Abstract
Nimodipine is a dihydropyridine calcium channel antagonist that blocks the flux of extracellular calcium through L-type, voltage-gated calcium channels. While nimodipine is FDA-approved for the prevention and treatment of neurological deficits in patients with aneurysmal subarachnoid hemorrhage (aSAH), it affects myriad cell types throughout the body, and thus, likely has more complex mechanisms of action than simple inhibition of cerebral vasoconstriction. Newer understanding of the pathophysiology of delayed ischemic injury after a variety of acute neurologic injuries including aSAH, traumatic brain injury (TBI) and ischemic stroke, coupled with advances in the drug delivery method for nimodipine, have reignited interest in refining its potential therapeutic use. In this context, this review seeks to establish a firm understanding of current data on nimodipine’s role in the mechanisms of delayed injury in aSAH, TBI, and ischemic stroke, and assess the extensive clinical data evaluating its use in these conditions. In addition, we will review pivotal trials using locally administered, sustained release nimodipine and discuss why such an approach has evaded demonstration of efficacy, while seemingly having the potential to significantly improve clinical care.
Keywords: Delayed cerebral ischemia, vasospasm, subarachnoid hemorrhage, L-type calcium channel, nimodipine, sustained release delivery
1. INTRODUCTION
The prototypical L-type calcium channel blocker nimodipine has been used in various contexts since the 1980s. The drug found a clinical indication in the treatment of aneurysmal subarachnoid hemorrhage (aSAH), initially thought to exert its effect by decreasing the ischemic effects of angiographic vasospasm and delayed cerebral ischemia (DCI) by relaxing cerebral vascular smooth muscle. However, these early pivotal studies showed seemingly paradoxical results where angiographic vasospasm was not significantly affected, but clinical outcomes were improved. This led to a hypothesis in the neurosurgical and neurocritical care world that nimodipine acted via “neuroprotective” or other mechanisms. Such efficacy has not been definitively demonstrated in other realms of neurologic injury including ischemic stroke and TBI. Advances in the understanding of the pathophysiology of DCI after aSAH and studies regarding the preclinical mechanisms particularly related to cortical spreading depolarization (SD) have led to a reconsideration of the potential role of L-type calcium channel antagonists. Coupled with these observations, recent advances in clinical sustained local delivery of nimodipine have shown safety and early evidence for improved outcomes despite the lack of a definite beneficial effect. Such an approach of local delivery has the potential to limit damaging hypotensive episodes, and the effect of local nimodipine in high-risk patients experiencing damaging SD is under investigation. These new data suggest a potential future role of nimodipine and other similar drugs in improving outcomes not only in patients with aSAH, but also a reevaluation of the extensive existing literature evaluating nimodipine for other neurologic indications such as ischemic stroke, TBI, and migraine. Modern improved understanding of mechanisms of secondary brain injury including the role of SD across these various conditions may further strengthen the rationale for future trials using newer local delivery techniques in select high-risk patients. In this review, we will discuss the current state of the use of nimodipine in aSAH, provide a detailed review of the mechanisms of action of nimodipine, review the clinical evidence across multiple neurologic conditions, and discuss potential strategies to improve the efficacy of nimodipine based on better recent understanding of the mechanism and delivery technologies.
2. PATHOPHYSIOLOGY OF ASAH/DELAYED CEREBRAL ISCHEMIA AND LIMITATIONS OF CURRENT TREATMENT
2.1. Overview of aSAH and the Burden of Disease
Non-traumatic subarachnoid hemorrhage is caused by the rupture of an intracranial aneurysm in 80% of cases; other causes include vascular malformations and vasculitis. aSAH accounts for 5% to 10% of all strokes in the United States and affected patients tend to be younger than those affected by other subtypes of stroke, resulting in a greater loss of productive life [1, 2]. ASAH is more common among women than men, and the incidence increases with age to a peak among persons in their 50s [3]. Despite the current treatment strategies, aSAH is associated with high morbidity and mortality [4]. Even after the initial event, multiple factors can contribute to worse outcomes in the subsequent weeks including delayed cerebral ischemia (DCI), vasospasm, seizures, hyperthermia, and other systemic insults such as pneumonia, deep venous thrombosis, etc. [5]. Among the patients with aSAH who survive, half suffer long-term neuropsychological effects and decreased quality of life [6]. Factors associated with an increased risk of aneurysm rupture include age, hypertension, history of aSAH, aneurysm size, aneurysm location, and geographical region [7].
2.2. Pathophysiology of aSAH (Including DCI)
DCI is the most common, potentially reversible complication of aSAH; however, to date, there is no effective treatment to prevent DCI. The mechanism by which aSAH causes DCI is not entirely understood, but multiple processes are hypothesized to contribute, including angiographic micro- and macrovasospasm, SD, microthromboembolism, loss of autoregulation, and capillary transit time heterogeneity [8, 9]. Consequences of DCI and early brain injury from aSAH may also impact other body systems, including the heart and lungs, particularly in the form of stress-induced cardiomyopathy and pulmonary edema [10].
2.3. Limitations of the Current Treatment
The only drug approved for aSAH in North America and Europe is the dihydropyridine, L-type calcium channel antagonist nimodipine. Possible mechanisms of action include reduction of angiographic vasospasm, increase in fibrinolytic activity, neuroprotection, inhibition of SD, and reduction of microthromboemboli [11]. A lack of significant effect of nimodipine on angiographic vasospasm in these trials [12] argues for the other actions listed above and emerging preclinical data have implicated SD targeting as one of the most likely mechanisms [13]. When nimodipine is administered orally or intravenously, the concentration of nimodipine in the cerebrospinal fluid (CSF) is at least 1 order of magnitude lower than the plasma concentration (about 2-7 nmol/L (0.84-3 ng/mL) vs 70-96 nmol/L (30-40 ng/mL), respectively). Hypotension occurs at plasma concentrations of 70 nmol/L (30 ng/mL) or higher, when CSF concentrations are still below the concentration that will dilate cerebral arteries. While higher doses of nimodipine may be more efficacious, their use is limited due to the risk of hypotension [8]. Additional support for use of high doses of dihydropyridines comes from reports of the use of intrathecal and intraventricular injections of nimodipine or nicardipine to treat angiographic vasospasm [14, 15]. Furthermore, intra-arterial infusion of nimodipine or other dihydropyridine calcium channel antagonists reversed established angiographic vasospasm and improved clinical condition in multiple retrospective reviews of uncontrolled patient series [16]. The limitations of local injections of native dihydropyridines include the risk of hypotension and the need for repeated or continuous injection due to a time-limited efficacy, which is technically difficult and invasive [17]. Intraoperative local delivery of sustained-release pellets of dihydropyridines, such as nicardipine, into the subarachnoid space has demonstrated improved outcome, but not without some limitations, such as the inability to use in patients with endovascular coiling [18, 19]. Despite the evidence of efficacy of nimodipine, there is a clear need for improvements in limiting systemic hypotension and improved delivery to the most vulnerable regions of the brain.
3. MECHANISM OF ACTION OF NIMODIPINE AND RECENT PRECLINICAL ADVANCES
Voltage-dependent calcium channels (VDCCs) are widely distributed throughout the body and regulate excitability and secretion of a diverse range of cell types [20]. There are multiple families of VDCCs, initially differentiated by distinct biophysical properties (e.g. voltage thresholds for activation and durations of opening) and subsequently supported by cloning of different pore-forming alpha-1 subunits. The first VDCCs blockers were identified in the early 1960s [21, 22], and were followed by the development of blockers selective for different classes of VDCCs in the late 1960s and early 1970s [20-23]. Nimodipine and other dihydropyridine blockers inhibit VDCCs that were first identified as “L-type” VDCCs due to their long-lasting inward currents, which were subsequently identified as the CaV1 family of VDCCs. Nimodipine thus binds to the alpha-1 subunits that contain the transmembrane pore and voltage sensor, and acts as negative allosteric modulator of channel function [24-26] Four different alpha1 isoforms have now been identified within the L-type class, Cav1.1, Cav1.2, Cav1.3, Cav1.4, and these are associated with different cellular distributions throughout the body [27]. Channels containing either the Cav1.2 or Cav1.3 subunits are of most relevance for this review, as these are widely expressed in the cardiovascular system, as well as neurons and perhaps other cell types that are relevant for central nervous system (CNS) pathophysiology. In contrast, Cav1.1 expression is limited to skeletal muscle and Cav1.4 containing channels appear restricted to retina. It is now recognized that most dihydropyridines show some preferential inhibition of Cav1.2 channels, as compared to Cav1.3. Whether or not nimodipine has any selectivity for Cav1.2 or Cav1.3 is currently unknown. Following the general distribution of L-type channels, nimodipine has the potential to influence a wide range of cell types throughout the body. Of particular interest are the multiple targets directly relevant to treatment of cerebral injury and neurodegeneration, such as cerebral arteries, neurons, other cell types involved in regulation of the neurovascular unit (Fig. 1) [28], and inflammatory processes. Recent data have suggested that the phenomenon of SD may play a central role in both injury progression after brain injury as well as being affected by nimodipine (Figs. 1 and 2).
Fig. (1).
Proposed model by which Nimodipine and other L-type calcium channel blockade may mitigate the harmful effects of SD through both direct mechanisms limiting SD initiation and propagation as well as the microvascular response to SD. This leads to less severe metabolic stress to vulnerable cells in compromised regions. The four vertical panels (separated by dashed lines) in the chart demonstrate the effects of SD (green arrows) across the ischemic spectrum of perfusion from irreversible core to normally perfused brain (See CBF chart at the top). As SD passes from initiation in core or penumbra into vulnerable brain, neurons and astrocytes are further depolarized and penetrating arterioles (red) transiently constrict (spreading ischemia), causing progression of injury. In normal brain, SD dissipates, causes reversible metabolic stress to neurons, and vasodilation (spreading hyperemia.) Under conditions of adequate l-type calcium channel blockade with nimodipine, SD is initiated less frequently and propagates less efficiently by neurons and astrocytes, leading to fewer and less severe SD. The microvascular constriction is reversed through a process that involves smooth muscle cells, but may also be mediated through effects in pericytes and perivascular astrocytes. Normal brain is therefore able to compensate with the metabolic challenge and even in conditions of vasospasm, this lack of metabolic stress to potentially vulnerable brain lessens the burden of delayed cerebral ischemia. Metabolic stress to neurons and astrocytes is demonstrated in each vulnerable zone before and after SD.
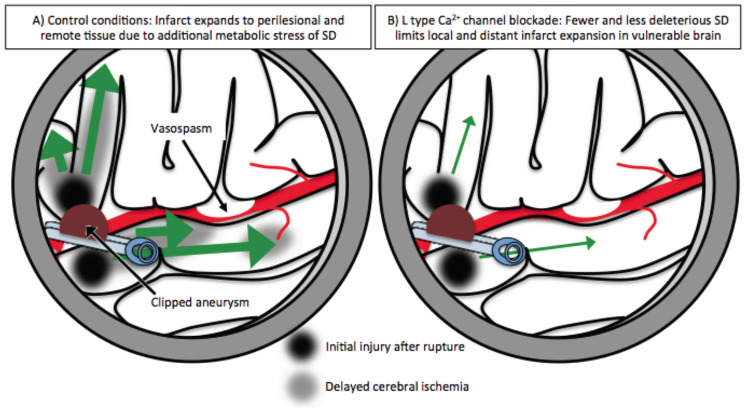
Fig. (2).
Mechanism for reduction of delayed cerebral ischemia (DCI) with nimodipine even in the presence of large vessel vasospasm. Such a model explains the clinically beneficial effect without consistently limiting large vessel vasospasm. Under control conditions (A), SD (green arrows) propagates from core (black zone) and vulnerable regions (grey zone), causing additional ischemia both adjacent to the core insult and in distant regions with compromised perfusion due to large vessel vasospasm. Under conditions of L-type calcium channel blockade with nimodipine (B), some SD may still be initiated but is less frequent and less severe as outlined in Fig. 1. This then leads to less risk of DCI (grey area) both locally and in remote tissue, even in the case of large vessel vasospasm.
