Abstract
Glutamate is the major excitatory neurotransmitter in the central nervous system, and its signaling is critical for excitatory synaptic transmission. The well-established glutamate system involves glutamate synthesis, presynaptic glutamate release, glutamate actions on the ionotropic glutamate receptors (NMDA, AMPA, and kainate receptors) and metabotropic glutamate receptors, and glutamate uptake by glutamate transporters. When the glutamate system becomes dysfunctional, it contributes to the pathogenesis of neurodegenerative and neuropsychiatric diseases such as Alzheimer's disease, Parkinson's disease, depression, epilepsy, and ischemic stroke. In this review, based on regulating glutamate signaling, we summarize the effects and underlying mechanisms of natural constituents from Chinese herbal medicines on neurological disorders. Natural constituents from Chinese herbal medicine can prevent the glutamate-mediated excitotoxicity via suppressing presynaptic glutamate release, decreasing ionotropic and metabotropic glutamate receptors expression in the excitatory synapse, and promoting astroglial glutamate transporter expression to increase glutamate clearance from the synaptic cleft. However, some natural constituents from Chinese herbal medicine have the ability to restore the collapse of excitatory synapses by promoting presynaptic glutamate release and increasing ionotropic and metabotropic glutamate receptors expression. These regulatory processes involve various signaling pathways, which lead to different mechanistic routes of protection against neurological disorders. Hence, our review addresses the underlying mechanisms of natural constituents from Chinese herbal medicines that regulate glutamate systems and serve as promising agents for the treatment of the above-mentioned neurological disorders.
Keywords: Neurological disorders, natural constituents, chinese herbal medicine, glutamate, glutamate receptors, glutamate transporters
1. INTRODUCTION
Glutamate is the predominant excitatory neurotransmitter involved neural differentiation, migration, survival, and synaptic plasticity in the mammalian central nervous system (CNS) [1, 2]. Glutamate mediates synaptic action by activating ionotropic and metabotropic receptors [2-4]. Ionotropic glutamate receptors are ligand-gated ion channels that are also referred to as the fast synaptic response to glutamate and include α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), N-methyl-D-aspartate (NMDA), and kainite (KA) receptors [5]. Glutamate also activates a family of G-protein-coupled receptors, known as metabotropic glutamate receptors (mGluRs), which are responsible for the slow modulatory response to glutamate [6]. Under the pathological conditions, excessive glutamate in the synaptic cleft can induce neuronal death by activating glutamate receptors [7]. High-affinity glutamate transporters, also known as excitatory amino acid transporters (EAATs), mainly located on astrocytes are responsible for the uptake of excess glutamate in the synaptic cleft to maintain levels at a relatively safe and low range. In addition, the global glutamate system plays a role in the maintenance of the normal excitatory synaptic network in the CNS [8].
In the past, as the most important therapeutic treatment, herbal medicines have been widely used in East Asian countries such as China, Japan, and Korea. Chinese herbal medicine, together with acupuncture, autonomy, and Chinese massage are called traditional Chinese medicine (TCM) therapies. Recently, TCM supports insightful strategies in treating neurologic diseases, and a variety of active ingredients of Chinese herbal medicine have been proven to specifically modulate glutamate targets for improving neurologic disorders [9-11]. To completely overview the effects of these natural constituents on neurological diseases through regulating glutamate signaling, we gathered the relevant literature since 2000, and a majority of these studies were concentrated around the last three years. First, we reviewed the overall glutamate signaling and synaptic transmission, which are mainly involved in glutamate synthesis, glutamate release, glutamate transmission, and glutamate clearance. Secondly, we draw forth the brief underlying mechanism of the dysfunctional glutamate system in neurological diseases such as Alzheimer's Disease (AD), Parkinson's Disease (PD), Amyotrophic Lateral Sclerosis (ALS), depression, and stroke. Afterwards, we described the active ingredients of Chinese herbal medicine targeting glutamate signaling in different processes (glutamate release, glutamate receptors, and glutamate transporters). Then, we reviewed the clinical use of natural constituents from Chinese herbal medicine in neurological diseases. In the end, we provide a summary and prospect for the potential usage of these natural constituents for the treatment of neurologic disorders.
2. GLUTAMATE METABOLISM, GLUTAMATE RECEPTORS, AND GLUTAMATE TRANSPORTERS
Generally, glutamate is produced from 2-oxoglutarate through two pathways, one involving the reductive amination of 2-oxoglutarate with ammonium via glutamate dehydrogenase (GLUD) and the other involving the glutamate synthase-catalyzed reductive amination of 2-oxoglutarate using glutamine as the nitrogen donor [12, 13]. Glutamine synthetase catalyzes the ATP-dependent amidation of glutamate using ammonium as a nitrogen source to produce glutamine, which mainly involves protein and lipid synthesis, and cellular energy. As the key compound, glutamate is essential for the cellular metabolism of many organisms, including nitrogen assimilation, amino acid biosynthesis, and cofactor production, and it also contributes to the biosynthesis of nonribosomal peptide and polyketide natural products [14, 15]. Glutamate is stored in presynaptic vesicles by an uptake system through vesicular glutamate transporters (vGluTs), and three vesicular glutamate transporters (vGluTs 1–3) have been identified [16]. Glutamate-loaded vesicles were removed through synapsin phosphorylation upon protein kinases A (PKA), protein kinases C (PKC), or CaM kinase II (CaMKII)-activated, and then these vesicles were transformed to the release-ready pool [17]. Subsequently, glutamate vesicle docking and fusion with the presynaptic plasma membrane are thought to be mediated by the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, and synaptotagmin mediates the Ca2+-dependent step of glutamate exocytosis to the synapses [18].
In the synapses, glutamate plays a critical role in the excitatory neurotransmission, and this action is mainly mediated by its binding to the ionotropic and metabotropic glutamate receptors. Generally, NMDA receptors mainly elicit long-term plasticity, such as long-term potentiation and long-term depression (LTP/LTD) [19], and these assemble as obligate heteromers to form GluN1, GluN2A, GluN2B, GluN2C, GluN2D, GluN3A, and GluN3B subunits. Besides glutamate, NMDA receptor activation also requires glycine or serine binding simultaneously to the channel, and these receptors are sensitive to pH, and relief of acute voltage-dependent block by Mg2+ [20]. In addition, the NMDA receptors have a relatively high permeability to Ca2+, allowing prolonged entry of Ca2+ into neurons [20]. AMPA receptors play a critical role in the fast excitatory synaptic transmission and plasticity [21], and these assemble as obligate homomers to form GluA1, GluA2, GluA3, and GluA4 subunits. In response to the fast excitatory transmission, the trafficking, single-channel conductance gating, and pharmacology of postsynaptic AMPA receptors are under dynamic regulation [22]. KA receptors also mediate a minor contribution to the synaptic transmission [23] and assemble as homomers or heteromers of GluK1 (also called GluR5), GluK2 (also called GluR6), or GluK3 (also called GluR7), and GluK4 and GluK5. The ionotropic signaling of KA receptors is mainly responsible for membrane depolarization and the synaptic current at postsynaptic synapses, whereas metabotropic signaling is mainly responsible for presynaptic facilitation and inhibition of neurotransmitter release [23]. In addition, glutamate also acts on the mGluRs, which activate G protein-coupled or G protein-independent pathways [6]. According to the sequence homology and downstream signaling activation, eight mGluRs subtypes have been characterized and divided into three subgroups. Group I mGluRs are formed by mGluR1 and mGluR5, which can induce Ca2+ release from intracellular stores. Group I mGluRs are mostly located at postsynaptic sites, and these are positively coupled to Gαq/11. These receptors mainly modulate neuronal excitability via activation of cellular PKC cascades [24, 25]. Group II mGluRs consist of mGluR2 and mGluR3, and Group III mGluRs comprise mGluR4, mGluR6, mGluR7, and mGluR8. Both Groups II and III mGluRs downregulate adenylyl cyclase via Gαi and function as autoreceptors that inhibit the presynaptic release of glutamate or Gamma-Aminobutyric Acid (GABA) [26].
Since the extracellular glutamate concentrations need to be kept at a low level to limit tonic activation of postsynaptic glutamate receptors, excessive glutamate in the synaptic cleft can induce the neuronal death due to overstimulation of glutamate receptors. The excessive glutamate is mainly taken up by the glutamate transporters located in astrocytes. Thus far, five mammalian EAATs have been characterized: GLAST (glutamate/aspartate transporter, also called EAAT1), GLT-1 (glutamate transporter-1, also called EAAT2), EAAC1 (excitatory amino acid carrier-1, also called EAAT3), EAAT4, and EAAT5 [27-29]. Both GLT-1 and GLAST are primarily located on astrocytes and are mainly responsible for the clearance of glutamate in the CNS [30-32]. Glutamate was then converted to glutathione and later synthesized glutamate in neuron through the glutathione metabolic pathway. As a neuronal glutamate transporter, EAAC1 is mainly expressed in the postsynaptic neuronal membrane and may have antioxidative effects [30]. EAAT4 is expressed at low levels by Purkinje cells in the cerebellar molecular layer, whereas EAAT5 is expressed at low levels in the neurons and astrocytes of the retina [30].
Homeostatic glutamate signaling is maintained by the normal regulation of glutamate metabolism, glutamate receptors, and glutamate transporters (Fig. 1). Excitotoxicity is specified by excitatory amino acids induced neuronal damage, and glutamate-mediated excitotoxic damage [33, 34]. Neuroscientists proposed the ionic mechanism of excitotoxicity, induced by glutamate overstimulating postsynaptic ionotropic glutamate receptors. Glutamate induces neuronal Ca2+ overload via overactivating the NMDA receptors, with the production of reactive oxygen species and reactive nitrogen radicals. Glutamate also induces Na+ influx by overactivating AMPA and KA receptors, with acute osmotic swelling of nerve cells [35, 36]. Both these harmful cascades then lead to neuronal death mainly through the dysfunction of calcium homeostasis, which plays a critical role in the process [34]. In addition, glutamate can also stimulate mGluRs to strengthen the calcium influx mediated by NMDA receptors [37]. Briefly, actions of glutamate on mGluRs activate the intracellular Phospholipase C (PLC) and induce the hydrolysis of PLC into inositol triphosphate (IP3) and diacylglycerol (DAG). Afterwards, IP3 induces intracellular Ca2+ release and DAG activates PKC, leading to additional dysfunction of calcium homeostasis. Thus, glutamate-mediated neurotoxicity mediates neuronal death [38, 39].
Fig. (1).
