Abstract
Background:
Natural phenolic compounds in medicinal herbs and dietary plants are antioxidants which play therapeutic or preventive roles in different pathological situations, such as oxidative stress and inflammation. One of the most studied phenolic compounds in the last decade is chlorogenic acid (CGA), which is a potent antioxidant found in certain foods and drinks.
Objective:
This review focuses on the anti-inflammatory and antinociceptive bioactivities of CGA, and the putative mechanisms of action are described. Ethnopharmacological reports related to these bioactivities are also reviewed.
Materials and Methods:
An electronic literature search was conducted by authors up to October 2019. Original articles were selected.
Results:
CGA has been shown to reduce inflammation and modulate inflammatory and neuropathic pain in animal models.
Conclusion:
The consensus of the literature search was that systemic CGA may facilitate pain management via bolstering antioxidant defenses against inflammatory insults.
Keywords: Chlorogenic acid, inflammation, pain, inflammatory, neuropathic, antihyperalgesic, antiallodynic
1. INTRODUCTION
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage [1]. Pain is a complex and subjective phenomenon, resulting from the interplay between systems signaling noxious events and pain-modulating processes [2]. More than 1.5 billion people worldwide suffer from chronic pain of varying degrees [3], with chronic pain affecting the lives of hundreds of millions [4]. Chronic pain may lead to deleterious effects on health, employment, economy, and quality of life [5]. The economic impact of chronic pain in the United States ranges from $560 billion to $635 billion a year [3], which is greater than the economic burden of heart disease, or the combined cost of cancer and diabetes.
Among all types of chronic pain, the relentless discomfort of neuropathic pain has the greatest impact on the quality of life, with approximately 3-4.5% of the global population affected [6]. Unfortunately, pain management therapies for neuropathic pain are inefficacious, problematic, or have abuse potential. Neuropathic pain is due to a dysfunction of, or damage to, a nerve or group of nerves [7]. While acute pain can be effectively managed with nonsteroidal anti-inflammatory drugs and opioids, these agents are less efficacious for chronic neuropathic pain associated with inflammation and nerve injury.
Growing evidence suggests that natural phenolic compounds play preventative and therapeutic roles in neurodegenerative diseases and inflammatory pathological states. The therapeutic potential of these bioactive compounds is due to their antioxidant and anti-inflammatory properties [8-11]. Chlorogenic acid (CGA), which is formed by the condensation of caffeic acid with quinic acid, is widely present in nature and is one of the most abundant polyphenols in the human diet [12]. CGA is found ubiquitously in plants, fruits, and vegetables [13]. The highest levels of CGA are found in green coffee beans [14, 15]. Coffee is widely consumed throughout the world and contains high levels of CGA. However, the roasting process reduces the CGA content. The CGA levels in a 200 ml cup of coffee have been reported to range from 70-350 mg [13]. It is unclear at this time if the levels of CGA present in brewed coffee made from roasted beans are of clinical significance.
Recently, CGA has been shown to have potent anti-inflammatory, antigenotoxic, and antioxidant activities [14-21]. Among these activities, the anti-inflammatory and antinociceptive effects are by far the least explored, but CGA-rich medicinal plants are used as traditional medicines to relieve pain in inflammatory processes in many countries [22-24]. In this review, we will summarize and interpret recent experiments in the field of phenolic compound research. This review summarizes the beneficial effects of CGA on inflammation and pain in vitro and in vivo.
2. ANTI-INFLAMMATORY PROPERTIES OF CHLOROGENIC ACID
Prolonged dysregulation of the immune system can lead to the development of non-communicable diseases, such as autoimmune disorders: 50 million Americans have autoimmune diseases [25]. The World Health Organization announced that the frequency of these diseases is accelerating across all geographic regions and socioeconomic classes. Moreover, chronic diseases are expected to account for 73% of all deaths and 60% of the global burden of disease by 2020 [26]. Immune system dysregulation has been implicated in numerous disorders. Cardiovascular diseases, cancer, chronic obstructive pulmonary disease, and type 2 diabetes are four of the most prominent chronic diseases, which may result from, or lead to, inflammation and/or oxidative stress. A considerable amount of literature suggests that oxidative stress and inflammation contribute to over 100 diseases, including arthritis, meningitis, lupus, multiple sclerosis, and Alzheimer’s disease [27].
Plant-based immunomodulators can be used to prevent inflammatory disease progression or frequency. Immunomodulators can either increase or decrease the magnitude of the immune response. The immunomodulatory effects (cytokine secretion, phagocytosis promotion, macrophage activation, and immunoglobulin production) of herbal remedies have recently gained the attention of researchers [28]. CGA has strong immunomodulatory effects, which might represent a promising approach for inflammatory disease management.
The inflammatory process has three major components: hemodynamic changes, leukocytic exudation, and chemical mediators with the related hormonal responses. These components include the modulation of vascular events, chemotaxis, macrophage activation, cytokine secretion, and immunoglobulin production [29, 30]. Inflammatory stimuli induce gene expression of cytokines, initiating the inflammatory response. Tumor necrosis factor-alpha (TNF-α) is a major cytokine involved in the initiation of the inflammatory response. Its actions include induction of other cytokines, such as interleukin (IL)-1 and IL-6, priming of polymorph nuclear leukocytes, and up-regulation of adhesion molecules. Stimulation of macrophages/monocytes, fibroblasts, and epithelial cells with IL-1β and TNF-α leads to prostaglandin (PG)-E2 production via arachidonic acid metabolism, which consequently leads to edema. Therefore, impairment of TNF-α synthesis/release or impairment of other pro-inflammatory cytokines are alternative methods of PGE2 inhibition, which can prevent edema by anti-inflammatory effects [31].
Various inflammatory stimuli and inflammatory cells such as macrophages can activate the nuclear factor kappa B (NF-κB) signaling pathway. This activation can lead to inflammation and cell proliferation [32, 33]. Following the translocation of NF-κB into the nucleus, the expression of specific genes involved in inflammation or immunomodulation is increased, leading to a cell survival response or cellular proliferation [34]. NF-κB activation also induces the transcription of inducible nitric oxide synthase, leading to nitric oxide (NO) production. NO is a pro-inflammatory mediator that contributes to the pathogenesis of inflammatory disorders [35, 36]. Overproduction of these pro-inflammatory mediators causes inflammation. Therefore, the characterization of new substances that modulate NF-κB and overproduction of pro-inflammatory mediators is a topic of considerable research interest [37]. CGA and its metabolites are being actively studied in the context of inflammation and related disorders caused by dysregulation of NF-κB.
2.1. CGA is Anti-inflammatory in Animal Models of Sepsis
During septic shock, lipopolysaccharide (LPS), the major component of external membranes in gram-negative bacteria, induces expression of TNF-α, IL-1, IL-6, NO, PGE2, and other pro-inflammatory mediators [38, 39]. Shan et al. showed that CGA significantly decreased LPS-induced cyclooxygenase (COX)-2 up-regulation and inhibited PGE2 release in RAW264.7 cells via attenuation of NF-κB and c-Jun N-terminal kinase (JNK)/activation protein-1 (AP-1) signaling pathway activation [40]. These results suggest that CGA may have anti-inflammatory effects by inhibiting PGE2 production. Further, CGA, in a concentration-dependent manner, is able to strongly inhibit the production of TNF-α and IL-6 by human peripheral blood mononuclear cells stimulated by staphylococcal exotoxins [41]. Further, CGA inhibits the synthesis of other mediators, such as IL-1β, interferon-γ, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α [41-43].
Additional mechanisms of CGA-mediate inhibition of inflammation include modulation of toll-like receptors (TLRs) and high mobility group box 1 in animal models of sepsis. TLRs are known to interact with pro-inflammatory mediators that are released during ischemia, and this interaction activates the innate immune system [44]. As shown in Fig. 1, the mechanisms underlying the action of CGA include attenuation of TLR-4 expression and suppression of sepsis-induced signaling pathways, such as JNK, p38-mitogen-activated protein kinase (MAPK) and NF-κB [45-47], suggesting that CGA modulates cytokine and chemokine release, and suppresses immune cell apoptosis [48]. In vivo investigations corroborate these results; chronic oral administration of CGA prevents acetaminophen-induced hepatotoxicity: TLR-3/4 and MyD88 expression, expression of phosphorylated p65 subunit of NF-κB, and serum levels and liver mRNA expression of TNF-α, IL-1β, IL-6, and monocyte chemoattractant protein-1, are all reduced [43]. Moreover, intraperitoneal injection of CGA lowers neutrophil infiltration in the liver through the modulation of TLR-4, TNF-α, and NF-κB signaling in LPS-treated mice [46]. CGA’s protective mechanisms extend to include inhibition of phosphorylation-mediated activation of JNK, p38-MAPK, extracellular signal-regulated kinase (ERK) 1/2, and upstream molecular signals [49]. High mobility group box 1, another important inflammatory factor, is released largely during immune system activation and inflammatory damage [50, 51]. Administration of CGA attenuates systemic HMGB-1 accumulation in vivo and prevents mortality induced by endotoxemia and polymicrobial sepsis [45, 52].