3.1. Cardiovascular System
L-type channels blockers are very well-established in clinical use for the treatment of hypertension, and their effects are due to block of VDCCs involved in both cardiac excitability and smooth muscle tone. CaV1.2 and CaV1.3 channels both have important actions on cardiac myocytes and pacemaking, whereas Cav1.2-containing channels underlie the well-established effects of nimodipine on vascular smooth muscle cells and effects on peripheral resistance. Like other dihydropyridines, nimodipine effectively inhibits transmembrane Ca2+influx following depolarization of smooth muscle cells, and thereby reduces Ca2+-dependent activation of contractile machinery [29]. Nimodipine crosses the blood brain barrier, and early studies demonstrated increases in cerebral blood flow (CBF) after intravenous or intra-arterial injection of nimodipine in rabbits, dogs, and cats [29]. More recent work with near infrared spectroscopy and transcranial Doppler ultrasound has demonstrated significant increases in cerebral perfusion in healthy human subjects [30], supporting a role for L-type channels in cerebrovascular tone.
As noted above, nimodipine was initially hypothesized to be of therapeutic benefit for aSAH and other CNS disorders by virtue of its ability to reverse persistent, pathological L-type channel activation on large cerebral arteries. Angiographic evidence for vasospasm in these patients was clear, and preclinical work showing improved perfusion cerebral flow made nimodipine a logical therapeutic candidate. However, the lack of correlation between improvement in angiographic persistent large vessel vasospasm and clinical outcomes with nimodipine cast doubt over whether the reversal of vasospasm is the primary mechanism of nimodipine’s benefit in aSAH [31, 32]. Recent work has suggested an appealing alternative mechanism of action for nimodipine, related to the dramatic vascular dynamics associated with SD in a range of pathological states.
Nimodipine is thought to prevent deleterious vascular responses to SD in the injured brain. Preclinical and clinical studies demonstrate that SDs cause damage in the brain tissue that has already been rendered vulnerable as a consequence of ischemia or injury. Neurovascular coupling is impaired, or even reversed in injured brain - leading to transient, inappropriate constriction of small arteries and arterioles, strictly time-linked to SD events [33]. This inappropriate constriction results in a “spreading ischemia” that follows the slow propagation of SD through the injured territory (Fig. 1). There is literature that has demonstrated the effectiveness of nimodipine at preventing SD and that spreading ischemia was in fact converted into hyperemia by nimodipine. In studies of anesthetized rats, SD normally gives rise to an appropriate hyperemic response. The application of hemoglobin to the brain surface (to model aspects of aSAH) converts the hyperemia to spreading ischemia, and the intravenous administration of nimodipine restores hyperemia. Neuronal injury mediated by SD is therefore reduced in this model [13, 34, 35] Similarly in normally perfused cortex, nimodipine may even attenuate the early hypoperfusion seen during SD [36]. In addition, the hyperemic response to SD can be augmented with nimodipine under ischemic conditions [37]. These observations imply an important role for L-type channels, presumably activated by K+ released during the event itself, and also contributed to by inappropriate release from astrocytes (see below). Nimodipine’s effectiveness in preventing K+-induced vascular constriction presumably explains correction of neurovascular coupling and allows greater restoration of metabolic substrates to brain tissue in the immediate wake of an SD event.
3.2. Neurons
L-type channels are extensively distributed on central neurons [38, 39], and play significant roles in dendritic and somatic Ca2+ accumulation [40-42], gene expression [43], and excitability [44]. While regulated Ca2+ accumulation underlies much physiological signaling, un-regulated neuronal Ca2+ overload has long been established as a mechanism of neuronal injury. Much interest in Ca2+-dependent neuronal injury has focused on consequences of glutamate receptor over-activation - usually termed “excitotoxicity” [45, 46]. While NMDA (N-methyl-D-aspartate)-receptor activation is a primary contributor to such injury, it is worth emphasizing that Ca2+ influx via these receptor channels is only a small fraction of the transmembrane current. NMDA-mediated Na+ flux contributes to substantial depolarization, and voltage-dependent calcium channel opening can thus make significant contributions to injury, secondary to NMDA receptor activation. Consistent with this idea, nimodipine was shown to protect against injury generated by exogenous application of NMDA to cultured cortical neurons [45]. In more complex slice preparations, exposure to low Mg2+ generates bursting activity due to relief of NMDA receptor block, and activation by endogenous glutamate. Under these conditions, neuronal Ca2+ spikes and injury were greatly reduced by nimodipine, and raised the possibility of nimodipine as a protective agent against neurotoxicity in a range of settings [47]. As noted previously [48], nimodipine has not proven particularly useful in clinical studies in conditions where calcium-mediated excitotoxicity might be thought to predominate (eg, ischemic stroke). However, failure in clinical trials or preclinical animal studies may bear some reevaluation, given the potential complications that are inevitable due to the traditional routes of administration and systemic effects of nimodipine (discussed in the next section). An additional consideration for the translation of studies may be age, as in neuronal cultures, nimodipine is protective against glutamate excitotoxicity in older neurons, with quite a limited contribution in young neurons [49, 50]. Whether or not there is an age-dependency for protection in clinical conditions is not yet clear.
While much historical focus has been on glutamate receptor-mediated excitotoxicity, L-type channels can contribute in more direct ways to neuronal vulnerability. For example, L-type channel opening makes a significant contribution to broad action potentials in substantia nigra neurons. L-type activity is important for maintaining the tonic firing activity of these cells, but the Ca2+ accumulation that results (from both transmembrane flux and associated release from intracellular stores) is large and appears likely to contribute to the vulnerability of these neurons to pathology underlying Parkinson disease [51]. Cav1.2 and Cav1.3 channels have both been implicated in substantia nigra Ca2+ transients, and there is a body of evidence to suggest that reducing flux through these channels is beneficial for Parkinson's disease. Supporting evidence includes epidemiological data comprising over 27 million subjects demonstrating a relative risk reduction of 0.80 (95% CI 0.65-0.98) for Parkinson's disease in patients on dihydropyridone calcium channel blockers [51, 52].
Neuronal injury following exposure to a range of toxins can also be prevented by L-type Ca2+ channel blockade. For example, nimodipine has been shown to protect against the behavioral consequences of methylmercury exposure in rats. Interestingly, nimodipine was more effective in younger, rather than older animals [53]. Beta amyloid exposure led to Ca2+-dependent toxicity in cortical neuronal cultures, and nimodipine reduced injury under these conditions [54]. Accumulating evidence suggests that neuronal Cav1.2 expression is enhanced as a consequence of L-type channel over-activation in models of Alzheimer’s disease and contribute to further pathologic Ca2+ loading in this condition, as well as other beneficial consequences downstream of L-type channel opening [55].
Finally, an under-appreciated mechanism of action of nimodipine on CNS neurons relates to the ability of L-type channels to effectively inhibit influx of Zn2+ ions [56]. Zn2+ is highly enriched in the brain and has essential roles in transcriptional regulation and intracellular signaling, and serves as a neurotransmitter and/or neuromodulator [57, 58]. Like Ca2+, over-accumulation of cytoplasmic Zn2+ is toxic, and deregulation of Zn2+ influx or intracellular sequestration kills neurons and has been implicated in brain injury and neurodegeneration models [59]. Nimodipine is clearly effective against Zn2+ toxicity in cell culture [60] but the extent to which stabilization of intracellular Zn2+ levels contributes to protective effects in many other studies (i.e., those generally attributed to Ca2+) is unknown. Neuronal Zn2+ toxicity could be of particular importance for therapeutic targeting of SD events in aSAH and other disorders, since there are large increases in extracellular Zn2+ during SD events in brain slices, due to release from presynaptic vesicles [61]. Synaptic Zn2+ appears to translocate into postsynaptic neurons during SD, with the transmembrane flux being carried by L-type channels. It is not yet known whether nimodipine directly limits SD-induced neuronal injury, due to limiting postsynaptic Zn2+ accumulation. However, nimodipine was shown to effectively prevent the initiation of SD events in brain slices under conditions that are relevant to brain injury, due to prevention of Zn2+ accumulation [62, 63]. For this reason, targeting of neuronal L-type channels could be an interesting adjunct therapeutic approach for SD. Even in models of KCl stimulation, nimodipine has been shown to decrease the frequency, latency, and amplitude of recurrent SD [64] and also reduces the potassium shift and rate of this shift [36]. Neuronal effects of nimodipine could help prevent the initiation of SD and associated toxicity in vulnerable tissue, in addition to targeting inappropriate neurovascular consequences described in the previous section. Whether or not nimodipine decreases the number of SDs observed in intensive care unit (ICU) patients or some subsets of particularly vulnerable patients is an intriguing question that remains to be tested. (Fig. 1).
3.3. Astrocytes
Astrocytes are critical participants in the regulation of vascular tone and neurovascular coupling [65]. Large intracellular Ca2+ waves are prominent features of astrocyte networks, and while L-type Ca2+ channels are prominent on astrocytes [66], there is currently little evidence for a central role of L-type channels in these waves. However, astrocyte Ca2+ waves do couple to vascular responses, in part due to activation of large conductance Ca2+-activated K+ channels (BK) in astrocyte end feet. In a study modeling aSAH in anesthetized rats, brain slices were taken from rats that had received injections of autologous arterial blood into the subarachnoid space. Perivascular astrocytes in aSAH preparations showed enhanced spontaneous astrocytic Ca2+ transients and were concluded to be sufficient to excessively activate Ca2+-activated K+ channels in end feet, elevate extracellular K+ and contribute to inappropriate vascular constriction via L-type Ca2+ channels [67]. From this model, nimodipine’s beneficial effects in neurovascular coupling would be attributed to effects on smooth muscle L-type channels as a downstream consequence of disrupted astrocyte Ca2+ signaling in aSAH.
Astrocyte phenotypes are not static, and especially after injury, these cells can take on an “activated” or reactive phenotype [68]. Toxicity due to glial activation has been implicated in neurodegenerative disorders, and nimodipine was reported to prevent toxicity due to activated astrocyte exposure in culture [69]. Reactive astrocytes can also have beneficial consequences in the context of brain injury. Astrocyte activation accompanies repetitive episodes of SD and likely contributes to the progressive elevation of the SD threshold in mice [70]. Deliberate activation of populations of astrocytes can completely prevent initiation of SD in brain slices [71]. Interestingly, L-type channels appear to contribute to astrocyte activation [72] (Fig. 1). Whether or not nimodipine reduces the degree of astrocyte activation associated with SD in injury models is not yet known. Likewise, further study is required to determine whether or not Ca2+ transients recorded in activated astrocytes are enhanced by L-type flux, and whether these could modify neurovascular coupling via excessive activation of BK channels. These issues are of potential significance to nimodipine’s actions in restoring neurovascular coupling and limiting neuronal injury after brain injury.
3.4. Pericytes
Recent work has revealed important and diverse roles for pericytes in cerebrovascular regulation. Pericyte processes that wrap around capillaries are contractile and make significant contributions to regulation of parenchymal blood flow. There is evidence that contraction of isolated pericytes involves L-type Ca2+ flux [73] and while it is assumed that voltage-dependent Ca2+ influx is responsible for contractile activity in vivo, this required direct demonstration. Pericyte constriction of capillaries has been demonstrated in ischemic conditions and suggested to be due to depletion of ATP and failure of pericyte Ca2+ extrusion mechanisms [74]. SD could provide another mechanism for inappropriate constriction of pericytes in injured brain, either as a direct result of local K+ elevations and voltage-dependent Ca2+ influx, or accumulation of 20-HETE, which constricts pericytes. In addition to Ca2+-regulated contraction, death of pericytes as a result of persistent capillary constriction has been suggested to cause long-lasting capillary constriction, which persists until removal of dead pericytes by microglia. Such persistent constriction could underlie the non-reflow phenomenon in severe ischemia [74, 75]. If these ideas are supported, then therapeutic approaches selectively targeting mechanisms of pericyte contraction (potentially including L-type Ca2+ influx) could be very valuable for restoring flow in a range of CNS injuries and disorders. It is not yet known whether nimodipine or other L-type blockers modify other important functions of pericytes, including the formation of glial scarring, maintenance of the blood-brain barrier and angiogenesis that are important for injury progression and repair.
