Abstract
Environmental agents, including socioeconomic condition, and host factors can act as causal agents and risk factors in disease. We use biomarkers and sociomarkers to study causal factors, such as overproduction of reactive oxygen species (ROS) which could play a role in disease through oxidative stress. It is therefore important to define the exact meaning of the biomarker we measure. In this review we attempt a classification of biomarkers related to oxidative stress based on their biological meaning. We define as type zero biomarkers the direct measurement of ROS in vivo in patients. Type 1 biomarkers are the most frequently used indicators of oxidative stress, represented by oxidized lipids, proteins or nucleic acids and their bases. Type 2 biomarkers are indicators of the activation of biochemical pathways that can lead to the formation of ROS. Type 3 biomarkers are host factors such as small-molecular weight antioxidants and antioxidant enzymes, while type 4 biomarkers measure genetic factors and mutations that could modify the susceptibility of an individual to oxidative stress. We also discuss whether biomarkers are actionable or not, that is if the specific blockade of these molecules can ameliorate disease or if they are just surrogate markers.
The proposed classification of biomarkers of oxidative stress based on their meaning and ambiguities, within the theoretical framework of the oxidative stress theory of disease may help identify those diseases, and individuals, where oxidative stress has a causal role, to allow targeted therapy and personalized medicine.
1. Introduction
Oxidative stress is considered a potential mechanism in the toxicity of several chemicals as well as in the aetiology of many diseases, where its causal role is often implied by the suggestion, frequently made in the literature, that antioxidant molecules could have a protective effect in those conditions.
In the following pages we will discuss the different positions of oxidative stress in the theoretical frameworks of disease causation, and how the different roles of oxidative stress can be studied using biomarkers. We will first summarize some basic concepts of causation. Then we will provide some examples of the causal role of oxidative stress in toxicology and in the aetiology of several diseases, giving some consideration to the concept of risk factor and the use of biological responses as biomarkers. In this context, we will focus on biomarkers used in human studies. Finally, we will discuss the problem of psychological stress and socioeconomic conditions (and sociomarkers) as an often overlooked component of the causal framework. We will conclude with some considerations on biomarker classification with respect to their actionability.
2. Causation in medicine
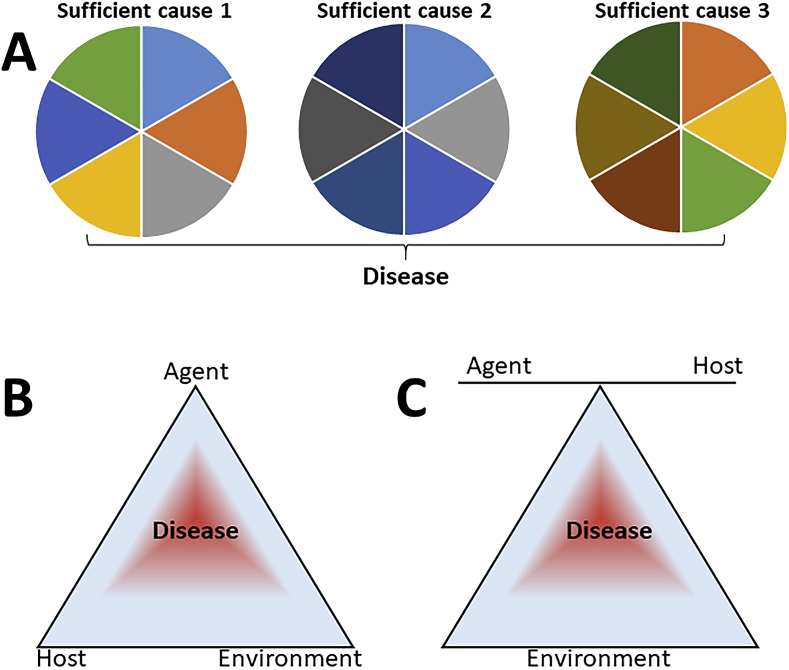
The modern concept of multiple causation in disease is well represented in the scheme described by Rothman [1] and shown in Fig. 1A. According to this model, a disease can be caused by several different sufficient causes (Rothman's pies), each composed of different component causes (the slices). Hence, a component cause (e.g. oxidative stress) could be a slice in the pie and, while not causing the disease alone, could do so in combination with other component causes. The limitation of the pie scheme is that it doesn't distinguish risk factors (such as crowding or immunosuppression, in the case of tuberculosis) and essential component causes (such as the presence of mycobacteria, in the case of tuberculosis).
Fig. 1.
Models of disease causation. A The Rothman pie [1]. B, C The epidemiological triad. From Centers for Disease Control and Prevention [Internet]. Atlanta. https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section8.html (public domain).
According to the World Health Organization, “A risk factor is any attribute, characteristic or exposure of an individual that increases the likelihood of developing a disease or injury. Some examples of the more important risk factors are underweight, unsafe sex, high blood pressure, tobacco and alcohol consumption, and unsafe water, sanitation and hygiene.” (https://www.who.int/topics/risk_factors/en/). The definition is, in reality, more complex, and a risk factor may be considered as such if it associated with an increased risk of disease but may not be, in itself, a cause.
Oxidative stress could participate in the causation of disease in three ways: 1) as a sufficient cause (when oxidative stress alone can induce the disease); 2) as insufficient but necessary component cause (oxidative stress will induce the disease only when combined with other component causes but, whatever the combination of causes is, oxidative stress has to be present); 3) as a non-necessary component cause (that is, in some patients oxidative stress will contribute in the disease but other patients may develop the same condition with a different set of component causes, even in the absence of oxidative stress). We have discussed elsewhere the implications of this model in determining the causal role of oxidative stress in disease and its therapeutic implications [2,3].
