BP in homeostatic range. Stretch-receptors share the same glossopharingeal parasympathetic afferents with baro-receptors, to convey information to the PVN. Both dehydration and hemorrhage, by unloading thoracic vessels, increase sympathetic outflow and VP release, in a volume dependent manner [104-110]. When extreme hypotension occurs during hemorrhagic shock, Peuler and associates [109] provided evidence that VP mediates renal sympatho-inhibition and bradycardia, adjusting the functioning of the kidneys and the heart to reduced circulation. This protective effect of VP during shock seems to involve V1aRs [78, 106]. Conversely, during hypervolemia, when circulating volume distends stretch-receptors, the activation of pre-sympathetic PVN neurons was observed [131, 132]. John Coote and collaborators [133-138] identified two pools of PVN neurons in the parvocellular division differentially affected by the stretch receptors. Both project to the intermediolateral column (IML) of the spinal cord near pre-ganglionic sympathetic neurons. One pool of neurons contains OT and terminate at the level of upper thoracic part of the spinal cord and mediate tachycardia by OTR stimulation. The other pool of neurons contain VP, project to lower parts of the spinal cord, bind to V1aR and induce renal symaptho-inhibition, vasodilatation and diuresis [66]. Thus, distension of stretch-receptors creates a unique pattern of the hemodynamic response in order to pump out superfluous volume.
In the aorta and the carotids, there are highly vascularized structures - carotid bodies, embedding specific chemo-receptors that detect changes of blood gases and pH. They share the same parasympathetic afferent pathways with baro-receptors to convey information to the brain. It has been shown that the PVN is a key nucleus that receives information about hypoxia, and then modulates respiration correspondingly, synchronizing it with sympathetic outflow to the cardiovascular system [111-116]. It has been documented that neurons from the parvocellular division of the PVN containing VP and OT, project to the pre-Bötzinger complex and phrenic motoneurons, while only PVN neurons containing VP project to the RVLM. In these medullary structures both OTRs and V1aRs were identified [40, 67, 139-141]. When hypoxia is detected by chemo-receptors, VP and OT neurons projecting to the pre-Bötzinger complex and RVLM will stimulate respiration and increase BP and HR [67, 140-143]. Japundndžić-Žigon and coworkers observed in freely moving rats concomitant increase of sympathetically- and respiratory-mediated BP variability during hemorrhage [78, 144], stress [39] and central cholinergic activation [146] involving central V1aR. It is worth noting that VP also affects respiration at the periphery in a complex manner, by the stimulation of V1aR located in area postrema [147] and in the carotid bodies [148].
4.2. Cardiovascular Response to Stress & Feed-forward Control
In everyday life, we are constantly exposed to environmental stressors, and, inappropriate responses cause distress. At long term, distress will increase cardiovascular morbidity and mortality [149-151]. It is now acknowledged that each type of stressor has characteristic neuorendocrine imprint involving, to a different extent, sympathoadrenal system (SAS) and hypothalamo-pituitary axis (HPA) [152, 153]. The role of VP, both as modulator of SAS and HPA axis in the acute response to stress is undisputable [154, 155], as well as in the process of adaptation [156, 157]. Acute stress induces baro-reflex desensitization that allows concomitant increase of BP and HR to prepare the animal for escape. Electrophysiological and pharmacological experimentation unraveled that stress-induced suppression of the baro-receptor reflex comes from PVN neurons projecting to NTS. Notably, centrally administered VP and VP microinjected in NTS blunts arterial baro-receptor reflex and increases sympathetic outflow to the cardiovascular system via V1aR [158-161]. In rats exposed to air-jet paradigm VP was shown to modulate neurogenic but not endocrine (ACTH – corticostrone) response [162, 163]. Collectively, these findings sharply contrast reports on baro-receptor reflex sensitization by VP in the area postrema. The dilemma was solved by Unger and coworkers who showed that VP, at the periphery and in the brain, acts differentially on baro-receptor reflex sensitivity, involving different VP receptor subtypes [164]. Milutinović-Smiljanić provided further pharmacological evidence in rats that baro-receptor reflex desensitization and tachycardia induced by immobilization involve central V1bR and putative V2 receptors [165]. It was further suggested that VP containing neurons in PVN projecting to NTS induce baro-receptor reflex desensitization during exercise [166, 167]. Experimental evidence was then provided that PVN neurons containing OT and project to the spinal cord mediate stress-induced tachycardia by the stimulation of OTR [168]. Finally, evidence accumulated to clarify the functioning of the PVN pre-sympathetic neurons under baseline conditions and stress. It was shown that under baseline physiological conditions gamma aminobutiryc acid (GABA) containing neurons surrounding PVN tonically inhibit excitatory glutamate inputs to pre-sympathetic neurons [169, 170].
The complexity of VP involvement in the hemodynamic response to stress was robustly depicted in the experimentation by Japundžić-Žigon and collaborators in freely moving rats using frequency analysis of BP and HR variability [39, 171]. They showed that VP contributes to the increase of sympathetically mediate (low-frequency) BP and HR variability during acute exposure to stress, and, that at the same time, VP stimulates respiration (high-frequency BP variability) and vagal outflow to the heart (high-frequency HR variability). The effect on the respiration and the heart are beneficial and may be lifesaving during shock, for at least three reasons. First, deeper breathing expands alveolar surface for more efficient blood oxygenation. Second, more negative intra-thoracic pressure produced by deep breathing provides greater blood aspiration and heart filling. Finally, at the heart level, VP preserves a balanced sympatho-vagal stimulation protecting the heart from detrimental, stress-induced sympathetic over-stimulation. They also found that effects of VP on respiration were mediated via medullary V1aR [40, 140, 164] and also via V1bR located in the limbic structures where VP exerts axiogenic effects indirectly impinging on respiration during stress [51, 172-176].
Experimental evidence [55-60] and data collected from humans [73, 177] suggest that OT diminishes emotional, hormonal, and cardiovascular response to stress. OT KO mice [82] and rats treated with OTR antagonist [60] were reported to exert accentuated BP and blood corticosterone response to stress. In contrast, lactating women with increased level of blood oxytocin were reported to exhibit reduced cardiovascular reactivity to stress [177] and increased vagal control of the heart [73]. The proposed anti-stress mechanisms also tortuously involve OT that induces feelings of love, trust and relaxation lowering the susceptibility to stress.
4.3. Somato-dendritic Release of VP & OT and Cardiovascular Reactivity to Stress
It is now well established that brain peptides have actions that are not restricted to synapses [13, 88]. Vesicles of peptidergic neurons are larger than vesicles of neurons carrying conventional transmitters, and have densely packaged cargo that may be released all along the neuron. Somato-dendritic release of peptides was confirmed by electron microscopy visualization of omega fusion profiles in the dendritic plasma membrane and of the dense-core vesicular content in the extracellular space [178]. Once in the extracellular space, peptides have long half-lives and use extracellular fluids (volume) for transmission to reach remote receptors for which they have high affinity. The end of peptide action is elicited by down regulation of receptors rather than degradation by non-specific peptidases in extracellular fluids [13].
VP and OT released from somata and dendrites of hypothalamic MCNs can occur independently from depolarization and axonal release, and more importantly it can impinge on axonal release of the peptides. It has been well established that OT containing MCNs express OTR [179] and activation of these auto-receptors has been shown to release calcium from intracellular stores that trigger self-sustained OT release [180] synchronizing milk ejection with suckling [180, 181]. VP secreting MCNs also express V1aR and their stimulation during dehydration has been found to induce the regularization of the firing rate of the whole population of MCNs enhancing VP release [182]. Notably, Son and colleagues [183] demonstrated that V1aR located on neurons in the parvocellular division of PVN are stimulated by VP released by MCNs in the magnocellular division to provide an integrated humoral and autonomic response to complex homeostatic challenge such as osmotic challenge.
Given that the PVN is the key site of integration of neuroendocrine, autonomic and behavioral response to stress [184], we addressed the question as to whether VP and OT receptors in the PVN affect the stress response, particularly neurocardiogenic variables. Stress normally reduces BRS to permit concomitant BP and HR increase, preparing the animal for escape [185]. By transgenic generation of distinct rat phenotypes [186, 187], those over-expressing OTR or V1aR in the PVN and by microinjecting selective blockers into the PVN, we found that the OTR rat phenotype has a blunted neurocardiogenic response to air-jet stress. OTR rats showed no baro-receptor desensitization and the increase of sympathetically-mediated BP variability was buffered compared to wild type controls. In OTR rats the HR variability profile uncovered concomitant sympathetic (harmful) and vagal (protective) activation, while wild type rats exhibited solely sympathetic cardiac activation [188]. In rats over-expressing V1aR [186, 187] in the PVN air-jet stress produced even greater baro-reflex desensitization than in wild type controls. In V1aR rats, the increase of sympathetically mediated BP variability and HR variability was greater than in wild type controls. Bearing in mind the fact that increased cardiovascular variability and decreased BRS are acknowledged risk markers in cardiology practice [117, 189], our results suggest that OTR rat phenotype is protected while V1aR rat phenotype is vulnerable to stress (Fig. 1). Although there is not yet convincing evidence that changes in V1aR, and OTR density in PVN mark cardiovascular diseases, our results corroborate well with the finding by Ribeiro and coworkers [190] who observed that V1aR in the PVN of normotensive rats mediate sympathetic activation during salt load.
Fig. (1).
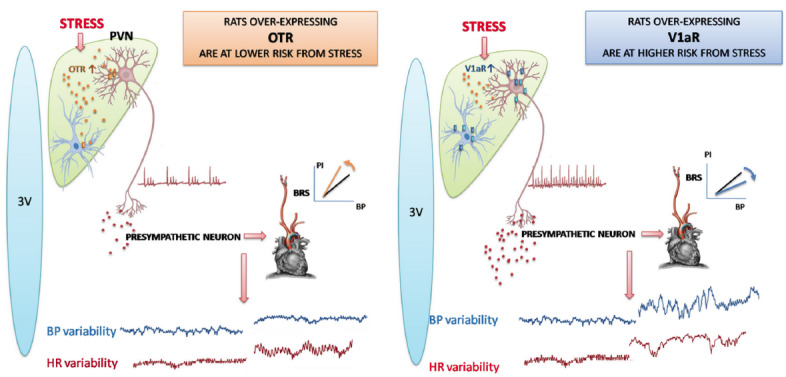
Hypothalamic rat phenotypes exhibiting distinct neurocardiogenic reactivity to stress. Left panel depicts a blunted response to acute stress by rats over-expressing oxytocin receptors (OTR) on neurons and glia of the hypothalamic paraventricular nucleus (PVN): increased baro-receptor reflex sensitivity (BRS) and decreased blood pressure (BP) variability and increased respiratory heart rate (HR) variability. Right panel illustrates a marked response to acute stress by rats over-expressing vasopressin V1a receptors (V1aR) on neurons and glia of the PVN: accentuated decrease of BRS and potentiated increase of sympathetically-mediated low-frequency component of BP and HR variability.
The role of V1bRs on pituitary corticotrophs is well known in stress. VP co-released with CRF in the hypothalamic-hypophiseal portal circulation during acute stress potentiates ACTH release [191]. V1bR are also found in the limbic system and pre-frontal cortex where they affect behavior during stress and contribute to the pathogenesis of anxiety and depression [52-54]. Both stress and depression are associated with increased risk of cardiovascular pathologies, and in this view El-Werfali and colleagues [192] proposed that V1bR in the PVN may be the link between these two pathologies. This assumption was based on their finding that nelivaptan, a selective V1bR antagonist microinjected in PVN blocked the increase of BP, HR and RSNA by exogenously applied VP.
In addition to auto-receptors, MCNs activity is modified by peptides co-released with OT and VP, especially in the stress response [193]. Both OT [193, 194] and VP [194, 195] MCNs have been shown to co-express apelin, a bioactive peptide [196, 197]. Furthermore, MCNs have been found to express apelin APJ receptors [198] suggesting a direct feed-back regulation of MCN neurons that co-release apelin. It has been suggested [195, 198] that apelin inhibits typical phasic firing pattern of VP MCNs thus decreasing systemic VP release and by doing so increases diuresis. In addition, these authors show that endogenous levels of VP and apelin are conversely regulated suggesting the crucial role played by apelin, along with VP, in the maintenance of body fluid balance during lactation and water deprivation.
5. VP & OT IN HYPERTENSION
Primary hypertension is a hyperadrenergic condition that primarily affects peripheral arterial resistance blood vessels leading to their narrowing and compromising blood flow to organs. Individuals with hypertension, generally symptomless, are at an increased risk for end-organ damage including heart, brain and kidneys. These complications are the leading global causes of mortality. Though many factors have been found to favor the occurrence of primary hypertension, its cause is still unknown.