3.5. Microglia and Inflammatory Mediators
Neuroinflammatory responses are increasingly attributed to a wide range of CNS disorders, and L-type channels (both Cav1.2 and Cav1.3) appear to be expressed on microglia [76]. Nimodipine has been shown to significantly modify neuroimmune signaling, as a mechanism to reduce neuronal injury. Li et al. [77] showed that dopamine neurons in culture were protected from LPS-induced degeneration by nimodipine, an effect likely due to prevention of microglial activation and NADPH oxidase activation in these cultures. Nimodipine also effectively prevented neurotoxicity mediated by the chemokine receptor ligand CLCX12 in mixed cultures from rat cortex [78]. Nimodipine reduced neuronal death in slice cultures, which was attributed to reduction of inflammatory processes [79]. Nimodipine also appears effective in in vivo animal models of CNS inflammation. Hopp et al. [80] showed that Ca2+ dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. Finally, nimodipine inhibits IL-1β release stimulated by amyloid β from microglia, both in vitro and in vivo [81]. Interestingly, recent work has identified close links between microglia and SD activation, including the suggestion that repetitive bouts of SD lead to microglial activation, which via TNF alpha and ROS lead to decreased SD threshold [82]. It is possible that activated microglia could contribute to repair of dendritic spines affected by SD or, alternatively, contribute to injury. Whether or not nimpdipine influences SD by actions on microglia could be another useful avenue to investigate [82].
In summary, the broad cellular targeting of nimodipine suggests that the early models which considered only persistent, large vessel vasoconstriction mechanisms are inadequate to understand the potential therapeutic effects. Among these implicated targets, the effects on SD offer some of the most interesting and relevant observations for potential therapeutic action, both related to improved microvascular response to SD (spreading ischemia) as well as potential inhibitory effects on neuronal or astrocytic SD initiation. These mechanisms are important to consider as we now review the clinical data both supporting and refuting the role of nimodipine in various conditions in order to try to improve perspective on if it is possible that there is a subgroup with a high degree of SD dependent secondary injury in whom nimodipine may be effective.
4. CLINICAL USE OF NIMODIPINE
4.1. Nimodipine for Reducing Stroke after Aneurysmal SAH
Nimodipine was approved for clinical use by the FDA initially for improvement of neurologic outcomes after aSAH in 1988 for good Hunt and Hess grade patients and then expanded in 2000 to include all Hunt and Hess grades based on 4 randomized controlled trials (RCTs) demonstrating efficacy [31, 83-85]. While these studies remain the backbone of clinical evidence for the efficacy of nimodipine, multiple randomized studies have since been conducted to elucidate the mechanisms and refine its use; however, there has been little progress in clinical practice. Though there are now multiple generic forms on the market, nimodipine remains the only FDA-approved treatment for aSAH. Nymalize (Arbor) is a formulation comprising a liquid filled capsule that could be injected into a nasogastric tube, however errors resulting in deaths from IV injection have lead to an FDA boxed warning limiting use to oral or via nasogastric tube (www.fda.gov). An IV formulation is available in Europe, but is not FDA approved.
The initial evidence of the efficacy of nimodipine was from ex-vivo animal data showing selectivity of nimodipine in inhibiting contractions of large cerebral arteries versus femoral arteries in dogs [86]. The first RCT demonstrated a statistically significant decrease in the primary outcome of stroke or death related to vasospasm for nimodipine versus placebo within 96 hours of aSAH (13.3% vs 1.8%) in 125 good (neurologically “normal”) Hunt and Hess grade subjects [83]. This improvement was attributed to mitigating the hemorrhage volume related dependency that was observed in the placebo arm. The second study, from a single center in France, conducted in 70 aSAH patients with generally good Hunt and Hess grade (I-III), also demonstrated significant reduction in death or severe neurologic deficit from vasospasm with nimodipine versus placebo (23.8% vs 5%) [84]. The third RCT enrolled 554 subjects of any clinical grade across 4 centers in the UK [85]. There was a significant reduction in incidences of infarction and poor outcomes with nimodipine versus placebo (33% vs 22%; 33% vs 20%, respectively). A study from Canada specifically evaluated the use of nimodipine in 188 subjects with poor neurologic grades (Hunt and Hess III and IV) and found significant improvement in good outcomes with nimodipine (29.2%) versus placebo (9.8%) [31]. Comparison of angiograms from admission and during the vasospasm interval did not show differences in moderate or severe angiographic vasospasm between groups, underscoring the points emphasized above, that while nimodipine seems to improve clinical outcome and decreases vasospasm related infarction, angiographic vasospasm itself is an imperfect surrogate and may not be directly affected. While these studies formed the basis for FDA approval, varying dosing between studies and inconsistencies in outcome raise concern that these might not be considered “adequate, well-controlled” studies by modern criteria.
Several additional trials in aSAH have been conducted. A single-center RCT with 75 patients with aSAH found a non-significant reduction in death with nimodipine (OR=0.52 for death or poor outcome, 95% CI= 0.26-1.01) [87]. There was no effect of nimodipine on blood pressure, and a non-significant, slight progressive decrease of cerebral blood flow (CBF) was observed in the nimodipine group over the treatment period, suggesting that nimodipine does not significantly augment CBF or decrease blood pressure, both contrary to predicted pre-clinical data. Another clinical study of 188 patients, who had already developed vasospasm, demonstrated a significant reduction in death or severe deficit from 49% in placebo to 19% with intravenous (IV) nimodipine [88]. A study with 213 subjects found that mixed IV/oral delivery of nimodipine was associated with a significant reduction in mortality in the early-surgery group but not in the late-surgery group (which experienced excess death from re-bleeding) [89]. A significant decrease in levels of thromboxane B2 release from platelets was also observed, which was postulated to improve microcirculation as a mechanism of neuroprotection [90]; an alternate explanation to large vessel vasospasm-mediated clinical benefit [90, 91]. A population-based longitudinal study with 208 patients who were evaluated before and after the approval of nimodipine showed an overall reduction in poor outcomes from 37% to 20% [92]. These studies provide reassuring confirmation that the clinical benefits observed in RCTs translate to measurable clinical improvements in routine patient care.
The most recent update to a Cochrane systematic review of calcium antagonists for aSAH [93] reported a significant overall reduction in relative risk of unfavorable outcome with any calcium channel antagonist (RR=0.81, 95% CI 0.72-0.92), with a number-needed-to-treat of 19 [93]. The effect was much stronger for oral nimodipine alone (RR=0.67, 95% CI 0.55-0.81), which was noted by the authors to be driven by one large clinical trial of oral nimodipine. Since then, 2 additional studies further supported the use of oral nimodipine. Two RCTs demonstrated no significant difference between oral versus IV nimodipine in either delayed ischemia or vasospasm [94, 95]. Though these studies were not powered as non-inferiority trials, the results suggest that oral administration should still be considered standard of care in most circumstances due to higher cost and potentially increased side effects with IV administration.A similar trial in 171 subjects was published in 2012, which also failed to show a significant additional benefit of IV administration, again underpowered as a non-inferiority study [95].
Alternative routes of administration, including prolonged intra-arterial infusion and local delivery (discussed in the following section), are now also being investigated. In a pilot study, 10 patients with severe refractory vasospasm had placement of intra-arterial catheters into the cervical internal carotid artery with continuous infusion of nimodipine at 50 mL/hour [96]. Radiographic and physiologic improvement was reported in all subjects while outcomes were reportedly all favorable. Finally, the results of a phase 1/2a clinical trial of a new formulation of sustained release intraventricular nimodipine demonstrated a favorable safety profile with some promise of efficacy [97].
4.2. Nimodipine for Ischemic Stroke
Second to aSAH, ischemic stroke has been one of the most well-studied conditions for potential effect of nimodipine. While there was some hope established from early RCTs, larger subsequent trials failed to convincingly demonstrate a benefit in ischemic stroke in general or in any pre-defined subgroup. In a 2012 Cochrane analysis of 34 trials (most with nimodipine but including other calcium channel antagonists) with 7731 patients, the pooled analysis of all calcium antagonists in acute ischemic stroke found no significant effect on primary outcome (RR=1.05; 95% CI 0.98-1.13) or death (RR=1.07, 95% CI 0.98-1.17) [98]. Higher doses of nimodipine were not associated with any signal of improvement and might in fact lead to worse outcomes. This analysis revealed, with a fairly high degree of confidence, that nimodipine does not improve outcomes after acute ischemic stroke. Nonetheless, based on new understanding of pathophysiologic mechanisms in stroke (such as SD) coupled with new methods of delivery of nimodipine, it seems reasonable to revisit this potential therapeutic target of nimodipine.
Early trials raised initial interest in the use of nimodipine for acute ischemic stroke, albeit with some methodological concerns and potential of fraudulent reporting. Gelmers et al reported an initial non-blinded, randomized trial of nimodipine [99] followed by a larger multicenter RCT in 186 subjects, reportedly demonstrating benefit only in male subjects [100] . Dr Gelmers was later found guilty of falsifying records (BMJ 2002; 325 doi: https://doi.org/10.1136/bmj.325.7367.734). Nonetheless, subsequent data seemed encouraging from other centers with a study of 41 subjects demonstrating a higher rate of improvement in neurologic outcomes with nimodipine within 12 hours of stroke [101], and additional data supporting a significant benefit in mortality and neurologic outcome in patients with mild stroke [102]. An assessment of memory up to 3 months after stroke in patients randomized into nimodipine or placebo found significant improvement in these measurements with time in the nimodipine group [103]. To determine the mechanism of action nimodipine in stroke, positron emission tomography studies of CBF were performed in 14 ischemic stroke subjects randomized to nimodipine or placebo [104]. A significant improvement in CBF in the densely ischemic region (penumbra), as well as improved metabolism (CMRO2), were both observed, but the study was not designed to detect any outcome difference.
Several large clinical trials have demonstrated a lack of benefit with nimodipine in ischemic stroke, even in patients with less severe stroke [105-110]. In one study, with 1215 patients, a trend towards worse outcomes was even observed [111]. Each study offered further tantalizing post-hoc analyses to attempt to identify a subgroup with benefits. The American Nimodipine Study Group found significant benefit in a group treated with 12 mg of nimodipine within 18 hours with a negative initial CT [107]. A subgroup analysis by Kaste et al. [109] found that nimodipine seemed to abolish infarct growth but only on smaller volume strokes where treatment was initiated within 24 h [112]. A study of 31 patients showed that, in the absence of an overall benefit, there was a significant improvement in relative neurologic deficit with nimodipine [111]. To further investigate the potential benefit in early intervention, the VENUS trial evaluated nimodipine versus placebo use started within 6 hours of stroke. The trial was stopped early because the first Cochrane systematic review did not show a benefit with nimodipine and likewise, in the 454 subjects enrolled, no beneficial effect was found with nimodipine, again with a trend toward harm [108]. Based on these data, including the Cochrane review, no further trials of nimodipine in stroke have been initiated [98]. Ongoing analysis of the previous trials has continued to underscore the importance of the blood pressure-lowering effect in predicting worse outcome in nimodipine treated subjects [113].
It was hypothesized that any potential benefits of nimodipine was masked by the detrimental effect of blood pressure reduction inducing (even moderate) hypotension [109]. To test this hypothesis prospectively, Ahmed et al. [114] conducted a study in 265 subjects receiving low-dose or high-dose nimodipine or placebo. There was a statistically significant reduction in systolic and diastolic blood pressure with nimodipine, which was significantly correlated with worse outcome, further bolstering the role of hypotension as a driving factor for poor outcome.
While the overall data have suggested that there is no overall effect of nimodipine in ischemic stroke, there certainly has been a suggestion of benefit. With a newer understanding of the role that SD may play in the early and subacute expansion of stroke coupled with the known deleterious effects of hypotension, there may be a rationale to revive this “previously closed” topic. A protocol to test the efficacy of nimodipine in improving cognitive impairment after stroke has been published, possibly paving the way for studies evaluating more nuanced outcomes compared to previous studies focused only on gross outcomes [115]. In addition to more nuanced outcome measurement, limiting deleterious effects of hypotension would be critical in considering any future study, potentially with local delivery techniques.
4.3. Nimodipine for Traumatic Brain Injury
The beneficial effect of nimodipine observed in aSAH led to an interest in whether nimodipine could affect the delayed injury phase in TBI (particularly in cases of traumatic SAH [tSAH]). While no convincing data based on meta-analyses currently supports routine use of nimodipine in TBI, the known detrimental effects of hypotension may contribute to these overall equivocal results. In the most current Cochrane review, a significant beneficial effect on death or severe disability in the subgroup with tSAH was demonstrated [116]. The conclusions, however, represented significant uncertainty, pointing out that the adverse reactions (particularly hypotension) could be harmful in some patients and have been challenged by a larger meta-analysis, which incorporated a large unpublished data set.
