Abstract
Background. Sleep is important for consolidation of motor learning, but brain injury may affect sleep continuity and therefore rehabilitation outcomes. Objective. This study aims to assess the relationship between sleep quality and motor recovery in brain injury patients receiving inpatient rehabilitation. Methods. Fifty-nine patients with brain injury were recruited from 2 specialist inpatient rehabilitation units. Sleep quality was assessed (up to 3 times) objectively using actigraphy (7 nights) and subjectively using the Sleep Condition Indicator. Motor outcome assessments included Action Research Arm test (upper limb function), Fugl-Meyer Assessment (motor impairment), and the Rivermead Mobility Index. The Functional Independence Measure (FIM) was assessed at admission and discharge by the clinical team. Fifty-five age- and gender-matched healthy controls completed one assessment. Results. Inpatients demonstrated lower self-reported sleep quality (P < .001) and more fragmented sleep (P < .001) than controls. For inpatients, sleep fragmentation explained significant additional variance in motor outcomes, over and above that explained by admission FIM score (P < .017), such that more disrupted sleep was associated with poorer motor outcomes. Using stepwise linear regression, sleep fragmentation was the only variable found to explain variance in rate of change in FIM (R2adj = 0.12, P = .027), whereby more disrupted sleep was associated with slower recovery. Conclusions. Inpatients with brain injury demonstrate impaired sleep quality, and this is associated with poorer motor outcomes and slower functional recovery. Further investigation is needed to determine how sleep quality can be improved and whether this affects outcome.
Keywords: sleep, brain injury, stroke, motor, recovery, functional independence
Introduction
Sleep disturbance is a common complaint after brain injury, including stroke, with a high proportion (30%-70%) of patients presenting with impaired subjective sleep quality and meeting the criteria for at least one sleep disorder.1-4 Sleep disturbance could be resulting from direct damage to brain areas, or due to secondary effects such as being in the hospital environment, depression, anxiety or pain, and could potentially have an impact on rehabilitation through reduced engagement or impaired learning and consolidation.5
There is some evidence for improvements in sleep quality from the acute to the chronic stage of stroke6,7; however, stroke survivors at the chronic stage continue to have impaired subjective and objective sleep quality and worse quality of life than controls.8,9 Interestingly, the longer the time since stroke, the worse the perceived daytime sleepiness becomes.10 This suggests that sleep disturbance may be persistent throughout the rehabilitation period for some, and changes within this time frame in patients with different types of brain injuries are yet to be determined.
The link between sleep quality and function after stroke and brain injury is currently emerging. Siccoli et al11 demonstrated a cross-sectional correlation between the National Institute for Health Stroke Scale (NIHSS) score and wake after sleep onset (WASO), in a small sample of acute stroke patients. A larger study12 found a cross-sectional relationship between subjective sleep quality and the functional ambulation score after stroke but had no objective sleep measures. Similarly, Kalmbach et al13 found that patients with subjective difficulties initiating sleep had lower function at multiple time-points over the first 6 months of recovery from traumatic brain injury (TBI). Sleep variables, such as total sleep time, WASO and daytime napping, have also been shown to explain significant variance in Barthel Index (BI) score at the acute stage of stroke,14,15 and the percentage of sleep stages I and rapid eye movement (REM) are negatively associated with NIHSS.16
However, there is little research to indicate whether sleep quality over the rehabilitation period correlates with outcome or change in function over time, and studies that are available are somewhat inconsistent in their findings. The presence of sleep-disordered breathing at the acute stage has been found to be associated with reduced modified Rankin scale (mRS) and BI at 6 weeks poststroke17 and other studies have demonstrated that stroke patients categorized with a “poor” functional outcome have a lower sleep efficiency, less REM sleep or a reduced REM sleep latency at the acute stage than those with a better outcome.16,18,19 In contrast, Joa et al20 found no difference in the change in NIHSS or BI between patients reporting sleep disturbance at 1 month poststroke and those reporting no disturbance. They did, however, find that the group reporting no sleep disturbance had a greater improvement in the Berg Balance Scale (BBS). This was particularly evident for the moderate-severe stroke patients compared with mild (on the basis of NIHSS score at 1 week poststroke), suggesting sleep may have a greater impact on functional recovery in those who have the most relearning to achieve. The studies by Iddagoda et al4 and Joa et al20 used only subjective sleep measures and many of the studies have divided participants into groups based on outcome or the presence/absence of sleep disturbance, rather than examining both sleep quality and outcome as a continuum which may be more sensitive to differences across participants. Studies that did assess objective sleep quality as a continuum are mixed in their findings. Bakken et al15 found no correlation between sleep variables in the acute stage and BI at 6 months poststroke whereas Vock et al7 found that higher WASO or lower sleep efficiency at the acute stage poststroke was associated with worse outcome (mRS or BI score) at discharge. Similarly, Huang et al14 demonstrate that total sleep time correlates positively, and sleep latency correlates negatively, with the change in BI with rehabilitation.
As there is no clear consensus on the relationship between sleep quality measures and the rate of recovery with rehabilitation, and it is unclear how sleep quality changes over the course of rehabilitation, we sought to conduct a prospective assessment of sleep quality in neurological inpatients and explore the relationship with neurorehabilitation outcomes. We therefore assessed objective and subjective sleep quality at up to 3 time-points throughout the rehabilitation period and examined the relationship between sleep quality and motor and functional outcome measures. Specifically, we aimed to address the following questions:
Does sleep quality at a single time-point correlate with function/impairment at that time-point?
Does sleep quality change over the inpatient rehabilitation period?
Does objective sleep quality averaged over the inpatient rehabilitation period explain variance in motor outcomes over that explained by baseline function?
Does objective or subjective sleep quality averaged over the inpatient rehabilitation period explain variance in the rate of recovery in addition to covariates such as initial independence, age, and time since injury?