Extensive experimental evidence supports VP involvement in the pathological process of hypertension, and V1aR located in blood vessels. In genetically, spontaneously hypertensive rats [199-204] and rats with endocrine deoxycorticosterone acetate (DOCA) - salt hypertension [205] V1aR antagonists applied peripherally have been found to reduce blood pressure. Moreover, spontaneously hypertensive rats and humans suffering from primary hypertension have been shown to have elevated plasma VP concentration [206] and to exhibit hypersensitivity of the vasculature to exogenously applied VP [207]. Although the elevation of VP concentration in blood of hypertensive animals and humans is insufficient to justify the increase of BP, it correlates well to the severity of disease [206]. Some earlier reports have suggested that chronic peripheral stimulation of vascular V1aRs in normotensive rat resulted in sustained hypertension [208, 209]. A more recent work by the same author [210] demonstrated that VP in sub-pressor dose produces sustained hypertension in Dahl salt sensitive rats with impaired renal medullary NO. Peripheral administration of VP antagonists uncovered a reduced buffering capacity of VP on BP short-term variability in spontaneously hypertensive rats [145]. This finding suggests that VP might be involved in the breakdown of neurogenic mechanisms in hypertension.
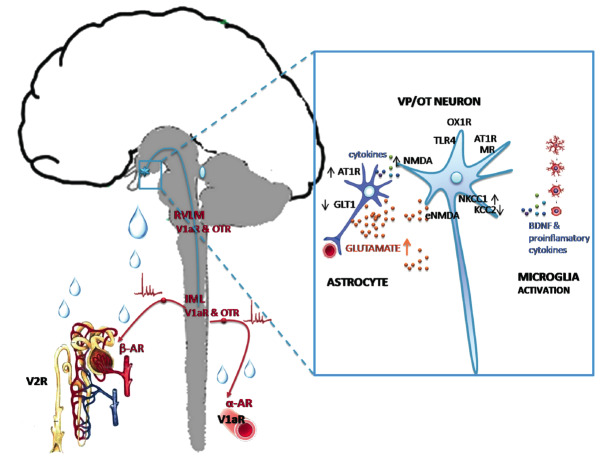
In many models of experimental hypertension (spontaneously hypertensive rats, DOCA-salt rats, renin transgenic hypertension and reno-vascular hypertension) an over-activation of the brain VP system and altered expression of VP receptors in the brain was described [211-213]. Yi and collaborators [214] reported increased VP synthesis in PVN and SON of spontaneously hypertensive rats that develop stroke. Coleman and colleagues [215] and Kc and colleagues [142] also described augmented activity of VP secreting MCNs in the PVN in the generation and/or maintenance of chronic intermittent hypoxia-induced hypertension. Several pathways have been suggested to enlighten pathophysiological mechanism involving VP secreting MCNs in hypertension (Fig. 2): V1aR-mediated peripheral vasoconstriction by VP released in peripheral circulation [216]; V1aR mediated PVN sympatho-excitation in the brainstem and spinal cord [217], brain RAS hyperactivity inducing VP release into the circulation and activation of V2R in the kidneys [216-218]. It is estimated that up to 2000 PVN neurons project to the brainstem and the spinal cord [219, 220], and terminate close to sympathetic preganglionic neurons [221, 222]. That these neurons co-express OT (up to 40%) and VP (up to 40%) was confirmed by several methods: retrograde and anterograde labelling, trans-synaptic tracing, immunohistochemistry and in situ hybridization [222-224]. The question is what drives these pre-sympathetic neurons in PVN to the hyperactivity leading to hypertension. Several mechanisms have been recently discovered (Fig. 2).
Fig. (2).
Hypothalamic targets in primary hypertension. Left: axonal projections of VP/OT neurons to neurohypophysis, rostral ventrolateral medulla (RVLM) and intermediolateral column (IML) of the spinal cord in dual, peripheral and central control of vascular resistance and the kidneys. Right: molecules implicated in dysregulation of VP/OT neurons. V1aR: vasopressin V1a receptor; OTR: oxytocin receptor; αAR: alpha adrenergic receptor; βAR: beta adrenergic receptor; AT1R: angiotensin II receptor type 1; MR: mineralocorticoid receptor; NMDA: N-methyl-d-aspartate synaptic receptors, eNMDA: extra-synaptic NMDA receptors; TLR-4: toll-like receptor type 4; NKCC1: Na+-K+-2Cl−cotransporter isoform 1; KCC2: K+-Cl- co-transporter isotype 2; OX1R: orexin type 1 receptor; GLT1: glutamate transporter type 1; BDNF: brain-derived neurotrophic factor.
Considerable findings point to angiotensin II receptor type 1 (AT-1R) and mineralocorticoid receptors (MR) located on VP secreting MCNs. Pietranera and coworkers [225] showed in spontaneously hypertensive rat that VP neurons in PVN over-express MR while Chen and colleagues [226] found that knock-down of both MR and AT-1R in PVN prevent reno-vascular 2-kidney 1-clip hypertension by decreasing vasopressinergic input to the brainstem involving V1aR. Recent evidence in high salt diet-fed Dahl sensitive rats points to another, novel mechanism whereby elevated orexin receptor type 1 (OX1R) activation in the PVN enhances VP signaling that maintains hypertension [227]. An impaired intracellular chloride homeostasis was observed in DOCA-salt hypertension. Namely, in VP MCNs there is an up-regulation of Cl- importer, Na+-K+-2Cl- cotransporter isoform 1 (NKCC1), and down-regulation of Cl- extruder, K+-2Cl- co-transporter isotype 2 (KCC2), which make a switch from inhibitory to excitatory GABA-A input. In these animals, GABA-A receptor mediated tonic inhibition of VP secreting MCNs in the PVN under baseline physiological conditions is abolished [228]. This alteration in inhibitory inputs drives excessive VP release in the blood and triggers hypertension via peripheral V1aR-mediated vasoconstriction [229]. Similar mechanism could be linking chronic stress with hypertension. Under chronic stress conditions it appears that neuron-specific KCC2 is down-regulated. Then the chloride gradient would reverse ion influx and GABA would mediate neuronal depolarization instead of hyperpolarization [230].
Neuroinflammation and cytokines derived from the periphery (vascular endothelial cells, white blood cells) and the brain (microglia and astrocytes) are now recognized mechanisms that can trigger over-activation of pre-sympathetic neurons in hypertension [231]. Microglia are the major sources to produce inflammatory molecules and neurotrophic factors. Pro-inflammatory molecules such as IL-1β and TNFα, as well as neurotrophic factors produced by microglia have been found to increase the activity of pre-sympathetic neurons in the parvocellular part of the PVN [232]. It was proposed that IL-1β and TNFα promote neuronal excitation by inducing over-expression of N-methyl-d-aspartate (NMDA) receptor in the post-synapse [233]. A similar mechanism of neuronal excitation (over-expression of post-synaptic NMDA receptors) is suggested for microglia derived brain neurotrophic factor (BDNF) [234]. It then seems logical that molecules such as IL-1β and TNFα and BDNF, that increase post-synaptic NMDA receptor density that have essential role in neuronal excitation and plasticity [235] can be key contributors driving hyper-activation of pre-sympathetic neurons in PVN. Shen and coworkers [236] provided further experimental evidence that no exacerbation of hypertension occurs when microglia are depleted in PVN, and that this is associated with down-regulation of NMDA receptors. Furthermore, a growing body of evidence supports an elevated glutamate tone [237] and increased glutamatergic innervations density [238] as well as increased expression of NMDARs [239] in the SON and PVN of hypertensive rats. Stern and collaborators [240] found that AT-1R receptor mRNA is expressed in PVN astrocytes and that angiotensin II inhibits glutamate transporter function, increasing in turn extracellular (non-synaptic) glutamate levels. This activates neuronal extra-synaptic NMDA receptors, increases pre-sympathetic neuronal activity, enhances sympatho-excitatory outflow, and increases blood pressure. Dange et al. [241] report that pathogen recognition receptors, toll-like receptor type 4, that are part of innate immune system, located on neurons and microglia in the PVN contribute to hypertension in spontaneously hypertensive rats.
The involvement of OT in the pathogenesis of hypertension is not so obvious. There is one report in spontaneously hypertensive rats by Petersson and coworkers [242]. Recently, exciting new evidence has been provided in rats that OT contributes to chronic intermittent hypoxia-induced hypertension [243], a recognized model of human obstructive sleep apnea (OSA) associated hypertension. The pathophysiological mechanisms connecting OSA and hypertension are well studied. Hypoxemia induced by periods of hypopnea and apnea during sleep causes systemic inflammation and oxidative stress injuring vascular endothelium and production of endothelial-derived NO. Hypoxemia also triggers chemo-reflex and increases sympathetic tone and catecholamine release [244] potentiating vasoconstriction. OSA-associated hypertension is resistant to treatment and has bad prognosis. Thus, treating OSA should prevent the incidence of hypertension and its major complications. Jamson and colleagues [243] reported that OT released from the PVN neurons into the brainstem is reduced in rats exposed to chronic intermittent hypoxia-hypercapnia. Using optogenetic tools to chronically activate this specific population of OT secreting PVN MCNs they prevented the development of hypertension. In line with this finding, Jain and coworkers [245] reported that intranasal OT administration in patients suffering from OSA reduces the frequency of hypopneas and duration of hypopneas and apneas, and increases cardiac parasympathetic activity, improving overall cardiorespiratory homeostasis, suggesting a role for OT in the pathophysiology of OSA.
6. VP & OT IN HEART FAILURE
While hypertension is characterized by increased peripheral resistance and euvolemia, HF is characterized by increased blood volume and increased peripheral resistance, not necessarily associated with hypertension. It is now well-established that VP contributes to the pathogenesis of HF. Enhanced release of VP in the circulation is initiated by decreased cardiac output (an important determinant of the level of BP) and subsequent stimulation of the baro-receptor reflex (Fig. 3). In the circulation, VP targets the kidneys to produce V2R mediated translocation of Aqp2 into the baso-lateral membrane of epithelial cells in the renal collecting ducts and conserve water which produces volume over-load. Another important peripheral target of VP in HF is arterial resistance vessels that are constricted via V1aR. Both hypervolemia and increased peripheral resistance are aimed at supporting circulation and compensate for reduced CO, but, they burden the heart and precipitate failure. Therefore, pharmacotherapy of HF (with reduced ejection fraction) is directed against neuroendocrine arousal. Drug treatment with loop diuretics, angiotensin converting enzyme (ACE) inhibitors, vasodilators and beta adrenergic blockers alleviate the symptoms and prolong survival [246]. However, in the late stage of HF, when excessive VP release produces water intoxication the use of selective V2R antagonist tolvaptan or nonselective V1aR and V2R antagonist conivaptan is recommended [247, 248]. And although the vaptans are effective in eliminating water in excess and restoring natremia, they regrettably, do not increase survival [247].
Fig. (3).
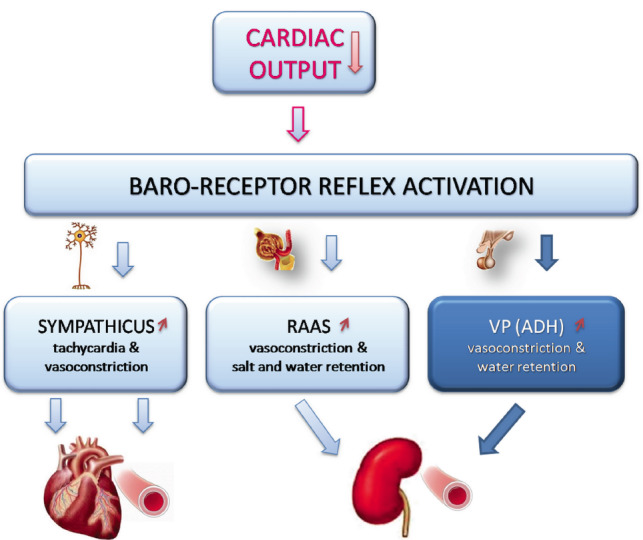
Neuroendocrine remodeling in congestive heart failure. Decompensated form of heart failure (HF) has decreased cardiac output. This triggers reflex sympathetic activation inducing tachycardia and vasoconstriction, renin-angiotensin-aldosterone (RAAS) and vasopressin (VP) synthesis and release contributing to vasoconstriction and producing hypervolemia, congestion and edema.