Considering the topic of this special issue, another limitation of the two-dimensional pie model is that it does not distinguish host factors (such as genetic risk factors, for instance lower levels of an antioxidant enzyme) and environmental factors.
Two other schemes could be useful here, representing two different views of the epidemiological triad in disease causation (Fig. 1B and C). This triad is typically used to represent causation in infectious disease. However, its use has been proposed in the interpretation of non-infectious conditions, such as injury [4] and diabetes [5].
We will try here to consider the relative role of oxidative stress as an agent, as part of the environmental component,s and as a host factor, and will provide examples based on the use of biomarkers to detect its various roles as a slice of causal pies or element in the causative triad, focusing on clinical data.
A prerequisite for the hypothesis of a causal role of oxidative stress in disease is the measurement of markers of oxidative stress in patients, and this review will discuss the differences between the various biomarkers of oxidative stress. However, it is important to distinguish between association and causation [[6], [7], [8]]. Specifically, the association of oxidative stress and disease (statistical association) could be explained not only by hypothesizing that oxidative stress causes the disease but also that it is the disease that causes oxidative stress (reverse causation) [9,10]. It is also possible that both the disease and oxidative stress are caused by a third factor (confounder); for instance, in an inflammatory disease, inflammation could cause both the disease and oxidative stress (such as through ROS production by phagocytes) [9,10]. While this has already been discussed elsewhere, this has to be kept in mind when considering the use of biomarkers either as prognostic/diagnostic indicators or in the formulation of causal theories.
3. Oxidative stress biomarkers
Oxidative stress is an expression used to define a status where the levels or ROS are increased, either due to increased production or impairment in the antioxidant systems [11]. However, the short half-life of ROS makes it very difficult to measure them in biological samples obtained from patients. It should be mentioned that there are a number of methodologies developed for measuring ROS directly, despite of their short half-lives, as mentioned in section 4.2. However, while they have been widely applied to in vitro or animal studies, they are not yet easily applicable to patients. For this reason, oxidative stress is usually assessed by measuring the products of the oxidation of cellular molecules by ROS in blood, urine or exhaled breath, depending on the biomarker (reviewed in Refs. [12,13]). These include biomarkers of lipid oxidation (such as malondialdehyde, MDA, or isoprostanes), protein oxidation (particularly protein carbonyls) and nucleic acid oxidation (particularly 8-hydroxydeoxyguanosine, 8-OH-dG). This is an important limitation as ROS, particularly H2O2, are also important signalling molecules. Thus, it could be argued that by measuring only terminal product of oxidation reactions we detect “oxidative damage” rather than “oxidative stress”.
4. Oxidative stress as a causal factor
We will examine here the cases where oxidative stress was suggested to act as the main, essential and sufficient, cause of disease. In our view, the only such examples are in the field of toxicology, namely the toxicity of the herbicide paraquat, hyperoxia, and ionizing radiation.
4.1. Paraquat
Paraquat (1,1′-dimethyl-4,4′-bipyridylium dichloride) is an herbicide that causes a significant number of deaths by pulmonary toxicity, due to its accumulation in the lung. The mechanism of toxicity has been well studied and is related to generation of ROS though redox cycling catalyzed by several oxidases (reviewed in Ref. [14]). Although this has been extensively studied in animal models, there is also evidence that paraquat poisoning is associated with increased biomarkers of oxidative stress in patients. Dinis-Oliveira et al. [14] have reviewed the studies on biomarkers of oxidative stress in paraquat-poisoned patients, and concluded that lipid peroxidation has been detected in studies on a few patients, either as serum malondialdehyde (MDA) levels or exhaled ethane.
4.2. Ionizing radiations
Ionizing radiations have long been known to generate ROS [15], and pulse radiolysis has been, historically, a means of generating superoxide radicals in vitro [16]. The efficacy of thiols as radioprotectant demonstrates a causative role for ROS in radiation-induced DNA damage [17]. Despite this, the few published studies in patients showed that radiotherapy results only in a small increase in serum MDA levels [18,19]. Indirect evidence for an increase in plasma hydroperoxides (measured by chemiluminescence or as conjugated dienes) was observed in workers involved in the clean-up following the Chernobyl accident who suffered from post-radiation syndrome [20,21].
4.3. Oxygen toxicity
Oxygen toxicity, such as that following exposure to hyperbaric oxygen, is also thought to be in part due to increased ROS formation with a mechanism similar to radiation toxicity [22]. However, the literature report contradictory results. Some studies reported that exposure of patients to hyperbaric oxygen increases levels of blood lipid hydroperoxides [23], MDA [24] and 8-isoprostane [25], while other studies showed a very small effect or no effect on these markers [26,27].
4.4. Oxidative stress following exposure to environmental chemicals and stressors
Several studies have measured markers of oxidative stress in relation to environmental chemicals. For instance, MDA levels in exhaled breath condensate was associated with air pollution in a study in Bejing [28] and in workers exposed to titanium dioxide nanoparticles [29]. A meta-analysis of over 50 studies has reached the conclusion that exposure to combustion particles due to air pollution (excluding occupational health) is associated with increased levels of biomarkers of DNA oxidation (such as 8-oxo-7,8-dihydroguanine or 8-oxo-7,8-dihydro-2′-deoxyguanosine) or of lipid oxidation (including MDA and isoprostanes) [30]. More specifically, a meta-analysis of studies using F2-isoprostane as a biomarker of oxidative stress has found a significant effect of exposure to asbestos, occupational exposure and silicosis [31]. It is important to note that these studies reported an association, and did not attempt to establish whether oxidative stress had a causal role in the mechanism of toxicity of xenobiotics or if it was associated with the severity of the toxic effects.