Methods
Participants
This was a prospective observational study, based in the Oxford Centre for Enablement Neurological Rehabilitation Unit (Oxford University Hospitals NHS Foundation Trust; June 2017 to October 2019) and the Oxfordshire Stroke Rehabilitation Unit (Oxford Health NHS Foundation Trust; May 2019 to October 2019). Potential participants were screened for eligibility by the clinical and research teams following admission and approximately weekly thereafter. Inclusion criteria were acquired brain injury with definitive onset (stroke, TBI, hemorrhage, hypoxic brain injury) requiring motor rehabilitation (upper and/or lower limb). Exclusion criteria were inability to provide informed consent, other neurological or psychiatric conditions (eg, Guillain-Barre syndrome, brain tumors, personality disorder, visual hallucinations), preexisting sleep disorder. Patients with aphasia or cognitive impairment limiting the ability to provide informed consent were considered weekly and approached only if sufficient improvement was made throughout their stay. This judgment was made by the multidisciplinary clinical team (including doctors, speech and language therapists, and psychologists). The study was approved by the National Research Ethics Service (11/H0605/12) and all participants provided written informed consent.
In total, 197 patients were screened between June 2017 and October 2019 (Figure 1). Of these, 121 were found to be ineligible, 14 declined to participate and 62 provided written informed consent. Of those providing consent, 3 withdrew without any usable data, leaving 59 for analysis. Diagnoses included TBI (n = 9), ischemic stroke (STROKE, n = 30), intracerebral hemorrhage (ICH, n = 10), subarachnoid hemorrhage (SAH, n = 4), and other brain injury (OTHER, n = 6). Patients were admitted to the units at variable times after their brain injury (median 29 days, range 3-247).
Figure 1.
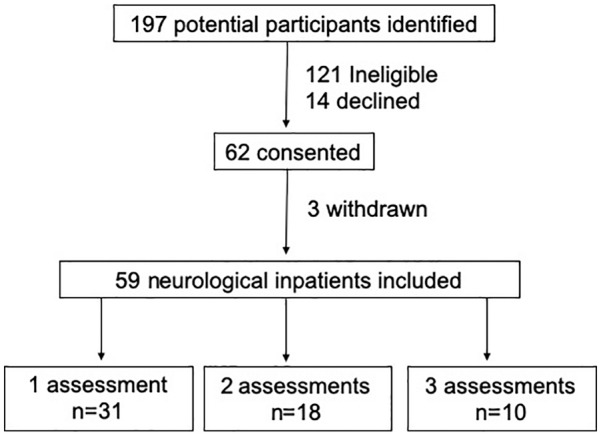
Recruitment flowchart.
Where possible, sleep quality and motor assessments were conducted as soon as possible after admission (EARLY; mean 22 days postadmission), at midpoint of their stay (MID; mean 59 days) and prior to discharge (LATE; mean 91 days). However, in some cases only 1 or 2 assessments were possible due to delays in recruitment after admission, short admissions or unexpected early discharge (Figure 1).
Additionally, sleep quality was assessed at one time point for a cohort of 55 age-matched (mean ± SD: 56.5 ± 17.6 years) and gender-matched (27 male, 28 female), community-dwelling, healthy controls in order to confirm that this cohort of inpatients demonstrated impaired sleep quality.
Assessments
Sleep quality was assessed through actigraphy (Motionwatch, Camntech Ltd), and the sleep condition indicator (SCI).21 A Motionwatch was placed on each wrist and worn continuously for 7 days and nights at each assessment time-point. The actigraph can be used to predict when the body is in periods of sleep in comparison to wake under the assumption of the body being motionless during deep sleep. Therefore, parameters such as sleep fragmentation can be calculated22 in the hospital environment. During this time, participants also completed a sleep diary to indicate what time they tried to go to sleep and what time they woke up each day. If they were unable to do so, then the researchers or the therapists assisted them, or approximate sleep/wake times were taken from clinical notes. The SCI was completed during the 7-day period (at the same time as the motor assessments) to provide a self-report measure of sleep quality and the impact of sleep disturbance on daytime function. Additionally, anxiety and depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) during the 7-day sleep monitoring period.
Motor assessments were also conducted by a researcher (MKF) during the 7-day sleep monitoring period. These included the action research arm test23 (ARAT; maximum score 57) to assess function of the upper limb, and the Fugl-Meyer Assessment24 (FMA; max score 100) to assess upper and lower limb motor impairment.
The Functional Independence Measure25 (maximum score 126) and the BI26,27 (maximum score 20) were used to measure function and independence in activities of daily living at admission and discharge. These measures are scored by the clinical team as a matter of clinical practice. Additionally, the Rivermead Mobility Index28 (RMI; maximum score 15) score obtained routinely by the clinical team for some patients was recorded where possible. The clinical team had no access to sleep quality measures of any of the participants.
Analysis
Based on a previous study, demonstrating a correlation between WASO early after stroke and BI at discharge,7 we estimated a likely correlation of 0.45, and therefore a sample size of 36 participants would be required (α = 0.05 and 80% power). However, as we sought to perform stepwise linear regression, we aimed to recruit a higher sample, if possible, over the 21 months.
Measures of sleep quality were taken from the Motionwatch attached to the wrist of the less-affected arm for inpatients or the nondominant arm of healthy controls, using Motionware software (Camntech Ltd). Data were averaged by the monitor into 30-second epochs. Sleep measures included assumed sleep (hours, minutes), actual sleep time (hours, minutes), WASO (hours, minutes), and the sleep fragmentation index.