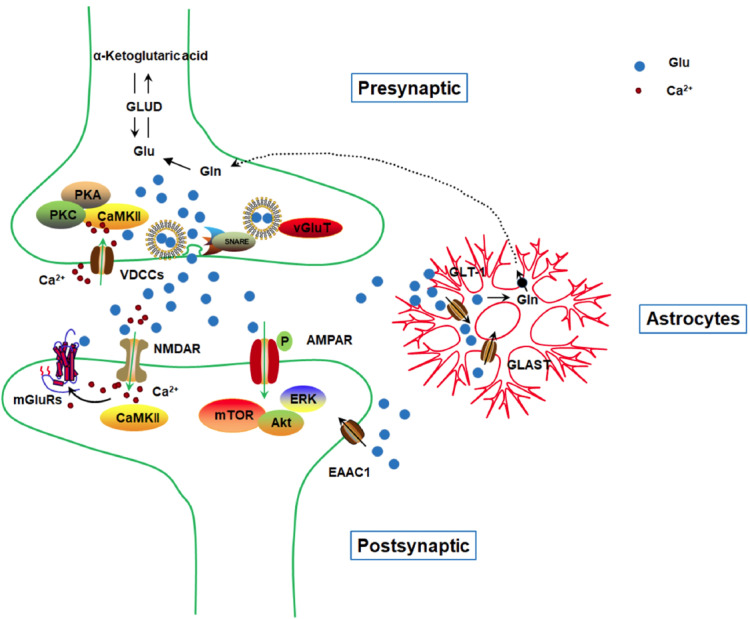
Glutamate metabolic pathways in the central nervous system. Upon presynaptic biosynthesis, glutamate is stored in vesicles through vGluTs, and glutamate-loaded vesicles were removed through activation of PKA, PKC, or CaMKII. Glutamate is released to the synapses through the SNARE complex. Then glutamate actions on postsynaptic glutamate receptors, ionotropic and metabotropic glutamate receptors (mainly NMDAR, AMPAR, and mGluRs), through direct binding or phosphorylation. Downstream signaling involves mTOR, ERK, Akt, or CaMKII. Excessive synaptic glutamate is uptaken through glutamate transporters located in astrocytes (mainly GLT-1 and GLAST), and to a less extent, through neuronal EAAC1. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. DYSFUNCTIONAL GLUTAMATE SYSTEM IN NEUROLOGICAL DISEASES
Dysfunctional glutamate system plays a crucial role in the pathogenesis of neurodegenerative diseases and neuropsychiatric diseases such as AD, PD, ALS, depression and stroke [40].
AD is the most common form of dementia, which accounts for 60-70% of cases in subjects over 65 years of age. The neuropathological characters of AD are extracellular β-Amyloid (Aβ) plaques and phosphorylated tau-associated intraneuronal neurofibrillary tangles (NFTs) [41]. It has been shown that excessive activation of NMDA receptors may enhance the localized vulnerability of neurons in a manner consistent with AD neuropathology [42]. The transcellular spread of Aβ could impair neuronal Ca2+ homeostasis and mitochondrial function, thus leading to neuronal vulnerability to glutamate excitotoxicity [43]. Aβ oligomers could affect glutamate receptors through several pathways, firstly, Aβ oligomers binding to synapses via interacting with AMPA receptors, NMDA receptors, or metabotropic glutamate receptors (such as mGluR5) [44-46]. These receptors may also integrate such multi-protein receptor complex to function as a receptor for Aβ oligomers. Secondly, Aβ oligomers change the surface expression of glutamate receptors via abnormal trafficking or degradation, which is detrimental to synaptic transmission and plasticity [47, 48]. Thirdly, Aβ oligomers cause dysfunctional glutamatergic transmission which involves Aβ oligomers increasing intracellular calcium levels through overactivation of NMDA receptors, and accumulation of glutamate at the synaptic cleft mediated by Aβ oligomers-reduced glutamate transporters expression and function [49-51]. Accumulation of glutamate could also overactivate NMDA receptors and induce cell death. Thus, pathogenic Aβ oligomers cause synapse failure and neuronal death by affecting glutamate receptors in AD [52, 53]. PD is a common chronic movement disorder in the elderly and the hallmarks include the progressive degeneration of dopaminergic (DA) neurons in the pars compacta of the substantia nigra (SNpc) and the formation of Lewy bodies. It has been substantially shown that motor incoordination and dyskinesias in PD were closely associated with the increased glutamate levels in the basal ganglia [54]. As mentioned above, glutamate excitotoxicity could induce DA neuron death. However, dopaminergic denervation also induces dysfunctional presynaptic glutamate release and further exacerbates DA neuron death [55]. ALS is an adult onset fatal neurodegenerative disease characterized by progressive muscle paralysis induced by motoneurons degeneration in the motor cortex brainstem and spinal cord. Glutamate excitotoxicity is considered to be the core factor in the ALS pathophysiology. It is well established that glutamate-mediated neurotoxicity contributes to motor neuron death, and motor neuron hyperexcitability is also obvious in the SOD1G93A ALS mouse models [56, 57]. Moreover, glutamate transporters, responsible for the glutamate clearance, were found to be significantly decreased in the spinal cord of ALS patients and animal models [58, 59]. Right now, riluzole, which possesses anti-glutamatergic properties, is the only drug approved as a neuroprotective agent for ALS.
Depression is a chronic and debilitating illness. It has been reported that a dysfunctional glutamatergic system, as well as malfunction in the glutamate clearance and metabolism, have been reported in the depression pathophysiology [60, 61]. Chronic stress, attributed to the experimental and clinical depression, promotes glutamate release. Previous studies have shown that treatment with antidepressants could decrease plasma glutamate levels in subjects with depression and regulate glutamate receptors via reducing the NMDA receptor function by decreasing the expression of its subunits and by potentiating AMPA receptor-mediated glutamate transmission [62]. Thus, treatments targeting NMDA receptors and the glutamate neurotransmitter system have shown benefits in treating depression [63]. Stroke is a leading cause of human mortality and disability, and most cases of stroke are considered ischemic. Usually, glutamate-induced excitotoxicity happens in minutes due to the fast increase of extracellular glutamate level [64]. As the sudden occlusion of blood flow, the neuronal and astroglial membrane potentials were broken down due to the lack of energy supply. In neurons, the subsequent membrane depolarization induces presynaptic glutamate release. While in astrocytes, energy depletion decreased the glutamate transporters expression. Both effects lead to an increase in extracellular glutamate [65]. Thus, the excess amount of synaptic glutamate over-stimulating its receptors leads to excitotoxicity and makes neurons susceptible to death [66].
Generally, we summarize the underlying mechanisms of the dysfunctional glutamate system in neurological diseases. In the early stages of neurological disease, glutamate-induced excitotoxicity contributes to neuronal death by overstimulating glutamate receptors, which subsequently results in Ca2+ overload. However, the amyloid plaques, phosphorylated tau, and α-synuclein may trigger synaptic loss and abnormal distribution of glutamate receptors [67]. As a result, the disruption of the normal glutamate signaling via glutamate receptors is implicated in a wide range of neurologic diseases [30, 68, 69]. Because of this, the glutamate system has ultimately emerged as a leading focus for novel drug discovery for neurological disorders. This review discusses the effects of the active ingredients from Chinese herbal medicine on neurological disorders by targeting glutamate signaling.
4. REGULATION OF GLUTAMATE-MEDIATED SIGNALING BY THE NATURAL CONSTITUENTS FROM CHINESE HERBAL MEDICINE
4.1. Chinese Herbal Medicine and Glutamate Receptors
4.1.1. Chinese Herbal Medicine and Ionotropic Glutamate Receptors
4.1.1.1. Chinese Herbal Medicine and NMDA Receptors
Huperzine A, a compound originally isolated from the Chinese medical herb Huperzia serrata, has been approved for the treatment of AD in China [70]. Although it has been proven to be a potent, reversible, and selective inhibitor of Acetylcholinesterase (AChE), huperzine A also modulates the glutamatergic system [71]. Previously, huperzine A pretreatment was found to reduce glutamate-induced calcium mobilization and neuronal death by activating the Brain-Derived Neurotrophic Factor (BDNF)/TrkB-dependent PI3K/Akt/mTOR signaling pathway [72, 73], and then several groups found huperzine A can attenuate glutamate or NMDA-induced excitotoxicity by blocking NMDA ion channels and subsequent Ca2+ mobilization [74, 75]. Moreover, bis(12)-hupyridone (B12H), derived from the natural compound huperzine A, has also been shown to be a mild NMDA blocker [76]. By blocking NMDA-induced excitotoxicity, huperzine A imparts neuroprotective effects on AD and status epilepticus [72, 75]. Kim et al. reported that ginsenosides (ginsenoside Rb1, Rc, Re, Rf, and Rg1) can inhibit Ca2+ currents and ΔCm in rat adrenal chromaffin cells [77]. Subsequently, their group revealed that ginsenoside Rg3 is a competitive NMDA receptor antagonist, and Rg3 elicits its effect in a glycine concentration-dependent manner [78, 79]. In addition, although weaker than Rg3, Rh2 has been suggested to inhibit intracellular Ca2+ [79], and S-isomer of Rh2 (SRh2) acts as a noncompetitive NMDA receptor antagonist at the polyamine-(or spermine-) binding site, unlike Rg3 that acts on the glycine-binding site [80]. In their study, 20(S)-ginsenoside Rh2 (20(S)-Rh2) along with 20(S)-ginsenoside Rg3 (20(S)-Rg3) showed the highest NMDA receptor inhibitory effect on cultured hippocampal neurons [80]. Radad et al. showed that although ginsenosides Rg1 and Rb1 can prevent glutamate-induced dopaminergic neuronal death, these compounds failed to antagonize NMDA receptor over-activation by glutamate at the early stage [81]. Our recent work exhibited that Rb1 may decrease the NR2B subunits expression to prevent excitotoxicity in the prefrontal cortex in a PD model [82]. The contradictory results may be due to the cellular model and the time point of glutamate addition. Moreover, Rb1 may also regulate the synaptic glutamate system via the α-synuclein and GABA system in PD [83, 84]. Ginsenoside Rb1, together with Rc and Rg5, has also been shown to protect primary medium spiny striatal neurons from glutamate-induced intracellular Ca2+ concentrations and glutamate-induced apoptosis [85]. As mentioned above, NR2A and NR2B receptors are the main forms of NMDA containing receptors in the CNS. However, they function differentially due to the higher affinity of NR2B than NR2A for glycine. It has been shown that NR2A is involved in cell survival and LTP, whereas NR2B activation leads to cell death and LTD [86, 87]. Additionally, NR2A is almost synaptic and NR2B is almost extrasynaptic [88, 89]. Notoginsenoside R1 (NTR1) is the main active ingredient in Panax notoginseng, and NTR1 has been shown to protect cultured neurons from glutamate-induced excitotoxicity by modulating NMDA receptors that are composed of NR1/NR2B subunits, rather than NR1/NR2A subunits [90]. Rhynchophylline (RIN), a significant active component of Uncaria rhynchophylla, can prevent Aβ1-42-induced excitotoxicity in the dentate gyrus region, which is mediated by the excessive activation of extrasynaptic NR2B expression, and subsequent Ca2+ overload [91]. These actions contribute to the neuroprotective effects of RIN on AD [91]. RIN could also inhibit the persistent sodium current (INaP) and NMDA receptor current by downregulating Nav1.6 and NR2B expression, and then RIN suppresses the neuronal hyperexcitability in several types of epilepsy [92]. Oxymatrine, which is derived from Sophora flavescens Ait, protects cortical neurons from NMDA-induced neurotoxicity by downregulating NR2B expression and calcium overload in a cerebral ischemic model [93], and this mechanism also contributes to the neuroprotective effects of senegenin, a component of Polygala tenuifolia in hepatic ischemia-reperfusion [94]. Magnesium lithospermate B (MLB) is a major component of the aqueous extract of Salvia miltiorrhiza Bunge, and MLB imparts neuroprotective effects on traumatic brain injury by inhibiting the NMDA receptor-mediated neurotoxicity through a cyclic adenosine monophosphate (cAMP)-dependent mechanism [95]. Isoliquiritigenin has been shown to bind to NMDA receptors and inhibit the glutamate-induced increase in Ca2+ influx and has been suggested to be a novel NMDA receptor antagonist in kampo medicine yokukansan for the treatment of AD, dementia with Lewy bodies, and other forms of senile dementia [96]. Gastrodin, an important component derived from the Chinese herb Gastrodia elata Blume, has been reported to reduce complete Freund's adjuvant (CFA)-induced upregulation of NR2A and NR2B receptors and CaMKII-α in the anterior cingulate cortex [97]. Moreover, gastrodin decreased the activation of astrocytes and microglia and the induction of TNF-α and IL-6 in CFA-injected mice, which contributes to its analgesic and anxiolytic effects [97].