Four large randomized studies using nimodipine (only 3 of which were published) and 2 studies using nicardipine have been conducted. The nimodipine studies are sequentially referred to as HIT (Head Injury Trial) I-IV. The HIT I trial enrolled 352 patients to IV nimodipine (2 mg/h for 7 days) and demonstrated a non-significant 8% improvement in a good outcome in the treated group [117]. HIT II enrolled 852 subjects within 24 hours of TBI and was powered to detect a 10% difference in good outcome as suggested by HIT I; however, the outcome was not significant. A post hoc analysis showed a significant effect in patients with tSAH [118]. However, a pooled analysis with HIT I did not confirm this hypothesis [119]. HIT III therefore was designed to test this group with tSAH specifically. The duration of treatment was longer, at 3 weeks, and the results demonstrated significantly less poor outcome in the nimodipine group [120]. The HIT IV trial also focused only on tSAH; however, the results of the study have not yet been published in their entirety. The unpublished data, which included 592 subjects with tSAH who had been enrolled before the trial was stopped, was analyzed in a systematic review and showed that there were significantly worse outcomes with nimodipine (OR=1.44, 95% CI 1.02-2.06). The 4 HIT studies and an additional study from India in 97 severe TBI subjects, which also did not show any benefit in the treatment group based on GOS [121], were analyzed together. This meta-analysis found no significant effect of nimodipine on outcome. While specific analysis in the tSAH group was not performed, the authors argued that since HIT IV included only tSAH patients, and this analysis comprised more than double the numbers reported in the Cochrane review (1074 versus 460), that even in tSAH, there was no significant beneficial effect [122].
In summary, these relatively limited data have yielded heterogeneous and conflicting results in TBI. This may be related to many factors including the heterogeneity of TBI itself, potential deleterious effects of hypotension, or lack of efficacy of the treatment. If a targeted high-risk population can be identified and hypotension can be mitigated, nimodipine may still hold promise to reduce delayed injury progression in TBI. SD occurs only in about 50-60% of TBI subjects, however, has been closely linked to worsened outcome [123]. If this subgroup can be predicted or targeted, such therapies may be worth reconsideration.
4.4. Nimodipine for Migraine
The “vascular theory” of migraine (i.e, there may be a role of vasoconstriction followed by vasodilation in the headache phase) led to the hypothesis that nimodipine may have efficacy for migraine, though after initial interest, nimodipine has been largely abandoned in favor of newer agents. However, further elucidation of the pathophysiology of migraine, and the involvement of SD as an underlying cause suggested that the efficacy of nimodipine prophylaxis could be related not only to vascular effects, but also neuronal effects of L-type calcium channels in limiting SD and therefore migraine initiation.
Several RCTs in migraine prophylaxis have demonstrated a reduction in the frequency and duration of migraine attacks with nimodipine [124-127]. One trial evaluating sublingual nimodipine for acute attacks failed to show a benefit, but had significant methodological problems [128]. Two relatively large studies from the migraine-nimodipine European study group were conducted for both common migraine (without aura) and classic migraine (with aura). In 192 patients with common migraine, no difference in treatment groups was noted. However, there was a significant placebo effect, which raised some uncertainty about the observed modest possible beneficial effect [129]. There was also no difference between in treatment groups in the second study that enrolled 89 patients with classic migraine, except that both groups had significant improvement over the 11 weeks of treatment [130]. In another study with a more robust design, significantly greater reductions in both frequency and duration of attacks were noted for nimodipine versus placebo [131]. Underlying methodological concerns may be relevant, because if adequate doses were delivered to the CNS, the migraine with aura group would be expected to respond more favorably if there is a significant effect on SD.
Despite these promising results, use and further investigation of nimodipine in migraine have largely faded, likely due to improved efficacy of alternative agents as well as limited availability and high cost of nimodipine. While these subjects would not be a likely group to benefit from local delivery, given better understanding of both the pathophysiology of migraine and the mechanisms of action of nimodipine, it may be a reasonable target to consider a reappraisal in this context.
4.5. Nimodipine for “Neuroprotection”
Nimodipine was proposed early in its evaluation to play a “neuroprotective” role and has therefore been studied in a wide range of conditions. The significant heterogeneity of these conditions and the lack of any repeated or large trials significantly limit drawing meaningful conclusions from these data other than to understand that there has been a wide range of applications with mixed reported effects.
A study of CBF after cardiac arrest found significantly higher blood flow with nimodipine compared to placebo with no effect on intracranial pressure or neurologic outcome [132]. Another study of 39 out-of-hospital cardiac arrests resuscitated with success showed slightly decreased intracranial pressure with nimodipine [133]. In a larger study of 155 patients after out-of-hospital ventricular fibrillation resuscitation, no significant difference in mortality was noted, except for a survival benefit with nimodipine in patients with delays longer than 10 minutes [92]. After cardiac surgery with cardiopulmonary bypass, there was also no difference in CBF between nimodipine and controls, except for possible subtle neuropsychological improvement [132]. A more extensive study of 400 patients with valve replacement was stopped early due to increased bleeding in the nimodipine group [134]. The lack of benefit in outcomes halted further interest in this line of study.
Regarding cognitive effects, initial studies found no effect of nimodipine on memory after electroconvulsive therapy or progression of dementia [135, 136]. However, a prophylactic benefit, in terms of delaying disease progression, was reported in a larger study in 227 patients with Alzheimer’s disease [137]. This was further supported by a multicenter study in 755 elderly patients with cognitive deterioration, where a significant improvement in cognitive function was reported [138]. A specific trial in multi-infarct dementia showed no overall effect, but post-hoc analysis demonstrated a possible effect in vascular dementia [139]. A Cochrane review from 2001 identified 14 trials, 9 with data available, and concluded that there were some short-term beneficial effects of nimodipine, but did not recommend routine use because of only relatively moderate effect in certain domains and only short-term outcome data [140]. Since then, another study similarly showed a possible moderate benefit for such conditions [139].
In motor neuron disease, nimodipine was not found to be effective to slow progression [141, 142]. During treatment with cisplatin for ovarian cancer, nimodipine did not show a neuroprotective effect [143]. Similarly, no significant effect was noted on cognitive function during treatment for HIV [144]. Other niche exploratory applications have included vascular depression [145], acute mania [146], various withdrawal syndromes [147-149], affective dysregulation [150], essential tremor [151], vertigo [152], as an adjunct to opiates [153], as an adjunct in epilepsy [154, 155], and to improve hearing and facial function after vestibular schwannoma surgery [156, 157].
In summary, nimodipine has been extensively studied in multiple conditions. While vascular effects in cerebral smooth muscle have been the underlying mechanism thought to provide benefits, harmful waves of SD and associated spreading ischemia may underlie at least some of the observed therapeutic efficacy. Conditions characterized by ischemia and particularly high frequent rate of SD may be a potential target group based on this improved mechanistic understanding. Techniques to mitigate the known deleterious effects of hypotension (such as local delivery) may further improve the safety profile in such revisited indications.
5. IMPROVING STANDARD OF CARE-IMPROVING THE EFFECTIVENESS OF NIMODIPINE AND LIMITING RISK OF HYPOTENSION
While clinical evidence has demonstrated the therapeutic benefits of oral nimodipine in improving outcomes in aSAH, the recurring concerns for systemic hypotension have continued to be problematic. A potential solution to this problem is a targeted drug delivery approach that increases local drug concentration while minimizing systemic concentrations, which may help to reduce the unwanted systemic side effects. This approach was investigated for aSAH in the intraoperative local delivery of sustained-release pellets of nicardipine into the subarachnoid space next to cerebral arteries [19, 158]. The pharmacology of nicardipine is very similar to nimodipine. In a Phase 2a study of 32 aSAH patients treated with nicardipine implants during surgery after clipping of the aneurysm, angiographic vasospasm was significantly reduced (73% control vs. 7% nicardipine), as well as the incidence of delayed ischemic lesions (47% control vs. 14% nicardipine) [159]. Similar outcomes have been observed in a larger study with 100 poor-grade aSAH patients [158]. Pellet-based therapeutics, however, are difficult to administer intraventricularly or non-surgically, which limits their use in patients who undergo endovascular coiling to repair ruptured aneurysms [18] In addition, the pellets would not be expected to circulate into the basal cisterns.
A new platform for localized, sustained-release administration of nimodipine, (EG-1962; Edge Therapeutics) is being investigated in clinical trials [160]. Pharmacokinetic evaluation showed nimodipine release after administration consists of an initial burst followed by sustained release over 21 days [161]. The plasma concentrations after aSAH in dogs are equivalent to oral nimodipine, although with less dose-dependent fluctuation, and are maintained for the entire time window when DCI may develop. Importantly, nimodipine concentrations are dramatically higher in the CSF compared to oral administration (Fig. 3) [161]. In addition, EG-1962 can be administered intraventricularly to patients with a ventricular catheter who undergo endovascular aneurysm repair or neurosurgical clipping. The safety and efficacy profiles of EG-1962 have been extensively studied in preclinical and clinical studies. In dogs, EG-1962 reduced angiographic vasospasm as compared with oral nimodipine and was not associated with adverse effects, systemic hypotension, or pathologic effects in the brain [8]. In rats that received intraventricular injections of nimodipine microparticles at various doses equivalent to human doses of up to 1200 mg, there were no effects of EG-1962 on clinical observations, neurobehavioral evaluations, body weight, food consumption, ophthalmoscopy, hematology, coagulation, clinical chemistry, urinalysis, organ weight, or macroscopic pathology [161]. In a beagle toxicity study, a granulomatous foreign body-type reaction that was dose related to EG-1962 in the ventricular system was observed [161]. In addition, inflammation, fibrosis, and cardiac myofiber degeneration and necrosis of the left ventricle and interventricular septum of the heart were also observed in several beagles receiving intracisternal injection and in an animal receiving 103 mg dose intraventricularly; these effects were partially resolved by day 29 [161].
Fig. (3).
Pharmakokinetic analysis of plasma and serum nimodipine concentrations in the NEWTON 1 study [97], averaged across all doses. Intrathecal delivery of EG-1962 is compared to standard of care oral nimodipine. Note that CSF concentration (A) of nimodipine is nearly unmeasurable in the standard of care oral administration group. In plasma (B), concentrations of nimodipine in the oral group were higher, potentially increasing the risk of hypotensive transient events. The inset to the right (C) is a scanning electron micrograph of the microparticles used for delivery of the nimodipine crystals, which are embedded in the clefts of the particles (white arrows). This allows for slow sustained release of the nimodipine as measured by persistent nimodipine presence in serum even to 30 days after subarachnoid hemorrhage [161].
The randomized, open-label, dose-finding, Phase 1/2a NEWTON study (Nimodipine Microparticles to Enhance Recovery While Reducing Toxicity After SubarachNoid Hemorrhage) was recently completed. The results showed that 800 mg was the maximum tolerated dose of EG-1962 when used as a single administration in humans [8, 97]. Among the 54 subjects who were randomized to EG-1962, 60% achieved favorable clinical outcomes on the extended Glasgow outcome scale (GOSE) at 90 days, as compared with 28% of subjects who received oral nimodipine. In addition, EG-1962 was associated with less angiographic vasospasm/DCI, infarction because of DCI, and use of rescue therapy compared with oral nimodipine. A higher incidence of hypotension was reported with oral nimodipine versus EG-1962, and for the latter, none of the hypotension was considered related to treatment. More frequent incidences of CNS events, including cerebral vasoconstriction, DCI, cerebral infarction, and intracranial hemorrhage were reported in the oral nimodipine group. Overall, treatment with EG-1962 was considered safe and tolerable up to 800 mg. Fig. 3 shows the pharmacokinetic analysis. IV nimodipine was not used due to variable approval and data discussed above regarding lack of independent proof of efficacy.
A pivotal phase 3, double-blind, double-placebo, RCT was conducted to compare the efficacy of intraventricular 600-mg dose of EG-1962 to the standard of care oral nimodipine in subjects with aSAH (NCT02790632; NEWTON-2). This study was conducted at centers in North America, Europe and Australasia. Treatment consisted of 1 dose of intraventricular EG-1962 versus standard oral nimodipine with either placebo controls for both arms. Patients were followed up for 90 days or until premature discontinuation from the study. The primary endpoint was the proportion of subjects with a favorable outcome measured on the GOSE at 90 days after study randomization [162].
