Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor regulating the expression of genes, for instance encoding the monooxygenases cytochrome P450 (CYP) 1A1 and CYP1A2, which are important enzymes in metabolism of xenobiotics. The AHR is activated upon binding of polycyclic aromatic hydrocarbons (PAHs), persistent organic pollutants (POPs), and related ubiquitous environmental chemicals, to mediate their biological and toxic effects. In addition, several endogenous and natural compounds can bind to AHR, thereby modulating a variety of physiological processes. In recent years, ambient particulate matter (PM) associated with traffic related air pollution (TRAP) has been found to contain significant amounts of PAHs. PM containing PAHs are of increasing concern as a class of agonists, which can activate the AHR. Several reports show that PM and AHR-mediated induction of CYP1A1 results in excessive generation of reactive oxygen species (ROS), causing oxidative stress. Furthermore, exposure to PM and PAHs induce inflammatory responses and may lead to chronic inflammatory diseases, including asthma, cardiovascular diseases, and increased cancer risk. In this review, we summarize findings showing the critical role that the AHR plays in mediating effects of environmental pollutants and stressors, which pose a risk of impacting the environment and human health.
Keywords: Aryl hydrocarbon receptor, Air pollution, Inflammation, Oxidative stress, Polycyclic aromatic hydrocarbons, Particulate matter
Graphical abstract
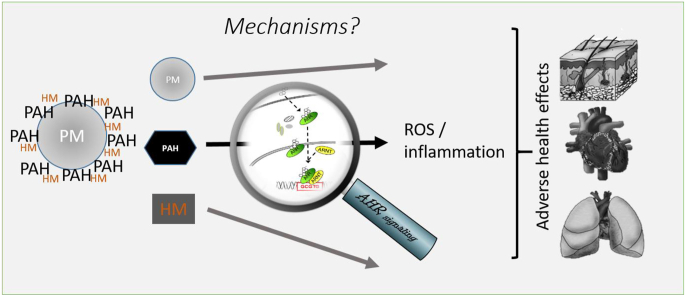
Particulate matter (PM) can adsorb polycyclic aromatic hydrocarbons (PAH), and heavy metals (HM). Particle cores and absorbed chemicals may trigger different cellular mechanisms, possibly resulting in adverse health effects. In this review, we discuss the effects of chemicals, which are ligands of the aryl hydrocarbon receptor (AHR), a latent transcription factor. PAHs activate the AHR, causing gene transcription and ultimately oxidative stress, inflammation and cellular changes. These are context dependent and may be different for different tissues and organs.
Highlights
-
•
PAHs present on ambient air pollution particles are ligands of the cellular AHR.
-
•
AHR-dependent induction of CYP1, AKR, NOX and COX-2 genes can be a source of ROS generation.
-
•
AHR signaling and NRF2 signaling interact to regulate the expression of antioxidant genes.
-
•
Air pollution and ROS can affect inflammation, which is partially triggered by AHR and associated immune responses.
-
•
Skin, lung, and the cardiovascular system are major target sites for air pollution-induced inflammation.