Finally, non-chemical environmental stressors can also be associated with oxidative stress, and increased biomarkers of oxidative stress in response to environmental noise could shed light on epidemiological evidence that traffic noise is associated with cardiovascular disease [32,33].
5. Oxidative stress as a causal component or risk factor in disease
Oxidative stress has been suggested as a causal component for almost any disease, and different expressions have been used for this in the literature, such as: plays a role, is important for, is implicated etc. A recent comprehensive meta-analysis summarizing the evidence for an increase in the levels of the lipid peroxidation products F2-isoprostane in disease lists about 50 different disease conditions [31].
5.1. The “redox status” as a causal component or a risk factor
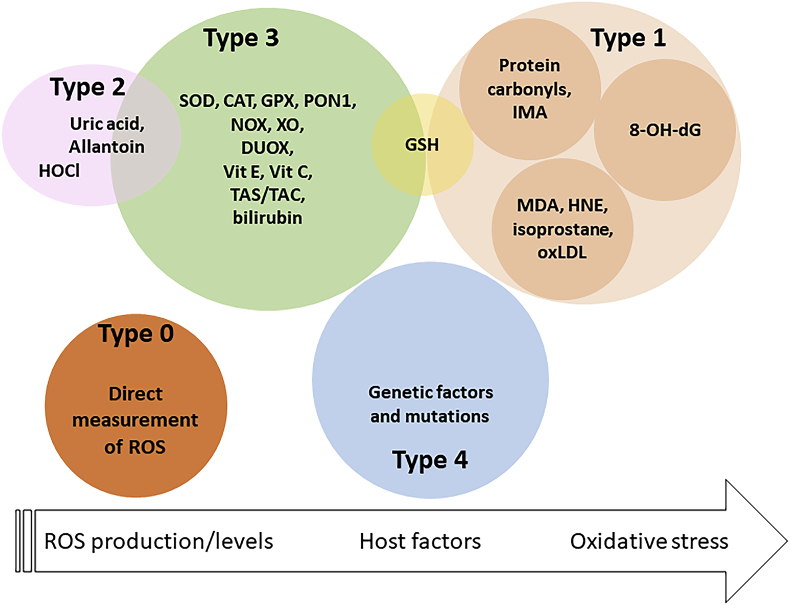
As mentioned above, it is not easy to distinguish a risk factor from a component cause for a disease. We can assume, although not with an absolute certainty, that oxidative stress is a cause, and probably a sufficient cause, of paraquat toxicity. However, the role of oxidative stress in disease is more complex. We searched the Web of Science on 23/10/2018 for “oxidative stress meta-analysis OR oxidative stress metaanalysis”. The aim of the search was to find all studies where one or more oxidative stress-related biomarkers were measured in patients with specific disease conditions compared to healthy controls. After excluding interventional studies we analyzed 198 studies. The “biomarkers” measured, which were used to conclude (or exclude) an association between disease and oxidative stress, are listed in Fig. 2.
Fig. 2.
Biomarkers related to oxidative stress and their meaning.
Abbreviations used: CAT, catalase; DUOX, dual oxidase; GPX, GSH peroxidase; GSH, glutathione; HNE, hydroxynonenal; IMA, ischemia-modified albumin; MPO, myeloperoxidase; PON1, paraoxonase 1 UA, uric acid; Vit, vitamin.
We can classify these in four types of biomarkers. The first type, that we and others defined as “biomarkers of oxidative stress”, are, in fact, biomarkers of “oxidative damage” as discussed in section 2, and include the oxidation products of lipid, proteins and nucleic acids. This group also includes molecules that are decreased following oxidation by ROS, such as glutathione (GSH).
A second type of biomarkers are indicators that ROS-generating enzymes have been activated. To our knowledge, the only biomarkers of this type are uric acid (UA) and allantoin, stable products of ROS-generating xanthine oxidase (XO). They also include hypochlorous acid (HOCl) that is an indicator of phagocytic H2O2 production via myeloperoxidase [34,35].
A third type are biomarkers measuring factors that determine the susceptibility to oxidative damage. We include in this group enzymes (such as superoxide dismutase, SOD, or catalase, CAT) and small-molecular weight molecules (e.g. vitamins C and E, bilirubin) that react with ROS and are often generically described as antioxidants or scavenges. This group also includes levels of enzymes that produce ROS (e.g. NADPH oxidases, NOX, and XO).
Finally, as this review focusses on biomarkers used to study causative mechanisms, we included a fourth type of biomarkers represented by the measure of mutations in the various enzymes implicated as ROS producers or scavengers, as they will provide information on the host factors determining the susceptibility to an environmental stressor, and thus indicate a role for oxidative stress in a specific disease. For instance, the association between mutations of the SOD2 gene and the risk of diabetes [36], or that of mutations in the gene encoding for Nrf2 with Parkinson's disease [37] could support the hypothesis that oxidative stress is somewhat implicated in these diseases.