Fig. (1).
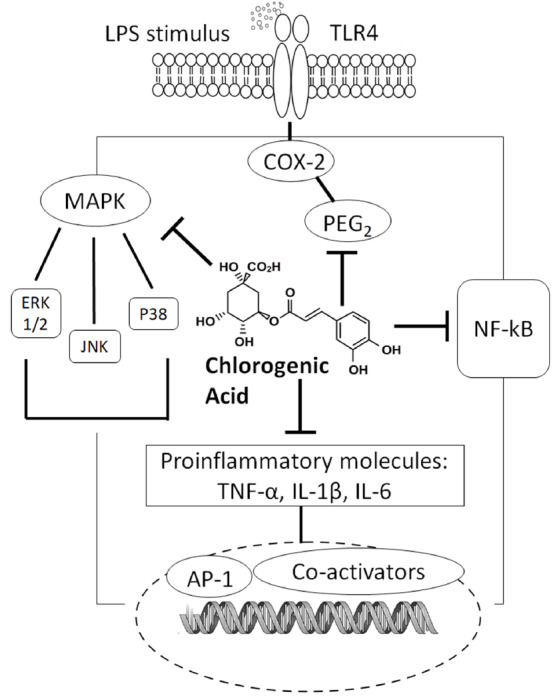
Possible mechanisms of chlorogenic acid in regulating inflammation. CGA has a broad range of anti-inflammatory effects relevant to pain and other health outcomes (neuroprotective, gastroprotective, renoprotective, antirheumatic, and anti-atherothrombotic effects). CGA and its metabolites appear to contribute to its pain-alleviating mechanisms by interrelated anti-inflammatory mechanisms.
The anti-inflammatory activity of CGA in animal models of sepsis may be related to its antioxidative properties [49, 53-56]. For example, as a free radical scavenger and antioxidant, CGA prevents chemically-induced damage in the liver and in primary cortical neurons by reducing oxidative damage and apoptosis [49, 55]. Integrating intracellular pathways with the inhibition of NF-kB activation and/or inhibition of pro-inflammatory cytokine release are similar components of both antioxidant and anti-inflammatory mechanisms [57-59].
2.2. CGA May Be Protective in Neurodegenerative Inflammatory Disease Models
Inflammation is a major contributor to the pathogenesis of chronic diseases such as diabetes, neurodegeneration, and cancer [60, 61]. CGA may have beneficial health outcomes (such as neuroprotective, gastroprotective, renoprotective, antirheumatic, and anti-atherothrombotic effects) against many inflammatory disorders [62-65]. The mechanism by which CGA is beneficial in inflammatory disease states may be due to its immunosuppressive effects; pro-inflammatory factors released by activated microglia may contribute to the progression of neurodegenerative diseases, whereas CGA prevented neurotoxicity caused by microglial activation and ultimately improved survival of dopaminergic neurons [66]. Notably, CGA protects dopaminergic neurons against neuro-inflammatory conditions associated with Alzheimer’s disease [67]. In addition, it has been reported that CGA was able to improve some measures of cognitive function [68]. CGA may show neuroprotective effects in the case of pro-inflammatory factor-mediated neurodegenerative disorders [66]. Possible mechanisms of immunosuppression in a model of LPS-stimulated primary microglial activation include suppressing: NO production, TNF-α release, and NF-κB translocation. Oral administration of coffee extract and CGA has been reported to protect against retinal degeneration as well [64, 65]. While optimistic, the results of these clinical studies would need to be replicated by independent researchers in order to conclude that CGA is a viable treatment for Alzheimer’s disease or other degenerative diseases.
Bioactive polyphenolic compounds have the potential to act as pro-oxidants under certain circumstances [69]. Notably, the concentration of the compounds determines their antioxidant or pro-oxidant activity. Pro-oxidant activity can induce damage to biomolecules such as DNA, lipids, or proteins [70]. Excess CGA intake for prolonged periods may cause pro-oxidative effects [71] on the liver, kidneys, and bone marrow [20, 56]. Intravenous CGA at high doses has been reported to cause DNA damage [72] and a range of inflammatory reactions in rats [73]. In humans, high doses of CGA can cause cardiovascular complications [74], headache, diarrhea, emesis, asthma, pruritus, anxiety, liver damage, and kidney damage [75-77]. However, CGA doses required to have pro-oxidative effects or induce an inflammatory response were in excess of the typical human diet. Therefore, dietary supplementation with CGA should be in moderation for optimal health.
2.3. CGA Metabolites Exhibit Anti-Inflammatory Properties
CGA has a broad range of anti-inflammatory effects relevant to pain. In vitro studies suggest that possible mechanisms of CGA’s anti-inflammatory effects involve inhibition of: NF-kB [40, 41, 47, 78], TNF-α [47], IL-1β [47], IL-6 [41], PGE2 [40, 41] and JNK/AP-1 signaling pathway activation [40]. Further, CGA inhibits the synthesis of other mediators, such as interferon-γ, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α [41-43]. Moreover, CGA metabolites caffeic acid [37, 79, 80] and ferulic acid [81, 82] have also been reported to have anti-inflammatory effects. These findings indicate that the anti-inflammatory effects of CGA are not limited to the parent molecule. The metabolites of CGA appear to contribute to its pain-alleviating mechanisms by interrelated anti-inflammatory mechanisms.
Caffeic acid is a major metabolite of CGA with extensively-documented anti-inflammatory activity. Caffeic acid decreased NO and PGE2 production and downregulated TNF-α, COX-2, and inducible nitric oxide synthase levels in LPS-stimulated RAW264.7 cells. Caffeic acid also suppressed the nuclear translocation of AP-1 family proteins and the related upstream signaling cascade composed of IL-1 receptor-associated kinase (IRAK)-1, IRAK4, transforming growth factor β-activated kinase 1, MAPK kinase 4/7, and JNK [80]. These results indicate that the antioxidative effect of caffeic acid and its restoration of redox balance are responsible for caffeic acid’s anti-inflammatory action [37].
Detailed examination of the minor CGA metabolite sinapic acid also shows anti-inflammatory properties. Sinapic acid was able to inhibit LPS-induced expression of NO, PGE2, TNFα, IL-1β, and NF-κB in a dose-dependent manner in vitro [83]. However, Jin et al. showed that a single dose of CGA was not sufficient to alter TNF-α levels in the supernatant of LPS-stimulated RAW cells [84]. On the other hand, the herbal formula of Rosae Multiflorae Fructus, which contains a remarkable amount of CGA, demonstrated anti-inflammatory properties in LPS-stimulated RAW264.7 cells due to its regulatory effects on the NF-κB and MAPK signaling pathways [78]. Ferulic acid, another metabolite of CGA, exerts anti-inflammatory effects via similar mechanisms as CGA [79, 82]. Such variations in findings between studies on metabolites and herbal extracts may be due to the use of different dosing regimens.
Table 1 lists a summary of CGA's anti-inflammatory effects in vitro. Table 2 summarizes CGA's anti-inflammatory effects in vivo.
Table 1. Chlorogenic acid modulates pain and inflammation in vitro.
| Compound | Method | Model | Dose | Response | Refs. | |
|---|---|---|---|---|---|---|
| Anti-inflammatory effects | CGA | Staphylococcal exotoxin-stimulated inflammation | Human peripheral blood mononuclear cells |
0.2, 2, 20, and 200 µg/ml | Anti-inflammatory; inhibited production of TNF-α and IL-6 |
[41] |
| CGA | LPS-induced inflammation | RAW264.7 cells | 37.5 µg/ml | Anti-inflammatory; decreased LPS-induced cyclooxygenase (COX)-2 upregulation, inhibited PGE2 release, attenuated activation of NF-κB and JNK/AP-1 signaling pathways |
[40] | |
| CGA | LPS-induced inflammation | Primary culture of microglia | 1-4 mM | Improved survival of dopaminergic neurons; suppressed NO production, TNF-α release, and NF-κB translocation |
[66] | |
| Caffeic acid | LPS-induced inflammation | RAW264.7 cells | 100-400 µM | Decreased NO and PGE2 production; downregulated TNF-α, COX-2, and iNOS levels; suppressed nuclear translocation of AP-1 family proteins, IL-1 receptor, IRAK-1, IRAK4, TGF-β,TAK1, MAPKK-4/7, JNK |
[80] | |
| Rosae Multiflorae Fructus extract | LPS-induced inflammation | RAW264.7 cells | 25, 50, 100, and 200 µg/ml | Anti-inflammatory; regulatory effects on NF-κB and MAPK signaling pathways |
[78] | |
| Pain modulatory effects | CGA | Whole-cell patch-clamp recordings in an inflammatory environment | Rat trigeminal ganglion neurons | 0.2 mmol | Promoted Kv channels activation and inactivation under inflammatory conditions | [122] |
| CGA | Acid stretch test | Rats; rat dorsal root ganglia neurons | 0.01, 0.1, 1, and 10 µM |
Ameliorated the acidosis-evoked pain; inhibited acid-sensing ion channels in rat dorsal root ganglia neurons |
[126] | |
| CGA | Extracellular single-unit recordings |
Rat trigeminal spinal nucleus caudalis neurons (SpVc) | 0.1-10 mM | Local CGA injection into the periphery suppressed SpVc neuron excitability | [127] | |
| CGA | Whole-cell patch-clamp recordings | Rat trigeminal ganglion neurons |
0.2 and 1 mmol |
Enhanced Kv activities | [121] |
Table 2. Chlorogenic acid modulates pain and inflammation in vivo in rodent models.