Abbreviations
- AD
atopic dermatitis
- AHR
aryl hydrocarbon receptor
- AKR
aldo-keto reducatse
- ARE
antioxidant response elements
- ARNT
aryl hydrocarbon receptor nuclear translocator
- ASCVD
atherosclerotic cardiovascular disease
- BaP
benzo[a]pyrene
- COPD
chronic obstructive pulmonary disease
- COX
cyclooxygenase
- CVD
cardiovascular disease
- CYP
cytochrome P450
- DC
dendritic cells
- GST
glutathione S-transferase
- HM
heavy metals
- KEAP1
Kelch-like ECH-associated protein
- LPS
lipopolysaccharide
- 3MC
3-methylcholanthrene
- MCP-1
monocyte chemotactic protein 1
- MMP
matrix metalloproteinases
- NF-κB
nuclear factor-κB
- NKX2.5
NK2 homeobox 5
- NOX
NADPH oxidase
- NQO1
NADPH:quinone oxidoreductase-1
- NRF2
nuclear factor erythroid 2-related factor 2
- PAH
polycyclic aromatic hydrocarbon
- PCB
polychlorinated biphenyl
- PFP
PAH rich PM
- PG
prostaglandin
- PM
particulate matter
- POP
persistent organic pollutant
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TCF21
transcription factor 21
- TRAP
traffic-related air pollution
- UGT
UDP-glucuronosyltransferase
- XRE
xenobiotic-responsive element
1. Introduction
Environmental exposure via air pollution and industrial chemicals found as contaminants in food and consumer products are increasing the risk of adverse health outcomes. The exposure to chemicals through ambient particulate matter (PM), consumer and food products may affect multiple cellular and physiological processes in our body including oxidative stress and inflammation. There is strong evidence that chronic inflammation is the basis of many adverse human health effects triggered by PM; especially the exposure to PM generated by combustion and accumulating in urban areas have been found to be associated with inflammatory lung and cardiovascular diseases [1,2]. In addition, evidence is accumulating that PM exposure contributes to the pathogenesis of neurodegenerative diseases, diabetes, and inflammatory skin disorders [[3], [4], [5]]. Traffic-related air pollution (TRAP) is a major source of ambient PM, which is based on the combustion of fossil fuels by motor vehicles and industry [6]. The adverse health effects of PM can result from their particulate characteristics (size, mass, even shape) or from the chemical components they adsorb, such as polycyclic aromatic hydrocarbon (PAHs), heavy metals (HM), or a combination thereof (see graphical abstract). PM derived from combustion contain significant amounts of PAHs compared to other PM sources [7]. PAHs are well-known activators of the aryl hydrocarbon receptor (AHR), a latent transcription factor with numerous roles in physiology. In addition, persistent organic pollutants (POPs), in particular dibenzo-p-dioxins, dibenzofurans and non-ortho substituted polychlorinated biphenyls (PCBs), are another class of high-affinity AHR ligands that can be found in PM fractions [8,9]. In contrast to PAHs, these organochlorines are metabolically stable and accumulate in body fat over time, thus evoking chronic toxicity [10,11]. In this review, we look at the role of the AHR signaling pathway as an important mechanism of PM mediated adverse health effects, especially regarding the generation of oxidative stress and the modulation of immunity. While obvious and often silently assumed, there is still a paucity of studies demonstrating experimentally the AHR-dependency of PM-mediated immune effects. (see Table 1)
Table 1.