Statistical analysis was performed with SPSS version 25 (IBM Corp) and Graphpad Prism 8.3.0 (GraphPad Software). Differences in characteristics and sleep quality between patients and controls were assessed using independent-samples t tests, or Mann-Whitney U tests as appropriate, with effect sizes (Cohen’s d) reported where significant differences were found. Group characteristics based on number of assessments or diagnosis group were compared using 1-way analysis of variance, Kruskal-Wallis or chi-square tests as appropriate. Where group differences were significant, post hoc comparisons were conducted with Bonferroni correction for multiple comparisons.
Cross-sectional relationships between sleep quality, motor function/impairment, and functional independence were assessed using data obtained from the first assessment only. Spearman correlations were conducted between objective sleep quality measures (WASO/sleep fragmentation index), and SCI score with ARAT and FMA at the first assessment and FIM at admission. An adjusted significance level of P < .005 was used to compensate for multiple correlations.
Changes in sleep quality (WASO, sleep fragmentation index, SCI score) over the rehabilitation period were assessed for participants with 2 or 3 assessments, using a linear mixed model with assessment time (EARLY, MID, DISCHARGE) as the fixed effect and participant as the random effect.
We also sought to determine whether objective sleep quality (WASO, sleep fragmentation index) averaged over the rehabilitation period explained variance in motor function (ARAT, FMA, RMI) at discharge from the rehabilitation unit, over and above baseline severity of injury (assessed as baseline FIM). To ensure that outliers did not influence the model, data greater than 2 standard deviations from the mean were removed prior to analysis (maximum 4 data-points in any 1 measure). A hierarchical regression analysis was used. Initially, FIM at admission was entered alone into the regression model to determine the proportion of variance explained by baseline severity. Then, WASO and sleep fragmentation were added using stepwise selection, to determine whether these variables increased the variance explained. An adjusted significance of P < .017 was used to compensate for 3 regression models.
Finally, we wanted to determine whether any sleep or demographic factors could explain variance in the rate of recovery of functional independence (FIM; calculated as [discharge − admission]/length of stay in days). To ensure that outliers did not influence the model, data greater than 2 standard deviations from the mean were removed prior to analysis (maximum 4 data-points in any 1 measure). Stepwise linear regression was conducted with the dependent variable of rate of change in FIM and independent variables of sleep quality (WASO, sleep fragmentation index, SCI score), diagnosis group, HADS, age, BI at admission and time since injury at admission. Pairwise deletion was utilized to enable associations between variables to be calculated in the case of missing data in one variable.
Results
Inpatient Characteristics
There were no differences in age (F2, 56 = 2.365, P = .103), sex (χ2(2) = 0.320, P = .852), days since injury at admission (χ2(2) = 3.958, P = .138), days since admission at recruitment (χ2(2) = 3.840, P = .147), diagnosis (χ2(8) = 8.873, P = .353), BI at admission (χ2(2) = 3.016, P = .221), or FIM at admission (F2, 56 = 0.346, P = .709) between those with 1, 2, or 3 assessments completed. There was a difference in the length of stay (χ2(2) = 16.657, P < .001), as patients with 1 assessment had a shorter length of stay than those with 2 or 3 assessments.
Participant characteristics for each diagnosis group are presented in Table 1. There were no differences in age (F4, 54 = 1.728, P = .157), FIM at admission or discharge (F4, 54 = 2.045, P = .101; F4, 54 = 0.252, P = .907), BI at admission or discharge (χ2(4) = 2.254, P = .689; χ2(4) = 1.128, P = .890), length of stay (χ2(4) = 3.056, P = .549), first assessment WASO (χ2(4) = 4.771, P = .312), sleep fragmentation (χ2(4) = 8.237, P = .083), or SCI (χ2(4) = 7.331, P = .119) between the different diagnosis groups. There was a significant difference for time since injury at admission (χ2(4) = 16.865, P = .002). Post hoc Mann-Whitney U tests found that those with ICH were admitted to the rehabilitation unit more quickly than either TBI (U = 9.5, P = .004) or SAH (U = 0.0, P = .005).
Table 1.
Inpatient Characteristics.a
| Stroke | TBI | ICH | SAH | Other | All | |
|---|---|---|---|---|---|---|
| N | 30 | 9 | 10 | 4 | 6 | 59 |
| Age (years) | ||||||
| Mean (SD) | 61 (20) | 49 (20) | 63 (19) | 56 (13) | 43 (21) | 57 (20) |
| Sex | ||||||
| Male:female | 14:16 | 8:1 | 7:3 | 4:0 | 5:1 | 38:21 |
| Time since injury at admission (days) | ||||||
| 22 | 43 | 14 | 75 | 43 | 29 | |
| (6-246) | (23-108)b | (3-40) | (46-247)b | (20-98) | (3-247) | |
| Time since admission at first assessment (days) | ||||||
| 20 | 34 | 28.5 | 26.5 | 36.5 | 24 | |
| (8-83) | (14-127) | (7-68) | (18-174) | (20-93) | (7-174) | |
| Length of stay (days) | ||||||
| 78 | 112 | 54.5 | 68 | 98 | 71 | |
| (20-169) | (14-146) | (13-101) | (33-181) | (33-125) | (13-181) | |
| SCI | ||||||
| 21 | 26 | 13 | 14 | 16 | 21 | |
| (4-30) | (12-30) | (2-24) | (9-24) | (10-32) | (2-32) | |
| WASO (minutes) | ||||||
| 84 | 87 | 68 | 58 | 66 | 76 | |
| (22-298) | (7-173) | (33-247) | (49-81) | (50-99) | (7-198) | |
| Sleep fragmentation index | ||||||
| 47 | 29 | 39 | 41 | 33 | 42 | |
| (25-131) | (14-68) | (26-96) | (20-44) | (19-65) | (14-131) | |
| Admission scores | ||||||
| FIM | 62 | 48 | 66 | 66 | 78.5 | 61 |
| (30-101) | (9-78) | (19-83) | (19-83) | (52-92) | (19-114)c | |
| BI | 6.5 | 6 | 67 | 8 | 10 | 7 |
| (2-15) | (3-8) | (1-16) | (2-14) | (2-17) | (1-17)c | |
| Discharge scores | ||||||
| FIM | 93.5 | 100 | 86.5 | 101.5 | 103 | 97 |
| (48-123) | (41-120) | (53-120) | (49-119) | (74-123) | (41-123) | |
| BI | 13 | 15 | 13.5 | 16.5 | 16.5 | 14 |
| (3-20) | (7-20) | (4-20) | (4-20) | (4-20) | (3-20) | |
Abbreviations: TBI, traumatic brain injury; ICH, intracerebral hemorrhage; SAH, subarachnoid hemorrhage; SCI, sleep condition indicator; WASO, wake after sleep onset; FIM, Functional Independence Measure; BI, Barthel Index.