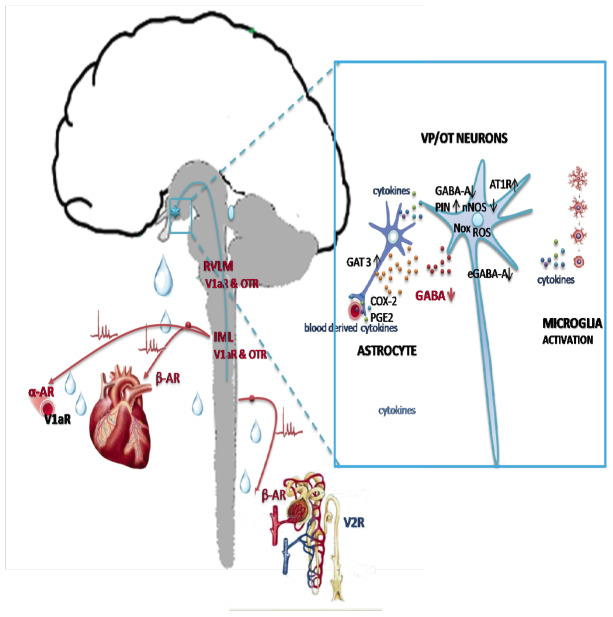
Accumulated experimental evidence suggests that PVN pre-sympathetic neurons elicit detrimental sympathetic over-activation in HF, and that multiple molecular mechanisms are involved (Fig. 4). As previously mentioned, the level of sympathetic nerve activity directed from PVN to the brainstem autonomic centers is dependent upon the integration of excitatory and inhibitory activation of pre-autonomic neurons by GABA-ergic and glutamatergic inter-neurons that surround the nucleus [238]. Under baseline physiological conditions, pre-autonomic neurons are under tonic GABAergic inhibition via GABA-A receptors and NO signaling [249]. When HF occurs, pre-autonomic neurons in PVN are disinhibited due to the domination of excitatory glutamate action [250]. This occurs because of the reduction of inhibitory GABA action upon GABA-A receptors whose density is decreased in the PVN [251]. It has been documented that, in the PVN, GABA-A receptors are expressed both post-synaptically and extra-synaptically, wherefrom they generate two types of inhibitory action. GABA-A receptors located in the synapse mediate conventional inhibitory post-synaptic current (IPSC), while extra-synaptic GABA-A receptors are responsible for persistent tonic inhibitory current (Itonic). In the experimental model of HF Itonic and extra-synaptic GABA-A receptors seem to have a major impact on neuronal excitability of PVN neurons projecting to the brainstem, where sympathetic outflow to the cardiovascular system is set [252, 253]. It has been well documented that GABA derived from glia is important in setting the level of Itonic in the brain [254]. This is counteracted by astroglial GABA clearance by GABA transporter (GAT) type 3. In experimental HF enhanced astroglial GABA uptake was recognized and associated with a deficit in GABAergic tonic inhibition of pre-sympathetic neurons that dictate sympathetic outflow to the heart. Pandit and colleagues showed that nonselective GAT blocker (nipecotic acid, NPA) and GAT-3 selective blocker (SNAP-5114), but not by GAT-1 blocker (NO-711) reverse attenuation of Itonic in HF, providing pharmacological evidence that astroglial GABA uptake is a key mechanism regulating Itonic of PVN-RVLM neurons in HF [252]. They also showed that GABA release is not affected by myocardial infarction and that blockade of GABA-releasing anion channel bestrophin-1 (Best-1) does not affect Itonic in these rats. Moreover, they found that there is reduced function of extrasynaptic GABA-A receptors containing δ subunits in HF. Their findings pinpoint to astroglial GABA clearance as new target for drug development aimed at reducing sympatho-excitation associated with HF. It is noteworthy mentioning that a recent report by Ferreira-Neto and associates [255] identifies small conductance Ca2+-activated K+(SK) channels on MCNs in SON and they found that they were down-regulated in an experimental model of HF.
Fig. (4).
Hypothalamic targets in heart failure. Left: axonal projections of VP/OT neurons to neurohypophysis and rostral ventrolateral medulla (RVLM) and intermediolateral column (IML) of the spinal cord involved in dual, peripheral and central control of the heart, vascular resistance and the kidneys. Right: molecules implicated in the dysregulation of VP and OT neurons. V1aR: vasopressin V1a receptor; OTR: oxytocin receptor; αAR: alpha adrenergic receptor; βAR: beta adrenergic receptor; AT1R: angiotensin II receptor type 1; GABA-gamma aminobutiryc acid; GABA-A: gamma aminobutiryc acid receptor type A at the synapse; eGABA-A: extra-synaptic GABA-A receptors; GAT 3: GABA transporter type 3; nNOS: neuronal NO synthase; PIN: protein inhibitor of nNOS; ROS: reactive oxygen species; Nox: NADPH oxidases; PGE2: prostaglandin E2; COX-2: cyclooxygenase type 2.
Nonetheless, the most studied mechanism involved in PVN-RVLM sympatho-excitation in experimental HF is by angiotensin II. In the PVN of rats subjected to myocardial infarction by arterial coronary ligation, ACE is up-regulated [256] along with the increase of AT-1 receptor (AT-1R) density [257, 258]. Thus, angiotensin II acting via over-expressed AT1R in PVN, up-regulates the expression of protein inhibitor of nNOS (PIN), and consequently, at post-translational level, down-regulates nNOS, disinhibiting sympathetic outflow to the infarcted heart [259]. An interesting finding by Sato and coworkers [260] suggests that apelin improves angiotensin II-induced cardiac disfunction and remodeling and antagonizes endogenous angiotensin II-induced impairment of heart contractility in elderly mice. Using in situ hybridization and transgenic mice Sandgren and colleagues [261] recently demonstrated that only in the SON but not in the PVN, do neurons co-express VP and AT-1AR mRNA and that these neurons are solely involved in the osmotic regulation of VP secretion. In addition, evidence has been provided that in HF, pre-sympathetic OT neurons are implicated. Using immunohistochemistry and retrograde labeling, Roy and colleagues [262] demonstrated that coronary artery ligation in rats activates a distinct population of PVN OT neurons located in the parvocellular part that project to the RVLM. They also noted that intravenous administration of the OTR antagonist, retosiban that crosses the blood brain barrier, prevented the myocardial infarction-induced increase in cardiac sympathetic nerve activity, which likely contributed to the reduced incidence of ventricular arrhythmias and improved survival of infarcted rats.
Following an acute myocardial infarction, considerable amount of pro-inflammatory cytokines is found in the blood, and being transported into the brain via the circumventricular organs. Within minutes TNF-α is observed in the brain of rats with myocardial infarction [263, 264]. Also, TNF-α and IL-1β have been shown to activate cyclooxygenase-2 (COX-2) in macrophages located outside the blood-brain barrier [265] and to increase prostaglandin E2 (PGE2) synthesis, that can enter the brain [266] and excite PVN neurons. Angiotensin II and pro-inflammatory cytokines are major activators of microglial cells, astrocytes and macrophages, which consequently produce more pro-inflammatory cytokines triggering ROS generation and neuronal cell death [267]. Of the many ROS-generating enzymes, activation of the NAD(P)H oxidase (or Nox enzymes) appears particularly involved in HF [268].
CONCLUSION
It is now well acknowledged that neurohypophysial peptides VP and OT control the cardiovascular system both at the periphery (kidneys, vasculature, heart) and in the brain. In the last decade, exciting new evidence emerged about the brain mechanisms controlling the synthesis and the release of VP and OT: autocrine and paracrine factors, neuronal co-transmission and neuro-inflammation. Collectively these new findings pinpoint potential targets in hypertension and heart failure for the development of new, centrally acting cardiovascular drugs.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATONS
- ACTH
Adrenocorticotrophic hormone
- Aqp2
Aquaporin water channel type 2
- AT1R
Angiotensin II type 1 receptor
- BDNF
Brain derived neurotophic factor
- BP
Blood pressure
- BRS
Baroreflex sensitivity
- COX-2
Cyclooxygenase type 2
- CRF
Corticotropin releasing factor
- CVLM
Caudal ventrolateral medulla
- DOCA
Deoxycorticostron acetate
- GABA
Gamma aminobutiryc acid
- GABA-A
GABA receptor type A
- GAT
GABA transporter
- HF
Heart failure
- HPA
Hypothalamic-pituitary axis
- HR
Heart rate
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- IML
Intermediolatral column
- IPSC
Inhibitory post synaptic current
- Itonic
Tonic inhibitory current
- KCC2
K+-2Cl- co-transporter isotype 2
- KCl2
K+-Cl- co-transporter isotype 2
- KO
Knockout
- MCN
Magnocellular neuron
- MR
Mineralocorticoid receptor
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NKCC1
Na+-K+-2Cl-cotransporter isoform 1
- NMDA
N-methyl-d-aspartate
- eNMDA
extrasynaptic NMDA
- nNOS
Neural nitric oxide synthase
- NO
Nitric oxide
- NTS
Nucleus of the solitary tract
- OSA
Obstructive sleep apnea
- OT
Oxytocin
- OTR
Oxytocin receptor
- PGE2
Prostaglandin E2
- PIN
Protein inhibitor of nNOS
- PVN
Paraventricular nucleus
- ROS
Reactive oxygen species
- RSNA
Renal sympathetic nerve activity
- RVLM
Rostral ventrolateral medulla
- SAS
Sympathoadrenal system
- SK
Small conductance calcium activated K+ channel
- SON
Supraoptic nucleus
- TNF-α
Tumor necrosis factor alpha
- V1aR
Vasopressin V1a receptor
- V1bR
Vasopressin V1b receptor
- V2R
Vasopressin V2 receptor
- VP
Vasopressin
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The original work described in this paper has been funded by Welcome Trust (UK), Royal Society (UK) and Ministry of Education and Science (RS, grant III41013).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Du Vigneaud V., Ressler C., Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J. Biol. Chem. 1953;205(2):949–957. [PubMed] [Google Scholar]
- 2.Duvigneaud V., Lawler H.C., Popenoe E.A. Enzymatic cleavage of glycinamide from vasopressin and a proposed structure for this pressor-antidiuretic hormone of the posterior pituitary. J. Am. Chem. Soc. 1953;75(19):4880–4881. doi: 10.1021/ja01115a554. [DOI] [Google Scholar]
- 3.Acher R., Chauvet J. The structure of bovine vasopressin. Biochim. Biophys. Acta. 1953;12(3):487–488. doi: 10.1016/0006-3002(53)90173-5. [DOI] [PubMed] [Google Scholar]
- 4.Barlow M. Vasopressin. Emerg. Med. (Fremantle) 2002;14(3):304–314. doi: 10.1046/j.1442-2026.2002.00349_2.x. [DOI] [PubMed] [Google Scholar]
- 5.Treschan T.A., Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006;105(3):599–612. doi: 10.1097/00000542-200609000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Ukor I.F., Walley K.R. Vasopressin in vasodilatory shock. Crit. Care Clin. 2019;35(2):247–261. doi: 10.1016/j.ccc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Gutkowska J., Jankowski M. Oxytocin revisited: its role in cardiovascular regulation. J. Neuroendocrinol. 2012;24(4):599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- 8.Jankowski M., Broderick T.L., Gutkowska J. Oxytocin and cardioprotection in diabetes and obesity. BMC Endocr. Disord. 2016;16(1):34. doi: 10.1186/s12902-016-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japundžić-Žigon N. Vasopressin and oxytocin in control of the cardiovascular system. Curr. Neuropharmacol. 2013;11(2):218–230. doi: 10.2174/1570159X11311020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bree F.M., Van Der Kleij A.A., Nijenhuis M., Zalm R., Murphy D., Burbach J.P. The hormone domain of the vasopressin prohormone is required for the correct prohormone trafficking through the secretory pathway. J. Neuroendocrinol. 2003;15(12):1156–1163. doi: 10.1111/j.1365-2826.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- 11.Burbach J.P., Luckman S.M., Murphy D., Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81(3):1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 12.Geerling J.C., Shin J.W., Chimenti P.C., Loewy A.D. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J. Comp. Neurol. 2010;518(9):1460–1499. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25(3-4):150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Brinton R.E., Gee K.W., Wamsley J.K., Davis T.P., Yamamura H.I. Regional distribution of putative vasopressin receptors in rat brain and pituitary by quantitative autoradiography. Proc. Natl. Acad. Sci. USA. 1984;81(22):7248–7252. doi: 10.1073/pnas.81.22.7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrowski N.L., Lolait S.J., Bradley D.J., O’Carroll A.M., Brownstein M.J., Young W.S. III Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992;131(1):533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowski N.L., Lolait S.J., Young W.S. III Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135(4):1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 17.Hirasawa A., Hashimoto K., Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur. J. Pharmacol. 1994;267(1):71–75. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 18.Hurbin A., Orcel H., Alonso G., Moos F., Rabié A. The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology. 2002;143(2):456–466. doi: 10.1210/endo.143.2.8643. [DOI] [PubMed] [Google Scholar]