Studies showing a link between air pollution, AHR-signaling, and adverse immune effect.
| Pollutant/substance | Target organ/cells | Immune effect | Reference | AHR dependency confirmeda |
|---|---|---|---|---|
| Mouse | ||||
| Ambient urban dust (SRM1649b) | Murine T cells in vivo | Severity of experimental autoimmune encephalitis; Th17, Th1, Treg cells |
[108] | AHR−/- mice; inhibitors |
| Organic fractions of ambient urban dust | Murine T cell differentiation in vitro | Enhancement of Th17 differentiation | [107] | Induction of CYP1A1 |
| Ambient air PM2.5 | Murine dendritic cells, T cells, in vitro | Activation dependent upregulation of IL1ß, co-stimulation molecules; IL17 and IL22 production by T cells | [117] | Luciferase reporter assays; cyp1a1 induction |
| MCP230 particles | Mouse lung in vivo, dendritic cells in vitro | Increase in Th17 cytokines (IL6, IL17A, IL22, IL1ß, IL33 etc.); oxidative stress | [115] | Inhibitors, cyp1a1 induction, luciferase reporter assay |
| Diesel exhaust samples differing in PAH content | Murine T cell differentiation in vitro, EAE in vivo | Effects depended on Diesel sample, i.e. Active PAH components matter | [122] | AHR−/- mice |
| Air pollutants | Murine skin/skin innervation | Hypersensitivity to pruritus, induction of artemin | [18] | AHR−/- mice |
| Diesel exhaust (ultrafine particles) | Murine airways in vivo; human monocytes, murine dendritic cells in vitro | Promotion of airway inflammation; activation of Jag1-Notch Cascade | [118] | Antagonists; luciferase reporter assay |
| Ambient Urban dust, diesel exhaust, cigarette smoke | Murine T cells in vitro, in vivo exposure of mice | Enhanced Th17 differentiation, IL17 secretion | [120] | AHR−/- mice |
| Human | ||||
| Diesel exhaust particles | Human primary bronchial epithelial from asthma patients | upregulation of IL-33, IL-25 and TSLP | [12] | Knock-down of AHR with siRNA |
| Ambient particulate matter and diesel exhaust particles | Human primary bronchial epithelial cells | Upregulation of TSLP mRNA and human microRNA (hsa-miR)-375; regulation of mRNA for AHR | [128] | Induction of AHR and CYP1A1 |
in the studies listed here an involvement of AHR has been demonstrated, e.g. by measuring induction of AHR target genes, inhibition or ablation of AHR in the target cells, or use of AHR-deficient mice. For more details, see the respective publication.
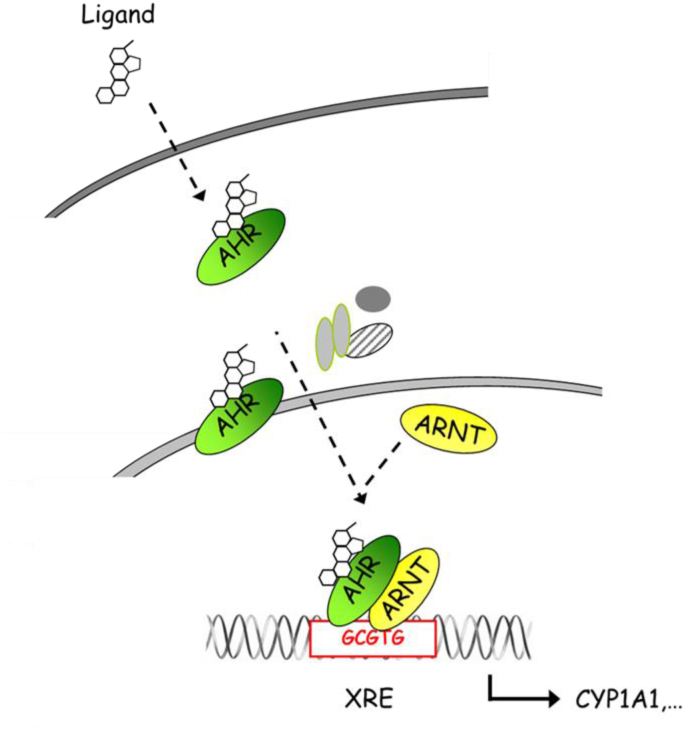
Numerous studies have shown that PM-associated PAHs and POPs are capable of binding to and activating AHR in various test systems [[12], [13], [14]]. The AHR is a ligand-activated transcription factor located in the cytoplasm forming a complex with heat-shock protein 90, AHR interacting protein and p23 [10,11]. After ligand binding, the AHR translocates to the nucleus, dimerizes with AHR nuclear translocator (ARNT) and binds on xenobiotic-responsive elements (XREs; a.k.a. dioxin-responsive elements) in the promoter of AHR target genes (Fig. 1). Targets of the AHR gene battery include xenobiotic-metabolizing phase I enzymes, such as CYP1A1, CYP1A2, CYP1B1, and phase II enzymes, including NADPH:quinone oxidoreductase (NQO1), glutathione S-transferase (GST) A2, and UDP-glucuronosyltransferase (UGT) 1A1 and UGT1A6 [10,11]. In addition to this canonical signaling pathway, AHR frequently interacts with other signaling pathways, such as nuclear factor-κB (NF-κB), nuclear factor erythroid 2-related factor 2 (NRF2) and estrogen receptor signaling [10,11].