Values are median (range) unless otherwise specified.
Significantly greater than for ICH group.
Significant improvement from admission to discharge.
At the first assessment, there was significantly more movement of the less-affected arm per 24-hour period than the more-affected arm (median (interquartile range) “Motionwatch units” – affected: 7 (3.5-26), less-affected: 40 (27.2-60.5), Z = 5.784, P < .001), suggesting overall less movement of the more impaired arm.
There was a significant overall improvement in FIM (Z = 6.681, P < .001, d = 1.56) and BI (Z = 6.630, P < .001, d = 1.54) scores from admission to discharge suggesting functional recovery over the rehabilitation period (Table 1).
Inpatients Demonstrate Poor Sleep Quality
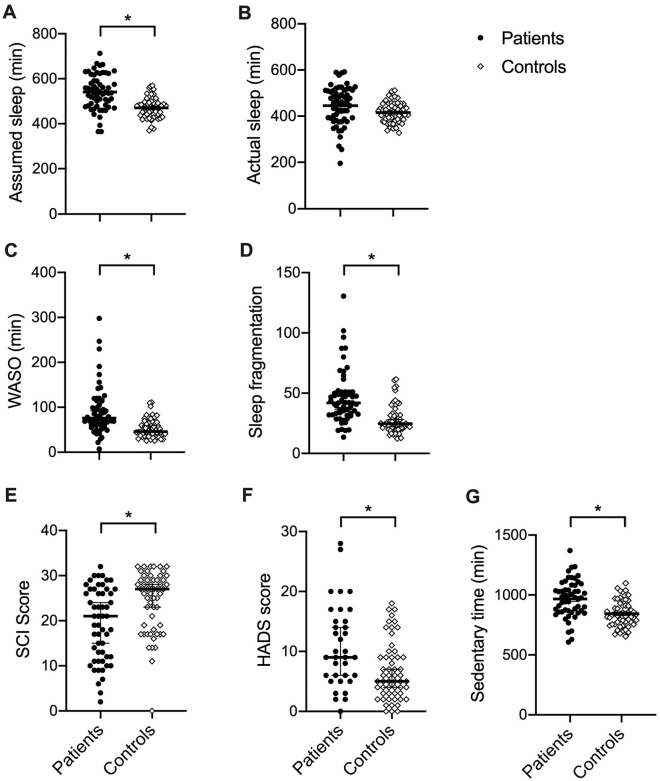
Initially we sought to confirm whether our inpatient cohort experienced poorer sleep than age-matched, community-dwelling healthy controls (Figure 2). There was no difference between groups for age (t(112) = 0.262, P = .794), or sex (χ2(1) = 2.725, P = .099). There was significantly higher assumed sleep duration for inpatients (t(96.8) = 5.957, P < .001, d = 1.1), but actual sleep duration did not differ significantly with the Bonferroni correction (t(88.6) = 2.396, P = .019). Inpatients were found to have more fragmented sleep (Z = −5.336, P < .001, d = 1.15) and a higher WASO (Z = −4.977, P < .001, d = 1.05), as well as poorer subjective sleep quality (SCI; Z = 3.497, P < .001, d = .707) compared with controls. Factors that may influence sleep quality were also found to differ between groups; inpatients had higher anxiety/depression (HADS; Z = −3.003, P = .003, d = .67) and more sedentary time (t(95.1) = 4.780, P < .001, d = 0.92) than controls. We therefore sought to examine whether these potential explanatory variables correlated with sleep quality for the inpatients. HADS score was found to negatively correlate with SCI score (r = −0.474, P = .003), such that more anxiety/depression was associated with poorer subjective sleep quality, but not with objective sleep quality (sleep fragmentation r = 0.178, P = .291, WASO r = −0.053, P = .757). Sedentary time did not correlate significantly with any of the sleep quality measures with the Bonferroni correction (SCI: r = 0.089, P = .531, sleep fragmentation index: r = −0.017, P = .903, WASO: r = −0.300, P = .026).
Figure 2.
Differences between inpatients and controls. (A) Inpatients show longer time in bed trying to sleep. (B) Actual time asleep does not differ significantly between groups. (C) Inpatients have a higher wake after sleep onset (WASO). (D) Inpatients show more fragmented sleep. (E) Subjective sleep quality (sleep condition indicator [SCI] score) is worse for inpatients. (F) Inpatients show significantly higher self-reported levels of anxiety and depression (Hospital Anxiety and Depression Score [HADS]). (G) Inpatients have significantly more sedentary time per 24-hour period. Black circles = patients, open diamonds = controls. Individual data points are shown with mean or median (black line) and standard error or the mean or 95% confidence interval as appropriate. *Mann-Whitney U test or independent-samples t test, P < .008.