4.1.1.2. Chinese Herbal Medicine and AMPA Receptors
As the major active agent of A. tatarinowii Schott, β-asarone has been proven to improve learning memory deficiency and suppress Aβ neurotoxicity in an APP/PS1 mice model of AD. Their study also clarified the neuroprotective effects of β-asarone, which involves the upregulation of synaptophysin and GluR1 [98]. ESP-102, an extract from Angelica gigas, Saururus chinensis, and Schisandra chinensis, has been used as herbal medicine and dietary supplement in Korea [99]. Kim et al. found that ESP-102 treatment significantly increases LTP induction in the hippocampal slice, and can also recover the scopolamine-suppressed BDNF and GluR2 protein levels, suggesting that ESP-102 is a novel herbal ingredient with memory-enhancing as well as neuroprotective effects [100]. As stated above, Rg1, which prevents glutamate-induced neuronal death, might not act by antagonizing NMDA receptors [81]. Zhu et al. reported that 30 days of ginsenoside Rg1 treatment enhances long-term memory and LTP induction in middle-aged animals and the underlying mechanism involves the upregulation of p-Akt, BDNF, and GluR1-AMPA receptor in the hippocampus [101]. Thus, the neuroprotective effects of Rg1 in improving memory function may mainly involve regulating AMPA receptor expression. Depression is a common neurological disorder that involves dysfunctional glutamate signaling [60], and several natural medicines derived from Chinese herbal medicine exert antidepressant effects by regulating glutamate signaling. Puerarin is an isoflavonoid derived from Radix puerariae, a traditional Chinese medicine with many clinical effects. Recently, Puerarin has been reported to activate AMPA receptors-induced mTOR signaling pathway, increase BDNF release, as well as increase GluR1 phosphorylation at its PKA site and facilitate AMPA receptors membrane insertion. These effects seem to suggest that it may be potentially used for the treatment of depression [102]. Total saikosaponins is the major ingredient of Bupleurum yinchowense. Recently, Sun et al. elucidated that total saikosaponins upregulated the phosphorylation expression of GluR1 Ser 845 and its downstream regulators ERK, Akt, and mTOR in the hippocampus [103]. The AMPA receptor-mTOR signaling pathway may thus be involved in the antidepressant-like and anxiolytic effects of total saikosaponins [103]. Lentinan is a polysaccharide isolated from the fruit body of Shiitake, and recently it has been reported that the antidepressant-like effects of lentinan were due to the enhanced prefrontal cortical expression of phosphorylation of AMPA GluR1 S845 and dendritic cell-associated C-type lectin-1 (Dectin-1), which is an immune regulator and receptor for lentinan [104]. 3’-Deoxyadenosine (3’-dA, Cordycepin), one of the major bioactive metabolites in the Cordyceps militaris, was identified to produce the rapid antidepressant effects by enhancing GluR1 S845 phosphorylation in the prefrontal cortex and hippocampus, and cordycepin modulates GluR1 synaptic localization in a subtype-specific and brain region-specific manner [105].
Other than regulating NMDA receptors, gastrodin can also inhibit CFA-induced inflammatory responses by downregulating GluR1-APMA receptor expression and then exert analgesic and anxiolytic effects on neurological disorders [97]. Dencichine is one of the non-protein amino acids in the roots of Panax notoginseng that can improve cytosolic calcium influx and thromboxane A2 (TXA2) release [94]. But it also partakes in the decrease of intracellular cAMP production, ultimately contributing to platelet aggregation [106]. Most importantly, the hemostatic effect of Dencichine is mainly mediated by AMPA receptors on platelets [106].
4.1.2. Chinese Herbal Medicine and Metabotropic Glutamate Receptors
Compared to ionotropic glutamate receptors, few studies have shown the effects of Chinese herbal medicine on the metabotropic glutamate receptors. Wang et al. identified several new benzofuran-type stilbene glycosides from Cortex Mori Radicis, and using the molecular-docking method, they found that these glycosides can bind to the mGluR1 via aromatic group and the hydroxyl group [107]. Thus, these compounds may be beneficial in the treatment of AD, PD, and other neurologic diseases [107]. As a major bioactive component of Radix Paeoniae alba, paeoniflorin has been proven to suppress the glutamate-induced elevation of intracellular Ca2+ levels and protect against glutamate-induced neuronal death [108]. Paeoniflorin-inhibited intracellular Ca2+ involves negatively modulation of mGluR5, rather than NMDA or AMPA receptors, and paeoniflorin may be potentially used in the treatment of febrile seizures [108]. Using allosteric modulators research, Jiang et al. determined that thesinine-4ʹ-O-β-d-glucoside, nigrolineaxanthone-P, and nodakenin may be potentially used as mGluR1 negative allosteric modulators. However, further investigations are necessary to confirm their neuroprotective effects using various neurological disease models [109].
4.2. Chinese Herbal Medicine and Glutamate Release
Ginsenosides Rg1 and Rb1, which are the active ingredients of Panax ginseng, can promote the glutamate release through different mechanisms. Although both Rg1 and Rb1 that promote glutamate release require the phosphorylation of synapsins, Rg1 undergoes a CaMKII-dependent signaling pathway, and Rb1 undergoes a PKA-dependent signaling pathway [110-112]. Thorajak et al. also reported that aged garlic extract (AGE), a garlic (Allium sativum L.) product, attenuates the impairment of the working memory by modulating cholinergic, glutamatergic, and GABAergic systems in an Aβ1-42-induced animal model [113]. AGE increases vGluT1, rather than vGluT2, in the hippocampus of the Aβ1-42-induced AD rat model, suggesting that AGE may affect presynaptic glutamate release by regulating the expression vesicular glutamate transporters [113]. Moreover, some compounds from herbal medicine inhibit presynaptic glutamate release, such as tanshinone IIA, a major active constituent of Salvia miltiorrhiza Bunge, which has been shown to suppress glutamate release from cortical synaptosomes [114]. Tanshinone IIA-mediated inhibition of glutamate release may be involved in the alteration of the synaptosomal plasma membrane potential and downstream modulation of Ca2+ influx [114]. In addition, tanshinone IIA may also directly regulate Ca2+ entry via Cav2.2 (N-type) and Cav2.1 (P/Q-type)-associated voltage-dependent Ca2+ channels (VDCCs) [114]. Echinacoside, a phenylethanoid glycoside extracted from Herba Cistanche. Lu et al. showed that echinacoside can diminish 4-aminopyridine (4-AP)-evoked glutamate release from rat cerebrocortical nerve terminals in a Ca2+-dependent manner, without altering the synaptosomal membrane potential [115]. Besides, echinacoside that inhibits Ca2+ entry also reduces PKC activity and may be utilized in the treatment of epilepsy [116]. Acacetin, a natural flavone derived from Clerodendrum inerme (L.) Gaertn has been proven to inhibit glutamate release via the Cav2.2 and Cav2.1 channels and can prevent kainic acid-induced neurotoxicity in the hippocampus [117]. Galangin is also the major component of flavonoids Alpinia officinarum Hance and has been shown to improve the neurological deficits in a middle cerebral artery occlusion (MCAO) model by decreasing serum glutamate concentrations [118]. In addition, galangin regulates GLUD1 to inhibit glutamate production and suppress glutamate excitotoxicity in ischemic stroke [118]. Oxyresveratrol from Smilacis chinae rhizome has been shown to prevent Aβ25-35-induced neuronal cell death by interfering with the glutamate release and inhibiting the elevation of cytosolic calcium concentrations in primary cultured cortical neurons [119]. Ban et al. also reported that other active components from Smilacis chinae rhizomes, catechin, and epicatechin, can also inhibit glutamate release in the same manner [120]. Besides promoting glutamate release, total ginsenosides extracted from Panax ginseng have also been shown to attenuate spatial memory impairment by decreasing glutamate and aspartic acid levels in the hippocampus and cortex in an AD animal model, suggesting that ginsenoside can regulate glutamate levels [121].
4.3. Chinese Herbal Medicine and Glutamate Transporters
Glutamate transporters are mainly responsible for the clearance of excess glutamate to suppress and prevent excitotoxicity. Xu et al. showed that schisantherin B, a bioactive of lignans isolated from Schisandra chinensis (Turcz.) Baill, could increase GLT-1 expression and decrease tau hyperphosphorylation to protect against Aβ1-42-induced cognitive decline in a mouse AD model [122]. Recently, this group reported that schisantherin B can increase GLT-1 expression via the PI3K/Akt/mTOR pathway to improve the short-term learning and memory impairment in depression [123]. MLB has also been shown to protect the rat brain from excitatory neurotoxicity during cerebral ischemia-reperfusion injury by regulating the miR-107/GLT-1 pathway [124]. Alpha-asarone, the effective component of Acorus tatarinowii (Schott), can promote glutamate uptake and inhibit EAAC1-mediated currents to reduce excitatory neuronal activity [125]. Moreover, both ginsenosides Rd and Rb1 have been shown to promote glutamate uptake and increase GLT-1 expression via PI3K/Akt/NF-κB signaling, and Rb1 can protect against glutamate excitotoxicity in PD and ischemic animal models [82, 126, 127]. Intriguingly, Ginsenoside Rd is the main hydrolyzed product of ginsenoside Rb1, Rb2, and Rc [128, 129], suggesting that Rb1 and Rd may share the essential domain regulating glutamate transporter GLT-1.
We list the potential underlying mechanisms of these natural constituents in various neurological disorders in Table 1.
Table 1. Natural constituents from chinese herbal medicine in regulating glutamate signaling in neurological disease.