Fig. 1.
Scheme of AHR signaling – The AHR resides in the cytosol within a complex of chaperones and other proteins. Lipophilic ligands (e.g. PAH) may cross the cell membrane and bind to the AHR. Due to the ensuing conformational change, the AHR sheds its complexing proteins and translocates to the nucleus, where it dimerizes with ARNT or other partners from other signaling pathways (not shown here). Finally, the AHR:ARNT complex binds to DNA at responsive promoter elements an can initiate transcription. Genes encoding for xenobiotic metabolizing enzymes were among the first AHR target genes described.
POPs, PAHs and related environmental AHR ligands are hazardous substances and may elicit a variety of adverse health effects. Specifically, the chronic exposure to PAHs is associated with the development of atherosclerosis [15,16], allergic inflammatory diseases (e.g. asthma and atopic dermatitis) [[17], [18], [19]], cancer [20], and premature tissue aging [21,22]. Accordingly, the AHR represents a sensor of environmental stimuli and stressors and acts as a mediator of their potential harmful health effects.
It is, however, worth mentioning that apart from environmental chemicals, numerous small molecular weight compounds have been identified to bind to AHR and modulate its activity. The growing list of AHR ligands includes endogenously generated tryptophan metabolites and indole derivatives, plant- and microbiota-derived metabolites as well as several pharmaceutical drugs [11,23]. Importantly, not all of these AHR ligands initiate similar molecular and cellular responses. In fact, numerous studies have reported that AHR modulation affects the expression of a multitude of direct and indirect target genes, not only in a cell- and species-specific fashion but also in a ligand-specific manner. In fact, transcriptome analyses have shown that (i) different AHR ligands induce a different transcriptome profile even within the same cell-type and (ii) the same AHR ligand can induce different transcriptome profiles in different cell-types and tissues [[24], [25], [26], [27]]. The molecular mechanisms underlying this ligand-specificity of AHR signaling are yet poorly understood and may include the induction of different conformational states of the AHR protein, for instance causing altered DNA binding behavior or recruitment of different transcriptional co-factors to AHR, as well as different toxicokinetic parameters of the ligands [28,29]. Ligand-specific modulation of AHR target gene expression may translate to the functional level and affect a multitude of processes, including apoptosis [30] and differentiation of T cell subsets [31,32].
Herein we review the current concepts of AHR activity concerning the generation of reactive oxygen species (ROS) and its interaction with NRF2, the key player in the cellular antioxidant response. We move on to review the literature regarding PM, AHR, and adverse health effects, which involve the immune system, and may affect the heart, lung, and skin.
2. AHR and the generation of ROS
Exposure to PM and PAHs is well known to cause ROS formation and induce oxidative damage to DNA, lipids and other cellular macromolecules. A study on Asian schoolchildren, for instance, revealed a synergistic positive association between outdoor PM concentration and urinary 1-hydroxypyrene (a biomarker for PAH exposure) on one side and urinary malondialdehyde levels (a biomarker for oxidative stress) on the other side [33]. One pathway through which PAHs may induce ROS formation is the AHR-dependent induction of CYP1 isoforms, including CYP1A1, CYP1A2, and CYP1B1. For instance, a study showed that overexpression of recombinant CYP1A1 and CYP1A2 in human lymphoblast-derived microsomes stimulated the production of ROS, such as superoxide anion radicals [34]. Along the same lines, treatment of murine Hepa1c1c7 hepatoma cells and C57BL/6J mice with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a prototypical ligand of AHR, resulted in an enhanced excretion of 8-hydroxyguanine and 8-hydroxydeoxyguanosine, two biomarkers of oxidative DNA damage [35,36]. Importantly, in the in vitro study, the excretion rate of these markers for oxidative DNA damage could be reduced by AHR antagonism and CYP1A1 inhibition [35]. Indeed, ROS production is tightly linked to the catalytic circle of CYP enzymes, more precisely to a phenomenon called “uncoupling” [37]. Briefly, in the presence of NADPH, CYP monooxygenases reduce molecular oxygen to introduce one oxygen equivalent into the substrate and the second one into water. By analyzing the stoichiometry of this reaction, it became evident that most CYP enzymes expend more oxygen than needed to monooxygenize their substrates and, in fact, small amounts of hydrogen peroxide are frequently formed as a by-product of this catalysis [[38], [39], [40]]. In particular, when compounds that are not oxidizable, such as TCDD, induce the formation of the oxygen-bound CYP complex, the lack of an electron acceptor may lead to autooxidation of the CYP enzyme and the subsequent release of superoxide [35,37]. Superoxide dismutates to form hydrogen peroxide and, by undergoing Fe2+-catalyzed Haber-Weiss and Fenton reactions, both superoxide anions and hydrogen peroxide may be converted to highly reactive hydroxyl radicals [39,41].