The study was halted in March 2018 by the independent Data Monitoring Committee after a review of interim data on 210 patients. A review of the data on these patients suggested that the study had a low probability of meeting its primary endpoint. Prespecified subgroup analysis of World Federation of Neurological Surgeons grades 3 and 4 patients reported that 43% (29/67) achieved favorable outcome on the GOSE compared to 29% (20/70) in the placebo group. This suggested a clinically meaningful potential benefit for these poor grade patients and is consistent with results of the phase 1/2a study. No safety concerns that would have halted the study or precluded further development were identified. While no other information is available now pending review and publication of the final results of the study, it is possible to speculate on some explanations for these results. Some theories previously expounded to explain negative results of aSAH trials include that rescue therapy works and increased use in placebo group, if true, would balance any benefits from the study drug. The outcome measures may not be sensitive enough to detect clinically meaningful outcome differences. No safety concerns were identified so it seems unlikely that EG-1962 adverse events counteracted its potential efficacy. It is also possible that enrollment of good grade patients with low probability of DCI could dilute an effect. Ongoing subgroup analysis, as well as exploratory analysis of the effect of local delivery of nimodipine on SD as a potential mechanism, is currently being explored. Such further analysis of the NEWON study data is necessary to understand the possible reasons for the study being halted early and hopefully will support conduct of additional studies of local drug delivery for aSAH. Promising results from these studies may help pave the way for the targeted, local drug delivery approach to be a new treatment option in aSAH that optimizes treatment outcomes by leveraging efficacy as it relates to novel mechanisms of injury such as SD, while minimizing the systemic drug side effects.
Finally, there may be other newer delivery approaches on the horizon that may offer additional methods to deliver nimodipine or similar drugs to tissues of interest while avoiding systemic side effects. A recent novel formulation has linked nimodipine with pH-sensitive chitosan nanoparticles. The goal using such a technique would be to preserve systemic administration, but limit release of the drug to tissues with decreased pH in the ischemic penumbra [163]. Though early in the developmental stage, such an approach represents an exciting advance to potentially target therapy to a patient’s pathological state.
CONCLUSION
Nimodipine is an effective treatment for the prevention of DCI after aSAH, however the mechanism of action and dose limiting hypotension remain major areas of uncertainty. Recent preclinical data have raised interesting additional mechanisms by which nimodipine may exert its action, particularly related to initiation of SD and mitigation of associated deleterious consequence (spreading ischemia). In addition, recent advances in delivery have demonstrated safety of local delivery with less hypotension and significantly higher concentration at the target organ. This site-specific, sustained-release delivery may increase concentrations of the nimodipine activity at the site it is needed most while avoiding additional deleterious effects related to systemic hypotension. While aSAH remains a major health problem and improved outcomes in these patients would be a major success, this better understanding of both the mechanisms of secondary brain injury and the ability to limit toxicity potentially opens the doors to a re-evaluation of such an approach in several other disease processes where nimodipine may have some therapeutic efficacy such as ischemic stroke and TBI. Detailed analysis of the EG-1962 phase 3 trial will certainly shed light to whether there is a significant effect that should be considered in such other conditions.
ACKNOWLEDGEMENTS
P-value was used for initial preparation. The study was funded by the Edge Therapeutics until the company was dissolved. Additional support for Carlson and Shuttleworth was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM109089.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
Dr. Macdonald receives grant support from the Physicians Services Incorporated Foundation, Brain Aneurysm Foundation and Ontario Genomics/Genome Canada and was previously an employee of EDGE therapeutics. Dr Carlson is a consultant for CerebroScope. Dr Hanggi was a consultant for EDGE therapeutics.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Johnston S.C., Selvin S., Gress D.R. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–1418. doi: 10.1212/WNL.50.5.1413. [DOI] [PubMed] [Google Scholar]
- 2.Rincon F., Rossenwasser R.H., Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73(2):217–222. doi: 10.1227/01.neu.0000430290.93304.33. [DOI] [PubMed] [Google Scholar]
- 3.Shea A.M., Reed S.D., Curtis L.H., Alexander M.J., Villani J.J., Schulman K.A. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery. 2007;61(6):1131–1137. doi: 10.1227/01.neu.0000306090.30517.ae. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwkamp D.J., Setz L.E., Algra A., Linn F.H.H., de Rooij N.K., Rinkel G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 5.Serrone J.C., Maekawa H., Tjahjadi M., Hernesniemi J. Aneurysmal subarachnoid hemorrhage: pathobiology, current treatment and future directions. Expert Rev. Neurother. 2015;15(4):367–380. doi: 10.1586/14737175.2015.1018892. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khindi T., Macdonald R.L., Schweizer T.A. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–e536. doi: 10.1161/STROKEAHA.110.581975. [DOI] [PubMed] [Google Scholar]
- 7.Greving J.P., Wermer M.J.H., Brown R.D., Jr, Morita A., Juvela S., Yonekura M., Ishibashi T., Torner J.C., Nakayama T., Rinkel G.J.E., Algra A. Development of the phases score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 8.Hänggi D., Etminan N., Macdonald R.L., Steiger H.J., Mayer S.A., Aldrich F., Diringer M.N., Hoh B.L., Mocco J., Strange P., Faleck H.J., Miller M.N. Nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhage. Neurocrit. Care. 2015;23(2):274–284. doi: 10.1007/s12028-015-0112-2. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014;10(1):44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 10.Malik A.N., Gross B.A., Rosalind L.P.M., Moses Z.B., Du R. Neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015;83(6):880–885. doi: 10.1016/j.wneu.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macdonald R.L. Origins of the concept of vasospasm. Stroke. 2016;47(1):e11–e15. doi: 10.1161/STROKEAHA.114.006498. [DOI] [PubMed] [Google Scholar]
- 12.Laskowitz D.T., Kolls B.J. Neuroprotection in subarachnoid hemorrhage. Stroke. 2010;41(10) Suppl.:S79–S84. doi: 10.1161/STROKEAHA.110.595090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreier J.P., Windmüller O., Petzold G., Lindauer U., Einhäupl K.M., Dirnagl U. Ischemia triggered by red blood cell products in the subarachnoid space is inhibited by nimodipine administration or moderate volume expansion/hemodilution in rats. Neurosurgery. 2002;51(6):1457–1465. doi: 10.1097/00006123-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Lu N., Jackson D., Luke S., Festic E., Hanel R.A., Freeman W.D. Intraventricular nicardipine for aneurysmal subarachnoid hemorrhage related vasospasm: assessment of 90 days outcome. Neurocrit. Care. 2012;16(3):368–375. doi: 10.1007/s12028-011-9659-8. [DOI] [PubMed] [Google Scholar]
- 15.Hänggi D., Beseoglu K., Turowski B., Steiger H-J. Feasibility and safety of intrathecal nimodipine on posthaemorrhagic cerebral vasospasm refractory to medical and endovascular therapy. Clin. Neurol. Neurosurg. 2008;110(8):784–790. doi: 10.1016/j.clineuro.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Biondi A., Ricciardi G.K., Puybasset L., Abdennour L., Longo M., Chiras J., Van Effenterre R. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. Am. J. Neuroradiol. 2004;25(6):1067–1076. [PMC free article] [PubMed] [Google Scholar]
- 17.Hänggi D., Turowski B., Beseoglu K., Yong M., Steiger H.J. Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid hemorrhage: influence on clinical course and cerebral perfusion. Am. J. Neuroradiol. 2008;29(6):1053–1060. doi: 10.3174/ajnr.A1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barth M., Pena P., Seiz M., Thomé C., Muench E., Weidauer S., Hattingen E., Kasuya H., Schmiedek P. Feasibility of intraventricular nicardipine prolonged release implants in patients following aneurysmal subarachnoid haemorrhage. Br. J. Neurosurg. 2011;25(6):677–683. doi: 10.3109/02688697.2010.548878. [DOI] [PubMed] [Google Scholar]
- 19.Kasuya H., Onda H., Sasahara A., Takeshita M., Hori T. Application of nicardipine prolonged-release implants: analysis of 97 consecutive patients with acute subarachnoid hemorrhage. Neurosurgery. 2005;56(5):895–902. [PubMed] [Google Scholar]
- 20.Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015;67(4):821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleckenstein A., Frey M., Fleckenstein-Grün G. Consequences of uncontrolled calcium entry and its prevention with calcium antagonists. Eur. Heart J. 1983;4(Suppl. H):43–50. doi: 10.1093/eurheartj/4.suppl_H.43. [DOI] [PubMed] [Google Scholar]
- 22.Meyer H., Wehinger E., Bossert F., Scherling D. cheminform abstract: nimodipine: synthesis and metabolic pathway. Chemischer Informationsdienst. 1983;14(20) doi: 10.1002/chin.198320206. [DOI] [PubMed] [Google Scholar]
- 23.Tsien R.W., Lipscombe D., Madison D.V., Bley K.R., Fox A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 24.Tang L., Gamal El-Din T.M., Swanson T.M., Pryde D.C., Scheuer T., Zheng N., Catterall W.A. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature. 2016;537(7618):117–121. doi: 10.1038/nature19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bean B.P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc. Natl. Acad. Sci. USA. 1984;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanguinetti M.C., Kass R.S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ. Res. 1984;55(3):336–348. doi: 10.1161/01.RES.55.3.336. [DOI] [PubMed] [Google Scholar]
- 27.Zuccotti A., Clementi S., Reinbothe T., Torrente A., Vandael D.H., Pirone A. Structural and functional differences between L-type calcium channels: crucial issues for future selective targeting. Trends Pharmacol. Sci. 2011;32(6):366–375. doi: 10.1016/j.tips.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Kisler K., Nelson A.R., Montagne A., Zlokovic B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017;18(7):419–434. doi: 10.1038/nrn.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scriabine A., Kerckhoff W. Pharmacology of nimodipine. Ann. N.Y. Acad. Sci. 1988;522(1 Calcium Antag):698–706. doi: 10.1111/j.1749-6632.1988.tb33415.x. [DOI] [PubMed] [Google Scholar]
- 30.Canova D., Roatta S., Micieli G., Bosone D. Extracranial circulation affects near infrared spectroscopy assessment of cerebral blood volume and oxygenation during neurovegetative tests. Auton. Neurosci. 2011;163(1-2):97. doi: 10.1016/j.autneu.2011.05.164. [DOI] [Google Scholar]