| Name | Source | Experimental Setting/Model | Effect/Mechanism | Refs. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ginsenoside Rg1 | Panax ginseng | Cerebral cortex from male SD rats | Promotion in glutamate release through the activation of PKA | [110] | |||||
| Primary hippocampal neuron from C57BL/6 mice | Promotion in glutamate release via a CaMKII-dependent signaling pathway | [111] | |||||||
| Hippocampal slice from male C57BL/6 mice | Enhancement in long-term memory and LTP through regulating the PI3K/Akt pathway | [101] | |||||||
| Pregnant OF1/SPF mice | Prevention in glutamate-induced dopaminergic neuronal death | [81] | |||||||
| Ginsenoside Rb1 | Panax ginseng | Cerebral cortex from male SD rats | Promotion in glutamate release through the activation of protein kinase A | [110] | |||||
| PC12 rat pheochromocytoma cells | Proliferation in PC12 cells via a mediation of adenylate cyclase–dependent PKA signaling pathway | [112] | |||||||
| MPTP induced PD mice model | Increase in GLT-1 and decrease in NR2B subunit to prevent the excitotoxicity in the prefrontal cortex | [82] | |||||||
| Regulation the synaptic glutamate system via α-synuclein and GABA system | [83, 84] | ||||||||
| Striatal medium spiny neurons from YAC128 mice | Inhibition in glutamate-induced apoptosis and glutamate-induced intracellular Ca2+ concentrations | [85] | |||||||
| Pregnant OF1/SPF mice | Prevention in glutamate-induced dopaminergic neuronal death | [81] | |||||||
| Cerebral ischemia model in SD rats | Increase GLT-1 expression, inhibit NMDAR expression and Ca2+ overload, and reduce the release of cytochrome C | [127] | |||||||
| Ginsenoside Rg3 | Panax ginseng | Cultured hippocampal neurons from 16-18-day-old SD rats | Protection of NMDA-induced neuronal death via competitive interaction with the glycine-binding site of NMDA receptors | [78] | |||||
| Ginsenoside Rh2 | Panax ginseng | Cultured hippocampal neurons from neonatal SD rats | Inhibition of intracellular Ca2+ | [79] | |||||
| 20(S)-Ginsenoside Rh2 | Panax ginseng | Cultured hippocampal neurons from 16-18-days SD rats | Inhibition NMDA receptor via its interaction with polyamine-binding sites | [80] | |||||
| Ginsenoside Rc | Panax ginseng | Striatal medium spiny neurons from YAC128 mice | Inhibition in glutamate-induced apoptosis and glutamate-induced intracellular Ca2+ concentrations | [85] | |||||
| Ginsenoside Rg5 | Panax ginseng | Striatal medium spiny neurons from YAC128 mice | Inhibition in glutamate-induced apoptosis and glutamate-induced intracellular Ca2+ concentrations | [85] | |||||
| Ginsenoside Rd | Panax ginseng | Focal cerebral ischemia model in SD rats | Promotion of glutamate clearance by upregulating GLT-1 expression through PI3K/Akt and ERK 1/2 pathways | [126] | |||||
| Total ginsenosides | Panax ginseng | AlCl3 and D-Galactose induced AD model in Wistar rats | Decrease in glutamate and aspartic acid levels in the hippocampus and cortex | [121] | |||||
| Notoginsenoside R1 | Panax notoginseng | Cortical neurons from 15-day-old ddY mouse embryos | Protection in glutamate-induced excitotoxicity by modulating NMDA receptor | [90] | |||||
| Aged Garlic Extract (AGE) |
Allium sativum L. | Aβ1-42-induced AD model in Wistar rats | Decrease in the impairment of working memory via modulating cholinergic, glutamatergic, and GABAergic systems | [113] | |||||
| Tanshinone IIA | Salvia miltiorrhiza Bunge | Cerebral cortex from SD rats | Suppression of glutamate release from cortical synaptosomes through the synaptosomal plasma membrane potential and downstream modulation of Ca2+ influx Regulation of Ca2+ entry via Cav2.2 and Cav2.1-associated VDCCs |
[114] | |||||
| Name | Source | Experimental Setting/Model | Effect/Mechanism | Refs. | |||||
| Echinacoside | Herba Cistanche | Cerebral cortex from SD rats | Decrease in 4-AP evoked glutamate release without altering the synaptosomal membrane potential | [115] | |||||
| Hippocampal slice from SD rats | Inhibition in Ca2+ entry through reducing PKC activity | [116] | |||||||
| Acacetin | Clerodendrum inerme (L.) Gaertn | Hippocampus from SD rats | Inhibition in glutamate release via Cav2.2 and Cav2.1 channel and prevention in kainic acid-induced neurotoxicity in the hippocampus | [117] | |||||
| Galangin | Alpinia officinarum Hance | Middle cerebral artery occlusion (MCAO) model in SD rats | Decrease in the glutamate concentration in the serum Regulation in glutamate dehydrogenase 1 to inhibit the glutamate production and suppress the glutamate excitotoxicity |
[118] | |||||
| Oxyresveratrol | Smilacis chinae rhizome | Aβ25-35-induced neurotoxicity on cultured cortical neurons from SD rats | Prevention in the Aβ25-35-induced neuronal cell damage by interfering with the increase of Ca2+ and inhibiting glutamate release | [119] | |||||
| Catechin/ Epicatechin | Smilacis chinae rhizome | Aβ25-35-induced neurotoxicity on cultured cortical neurons from SD rats | Inhibition in glutamate release | [120] | |||||
| Huperzine A | Huperzia serrata | Immortalized mouse hippocampal HT22 cells | Reduction in glutamate-induced calcium mobilization and neuronal death via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway | [72-73] | |||||
| NMDA seizure model in rats | Attenuation in glutamate or NMDA-induced excitotoxicity by blocking the NMDA ion channel and subsequent Ca2+ mobilization | [74-75] | |||||||
| Bis (12)-hupyridone (B12H) | Huperzine A | Primary hippocampal neurons from 18-day-old SD rat embryos | Protection of CGNs against glutamate-induced neuronal toxicity via activating the a7nAChR/PI3-K/Akt pathway | [76] | |||||
| Rhynchophylline | Uncaria rhynchophylla | Aβ1-42-induced impairment in SD rats | Activation of extrasynaptic GluN2B-NMDAR expression, and subsequent Ca2+ overload | [91] | |||||
| Lithium–pilocarpine-induced status epilepticus model in SD rats | Inhibition in the INaP and NMDA receptor current via downregulating Nav1.6 and NR2B expression | [92] | |||||||
| Oxymatrine | Sophora flavescens Ait | Middle cerebral artery occlusion model in C57BL/6 mice | Neuroprotective effect via down-regulation of NR2B containing NMDA receptors and up-regulation of Bcl2 family | [93] | |||||
| Senegenin | Polygala tenuifolia | Hepatic ischemia-reperfusion model in SD rats | Neuroprotective effect through increasing NR2B expression | [94] | |||||
| Magnesium lithospermate B | Salvia miltiorrhiza Bunge | Weight-drop device-induced traumatic injury model in SD rats | Neuroprotective effect through inhibiting the NMDA receptor-mediated neurotoxicity | [95] | |||||
| Isoliquiritigenin | Kampo medicine yokukansan | Cortical neurons from 18-day-old SD rat embryos | Inhibition of NMDA receptors and glutamate-induced increase in Ca2+ influx | [96] | |||||
| Gastrodin | Gastrodia elata Blume | Complete Freund’s adjuvant-injected model in mice | Reduction in the activation of astrocyte and microglia and the induction of TNF-α and IL-6 Analgesic and anxiolytic effect of inhibition inflammatory response via downregulating GluR1-AMPA receptor |
[97] | |||||
| β-Asarone | A. tatarinowii Schott | APPswe/PS1dE9 double transgenic male mice | Neuroprotective effect via upregulating of synaptophysin and GluR1 expression | [98] | |||||
| ESP-102 | Angelica gigas, Saururus chinensis and Schisandra chinensis | Male ICR mice and cortical neurons from SD rats | Neuroprotective effect against neuronal cell death and cognitive impairment | [99] | |||||
| Cortical neurons and hippocampal slice from fetal SD rats | Recover in the scopolamine-suppressed BDNF and GluR2 protein levels | [100] | |||||||
| Name | Source | Experimental Setting/Model | Effect/Mechanism | Refs. | |||||
| Puerarin | Radix puerariae | Male C57BL/6J mice | Antidepressant effect in activation of AMPA receptors-induced mTOR signaling pathway via increasing BDNF release and facilitating AMPA receptors membrane insertion | [102] | |||||
| Total saikosaponins | Bupleurum yinchowense | Chronic corticosterone treatment model in ICR and C57BL/6J mice | Antidepressant-like and anxiolytic effects and increase in synaptic proteins expression via inducing AMPA receptor and subsequent mTOR signaling pathway | [103] | |||||
| Lentinan | Lentinus edodes (Berk) Sing | Male CD-1 mice used as the rapid and robust antidepressant model | Antidepressant-like effect via enhancing the prefrontal cortical expression of phosphorylation of AMPA GluR1 S845 and Dectin-1 | [104] | |||||
| 3’-Deoxyadenosine | Cordyceps Militaris | Male CD-1 mice used as the rapid and robust antidepressant model | Antidepressant-like effect via enhancing GluR1 S845 phosphorylation in the prefrontal cortex and hippocampus | [105] | |||||
| Dencichine | Panax notoginseng | Tail bleeding model in male Wistar rats | Elevation of the cytoplasmic concentration of calcium, and secretion of TXA2 and decrease in the level of intracellular of AMPA | [106] | |||||
| Benzofurantype stilbene glycosides | Cortex Mori Radicis | Molecular-docking method | Bind with the mGluR1 via aromatic group and the hydroxyl group | [107] | |||||
| Paeoniflorin | Radix Paeoniae alba | Febrile seizures model in male Lewis rats | Suppression in glutamate-induced elevation of intracellular Ca2+ through inhibiting mGluR5 | [108] | |||||
| Thesinine-4ʹ-O-β-d-glucoside | Borago officinalis (Boraginaceae) | Allosteric modulators research | Potential mGluR1 negative allosteric modulators | [109] | |||||
| Nodakenin | Angelica decursiva | Allosteric modulators research | Potential mGluR1 negative allosteric modulators | [109] | |||||
| Schisantherin B | Schisandra chinensis (Turcz.) Baill | Aβ1-42-induced AD model in mice | Increase in GLT-1 expression and decrease in the tau hyperphosphorylation | [122] | |||||
| Chronic unpredictable mild stress depression model in male KM mice | Increase GLT-1 expression via PI3K/Akt/mTOR pathway | [123] | |||||||
| Magnesium lithospermate B | Salvia miltiorrhiza Bge. | Focal cerebral I/R injury model in male SD rats | Neuroprotective effect in excitatory neurotoxicity via regulation of the miR-107/GLT-1 pathway | [124] | |||||
| Alpha-asarone | Acorus tatarinowii (Schott) | Females clawed toad Xenopus laevis | Promotion in glutamate uptake and inhibition of EAAC1-mediated current to reduce excitatory neuronal activity | [125] | |||||
Thus, evidence of neuroprotection observed in these natural constituents from Chinese herbal medicine may suggest their action on the glutamate signaling in neurological disorders. It is evident that excitotoxicity is related to the generation of free radicals produced as consequences of activation of nitric oxide synthase (NOS), and oxidative stress in mitochondria. Nitric oxide (NO) is the free radical and messenger molecule in the CNS, and several studies indicated that NO is linked to neuronal cell death [130, 131]. Usually, NO combines with the superoxide anion to form peroxynitrite (ONOO-) that damages DNA and results in neuronal death. Also, glutamate receptor-mediated calcium influx provides a mechanistic link between excitotoxic damage and NO-mediated cell death [132]. Moreover, glutamate receptor over-stimulation is the main mediator to intracellular oxidative stress [133]. Neuroinflammation is a key pathological process in neurological disorder [134, 135], and strategies against neuroinflammation showed neuroprotective outcomes [136-138]. Elevations in extracellular glutamate can trigger chronic neuroinflammatory reactions, leading to secondary damage to the brain [139]. The natural constituents from Chinese herbal medicine also show neuroprotective roles against nitric oxide, oxidative stress, and inflammation via multiple pathways. For example, berberine could attenuate depressive-like behaviors via inhibiting NF-κB-mediated neuroinflammation and downstream iNOS [140] and isoacteoside (a phenylethanoid isolated from Monochasma savatieri Franch. ex Maxim) could suppress iNOS-induced inflammation via blocking toll-like receptor 4 dimerization, which activates the MyD88-TAK1-NF-κB/MAPK signaling pathway [141]. Furthermore, icariin (a natural flavonoid compound isolated from famous Chinese herb Epimedium brevicornum) could suppress pro-inflammatory factors, oxidative stress, and neuronal apoptosis via mitochondrial apoptotic pathway [142].
5. CLINICAL USE OF NATURAL CONSTITUENTS FROM CHINESE HERBAL MEDICINE
It is reported that natural constituents derived from herbal medicine showed neuroprotective effects in various neurological disorders by targeting the glutamate system. However, these natural constituents studies based on clinical trials have been very limited. Here, we provide the currently available clinical trials on these natural constituents.