Recent studies confirm that AHR-dependent induction of CYP1A is a major source of ROS formation in TCDD-treated hepatocytes [42]. This study also showed that a significant and sustained increase of CYP1A1 expression and 7-ethoxyresorufin-O-deethylase enzyme activity over 48 h generates ROS and 8-hydroxydeoxyguanosine in an AHR-dependent manner. In contrast, PM-mediated generation of ROS may involve AHR-independent pathways, which are based on the presence of metals (e.g. iron, nickel, copper), the matrix or other components of PM. The AHR-independent production of ROS is supported by the direct oxidative potential of PM and the rapid generation of ROS, which can be measured in cell free assays [43,44]. It is important to note that the direct oxidative potential of PM is distinctive from the intracellular production of ROS, which can be mediated via activation of AHR by PAHs present in PM from urban areas. The importance of ROS generation for the toxicity induced by PM exposure, however, is unclear and seems to be limited based on a literature review [45].
Besides CYP1 isoforms, aldo-keto reductases (AKR) are involved in the metabolic activation of PAHs and associated with ROS formation. AKRs are cytosolic NADPH-dependent oxidoreductase that convert carbonyl groups to primary and secondary alcohols [46]. AKRs, in particular human AKR1A1 and AKR1C1 – AKR1C4, can oxidize trans-dihydrodiols, intermediates derived from CYP1-mediated oxidation of PAHs, to respective catechols [47,48]. Benzo[a]pyrene (BaP), for instance, is oxidized by CYP1A1 to BaP-7,8-epoxide [49]. In a dihydroxylation reaction, microsomal epoxide hydrolase-1 converts this epoxide to BaP-7,8-trans-dihydrodiol, which is either detoxified by phase II enzymes, again oxidized by CYP1 isoforms, or converted by AKR enzymes to BaP-7,8-catechol [46,49]. In the presence of oxygen, BaP-7,8-catechol undergoes a one-electron oxidation to form an o-semiquinone anion radical, a reaction which releases hydrogen peroxide [50]. If not detoxified by catechol-O-methyltransferases or conjugating phase II enzymes, another one-electron oxidation leads to the formation of BaP-7,8-dione (o-quinone) and superoxide anion radicals [50]. BaP-7,8-dione is highly reactive and either forms DNA adducts [51] or undergoes redox-cycling, i.e. in presence of NADPH it is reduced back to BaP-7,8-catechol [52]. Hence, AKR-mediated metabolism of PAHs contributes to oxidative damage and genotoxicity. Notably, the role of AHR in regulating AKR1 gene expression is not clear yet. An increased expression of AKR1C enzymes was observed in BaP-exposed cell-lines, including human hepatoma, colon carcinoma and breast cancer cells [[53], [54], [55]]. Moreover, knockdown of AHR in MDA-MB-231 breast cancer cells dramatically reduced both basal as well as 3-methylcholanthrene (3MC)-induced expression of AKR1C3 [55]. However, the AHR prototype ligand TCDD has been shown to be ineffective in terms of AKR1C induction and the promoter sequences of the human AKR1C-encoding genes do not contain responsive XRE [53,55], arguing against a regulation of these enzymes through the canonical AHR/ARNT signaling pathway.