To be clear, one would expect that if we induce oxidative stress, for instance with paraquat, the levels of the first type of biomarkers will increase (or decrease in the case of GSH) but the level of risk factors will not change, at least in the early phases of intoxication (it may increase later as an adaptive response, as we will discuss in section 4.2 below). On the other hand, the levels of risk factors pre-existing paraquat exposure can be important in determining the susceptibility to the oxidative stress-mediated toxic action of paraquat, and the same will be true for genetic factors.
In essence, type 3 and 4 biomarkers are not part of the exposome as they are not affected by oxidative stress, but rather host factors. Of course, what we are missing in clinical studies are “type zero” biomarkers, that is a direct, unambiguous, measurement of ROS in patients. However, imaging techniques have been developed that may allow this in the near future [38,39].
Of note, some of these biomarkers are ambiguous and can belong to more than one classification. For instance decreased levels of GSH can be a sign of oxidative stress as GSH is oxidized by H2O2. However, low pre-exposure levels of GSH in an individual can increase the susceptibility to oxidative stress-mediated injury. High levels of UA can be a marker positively associated with oxidative stress as it is an indicator of the activation of ROS-producing xanthine oxidase. However, UA is also an antioxidant molecule [40], and high levels could provide resistance to oxidative stress.
5.2. Adaptive response as a biomarker: nuclear factor (erythroid-derived 2)-like2 (Nrf2) and the antioxidant/electrophile response
There is an important caveat to our last paragraph. In fact, exposure to a xenobiotic that induces oxidative stress, or that is a direct electrophile, will result in an adaptive response mediated by the activation of Nrf2 through oxidation of its redox- and electrophile-sensor, Keap1 (reviewed in Ref. [41]) and consequently increase the levels of its target genes including the GSH synthetic enzyme glutamate-cysteine ligase, NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), superoxide dismutase (SOD) and thioredoxin.
This highlights the possible use of biomarkers of response as an indication that oxidative stress has taken place. It is not unusual to assess a secondary biological response as an indicator of the activation of a causal mechanism in disease. As we noted elsewhere [3], the most used biomarker of inflammation is not represented by inflammatory cytokines (that have a causal role in the disease) but by C-reactive protein (CRP), whose production in the liver is increased as a secondary effect of the cytokine interleukin 6 (IL-6).
Measuring a biological response to oxidative stress can have the advantage or offering biomarkers with a more favourable kinetics over biomarkers of oxidative stress (our type 1) and would have the added value of measuring an occurrence of oxidative stress that has been biologically relevant. On the other hand, biomarkers of Nrf2 activation are also ambiguous because not only they are indicators of oxidative stress but they also behave as protective factors that detoxify ROS (negative risk factors).
These types of biomarkers are already being used in environmental toxicology. For instance, activation of Nrf2 has been reported in fish treated with environmental pollutants [42,43], and a study on over 500 women has found an association between the expression of Nrf2 target genes and the level of exposure to biomass fuel smoke [44]. The Keap1-Nrf2 redox sensor is of particular importance in the context of environmental chemicals as it regulates several pathways implicated in the detoxification of xenobiotics. Finally, there are other biological responses that can help detect oxidative stress, such as changes in the circadian clock [45].
It is important to note a limitation in the use of Nrf2 as a biomarker of oxidative stress when studying its association with a disease. In fact, while Nrf2 activation is an indicator of oxidative stress, and thus might correlate with disease severity, it also reflect an adaptive-protective response and thus negatively correlate with disease severity. Its meaning is, therefore, ambiguous.
6. Role of psychosocial factors and sociomarkers
We often consider social and psychological factors as additional variables, independent of strictly biological ones like oxidative stress. However, it is probably wrong to see those as independent. The problem of the social determinants of health has been well summarized in 2005 by Marmot [46]. Socioeconomic factors not only affect health through differences in the access to healthcare but an integrated framework has also been proposed where socioeconomic factors influence health through psychosocial factors such as health behaviours and stress [47,48].
Studies have shown that lower education levels are associated with higher levels of inflammatory biomarkers, lower GSH levels [49] and higher isoprostanes levels [50,51]. Women under financial strain have higher serum protein carbonyl levels [52]. Biomarkers of oxidative stress are also elevated in conditions of adverse childhood experiences [53,54], with increased perceived workload [55] and in victims of violence against women [56]. Finally, there is an abundant literature on oxidative stress in psychiatric disorders (see for instance Refs. [[57], [58], [59]] although, as usual, it is unclear whether this is a cause or a consequence of the disease.
These findings have led to definition of “sociomarkers” as recently defined by Ghiara and Russo [60]. Researchers are starting considering the correlations of sociomarkers with mechanisms of disease such as inflammation [61] and the use of sociomarkers, along with biomarkers, as prognostic indicators, for instance in asthma [62]. We could make many hypotheses on how social markers could induce oxidative stress. This could be in part due to psychological stress, and there are studies showing that stress hormones, particularly glucocorticoids, induce oxidative stress [63,64]. Socioeconomic factors are also associated with health behaviours such as smoking, lower exercise and unhealthy diet with consequent obesity [65,66], all of whom may impact on oxidative stress.
However, sociomarkers should be considered not only as surrogate markers of health behaviour (such as dietary habits and malnutrition) but also as an integral part of the causal pathways by which environmental factors affect health. In fact, in addition to factors such as stress due to adverse childhood experiences [[67], [68], [69]], socioeconomic status may affect access to healthcare [70], and this may be particularly important for psychiatric disorders [71].