- 19.Kato Y., Igarashi N., Hirasawa A., Tsujimoto G., Kobayashi M. Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation. 1995;59(3):163–169. doi: 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- 20.Hernando F., Schoots O., Lolait S.J., Burbach J.P. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142(4):1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- 21.Vargas K.J., Sarmiento J.M., Ehrenfeld P., Añazco C.C., Villanueva C.I., Carmona P.L., Brenet M., Navarro J., Müller-Esterl W., González C.B. Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation. 2009;77(4):377–385. doi: 10.1016/j.diff.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barberis C., Mouillac B., Durroux T. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol. 1998;156(2):223–229. doi: 10.1677/joe.0.1560223. [DOI] [PubMed] [Google Scholar]
- 23.Birnbaumer M. Vasopressin receptors. Trends Endocrinol. Metab. 2000;11(10):406–410. doi: 10.1016/S1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- 24.Gimpl G., Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 25.Thibonnier M., Coles P., Thibonnier A., Shoham M. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu. Rev. Pharmacol. Toxicol. 2001;41:175–202. doi: 10.1146/annurev.pharmtox.41.1.175. [DOI] [PubMed] [Google Scholar]
- 26.Tribollet E., Barberis C., Jard S., Dubois-Dauphin M., Dreifuss J.J. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442(1):105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- 27.Manning M., Misicka A., Olma A., Bankowski K., Stoev S., Chini B., Durroux T., Mouillac B., Corbani M., Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012;24(4):609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong L.L., Verbalis J.G. Vasopressin V2 receptor antagonists. Cardiovasc. Res. 2001;51(3):391–402. doi: 10.1016/S0008-6363(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T., Tanizawa O., Mori K., Brownstein M.J., Okayama H. Structure and expression of a human oxytocin receptor. Nature. 1992;356(6369):526–529. doi: 10.1038/356526a0. [DOI] [PubMed] [Google Scholar]
- 30.Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- 31.Lolait S.J., O’Carroll A.M., McBride O.W., Konig M., Morel A., Brownstein M.J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 32.Morel A., O’Carroll A.M., Brownstein M.J., Lolait S.J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto T., Saito M., Mochizuki S., Watanabe Y., Hashimoto S., Kawashima H. Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J. Biol. Chem. 1994;269(43):27088–27092. [PubMed] [Google Scholar]
- 34.Thibonnier M., Auzan C., Madhun Z., Wilkins P., Berti-Mattera L., Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J. Biol. Chem. 1994;269(5):3304–3310. [PubMed] [Google Scholar]
- 35.Wacker D., Ludwig M. The role of vasopressin in olfactory and visual processing. Cell Tissue Res. 2019;375(1):201–215. doi: 10.1007/s00441-018-2867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgiev T., Tolekova A., Kalfin R., Hadzhibozheva P. Short-term administration of melatonin or ghrelin on diabetic rats: effects on angiotensin II and vasopressin-induced uterine contractility. Physiol. Res. 2017;66(1):125–133. doi: 10.33549/physiolres.933337. [DOI] [PubMed] [Google Scholar]
- 37.Gizowski C., Trudel E., Bourque C.W. Central and peripheral roles of vasopressin in the circadian defense of body hydration. Best Pract. Res. Clin. Endocrinol. Metab. 2017;31(6):535–546. doi: 10.1016/j.beem.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Hernández V.S.H.O., Hernández O.R., Perez de la Mora M., Gómora M.J., Fuxe K., Eiden L.E., Zhang L. Hypothalamic vasopressinergic projections innervate central amygdala gabaergic neurons: Implications for anxiety and stress coping. Front. Neural Circuits. 2016;10(92):92. doi: 10.3389/fncir.2016.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milutinović S., Murphy D., Japundzić-Zigon N. The role of central vasopressin receptors in the modulation of autonomic cardiovascular controls: a spectral analysis study. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291(6):R1579–R1591. doi: 10.1152/ajpregu.00764.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kc P., Haxhiu M.A., Tolentino-Silva F.P., Wu M., Trouth C.O., Mack S.O. Paraventricular vasopressin-containing neurons project to brain stem and spinal cord respiratory-related sites. Respir. Physiol. Neurobiol. 2002;133(1-2):75–88. doi: 10.1016/S1569-9048(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 41.Altura B.M., Altura B.T. Actions of vasopressin, oxytocin, and synthetic analogs on vascular smooth muscle. Fed. Proc. 1984;43(1):80–86. [PubMed] [Google Scholar]
- 42.Aperia A., Sahlgren B., Eklöf A.C., Lundin S., Melin P. Role of arginine-vasopressin for the development of hypertension following aortic constriction. Acta Physiol. Scand. 1986;128(4):495–499. doi: 10.1111/j.1748-1716.1986.tb08004.x. [DOI] [PubMed] [Google Scholar]
- 43.Mancinelli R., Franchitto A., Glaser S., Vetuschi A., Venter J., Sferra R., Pannarale L., Olivero F., Carpino G., Alpini G., Onori P., Gaudio E. Vasopressin regulates the growth of the biliary epithelium in polycystic liver disease. Lab. Invest. 2016;96(11):1147–1155. doi: 10.1038/labinvest.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eugenín E.A.G.H., González H., Sáez C.G., Sáez J.C. Gap junctional communication coordinates vasopressin-induced glycogenolysis in rat hepatocytes. Am. J. Physiol. 1998;274(6):G1109–G1116. doi: 10.1152/ajpgi.1998.274.6.G1109. [DOI] [PubMed] [Google Scholar]
- 45.Aoyagi T., Koshimizu T.A., Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney Int. 2009;76(10):1035–1039. doi: 10.1038/ki.2009.319. [DOI] [PubMed] [Google Scholar]
- 46.Bharati K.P.P.U., Prashanth U.R. Von Willebrand disease: an overview. Indian J. Pharm. Sci. 2011;73(1):7–16. doi: 10.4103/0250-474X.89751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung H.J., Kwon T.H. Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am. J. Physiol. Renal Physiol. 2016;311(6):F1318–F1328. doi: 10.1152/ajprenal.00485.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bankir L. Antidiuretic action of vasopressin: quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc. Res. 2001;51(3):372–390. doi: 10.1016/S0008-6363(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 49.Rivier C., Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;113(3):939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- 50.Lolait S.J., O’Carroll A.M., Mahan L.C., Felder C.C., Button D.C., Young W.S., III, Mezey E., Brownstein M.J. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc. Natl. Acad. Sci. USA. 1995;92(15):6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stemmelin J., Lukovic L., Salome N., Griebel G. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30(1):35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- 52.Griebel G., Simiand J., Serradeil-Le Gal C., Wagnon J., Pascal M., Scatton B., Maffrand J.P., Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl. Acad. Sci. USA. 2002;99(9):6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iijima M., Yoshimizu T., Shimazaki T., Tokugawa K., Fukumoto K., Kurosu S., Kuwada T., Sekiguchi Y., Chaki S. Antidepressant and anxiolytic profiles of newly synthesized arginine vasopressin V1B receptor antagonists: TASP0233278 and TASP0390325. Br. J. Pharmacol. 2014;171(14):3511–3525. doi: 10.1111/bph.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brunner S.M., Farzi A., Locker F., Holub B.S., Drexel M., Reichmann F., Lang A.A., Mayr J.A., Vilches J.J., Navarro X., Lang R., Sperk G., Holzer P., Kofler B. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc. Natl. Acad. Sci. USA. 2014;111(19):7138–7143. doi: 10.1073/pnas.1318066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grippo A.J., Trahanas D.M., Zimmerman R.R., II, Porges S.W., Carter C.S. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34(10):1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause E.G., de Kloet A.D., Flak J.N., Smeltzer M.D., Solomon M.B., Evanson N.K., Woods S.C., Sakai R.R., Herman J.P. Hydration state controls stress responsiveness and social behavior. J. Neurosci. 2011;31(14):5470–5476. doi: 10.1523/JNEUROSCI.6078-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee P.R., Brady D.L., Shapiro R.A., Dorsa D.M., Koenig J.I. Social interaction deficits caused by chronic phencyclidine administration are reversed by oxytocin. Neuropsychopharmacology. 2005;30(10):1883–1894. doi: 10.1038/sj.npp.1300722. [DOI] [PubMed] [Google Scholar]
- 58.Windle R.J., Shanks N., Lightman S.L., Ingram C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 59.Windle R.J., Kershaw Y.M., Shanks N., Wood S.A., Lightman S.L., Ingram C.D. Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J. Neurosci. 2004;24(12):2974–2982. doi: 10.1523/JNEUROSCI.3432-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wsol A., Cudnoch-Jedrzejewska A., Szczepanska-Sadowska E., Kowalewski S., Puchalska L. Oxytocin in the cardiovascular responses to stress. J. Physiol. Pharmacol. 2008;59(8):123–127. [PubMed] [Google Scholar]
- 61.Mileva-Seitz V., Steiner M., Atkinson L., Meaney M.J., Levitan R., Kennedy J.L., Sokolowski M.B., Fleming A.S. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One. 2013;8(4):e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dumais K.M., Veenema A.H. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bredewold R., Veenema A.H. Sex differences in the regulation of social and anxiety-related behaviors: Insights from vasopressin and oxytocin brain systems. Curr. Opin. Neurobiol. 2018;49:132–140. doi: 10.1016/j.conb.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKinley M.J., Mathai M.L., McAllen R.M., McClear R.C., Miselis R.R., Pennington G.L., Vivas L., Wade J.D., Oldfield B.J. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. J. Neuroendocrinol. 2004;16(4):340–347. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- 65.Hasser E.M., Bishop V.S., Hay M. Interactions between vasopressin and baroreflex control of the sympathetic nervous system. Clin. Exp. Pharmacol. Physiol. 1997;24(1):102–108. doi: 10.1111/j.1440-1681.1997.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 66.Coote J.H. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp. Physiol. 2005;90(2):169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 67.Kc P., Dick T.E. Modulation of cardiorespiratory function mediated by the paraventricular nucleus. Respir. Physiol. Neurobiol. 2010;174(1-2):55–64. doi: 10.1016/j.resp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirsch A.T., Dzau V.J., Majzoub J.A., Creager M.A. Vasopressin-mediated forearm vasodilation in normal humans. Evidence for a vascular vasopressin V2 receptor. J. Clin. Invest. 1989;84(2):418–426. doi: 10.1172/JCI114182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russ R.D., Resta T.C., Walker B.R. Pulmonary vasodilatory response to neurohypophyseal peptides in the rat. J. Appl. Physiol. 1992;73(2):473–478. doi: 10.1152/jappl.1992.73.2.473. [DOI] [PubMed] [Google Scholar]
- 70.Aki Y., Tamaki T., Kiyomoto H., He H., Yoshida H., Iwao H., Abe Y. Nitric oxide may participate in V2 vasopressin-receptor-mediated renal vasodilation. J. Cardiovasc. Pharmacol. 1994;23(2):331–336. doi: 10.1097/00005344-199402000-00023. [DOI] [PubMed] [Google Scholar]
- 71.Cowley A.W., Jr Control of the renal medullary circulation by vasopressin v1 and v2 receptors in the rat. Exp. Physiol. 2000:223S–231S. doi: 10.1111/j.1469-445x.2000.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 72.Russ R.D., Walker B.R. Role of nitric oxide in vasopressinergic pulmonary vasodilatation. Am. J. Physiol. 1992;262(3 Pt 2):H743–H747. doi: 10.1152/ajpheart.1992.262.3.H743. [DOI] [PubMed] [Google Scholar]