| Compound | Method | Model | Dose | Response | Refs. | |
|---|---|---|---|---|---|---|
| Anti-inflammatory effects | CGA | Piroxicam (NSAID)-induced ulcer | Rats | 5, 25, and 50 mg/kg | Gastroprotective effect without altering the secretory functions; inhibited neutrophil migration; restored levels of antioxidant enzymes; blocked increase in TNF-α and leukotriene β4; did not restore prostaglandin levels | [148] |
| CGA | Acetaminophen-induced hepatotoxicity | Mice | 10, 20, and 40 mg/kg | Reduced acetaminophen-induced TLR-3/4 and MyD88 expression; attenuated serum levels and liver mRNA expression of TNF-α, IL-1β, and IL-6 | [43] | |
| CGA | LPS-induced inflammation | Mice | 50 mg/kg | Decreased neutrophil infiltration in the liver; modulated TLR-4, TNF-α, and NF-κB signaling | [44] | |
| Pain modulatory effects | CGA | CCI | Rats | 0.5, 1, and 2 mg in 10 μL | Reduced mechanical and cold hyperalgesia; no effect on thermal hyperalgesia | [96] |
| CGA | Streptozotocin-induced diabetic neuropathy | Rats | 100 mg/kg | Antihyperalgesic; chronic treatment reduced diabetes-induced hyperalgesia | [94] | |
| CGA | CCI | Rats | 50, 100, and 200 mg/kg | Inhibited mechanical hyperalgesia; antihyperalgesic activity without impairing motor coordination | [95] | |
| CGA | Carrageenan-induced paw edema, formalin test | Rats | 10, 50, and 100 mg/kg | Anti-inflammatory and anti-nociceptive | [17] | |
| Caffeic acid | Carrageenan-induced inflammation | Mice and Rats | 200 mg/kg | Antihyperalgesic; reduced neutrophil-, free radical-, and nitric oxide-mediated hypernociception | [114] | |
| Ferulic acid | CCI | Rats | 50 mg/kg | Antihyperalgesic; decreased P2X3 receptor-mediated primary afferent sensitization | [115] | |
| CGA isolated from aqueous fraction of Bidens pilosa | LPS-induced knee joint inflammation, CFA-induced arthritis |
Rats | 2.5, 5, 10, 20, and 40 mg/kg | Anti-inflammatory; inhibited TNF-α and IL-1β production | [97] | |
| Methanol fraction of Cheilanthes farinose | Carrageenan-induced inflammation, formalin test, tail-flick test | Mice | 200 and 400 mg/kg | Strong anti-inflammatory and antinociceptive | [24] | |
|
Cnidium officinale extracts |
Spared nerve injury, plantar incision, and ovariectomy rat model of menopausal pain | Rats | 30, 100, and 300 mg/kg | Antihyperalgesic; attenuated hypersensitivity in all pain models; decreased mechanical hyperalgesia; inhibited proinflammatory cytokines and calpain-3 in dorsal root ganglia neurons |
[91] | |
| Mansoa alliacea extract | CFA-induced inflammatory pain model | Mice | 10 and 100 mg/kg | Antihyperalgesic and antinociceptive; reversed thermal hyperalgesia, but did not reduce the CFA-induced edema nor myeloperoxidase activity | [93] | |
| Ethanolic fraction of Urtica circularis |
Formalin test, hot plate test, acetic acid stretch test | Mice | 10–300 mg/kg (intraperitoneal) 250 mg/kg and 500 mg/kg (per os) | Antinociceptive in the acid stretch test and formalin test | [20] |
3. EFFECTS OF CHLOROGENIC ACID IN INFLAMMATORY AND NEUROPATHIC PAIN MODELS
Primary afferents in the periphery transduce information about noxious stimuli by synapsing onto second-order neurons in the dorsal horn of the spinal cord. The second- order neurons decussate the spinal cord, ascend, and project to the thalamus. In the thalamus, the second-order neurons synapse onto the third-order neurons, which project to the somatosensory cortex. Modulation of any part of the spinothalamic pathway, especially the dorsal horn, can result in changes in pain transmission, and ultimately pain perception [7]. For example, peripheral nerve injuries cause neural plasticity, which leads to central sensitization of the spinal neurons and enhancement of nociceptive transmission [7, 85-87].
Both peripheral and central sensitizations are involved in neuropathic pain, and the mechanisms are very complex. Neuropathic pain results from tissue damage, inflammation, or injury of the nervous system. Neuropathic pain is characterized by three sensory abnormalities: (i) increased sensitivity to painful stimuli (hyperalgesia); (ii) perception of innocuous stimuli as painful (allodynia); and (iii) spontaneous pain [88]. Nerve injury leads to an inflammatory response, prompting the release of several ions, histamine, TNF-α, ATP, PGs, leukotrienes, cytokines, and nerve growth factor. During this inflammatory response, macrophages release a variety of inflammatory mediators, and the expression of these mediators is regulated by different intracellular signaling pathways, such as NF-κB [89]. This cocktail of mediators serves as a mechanism of enhanced inflammatory response to an injured nerve and contributes to neuropathic pain [88].
Ethnopharmacological use of medicinal plants with anti-inflammatory properties may be a starting point for the discovery of new classes of analgesics for neuropathic pain. As we have explained, neuropathic pain is modulated by the immune and inflammatory systems. Therefore, there has been considerable interest in investigating the antinociceptive and anti-inflammatory effects of phenolic compound-rich plants, such as those with CGA. For example, Cnidium officinale is traditionally used in Korea to attenuate pain and increase stamina [90]. Postoperative, neuropathic, and menopausal pain models confirmed that C. officinale extracts attenuate hypersensitivity [91]. Further, in the rat spared nerve injury model, C. officinale inhibited the induction of the proinflammatory cytokines and calpain-3 in dorsal root ganglion neurons, which may be due to its CGA and ferulic acid contents. Similarly, Mansoa alliacea, native to the Brazilian Amazon, is used in the treatment of fever, convulsions, and head and neck pain [92]. Phytochemical screening of M. alliacea revealed the presence of several phenolic compounds, such as ρ-coumaric acid, luteolin, apigenin, ferulic acid, and CGA. M. alliacea extracts exhibit antinociceptive activity in the CFA model of inflammatory pain model, which may be δ-opioid receptor-mediated, in addition to CGA’s anti-inflammatory properties [93]. The consensus of the literature is that extracts derived from CGA-containing plants produce antinociception in animal models [22-24, 93]. While pure CGA is not effective for the treatment of acute pain [94], it may possess antinociceptive activity in tonic and inflammatory pain models, such as formalin- and carrageenan-induced pain [17] and chronic neuropathic pain models [94-96]. CGA’s effectiveness in chronic neuropathic pain models may be due to CGA’s anti-inflammatory properties. Dos Santos et al. suggested that the antinociceptive effect of CGA in inflammatory pain is associated with its inhibitory activity on peripheral TNF-α and NO [17]. Other studies using different pain models reported that CGA-rich fractions of the medicinal plants Urtica urens, Urtica circularis, and Cheilanthes farinosa exhibit antinociceptive effects, which were attributed to their CGA content [22-24]. These studies posited that the effect of CGA on various animal models of pain was due to CGA’s anti-inflammatory activity. Many authors noted that CGA has an inhibitory effect on peripheral synthesis or release of select inflammatory mediators, including TNF-α, NO, and several interleukins [17, 22, 97-99].
Chronic constrictive nerve injury (CCI) is a peripheral neuropathic pain model that initiates an inflammatory cascade [100, 101]. Both acute and chronic schedules of intraperitoneal CGA treatment inhibits mechanical hyperalgesia in CCI-induced neuropathic pain [95]. Moreover, CGA-mediated antinociception did not affect motor performance, suggesting that CGA is not psychoactive [95]. While intrathecal CGA had no effect on thermal hyperalgesia, CGA was able to reduce mechanical and cold hypersensitivity in the rat CCI model [96]. Histopathological analysis of the sciatic nerve confirmed that the antihyperalgesic effects of CGA in the CCI model was due to attenuation of the inflammatory cascade [95].