Further, there is accumulating evidence in the literature that PM and PAHs may stimulate ROS production by NADPH oxidases (NOX), in particular NOX2 and NOX4 [[56], [57], [58], [59], [60], [61]]. These membrane-bound enzyme complexes can be found in the plasma membrane of various cell types, such as phagocytic cells, endothelial and epithelial cells [62]. In its inactive state, the NOX subunits, three cytoplasmic (Rac1, p47phox, p67phox) and two intrinsic membrane (p22phox, gp91phox) components, are unassembled [62]. Upon activation by cytokines, opsonized bacteria, bacterial lipopolysaccharides (LPS) or other stimuli, the complex assembles and the catalytic subunit, i.e. the heterodimeric flavocytochrome consisting of gp91phox and p22phox, transfers one electron from NADPH to molecular oxygen thereby generating superoxide anions, which then dismutate to hydrogen peroxide [62]. Notably, additional proteins, such as p40phox, probably also play a role for NOX activity, in particular for activation of the complex [62]. In the literature, several ways of interaction between NOX and AHR signaling are described. In epidermal keratinocytes, for instance, PM-bound PAHs caused NOX2-mediated ROS formation by stimulating the phosphorylation of p47phox (also known as neutrophil cytosolic factor 1), a signal for NOX2 complex assembly at the plasma membrane, in an AHR-dependent manner [58]. Subsequently generated ROS initiate MAPK signal transduction resulting in the activation of AP-1 and NF-κB transcription factors and downstream pro-inflammatory responses [58]. In human and rat macrophages, BaP treatment led to a transcriptional upregulation of p47phox through direct binding to a responsive XRE in the promoter region of the gene. In addition, BaP facilitated the translocation of the p47phox protein to the cell membrane of the macrophages and potentiated phorbol myristate acetate-induced superoxide anion production [57]. Another study observed a 3MC-induced induction of the NOX subunit p40phox in liver of C57BL/6J mice, which was absent in the hepatic tissue of AHR liver-specific knockout mice [61]. Corresponding mechanistic analyses in Hepa1c1c7 cells revealed the presence of a functional XRE in the promoter of the murine p40phox gene and demonstrated the critical role of p40phox for the 3MC-induced increase in NADPH oxidase activity and ROS formation [61]. Interestingly, activation of NOX4 by thiol-reactive agents, such as cadmium, arsenic, nickel and mercury, was reported to interfere with AHR signaling [63]. Treatment of human HaCaT keratinocytes with arsenic resulted in a NOX4-dependent generation of oxidative stress. Subsequently, ROS inhibited the catalytic activity of CYP1A1, leading to an accumulation of the endogenous AHR ligand 6-formylindolo[3,2-b]carbazole and an AHR-dependent transcriptional upregulation of CYP1A1 [63,64], which has been previously observed in arsenic-treated cells and mice, too [65,66]. Notably, an interference of ROS with the metabolic degradation of AHR ligands may also account for the enhanced transcriptional activity of AHR observed in glutathione-depleted normal and malignant mammary cells [67].