- 73.Altemus M., Redwine L.S., Leong Y.M., Frye C.A., Porges S.W., Carter C.S. Responses to laboratory psychosocial stress in postpartum women. Psychosom. Med. 2001;63(5):814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 74.Loichot C., Krieger J.P., De Jong W., Nisato D., Imbs J.L., Barthelmebs M. High concentrations of oxytocin cause vasoconstriction by activating vasopressin V1A receptors in the isolated perfused rat kidney. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363(4):369–375. doi: 10.1007/s002100000372. [DOI] [PubMed] [Google Scholar]
- 75.Thibonnier M., Conarty D.M., Preston J.A., Plesnicher C.L., Dweik R.A., Erzurum S.C. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140(3):1301–1309. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- 76.Katusic Z.S., Shepherd J.T., Vanhoutte P.M. Oxytocin causes endothelium-dependent relaxations of canine basilar arteries by activating V1-vasopressinergic receptors. J. Pharmacol. Exp. Ther. 1986;236(1):166–170. [PubMed] [Google Scholar]
- 77.Miller M.E., Davidge S.T., Mitchell B.F. Oxytocin does not directly affect vascular tone in vessels from nonpregnant and pregnant rats. Am. J. Physiol. Heart Circ. Physiol. 2002;282(4):H1223–H1228. doi: 10.1152/ajpheart.00774.2001. [DOI] [PubMed] [Google Scholar]
- 78.Japundzic-Zigon N. Effects of nonpeptide V1a and V2 antagonists on blood pressure fast oscillations in conscious rats. Clin. Exp. Hypertens. 2001;23(4):277–292. doi: 10.1081/CEH-100102667. [DOI] [PubMed] [Google Scholar]
- 79.Petersson M., Alster P., Lundeberg T., Uvnäs-Moberg K. Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol. Behav. 1996;60(5):1311–1315. doi: 10.1016/S0031-9384(96)00261-2. [DOI] [PubMed] [Google Scholar]
- 80.Zerbe R.L.F.G., Meyer D.K., Kopin I.J. Cardiovascular, sympathetic, and renin-angiotensin system responses to hemorrhage in vasopressin-deficient rats. Endocrinology. 1982;111:608–613. doi: 10.1210/endo-111-2-608. [DOI] [PubMed] [Google Scholar]
- 81.Koshimizu T.A., Nasa Y., Tanoue A., Oikawa R., Kawahara Y., Kiyono Y., Adachi T., Tanaka T., Kuwaki T., Mori T., Takeo S., Okamura H., Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc. Natl. Acad. Sci. USA. 2006;103(20):7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bernatova I., Rigatto K.V., Key M.P., Morris M. Stress-induced pressor and corticosterone responses in oxytocin-deficient mice. Exp. Physiol. 2004;89(5):549–557. doi: 10.1113/expphysiol.2004.027714. [DOI] [PubMed] [Google Scholar]
- 83.Pelletier J.S.D.B., Bigam D., Cheung P.Y. Cardiac effects of vasopressin. Cardiovasc Pharmacol. 2014;34:100–107. doi: 10.1097/FJC.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 84.Conrad K.P., Gellai M., North W.G., Valtin H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann. N. Y. Acad. Sci. 1993;689:346–362. doi: 10.1111/j.1749-6632.1993.tb55559.x. [DOI] [PubMed] [Google Scholar]
- 85.Gutkowska J., Jankowski M., Lambert C., Mukaddam-Daher S., Zingg H.H., McCann S.M. Oxytocin releases atrial natriuretic peptide by combining with oxytocin receptors in the heart. Proc. Natl. Acad. Sci. USA. 1997;94(21):11704–11709. doi: 10.1073/pnas.94.21.11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jankowski M., Hajjar F., Kawas S.A., Mukaddam-Daher S., Hoffman G., McCann S.M., Gutkowska J. Rat heart: a site of oxytocin production and action. Proc. Natl. Acad. Sci. USA. 1998;95(24):14558–14563. doi: 10.1073/pnas.95.24.14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jankowski M., Wang D., Hajjar F., Mukaddam-Daher S., McCann S.M., Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc. Natl. Acad. Sci. USA. 2000;97(11):6207–6211. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leng G., Russell J.A. The osmoresponsiveness of oxytocin and vasopressin neurones: Mechanisms, allostasis and evolution. J. Neuroendocrinol. 2019;31(3):e12662. doi: 10.1111/jne.12662. [DOI] [PubMed] [Google Scholar]
- 89.Andersen L.J., Norsk P., Johansen L.B., Christensen P., Engstrom T., Bie P. Osmoregulatory control of renal sodium excretion after sodium loading in humans. Am. J. Physiol. 1998;275(6):R1833–R1842. doi: 10.1152/ajpregu.1998.275.6.R1833. [DOI] [PubMed] [Google Scholar]
- 90.Rasmussen M.S., Simonsen J.A., Sandgaard N.C., Høilund-Carlsen P.F., Bie P. Mechanisms of acute natriuresis in normal humans on low sodium diet. J. Physiol. 2003;546(Pt 2):591–603. doi: 10.1113/jphysiol.2002.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jankowski M., Danalache B., Wang D., Bhat P., Hajjar F., Marcinkiewicz M., Paquin J., McCann S.M., Gutkowska J. Oxytocin in cardiac ontogeny. Proc. Natl. Acad. Sci. USA. 2004;101(35):13074–13079. doi: 10.1073/pnas.0405324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oyama T., Nagai T., Wada H., Naito A.T., Matsuura K., Iwanaga K., Takahashi T., Goto M., Mikami Y., Yasuda N., Akazawa H., Uezumi A., Takeda S., Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J. Cell Biol. 2007;176(3):329–341. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsuura K., Nagai T., Nishigaki N., Oyama T., Nishi J., Wada H., Sano M., Toko H., Akazawa H., Sato T., Nakaya H., Kasanuki H., Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J. Biol. Chem. 2004;279(12):11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 94.Mukaddam-Daher S., Yin Y.L., Roy J., Gutkowska J., Cardinal R. Negative inotropic and chronotropic effects of oxytocin. Hypertension. 2001;38(2):292–296. doi: 10.1161/01.HYP.38.2.292. [DOI] [PubMed] [Google Scholar]
- 95.Das B., Sarkar C. Is preconditioning by oxytocin administration mediated by iNOS and/or mitochondrial K(ATP) channel activation in the in vivo anesthetized rabbit heart? Life Sci. 2012;90(19-20):763–769. doi: 10.1016/j.lfs.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez-Reyes A., Menaouar A., Yip D., Danalache B., Plante E., Noiseux N., Gutkowska J., Jankowski M. Molecular mechanisms underlying oxytocin-induced cardiomyocyte protection from simulated ischemia-reperfusion. Mol. Cell. Endocrinol. 2015;412:170–181. doi: 10.1016/j.mce.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 97.Işeri S.O., Sener G., Saglam B., Gedik N., Ercan F., Yegen B.C. Oxytocin protects against sepsis-induced multiple organ damage: role of neutrophils. J. Surg. Res. 2005;126(1):73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 98.Jankowski M., Bissonauth V., Gao L., Gangal M., Wang D., Danalache B., Wang Y., Stoyanova E., Cloutier G., Blaise G., Gutkowska J. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res. Cardiol. 2010;105(2):205–218. doi: 10.1007/s00395-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 99.Jung C., Wernly B., Bjursell M., Wiseman J., Admyre T., Wikström J., Palmér M., Seeliger F., Lichtenauer M., Franz M., Frick C., Andersson A.K., Elg M., Pernow J., Sjöquist P.O., Bohlooly-Y M., Wang Q.D. Cardiac-specific overexpression of oxytocin receptor leads to cardiomyopathy in mice. J. Card. Fail. 2018;24(7):470–478. doi: 10.1016/j.cardfail.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 100.Undesser K.P., Hasser E.M., Haywood J.R., Johnson A.K., Bishop V.S. Interactions of vasopressin with the area postrema in arterial baroreflex function in conscious rabbits. Circ. Res. 1985;56(3):410–417. doi: 10.1161/01.RES.56.3.410. [DOI] [PubMed] [Google Scholar]
- 101.Brizzee B.L., Walker B.R. Vasopressinergic augmentation of cardiac baroreceptor reflex in conscious rats. Am. J. Physiol. 1990;258(4 Pt 2):R860–R868. doi: 10.1152/ajpregu.1990.258.4.R860. [DOI] [PubMed] [Google Scholar]
- 102.Cowley A.W., Jr, Monos E., Guyton A.C. Interaction of vasopressin and the baroreceptor reflex system in the regulation of arterial blood pressure in the dog. Circ. Res. 1974;34(4):505–514. doi: 10.1161/01.RES.34.4.505. [DOI] [PubMed] [Google Scholar]
- 103.Bishop V.S., Hasser E.M., Nair U.C. Baroreflex control of renal nerve activity in conscious animals. Circ. Res. 1987;61(4 Pt 2):I76–I81. [PubMed] [Google Scholar]
- 104.Rocha E.S.M., Jr, Rosenberg M. The release of vasopressin in response to haemorrhage and its role in the mechanism of blood pressure regulation. J. Physiol. 1969;202(3):535–557. doi: 10.1113/jphysiol.1969.sp008826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laycock J.F., Penn W., Shirley D.G., Walter S.J. The role of vasopressin in blood pressure regulation immediately following acute haemorrhage in the rat. J. Physiol. 1979;296:267–275. doi: 10.1113/jphysiol.1979.sp013004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujisawa Y., Miyatake A., Hayashida Y., Aki Y., Kimura S., Tamaki T., Abe Y. Role of vasopressin on cardiovascular changes during hemorrhage in conscious rats. Am. J. Physiol. 1994;267(5 Pt 2):H1713–H1718. doi: 10.1152/ajpheart.1994.267.5.H1713. [DOI] [PubMed] [Google Scholar]
- 107.Imai Y., Kim C.Y., Hashimoto J., Minami N., Munakata M., Abe K. Role of vasopressin in neurocardiogenic responses to hemorrhage in conscious rats. Hypertension. 1996;27(1):136–143. doi: 10.1161/01.HYP.27.1.136. [DOI] [PubMed] [Google Scholar]
- 108.Kakiya S., Arima H., Yokoi H., Murase T., Yambe Y., Oiso Y. Effects of acute hypotensive stimuli on arginine vasopressin gene transcription in the rat hypothalamus. Am. J. Physiol. Endocrinol. Metab. 2000;279(4):E886–E892. doi: 10.1152/ajpendo.2000.279.4.E886. [DOI] [PubMed] [Google Scholar]
- 109.Peuler J.D., Schmid P.G., Morgan D.A., Mark A.L. Inhibition of renal sympathetic activity and heart rate by vasopressin in hemorrhaged diabetes insipidus rats. Am. J. Physiol. 1990;258(3 Pt 2):H706–H712. doi: 10.1152/ajpheart.1990.258.3.H706. [DOI] [PubMed] [Google Scholar]
- 110.Julien C., Chapuis B., Cheng Y., Barrès C. Dynamic interactions between arterial pressure and sympathetic nerve activity: role of arterial baroreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285(4):R834–R841. doi: 10.1152/ajpregu.00102.2003. [DOI] [PubMed] [Google Scholar]
- 111.Yang Z., Wheatley M., Coote J.H. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp. Physiol. 2002;87(6):663–674. doi: 10.1113/eph8702439. [DOI] [PubMed] [Google Scholar]
- 112.Redgate E.S. Hypothalamic influence on respiration. Ann. N. Y. Acad. Sci. 1963;109:606–618. doi: 10.1111/j.1749-6632.1963.tb13491.x. [DOI] [PubMed] [Google Scholar]
- 113.Duan Y.F., Winters R., McCabe P.M., Green E.J., Huang Y., Schneiderman N. Cardiorespiratory components of defense reaction elicited from paraventricular nucleus. Physiol. Behav. 1997;61(2):325–330. doi: 10.1016/S0031-9384(96)00410-6. [DOI] [PubMed] [Google Scholar]
- 114.Schlenker E., Barnes L., Hansen S., Martin D. Cardiorespiratory and metabolic responses to injection of bicuculline into the hypothalamic paraventricular nucleus (PVN) of conscious rats. Brain Res. 2001;895(1-2):33–40. doi: 10.1016/S0006-8993(01)02011-X. [DOI] [PubMed] [Google Scholar]
- 115.Swanson L.W., Sawchenko P.E. Hypothalamic integration: Organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 116.Koshiya N., Guyenet P.G. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am. J. Physiol. 1996;270(6 Pt 2):R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 117.Narkiewicz K., Grassi G. Impaired baroreflex sensitivity as a potential marker of cardiovascular risk in hypertension. J. Hypertens. 2008;26(7):1303–1304. doi: 10.1097/HJH.0b013e328305e1a5. [DOI] [PubMed] [Google Scholar]
- 118.Dampney R.A.L. Resetting of the baroreflex control of sympathetic vasomotor activity during natural behaviors: Description and conceptual model of central mechanisms. Front. Neurosci. 2017;11:461. doi: 10.3389/fnins.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Montani J.P., Liard J.F., Schoun J., Möhring J. Hemodynamic effects of exogenous and endogenous vasopressin at low plasma concentrations in conscious dogs. Circ. Res. 1980;47(3):346–355. doi: 10.1161/01.RES.47.3.346. [DOI] [PubMed] [Google Scholar]