The results of these CCI studies suggest that the site of action of CGA’s antihyperalgesic effects may be in spinal or supraspinal pathways. Supporting the hypothesis of a central site of action, intrathecal studies with a wide array of compounds determined that CGA’s effects are, at least in part, mediated spinally by gamma-amino butyric acid A (GABAA) receptors. GABAergic transmission in the spinal cord has been demonstrated to modulate pain processing [102, 103]. GABAA receptor agonists have been shown to attenuate hyperalgesia and allodynia induced by nerve injury [104-106]. The antihyperalgesic effects of CGA were partially reversed by GABAA receptor antagonist bicuculline [106]. This finding suggests that CGA is effective against mechanical and cold hyperalgesia due to its activation of GABAergic transmission in the spinal cord. Conversely, a variety of antagonists, such as strychnine (glycinergic), yohimbine (adrenergic), naloxone (mu opioidergic), and methysergide and ondansetron (serotonergic) failed to reverse the antihyperalgesic effects of CGA [96]. The antinociceptive effects of CGA in inflammatory and neuropathic pain are also related to its inhibitory effects on the release or synthesis of inflammatory mediators, such as TNF-α, NO, and ILs [17, 97, 107]. While the exact mechanisms underlying CGA’s role against neuro-inflammation are largely unknown, one possible explanation is linked to the suppressor role of CGA on the release of NO from LPS/interferon-γ-stimulated C6 astrocyte cells [17].
Oxidative stress is a key mediator in all phases of painful neuropathy, including the development, maintenance, and resolution of neuropathy [108, 109]. Peripheral nerve injury initiates an inflammatory process, which is often associated with free radical damage. In vivo and in vitro studies have shown that the antioxidant activities of CGA occur by inhibiting the formation of reactive oxygen species (ROS) or by scavenging them [18, 98]. Thus, CGA may have a beneficial and important role in the prevention of oxidative stress. Recent studies suggest that ROS may contribute to the development of neuropathic and inflammatory pain [88, 110-113]. Additionally, various phenolic antioxidants have been shown to exhibit antinociceptive activities in ROS-related pain [107, 114, 115]. Presumably, CGA exhibits this activity due to its antioxidant activities of inhibiting and/or scavenging ROS.
3.1. Effects of Chlorogenic Acid on Ion Channels Involved in Neuropathic Pain
Voltage-gated potassium channels (Kvs) are physiological regulators of membrane potential in sensory neurons. Kv 1.4 channels present on small diameter nociceptive neurons (Aδ and C fibers) in the dorsal root ganglia [111] regulate the activity of those neurons [117]. The inhibition of the Kv 1.4-type channel leads to hyper-excitability and hyperalgesia [118]. Clinically, Kv malfunctions lead to neuronal excitability in various pathologic conditions, such as chronic pain, migraine, and multiple sclerosis [119, 120]. Notably, CGA strongly enhances Kv activities in rat trigeminal ganglion neurons during treatment naïve [121] and PGE2-induced inflammatory conditions [116], resulting in a gradual decrease of the excitability of neurons involved in signaling neuropathic and inflammatory pain [118, 121, 123-125]. Hyperpolarization of sensory ganglia may be an alternative explanation for CGA’s antinociceptive effects in animal models. Therefore, Kvs are a putative therapeutic target for inflammation and neuropathic pain disorders [121, 125].
In addition to Kvs, peripheral acid-sensing ion channels have been suggested to be involved in various pain conditions. CGA was able to inhibit acid-sensing ion channels in rat dorsal root ganglia neurons and trigeminal ganglion neurons [126, 127], which indicates yet another novel peripheral antinociceptive mechanism of CGA. Further, peripheral application of CGA attenuated acidosis-induced pain [126] and trigeminal nociceptive pain [128].
3.2. Use of CGA and Active Metabolites as Complementary and Alternative Medicine for the Treatment of Rheumatoid Arthritis
Phenolic compounds and flavonoids are widely used in complementary and alternative medicine as treatments for arthritic diseases [128-130]. Rheumatoid arthritis (RA) is the most common inflammatory arthritis that leads to disability. Prolonged RA affects multiple joints, which are often affected in a fairly symmetrical fashion. Due to pathological progress, the inflammatory activity causes tendon tethering and erosion, and destruction of the joint surfaces, leading to an impaired range of movement and deformity [131]. Herbal remedies have been used in the treatment of the fever, pain, and inflammation related to RA in Asian and Mediterranean countries [131-134]. Herbal remedies aim to alleviate inflammatory mediators of RA, such as TNF-α, IL-1, IL-17, PGE2, and NO.
As briefly mentioned above, the first stage in the metabolism of CGA is hydrolysis to caffeic and quinic acids. Caffeic acid is subsequently metabolized to ferulic and vanillic acids [98]. Caffeic acid exerts antihyperalgesic activity in carrageenan-induced inflammatory pain in mice and rats by reducing neutrophils, free radicals, and nitric oxide-mediated hypernociception [114]. In addition, sodium ferulate, the sodium salt of ferulic acid, exhibits antihyperalgesic effects on CCI-induced neuropathic pain by decreasing P2X3 receptor-mediated primary afferent sensitization [107, 115]. These findings suggest that the antinociceptive effects of CGA may be a result of action by CGA itself and/or by its metabolites. Therefore, either CGA or its metabolites may alleviate RA.
Porana sinensis, Erycibe obtusifolia, and Erycibe schmidtii are CGA-containing plants widely used in traditional medicine for the treatment of joint pain and RA. The efficacy of CGA extracts from these plants has recently been validated in rodent models of tonic, visceral, and inflammatory pain [135]. The mechanism underlying these effects has been proposed to involve the inhibitory activity of CGA on PGE2 synthesis [135]. Further, CGA isolated from the aqueous fraction of Bidens pilosa showed anti-inflammatory effects in LPS-induced knee joint inflammation and complete freund's adjuvant induced arthritis by inhibiting TNF-α and IL-1β production [97].
Hypericum perforatum (H. perforatum) is used in traditional medicine in Europe as an agent to reduce inflammation and promote healing. H. perforatum is commercially available for therapeutic use in Brazil and strongly modulates inflammation and oxidative stress [136]. H. perforatum and other hypericum species have a well-documented antinociceptive role in rodents in different pain models [137-142]. The Iowa Center for Research on Botanical Dietary Supplements investigated the bioactivities of the constituents extracted from H. perforatum (including hyperforin, hypericin, pseudohypericin, quercetin, quercitrin, isoquercitrin, rutin, amentoflavone and CGA) in regards to their role in LPS-induced PGE2 production [143]. While extracts did not produce high enough concentrations of the target compounds to inhibit inflammation, amentoflavone, CGA, pseudohypericin, and quercetin inhibited LPS-induced PGE2 activity in their pure forms [143, 144]. CGA was less effective than the other bioactive compounds identified in H. perforatum extracts [144]. However, CGA reduced the proliferation of IL-1β-induced fibroblast-like synoviocytes by regulating the activation of the NF-κB and Janus-family tyrosine kinase/signal transducer and activator of transcription-signaling pathways (JAK/STAT) [145, 148]. Together, these studies suggest that pure CGA, or in combination with other bioactive extracts, may be a treatment for arthritis.
Much like the reports of CGA’s efficacy in neuroinflammatory diseases [62-65], the findings of these complementary and alternative medicine studies may be overly optimistic; double-blind, placebo-controlled clinical trials are needed in order to confirm the validity of CGA as a putative treatment for human pain conditions. One randomized placebo-controlled trial demonstrated that oral consumption of either CGA-weak (420 mg CGA) and CGA-rich (780 mg CGA) coffee was sufficient to increase plasma antioxidant capacity greater than baseline levels, which was greater than placebo [146]. Similar results were reported by Corrêa et al. [147], who found increased plasma antioxidant capacity after subjects drank coffee for four weeks. In a metabolic analysis, coffee consumption led to an increase in serum coffee-derived compounds, such as caffeine, chlorogenic acid, and caffeic acid metabolites. Significant changes were also observed for serum concentrations of interleukin IL-18, 8-isoprostane, and adiponectin. These observations suggest that coffee consumption may have therapeutic utility as an antioxidant, though the clinical efficacy of CGA for the treatment and management of pain in humans remains to be elucidated.
Ex vivo pain-related experiments are summarized in Table 1. The effects of CGA on in vivo pain models are summarized in Table 2.
CONCLUSION
As summarized in Fig. 2, CGA has the therapeutic potential to act against inflammatory and neuropathic pain via a variety of mechanisms; however, CGA does not alleviate acute pain. The anti-inflammatory, antioxidant, neuroprotective, and neurotrophic activities of CGA most likely underlie its antinociceptive effects. Furthermore, CGA-mediated antinociception does not affect motor performance, suggesting that CGA is not psychoactive.
Fig. (2).