Additionally, an alternate enzyme for chemical oxidation exists in form of prostaglandin-endoperoxide synthase-2, also known as cyclooxygenase (COX)-2. COX-2 may exemplify an alternate enzyme of xenobiotic metabolism in extrahepatic tissues [68,69]. Previously, it has been shown that AHR activation by TCDD induced the expression and activity of COX-2 [70,71]. Unlike COX-1, the expression of COX-2 is inducible by various stimuli, such as growth factors and cytokines [72]. An elevated expression of COX-2 has been found to be associated with chronic inflammation and carcinogenesis [[73], [74], [75]]. COX-2 converts arachidonic acid to prostaglandin (PG) G2, which undergoes peroxidation to PGH2. In this two-step enzymatic process, which generates ROS, COX represents the rate-limiting enzyme to produce PGs [76,77]. Whereas TCDD and other AHR ligands mediate the expression of CYP1A1 and CYP1A2 via the canonical AHR/ARNT pathway, the TCDD-induced expression of COX-2 involves non-canonical AHR signaling pathways, including activation of c-Src and downstream CCAAT/enhancer binding protein-β [78] and MAPK signal transduction [58,79]. The increased expression of COX-2 may contribute to an enhanced production of ROS. Interestingly, reports demonstrated that elevated levels of COX-2 and ROS cause vasoconstriction and renal endothelial dysfunction [80]. Another study suggested that LPS induces the inhibition of the endothelium-dependent vasodilator response in middle cerebral arteries from normotensive rats [81]. The LPS effect was mediated by the release of superoxide anion generated at least in part via the LPS-activated expression of COX-2.
In summary, several enzyme systems including CYP1A, AKR1, NOX, and COX-2 are regulated through the AHR signaling pathway with the ability to generate ROS in various cell types and tissues.
3. AHR mediated antioxidant response and interaction with NRF2
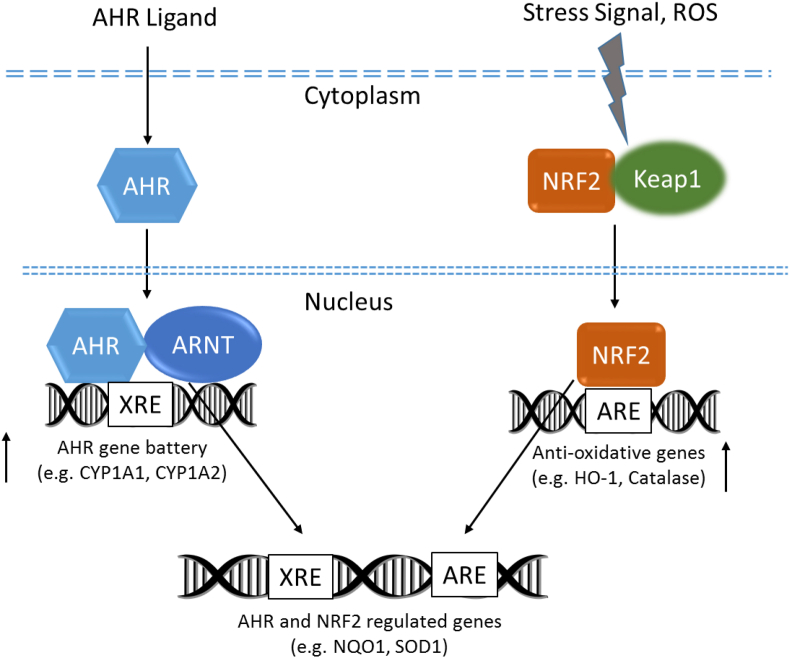
In contrast to AHR-dependent and CYP1A-mediated production of intracellular ROS, the AHR signaling pathway also regulates the expression of genes involved in antioxidant responses. Besides AHR signaling, NRF2 is another important transcription factor regulating genes that are critically involved in the metabolism of xenobiotics as well as endogenous compounds (Fig. 2). Both signaling pathways respond to environmental and endogenous stressors. Even though, AHR and NRF2 are clearly separated signaling pathways, recent reports demonstrate the cross-regulation between these two signaling axes suggesting that a tight connection of the AHR and NRF2 pathways provides an integrated response to environmental stressors [82].
Fig. 2.
AHR- and NRF2-mediated regulation of genes of CYP1A genes and anti-oxidant genes, respectively. AHR and NRF2 can interact and regulate the expression of antioxidant genes containing DNA response elements for both pathways. Possible antioxidant mechanisms are triggered by activation of AHR and NRF2 inducing NQO1 and SOD1.