7. Actionability of biomarkers
As we noted elsewhere, some biomarkers measure molecules that are in the causal pathway of disease [3,72]. For instance, in the case of inflammatory diseases, inflammatory cytokines such as IL-6 are not only indicators of inflammation but also pharmacological targets and blocking IL-6, for instance with antibodies, will improve the disease [73]. Other biomarkers of inflammation, such as CRP, are clinically recognized as better diagnostic biomarkers, but blocking them will not ameliorate disease because they are not involved in the causal pathways. We could then say that IL-6 is an actionable biomarker, while CRP is not.
The problem with oxidative stress biomarkers is that few of them have a role as causal components of disease. The oxidative stress theory of disease implies ROS as the noxious agent. However, ROS have an extremely short half-life making their measurements in patients very difficult, it very difficult, at least with the existing technologies. We therefore resort to measure non-actionable biomarkers such as the end products of their reaction with biological molecules. There is a caveat to this, however, as some of the terminal products of lipid peroxidation, typically aldehydes, can have toxic properties and/or react with proteins to produce protein carbonyls (e.g. HNE) [74].
8. Conclusions
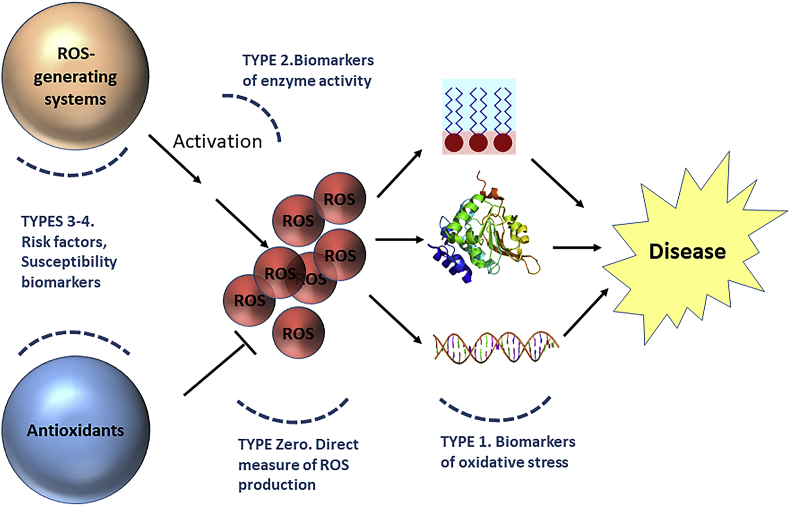
The scheme in Fig. 3 shows how we could fit the biomarkers as classified in Fig. 2 in the causal hypothesis where ROS are a component cause in disease. We suggest that the different role of biomarkers in the causal pathway as well as their actionability should be taken into account when attempting to draw the big picture of biomarkers of oxidative stress in relation to the exposome.
Fig. 3.
The oxidative stress theory of disease. Participating factors and their biomarkers.
Image sources (CC BY-SA 3.0): https://commons.wikimedia.org/wiki/File:Double_stranded_DNA_with_coloured_bases.png, https://commons.wikimedia.org/wiki/File:Protein_PCMT1_PDB_1i1n.png, https://commons.wikimedia.org/wiki/File:Lipid_bilayer_and_micelle.svg.
The proposed classification of biomarkers of oxidative stress is not just an attempt to build a theoretical framework but could help in making conclusions on the causal role of oxidative stress in disease. In fact, if different biomarkers have different meanings, it would then be important to distinguish, for instance, indicators that oxidative stress has occurred from risk factors. This would also be important in meta-analyses, where only biomarkers with the same meaning should be analyzed together.
A classification of biomarkers could also be useful when considering the different use of biomarkers. While this reviews has focused on their importance when studying the causal mechanism of the disease, biomarkers are also used for diagnostic or prognostic purposes. We discuss elsewhere the possibility that, when oxidative stress is not a cause of a disease but its consequence, biomarkers showing evidence of oxidative stress (indicated as type 1 or type 0 here), could be used as surrogate markers of disease [9]. The challenge for the future will be to extend the research on the role of socioeconomic factors and to put sociomarkers in the context of the causal determinants of disease.