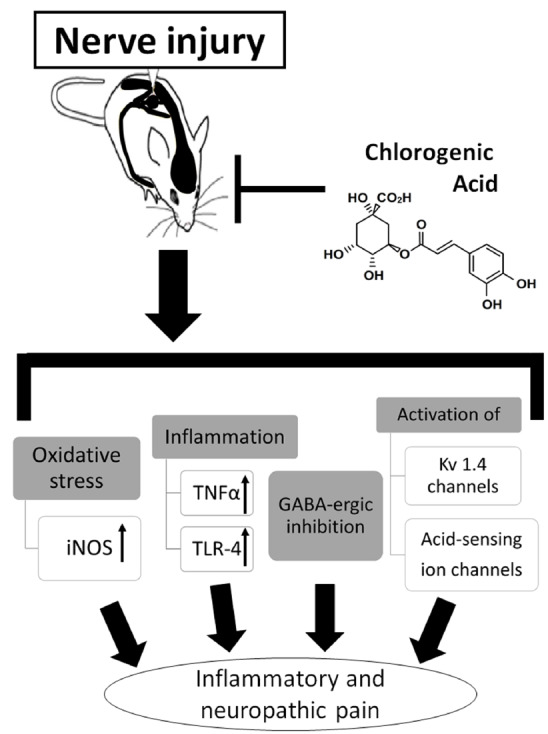
Possible mechanisms of chlorogenic acid in regulating inflammatory and neuropathic pain. CGA inhibits inflammatory and neuropathic pain; however, CGA does not alleviate acute pain. The anti-inflammatory, antioxidant, neuroprotective, and neurotrophic activities of CGA most likely underlie its antinociceptive effects. Furthermore, CGA-mediated antinociception does not affect motor performance, suggesting that CGA is not psychoactive.
Non-addictive pain treatment options with fewer side effects are needed in order to curb the ever-increasing wave of pain patients who become opioid addicts. The integration of ethnopharmacology with modern technologies has the potential to lead to the discovery of new analgesic medicines. Overall, CGA is an intriguing candidate for analgesic development. Indeed, a recent patent application (Pub. No. US2019/0255007 A1) aims to capitalize on the therapeutic potential of CGA in pain. The recent surge in popularity of green coffee dietary supplements, in addition to the ubiquitous nature of CGA in the diet, warrants future studies into the extent to which CGA modulates biochemical responses in the human body.
ACKNOWLEDGEMENTS
We apologize in advance to colleagues whose work could not be cited in this mini-review.
LIST OF ABBREVIATIONS
- AP-1
Activator protein 1 (c-jun and c-fos)
- CCI
Chronic constrictive nerve injury
- CGA
Chlorogenic acid
- COX
Cyclooxygenase
- ERK
Extracellular signal-regulated kinase
- GABA
Gamma-Aminobutyric acid
- IL
Interleukin
- IRAK
Interleukin 1 receptor-associated kinase 1
- JNK
Jun N-terminal kinase
- Kv
Voltage-gated potassium channel
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- NF-ĸB
Nuclear factor kappa B
- NO
Nitric oxide
- PG
Prostaglandin
- RA
Rheumatoid arthritis
- ROS
Reactive oxygen species
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Merskey H., Bogduk N. Classification of chronic pain, descriptions of chronic pain syndromes and definitions of pain terms. 2nd ed. Seattle: Iasp Press; 1994. [Google Scholar]
- 2.Stannard C.F., Booth S. Churchill’s Pocketbook of Pain. 2nd ed. Churchill; Livingstone: 1998. [Google Scholar]
- 3.Interagency Pain Research Coordinating Committee. National pain strategy: a comprehensive population health-level strategy for pain. https://iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf201 (Accessed May 5, 2019)
- 4.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B.H., Elliott M., Chambers W., Smith W.C., Hannaford P.C., Penny K. The impact of chronic pain in the community. Fam. Pract. 2001;18:292–299. doi: 10.1093/fampra/18.3.292. [DOI] [PubMed] [Google Scholar]
- 6.Bouhassira D., Lantéri-Minet M., Attal N., Laurent B., Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi: 10.1016/j.pain.2007.08.013f. [DOI] [PubMed] [Google Scholar]
- 7.Millan M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999;57:1–164. doi: 10.1016/S0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang W.Y., Cai Y.Z., Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential Use for cancer Prevention. Nutr. Cancer. 2009;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 9.Kim Y.C. Neuroprotective phenolics in medicinal plants. Arch. Pharm. Res. 2010;33:1611–1632. doi: 10.1007/s12272-010-1011-x. [DOI] [PubMed] [Google Scholar]
- 10.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 12.El-Seedi H.R., El-Said A.M., Khalifa S., Göransson U., Bohlin L., Borg-Karlson A.K., Verpoorte R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012;60:10877–10895. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 13.Clifford M.N. Review Chlorogenic acids and other cinnamates - nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 1999;79:362–372. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1033:AID-JSFA595>3.0.CO;2-T. [DOI] [Google Scholar]
- 14.Del Rio D., Stalmach A., Calani L., Crozier A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients. 2010;2:820–833. doi: 10.3390/nu2080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig I., Clifford M.N., Lean M.E.J., Ashihara H., Crozier A. Coffee: biochemistry and potential impact on health. Food Funct. 2014;5:1695–1717. doi: 10.1039/c4fo00042k. [DOI] [PubMed] [Google Scholar]
- 16.Abraham S.K., Schupp N., Schmid U., Stopper H. Antigenotoxic effects of the phytoestrogen pelargonidin chloride and the polyphenol chlorogenic acid. Mol. Nutr. Food Res. 2007;51:880–887. doi: 10.1002/mnfr.200600214. [DOI] [PubMed] [Google Scholar]
- 17.dos Santos M.D., Almeida M.C., Lopes N.P., de Souza G.E.P. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006;29:2236–2240. doi: 10.1248/bpb.29.2236. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y., Itagaki S., Kurokawa T., Ogura J., Kobayashi M., Hirano T., Sugawara M., Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011;403:136–138. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Gul Z., Demircan D., Bagdas D., Buyukuysal R.L. Protective effects of chlorogenic acid and its metabolites on hydrogen peroxide-induced alterations in rat brain slices: a comparative study with resveratrol. Neurochem. Res. 2016;41:2075–2085. doi: 10.1007/s11064-016-1919-8. [DOI] [PubMed] [Google Scholar]
- 20.Bagdas D., Cam, Etoz B., Gul Z., et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015;81:54–61. doi: 10.1016/j.fct.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Bagdas D., Cam, Etoz B., Inan, Ozturkoglu S., et al. Effects of systemic chlorogenic acid on random-pattern dorsal skin flap survival in diabetic rats. Biol. Pharm. Bull. 2014;37:361–370. doi: 10.1097/SAP.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 22.European Medicines Agency (EMEA) Evaluation of Medicines for Humen Use Community herbal monograph on Urtica dioca L. and Urtica urens L. Herba. Doc. 2008 Ref. EMEA/HMPC/170261/2006. [Google Scholar]
- 23.Domínguez J.A. Peuser. Buenos Aires, Argentina: 1982. Contribuciones a la Materia Médica Argentina. [Google Scholar]
- 24.Yonathan M., Asres K., Assefa A., Bucar F. In vivo antiinflammatory and anti-nociceptive activities of Cheilanthes farinosa. J. Ethnopharmacol. 2006;108:462–70. doi: 10.1016/j.jep.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.American Autoimmune related diseases association. https://www.aarda.org/ (Accessed May 5, 2019)
- 26.Integrated chronic disease prevention and control. https://www.who.int/chp/about/integrated_cd/en/ (Accessed May 5, 2019)
- 27.Lenart N., Brough D., Denes A. Inflammasomes link vascular disease with neuroinflammation and brain disorders. J. Cereb. Blood Flow Metab. 2016;36:1668–1685. doi: 10.1177/0271678X16662043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jantan I., Ahmad W., Bukhari S.N.A. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015;6:1–18. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark R.A. Cutaneous tissue repair: basic biologic considerations. I. J. Am. Acad. Dermatol. 1985;13:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- 30.Stadelmann W.K., Digenis A.G., Tobin G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998;176:26S–38S. doi: 10.1016/s0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 31.Nantel F., Denis D., Gordon R., Northey A., Cirino M., Metters K.M., Chan C.C. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br. J. Pharmacol. 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiDonato J.A., Mercurio F., Karin M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 33.Vitiello M., Galdiero M., Finamore E., Galdiero S., Galdiero M. NF-κB as a potential therapeutic target in microbial diseases. Mol. Biosyst. 2012;8:1108–1120. doi: 10.1039/c2mb05335g. [DOI] [PubMed] [Google Scholar]
- 34.Kopf M., Bachmann M.F., Marsland B.J. Averting inflammation by targeting the cytokine environment. Nat. Rev. Drug Discov. 2010;9:703–718. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 35.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 36.Zamora R., Vodovotz Y., Billiar T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S.R., Jung Y.R., Kim D.H., An H.J., Kim M.K., Kim N.D., Chung H.Y. Caffeic acid regulates LPS-induced NF-κB activation through NIK/IKK and c-Src/ERK signaling pathways in endothelial cells. Arch. Pharm. Res. 2014;37:539–547. doi: 10.1007/s12272-013-0211-6. [DOI] [PubMed] [Google Scholar]