- 31.Petruk K.C., West M., Mohr G., Weir B.K., Benoit B.G., Gentili F., Disney L.B., Khan M.I., Grace M., Holness R.O. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J. Neurosurg. 1988;68(4):505–517. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- 32.Feigin V.L., Rinkel G.J.E., Algra A., Vermeulen M., van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology. 1998;50(4):876–883. doi: 10.1212/WNL.50.4.876. [DOI] [PubMed] [Google Scholar]
- 33.Dreier J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 34.Dreier J.P., Victorov I.V., Petzold G.C., Major S., Windmüller O., Fernández-Klett F., Kandasamy M., Dirnagl U., Priller J. Electrochemical failure of the brain cortex is more deleterious when it is accompanied by low perfusion. Stroke. 2013;44(2):490–496. doi: 10.1161/STROKEAHA.112.660589. [DOI] [PubMed] [Google Scholar]
- 35.Windmüller O., Lindauer U., Foddis M., Einhäupl K.M., Dirnagl U., Heinemann U., Dreier J.P. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of NO. Brain. 2005;128(Pt 9):2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- 36.Menyhárt Á., Farkas A.E., Varga D.P., Frank R., Tóth R., Bálint A.R., Makra P., Dreier J.P., Bari F., Krizbai I.A., Farkas E. Large-conductance Ca2+-activated potassium channels are potently involved in the inverse neurovascular response to spreading depolarization. Neurobiol. Dis. 2018;119:41–52. doi: 10.1016/j.nbd.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Szabó Í., M Tóth O., Török Z., Varga D.P., Menyhárt A., Frank R., Hantosi D., Hunya A., Bari F., Horváth I., Vigh L., Farkas E. The impact of dihydropyridine derivatives on the cerebral blood flow response to somatosensory stimulation and spreading depolarization. Br. J. Pharmacol. 2019;176(9):1222–1234. doi: 10.1111/bph.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ludwig A., Flockerzi V., Hofmann F. Regional expression and cellular localization of the α1 and β subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 1997;17(4):1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hell J.W., Westenbroek R.E., Warner C., Ahlijanian M.K., Prystay W., Gilbert M.M., Snutch T.P., Catterall W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993;123(4):949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magee J.C., Johnston D. Synaptic activation of voltage-gated channels in the dendrites of hippocampal pyramidal neurons. Science. 1995;268(5208):301–304. doi: 10.1126/science.7716525. [DOI] [PubMed] [Google Scholar]
- 41.Helton T.D., Xu W., Lipscombe D. Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci. 2005;25(44):10247–10251. doi: 10.1523/JNEUROSCI.1089-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topolnik L., Chamberland S., Pelletier J.G., Ran I., Lacaille J.C. Activity-dependent compartmentalized regulation of dendritic Ca2+ signaling in hippocampal interneurons. J. Neurosci. 2009;29(14):4658–4663. doi: 10.1523/JNEUROSCI.0493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bengtson C.P., Bading H. Synaptic Plasticity, Springer Vienna. 2012. Nuclear calcium signaling. pp. 377–405. [DOI] [PubMed] [Google Scholar]
- 44.Vandael D.H., Marcantoni A., Mahapatra S., Caro A., Ruth P., Zuccotti A., Knipper M., Carbone E. Ca(v)1.3 and BK channels for timing and regulating cell firing. Mol. Neurobiol. 2010;42(3):185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- 45.Abele A.E., Scholz K.P., Scholz W.K., Miller R.J. Excitotoxicity induced by enhanced excitatory neurotransmission in cultured hippocampal pyramidal neurons. Neuron. 1990;4(3):413–419. doi: 10.1016/0896-6273(90)90053-I. [DOI] [PubMed] [Google Scholar]
- 46.Choi D.W. Excitotoxic cell death. J. Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 47.McLeod J.R., Jr, Shen M., Kim D.J., Thayer S.A. Neurotoxicity mediated by aberrant patterns of synaptic activity between rat hippocampal neurons in culture. J. Neurophysiol. 1998;80(5):2688–2698. doi: 10.1152/jn.1998.80.5.2688. [DOI] [PubMed] [Google Scholar]
- 48.Rowland M.J., Hadjipavlou G., Westbrook J., Pattinson K.T.S. Delayed cerebral ischaemia after subarachnoid haemorrhage. Surv. Anesthesiol. 2013;57(3):119–120. doi: 10.1097/01.UC.0000428883.72053.4e. [DOI] [PubMed] [Google Scholar]
- 49.Porter N.M., Thibault O., Thibault V., Chen K-C., Landfield P.W. Calcium channel density and hippocampal cell death with age in long-term culture. J. Neurosci. 1997;17(14):5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewer L.D., Thibault O., Staton J., Thibault V., Rogers J.T., Garcia-Ramos G., Kraner S., Landfield P.W., Porter N.M. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 51.Surmeier D.J. Calcium Homeostatasis and Mitochondrial Dysfunction in Dopaminergic Neurons of the Substantia Nigra. Defense Technical Information Center; 2010. [Google Scholar]
- 52.Gudala K., Kanukula R., Bansal D. Reduced risk of parkinson’s disease in users of calcium channel blockers: A meta-analysis. Int. J. Chronic Dis. 2015;2015: 697404. doi: 10.1155/2015/697404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen A.N., Cummings C., Hoffman D., Pope D., Arnold M., Newland M.C. Aging, motor function, and sensitivity to calcium channel blockers: An investigation using chronic methylmercury exposure. Behav. Brain Res. 2016;315:103–114. doi: 10.1016/j.bbr.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 54.Weiss J.H., Pike C.J., Cotman C.W. Ca2+ channel blockers attenuate β-amyloid peptide toxicity to cortical neurons in culture. J. Neurochem. 1994;62(1):372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- 55.Anekonda T.S., Quinn J.F. Calcium channel blocking as a therapeutic strategy for Alzheimer’s disease: the case for isradipine. Biochim. Biophys. Acta. 2011;1812(12):1584–1590. doi: 10.1016/j.bbadis.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerchner G.A., Canzoniero L.M.T., Yu S.P., Ling C., Choi D.W. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 2000;528(Pt 1):39–52. doi: 10.1111/j.1469-7793.2000.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frederickson C.J., Koh J-Y., Bush A.I. The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 2005;6(6):449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 58.Sensi S.L., Paoletti P., Bush A.I., Sekler I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009;10(11):780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 59.Shuttleworth C.W., Weiss J.H. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011;32(8):480–486. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss J.H., Hartley D.M., Koh J.Y., Choi D.W. AMPA receptor activation potentiates zinc neurotoxicity. Neuron. 1993;10(1):43–49. doi: 10.1016/0896-6273(93)90240-R. [DOI] [PubMed] [Google Scholar]
- 61.Carter R.E., Aiba I., Dietz R.M., Sheline C.T., Shuttleworth C.W. Spreading depression and related events are significant sources of neuronal Zn2+ release and accumulation. J. Cereb. Blood Flow Metab. 2010;31(4):1073–1084. doi: 10.1038/jcbfm.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dietz R.M., Weiss J.H., Shuttleworth C.W. Zn2+ influx is critical for some forms of spreading depression in brain slices. J. Neurosci. 2008;28(32):8014–8024. doi: 10.1523/JNEUROSCI.0765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietz R.M., Weiss J.H., Shuttleworth C.W. Contributions of Ca2+ and Zn2+ to spreading depression-like events and neuronal injury. J. Neurochem. 2009;109(Suppl. 1):145–152. doi: 10.1111/j.1471-4159.2009.05853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Richter F., Ebersberger A., Schaible H.G. Blockade of voltage-gated calcium channels in rat inhibits repetitive cortical spreading depression. Neurosci. Lett. 2002;334(2):123–126. doi: 10.1016/S0304-3940(02)01120-5. [DOI] [PubMed] [Google Scholar]
- 65.Attwell D., Buchan A.M., Charpak S., Lauritzen M., Macvicar B.A., Newman E.A. Glial and neuronal control of brain blood flow. Nature. 2010;468(7321):232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Latour I., Hamid J., Beedle A.M., Zamponi G.W., Macvicar B.A. Expression of voltage-gated Ca2+ channel subtypes in cultured astrocytes. Glia. 2003;41(4):347–353. doi: 10.1002/glia.10162. [DOI] [PubMed] [Google Scholar]
- 67.Koide M., Bonev A.D., Nelson M.T., Wellman G.C. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc. Natl. Acad. Sci. USA. 2012;109(21):E1387–E1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seidel J.L., Escartin C., Ayata C., Bonvento G., Shuttleworth C.W. Multifaceted roles for astrocytes in spreading depolarization: A target for limiting spreading depolarization in acute brain injury? Glia. 2016;64(1):5–20. doi: 10.1002/glia.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hashioka S., Klegeris A., McGeer P.L. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63(4):685–691. doi: 10.1016/j.neuropharm.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 70.Sukhotinsky I., Dilekoz E., Wang Y., Qin T., Eikermann-Haerter K., Waeber C., Ayata C. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia. 2011;31(16):1601–1608. doi: 10.1177/0333102411425865. [DOI] [PubMed] [Google Scholar]
- 71.Seidel J.L., Faideau M., Aiba I., Pannasch U., Escartin C., Rouach N., Bonvento G., Shuttleworth C.W. Ciliary neurotrophic factor (CNTF) activation of astrocytes decreases spreading depolarization susceptibility and increases potassium clearance. Glia. 2015;63(1):91–103. doi: 10.1002/glia.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheli V.T., Santiago G.D.A., Smith J., Spreuer V., Murphy G.G., Paez P.M. L-type voltage-operated calcium channels contribute to astrocyte activation In vitro. Glia. 2016;64(8):1396–1415. doi: 10.1002/glia.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamouchi M., Kitazono T., Ago T., Wakisaka M., Ooboshi H., Ibayashi S., Iida M. Calcium influx pathways in rat CNS pericytes. Brain Res. Mol. Brain Res. 2004;126(2):114–120. doi: 10.1016/j.molbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Cheng J., Korte N., Nortley R., Sethi H., Tang Y., Attwell D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018;136(4):507–523. doi: 10.1007/s00401-018-1893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai W., Liu H., Zhao J., Chen L.Y., Chen J., Lu Z., Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl. Stroke Res. 2017;8(2):107–121. doi: 10.1007/s12975-016-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Espinosa-Parrilla J.F., Martínez-Moreno M., Gasull X., Mahy N., Rodríguez M.J. The L-type voltage-gated calcium channel modulates microglial pro-inflammatory activity. Mol. Cell. Neurosci. 2015;64:104–115. doi: 10.1016/j.mcn.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Hu X., Liu Y., Bao Y., An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez A.B., Medders K.E., Maung R., Sánchez-Pavón P., Ojeda-Juárez D., Kaul M. CXCL12-induced neurotoxicity critically depends on NMDA receptor-gated and L-type Ca2+ channels upstream of p38 MAPK. J. Neuroinflammation. 2016;13(1):252. doi: 10.1186/s12974-016-0724-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daschil N., Humpel C. Nifedipine and nimodipine protect dopaminergic substantia nigra neurons against axotomy-induced cell death in rat vibrosections via modulating inflammatory responses. Brain Res. 2014;1581:1–11. doi: 10.1016/j.brainres.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hopp S.C., D’Angelo H.M., Royer S.E., Kaercher R.M., Crockett A.M., Adzovic L., Wenk G.L. Calcium dysregulation via L-type voltage-dependent calcium channels and ryanodine receptors underlies memory deficits and synaptic dysfunction during chronic neuroinflammation. J. Neuroinflammation. 2015;12(1):56. doi: 10.1186/s12974-015-0262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanz J.M., Chiozzi P., Colaianna M., Zotti M., Ferrari D., Trabace L., Zuliani G., Di Virgilio F. Nimodipine inhibits IL-1β release stimulated by amyloid β from microglia. Br. J. Pharmacol. 2012;167(8):1702–1711. doi: 10.1111/j.1476-5381.2012.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shibata M., Suzuki N. Exploring the role of microglia in cortical spreading depression in neurological disease. J. Cereb. Blood Flow Metab. 2017;37(4):1182–1191. doi: 10.1177/0271678X17690537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen G.S., Ahn H.S., Preziosi T.J., Battye R., Boone S.C., Boone S.C., Chou S.N., Kelly D.L., Weir B.K., Crabbe R.A., Lavik P.J., Rosenbloom S.B., Dorsey F.C., Ingram C.R., Mellits D.E., Bertsch L.A., Boisvert D.P., Hundley M.B., Johnson R.K., Strom J.A., Transou C.R. Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N. Engl. J. Med. 1983;308(11):619–624. doi: 10.1056/NEJM198303173081103. [DOI] [PubMed] [Google Scholar]