- 120.Leslie R.A., Gwyn D.G. Neuronal connections of the area postrema. Fed. Proc. 1984;43(15):2941–2943. [PubMed] [Google Scholar]
- 121.Shapiro R.E., Miselis R.R. The central neural connections of the area postrema of the rat. J. Comp. Neurol. 1985;234(3):344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- 122.Elliott J.M., West M.J., Chalmers J. Effects of vasopressin on heart rate in conscious rabbits. J. Cardiovasc. Pharmacol. 1985;7(1):6–11. doi: 10.1097/00005344-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Michelini L.C., Bonagamba L.G. Baroreceptor reflex modulation by vasopressin microinjected into the nucleus tractus solitarii of conscious rats. Hypertension. 1988;11(2 Pt 2):I75–I79. doi: 10.1161/01.HYP.11.2_Pt_2.I75. [DOI] [PubMed] [Google Scholar]
- 124.Suzuki S., Takeshita A., Imaizumi T., Hirooka Y., Yoshida M., Ando S., Nakamura M. Central nervous system mechanisms involved in inhibition of renal sympathetic nerve activity induced by arginine vasopressin. Circ. Res. 1989;65(5):1390–1399. doi: 10.1161/01.RES.65.5.1390. [DOI] [PubMed] [Google Scholar]
- 125.Hasser E.M., Bishop V.S. Reflex effect of vasopressin after blockade of V1 receptors in the area postrema. Circ. Res. 1990;67(2):265–271. doi: 10.1161/01.RES.67.2.265. [DOI] [PubMed] [Google Scholar]
- 126.Imai Y., Nolan P.L., Johnston C.I. Endogenous vasopressin modulates the baroreflex sensitivity in rats. Clin. Exp. Pharmacol. Physiol. 1983;10(3):289–292. doi: 10.1111/j.1440-1681.1983.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 127.Sampey D.B., Burrell L.M., Widdop R.E. Vasopressin V2 receptor enhances gain of baroreflex in conscious spontaneously hypertensive rats. Am. J. Physiol. 1999;276(3):R872–R879. doi: 10.1152/ajpregu.1999.276.3.R872. [DOI] [PubMed] [Google Scholar]
- 128.Nakayama Y., Takano Y., Eguchi K., Migita K., Saito R., Tsujimoto G., Kamiya H. Modulation of the arterial baroreceptor reflex by the vasopressin receptor in the area postrema of the hypertensive rats. Neurosci. Lett. 1997;226(3):179–182. doi: 10.1016/S0304-3940(97)00274-7. [DOI] [PubMed] [Google Scholar]
- 129.Oikawa R., Nasa Y., Ishii R., Kuwaki T., Tanoue A., Tsujimoto G., Takeo S. Vasopressin V1A receptor enhances baroreflex via the central component of the reflex arc. Eur. J. Pharmacol. 2007;558(1-3):144–150. doi: 10.1016/j.ejphar.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 130.Fujiwara Y., Tanoue A., Tsujimoto G., Koshimizu T.A. The roles of V1a vasopressin receptors in blood pressure homeostasis: a review of studies on V1a receptor knockout mice. Clin. Exp. Nephrol. 2012;16(1):30–34. doi: 10.1007/s10157-011-0497-y. [DOI] [PubMed] [Google Scholar]
- 131.Lovick T.A., Malpas S., Mahony M.T. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J. Auton. Nerv. Syst. 1993;43(3):247–255. doi: 10.1016/0165-1838(93)90331-N. [DOI] [PubMed] [Google Scholar]
- 132.Haselton J.R., Goering J., Patel K.P. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J. Auton. Nerv. Syst. 1994;50(1):1–11. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 133.Deng Y., Kaufman S. Effect of pregnancy on activation of central pathways following atrial distension. Am. J. Physiol. 1995;269(3 Pt 2):R552–R556. doi: 10.1152/ajpregu.1995.269.3.R552. [DOI] [PubMed] [Google Scholar]
- 134.Lovick T.A., Coote J.H. Circulating atrial natriuretic factor activates vagal afferent inputs to paraventriculo-spinal neurones in the rat. J. Auton. Nerv. Syst. 1989;26(2):129–134. doi: 10.1016/0165-1838(89)90161-6. [DOI] [PubMed] [Google Scholar]
- 135.Lovick T.A., Coote J.H. Effects of volume loading on paraventriculo-spinal neurones in the rat. J. Auton. Nerv. Syst. 1988;25(2-3):135–140. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 136.Lovick T.A., Coote J.H. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res. 1988;454(1-2):123–130. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- 137.Strack A.M., Sawyer W.B., Hughes J.H., Platt K.B., Loewy A.D. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491(1):156–162. doi: 10.1016/0006-8993(89)90098-X. [DOI] [PubMed] [Google Scholar]
- 138.Schramm L.P., Strack A.M., Platt K.B., Loewy A.D. Peripheral and central pathways regulating the kidney: a study using pseudorabies virus. Brain Res. 1993;616(1-2):251–262. doi: 10.1016/0006-8993(93)90216-A. [DOI] [PubMed] [Google Scholar]
- 139.Reddy M.K., Patel K.P., Schultz H.D. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289(3):R789–R797. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 140.Yeh E.R., Erokwu B., LaManna J.C., Haxhiu M.A. The paraventricular nucleus of the hypothalamus influences respiratory timing and activity in the rat. Neurosci. Lett. 1997;232(2):63–66. doi: 10.1016/S0304-3940(97)00579-X. [DOI] [PubMed] [Google Scholar]
- 141.Mack S.O., Kc P., Wu M., Coleman B.R., Tolentino-Silva F.P., Haxhiu M.A. Paraventricular oxytocin neurons are involved in neural modulation of breathing. J. Appl. Physiol. 2002;92(2):826–834. doi: 10.1152/japplphysiol.00839.2001. [DOI] [PubMed] [Google Scholar]
- 142.Kc P., Balan K.V., Tjoe S.S., Martin R.J., Lamanna J.C., Haxhiu M.A., Dick T.E. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J. Physiol. 2010;588(Pt 4):725–740. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mack S.O., Wu M., Kc P., Haxhiu M.A. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-botzinger complex. J. Appl. Physiol. 2007;102(1):189–199. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Japundzic-Zigon N. Physiological mechanisms in regulation of blood pressure fast frequency variations. Clin. Exp. Hypertens. 1998;20(4):359–388. doi: 10.3109/10641969809053219. [DOI] [PubMed] [Google Scholar]
- 145.Japundzić-Zigon N., Milutinović S., Jovanović A. Effects of nonpeptide and selective V1 and V2 antagonists on blood pressure short-term variability in spontaneously hypertensive rats. J. Pharmacol. Sci. 2004;95(1):47–55. doi: 10.1254/jphs.95.47. [DOI] [PubMed] [Google Scholar]
- 146.Milutinović S., Murphy D., Japundzić-Zigon N. Central cholinergic modulation of blood pressure short-term variability. Neuropharmacology. 2006;50(7):874–883. doi: 10.1016/j.neuropharm.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 147.Yang S.J., Lee K.Z., Wu C.H., Lu K.T., Hwang J.C. Vasopressin produces inhibition on phrenic nerve activity and apnea through V(1A) receptors in the area postrema in rats. Chin. J. Physiol. 2006;49(6):313–325. [PubMed] [Google Scholar]
- 148.Zera T., Przybylski J., Grygorowicz T., Kasarello K., Podobinska M., Mirowska-Guzel D., Cudnoch-Jedrzejewska A. Vasopressin v1a receptors are present in the carotid body and contribute to the control of breathing in male sprague-dawley rats. Peptides. 2018;102:68–74. doi: 10.1016/j.peptides.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 149.Brotman D.J., Golden S.H., Wittstein I.S. The cardiovascular toll of stress. Lancet. 2007;370(9592):1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- 150.Japundžić-Žigon N., Šarenac O., Lozić M., Vasić M., Tasić T., Bajić D., Kanjuh V., Murphy D. Sudden death: Neurogenic causes, prediction and prevention. Eur. J. Prev. Cardiol. 2018;25(1):29–39. doi: 10.1177/2047487317736827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Šarenac O., Lozic M., Drakulić S., Bajić D., Paton J.F., Murphy D., Japundžić-Žigon N. Autonomic mechanisms underpinning the stress response in borderline hypertensive rats. Exp. Physiol. 2011;96:574–589. doi: 10.1113/expphysiol.2010.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sawchenko P.E., Li H.Y., Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: A tale of two paradigms. Prog. Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- 153.Carrasco G.A., Van de Kar L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003;463(1-3):235–272. doi: 10.1016/S0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 154.Scantamburlo G., Ansseau M., Legros J.J. Role of the neurohypophysis in psychological stress. Encephale. 2001;27(3):245–259. [PubMed] [Google Scholar]
- 155.Benarroch E.E. Paraventricular nucleus, stress response, and cardiovascular disease. Clin. Auton. Res. 2005;15(4):254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 156.Carter R.N., Pinnock S.B., Herbert J. Does the amygdala modulate adaptation to repeated stress? Neuroscience. 2004;126(1):9–19. doi: 10.1016/j.neuroscience.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 157.Gray M., Innala L., Viau V. Central vasopressin V1A receptor blockade impedes hypothalamic-pituitary-adrenal habituation to repeated restraint stress exposure in adult male rats. Neuropsychopharmacology. 2012;37(12):2712–2719. doi: 10.1038/npp.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ciriello J., Calaresu F.R. Role of paraventricular and supraoptic nuclei in central cardiovascular regulation in the cat. Am. J. Physiol. 1980;239(1):R137–R142. doi: 10.1152/ajpregu.1980.239.1.R137. [DOI] [PubMed] [Google Scholar]
- 159.Matsuguchi H., Sharabi F.M., Gordon F.J., Johnson A.K., Schmid P.G. Blood pressure and heart rate responses to microinjection of vasopressin into the nucleus tractus solitarius region of the rat. Neuropharmacology. 1982;21(7):687–693. doi: 10.1016/0028-3908(82)90012-0. [DOI] [PubMed] [Google Scholar]
- 160.Schmid P.G., Sharabi F.M., Guo G.B., Abboud F.M., Thames M.D. Vasopressin and oxytocin in the neural control of the circulation. Fed. Proc. 1984;43(1):97–102. [PubMed] [Google Scholar]
- 161.Brattström A., de Jong W., De Wied D. Central vasopressin impairs the baroreceptor heart rate reflex in conscious rats. J. Cardiovasc. Pharmacol. 1990;15(1):114–117. doi: 10.1097/00005344-199001000-00018. [DOI] [PubMed] [Google Scholar]
- 162.Stojicić S., Milutinović S., Sarenac O., Zivković S., Japundzić-Zigon N. Central vasopressin V(1a) and V(1b) receptors modulate the cardiovascular response to air-jet stress in conscious rats. Biomed. Tech. (Berl.) 2006;51(4):268–271. doi: 10.1515/BMT.2006.053. [DOI] [PubMed] [Google Scholar]
- 163.Stojicić S., Milutinović-Smiljanić S., Sarenac O., Milosavljević S., Paton J.F., Murphy D., Japundzić-Zigon N. Blockade of central vasopressin receptors reduces the cardiovascular response to acute stress in freely moving rats. Neuropharmacology. 2008;54(5):824–836. doi: 10.1016/j.neuropharm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 164.Unger T., Rohmeiss P., Demmert G., Ganten D., Lang R.E., Luft F.C. Differential modulation of the baroreceptor reflex by brain and plasma vasopressin. Hypertension. 1986;8(6 Pt 2):II157–II162. doi: 10.1161/01.HYP.8.6_Pt_2.II157. [DOI] [PubMed] [Google Scholar]
- 165.Milutinović-Smiljanić S., Šarenac O., Lozić-Djurić M., Murphy D., Japundžić-Žigon N. Evidence for involvement of central vasopressin V1b and V2 receptors in stress-induced baroreflex desensitization. Br. J. Pharmacol. 2013;169:900–908. doi: 10.1111/bph.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Michelini L.C. Endogenous vasopressin and the central control of heart rate during dynamic exercise. Braz. J. Med. Biol. Res. 1998;31(9):1185–1195. doi: 10.1590/S0100-879X1998000900012. [DOI] [PubMed] [Google Scholar]
- 167.Dufloth D.L., Morris M., Michelini L.C. Modulation of exercise tachycardia by vasopressin in the nucleus tractus solitarii. Am. J. Physiol. 1997;273(4):R1271–R1282. doi: 10.1152/ajpregu.1997.273.4.R1271. [DOI] [PubMed] [Google Scholar]
- 168.Morris M., Callahan M.F., Li P., Lucion A.B. Central oxytocin mediates stress-induced tachycardia. J. Neuroendocrinol. 1995;7(6):455–459. doi: 10.1111/j.1365-2826.1995.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 169.Li Y.F., Mayhan W.G., Patel K.P. NMDA-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2001;281(6):H2328–H2336. doi: 10.1152/ajpheart.2001.281.6.H2328. [DOI] [PubMed] [Google Scholar]