Cross-Sectional Relationships Between Sleep Quality and Function for Inpatients
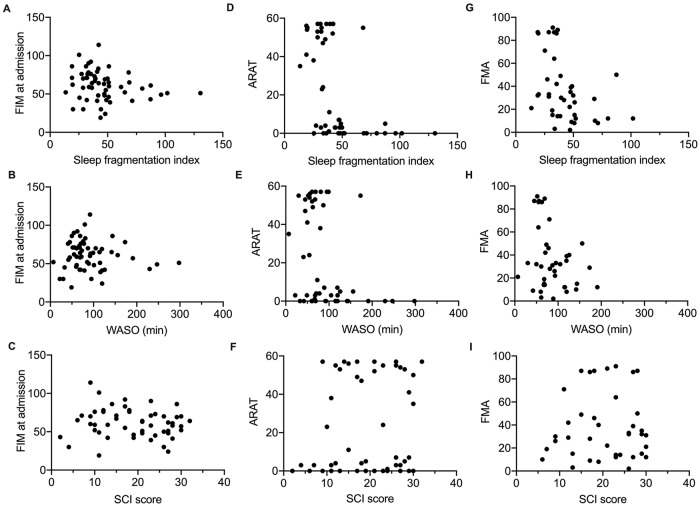
We then sought to assess, for inpatients, whether sleep quality at the first assessment was dependent on severity, assessed as FIM at admission and ARAT or FMA at the first assessment (Figure 3).
Figure 3.
Cross-sectional correlations between sleep quality measures and functional independence at admission, motor function and impairment at the first assessment for all inpatients. FIM = Functional Independence Measure at admission, higher scores indicate more functional independence (less severe brain injury). ARAT = Action Research Arm Test; higher values indicate greater upper limb function. FMA = Total Fugl-Meyer Assessment score, higher scores indicate less motor impairment (upper and lower limb). Higher sleep fragmentation and wake after sleep onset (WASO) indicate poorer sleep quality. Higher Sleep Condition Indicator (SCI) indicates better perceived sleep quality. Significant negative Spearman correlations were found for (E) and (G) (P < .008).
For FIM at admission (Figure 3A-C), there was a tendency for a negative correlation with sleep fragmentation (r = −0.259, P = .048), which was not significant with correction for multiple correlations. There was no correlation between FIM at admission and either WASO (r = −0.038, P = .775) or SCI (r = −0.148, P = .280) at the first assessment.
For ARAT at first assessment (Figure 3D-F), there was a negative correlation with WASO (r = −0.577, P < .001), such that inpatients with more time awake overnight have worse (lower) ARAT scores. There was a tendency for a negative correlation between ARAT and sleep fragmentation (r = −0.312, P = .027) and no correlation between ARAT and SCI (r = 0.106, P = .106).
For FMA at first assessment (Figure 3G-I), there was a negative correlation with sleep fragmentation (r = −0.484, P = .002), such that inpatients with more disrupted sleep have worse motor impairment (lower FMA score), but not for WASO (r = −0.256, P = .111) or SCI (r = 0.027, P = .867).
No Change in Sleep Quality Over Time
For patients with multiple assessments, we wanted to determine whether there was a change in sleep quality alongside recovery. Linear mixed model analysis showed no significant effect of assessment time (EARLY, MID, DISCHARGE) on any of the sleep quality measures (sleep fragmentation index F1.6, 28.72 = 1.693, P = .205; WASO F1.7, 30.8 = 1.007, P = .366; SCI F1.0, 17.5 = 1.894, P = .186; Figure 4).
Figure 4.
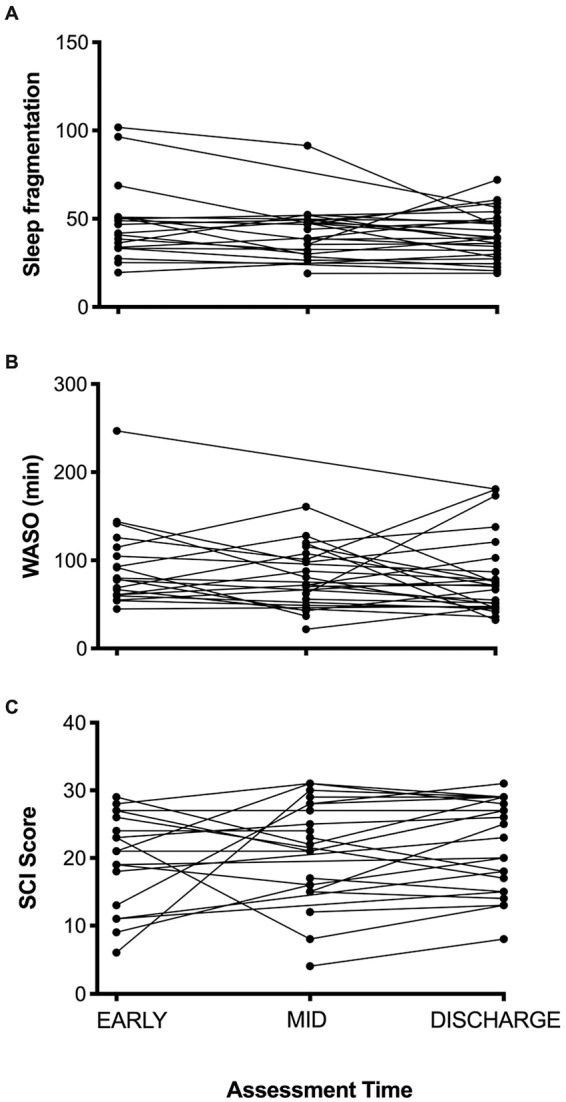
Sleep quality over each assessment for participants with 2 or 3 assessments over the inpatient stay. (A) Sleep fragmentation index (lower values indicate better sleep quality). (B) Wake after sleep onset (WASO; lower values indicate better sleep quality). (C) Sleep condition indicator (SCI; higher values indicate better perceived sleep quality). There was no effect of assessment time on any of the sleep quality variables, suggesting no change in subjective or objective sleep quality over the rehabilitation period.