- 84.Philippon J., Grob R., Dagreou F., Guggiari M., Rivierez M., Viars P. Prevention of vasospasm in subarachnoid haemorrhage. A controlled study with nimodipine. Acta Neurochir. (Wien) 1986;82(3-4):110–114. doi: 10.1007/BF01456369. [DOI] [PubMed] [Google Scholar]
- 85.Pickard J.D., Murray G.D., Illingworth R., Shaw M.D., Teasdale G.M., Foy P.M., Humphrey P.R., Lang D.A., Nelson R., Richards P. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ. 1989;298(6674):636–642. doi: 10.1136/bmj.298.6674.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allen G.S. Role of calcium antagonists in cerebral arterial spasm. Am. J. Cardiol. 1985;55(3):149B–153B. doi: 10.1016/0002-9149(85)90624-1. [DOI] [PubMed] [Google Scholar]
- 87.Neil-Dwyer G., Mee E., Dorrance D., Lowe D. Early intervention with nimodipine in subarachnoid haemorrhage. Eur. Heart J. 1987;8(Suppl. K):41–47. doi: 10.1093/eurheartj/8.suppl_K.41. [DOI] [PubMed] [Google Scholar]
- 88.Jan M., Buchheit F., Tremoulet M. Therapeutic trial of intravenous nimodipine in patients with established cerebral vasospasm after rupture of intracranial aneurysms. Neurosurgery. 1988;23(2):154–157. doi: 10.1227/00006123-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 89.Ohman J., Heiskanen O. Effect of nimodipine on the outcome of patients after aneurysmal subarachnoid hemorrhage and surgery. J. Neurosurg. 1988;69(5):683–686. doi: 10.3171/jns.1988.69.5.0683. [DOI] [PubMed] [Google Scholar]
- 90.Zhang S., Chang A.M., Li C.F., Li Z.J., Yin Z.J., Zhao X., Liang S.L. Mechanism of the therapeutic effect of anisodamine in disseminated intravascular coagulation: study of platelet adhesion and aggregation, malondialdehyde, thromboxane B2, 6-keto-prostaglandin F1 alpha, and microcirculation. Exp. Hematol. 1987;15(1):65–71. [PubMed] [Google Scholar]
- 91.Juvela S., Kaste M., Hillbom M. Effect of nimodipine on platelet function in patients with subarachnoid hemorrhage. Stroke. 1990;21(9):1283–1288. doi: 10.1161/01.STR.21.9.1283. [DOI] [PubMed] [Google Scholar]
- 92.Roine R.O., Kaste M., Kinnunen A., Nikki P., Sarna S., Kajaste S. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. A placebo-controlled, double-blind, randomized trial. JAMA. 1990;264(24):3171–3177. doi: 10.1001/jama.1990.03450240073043. [DOI] [PubMed] [Google Scholar]
- 93.Dorhout M.S.M., Rinkel G.J.E., Feigin V.L., Algra A., van den Bergh W.M., Vermeulen M., van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007;(3):CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kronvall E., Undrén P., Romner B., Säveland H., Cronqvist M., Nilsson O.G. Nimodipine in aneurysmal subarachnoid hemorrhage: a randomized study of intravenous or peroral administration. J. Neurosurg. 2009;110(1):58–63. doi: 10.3171/2008.7.JNS08178. [DOI] [PubMed] [Google Scholar]
- 95.Soppi V., Karamanakos P.N., Koivisto T., Kurki M.I., Vanninen R., Jaaskelainen J.E., Rinne J. A randomized outcome study of enteral versus intravenous nimodipine in 171 patients after acute aneurysmal subarachnoid hemorrhage. World Neurosurg. 2012;78(1-2):101–109. doi: 10.1016/j.wneu.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 96.Hockel K., Diedler J., Steiner J., Birkenhauer U., Danz S., Ernemann U., Schuhmann M.U. Long-term, continuous intra-arterial nimodipine treatment of severe vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2016;88:104–112. doi: 10.1016/j.wneu.2015.11.081. [DOI] [PubMed] [Google Scholar]
- 97.Hänggi D., Etminan N., Aldrich F., Steiger H.J., Mayer S.A., Diringer M.N., Hoh B.L., Mocco J., Faleck H.J., Macdonald R.L. Investigators, N. Randomized, open-label, phase 1/2a study to determine the maximum tolerated dose of intraventricular sustained release nimodipine for subarachnoid hemorrhage (NEWTON [nimodipine microparticles to enhance recovery while reducing toxicity after subarachnoid hemorrhagE]). Stroke. 2017;48(1):145–151. doi: 10.1161/STROKEAHA.116.014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang J., Yang J., Zhang C., Jiang X., Zhou H., Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst. Rev. 2012;(5):CD001928. doi: 10.1002/14651858.CD001928.pub2. [DOI] [PubMed] [Google Scholar]
- 99.Gelmers H.J. The effects of nimodipine on the clinical course of patients with acute ischemic stroke. Acta Neurol. Scand. 1984;69(4):232–239. doi: 10.1111/j.1600-0404.1984.tb07806.x. [DOI] [PubMed] [Google Scholar]
- 100.Gelmers H.J., Gorter K., de Weerdt C.J., Wiezer H.J. A controlled trial of nimodipine in acute ischemic stroke. N. Engl. J. Med. 1988;318(4):203–207. doi: 10.1056/NEJM198801283180402. [DOI] [PubMed] [Google Scholar]
- 101.Paci A., Ottaviano P., Trenta A., Iannone G., De Santis L., Lancia G., Moschini E., Carosi M., Amigoni S., Caresia L. Nimodipine in acute ischemic stroke: a double-blind controlled study. Acta Neurol. Scand. 1989;80(4):282–286. doi: 10.1111/j.1600-0404.1989.tb03879.x. [DOI] [PubMed] [Google Scholar]
- 102.Martínez-Vila E., Guillén F., Villanueva J.A., Matías-Guiu J., Bigorra J., Gil P., Carbonell A., Martínez-Lage J.M. Placebo-controlled trial of nimodipine in the treatment of acute ischemic cerebral infarction. Stroke. 1990;21(7):1023–1028. doi: 10.1161/01.STR.21.7.1023. [DOI] [PubMed] [Google Scholar]
- 103.Sze K.H., Sim T.C., Wong E., Cheng S., Woo J. Effect of nimodipine on memory after cerebral infarction. Acta Neurol. Scand. 1998;97(6):386–392. doi: 10.1111/j.1600-0404.1998.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 104.Hakim A.M., Evans A.C., Berger L., Kuwabara H., Worsley K., Marchal G., Biel C., Pokrupa R., Diksic M., Meyer E. The effect of nimodipine on the evolution of human cerebral infarction studied by PET. J. Cereb. Blood Flow Metab. 1989;9(4):523–534. doi: 10.1038/jcbfm.1989.76. [DOI] [PubMed] [Google Scholar]
- 105.Bogousslavsky J., Regli F., Zumstein V., Köbberling W. Double-blind study of nimodipine in non-severe stroke. Eur. Neurol. 1990;30(1):23–26. doi: 10.1159/000116620. [DOI] [PubMed] [Google Scholar]
- 106.Rosselli A., Landini G., Castagnoli A., Vannucchi L., Mugnaini C., Calacoci S., Regoli G., Francesconi A., Turchi A., Agostino I. The nimodipine therapy of acute focal cerebral ischemia (minor stroke). A clinical study with an assessment of regional cerebral blood flow by SPECT. Recenti Prog. Med. 1992;83(2):85–88. [PubMed] [Google Scholar]
- 107.Clinical trial of nimodipine in acute ischemic stroke. Stroke. 1992;23(1):3–8. doi: 10.1161/01.STR.23.1.3. [DOI] [PubMed] [Google Scholar]
- 108.Horn J., de Haan R.J., Vermeulen M., Limburg M. Very Early Nimodipine Use in Stroke (VENUS): a randomized, double-blind, placebo-controlled trial. Stroke. 2001;32(2):461–465. doi: 10.1161/01.STR.32.2.461. [DOI] [PubMed] [Google Scholar]
- 109.Kaste M., Fogelholm R., Erilä T., Palomäki H., Murros K., Rissanen A., Sarna S. A randomized, double-blind, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke. 1994;25(7):1348–1353. doi: 10.1161/01.STR.25.7.1348. [DOI] [PubMed] [Google Scholar]
- 110.Nag D., Garg R.K., Varma M. A randomized double-blind controlled study of nimodipine in acute cerebral ischemic stroke. Indian J. Physiol. Pharmacol. 1998;42(4):555–558. [PubMed] [Google Scholar]
- 111.Trust Study Group. Randomised, double-blind, placebo-controlled trial of nimodipine in acute stroke. Lancet. 1990;336(8725):1205–1209. doi: 10.1016/0140-6736(90)92829-7. [DOI] [PubMed] [Google Scholar]
- 112.Fogelholm R., Erilä T., Palomäki H., Murros K., Kaste M. Effect of nimodipine on final infarct volume after acute ischemic stroke. Cerebrovasc. Dis. 2000;10(3):189–193. doi: 10.1159/000016055. [DOI] [PubMed] [Google Scholar]
- 113.Fogelholm R., Palomäki H., Erilä T., Rissanen A., Kaste M. Blood pressure, nimodipine, and outcome of ischemic stroke. Acta Neurol. Scand. 2004;109(3):200–204. doi: 10.1034/j.1600-0404.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 114.Ahmed N., Näsman P., Wahlgren N.G. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000;31(6):1250–1255. doi: 10.1161/01.STR.31.6.1250. [DOI] [PubMed] [Google Scholar]
- 115.Wang P., Wang Y., Feng T., Zhao X., Zhou Y., Wang Y., Shi W., Ju Y. Rationale and design of a double-blind, placebo-controlled, randomized trial to evaluate the safety and efficacy of nimodipine in preventing cognitive impairment in ischemic cerebrovascular events (NICE). BMC Neurol. 2012;12(1):88. doi: 10.1186/1471-2377-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Langham J., Goldfrad C., Teasdale G., Shaw D., Rowan K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst. Rev. 2003;(4): CD000565. doi: 10.1002/14651858.CD000565. [DOI] [PubMed] [Google Scholar]
- 117.Teasdale G., Bailey I., Bell A., Gray J., Gullan R., Heiskanan U., Marks P.V., Marsh H., Mendelow A.D., Murray G. The effect of nimodipine on outcome after head injury: a prospective randomised control trial. Acta Neurochir. Suppl. (Wien) 1990;51:315–316. doi: 10.1007/978-3-7091-9115-6_106. [DOI] [PubMed] [Google Scholar]
- 118.The European Study Group. A multicenter trial of the efficacy of nimodipine on outcome after severe head injury. J. Neurosurg. 1994;80(5):797–804. doi: 10.3171/jns.1994.80.5.0797. [DOI] [PubMed] [Google Scholar]
- 119.Murray G.D., Teasdale G.M., Schmitz H. Nimodipine in traumatic subarachnoid haemorrhage: a re-analysis of the HIT I and HIT II trials. Acta Neurochir. (Wien) 1996;138(10):1163–1167. doi: 10.1007/BF01809745. [DOI] [PubMed] [Google Scholar]
- 120.Harders A., Kakarieka A., Braakman R. Traumatic subarachnoid hemorrhage and its treatment with nimodipine. German tSAH Study Group. J. Neurosurg. 1996;85(1):82–89. doi: 10.3171/jns.1996.85.1.0082. [DOI] [PubMed] [Google Scholar]
- 121.Pillai S.V., Kolluri V.R., Mohanty A., Chandramouli B.A. Evaluation of nimodipine in the treatment of severe diffuse head injury: a double-blind placebo-controlled trial. Neurol. India. 2003;51(3):361–363. [PubMed] [Google Scholar]
- 122.Vergouwen M.D., Vermeulen M., Roos Y.B. Effect of nimodipine on outcome in patients with traumatic subarachnoid haemorrhage: a systematic review. Lancet Neurol. 2006;5(12):1029–1032. doi: 10.1016/S1474-4422(06)70582-8. [DOI] [PubMed] [Google Scholar]
- 123.Hartings J.A., Bullock M.R., Okonkwo D.O., Murray L.S., Murray G.D., Fabricius M., Maas A.I., Woitzik J., Sakowitz O., Mathern B., Roozenbeek B., Lingsma H., Dreier J.P., Puccio A.M., Shutter L.A., Pahl C., Strong A.J. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12):1058–1064. doi: 10.1016/S1474-4422(11)70243-5. [DOI] [PubMed] [Google Scholar]