References
- 1.Rothman K.J. Causes. Am. J. Epidemiol. 1976;104(6):587–592. doi: 10.1093/oxfordjournals.aje.a112335. [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174:1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghezzi P., Davies K., Delaney A., Floridi L. Theory of signs and statistical approach to big data in assessing the relevance of clinical biomarkers of inflammation and oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2018;115(10):2473–2477. doi: 10.1073/pnas.1719807115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddon W., Jr. Advances in the epidemiology of injuries as a basis for public policy. Publ. Health Rep. 1980;95(5):411–421. [PMC free article] [PubMed] [Google Scholar]
- 5.Egger G., Swinburn B., Rossner S. Dusting off the epidemiological triad: could it work with obesity? Obes. Rev. 2003;4(2):115–119. doi: 10.1046/j.1467-789x.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 6.Illari P., Russo F., Williamson J. OUP Oxford; 2011. Causality in the Sciences. [Google Scholar]
- 7.Howick J.H. John Wiley & Sons; 2011. The Philosophy of Evidence-Based Medicine. [Google Scholar]
- 8.Parkkinen V.-P., Wallmann C., Wilde M., Clarke B., Illari P., Kelly M.P., Norell C., Russo F., Shaw B., Williamson J. Springer; Cham, Switzerland: 2018. Evaluating Evidence of Mechanisms in Medicine: Principles and Procedures. [PubMed] [Google Scholar]
- 9.Ghezzi P., Ghiara V., Davies K. Academic Press; London: 2020. Epistemological Challenges of the Oxidative Stress Theory of Disease and the Problem of Biomarkers, Oxidative Stress; pp. 13–27. [Google Scholar]
- 10.Ghezzi P., Jaquet V., Marcucci F., Schmidt H.H. The oxidative stress theory of disease: levels of evidence and epistemological aspects. Br. J. Pharmacol. 2017;174(12):1784–1796. doi: 10.1111/bph.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe F. Biomarkers of oxidative stress. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag, Berlin-Heidelberg; 2014. pp. 65–87. [Google Scholar]
- 13.Frijhoff J., Winyard P.G., Zarkovic N., Davies S.S., Stocker R., Cheng D., Knight A.R., Taylor E.L., Oettrich J., Ruskovska T., Gasparovic A.C., Cuadrado A., Weber D., Poulsen H.E., Grune T., Schmidt H.H., Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxidants Redox Signal. 2015;23(14):1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinis-Oliveira R.J., Duarte J.A., Sanchez-Navarro A., Remiao F., Bastos M.L., Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 15.Riley P.A. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994;65(1):27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 16.Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J. Biol. Chem. 1972;247(15):4839–4842. [PubMed] [Google Scholar]
- 17.Held K.D., Harrop H.A., Michael B.D. Effects of oxygen and sulphydryl-containing compounds on irradiated transforming DNA. Part I. Actions of dithiothreitol. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981;40(6):613–622. doi: 10.1080/09553008114551601. [DOI] [PubMed] [Google Scholar]
- 18.Shariff A.K., Patil S.R., Shukla P.S., Sontakke A.V., Hendre A.S., Gudur A.K. Effect of oral antioxidant supplementation on lipid peroxidation during radiotherapy in head and neck malignancies. Indian J. Clin. Biochem. 2009;24(3):307–311. doi: 10.1007/s12291-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabitha K.E., Shyamaladevi C.S. Oxidant and antioxidant activity changes in patients with oral cancer and treated with radiotherapy. Oral Oncol. 1999;35(3):273–277. doi: 10.1016/s1368-8375(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 20.Souchkevitch G., Lyasko L. Investigation of the impact of radiation dose on hormones, biologically active metabolites and immunoglobulins in Chernobyl accident recovery workers. Stem Cell. 1997;15(Suppl 2):151–154. doi: 10.1002/stem.5530150722. [DOI] [PubMed] [Google Scholar]
- 21.Kumerova A.O., Lece A.G., Skesters A.P., Orlikov G.A., Seleznev J.V., Rainsford K.D. Antioxidant defense and trace element imbalance in patients with postradiation syndrome: first report on phase I studies. Biol. Trace Elem. Res. 2000;77(1):1–12. doi: 10.1385/BTER:77:1:1. [DOI] [PubMed] [Google Scholar]
- 22.Gerschman R., Gilbert D.L., Nye S.W., Dwyer P., Fenn W.O. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119(3097):623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 23.Alleva R., Nasole E., Di Donato F., Borghi B., Neuzil J., Tomasetti M. alpha-Lipoic acid supplementation inhibits oxidative damage, accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy. Biochem. Biophys. Res. Commun. 2005;333(2):404–410. doi: 10.1016/j.bbrc.2005.05.119. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti S., Lamorgese A., Piersantelli M., Pagliarani S., Benvenuti F., Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin. Biochem. 2004;37(4):312–317. doi: 10.1016/j.clinbiochem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Gurdol F., Cimsit M., Oner-Iyidogan Y., Korpinar S., Yalcinkaya S., Kocak H. Early and late effects of hyperbaric oxygen treatment on oxidative stress parameters in diabetic patients. Physiol. Res. 2008;57(1):41–47. doi: 10.33549/physiolres.931139. [DOI] [PubMed] [Google Scholar]