- 38.Remick D.G., Strieter R.M., Eskandari M.K., Nguyen D.T., Genord M.A., Raiford C.L., Kunkel S.L. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am. J. Pathol. 1990;136:49–60. [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu H.Y., Wen M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 40.Shan J., Fu J., Zhao Z., Kong X., Huang H., Luo L., Yin Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009;9:1042–1048. doi: 10.1016/j.intimp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Krakauer T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002;24:113–119. doi: 10.1081/IPH-120003407. [DOI] [PubMed] [Google Scholar]
- 42.Kang T.Y., Yang H.R., Zhang J., Li D., Lin J., Wang L., Xu X. The studies of chlorogenic Acid antitumor mechanism by gene chip detection: the immune pathway gene expression. J. Anal. Methods Chem. 2013;617243 doi: 10.1155/2013/617243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zheng Z., Sheng Y., Lu B., Ji L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015;238:93–101. doi: 10.1016/j.cbi.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Bianchi M.E., Manfredi A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee C.H., Yoon S.J., Lee S.M. Chlorogenic acid attenuates high mobility group box 1 (HMGB1) and enhances host defense mechanisms in murine sepsis. Mol. Med. 2012;18:1437–1448. doi: 10.2119/molmed.2012.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y., Chen J., Yu X., Tao W., Jiang F., Yin Z., Liu C. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm. Res. 2010;59:871–877. doi: 10.1007/s00011-010-0199-z. [DOI] [PubMed] [Google Scholar]
- 47.Yun N., Kang J.W., Lee S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012;23:1249–1255. doi: 10.1016/j.jnutbio.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Xie H., Chen D., Yu B., Mao X., Zheng P., Yu P., Luo Y., Luo J., He J. Chlorogenic acid improves intestinal development via suppressing Mucosa inflammation and cell apoptosis in Weaned Pigs. ACS Omega. 2018;3:2211–2219. doi: 10.1021/acsomega.7b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji L., Jiang P., Lu B., Sheng Y., Wang X., Wang Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013;24:1911–1919. doi: 10.1016/j.jnutbio.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Gong Y., Jin X., Wang Q.S., Wei S.H., Hou B.K., Li H.Y., Zhang M.N., Li Z.H. The involvement of high mobility group 1 cytokine and phospholipases A2 in diabetic retinopathy. Lipids Health Dis. 2014;13:156. doi: 10.1186/1476-511X-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nogueira-Machado J.A., de Oliveira Volpe C.M. HMGB-1 as a target for inflammation controlling. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012;6:201–209. doi: 10.2174/187221412802481784. [DOI] [PubMed] [Google Scholar]
- 52.Park S.H., Baek S.I., Yun J., Lee S., Yoon D.Y., Jung J.K., Jung S.H., Hwang B.Y., Hong J.T., Han S.B., Kim Y. IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. J. Immunol. 2015;194:1122–1130. doi: 10.4049/jimmunol.1402101. [DOI] [PubMed] [Google Scholar]
- 53.Lou Z., Wang H., Zhu S., Ma C., Wang Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011;76:398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- 54.Shibata H., Sakamoto Y., Oka M., Kono Y. Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci. Biotechnol. Biochem. 1999;63:1295–1297. doi: 10.1271/bbb.63.1295. [DOI] [PubMed] [Google Scholar]
- 55.Kim J., Lee S., Shim J., Kim H.W., Kim J., Jang Y.J., Yang H., Park J., Choi S.H., Yoon J.H., Lee K.W., Lee H.J. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012;60:466–474. doi: 10.1016/j.neuint.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Bagdas D., Gul N.Y., Topal A., Tas S., Ozyigit M.O., Cinkilic N., Gul Z., Etoz B.C., Ziyanok S., Inan S., Turacozen O., Gurun M.S. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn Schmiedebergs Arch. Pharmacol. 2014;387:1101–1116. doi: 10.1007/s00210-014-1034-9. [DOI] [PubMed] [Google Scholar]
- 57.Domitrović R., Jakovac H., Romić Z., Rahelić D., Tadić Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J. Ethnopharmacol. 2010;130:569–577. doi: 10.1016/j.jep.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 58.Hwang S.J., Kim Y.W., Park Y., Lee H.J., Kim K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014;63:81–90. doi: 10.1007/s00011-013-0674-4. [DOI] [PubMed] [Google Scholar]
- 59.Shi H., Dong L., Jiang J., Zhao J., Zhao G., Dang X., Lu X., Jia M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology. 2013;303:107–114. doi: 10.1016/j.tox.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y., Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J. Immunother. 2012;35:299–308. doi: 10.1097/CJI.0b013e3182518e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osborn O., Olefsky J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 62.Chen W.P., Wu L.D. Chlorogenic acid suppresses interleukin-1β-induced inflammatory mediators in human chondrocytes. Int. J. Clin. Exp. Pathol. 2014;7:8797–8801. [PMC free article] [PubMed] [Google Scholar]
- 63.Bagdas D., Cam E.B., Gul Z., Ozyigit M.O., Cinkilic N., Inan O.S., Isbil B.N., Ozluk K., Gurun M.S. Chlorogenic Acid enhances abdominal skin flap survival based on superficial inferior epigastric artery in nondiabetic and diabetic rats. Ann. Plast. Surg. 2016;77:21–25. doi: 10.1097/SAP.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 64.Jang H., Ahn H.R., Jo H., Kim K.A., Lee E.H., Lee K.W., Jung S.H., Lee C.Y. Chlorogenic acid and coffee prevent hypoxia-induced retinal degeneration. J. Agric. Food Chem. 2014;62:182–191. doi: 10.1021/jf404285v. [DOI] [PubMed] [Google Scholar]
- 65.Jang H., Choi Y., Ahn H.R., Jung S.H., Lee C.Y. Effects of phenolic acid metabolites formed after chlorogenic acid consumption on retinal degeneration in vivo. Mol. Nutr. Food Res. 2015;59:1918–1929. doi: 10.1002/mnfr.201400897. [DOI] [PubMed] [Google Scholar]
- 66.Shen W., Qi R., Zhang J., Wang Z., Wang H., Hu C., Zhao Y., Bie M., Wang Y., Fu Y., Chen M., Lu D. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012;8:487–494. doi: 10.1016/j.brainresbull.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Oboh G., Agunloye O.M., Akinyemi A.J., Ademiluyi A.O., Adefegha S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013;38:413–419. doi: 10.1007/s11064-012-0935-6. [DOI] [PubMed] [Google Scholar]
- 68.Saitou K., Ochiai R., Kozuma K., Sato H., Koikeda T., Osaki N., Katsuragi Y. Effect of chlorogenic acids on cognitive function: A randomized, double-blind, placebo-controlled trial. Nutrients. 2018;10:1337. doi: 10.3390/nu10101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poljsak B., Milisav I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012;2012:1–12. doi: 10.1155/2012/480895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yordi E.G., Pérez E.M., Matos M.J., Villares E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. Jaouad Bouayed and Torsten Bohn, IntechOpen; 2012. [DOI] [Google Scholar]
- 71.Sakihama Y., Cohen M.F., Grace S.C., Yamasaki H. Plant phenolic antioxidant and prooxidant activities: Phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 72.Burgos-Morón E., Calderón-Montaño J.M., Orta M.L., Pastor N., Pérez-Guerrero C., Austin C., López-Lázaro M. The coffee constituent chlorogenic acid induces cellular DNA damage and formation of topoisomerase I-and II-DNA complexes in cells. J. Agric. Food Chem. 2012;60:7384–7391. doi: 10.1021/jf300999e. [DOI] [PubMed] [Google Scholar]
- 73.Du W.Y., Chang C., Zhang Y., Liu Y.Y., Sun K., Wang C.S., Wang M.X., Liu Y., Wang F., Fan J.Y. High-dose chlorogenic acid induces inflammation reactions and oxidative stress injury in rats without implication of mast cell degranulation. J. Ethnopharmacol. 2013;147:74–83. doi: 10.1016/j.jep.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 74.Olthof M.R., Hollman P.C., Zock P.L., Katan M.B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am. J. Clin. Nutr. 2001;73:532–538. doi: 10.1093/ajcn/73.3.532. [DOI] [PubMed] [Google Scholar]
- 75.Onakpoya I., Terry R., Ernst E. The use of green coffee extract as a weight loss supplement: a systematic review and meta-analysis of randomised clinical trials. Gastroenterol. Res. Pract. 2011;2011(382852):1–6. doi: 10.1155/2011/382852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajan R.K., Hussein M.Z., Fakurazi S., Yusoff K., Masarudin M.J. Increased ROS scavenging and antioxidant efficiency of chlorogenic acid compound delivered via a chitosan nanoparticulate system for efficient In Vitro visualization and accumulation in human renal adenocarcinoma cells. Int. J. Mol. Sci. 2019;20:4667. doi: 10.3390/ijms20194667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barahuie F., Hussein M.Z., Arulselvan P., Fakurazi S., Zainal Z. Controlled in vitro release of the anticancer drug chlorogenic acid using magnesium/aluminium-layered double hydroxide as a nanomatrix. Sci. Adv. Mater. 2016;8:501–513. [Google Scholar]