5.1. Huperzine A
As we told above, huperzine A can attenuate glutamate or NMDA-induced excitotoxicity and is now approved for the treatment of AD in China. Several randomized clinical trials (RCTs) of huperzine A for the treatment of AD indicated that it has some beneficial effects on the improvement of cognitive function in patients with AD by valuating the Mini-Mental State Examination (MMSE), Hastgawa Dementia Scale (HDS), and other scales [143-146]. Moreover, huperzine A can improve daily life activities, and global clinical assessment in participants with AD. There are no previous trails that have reported severe adverse events of huperzine A. Overall, these studies suggest huperzine A is a potential treatment for AD.
5.2. Ginsenoside Rb1
Recently, we, along with other groups, indicated that ginsenoside Rb1 protects neurons from glutamate-induced excitotoxicity, and shows benefits in PD mouse models [82-84]. However, only a few clinical trials of Rb1 have been reported. Kim et al. performed the pharmacokinetic analysis of Rb1 and compound K by orally administering healthy human individuals with red ginseng extract. They found the maximum plasma levels of compound K were significantly higher than those of Rb1, which provides evidence that intestinal microflora plays a pivotal role in transforming Rb1 to compound K [147]. Though some groups investigated the effect of Rb1 on insulin secretion and plasma glucose levels in healthy human individuals [148], there is still a dearth of clinical trials investigating the effect of Rb1 in neurological diseases. Recently, our groups have started the clinical trials on Rb1 in patients with PD, and we hope to provide some significant information for treating the neurodegenerative disease like PD.
5.3. Beta-asarone
There is only one clinical study investigating the effects of β-asarone in AD as of now. In this study, Chang et al. reported that the combined application of β-asarone and tenuigenin could improve the efficacy of memantine in promoting the cognitive function of moderate-to-severe AD patients [149]. However, this is a small-scale clinical trial, and they did not identify the specific role of β-asarone in this study. Though interesting, further large-scale, multicenter, and randomized studies are needed.
5.4. Tanshinone IIA
Several RCTs investigated the effects of tanshinone IIA on the stroke and coronary artery disease (CAD) patients. They reported that tanshinone IIA treatment could improve neurologic functional outcomes for acute ischemic stroke patients following recombinant tissue plasminogen activator (rt-PA) treatment by reducing the blood-brain barrier (BBB) leakage and damage [150]. Furthermore, tanshinone IIA can reduce elevated high-sensitivity C-reactive protein (hs-CRP)
and other circulating inflammation markers in CAD patients [151, 152].
5.5. Puerarin
As we mentioned above, puerarin could activate AMPA receptors-induced mTOR signaling pathway. Recently, Zheng et al. evaluated the efficacy and safety of the puerarin injection for the treatment of acute ischemic stroke. They reported that puerarin injection could improve the neurological deficit and decrease the hemorheology index and fibrinogen in patients with acute ischemic stroke [153]. Their study suggests that puerarin injection may be effective and relatively safe in the clinic for treating acute ischemic stroke [153]. However, Liu et al. reported there was no enough evidence to show puerarin is effective in treating patients with ischemic stroke [154].
Though the above natural constituents have been studied in various RCTs, we have to say that there is still a dearth of clinical trials investigating the effect of other natural constituents from herbal medicine on various neurological disorders. Therefore, further large-scale RCTs on these natural constituents with long-term follow-up are needed to address these issues.
CONCLUSION AND PERSPECTIVES
For centuries, herbal medicine was widely used for disease treatments in many countries. The use of medicinal plants predates ordinary medicines (especially synthetic drugs) synthesized by laboratories and pharmaceutical companies. Herbal and synthetic drugs have distinctive qualities for treating medical conditions. There are some clear advantages to the use of natural products. First, these natural products represent chemical novelties and they can generate lead drug candidates for complex targets as compared with other sources. Secondly, these natural constituents possess a chemical diversity unmatched by any synthetic chemical collection, and usually, they can possess bi- and tri-dimensional complex structures yet could be absorbed and metabolized by the human body [155]. Thirdly, the consumption of naturally derived constituents will promote fewer side effects because of the natural antioxidant properties [156]. Antioxidants are known to have protective roles against free radicals and help in decreasing toxicity levels. In contrast, synthetic drugs usually possess highly processed chemicals that our bodies may not recognize potentially leading detrimental reactions. Last but not least, the advantage of using herbal medicine is its cost-effectiveness. Generally, herbal medicine costs less for companies to produce and for patients to buy, while synthetic compounds cost more to make. Nevertheless, the use of naturally derived molecules as a source of new medicines also presents some challenges [157]. Compared with synthetic compounds, it may be physically difficult to have access to the natural habitats and the processes of isolating, purifying, and chemically characterizing active compounds are time-consuming. Plants may only thrive in certain regions making its accessibility geographically limiting. Secondly, compared with the chemical structure of synthetic compounds, the great structural complexity of natural molecules makes it difficult to synthesize analogous lead compounds. Lastly, the medicinal plants are usually collected from wild environmental populations. Therefore, quality control is an important issue, which directly influences the purity of the herbal ingredient being extracted [158]. In theory, extraction processes may potentially lead to a loss or reduction of the desired compound.
This review summarizes the active ingredients from Chinese herbal medicine that have been reported to protect against glutamate-induced excitotoxicity in various neurological disorders. These natural medicines achieve their neuroprotective effects mainly through regulating glutamate-associated signaling, including presynaptic glutamate release and ionotropic and metabotropic glutamate receptors’ activity and expression. Majority of the studies reporting the herbal effects on glutamate signaling are related to memory, cognition, and mentation, suggesting that these natural constituents from Chinese herbal medicine may serve as the candidates for the treatment of neurodegenerative and neuropsychiatric diseases, such as AD, PD, depression, epilepsy, and ischemic stroke. In this study, we reviewed the recent RCTs on these natural constituents in neurological disorders. We noticed that several studies demonstrated that natural constituents showed benefits in improving the cognitive function and neurological deficit in patients with AD or stoke. These RCTs results are consistent with the experimental results we mentioned in this study, suggesting these natural constituents may be potential drugs in treating neurological diseases. However, we should also point out that the quality of most clinical studies using natural constituents is relatively poor, and the scale of some RCTs is quite small. Hence, further systemic and large-scale RCTs on these natural constituents are required. Intriguingly, since our series of studies reported the neuroprotective of ginsenoside Rb1 in the PD mouse model recently [82-84], we have started multicenter, randomized, and double-blind clinical trials on Rb1 in patients with PD in China. We hope to provide insights for
developing herbal constituents, like ginsenosides Rb1, as efficacious and safe drugs for PD in the future. However, there are still several essential issues to overcome before these natural molecules from Chinese herbal medicine could be accepted as therapeutics for neurological disorders. How can the lead compound be precisely screened from the massive Chinese herbal medicines targeting glutamate signaling in different neurological disorders? How do the natural constituents pass through the BBB to enhance their efficacies? Are the natural constituents from Chinese herbal medicine disease-specific, brain region-specific, or glutamate receptors subtype-specific? Moreover, we think it is rational and necessary to explore the molecular interactions between the molecules and the various binding sites on the glutamate receptor subunits. Hence, the natural constituents from Chinese herbal medicine are promising therapeutics in treating neurological diseases.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- BDNF
Brain derived neurotrophic factor
- CaMKII
CaM kinase II
- cAMP
Cyclic adenosine monophosphate
- EAATs
Excitatory amino acid transporters
- GLUD
Glutamate dehydrogenase
- LTP
Long-term potentiation
- mGluRs
Metabotropic glutamate receptors
- NMDA
N-methyl-D-aspartate
- PKA
Protein kinases A
- PKC
Protein kinases C
- TCM
Traditional Chinese medicine
- VDCCs
Voltage-dependent Ca2+ channels
- vGluTs
Vesicular glutamate transporters
AUTHORS CONTRIBUTIONS
Y.L. and S.W. contributed equally to this work. Y.Z., Y.L., and S.W. made the most contributions to writing the draft. J.K. and J.Z. collected the literature. L.Z. checked the manuscript. Y.Z. and Y.H. conceived the project and modified the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The National Natural Science Foundation of China (No. 81704130 to YZ), the Science and Technology Planning Project of Guangzhou (No. 201904010238 to YZ), the Natural Science Foundation of Guangdong Province of China (No. 2017A030310643 to YZ), the Natural Science Foundation of Fujian Province of China (No. 2017J05139 to YZ), and the Startup Research Fund of Guangzhou Medical University (No. B195002002045 to YZ) supported this project.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Krnjević K. Glutamate and gamma-aminobutyric acid in brain. Nature. 1970;228(5267):119–124. doi: 10.1038/228119a0. [DOI] [PubMed] [Google Scholar]
- 2.Volk L., Chiu S.L., Sharma K., Huganir R.L. Glutamate synapses in human cognitive disorders. Annu. Rev. Neurosci. 2015;38:127–149. doi: 10.1146/annurev-neuro-071714-033821. [DOI] [PubMed] [Google Scholar]
- 3.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang P.K., Verbich D., McKinney R.A. AMPA receptors as drug targets in neurological disease--advantages, caveats, and future outlook. Eur. J. Neurosci. 2012;35(12):1908–1916. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- 5.Reiner A., Levitz J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron. 2018;98(6):1080–1098. doi: 10.1016/j.neuron.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro F.M., Vieira L.B., Pires R.G., Olmo R.P., Ferguson S.S. Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol. Res. 2017;115:179–191. doi: 10.1016/j.phrs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Butcher S.P., Hamberger A. In vivo studies on the extracellular, and veratrine-releasable, pools of endogenous amino acids in the rat striatum: effects of corticostriatal deafferentation and kainic acid lesion. J. Neurochem. 1987;48(3):713–721. doi: 10.1111/j.1471-4159.1987.tb05575.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. Curr. Opin. Pharmacol. 2006;6(1):103–107. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Sreenivasmurthy S.G., Liu J.Y., Song J.X., Yang C.B., Malampati S., Wang Z.Y., Huang Y.Y., Li M. Neurogenic traditional Chinese medicine as a promising strategy for the treatment of Alzheimer’s disease. Int. J. Mol. Sci. 2017;18(2):E272. doi: 10.3390/ijms18020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ke Z., Zhang X., Cao Z., Ding Y., Li N., Cao L., Wang T., Zhang C., Ding G., Wang Z., Xu X., Xiao W. Drug discovery of neurodegenerative disease through network pharmacology approach in herbs. Biomed. Pharmacother. 2016;78:272–279. doi: 10.1016/j.biopha.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Liang W., Lam W.P., Tang H.C., Leung P.C., Yew D.T. Current evidence of chinese herbal constituents with effects on NMDA receptor blockade. Pharmaceuticals (Basel) 2013;6(8):1039–1054. doi: 10.3390/ph6081039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker M.C., van der Donk W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016;43(2-3):419–430. doi: 10.1007/s10295-015-1665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umbarger H.E. Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- 14.Sieber S.A., Marahiel M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem. Rev. 2005;105(2):715–738. doi: 10.1021/cr0301191. [DOI] [PubMed] [Google Scholar]