- 26.Paprocki J., Sutkowy P., Piechocki J., Wozniak A. Markers of oxidant-antioxidant equilibrium in patients with sudden sensorineural hearing loss treated with hyperbaric oxygen therapy. Oxid. Med. Cell. Longev. 2019;2019:8472346. doi: 10.1155/2019/8472346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corcoran T., Ting S., Mas E., Phillips M., O'Loughlin E., Barden A., Mori T.A. Hyperbaric oxygen therapy is not associated with oxidative stress assessed using plasma F2-isoprostanes and isofurans. Prostaglandins Leukot. Essent. Fatty Acids. 2017;127:16–19. doi: 10.1016/j.plefa.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Gong J., Zhu T., Kipen H., Wang G., Hu M., Ohman-Strickland P., Lu S.E., Zhang L., Wang Y., Zhu P., Rich D.Q., Diehl S.R., Huang W., Zhang J.J. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. J. Expo. Sci. Environ. Epidemiol. 2013;23(3):322–327. doi: 10.1038/jes.2012.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelclova D., Zdimal V., Kacer P., Komarc M., Fenclova Z., Vlckova S., Zikova N., Schwarz J., Makes O., Navratil T., Zakharov S., Bello D. Markers of lipid oxidative damage among office workers exposed intermittently to air pollutants including nanoTiO2 particles. Rev. Environ. Health. 2017;32(1–2):193–200. doi: 10.1515/reveh-2016-0030. [DOI] [PubMed] [Google Scholar]
- 30.Moller P., Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ. Health Perspect. 2010;118(8):1126–1136. doi: 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van 't Erve T.J., Kadiiska M.B., London S.J., Mason R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: a meta-analysis. Redox Biol. 2017;12:582–599. doi: 10.1016/j.redox.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog J., Schmidt F.P., Hahad O., Mahmoudpour S.H., Mangold A.K., Garcia Andreo P., Prochaska J., Koeck T., Wild P.S., Sorensen M., Daiber A., Munzel T. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res. Cardiol. 2019;114(6):46. doi: 10.1007/s00395-019-0753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munzel T., Sorensen M., Schmidt F., Schmidt E., Steven S., Kroller-Schon S., Daiber A. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxidants Redox Signal. 2018;28(9):873–908. doi: 10.1089/ars.2017.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 35.Babior B.M. Phagocytes and oxidative stress. Am. J. Med. 2000;109(1):33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 36.Tian C., Fang S., Du X., Jia C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia. 2011;54(4):803–811. doi: 10.1007/s00125-010-2004-5. [DOI] [PubMed] [Google Scholar]
- 37.von Otter M., Bergstrom P., Quattrone A., De Marco E.V., Annesi G., Soderkvist P., Wettinger S.B., Drozdzik M., Bialecka M., Nissbrandt H., Klein C., Nilsson M., Hammarsten O., Nilsson S., Zetterberg H. Genetic associations of Nrf2-encoding NFE2L2 variants with Parkinson's disease - a multicenter study. BMC Med. Genet. 2014;15:131. doi: 10.1186/s12881-014-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berkowitz B.A. Oxidative stress measured in vivo without an exogenous contrast agent using QUEST MRI. J. Magn. Reson. 2018;291:94–100. doi: 10.1016/j.jmr.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhl A., Dixon A., Hali M., Apawu A.K., Muca A., Sinan M., Warila J., Braun R.D., Berkowitz B.A., Genene Holt A. Novel QUEST MRI in vivo measurement of noise-induced oxidative stress in the cochlea. Sci. Rep. 2019;9(1):16265. doi: 10.1038/s41598-019-52439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ames B.N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U. S. A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuadrado A., Manda G., Hassan A., Alcaraz M.J., Barbas C., Daiber A., Ghezzi P., Leon R., Lopez M.G., Oliva B., Pajares M., Rojo A.I., Robledinos-Anton N., Valverde A.M., Guney E., Schmidt H. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol. Rev. 2018;70(2):348–383. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 42.Yin J., Wang A.P., Li W.F., Shi R., Jin H.T., Wei J.F. Sensitive biomarkers identification for differentiating Cd and Pb induced toxicity on zebrafish embryos. Environ. Toxicol. Pharmacol. 2017;56:340–349. doi: 10.1016/j.etap.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Yadetie F., Karlsen O.A., Lanzen A., Berg K., Olsvik P., Hogstrand C., Goksoyr A. Global transcriptome analysis of Atlantic cod (Gadus morhua) liver after in vivo methylmercury exposure suggests effects on energy metabolism pathways. Aquat. Toxicol. 2013;126:314–325. doi: 10.1016/j.aquatox.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Mondal N.K., Saha H., Mukherjee B., Tyagi N., Ray M.R. Inflammation, oxidative stress, and higher expression levels of Nrf2 and NQO1 proteins in the airways of women chronically exposed to biomass fuel smoke. Mol. Cell. Biochem. 2018;447(1–2):63–76. doi: 10.1007/s11010-018-3293-0. [DOI] [PubMed] [Google Scholar]
- 45.Li H., Kilgallen A.B., Munzel T., Wolf E., Lecour S., Schulz R., Daiber A., Van Laake L.W. Influence of mental stress and environmental toxins on circadian clocks - implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 47.House J.S. Understanding social factors and inequalities in health: 20th century progress and 21st century prospects. J. Health Soc. Behav. 2002;43(2):125–142. [PubMed] [Google Scholar]
- 48.Solar O., Irwin A. World Health Organization; Geneva: 2010. A Conceptual Framework for Action on the Social Determinants of Health. Social Determinants of Health Discussion Paper 2 (Policy and Practice) [Google Scholar]
- 49.Kelli H.M., Awad M., Hammadah M., Haider M., Ahmed H., Topel M., Hayek S., Patel K., Gray B., Mohammed K. Education level is associated with cardiovascular risk factors, systemic inflammation, arterial stiffness and oxidative stress. J. Am. Coll. Cardiol. 2016;67(13 Supplement):1882. [Google Scholar]