- 78.Cheng B.C.Y., Ma X.Q., Kwan H.Y., Tse K.W., Cao H.H., Su T., Shu X., Wu Z., Yu Z. A herbal formula consisting of Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2014;153:922–7. doi: 10.1016/j.jep.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 79.Búfalo M.C., Ferreira I., Costa G., Francisco V., Liberal J., Cruz M.T., Lopes M.C., Batista M.T., Sforcin J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013;149:84–92. doi: 10.1016/j.jep.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Yang W.S., Jeong D., Yi Y.S., Park J.G., Seo H., Moh S.H., Hong S., Cho J.Y. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediators Inflamm. 2013:518183. doi: 10.1155/2013/518183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Das U., Manna K., Sinha M., Datta S., Das D.K., Chakraborty A., Ghosh M., Saha K.D., Dey S. Role of ferulic acid in the amelioration of ionizing radiation induced inflammation: a murine model. PLoS One. 2014;9: e97599. doi: 10.1371/journal.pone.0097599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Navarrete S., Alarcón M., Palomo I. Aqueous extract of tomato (Solanum lycopersicum L.) and ferulic acid reduce the expression of TNF-α and IL-1β in LPS-activated macrophages. Molecules. 2015;20:15319–15329. doi: 10.3390/molecules200815319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yun K.J., Koh D.J., Kim S.H., Park S.J., Ryu J.H., Kim D.G., Lee J.Y., Lee K.T. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J. Agric. Food Chem. 2008;56:10265–10272. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 84.Jin X.H., Ohgami K., Shiratori K., Suzuki Y., Koyama Y., Yoshida K., Ilieva I., Tanaka T., Onoe K., Ohno S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2006;82:860–867. doi: 10.1016/j.exer.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 85.Costigan M., Scholz J., Woolf C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Price T.J., Cervero F., Gold M.S., Hammond D.L., Prescott S.A. Chloride regulation in the pain pathway. Brain Res. Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woolf C.J. Overcoming obstacles to developing new analgesics. Nat. Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 88.Moalem G., Tracey D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 89.O’Neill L.A.J. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- 90.Kim H., Song M.J. Oral traditional plant-based therapeutic applications for pain relief recorded in North Jeolla province, Korea. Indian J. Tradit. Knowl. 2013;12, 4:573–584. [Google Scholar]
- 91.Lim E.Y., Kim J.G., Lee J., Lee C., Shim J., Kim Y.T. Analgesic effects of Cnidium officinale extracts on postoperative, neuropathic, and menopausal pain in rat models. Evid. Based Complement. Alternat. Med. 2019;9698727:1–8. doi: 10.1155/2019/9698727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pagani E., Santos J.F.L., Rodrigues E. Culture-Bound syndromes of a Brazilian Amazon riverine population : tentative correspondence between traditional and conventional medicine terms and possible ethnopharmacological implications. J. Ethnopharmacol. 2017;203:80–89. doi: 10.1016/j.jep.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 93.Hamanna F.R., Bruscoa I., Severo G.C., Carvalho L.M., Faccin H., Gobo L., Oliveira S.M., Rubin M.A. Mansoa alliacea extract presents antinociceptive effect in a chronic inflammatory pain model in mice through opioid mechanisms. Neurochem. Int. 2019;122:157–169. doi: 10.1016/j.neuint.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Bagdas D., Ozboluk H.Y., Cinkilic N., Gurun M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J. Med. Food. 2014;17:730–732. doi: 10.1089/jmf.2013.2966. [DOI] [PubMed] [Google Scholar]
- 95.Bagdas D., Cinkilic N., Ozboluk H.Y., Ozyigit M.O., Gurun M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2013;67:698–704. doi: 10.1007/s11418-012-0726-z. [DOI] [PubMed] [Google Scholar]
- 96.Hara K., Haranishi Y., Kataoka K., Takahashi Y., Terada T., Nakamura M., Sata T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014;723:459–64. doi: 10.1016/j.ejphar.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 97.Chauhan P.S., Satti N.K., Sharma P., Sharma V.K., Suri K.A., Bani S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 2012;26:1156–1165. doi: 10.1002/ptr.3684. [DOI] [PubMed] [Google Scholar]
- 98.Morishita H., Ohnishi M. Bioactive natural products (Part F). Stud. Nat. Prod. Chem. 2001;25:919–953. doi: 10.1016/S1572-5995(01)80024-7. [DOI] [Google Scholar]
- 99.Zhang X., Huang H., Yang T., Ye Y., Shan J., Yin Z., Luo L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury. 2010;41:746–752. doi: 10.1016/j.injury.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 100.Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 101.Muthuraman A., Singh N. Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: an evidence of anti-oxidative, anti-inflammatory, neuroprotective and calcium inhibitory effects. BMC Complement. Altern. Med. 2011;11:14. doi: 10.1186/1472-6882-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Enna S.J., McCarson K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006;54:1–27. doi: 10.1016/S1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 103.Zeilhofer H.U., Benke D., Yevenes G.E. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu. Rev. Pharmacol. Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 104.Hwang J.H., Hwang K.S., Kim J.U., Choi I.C., Park P.H., Han S.M. The interaction between intrathecal neostigmine and GABA receptor agonists in rats with nerve ligation Injury. Anesth. Analg. 2001;93:1297–1303. doi: 10.1097/00000539-200111000-00054. [DOI] [PubMed] [Google Scholar]
- 105.Hwang J.H., Yaksh T.L. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/S0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 106.Malan T.P., Mata H.P., Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–1167. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 107.Zhang A., Gao Y., Zhong X., Xu C., Li G., Liu S., Lin J., Li X., Zhang Y., Liu H., Linag S. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. 2010;1313:215–221. doi: 10.1016/j.brainres.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 108.Carrasco C., Naziroǧlu M., Rodríguez A.B., Pariente J.A. Neuropathic pain: Delving into the oxidative origin and the possible Implication of transient receptor potential channels. Front. Physiol. 2018;14:9–95. doi: 10.3389/fphys.2018.00095. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duggett N.A., Griffiths L.A., McKenna O.E., de Santis V., Yongsanguanchai N., Mokori E.B., Flatters S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience. 2016;333:13–26. doi: 10.1016/j.neuroscience.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao X., Kim H.K., Chung J.M., Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim H.K., Park S.K., Zhou J.L., Taglialatela G., Chung K., Coggeshall R.E., Chung J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Park E.S., Gao X., Chung J.M., Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 113.Yowtak J., Lee K.Y., Kim H.Y., Wang J., Kim H.K., Chung K., Chung J.M. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mehrotra A., Shanbhag R., Chamallamudi M.R., Singh V.P., Mudgal J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur. J. Pharmacol. 2011;666:80–86. doi: 10.1016/j.ejphar.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 115.Zhang A., Xu C., Liang S., Gao Y., Li G., Wei J., Wan F., Liu S., Lin J. Role of sodium ferulate in the nociceptive sensory facilitation of neuropathic pain injury mediated by P2X3 receptor. Neurochem. Int. 2008;53:278–282. doi: 10.1016/j.neuint.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 116.Rasband M.N., Park E.W., Vanderah T.W., Lai J., Porreca F., Trimmer J.S. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13373–8. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takeda M., Tanimoto T., Ikeda M., Kadoi J., Nasu M., Matsumoto S. Opioidergic modulation of excitability of rat trigeminal root ganglion neuron projections to the superficial layer of cervical dorsal horn. Neuroscience. 2004;125:995–1008. doi: 10.1016/j.neuroscience.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 118.Pearce R.J., Duchen M.R. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience. 1994;63:1041–1056. doi: 10.1016/0306-4522(94)90571-1. [DOI] [PubMed] [Google Scholar]