- 15.Moore B.S., Hertweck C. Biosynthesis and attachment of novel bacterial polyketide synthase starter units. Nat. Prod. Rep. 2002;19(1):70–99. doi: 10.1039/b003939j. [DOI] [PubMed] [Google Scholar]
- 16.Shigeri Y., Seal R.P., Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Brain Res. Rev. 2004;45(3):250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Q., Song S.H., Augustine G.J. Molecular mechanisms of Short-term plasticity: Role of synapsin Phosphorylation in augmentation and potentiation of spontaneous glutamate release. Front. Synaptic Neurosci. 2018;10:33. doi: 10.3389/fnsyn.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackett J.T., Ueda T. Glutamate release. Neurochem. Res. 2015;40(12):2443–2460. doi: 10.1007/s11064-015-1622-1. [DOI] [PubMed] [Google Scholar]
- 19.Lüscher C., Malenka R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012;4(6):a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vyklicky V., Korinek M., Smejkalova T., Balik A., Krausova B., Kaniakova M., Lichnerova K., Cerny J., Krusek J., Dittert I., Horak M., Vyklicky L. Structure, function, and pharmacology of NMDA receptor channels. Physiol. Res. 2014;63(Suppl. 1):S191–S203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- 21.Anggono V., Huganir R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diering G.H., Huganir R.L. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100(2):314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerma J., Marques J.M. Kainate receptors in health and disease. Neuron. 2013;80(2):292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Conn P.J., Pin J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 25.Gerber U., Gee C.E., Benquet P. Metabotropic glutamate receptors: Intracellular signaling pathways. Curr. Opin. Pharmacol. 2007;7(1):56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Schoepp D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299(1):12–20. [PubMed] [Google Scholar]
- 27.Kanai Y., Hediger M.A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 28.Arriza J.L., Fairman W.A., Wadiche J.I., Murdoch G.H., Kavanaugh M.P., Amara S.G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14(9):5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pines G., Danbolt N.C., Bjørås M., Zhang Y., Bendahan A., Eide L., Koepsell H., Storm-Mathisen J., Seeberg E., Kanner B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360(6403):464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Tan F., Xu P., Qu S. Recent advance in the relationship between excitatory amino acid transporters and Parkinson’s disease. Neural Plast. 2016;2016:8941327. doi: 10.1155/2016/8941327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., He X., Meng X., Wu X., Tong H., Zhang X., Qu S. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death Dis. 2017;8(2):e2574. doi: 10.1038/cddis.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., He X., Wu X., Lei M., Wei Z., Zhang X., Wen L., Xu P., Li S., Qu S. Rapamycin upregulates glutamate transporter and IL-6 expression in astrocytes in a mouse model of Parkinson’s disease. Cell Death Dis. 2017;8(2):e2611. doi: 10.1038/cddis.2016.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olney J. W. Excitotoxicity: an overview. Canada diseases weekly report = Rapport hebdomadaire des maladies au Canada. 1990;16(Suppl 1E):47–57.. [PubMed] [Google Scholar]
- 34.Lai T.W., Zhang S., Wang Y.T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Prog. Neurobiol. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Mehta A., Prabhakar M., Kumar P., Deshmukh R., Sharma P.L. Excitotoxicity: bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013;698(1-3):6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Tehse J., Taghibiglou C. The overlooked aspect of excitotoxicity: Glutamate-independent excitotoxicity in traumatic brain injuries. Eur. J. Neurosci. 2019;49(9):1157–1170. doi: 10.1111/ejn.14307. [DOI] [PubMed] [Google Scholar]
- 37.Hermans E., Challiss R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359(Pt 3):465–484. doi: 10.1042/bj3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire M.A. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PubMed] [Google Scholar]
- 39.Kaur P., Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 2018;16(8):1224–1238. doi: 10.2174/1570159X15666170613083606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magi S., Piccirillo S., Amoroso S. The dual face of glutamate: from a neurotoxin to a potential survival factor-metabolic implications in health and disease. Cell. Mol. Life Sci. 2019;76(8):1473–1488. doi: 10.1007/s00018-018-3002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucey B.P., Fagan A.M., Holtzman D.M., Morris J.C., Bateman R.J. Diurnal oscillation of CSF Aβ and other AD biomarkers. Mol. Neurodegener. 2017;12(1):36. doi: 10.1186/s13024-017-0161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hynd M.R., Scott H.L., Dodd P.R. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2004;45(5):583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Eitan E., Hutchison E.R., Marosi K., Comotto J., Mustapic M., Nigam S.M., Suire C., Maharana C., Jicha G.A., Liu D., Machairaki V., Witwer K.W., Kapogiannis D., Mattson M.P. Extracellular vesicle-associated Aβ mediates trans-neuronal bioenergetic and Ca2+-Handling deficits in Alzheimer’s disease models. NPJ Aging Mech. Dis. 2016;2:2. doi: 10.1038/npjamd.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao W.Q., Santini F., Breese R., Ross D., Zhang X.D., Stone D.J., Ferrer M., Townsend M., Wolfe A.L., Seager M.A., Kinney G.G., Shughrue P.J., Ray W.J. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J. Biol. Chem. 2010;285(10):7619–7632. doi: 10.1074/jbc.M109.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decker H., Lo K.Y., Unger S.M., Ferreira S.T., Silverman M.A. Amyloid-beta peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3beta in primary cultured hippocampal neurons. J. Neurosci. 2010;30(27):9166–9171. doi: 10.1523/JNEUROSCI.1074-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renner M., Lacor P.N., Velasco P.T., Xu J., Contractor A., Klein W.L., Triller A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priller C., Mitteregger G., Paluch S., Vassallo N., Staufenbiel M., Kretzschmar H.A., Jucker M., Herms J. Excitatory synaptic transmission is depressed in cultured hippocampal neurons of APP/PS1 mice. Neurobiol. Aging. 2009;30(8):1227–1237. doi: 10.1016/j.neurobiolaging.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Um J.W., Nygaard H.B., Heiss J.K., Kostylev M.A., Stagi M., Vortmeyer A., Wisniewski T., Gunther E.C., Strittmatter S.M. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012;15(9):1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Felice F.G., Velasco P.T., Lambert M.P., Viola K., Fernandez S.J., Ferreira S.T., Klein W.L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 50.Jacob C.P., Koutsilieri E., Bartl J., Neuen-Jacob E., Arzberger T., Zander N., Ravid R., Roggendorf W., Riederer P., Grünblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J. Alzheimers Dis. 2007;11(1):97–116. doi: 10.3233/JAD-2007-11113. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K., Kong Q., Lin Y., Stouffer N., Schulte D.A., Lai L., Liu Q., Chang L.C., Dominguez S., Xing X., Cuny G.D., Hodgetts K.J., Glicksman M.A., Lin C.L. Restored glial glutamate transporter EAAT2 function as a potential therapeutic approach for Alzheimer’s disease. J. Exp. Med. 2015;212(3):319–332. doi: 10.1084/jem.20140413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viola K.L., Klein W.L. Amyloid β oligomers in Alzheimer’s disease pathogenesis, treatment, and diagnosis. Acta Neuropathol. 2015;129(2):183–206. doi: 10.1007/s00401-015-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paula-Lima A.C., Brito-Moreira J., Ferreira S.T. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. J. Neurochem. 2013;126(2):191–202. doi: 10.1111/jnc.12304. [DOI] [PubMed] [Google Scholar]
- 54.Sgambato-Faure V., Cenci M.A. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson’s disease. Prog. Neurobiol. 2012;96(1):69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Garcia B.G., Neely M.D., Deutch A.Y. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb. Cortex. 2010;20(10):2423–2432. doi: 10.1093/cercor/bhp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothstein J.D., Tsai G., Kuncl R.W., Clawson L., Cornblath D.R., Drachman D.B., Pestronk A., Stauch B.L., Coyle J.T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1990;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs A., Kutterer S., Mühling T., Duda J., Schütz B., Liss B., Keller B.U., Roeper J. Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis. J. Physiol. 2013;591(10):2723–2745. doi: 10.1113/jphysiol.2012.247981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothstein J.D., Martin L.J., Kuncl R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 1992;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 59.Pardo A.C., Wong V., Benson L.M., Dykes M., Tanaka K., Rothstein J.D., Maragakis N.J. Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp. Neurol. 2006;201(1):120–130. doi: 10.1016/j.expneurol.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 60.Murrough J.W., Abdallah C.G., Mathew S.J. Targeting glutamate signalling in depression: progress and prospects. Nat. Rev. Drug Discov. 2017;16(7):472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 61.Gerhard D.M., Wohleb E.S., Duman R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today. 2016;21(3):454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubio-Casillas A., Fernández-Guasti A. The dose makes the poison: from glutamate-mediated neurogenesis to neuronal atrophy and depression. Rev. Neurosci. 2016;27(6):599–622. doi: 10.1515/revneuro-2015-0066. [DOI] [PubMed] [Google Scholar]
- 63.Jaso B.A., Niciu M.J., Iadarola N.D., Lally N., Richards E.M., Park M., Ballard E.D., Nugent A.C., Machado-Vieira R., Zarate C.A. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr. Neuropharmacol. 2017;15(1):57–70. doi: 10.2174/1570159X14666160321123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/S0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 65.Graham S.H., Shiraishi K., Panter S.S., Simon R.P., Faden A.I. Changes in extracellular amino acid neurotransmitters produced by focal cerebral ischemia. Neurosci. Lett. 1990;110(1-2):124–130. doi: 10.1016/0304-3940(90)90799-F. [DOI] [PubMed] [Google Scholar]
- 66.Lewerenz J., Maher P. Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front. Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hettinger J.C., Lee H., Bu G., Holtzman D.M., Cirrito J.R. AMPA-ergic regulation of amyloid-β levels in an Alzheimer’s disease mouse model. Mol. Neurodegener. 2018;13(1):22. doi: 10.1186/s13024-018-0256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang R., Reddy P.H. Role of glutamate and NMDA receptors in Alzheimer’s disease. J. Alzheimers Dis. 2017;57(4):1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blasco H., Mavel S., Corcia P., Gordon P.H. The glutamate hypothesis in ALS: pathophysiology and drug development. Curr. Med. Chem. 2014;21(31):3551–3575. doi: 10.2174/0929867321666140916120118. [DOI] [PubMed] [Google Scholar]