Longitudinal Relationships Between Objective Sleep Quality, Baseline Functional Independence, and Discharge Function
For ARAT at discharge, FIM at admission was not found to be a significant predictor (R2 = 0.132, F1, 26 = 3.952, P = .057). Adding sleep fragmentation increased the variance explained to 29.2% (ΔR2 = 0.160, F1, 25 = 5.647, P = .025), but this did not reach significance with Bonferroni correction. WASO did not contribute to the model.
For FMA at discharge, FIM at admission was found to explain 21.1% of the variance (R2 = 0.211, F1,21 = 5.622, P = .031), but this was not significant with Bonferroni correction. Adding sleep fragmentation significantly increased the variance explained to 48.5% (ΔR2 = 0.274, F1, 19 = 10.1, P = .005), such that higher functional independence on admission and less disrupted sleep over the rehabilitation period was associated with lower motor impairment (higher FMA) at discharge. WASO did not contribute to the model.
For RMI at discharge, FIM at admission explained 18.2% of the variance (R2 = 0.182, F1, 32 = 7.115, P = .012). Adding sleep fragmentation significantly increased the variance explained to 43.1% (ΔR2 = 0.249, F1, 31 = 13.557, P = .001), such that higher functional independence at admission and less disrupted sleep over the rehabilitation period was associated with better mobility at discharge. WASO did not contribute to the model.
If a stepwise regression was used, rather than a hierarchical regression, then sleep fragmentation alone was consistently found to explain significant variance in outcome (ARAT: R2 = 0.212, F1, 26 = 6.978, P = .014. FMA: R2 = 0.359, F1, 20 = 11.188, P = .003. RMI: R2 = 0.324, F1, 32 = 15.316, P < .001).
Longitudinal Relationships Between Sleep Quality and Rate of Change in Functional Independence
Sleep fragmentation, averaged over the inpatient stay, accounted for 12% of the variance in rate of change in FIM (R2adj = 0.120, P = .027, Figure 5), such that inpatients with more fragmented sleep showed slower rates of functional recovery. SCI score, WASO, HADS, as well as diagnosis group, age, baseline BI, or time since injury at admission, did not significantly contribute to the model.
Figure 5.
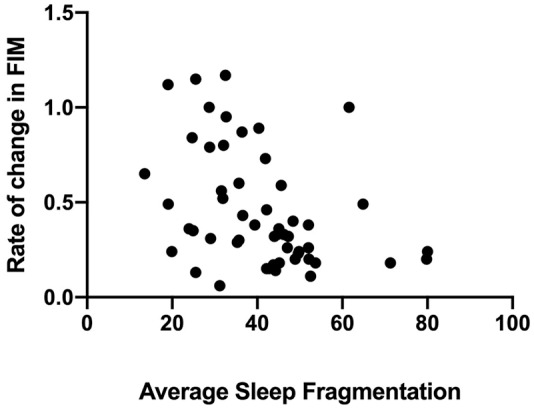
Rate of change in Functional Independence Measure (FIM) as a function of sleep fragmentation averaged over the inpatient stay. FIM was found to explain 12% of the variance in recovery.
Discussion
This study demonstrates that people requiring specialist inpatient rehabilitation after brain injury, including stroke, experience significantly worse sleep quality than age-matched community dwelling healthy controls, and that more fragmented sleep is associated with poorer motor outcomes and slower recovery of functional independence throughout rehabilitation.
The inpatients demonstrated clear impairments in both objective and subjective (self-reported) sleep quality in comparison with healthy controls. This is perhaps not surprising given that hospital environments are known to be quite disruptive to sleep patterns, particularly shared rooms or units with severely disturbed patients who may call out during the night. Nevertheless, other studies have also demonstrated impairment in aspects of sleep quality in comparison with people hospitalized for nonneurological reasons,29 and therefore the disruption to sleep is unlikely to be solely due to the environment of the rehabilitation unit.
Sleep fragmentation may play a key role in explaining variance in outcome and recovery, as this was the only measure that was consistently found to contribute to regression models. Sleep fragmentation increased the proportion of variance in motor outcomes explained, indicating that patients with poor sleep demonstrate worse outcomes, even when baseline severity (FIM at admission) is taken into account. The results of the regression analysis for rate of recovery of FIM suggest that sleep fragmentation explains variance in recovery that cannot be explained by age, type of brain injury (diagnosis group), depression and anxiety, or baseline independence in activities of daily living (BI at admission). These findings are generally consistent with previous observations that poorer functional outcome is associated with impaired sleep quality16,18,19 and extends these to motor outcomes in addition to functional independence. To our knowledge this is the first study to observe significant relationships between the sleep fragmentation index and outcome/recovery assessed as a continuum rather than categorizing patients as having a “good” or “poor” outcome. Furthermore, to our knowledge this is the first study to relate sleep fragmentation averaged over the rehabilitation period to the rate of change in functional independence, suggesting that those with more disrupted sleep may recover more slowly. However, as FIM was only measured at admission and discharge, rather than multiple time-points, it was not possible to ascertain whether patients had reached a plateau in their recovery. It is also not possible to fully understand the causal nature of the relationship between sleep disturbance and outcome with this data. Therefore, it remains to be seen whether improving sleep quality could improve rehabilitation outcomes.