- 119.Judge S., Lee J.M., Bever C.T., Hoffman P.M. Voltage-gated potassium channels in multiple sclerosis: Overview and new implications for treatment of central nervous system inflammation and degeneration. J. Rehabil. Res. Dev. 2006;43:111–122. doi: 10.1682/JRRD.2004.09.0116. [DOI] [PubMed] [Google Scholar]
- 120.Du X., Gamper N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013;11:621–640. doi: 10.2174/1570159X113119990042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y.J., Lu X.W., Song N., Kou L., Wu M.K., Liu F., Wang H., Shen J.F. Chlorogenic acid alters the voltage-gated potassium channel currents of trigeminal ganglion neurons. Int. J. Oral Sci. 2014;6:233–240. doi: 10.1038/ijos.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu F., Lub X.W., Zhang Y.J., Koub L., Songa N., Wua M.K., Wanga M., Wanga H., Shen J.F. Effects of chlorogenic acid on voltage-gated potassium channels of trigeminal ganglion neurons in an inflammatory environment. Brain Res. Bull. 2016;127:119–125. doi: 10.1016/j.brainresbull.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 123.Birinyi-Strachan L.C., Gunning S.J., Lewis R.J., Nicholson G.M. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. oxicol. Appl. Pharmacol. 2005;204:175–86. doi: 10.1016/j.taap.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 124.Everill B., Kocsis J.D. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J. Neurophysiol. 1999;82:700–8. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- 125.Harriott A.M., Gold M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009;13:197–207. doi: 10.1007/s11916-009-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qu Z.W., Liu T.T., Qiu C.Y., Li J.D., Hu W.P. Inhibition of acid-sensing ion channels by chlorogenic acid in rat dorsal root ganglion neurons. Neurosci. Lett. 2014;567:35–39. doi: 10.1021/acsomega.7b01971. [DOI] [PubMed] [Google Scholar]
- 127.Kakita K., Tsubouchi H., Adachi M., Takehana S., Shimazu Y., Takeda M. Local subcutaneous injection of chlorogenic acid inhibits the nociceptive trigeminal spinal nucleus caudalis neurons in rats. Neurosci. Res. 2018;134:49–55. doi: 10.1016/j.neures.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 128.Mahomoodally M.F. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid. Based Complement. Alternat. Med. 2013:Article ID, 617459, 14. doi: 10.1155/2013/617459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Akhtar N., Haqqi T.M. Current nutraceuticals in the management of osteoarthritis: a review. Ther. Adv. Musculoskelet. Dis. 2012;4:181–207. doi: 10.1177/1759720X11436238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang M., Li K., Nie Y., Wei Y., Li X. Antirheumatoid arthritis Activities and chemical compositions of phenolic compounds-rich fraction from Urtica atrichocaulis, an endemic plant to China. Evid. Based Complement. Alternat. Med. 2012;818230 doi: 10.1155/2012/818230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang C.L.H., Or T.C.T., Ho M.H.K., Lau A.S.Y. Scientific basis of botanical medicine as alternative remedies for rheumatoid Arthritis. Clin. Rev. Allergy Immunol. 2013;44:284–300. doi: 10.1007/s12016-012-8329-8. [DOI] [PubMed] [Google Scholar]
- 132.Dion C., Haug C., Guan H., Ripoll C., Spiteller P., Coussaert A., Boulet E., Schmidt D., Wei J., Zhou Y., Lamottke K. Evaluation of the anti-inflammatory and antioxidative potential of four fern species from China intended for use as food supplements. Nat. Prod. Commun. 2015;10:597–603. [PubMed] [Google Scholar]
- 133.Sales C., Oliviero F., Spinella P. The mediterranean diet model in inflammatory rheumatic diseases. Reumatismo. 2009;61:10–14. doi: 10.4081/reumatismo.2009.10. [DOI] [PubMed] [Google Scholar]
- 134.Setty A.R., Sigal L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005;34:773–784. doi: 10.1016/j.semarthrit.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 135.Chen Z., Liao L., Zhang Z., Wu L., Wang Z. Comparison of active constituents, acute toxicity, anti-nociceptive and anti-inflammatory activities of Porana sinensis Hemsl., Erycibe obtusifolia Benth. and Erycibe schmidtii Craib. J. Ethnopharmacol. 2013;150:501–506. doi: 10.1016/j.jep.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 136.Hohmann M.S.N., Cardoso R.D.R., Fattori V., Arakawa N.S., Tomaz J.C., Lopes N.P., Casagrande R., Verri W.A. Hypericum perforatum reduces paracetamol-induced hepatotoxicity and lethality in mice by modulating inflammation and oxidative stress. Phytother. Res. 2015;29:1097–1101. doi: 10.1002/ptr.5350. [DOI] [PubMed] [Google Scholar]
- 137.Abdel-Salam O.M.E. Anti-inflammatory, antinociceptive, and gastric effects of Hypericum perforatum in rats. Sci World J. 2005;5:586–595. doi: 10.1100/tsw.2005.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Apaydin S., Zeybek U., Ince I., Elgin G., Karamenderes C., Ozturk B., Tuglular I. Hypericum triquetrifolium Turra. extract exhibits antinociceptive activity in the mouse. J. Ethnopharmacol. 1999;67:307–312. doi: 10.1016/S0378-8741(99)00071-9. [DOI] [PubMed] [Google Scholar]
- 139.Bukhari I.A., Dar A., Khan R.A. Antinociceptive activity of methanolic extracts of St. John’s Wort (Hypericum perforatum) preparation. Pak. J. Pharm. Sci. 2004;17:13–19. [PubMed] [Google Scholar]
- 140.Subhan F., Khan M., Ibrar M. Nazar-ul-Islam, Khan, A.; Gilani, A.H. Antagonism of antinociceptive effect of hydro-ethanolic extract of Hypericum perforatum Linn. by a non selective opioid receptor antagonist, naloxone. Pak. J. Biol. Sci. 2007;10:792–796. doi: 10.3923/pjbs.2007.792.796. [DOI] [PubMed] [Google Scholar]
- 141.Uchida S., Hirai K., Hatanaka J., Hanato J., Umegaki K., Yamada S. Antinociceptive effects of St. John’s wort, Harpagophytum procumbens extract and Grape seed proanthocyanidins extract in mice. Biol. Pharm. Bull. 2008;31:240–5. doi: 10.1248/bpb.31.240. [DOI] [PubMed] [Google Scholar]
- 142.Viana A.F., Heckler A.P., Fenner R., Rates S.M.K. Antinociceptive activity of Hypericum caprifoliatum and Hypericum polyanthemum (Guttiferae). Braz. J. Med. Biol. Res. 2003;36:631–634. doi: 10.1590/s0100-879x2003000500011. [DOI] [PubMed] [Google Scholar]
- 143.Hammer K.D.P., Hillwig M.L., Neighbors J.D., Sim Y.J., Kohut M.L., Wiemer D.F., Wurtele E.S., Birt D.F. Pseudohypericin is necessary for the light-activated inhibition of prostaglandin E2 pathways by a 4 component system mimicking an Hypericum perforatum fraction. Phytochemistry. 2008;69:2354–2362. doi: 10.1016/j.phytochem.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Birt D.F., Widrlechner M.P., Hammer K.D.P., Hillwig M.L., Wei J., Kraus G., Murphy P., Mccoy J., Eve S., Neighbors J.D., Wiemer D.F., Maury W.J., Jason P. Hypericum in infection: Identification of anti-viral and anti- inflammatory constituents. Pharm. Biol. 2009;47:774–782. doi: 10.1080/13880200902988645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lou L., Liu Y., Zhou J., Wei Y., Deng J., Dong B., Chai L. Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1 β -induced fibroblast-like synoviocytes through regulating the activation of NF-κB and JAK/STAT-signaling pathways. Immunopharmacol. Immunotoxicol. 2015;3973:1–9. doi: 10.3109/08923973.2015.1095763. [DOI] [PubMed] [Google Scholar]
- 146.Agudelo-Ochoa G.M., Pulgarín-Zapata I.C., Velásquez-Rodriguez C.M., Duque-Ramírez M., Naranjo-Cano M., Quintero-Ortiz M.M., Lara-Guzmán O.J., Muñoz-Durango K. Coffee consumption increases the antioxidant capacity of plasma and has no effect on the lipid profile or vascular function in healthy adults in a randomized controlled trial. J. Nutr. 2016;146:524–531. doi: 10.3945/jn.115.224774. [DOI] [PubMed] [Google Scholar]
- 147.Corrêa T.A., Monteiro M.P., Mendes T.M., de Oliveira D.M., Rogero M.M., Benites C.I., Vinagre C.G., Mioto B.M., Tarasoutchi D., Tuda V.L. Medium light and medium roast paper-filtered coffee increased antioxidant capacity in healthy volunteers: results of a randomized trial. Plant Foods Hum. Nutr. 2012;67:277–282. doi: 10.1007/s11130-012-0297-x. [DOI] [PubMed] [Google Scholar]
- 148.Shimoyama A.T., Santin J.R., Machado I.D. de Oliveira e Silva, A.M.; de Melo, I.L.P.; Mancini-Filho, J.; Farsky, S.H.P. Antiulcerogenic activity of chlorogenic acid in different models of gastric ulcer. Naunyn Schmiedebergs Arch. Pharmacol. 2013;386:5–14. doi: 10.1007/s00210-012-0807-2. [DOI] [PubMed] [Google Scholar]