- 70.Wang R., Tang X.C. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer’s disease. Neurosignals. 2005;14(1-2):71–82. doi: 10.1159/000085387. [DOI] [PubMed] [Google Scholar]
- 71.Wang R., Yan H., Tang X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006;27(1):1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- 72.Ved H.S., Koenig M.L., Dave J.R., Doctor B.P. Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. Neuroreport. 1997;8(4):963–968. doi: 10.1097/00001756-199703030-00029. [DOI] [PubMed] [Google Scholar]
- 73.Mao X.Y., Zhou H.H., Li X., Liu Z.Q., Huperzine A. Huperzine A alleviates oxidative glutamate toxicity in hippocampal HT22 Cells via activating BDNF/TrkB-dependent PI3K/Akt/mTOR signaling pathway. Cell. Mol. Neurobiol. 2016;36(6):915–925. doi: 10.1007/s10571-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gordon R.K., Nigam S.V., Weitz J.A., Dave J.R., Doctor B.P., Ved H.S. The NMDA receptor ion channel: a site for binding of huperzine A. J. Appl. Toxicol. 2001;21(Suppl. 1):S47–S51. doi: 10.1002/jat.805. [DOI] [PubMed] [Google Scholar]
- 75.Coleman B.R., Ratcliffe R.H., Oguntayo S.A., Shi X., Doctor B.P., Gordon R.K., Nambiar M.P. [+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats. Chem. Biol. Interact. 2008;175(1-3):387–395. doi: 10.1016/j.cbi.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Cui W., Hu S., Chan H.H., Luo J., Li W., Mak S., Choi T.C., Rong J., Carlier P.R., Han Y. Bis(12)-hupyridone, a novel acetylcholinesterase inhibitor, protects against glutamate-induced neuronal excitotoxicity via activating α7 nicotinic acetylcholine receptor/phosphoinositide 3-kinase/Akt cascade. Chem. Biol. Interact. 2013;203(1):365–370. doi: 10.1016/j.cbi.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Kim H.S., Lee J.H., Goo Y.S., Nah S.Y. Effects of ginsenosides on Ca2+ channels and membrane capacitance in rat adrenal chromaffin cells. Brain Res. Bull. 1998;46(3):245–251. doi: 10.1016/S0361-9230(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 78.Kim S., Kim T., Ahn K., Park W.K., Nah S.Y., Rhim H. Ginsenoside Rg3 antagonizes NMDA receptors through a glycine modulatory site in rat cultured hippocampal neurons. Biochem. Biophys. Res. Commun. 2004;323(2):416–424. doi: 10.1016/j.bbrc.2004.08.106. [DOI] [PubMed] [Google Scholar]
- 79.Kim S., Ahn K., Oh T.H., Nah S.Y., Rhim H. Inhibitory effect of ginsenosides on NMDA receptor-mediated signals in rat hippocampal neurons. Biochem. Biophys. Res. Commun. 2002;296(2):247–254. doi: 10.1016/S0006-291X(02)00870-7. [DOI] [PubMed] [Google Scholar]
- 80.Lee E., Kim S., Chung K.C., Choo M.K., Kim D.H., Nam G., Rhim H. 20(S)-ginsenoside Rh2, a newly identified active ingredient of ginseng, inhibits NMDA receptors in cultured rat hippocampal neurons. Eur. J. Pharmacol. 2006;536(1-2):69–77. doi: 10.1016/j.ejphar.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 81.Radad K., Gille G., Moldzio R., Saito H., Rausch W.D. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021(1):41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y.L., Liu Y., Kang X.P., Dou C.Y., Zhuo R.G., Huang S.Q., Peng L., Wen L. Ginsenoside Rb1 confers neuroprotection via promotion of glutamate transporters in a mouse model of Parkinson’s disease. Neuropharmacology. 2018;131:223–237. doi: 10.1016/j.neuropharm.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 83.Qu S., Meng X., Liu Y., Zhang X., Zhang Y. Ginsenoside Rb1 prevents MPTP-induced changes in hippocampal memory via regulation of the α-synuclein/PSD-95 pathway. Aging (Albany NY) 2019;11(7):1934–1964. doi: 10.18632/aging.101884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y., Zong X., Huang J., Guan Y., Li Y., Du T., Liu K., Kang X., Dou C., Sun X., Wu R., Wen L., Zhang Y. Ginsenoside Rb1 regulates prefrontal cortical GABAergic transmission in MPTP-treated mice. Aging (Albany NY) 2019;11(14):5008–5034. doi: 10.18632/aging.102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu J., Jeong H.K., Bulin S.E., Kwon S.W., Park J.H., Bezprozvanny I. Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J. Neurosci. Res. 2009;87(8):1904–1912. doi: 10.1002/jnr.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Wong T.P., Aarts M., Rooyakkers A., Liu L., Lai T.W., Wu D.C., Lu J., Tymianski M., Craig A.M., Wang Y.T. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu L., Wong T.P., Pozza M.F., Lingenhoehl K., Wang Y., Sheng M., Auberson Y.P., Wang Y.T. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304(5673):1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 88.Parsons M.P., Raymond L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron. 2014;82(2):279–293. doi: 10.1016/j.neuron.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 89.Thomas C.G., Miller A.J., Westbrook G.L. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006;95(3):1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- 90.Gu B., Nakamichi N., Zhang W.S., Nakamura Y., Kambe Y., Fukumori R., Takuma K., Yamada K., Takarada T., Taniura H., Yoneda Y. Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-D-aspartate receptors composed of an NR1/NR2B subunit assembly. J. Neurosci. Res. 2009;87(9):2145–2156. doi: 10.1002/jnr.22021. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y., Ji W.G., Zhu Z.R., Wu Y.L., Zhang Z.Y., Qu S.C. Rhynchophylline suppresses soluble Aβ1-42-induced impairment of spatial cognition function via inhibiting excessive activation of extrasynaptic NR2B-containing NMDA receptors. Neuropharmacology. 2018;135:100–112. doi: 10.1016/j.neuropharm.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Shao H., Yang Y., Mi Z., Zhu G.X., Qi A.P., Ji W.G., Zhu Z.R. Anticonvulsant effect of rhynchophylline involved in the inhibition of persistent sodium current and NMDA receptor current in the pilocarpine rat model of temporal lobe epilepsy. Neuroscience. 2016;337:355–369. doi: 10.1016/j.neuroscience.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 93.Zhang K., Li Y.J., Yang Q., Gerile O., Yang L., Li X.B., Guo Y.Y., Zhang N., Feng B., Liu S.B., Zhao M.G. Neuroprotective effects of oxymatrine against excitotoxicity partially through down-regulation of NR2B-containing NMDA receptors. Phytomedicine. 2013;20(3-4):343–350. doi: 10.1016/j.phymed.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 94.Xie W., Yang Y., Gu X., Zheng Y., Sun Y.E., Liang Y., Bo J., Ma Z. Senegenin attenuates hepatic ischemia-reperfusion induced cognitive dysfunction by increasing hippocampal NR2B expression in rats. PLoS One. 2012;7(9):e45575. doi: 10.1371/journal.pone.0045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang C.Z., Wu S.C., Kwan A.L., Lin C.L. Magnesium Lithospermate B Implicates 3′-5′-Cyclic Adenosine monophosphate/protein kinase a pathway and N-Methyl-d-aspartate receptors in an experimental traumatic brain injury. World Neurosurg. 2015;84(4):954–963. doi: 10.1016/j.wneu.2015.05.075. [DOI] [PubMed] [Google Scholar]
- 96.Kawakami Z., Ikarashi Y., Kase Y. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell. Mol. Neurobiol. 2011;31(8):1203–1212. doi: 10.1007/s10571-011-9722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun T., Wang J., Li X., Li Y.J., Feng D., Shi W.L., Zhao M.G., Wang J.B., Wu Y.M. Gastrodin relieved complete Freund’s adjuvant-induced spontaneous pain by inhibiting inflammatory response. Int. Immunopharmacol. 2016;41:66–73. doi: 10.1016/j.intimp.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 98.Liu S.J., Yang C., Zhang Y., Su R.Y., Chen J.L., Jiao M.M., Chen H.F., Zheng N., Luo S., Chen Y.B., Quan S.J., Wang Q. Neuroprotective effect of β-asarone against Alzheimer’s disease: regulation of synaptic plasticity by increased expression of SYP and GluR1. Drug Des. Devel. Ther. 2016;10:1461–1469. doi: 10.2147/DDDT.S93559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang S.Y., Lee K.Y., Koo K.A., Yoon J.S., Lim S.W., Kim Y.C., Sung S.H. ESP-102, a standardized combined extract of Angelica gigas, Saururus chinensis and Schizandra chinensis, significantly improved scopolamine-induced memory impairment in mice. Life Sci. 2005;76(15):1691–1705. doi: 10.1016/j.lfs.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 100.Kim H.B., Hwang E.S., Choi G.Y., Lee S., Park T.S., Lee C.W., Lee E.S., Kim Y.C., Kim S.S., Lee S.O., Park J.H. Combined herbal extract of Angelica gigas, Saururus chinensis, and Schisandra chinensis, changes synaptic pPlasticity and attenuates scopolamine-induced memory impairment in Rat hippocampus tissue. Evid. Based Complement. Alternat. Med. 2016 doi: 10.1155/2016/8793095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu G., Wang Y., Li J., Wang J. Chronic treatment with ginsenoside Rg1 promotes memory and hippocampal long-term potentiation in middle-aged mice. Neuroscience. 2015;292:81–89. doi: 10.1016/j.neuroscience.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 102.Huang C.C., Tsai M.H., Wu Y.C., Chen K.T., Chuang H.W., Chen Y., Tseng G.W., Fu P.I., Wei I.H. Activity dependent mammalian target of rapamycin pathway and brain derived neurotrophic factor release is required for the Rapid Antidepressant Effects of Puerarin. Am. J. Chin. Med. 2018;4:1–16. doi: 10.1142/S0192415X18500787. [DOI] [PubMed] [Google Scholar]
- 103.Sun X., Li X., Pan R., Xu Y., Wang Q., Song M. Total Saikosaponins of Bupleurum yinchowense reduces depressive, anxiety-like behavior and increases synaptic proteins expression in chronic corticosterine-treated mice. BMC Complement. Altern. Med. 2018;18(1):117. doi: 10.1186/s12906-018-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bao H., Sun L., Zhu Y., Ran P., Hu W., Zhu K., Li B., Hou Y., Nie J., Gao T., Shan L., Du K., Zheng S., Zheng B., Xiao C., Du J. Lentinan produces a robust antidepressant-like effect via enhancing the prefrontal dectin-1/AMPA receptor signaling pathway. Behav. Brain Res. 2017;317:263–271. doi: 10.1016/j.bbr.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 105.Li B., Hou Y., Zhu M., Bao H., Nie J., Zhang G.Y., Shan L., Yao Y., Du K., Yang H., Li M., Zheng B., Xu X., Xiao C., Du J. 3′-Deoxyadenosine (Cordycepin) produces a rapid and robust antidepressant effect via enhancing Prefrontal AMPA Receptor signaling pathway. Int. J. Neuropsychopharmacol. 2016;19(4):pyv112. doi: 10.1093/ijnp/pyv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang L.F., Shi H.L., Gao B., Wu H., Yang L., Wu X.J., Wang Z.T. Decichine enhances hemostasis of activated platelets via AMPA receptors. Thromb. Res. 2014;133(5):848–854. doi: 10.1016/j.thromres.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 107.Wang Y.N., Liu M.F., Hou W.Z., Xu R.M., Gao J., Lu A.Q., Xie M.P., Li L., Zhang J.J., Peng Y., Ma L.L., Wang X.L., Shi J.G., Wang S.J. Bioactive benzofuran derivatives from cortex mori radicis, and their neuroprotective and analgesic activities mediated by mGluR1. Molecules. 2017;22(2):E236. doi: 10.3390/molecules22020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hino H., Takahashi H., Suzuki Y., Tanaka J., Ishii E., Fukuda M. Anticonvulsive effect of paeoniflorin on experimental febrile seizures in immature rats: possible application for febrile seizures in children. PLoS One. 2012;7(8):e42920. doi: 10.1371/journal.pone.0042920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang L., Zhang X., Chen X., He Y., Qiao L., Zhang Y., Li G., Xiang Y. Virtual screening and molecular dynamics Study of Potential negative allosteric modulators of mGluR1 from chinese herbs. Molecules. 2015;20(7):12769–12786. doi: 10.3390/molecules200712769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang Y., Huang W.J., Tien L.T., Wang S.J. Ginsenosides Rg1 and Rb1 enhance glutamate release through activation of protein kinase A in rat cerebrocortical nerve terminals (synaptosomes). Eur. J. Pharmacol. 2008;578(1):28–36. doi: 10.1016/j.ejphar.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 111.Liu Z.J., Zhao M., Zhang Y., Xue J.F., Chen N.H. Ginsenoside Rg1 promotes glutamate release via a calcium/calmodulin-dependent protein kinase II-dependent signaling pathway. Brain Res. 2010;1333:1–8. doi: 10.1016/j.brainres.2010.03.096. [DOI] [PubMed] [Google Scholar]