- 50.Black C.N., Bot M., Scheffer P.G., Penninx B.W. Sociodemographic and lifestyle determinants of plasma oxidative stress markers 8-OHdG and F2-isoprostanes and associations with metabolic syndrome. Oxid. Med. Cell. Longev. 2016;2016:7530820. doi: 10.1155/2016/7530820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eick S.M., Meeker J.D., Brown P., Swartzendruber A., Rios-McConnell R., Shen Y., Milne G.L., Velez Vega C., Rosario Z., Alshawabkeh A., Cordero J.F., Ferguson K.K. Associations between socioeconomic status, psychosocial stress, and urinary levels of 8-iso-prostaglandin-F2alpha during pregnancy in Puerto Rico. Free Radic. Biol. Med. 2019;143:95–100. doi: 10.1016/j.freeradbiomed.2019.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palta P., Szanton S.L., Semba R.D., Thorpe R.J., Varadhan R., Fried L.P. Financial strain is associated with increased oxidative stress levels: the Women's Health and Aging Studies. Geriatr. Nurs. 2015;36(2 Suppl):S33–S37. doi: 10.1016/j.gerinurse.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fanning J.R., Lee R., Gozal D., Coussons-Read M., Coccaro E.F. Childhood trauma and parental style: relationship with markers of inflammation, oxidative stress, and aggression in healthy and personality disordered subjects. Biol. Psychol. 2015;112:56–65. doi: 10.1016/j.biopsycho.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Koenig A.M., Karabatsiakis A., Stoll T., Wilker S., Hennessy T., Hill M.M., Kolassa I.T. Serum profile changes in postpartum women with a history of childhood maltreatment: a combined metabolite and lipid fingerprinting study. Sci. Rep. 2018;8(1):3468. doi: 10.1038/s41598-018-21763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irie M., Asami S., Nagata S., Miyata M., Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int. Arch. Occup. Environ. Health. 2001;74(2):153–157. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- 56.Kim J.Y., Lee J.H., Song H.J., Kim D.G., Yim Y.S. Relationships between psychosocial difficulties and oxidative stress biomarkers in women subject to intimate partner violence. Health Soc. Work. 2017;42(1):41–47. doi: 10.1093/hsw/hlw053. [DOI] [PubMed] [Google Scholar]
- 57.Black C.N., Bot M., Scheffer P.G., Cuijpers P., Penninx B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 58.Brown N.C., Andreazza A.C., Young L.T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatr. Res. 2014;218(1–2):61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Guney E., Fatih Ceylan M., Tektas A., Alisik M., Ergin M., Goker Z., Senses Dinc G., Ozturk O., Korkmaz A., Eker S., Kizilgun M., Erel O. Oxidative stress in children and adolescents with anxiety disorders. J. Affect. Disord. 2014;156:62–66. doi: 10.1016/j.jad.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Ghiara V., Russo F. Reconstructing the mixed mechanisms of health: the role of bio-and sociomarkers. Longitudinal Life Course Stud. 2019;10(1):7–25. [Google Scholar]
- 61.Castagne R., Delpierre C., Kelly-Irving M., Campanella G., Guida F., Krogh V., Palli D., Panico S., Sacerdote C., Tumino R., Kyrtopoulos S., Hosnijeh F.S., Lang T., Vermeulen R., Vineis P., Stringhini S., Chadeau-Hyam M. A life course approach to explore the biological embedding of socioeconomic position and social mobility through circulating inflammatory markers. Sci. Rep. 2016;6:25170. doi: 10.1038/srep25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin E.K., Mahajan R., Akbilgic O., Shaban-Nejad A. Sociomarkers and biomarkers: predictive modeling in identifying pediatric asthma patients at risk of hospital revisits. NPJ Digit. Media. 2018;1:50. doi: 10.1038/s41746-018-0056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costantini D., Marasco V., Moller A.P. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J. Comp. Physiol. B. 2011;181(4):447–456. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- 64.Haussmann M.F., Longenecker A.S., Marchetto N.M., Juliano S.A., Bowden R.M. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc. Biol. Sci. 2012;279(1732):1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banks J., Marmot M., Oldfield Z., Smith J.P. Disease and disadvantage in the United States and in england. J. Am. Med. Assoc. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 66.Pampel F.C., Krueger P.M., Denney J.T. Socioeconomic disparities in health behaviors. Annu. Rev. Sociol. 2010;36:349–370. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deighton S., Neville A., Pusch D., Dobson K. Biomarkers of adverse childhood experiences: a scoping review. Psychiatr. Res. 2018;269:719–732. doi: 10.1016/j.psychres.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 68.Loria H., Caughy M. Prevalence of adverse childhood experiences in low-income latino immigrant and nonimmigrant children. J. Pediatr. 2018;192:209–215 e1. doi: 10.1016/j.jpeds.2017.09.056. [DOI] [PubMed] [Google Scholar]
- 69.Bethell C.D., Newacheck P., Hawes E., Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff. 2014;33(12):2106–2115. doi: 10.1377/hlthaff.2014.0914. [DOI] [PubMed] [Google Scholar]
- 70.Saver B.G., Doescher M.P., Jackson J.E., Fishman P. Seniors with chronic health conditions and prescription drugs: benefits, wealth, and health. Value Health. 2004;7(2):133–143. doi: 10.1111/j.1524-4733.2004.72325.x. [DOI] [PubMed] [Google Scholar]
- 71.Muntaner C., Eaton W.W., Diala C., Kessler R.C., Sorlie P.D. Social class, assets, organizational control and the prevalence of common groups of psychiatric disorders. Soc. Sci. Med. 1998;47(12):2043–2053. doi: 10.1016/s0277-9536(98)00309-8. [DOI] [PubMed] [Google Scholar]
- 72.Ghezzi P., Floridi L., Boraschi D., Cuadrado A., Manda G., Levic S., D'Acquisto F., Hamilton A., Athersuch T.J., Selley L. Antioxid Redox Signal; 2017. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. [DOI] [PubMed] [Google Scholar]
- 73.Emery P., Keystone E., Tony H.P., Cantagrel A., van Vollenhoven R., Sanchez A., Alecock E., Lee J., Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann. Rheum. Dis. 2008;67(11):1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Comporti M. Lipid peroxidation and biogenic aldehydes: from the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic. Res. 1998;28(6):623–635. doi: 10.3109/10715769809065818. [DOI] [PubMed] [Google Scholar]