- 170.Li Y.F., Jackson K.L., Stern J.E., Rabeler B., Patel K.P. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Heart Circ. Physiol. 2006;291(6):H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- 171.Japundzic N., Grichois M.L., Zitoun P., Laude D., Elghozi J.L. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J. Auton. Nerv. Syst. 1990;30(2):91–100. doi: 10.1016/0165-1838(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 172.Liebsch G., Wotjak C.T., Landgraf R., Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci. Lett. 1996;217(2-3):101–104. doi: 10.1016/0304-3940(96)13069-X. [DOI] [PubMed] [Google Scholar]
- 173.Ebner K., Wotjak C.T., Landgraf R., Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur. J. Neurosci. 2002;15(2):384–388. doi: 10.1046/j.0953-816x.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- 174.Lolait S.J., Stewart L.Q., Jessop D.S., Young W.S., III, O’Carroll A.M. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148(2):849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roper J.A., Craighead M., O’Carroll A.M., Lolait S.J. Attenuated stress response to acute restraint and forced swimming stress in arginine vasopressin 1b receptor subtype (Avpr1b) receptor knockout mice and wild-type mice treated with a novel Avpr1b receptor antagonist. J. Neuroendocrinol. 2010;22(11):1173–1180. doi: 10.1111/j.1365-2826.2010.02070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stewart L.Q., Roper J.A., Young W.S., III, O’Carroll A.M., Lolait S.J. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. J. Neuroendocrinol. 2008;20(5):597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 177.Grewen K.M., Light K.C. Plasma oxytocin is related to lower cardiovascular and sympathetic reactivity to stress. Biol. Psychol. 2011;87(3):340–349. doi: 10.1016/j.biopsycho.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pow D.V., Morris J.F. Membrane routing during exocytosis and endocytosis in neuroendocrine neurones and endocrine cells: use of colloidal gold particles and immunocytochemical discrimination of membrane compartments. Cell Tissue Res. 1991;264(2):299–316. doi: 10.1007/BF00313967. [DOI] [PubMed] [Google Scholar]
- 179.Freund-Mercier M.J., Stoeckel M.E., Klein M.J. Oxytocin receptors on oxytocin neurones: Histoautoradiographic detection in the lactating rat. J. Physiol. 1994;480(1):155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ludwig M., Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006;7(2):126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 181.Rossoni E., Feng J., Tirozzi B., Brown D., Leng G., Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLOS Comput. Biol. 2008;4(7):e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Leng G., Brown C., Sabatier N., Scott V. Population dynamics in vasopressin cells. Neuroendocrinology. 2008;88(3):160–172. doi: 10.1159/000149827. [DOI] [PubMed] [Google Scholar]
- 183.Son S.J., Filosa J.A., Potapenko E.S., Biancardi V.C., Zheng H., Patel K.P., Tobin V.A., Ludwig M., Stern J.E. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78(6):1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sladek C.D.M.L., Michelini L.C., Stachenfeld N.S., Stern J.E., Urban J.H. Endocrine-autonomic linkages. Compr. Physiol. 2015;5(3):1281–1323. doi: 10.1002/cphy.c140028. [DOI] [PubMed] [Google Scholar]
- 185.Hilton S. The defence-arousal system and its relevance for circulatory and respiratory control. J. Exp. Biol. 1982;100:159–174. doi: 10.1242/jeb.100.1.159. [DOI] [PubMed] [Google Scholar]
- 186.Lozic M., Tasic T., Martin A., Greenwood M., Sarenac O., Hindmarch C., Paton J.F., Murphy D., Japundzic-Zigon N. Over-expression of v1a receptors in pvn modulates autonomic cardiovascular control. Pharmacol. Res. 2016;114:185–195. doi: 10.1016/j.phrs.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 187.Murphy D., Konopacka A., Hindmarch C., Paton J.F., Sweedler J.V., Gillette M.U., Ueta Y., Grinevich V., Lozic M., Japundzic-Zigon N. The hypothalamic-neurohypophyseal system: from genome to physiology. J. Neuroendocrinol. 2012;24(4):539–553. doi: 10.1111/j.1365-2826.2011.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lozić M., Greenwood M., Šarenac O., Martin A., Hindmarch C., Tasić T., Paton J., Murphy D., Japundžić-Žigon N. Overexpression of oxytocin receptors in the hypothalamic PVN increases baroreceptor reflex sensitivity and buffers BP variability in conscious rats. Br. J. Pharmacol. 2014;171(19):4385–4398. doi: 10.1111/bph.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mehlum M.H., Liestøl K., Kjeldsen S.E., Julius S., Hua T.A., Rothwell P.M., Mancia G., Parati G., Weber M.A., Berge E. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur. Heart J. 2018;39(24):2243–2251. doi: 10.1093/eurheartj/ehx760. [DOI] [PubMed] [Google Scholar]
- 190.Ribeiro N. Panizza, Hdo.N.; Santos, K.M.; Ferreira-Neto, H.C.; Antunes, V.R. Salt-induced sympathoexcitation involves vasopressin V1a receptor activation in the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(11):R1369–R1379. doi: 10.1152/ajpregu.00312.2015. [DOI] [PubMed] [Google Scholar]
- 191.Volpi S., Rabadan-Diehl C., Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7(2):75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- 192.El-Werfali W., Toomasian C., Maliszewska-Scislo M., Li C., Rossi N.F. Haemodynamic and renal sympathetic responses to V1b vasopressin receptor activation within the paraventricular nucleus. Exp. Physiol. 2015;100(5):553–565. doi: 10.1113/expphysiol.2014.084426. [DOI] [PubMed] [Google Scholar]
- 193.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Brailoiu G.C., Dun S.L., Yang J., Ohsawa M., Chang J.K., Dun N.J. Apelin-immunoreactivity in the rat hypothalamus and pituitary. Neurosci. Lett. 2002;327(3):193–197. doi: 10.1016/S0304-3940(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 195.De Mota N., Reaux-Le Goazigo A., El Messari S., Chartrel N., Roesch D., Dujardin C., Kordon C., Vaudry H., Moos F., Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl. Acad. Sci. USA. 2004;101(28):10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.X., Kawamata Y., Fukusumi S., Hinuma S., Kitada C., Kurokawa T., Onda H., Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 197.O’Dowd B.F., Heiber M., Chan A., Heng H.H., Tsui L.C., Kennedy J.L., Shi X., Petronis A., George S.R., Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1-2):355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 198.O’Carroll A.M., Lolait S.J. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraopric nuclei by osmotic stimuli. J. Neuroendocrinol. 2003;15(7):661–666. doi: 10.1046/j.1365-2826.2003.01044.x. [DOI] [PubMed] [Google Scholar]
- 199.Burrell L.M., Phillips P.A., Risvanis J., Aldred K.L., Hutchins A.M., Johnston C.I. Attenuation of genetic hypertension after short-term vasopressin V1A receptor antagonism. Hypertension. 1995;26(5):828–834. doi: 10.1161/01.HYP.26.5.828. [DOI] [PubMed] [Google Scholar]
- 200.Crofton J.T., Share L., Shade R.E., Allen C., Tarnowski D. Vasopressin in the rat with spontaneous hypertension. Am. J. Physiol. 1978;235(4):H361–H366. doi: 10.1152/ajpheart.1978.235.4.H361. [DOI] [PubMed] [Google Scholar]
- 201.Lo M., Julien C., Barres C., Medeiros I., Allevard A.M., Vincent M., Sassard J. Blood pressure maintenance in hypertensive sympathectomized rats. II. Renin-angiotensin system and vasopressin. Am. J. Physiol. 1991;261(4 Pt 2):R1052–R1056. doi: 10.1152/ajpregu.1991.261.4.R1052. [DOI] [PubMed] [Google Scholar]
- 202.Ogura T., Mitsui T., Yamamoto I., Katayama E., Ota Z., Ogawa N. Differential changes in atrial natriuretic peptide and vasopressin receptor bindings in kidney of spontaneously hypertensive rat. Life Sci. 1987;40(3):233–238. doi: 10.1016/0024-3205(87)90337-7. [DOI] [PubMed] [Google Scholar]
- 203.Vågnes O., Feng J.J., Iversen B.M., Arendshorst W.J. Upregulation of V(1) receptors in renal resistance vessels of rats developing genetic hypertension. Am. J. Physiol. Renal Physiol. 2000;278(6):F940–F948. doi: 10.1152/ajprenal.2000.278.6.F940. [DOI] [PubMed] [Google Scholar]
- 204.Yamada Y., Yamamura Y., Chihara T., Onogawa T., Nakamura S., Yamashita T., Mori T., Tominaga M., Yabuuchi Y. OPC-21268, a vasopressin V1 antagonist, produces hypotension in spontaneously hypertensive rats. Hypertension. 1994;23(2):200–204. doi: 10.1161/01.HYP.23.2.200. [DOI] [PubMed] [Google Scholar]
- 205.Crofton J.T., Share L., Shade R.E., Lee-Kwon W.J., Manning M., Sawyer W.H. The importance of vasopressin in the development and maintenance of DOC-salt hypertension in the rat. Hypertension. 1979;1(1):31–38. doi: 10.1161/01.HYP.1.1.31. [DOI] [PubMed] [Google Scholar]
- 206.Johnston C.I. Vasopressin in circulatory control and hypertension. J. Hypertens. 1985;3(6):557–569. doi: 10.1097/00004872-198512000-00001. [DOI] [PubMed] [Google Scholar]
- 207.Möhring J., Kintz J., Schoun J., McNeill J.R. Pressor responsiveness and cardiovascular reflex activity in spontaneously hypertensive and normotensive rates during vasopressin infusion. J. Cardiovasc. Pharmacol. 1981;3(5):948–957. doi: 10.1097/00005344-198109000-00004. [DOI] [PubMed] [Google Scholar]
- 208.Cowley A.W., Jr Szczepanska-Sadowska, E.; Stepniakowski, K.; Mattson, D. Chronic intravenous administration of V1 arginine vasopressin agonist results in sustained hypertension. Am. J. Physiol. 1994;267(2 Pt 2):H751–H756. doi: 10.1152/ajpheart.1994.267.2.H751. [DOI] [PubMed] [Google Scholar]
- 209.Szczepanska-Sadowska E., Stepniakowski K., Skelton M.M., Cowley A.W., Jr Prolonged stimulation of intrarenal V1 vasopressin receptors results in sustained hypertension. Am. J. Physiol. 1994;267(5 Pt 2):R1217–R1225. doi: 10.1152/ajpregu.1994.267.5.R1217. [DOI] [PubMed] [Google Scholar]
- 210.Yuan B., Cowley A.W., Jr Evidence that reduced renal medullary nitric oxide synthase activity of dahl s rats enables small elevations of arginine vasopressin to produce sustained hypertension. Hypertension. 2001;37(2 Pt 2):524–528. doi: 10.1161/01.HYP.37.2.524. [DOI] [PubMed] [Google Scholar]
- 211.Swords B.H., Wyss J.M., Berecek K.H. Central vasopressin receptors are upregulated by deoxycorticosterone acetate. Brain Res. 1991;559(1):10–16. doi: 10.1016/0006-8993(91)90280-9. [DOI] [PubMed] [Google Scholar]
- 212.Szczepańska-Sadowska E., Paczwa P., Loń S., Ganten D. Increased pressor function of central vasopressinergic system in hypertensive renin transgenic rats. J. Hypertens. 1998;16(10):1505–1514. doi: 10.1097/00004872-199816100-00016. [DOI] [PubMed] [Google Scholar]
- 213.Jackiewicz E., Szczepanska-Sadowska E., Dobruch J. Altered expression of angiotensin AT1a and vasopressin V1a receptors and nitric oxide synthase mRNA in the brain of rats with renovascular hypertension. J. Physiol. Pharmacol. 2004;55(4):725–737. [PubMed] [Google Scholar]
- 214.Yi S.S., Kim H.J., Do S.G., Lee Y.B., Ahn H.J., Hwang I.K., Yoon Y.S. Arginine vasopressin (AVP) expressional changes in the hypothalamic paraventricular and supraoptic nuclei of stroke-prone spontaneously hypertensive rats. Anat. Cell Biol. 2012;45(2):114–120. doi: 10.5115/acb.2012.45.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Coleman C.G., Wang G., Park L., Anrather J., Delagrammatikas G.J., Chan J., Zhou J., Iadecola C., Pickel V.M. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J. Neurosci. 2010;30(36):12103–12112. doi: 10.1523/JNEUROSCI.3367-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Littlejohn N.K., Siel R.B., Jr, Ketsawatsomkron P., Pelham C.J., Pearson N.A., Hilzendeger A.M., Buehrer B.A., Weidemann B.J., Li H., Davis D.R., Thompson A.P., Liu X., Cassell M.D., Sigmund C.D., Grobe J.L. Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304(10):R818–R828. doi: 10.1152/ajpregu.00082.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shafton A.D., Ryan A., Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801(1-2):239–243. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 218.Pyner S., Coote J.H. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100(3):549–556. doi: 10.1016/S0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 219.Motawei K., Pyner S., Ranson R.N., Kamel M., Coote J.H. Terminals of paraventricular spinal neurones are closely associated with adrenal medullary sympathetic preganglionic neurones: immunocytochemical evidence for vasopressin as a possible neurotransmitter in this pathway. Exp. Brain Res. 1999;126(1):68–76. doi: 10.1007/s002210050717. [DOI] [PubMed] [Google Scholar]