- 112.Lee S.D., Lo M.J. Ginsenoside Rb1 promotes PC12 cell cycle kinetics through an adenylate cyclase-dependent protein kinase A pathway. Nutr. Res. 2010;30(9):660–666. doi: 10.1016/j.nutres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Thorajak P., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with Regard to cognitive impairment in Aβ-Induced rats. Nutrients. 2017;9(7):E686. doi: 10.3390/nu9070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin T.Y., Lu C.W., Huang S.K., Wang S.J. Tanshinone IIA, a constituent of danshen, inhibits the release of glutamate in rat cerebrocortical nerve terminals. J. Ethnopharmacol. 2013;147(2):488–496. doi: 10.1016/j.jep.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 115.Lu C.W., Lin T.Y., Huang S.K., Wang S.J. Echinacoside Inhibits glutamate release by suppressing voltage-dependent Ca(2+) entry and protein kinase C in rat cerebrocortical nerve terminals. Int. J. Mol. Sci. 2016;17(7):E1006. doi: 10.3390/ijms17071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu C.W., Huang S.K., Lin T.Y., Wang S.J. Echinacoside, an active constituent of Herba Cistanche, suppresses epileptiform activity in hippocampal CA3 pyramidal neurons. Korean J. Physiol. Pharmacol. 2018;22(3):249–255. doi: 10.4196/kjpp.2018.22.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin T.Y., Huang W.J., Wu C.C., Lu C.W., Wang S.J. Acacetin inhibits glutamate release and prevents kainic acid-induced neurotoxicity in rats. PLoS One. 2014;9(2):e88644. doi: 10.1371/journal.pone.0088644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang R., Chen K., Zhao Y., Tian P., Duan F., Sun W., Liu Y., Yan Z., Li S. Analysis of potential amino acid biomarkers in brain tissue and the effect of galangin on cerebral ischemia. Molecules. 2016;21(4):438. doi: 10.3390/molecules21040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ban J.Y., Jeon S.Y., Nguyen T.T., Bae K., Song K.S., Seong Y.H. Neuroprotective effect of oxyresveratrol from smilacis chinae rhizome on amyloid Beta protein (25-35)-induced neurotoxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2006;29(12):2419–2424. doi: 10.1248/bpb.29.2419. [DOI] [PubMed] [Google Scholar]
- 120.Ban J.Y., Jeon S.Y., Bae K., Song K.S., Seong Y.H. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79(24):2251–2259. doi: 10.1016/j.lfs.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y., Pi Z., Song F., Liu Z. Ginsenosides attenuate d-galactose- and AlCl3-inducedspatial memory impairment by restoring the dysfunction of the neurotransmitter systems in the rat model of Alzheimer’s disease. J. Ethnopharmacol. 2016;194:188–195. doi: 10.1016/j.jep.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Xu M., Dong Y., Wan S., Yan T., Cao J., Wu L., Bi K., Jia Y. Schisantherin B ameliorates Aβ1-42-induced cognitive decline via restoration of GLT-1 in a mouse model of Alzheimer’s disease. Physiol. Behav. 2016;167:265–273. doi: 10.1016/j.physbeh.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 123.Xu M., Xiao F., Wang M., Yan T., Yang H., Wu B., Bi K., Jia Y. Schisantherin, B improves the pathological manifestations of mice caused by behavior desperation in different ages-depression with Cognitive Impairment. Biomol. Ther. (Seoul) 2019;27(2):160–167. doi: 10.4062/biomolther.2018.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Z.B., Luo X.J., Ren K.D., Peng J.J., Tan B., Liu B., Lou Z., Xiong X.M., Zhang X.J., Ren X., Peng J. Beneficial effect of magnesium lithospermate B on cerebral ischemia-reperfusion injury in rats involves the regulation of miR-107/glutamate transporter 1 pathway. Eur. J. Pharmacol. 2015;766:91–98. doi: 10.1016/j.ejphar.2015.09.042. [DOI] [PubMed] [Google Scholar]
- 125.Gu Q., Du H., Ma C., Fotis H., Wu B., Huang C., Schwarz W. Effects of alpha-asarone on the glutamate transporter EAAC1 in Xenopus oocytes. Planta Med. 2010;76(6):595–598. doi: 10.1055/s-0029-1240613. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X., Shi M., Bjørås M., Wang W., Zhang G., Han J., Liu Z., Zhang Y., Wang B., Chen J., Zhu Y., Xiong L., Zhao G. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front. Pharmacol. 2013;4:152. doi: 10.3389/fphar.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang S., Li M., Guo Y., Li C., Wu L., Zhou X.F., Luo Y., An D., Li S., Luo H., Pu L. Effects of Panax notoginseng ginsenoside Rb1 on abnormal hippocampal microenvironment in rats. J. Ethnopharmacol. 2017;202:138–146. doi: 10.1016/j.jep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Son J.W., Kim H.J., Oh D.K. Ginsenoside Rd production from the major ginsenoside Rb(1) by beta-glucosidase from Thermus caldophilus. Biotechnol. Lett. 2008;30(4):713–716. doi: 10.1007/s10529-007-9590-4. [DOI] [PubMed] [Google Scholar]
- 129.Hong H., Cui C.H., Kim J.K., Jin F.X., Kim S.C., Im W.T. Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J. Ginseng Res. 2012;36(4):418–424. doi: 10.5142/jgr.2012.36.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Keynes R.G., Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr. Mol. Med. 2004;4(2):179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- 131.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 132.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9(1):119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kamat P.K., Kalani A., Rai S., Swarnkar S., Tota S., Nath C., Tyagi N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol. Neurobiol. 2016;53(1):648–661. doi: 10.1007/s12035-014-9053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Streit W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 135.Cao W., Zheng H. Peripheral immune system in aging and Alzheimer’s disease. Mol. Neurodegener. 2018;13(1):51. doi: 10.1186/s13024-018-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bye N., Habgood M.D., Callaway J.K., Malakooti N., Potter A., Kossmann T., Morganti-Kossmann M.C. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp. Neurol. 2007;204(1):220–233. doi: 10.1016/j.expneurol.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 137.Guimarães J.S., Freire M.A., Lima R.R., Picanço-Diniz C.W., Pereira A., Gomes-Leal W. Minocycline treatment reduces white matter damage after excitotoxic striatal injury. Brain Res. 2010;1329:182–193. doi: 10.1016/j.brainres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 138.Lopes R.S., Cardoso M.M., Sampaio A.O., Barbosa M.S., Jr, Souza C.C., Silva D.A. M.C.; Ferreira, E.M.; Freire, M.A.; Lima, R.R.; Gomes-Leal, W. Indomethacin treatment reduces microglia activation and increases numbers of neuroblasts in the subventricular zone and ischaemic striatum after focal ischaemia. J. Biosci. 2016;41(3):381–394. doi: 10.1007/s12038-016-9621-1. [DOI] [PubMed] [Google Scholar]
- 139.Burd I., Welling J., Kannan G., Johnston M.V. Excitotoxicity as a common mechanism for fetal neuronal injury with hypoxia and intrauterine inflammation. Adv. Pharmacol. 2016;76:85–101. doi: 10.1016/bs.apha.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 140.Liu Y.M., Niu L., Wang L.L., Bai L., Fang X.Y., Li Y.C., Yi L.T. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res. Bull. 2017;134:220–227. doi: 10.1016/j.brainresbull.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 141.Gao H., Cui Y., Kang N., Liu X., Liu Y., Zou Y., Zhang Z., Li X., Yang S., Li J., Wang C., Xu Q.M., Chen X. Isoacteoside, a dihydroxyphenylethyl glycoside, exhibits anti-inflammatory effects through blocking toll-like receptor 4 dimerization. Br. J. Pharmacol. 2017;174(17):2880–2896. doi: 10.1111/bph.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li H., Zhang X., Zhu X., Qi X., Lin K., Cheng L. The effects of Icariin on enhancing motor recovery through attenuating pro-inflammatory factors and oxidative stress via mitochondrial apoptotic pathway in the mice model of spinal cord injury. Front. Physiol. 2018;9:1617. doi: 10.3389/fphys.2018.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang G., Wang Y., Tian J., Liu J.P. Huperzine A for Alzheimer’s disease: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8(9):e74916. doi: 10.1371/journal.pone.0074916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ha G.T., Wong R.K., Zhang Y. Huperzine a as potential treatment of Alzheimer’s disease: an assessment on chemistry, pharmacology, and clinical studies. Chem. Biodivers. 2011;8(7):1189–1204. doi: 10.1002/cbdv.201000269. [DOI] [PubMed] [Google Scholar]
- 145.Rafii M.S., Walsh S., Little J.T., Behan K., Reynolds B., Ward C., Jin S., Thomas R., Aisen P.S. A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology. 2011;76(16):1389–1394. doi: 10.1212/WNL.0b013e318216eb7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jiang H., Luo X., Bai D. Progress in clinical, pharmacological, chemical and structural biological studies of huperzine A: a drug of traditional chinese medicine origin for the treatment of Alzheimer’s disease. Curr. Med. Chem. 2003;10(21):2231–2252. doi: 10.2174/0929867033456747. [DOI] [PubMed] [Google Scholar]
- 147.Kim H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J. Ginseng Res. 2013;37(4):451–456. doi: 10.5142/jgr.2013.37.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chang W.H., Tsai Y.L., Huang C.Y., Hsieh C.C., Chaunchaiyakul R., Fang Y., Lee S.D., Kuo C.H. Null effect of ginsenoside Rb1 on improving glycemic status in men during a resistance training recovery. J. Int. Soc. Sports Nutr. 2015;12:34. doi: 10.1186/s12970-015-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chang W., Teng J. Combined application of tenuigenin and β-asarone improved the efficacy of memantine in treating moderate-to-severe Alzheimer’s disease. Drug Des. Devel. Ther. 2018;12:455–462. doi: 10.2147/DDDT.S155567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ji B., Zhou F., Han L., Yang J., Fan H., Li S., Li J., Zhang X., Wang X., Chen X., Xu Y. Sodium tanshinone IIA sulfonate enhances effectiveness Rt-PA treatment in acute ischemic stroke patients associated with ameliorating blood-brain barrier damage. Transl. Stroke Res. 2017;8(4):334–340. doi: 10.1007/s12975-017-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li S., Jiao Y., Wang H., Shang Q., Lu F., Huang L., Liu J., Xu H., Chen K. Sodium tanshinone IIA sulfate adjunct therapy reduces high-sensitivity C-reactive protein level in coronary artery disease patients: a randomized controlled trial. Sci. Rep. 2017;7(1):17451. doi: 10.1038/s41598-017-16980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tan D., Wu J.R., Zhang X.M., Liu S., Zhang B. Sodium Tanshinone II A sulfonate injection as adjuvant treatment for unstable Angina Pectoris: A meta-analysis of 17 randomized controlled trials. Chin. J. Integr. Med. 2018;24(2):156–160. doi: 10.1007/s11655-017-2424-x. [DOI] [PubMed] [Google Scholar]
- 153.Zheng Q.H., Li X.L., Mei Z.G., Xiong L., Mei Q.X., Wang J.F., Tan L.J., Yang S.B., Feng Z.T. Efficacy and safety of puerarin injection in curing acute ischemic stroke: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96(1):e5803. doi: 10.1097/MD.0000000000005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu B., Tan Y., Wang D., Liu M. Puerarin for ischaemic stroke. Cochrane Database Syst. Rev. 2016;2:CD004955. doi: 10.1002/14651858.CD004955.pub3. [DOI] [PubMed] [Google Scholar]
- 155.Strohl W.R. The role of natural products in a modern drug discovery program. Drug Discov. Today. 2000;5(2):39–41. doi: 10.1016/S1359-6446(99)01443-9. [DOI] [PubMed] [Google Scholar]
- 156.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015;4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 157.Li J.W., Vederas J.C. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325(5937):161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 158.Wills R.B., Bone K., Morgan M. Herbal products: active constituents, modes of action and quality control. Nutr. Res. Rev. 2000;13(1):47–77. doi: 10.1079/095442200108729007. [DOI] [PubMed] [Google Scholar]