This study included people with a range of neurological conditions admitted to the same rehabilitation units and receiving similar multidisciplinary therapy input. Unfortunately, it was not possible to gain access to clinical brain imaging to ascertain whether sleep disturbance was related to lesion extent or location. We were also not specifically aiming to assess whether sleep quality or outcome depended on the type of brain injury, though baseline comparisons suggested no clear differences in WASO, sleep fragmentation index, SCI, or the time spent in rehabilitation between the brain injury subtypes and FIM and BI at discharge did not differ significantly across groups. Additionally, diagnosis group was not found to contribute to the model explaining variance in rate of change in FIM. Bakken et al30 similarly demonstrated that actigraphy variables (total sleep time, WASO, number of wakenings) did not differ between stroke types (ischemic, hemorrhagic, chronic cerebral ischemia, and negative findings on computed tomography) or between left, right, and bilateral strokes. Nevertheless, it would be important for future studies to investigate whether lesion characteristics influence sleep quality or the nature of sleep disturbance. It also would be important for future studies to explore whether the link between sleep disturbance and outcome is affected by cognitive impairment as we did not have a specific cognitive assessment. Falck et al31 found that stroke survivors with disrupted sleep demonstrated larger impairments in cognitive performance relative to controls than those with low sleep fragmentation, suggesting that sleep disturbance may exacerbate cognitive problems after brain injury.
We were surprised to find no improvement in objective or subjective sleep quality over the course of the rehabilitation period. One previous study6 showed a significant improvement in WASO, sleep efficiency and apnea-hypopnea index from acute to 3 months post stroke/TIA, but many sleep architecture measures (eg, N1%, REM%) were unchanged and the percentage of patients with a periodic limb movement index ≥10 was actually found to get worse. Another study7 found improvements in WASO and sleep efficiency from the acute to the chronic stage of stroke, although their sample at the chronic stage was just 15 patients and none of their patients were older than 75 years, which may not be particularly representative of the stroke population. Overall, our finding therefore suggests that there may not be clear improvements in sleep over the early stages of recovery, and this may indicate that sleep disturbance is largely due to environmental issues, or that the neurological aspects that affect sleep quality are slow to recover.
Sleep disturbance could potentially affect rehabilitation through a reduced ability to engage in therapy activities. Worthington and Melia32 report that rehabilitation unit staff feel that rehabilitation and daily activities are frequently affected for patients with acquired brain injury who demonstrate arousal disturbance. Furthermore, more time in bed at night has been found to be associated with less daytime activity after stroke.33 We found significantly higher sedentary time for inpatients compared with controls, consistent with a study in chronic stroke survivors.34 However, in our cohort there was no correlation between sleep quality and total sedentary time, and as such there is no clear indication that those with poor sleep are engaging in rehabilitation any less than those with better sleep.
Conclusion/Implications
Overall, this study provides evidence for a relationship between sleep fragmentation and motor outcomes as well as recovery of functional independence during neurorehabilitation. Future studies should explore factors affecting sleep quality and develop interventions to see whether sleep can be improved in this environment, and whether this leads to improvements in recovery. Potential nonpharmacological options for targeting sleep include cognitive behavioral therapy for insomnia, noninvasive brain stimulation, light therapy, or potentially changes to the hospital environment to promote good sleep hygiene, though the feasibility and efficacy of these approaches in this population requires future research.
Acknowledgments
Thanks to all staff at the Oxford Centre for Enablement and the Oxfordshire Stroke Rehabilitation Unit for assistance with running the study. Thank you to Ximena Omlin for advice on actigraphy analysis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by the Wellcome Trust (Principal Research Fellowship to HJB) 110027/Z/15/Z and supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust 203139/Z/16/Z.
ORCID iD: Melanie K. Fleming  https://orcid.org/0000-0003-2232-9598
https://orcid.org/0000-0003-2232-9598
References
- 1. Gardani M, Morfiri E, Thomson A, O’Neill B, McMillan TM. Evaluation of sleep disorders in patients with severe traumatic brain injury during rehabilitation. Arch Phys Med Rehabil. 2015;96:1691-1697.e3. doi: 10.1016/j.apmr.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 2. Sterr A, Kuhn M, Nissen C, et al. Post-stroke insomnia in community-dwelling patients with chronic motor stroke: physiological evidence and implications for stroke care. Sci Rep. 2018;8:8409. doi: 10.1038/s41598-018-26630-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Ameri LT, Mohsin TS, Wahid ATA. Sleep disorders following mild and moderate traumatic brain injury. Brain Sci. 2019;9:E10. doi: 10.3390/brainsci9010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iddagoda MT, Inderjeeth CA, Chan K, Raymond WD. Post-stroke sleep disturbances and rehabilitation outcomes: a prospective cohort study. Intern Med J. 2020;50:208-213. doi: 10.1111/imj.14372 [DOI] [PubMed] [Google Scholar]
- 5. Gudberg C, Johansen-Berg H. Sleep and motor learning: implications for physical rehabilitation after stroke. Front Neurol. 2015;6:241. doi: 10.3389/fneur.2015.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manconi M, Fanfulla F, Ferri R, et al. Periodic limb movements during sleep in stroke/TIA. Neurology. 2018;90:e1663-e1672. doi: 10.1212/WNL.0000000000005471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vock J, Achermann P, Bischof M, et al. Evolution of sleep and sleep EEG after hemispheric stroke. J Sleep Res. 2002;11:331-338. doi: 10.1046/j.1365-2869.2002.00316.x [DOI] [PubMed] [Google Scholar]