- 124.Bussone G., Baldini S., D’Andrea G., Cananzi A., Frediani F., Caresia L., Ferro Milone F., Boiardi A. Nimodipine versus flunarizine in common migraine: a controlled pilot trial. Headache. 1987;27(2):76–79. doi: 10.1111/j.1526-4610.1987.hed2702076.x. [DOI] [PubMed] [Google Scholar]
- 125.Gelmers H.J. Nimodipine, a new calcium antagonist, in the prophylactic treatment of migraine. Headache. 1983;23(3):106–109. doi: 10.1111/j.1526-4610.1983.hed2303106.x. [DOI] [PubMed] [Google Scholar]
- 126.Havanka-Kanniainen H., Hokkanen E., Myllylä V.V. Efficacy of nimodipine in the prophylaxis of migraine. Cephalalgia. 1985;5(1):39–43. doi: 10.1046/j.1468-2982.1985.0501039.x. [DOI] [PubMed] [Google Scholar]
- 127.Havanka-Kanniainen H., Hokkanen E., Myllylä V.V. Efficacy of nimodipine in comparison with pizotifen in the prophylaxis of migraine. Cephalalgia. 1987;7(1):7–13. doi: 10.1046/j.1468-2982.1987.0701007.x. [DOI] [PubMed] [Google Scholar]
- 128.Jensen K., Tfelt-Hansen P., Lauritzen M., Olesen J. Clinical trial of nimodipine for single attacks of classic migraine. Cephalalgia. 1985;5(3):125–131. doi: 10.1046/j.1468-2982.1985.0503125.x. [DOI] [PubMed] [Google Scholar]
- 129.European Study Group. European multicenter trial of nimodipine in the prophylaxis of common migraine (migraine without aura). Headache. 1989;29(10):633–638. doi: 10.1111/j.1526-4610.1989.hed2910633.x. [DOI] [PubMed] [Google Scholar]
- 130.European Study Group. European multicenter trial of nimodipine in the prophylaxis of classic migraine (migraine with aura). Headache. 1989;29(10):639–642. doi: 10.1111/j.1526-4610.1989.hed2910639.x. [DOI] [PubMed] [Google Scholar]
- 131.Battistella P.A., Ruffilli R., Moro R., Fabiani M., Bertoli S., Antolini A., Zacchello F. A placebo-controlled crossover trial of nimodipine in pediatric migraine. Headache. 1990;30(5):264–268. doi: 10.1111/j.1526-4610.1990.hed3005264.x. [DOI] [PubMed] [Google Scholar]
- 132.Forsman M., Aarseth H.P., Nordby H.K., Skulberg A., Steen P.A. Effects of nimodipine on cerebral blood flow and cerebrospinal fluid pressure after cardiac arrest: correlation with neurologic outcome. Anesth. Analg. 1989;68(4):436–443. doi: 10.1213/00000539-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 133.Gueugniaud P-Y., Gaussorgues P., Garcia-Darennes F., Bancalari G., Roux H., Robert D., Petit P. Early effects of nimodipine on intracranial and cerebral perfusion pressures in cerebral anoxia after out-of-hospital cardiac arrest. Resuscitation. 1990;20(3):203–212. doi: 10.1016/0300-9572(90)90003-W. [DOI] [PubMed] [Google Scholar]
- 134.Legault C., Furberg C.D., Wagenknecht L.E., Rogers A.T., Stump D.A., Coker L., Troost B.T., Hammon J.W. Nimodipine neuroprotection in cardiac valve replacement: report of an early terminated trial. Stroke. 1996;27(4):593–598. doi: 10.1161/01.STR.27.4.593. [DOI] [PubMed] [Google Scholar]
- 135.Ban T.A., Morey L., Aguglia E., Azzarelli O., Balsano F., Marigliano V., Caglieris N., Sterlicchio M., Capurso A., Tomasi N.A., Crepaldi G., Volpe D., Palmieri G., Ambrosi G., Polli E., Cortellaro M., Zanussi C., Froldi M. Nimodipine in the treatment of old age dementias. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14(4):525–551. doi: 10.1016/0278-5846(90)90005-2. [DOI] [PubMed] [Google Scholar]
- 136.Cohen M.R., Swartz C.M. Absence of nimodipine premedication effect on memory after electroconvulsive therapy. Neuropsychobiology. 1990-1991;24(4):165–168. doi: 10.1159/000119480. [DOI] [PubMed] [Google Scholar]
- 137.Tollefson G.D. Short-term effects of the calcium channel blocker nimodipine (Bay-e-9736) in the management of primary degenerative dementia. Biol. Psychiatry. 1990;27(10):1133–1142. doi: 10.1016/0006-3223(90)90050-C. [DOI] [PubMed] [Google Scholar]
- 138.Parnetti L., Senin U., Carosi M., Baasch H. Mental deterioration in old age: results of two multicenter, clinical trials with nimodipine. Clin. Ther. 1993;15(2):394–406. [PubMed] [Google Scholar]
- 139.Pantoni L., del Ser T., Soglian A.G., Amigoni S., Spadari G., Binelli D., Inzitari D. Efficacy and safety of nimodipine in subcortical vascular dementia: a randomized placebo-controlled trial. Stroke. 2005;36(3):619–624. doi: 10.1161/01.STR.0000155686.73908.3e. [DOI] [PubMed] [Google Scholar]
- 140.López-Arrieta J.M., Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002;(3):CD000147. doi: 10.1002/14651858.CD000147. [DOI] [PubMed] [Google Scholar]
- 141.Miller R.G., Shepherd R., Dao H., Khramstov A., Mendoza M., Graves J., Smith S. Controlled trial of nimodipine in amyotrophic lateral sclerosis. Neuromuscul. Disord. 1996;6(2):101–104. doi: 10.1016/0960-8966(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 142.Ziv I., Achiron A., Djaldetti R., Abraham M., Melamed E. Can nimodipine affect progression of motor neuron disease? A double-blind pilot study. Clin. Neuropharmacol. 1994;17(5):423–428. doi: 10.1097/00002826-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 143.Cassidy J., Paul J., Soukop M., Habeshaw T., Reed N.S., Parkin D., Kaye S.B. Clinical trials of nimodipine as a potential neuroprotector in ovarian cancer patients treated with cisplatin. Cancer Chemother. Pharmacol. 1998;41(2):161–166. doi: 10.1007/s002800050723. [DOI] [PubMed] [Google Scholar]
- 144.Navia B.A., Dafni U., Simpson D., Tucker T., Singer E., McArthur J.C., Yiannoutsos C., Zaborski L., Lipton S.A. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology. 1998;51(1):221–228. doi: 10.1212/WNL.51.1.221. [DOI] [PubMed] [Google Scholar]
- 145.Taragano F.E., Allegri R., Vicario A., Bagnatti P., Lyketsos C.G. A double blind, randomized clinical trial assessing the efficacy and safety of augmenting standard antidepressant therapy with nimodipine in the treatment of ‘vascular depression’. Int. J. Geriatr. Psychiatry. 2001;16(3):254–260. doi: 10.1002/gps.340. [DOI] [PubMed] [Google Scholar]
- 146.Brunet G., Cerlich B., Robert P., Dumas S., Souetre E., Darcourt G. Open trial of a calcium antagonist, nimodipine, in acute mania. Clin. Neuropharmacol. 1990;13(3):224–228. doi: 10.1097/00002826-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 147.Banger M., Benkert O., Röschke J., Herth T., Hebenstreit M., Philipp M., Aldenhoff J.B. Nimodipine in acute alcohol withdrawal state. J. Psychiatr. Res. 1992;26(2):117–123. doi: 10.1016/0022-3956(92)90003-7. [DOI] [PubMed] [Google Scholar]
- 148.Jiménez-Lerma J.M., Landabaso M., Iraurgi L., Calle R., Sanz J., Gutiérrez-Fraile M. Nimodipine in opiate detoxification: a controlled trial. Addiction. 2002;97(7):819–824. doi: 10.1046/j.1360-0443.2002.00196.x. [DOI] [PubMed] [Google Scholar]
- 149.Rosse R.B., Alim T.N., Fay-McCarthy M., Collins J.P., Jr, Vocci F.J., Jr, Lindquist T., Jentgen C., Hess A.L., Deutsch S.I. Nimodipine pharmacotherapeutic adjuvant therapy for inpatient treatment of cocaine dependence. Clin. Neuropharmacol. 1994;17(4):348–358. doi: 10.1097/00002826-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 150.Pazzaglia P.J., Post R.M., Ketter T.A., George M.S., Marangell L.B. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993;49(3):257–272. doi: 10.1016/0165-1781(93)90066-P. [DOI] [PubMed] [Google Scholar]
- 151.Biary N., Bahou Y., Sofi M.A., Thomas W., al Deeb S.M. The effect of nimodipine on essential tremor. Neurology. 1995;45(8):1523–1525. doi: 10.1212/WNL.45.8.1523. [DOI] [PubMed] [Google Scholar]
- 152.Lisbeth M., Consuelo P., Glenda C., Elena C., Carlos R.J., Alejandro R., Maria G-Y. Evaluation of the effect of nimodipine o.d. (extended release) vs nimodipine t.i.d. in the treatment of peripheral vertigo. Curr. Drug Deliv. 2013;10(3):343–347. doi: 10.2174/1567201811310030011. [DOI] [PubMed] [Google Scholar]
- 153.Santillán R., Hurlé M.A., Armijo J.A., de los Mozos R., Flórez J. Nimodipine-enhanced opiate analgesia in cancer patients requiring morphine dose escalation: a double-blind, placebo-controlled study. Pain. 1998;76(1-2):17–26. doi: 10.1016/S0304-3959(98)00019-0. [DOI] [PubMed] [Google Scholar]
- 154.Larkin J.G., McKee P.J., Blacklaw J., Thompson G.G., Morgan I.C., Brodie M.J. Nimodipine in refractory epilepsy: a placebo-controlled, add-on study. Epilepsy Res. 1991;9(1):71–77. doi: 10.1016/0920-1211(91)90049-L. [DOI] [PubMed] [Google Scholar]
- 155.Meyer F.B., Cascino G.D., Whisnant J.P., Sharbrough F.W., Ivnik R.J., Gorman D.A., Windschitl W.L., So E.L., O’Fallon W.M. Nimodipine as an add-on therapy for intractable epilepsy. Mayo Clin. Proc. 1995;70(7):623–627. doi: 10.4065/70.7.623. [DOI] [PubMed] [Google Scholar]
- 156.Scheller C., Wienke A., Tatagiba M., Gharabaghi A., Ramina K.F., Ganslandt O., Bischoff B., Zenk J., Engelhorn T., Matthies C., Westermaier T., Antoniadis G., Pedro M.T., Rohde V., von Eckardstein K., Kretschmer T., Kornhuber M., Steighardt J., Richter M., Barker F.G., II, Strauss C. Prophylactic nimodipine treatment for cochlear and facial nerve preservation after vestibular schwannoma surgery: a randomized multicenter Phase III trial. J. Neurosurg. 2016;124(3):657–664. doi: 10.3171/2015.1.JNS142001. [DOI] [PubMed] [Google Scholar]
- 157.Strauss C., Bischoff B., Neu M., Berg M., Fahlbusch R., Romstöck J. Vasoactive treatment for hearing preservation in acoustic neuroma surgery. J. Neurosurg. 2001;95(5):771–777. doi: 10.3171/jns.2001.95.5.0771. [DOI] [PubMed] [Google Scholar]
- 158.Krischek B., Kasuya H., Onda H., Hori T. Nicardipine prolonged-release implants for preventing cerebral vasospasm after subarachnoid hemorrhage: effect and outcome in the first 100 patients. Neurol. Med. Chir. (Tokyo) 2007;47(9):389–394. doi: 10.2176/nmc.47.389. [DOI] [PubMed] [Google Scholar]
- 159.Barth M., Capelle H-H., Weidauer S., Weiss C., Münch E., Thomé C., Luecke T., Schmiedek P., Kasuya H., Vajkoczy P. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind phase IIa study. Stroke. 2007;38(2):330–336. doi: 10.1161/01.STR.0000254601.74596.0f. [DOI] [PubMed] [Google Scholar]
- 160.Etminan N., Macdonald R.L., Davis C., Burton K., Steiger H-J., Hänggi D. Intrathecal application of the nimodipine slow-release microparticle system EG-1962 for prevention of delayed cerebral ischemia and improvement of outcome after aneurysmal subarachnoid hemorrhage. Neurovascular Events After Subarachnoid Hemorrhage. Springer International Publishing; 2014. pp. 281–286. [DOI] [PubMed] [Google Scholar]
- 161.Hänggi D., Etminan N., Steiger H.J., Johnson M., Peet M.M., Tice T., Burton K., Hudson B., Turner M., Stella A., Heshmati P., Davis C., Faleck H.J., Macdonald R.L., Site-Specific A. A site-specific, sustained-release drug delivery system for aneurysmal subarachnoid hemorrhage. Neurotherapeutics. 2016;13(2):439–449. doi: 10.1007/s13311-016-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hanggi D., Etminan N., Mayer S.A., Aldrich E.F., Diringer M.N., Schmutzhard E., Faleck H.J., Ng D., Saville B.R., Macdonald R.L., Investigators N. Clinical Trial Protocol: Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study Comparing EG-1962 to Standard of Care Oral Nimodipine in Adults with Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care. 2018 doi: 10.1007/s12028-018-0575-z. [NEWTON-2(Nimodipine Microparticles to Enhance Recovery While Reducing TOxicity After SubarachNoid Hemorrhage)]. [DOI] [PubMed] [Google Scholar]
- 163.Janovák L., Turcsányi Á., Bozó É., Deák Á., Mérai L., Sebők D., Juhász Á., Csapó E., Abdelghafour M.M., Farkas E., Dékány I., Bari F. Preparation of novel tissue acidosis-responsive chitosan drug nanoparticles: Characterization and in vitro release properties of Ca2+ channel blocker nimodipine drug molecules. Eur. J. Pharm. Sci. 2018;123:79–88. doi: 10.1016/j.ejps.2018.07.031. [DOI] [PubMed] [Google Scholar]