- 220.Ranson R.N., Motawei K., Pyner S., Coote J.H. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp. Brain Res. 1998;120(2):164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- 221.Hallbeck M., Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J. Comp. Neurol. 1999;411(2):201–211. doi: 10.1002/(SICI)1096-9861(19990823)411:2<201:AID-CNE3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 222.Hallbeck M., Larhammar D., Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J. Comp. Neurol. 2001;433(2):222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 223.Hallbeck M. Dynorphin mRNA-expressing neurons in the rat paraventricular hypothalamic nucleus project to the spinal cord. Neurosci. Lett. 2000;285(3):161–164. doi: 10.1016/S0304-3940(00)01093-4. [DOI] [PubMed] [Google Scholar]
- 224.Pyner S. Neurochemistry of the paraventricular nucleus of the hypothalamus: Implications for cardiovascular regulation. J. Chem. Neuroanat. 2009;38(3):197–208. doi: 10.1016/j.jchemneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 225.Pietranera L., Brocca M.E., Cymeryng C., Gomez-Sanchez E., Gomez-Sanchez C.E., Roig P., Lima A., De Nicola A.F. Increased expression of the mineralocorticoid receptor in the brain of spontaneously hypertensive rats. J. Neuroendocrinol. 2012;24(9):1249–1258. doi: 10.1111/j.1365-2826.2012.02332.x. [DOI] [PubMed] [Google Scholar]
- 226.Chen A., Huang B.S., Wang H.W., Ahmad M., Leenen F.H. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J. Physiol. 2014;592(16):3523–3536. doi: 10.1113/jphysiol.2014.275560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Huber M.J., Fan Y., Jiang E., Zhu F., Larson R.A., Yan J., Li N., Chen Q.H., Shan Z. Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2017;313(6):H1075–H1086. doi: 10.1152/ajpheart.00822.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim Y.B., Kim Y.S., Kim W.B., Shen F.Y., Lee S.W., Chung H.J., Kim J.S., Han H.C., Colwell C.S., Kim Y.I. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ. Res. 2013;113(12):1296–1307. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 229.Choe K.Y., Han S.Y., Gaub P., Shell B., Voisin D.L., Knapp B.A., Barker P.A., Brown C.H., Cunningham J.T., Bourque C.W. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron. 2015;85(3):549–560. doi: 10.1016/j.neuron.2014.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hewitt S.A., Wamsteeker J.I., Kurz E.U., Bains J.S. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 2009;12(4):438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- 231.Khor S., Cai D. Hypothalamic and inflammatory basis of hypertension. Clin. Sci. (Lond.) 2017;131(3):211–223. doi: 10.1042/CS20160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ferri C.C., Ferguson A.V. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J. Neuroendocrinol. 2003;15(2):126–133. doi: 10.1046/j.1365-2826.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 233.Wheeler D., Knapp E., Bandaru V.V., Wang Y., Knorr D., Poirier C., Mattson M.P., Geiger J.D., Haughey N.J. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J. Neurochem. 2009;109(5):1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., III, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li D.P., Zhu L.H., Pachuau J., Lee H.A., Pan H.L. mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J. Neurosci. 2014;34(12):4309–4317. doi: 10.1523/JNEUROSCI.4295-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Shen X.Z., Li Y., Li L., Shah K.H., Bernstein K.E., Lyden P., Shi P. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66(2):309–316. doi: 10.1161/HYPERTENSIONAHA.115.05333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang M., Biancardi V.C., Stern J.E. An increased extrasynaptic NMDA tone inhibits A-type K+ current and increases excitability of hypothalamic neurosecretory neurons in hypertensive rats. J. Physiol. 2017;595(14):4647–4661. doi: 10.1113/JP274327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Biancardi V.C., Campos R.R., Stern J.E. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J. Comp. Neurol. 2010;518(5):567–585. doi: 10.1002/cne.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Li D.P., Pan H.L. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007;49(4):916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 240.Stern J.E., Son S., Biancardi V.C., Zheng H., Sharma N., Patel K.P. Astrocytes contribute to angiotensin ii stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68(6):1483–1493. doi: 10.1161/HYPERTENSIONAHA.116.07747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dange R.B., Agarwal D., Teruyama R., Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J. Neuroinflammation. 2015;12:31. doi: 10.1186/s12974-015-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Petersson M., Lundeberg T., Uvnäs-Moberg K. Oxytocin decreases blood pressure in male but not in female spontaneously hypertensive rats. J. Auton. Nerv. Syst. 1997;66(1-2):15–18. doi: 10.1016/S0165-1838(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 243.Jameson H., Bateman R., Byrne P., Dyavanapalli J., Wang X., Jain V., Mendelowitz D. Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats. Am. J. Physiol. Heart Circ. Physiol. 2016;310(11):H1549–H1557. doi: 10.1152/ajpheart.00808.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cai A., Wang L., Zhou Y. Hypertension and obstructive sleep apnea. Hypertens. Res. 2016;39(6):391–395. doi: 10.1038/hr.2016.11. [DOI] [PubMed] [Google Scholar]
- 245.Jain V., Marbach J., Kimbro S., Andrade D.C., Jain A., Capozzi E., Mele K., Del Rio R., Kay M.W., Mendelowitz D. Benefits of oxytocin administration in obstructive sleep apnea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017;313(5):L825–L833. doi: 10.1152/ajplung.00206.2017. [DOI] [PubMed] [Google Scholar]
- 246.Burnett H., Earley A., Voors A.A., Senni M., McMurray J.J., Deschaseaux C., Cope S. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: A network meta-analysis. Circ Heart Fail. 2017;10(1):e003529. doi: 10.1161/CIRCHEARTFAILURE.116.003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Konstam M.A.G.M., Gheorghiade M., Burnett J.C., Jr, Grinfeld L., Maggioni A.P., Swedberg K., Udelson J.E., Zannad F., Cook T., Ouyang J., Zimmer C., Orlandi C. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 248.Annane D., Decaux G., Smith N. Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am. J. Med. Sci. 2009;337(1):28–36. doi: 10.1097/MAJ.0b013e31817b8148. [DOI] [PubMed] [Google Scholar]
- 249.Li D.P., Chen S.R., Finnegan T.F., Pan H.L. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J. Physiol. 2004;554(Pt 1):100–110. doi: 10.1113/jphysiol.2003.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Li Y.F., Cornish K.G., Patel K.P. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ. Res. 2003;93(10):990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 251.Carillo B.A., Oliveira-Sales E.B., Andersen M., Tufik S., Hipolide D., Santos A.A., Tucci P.J., Bergamaschi C.T., Campos R.R. Changes in GABAergic inputs in the paraventricular nucleus maintain sympathetic vasomotor tone in chronic heart failure. Auton. Neurosci. 2012;171(1-2):41–48. doi: 10.1016/j.autneu.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 252.Pandit S., Jo J.Y., Lee S.U., Lee Y.J., Lee S.Y., Ryu P.D., Lee J.U., Kim H.W., Jeon B.H., Park J.B. Enhanced astroglial GABA uptake attenuates tonic GABAA inhibition of the presympathetic hypothalamic paraventricular nucleus neurons in heart failure. J. Neurophysiol. 2015;114(2):914–926. doi: 10.1152/jn.00080.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Park J.B., Skalska S., Son S., Stern J.E. Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J. Physiol. 2007;582(Pt 2):539–551. doi: 10.1113/jphysiol.2007.133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lee S., Yoon B.E., Berglund K., Oh S.J., Park H., Shin H.S., Augustine G.J., Lee C.J. Channel-mediated tonic GABA release from glia. Science. 2010;330(6005):790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 255.Ferreira-Neto H.C., Biancardi V.C., Stern J.E. A reduction in SK channels contributes to increased activity of hypothalamic magnocellular neurons during heart failure. J. Physiol. 2017;595(20):6429–6442. doi: 10.1113/JP274730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tan J., Wang H., Leenen F.H. Increases in brain and cardiac AT1 receptor and ACE densities after myocardial infarct in rats. Am. J. Physiol. Heart Circ. Physiol. 2004;286(5):H1665–H1671. doi: 10.1152/ajpheart.00858.2003. [DOI] [PubMed] [Google Scholar]
- 257.Wei S.G., Yu Y., Zhang Z.H., Weiss R.M., Felder R.B. Mitogen-activated protein kinases mediate upregulation of hypothalamic angiotensin II type 1 receptors in heart failure rats. Hypertension. 2008;52(4):679–686. doi: 10.1161/HYPERTENSIONAHA.108.113639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Zhu G.Q., Gao L., Patel K.P., Zucker I.H., Wang W. Ang ii in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J. Appl. Physiol. 2004;97(5):1746–1754. doi: 10.1152/japplphysiol.00573.2004. [DOI] [PubMed] [Google Scholar]
- 259.Sharma N.M., Llewellyn T.L., Zheng H., Patel K.P. Angiotensin II-mediated posttranslational modification of nNOS in the PVN of rats with CHF: role for PIN. Am. J. Physiol. Heart Circ. Physiol. 2013;305(6):H843–H855. doi: 10.1152/ajpheart.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sato T., Kadowaki A., Suzuki T., Ito H., Watanabe H., Imai Y., Kuba K. Loss of apelin augments angiotensin ii-induced cardiac dysfunction and pathological remodeling. Int. J. Mol. Sci. 2019;20(2):E239. doi: 10.3390/ijms20020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sandgren J.A., Linggonegoro D.W., Zhang S.Y., Sapouckey S.A., Claflin K.E., Pearson N.A., Leidinger M.R., Pierce G.L., Santillan M.K., Gibson-Corley K.N., Sigmund C.D., Grobe J.L. Angiotensin AT1A receptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314(6):R770–R780. doi: 10.1152/ajpregu.00435.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Roy R.K., Augustine R.A., Brown C.H., Schwenke D.O. Activation of oxytocin neurons in the paraventricular nucleus drives cardiac sympathetic nerve activation following myocardial infarction in rats. Commun. Biol. 2018;1:160. doi: 10.1038/s42003-018-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Francis J., Chu Y., Johnson A.K., Weiss R.M., Felder R.B. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am. J. Physiol. Heart Circ. Physiol. 2004;286(6):H2264–H2271. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 264.Francis J., Zhang Z.H., Weiss R.M., Felder R.B. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2004;287(2):H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- 265.Engblom D., Ek M., Saha S., Ericsson-Dahlstrand A., Jakobsson P.J., Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J. Mol. Med. (Berl.) 2002;80(1):5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 266.Schiltz J.C., Sawchenko P.E. Signaling the brain in systemic inflammation: The role of perivascular cells. Front. Biosci. 2003;80:s1321–s1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 267.Han Y., Shi Z., Zhang F., Yu Y., Zhong M.K., Gao X.Y., Wang W., Zhu G.Q. Reactive oxygen species in the paraventricular nucleus mediate the cardiac sympathetic afferent reflex in chronic heart failure rats. Eur. J. Heart Fail. 2007;9(10):967–973. doi: 10.1016/j.ejheart.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 268.Guggilam A., Cardinale J.P., Mariappan N., Sriramula S., Haque M., Francis J. Central TNF inhibition results in attenuated neurohumoral excitation in heart failure: a role for superoxide and nitric oxide. Basic Res. Cardiol. 2011;106(2):273–286. doi: 10.1007/s00395-010-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]