- 8. Cavalcanti P, Campos T, Araujo J. Actigraphic analysis of the sleep-wake cycle and physical activity level in patients with stroke: implications for clinical practice. Chronobiol Int. 2012;29:1267-1272. doi: 10.3109/07420528.2012.719960 [DOI] [PubMed] [Google Scholar]
- 9. Cavalcanti PRA, Campos TF, Araüjo JF. Circadian and homeostatic changes of sleep-wake and quality of life in stroke: implications for neurorehabilitation. Neurorehabilitation. 2013;32:337-343. doi: 10.3233/NRE-130853 [DOI] [PubMed] [Google Scholar]
- 10. Sterr A, Herron K, Dijk DJ, Ellis J. Time to wake-up: sleep problems and daytime sleepiness in long-term stroke survivors. Brain Inj. 2008;22:575-579. doi: 10.1080/02699050802189727 [DOI] [PubMed] [Google Scholar]
- 11. Siccoli MM, Rölli-Baumeler N, Achermann P, Bassetti CL. Correlation between sleep and cognitive functions after hemispheric ischaemic stroke. Eur J Neurol. 2008;15:565-572. doi: 10.1111/j.1468-1331.2008.02119.x [DOI] [PubMed] [Google Scholar]
- 12. Sonmez I, Karasel S. Poor sleep quality I related to impaired functional status following stroke. J Stroke Cerebrovasc Dis. 2019;28:104349. doi: 10.1016/j.jstrokecerebrovasdis.2019.104349 [DOI] [PubMed] [Google Scholar]
- 13. Kalmbach DA, Conroy DA, Falk H, et al. Poor sleep is linked to impeded recovery from traumatic brain injury. Sleep. 2018;41. doi: 10.1093/sleep/zsy147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang RJ, Lai CH, Lee SD, et al. Objective sleep measures in inpatients with subacute stroke associated with levels and improvements in activities of daily living. Arch Phys Med Rehabil. 2018;99:699-706. doi: 10.1016/j.apmr.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 15. Bakken LN, Kim HS, Finset A, Lerdal A. Stroke patients’ functions in personal activities of daily living in relation to sleep and socio-demographic and clinical variables in the acute phase after first-time stroke and at six months of follow-up. J Clin Nurs. 2012;21:1886-1895. doi: 10.1111/j.1365-2702.2011.04014.x [DOI] [PubMed] [Google Scholar]
- 16. Terzoudi A, Vorvolakos T, Heliopoulos I, Livaditis M, Vadikolias K, Piperidou H. Sleep architecture in stroke and relation to outcome. Eur Neurol. 2009;61:16-22. doi: 10.1159/000165344 [DOI] [PubMed] [Google Scholar]
- 17. Kumar R, Suri JC, Manocha R. Study of association of severity of sleep disordered breathing and functional outcome in stroke patients. Sleep Med. 2017;34:50-56. doi: 10.1016/j.sleep.2017.02.025 [DOI] [PubMed] [Google Scholar]
- 18. Pace M, Camilo MR, Seiler A, et al. Rapid eye movements sleep as a predictor of functional outcome after stroke: a translational study. Sleep. 2018;41. doi: 10.1093/sleep/zsy138 [DOI] [PubMed] [Google Scholar]
- 19. Giubilei F, Iannilli M, Vitale A, et al. Sleep patterns in acute ischemic stroke. Acta Neurol Scand. 1992;86:567-571. doi: 10.1111/j.1600-0404.1992.tb05488.x [DOI] [PubMed] [Google Scholar]
- 20. Joa KL, Kim WH, Choi HY, et al. The effect of sleep disturbances on the functional recovery of rehabilitation inpatients following mild and moderate stroke. Am J Phys Med Rehabil. 2017;96:734-740. doi: 10.1097/PHM.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 21. Espie CA, Kyle SD, Hames P, Gardani M, Fleming L, Cape J. The sleep condition indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. 2014;4:e004183. doi: 10.1136/bmjopen-2013-004183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259-267. doi: 10.1016/j.smrv.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 23. Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair. 2008;22:78-90. doi: 10.1177/1545968307305353 [DOI] [PubMed] [Google Scholar]
- 24. Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13-31. doi: 10.1038/35081184 [DOI] [PubMed] [Google Scholar]
- 25. Turner-Stokes L, Nyein K, Turner-Stokes T, Gatehouse C. The UK FIM+FAM: development and evaluation. Clin Rehabil. 1999;13:277-287. doi: 10.1191/026921599676896799 [DOI] [PubMed] [Google Scholar]
- 26. Collin C, Wade D, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61-63. [DOI] [PubMed] [Google Scholar]
- 27. Turner-Stokes L, Williams H, Rose H, Harris S, Jackson D. Deriving a Barthel Index from the Northwick Park Dependency Scale and the functional independence measure: are they equivalent? Clin Rehabil. 2010;24:1121-1126. doi: 10.1177/0269215510375904 [DOI] [PubMed] [Google Scholar]
- 28. Colleen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. Int Disabil Stud. 1991;13:50-54. [DOI] [PubMed] [Google Scholar]
- 29. Baglioni C, Nissen C, Schweinoch A, et al. Polysomnographic characteristics of sleep in stroke: A Systematic review and meta- analysis. PLoS One. 2016;11:e0148496. doi: 10.1371/journal.pone.0148496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bakken LN, Lee KA, Kim HS, Finset A, Lerdal A. Sleep-wake patterns during the acute phase after first-ever stroke. Stroke Res Treat. 2011;2011:936298. doi: 10.4061/2011/936298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falck RS, Best JR, Davis JC, et al. Sleep and cognitive function in chronic stroke: a comparative cross-sectional study. Sleep. 2019;42:zsz040. doi: 10.1093/sleep/zsz040 [DOI] [PubMed] [Google Scholar]
- 32. Worthington AD, Melia Y. Rehabilitation is compromised by arousal and sleep disorders: results of a survey of rehabilitation centres. Brain Inj. 2006;20:327-332. doi: 10.1080/02699050500488249 [DOI] [PubMed] [Google Scholar]
- 33. Ezeugwu VE, Manns PJ. Sleep duration, sedentary behavior, physical activity, and quality of life after inpatient stroke rehabilitation. J Stroke Cerebrovasc Dis. 2017;26:2004-2012. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 34. Paul L, Brewster S, Wyke S, et al. Physical activity profiles and sedentary behavior in people following stroke: a cross-sectional study. Disabil Rehabil. 2016;38:362-367. doi: 10.3109/09638288.2015.1041615 [DOI] [PubMed] [Google Scholar]