NRF2 is bound to the Kelch-like ECH-associated protein (KEAP1) in the cytoplasm and is activated by stress signals, like electrophilic molecules or free radicals. After dissociation of KEAP1, NRF2 binds to antioxidant response elements (AREs) inducing antioxidative enzymes, such as heme oxygenase-1, NQO1, superoxide dismutase (SOD), catalase, and thioredoxin-1 to protect against oxidative stress. As shown previously, the promoter region of the target genes NQO1 and SOD1 contain XRE as well as ARE elements and are regulated by both the AHR and NRF2 signaling pathways [[83], [84], [85], [86]]. Furthermore, besides generation of ROS via CYP1A1, AKR1 and COX-2, other mechanisms exist which may contribute to AHR-mediated induction of NRF2-regulated genes. First, Miao et al. (2005) have shown that the promoter of NRF2 contains several XRE elements [87] and that activation of AHR transcriptionally activates NRF2, leading to increases of NRF2 mRNA and protein [88]. Our recent data show the activation of NRF2 in a luciferase reporter assay after treatment with TCDD and diesel emission samples [14]. However, ligand-dependent activation of AHR by TCDD did not induce NRF2 mRNA expression in primary macrophages derived from mouse or human monocytes (unpublished results), indicating a cell type specific induction of NRF2 mRNA expression via AHR. Interestingly, treatment of human epidermal keratinocytes with the antifungal agent ketoconazole has been reported to induce both, the nuclear translocation of NRF2 and downstream NQO1 expression, in an AHR-dependent manner [89], suggesting that non-canonical AHR signaling pathways may activate protein kinases that control KEAP1 stability and thus NRF2 activation [90]. Interestingly, sustained activation of NRF2 in keratinocytes may lead to a chloracne-like skin phenotype in mice [91]. The authors reported that a similar NRF2-dependent mechanism is likely to be involved in dioxin-induced chloracne. Chloracne, a certain type of acne-like skin disease, is a very specific and sensitive biomarker of dioxin exposure in human [92].
4. Activation of AHR via PM, PAHs and POPs associated with adverse health effects and the immune system
Inflammation is a major defense mechanism of the immune system, but if inflammation gets chronic or somehow awry it can become a major cause of adverse health issues. Very early work on AHR and the immune system, mostly using TCDD as a ligand, described the AHR-dependent expression of pro-inflammatory cytokines, such as IL-1β or transforming growth factor-β [[93], [94], [95]], which later expanded to other cytokines, such as IL-6 or IL-22, as well [96,97]. At that time, research on AHR and immunity largely studied TCDD's immunosuppressive effects. Only much later, research on AHR and cytokine induction, and more generally AHR's role in the physiology of the immune system took off. This was due to the initial discovery that AHR is necessary for the differentiation and function of Th17 cells and expression of their signatory cytokine, the pro-inflammatory IL-22. Consequently, induction of AHR signaling by a high-affinity ligand 6-formylindolo[3,2-b]carbazole could exacerbate autoimmunity [31,98]. Other reports demonstrated that AHR-signaling balances the Th17 vs. regulatory T cell differentiation [31,99,100]. Moreover, the concept gained traction that, beyond being a mediator of toxicity, AHR is critical for a meaningful immune response against pathogens and in the context of barrier integrity of, e.g., the skin, gut, or lungs and also for the brain (reviewed in Ref. [11,101,102]). A major discussion point has been which parameters are critical for a beneficial vs. adverse outcome for AHR ligand exposure: source, chemistry, dose, exposure routes, cellular context or a combination thereof. This important issue has not yet been satisfactorily resolved, albeit the Janus-faced role of AHR signaling is an accepted concept by now, including regarding its role mediating effects of air pollution [103,104]. Halogenated aromatic hydrocarbons and PAHs adsorb to airborne particulate matter and thereby become a relevant source of environmental AHR ligands to which humans are exposed [105,106]. By now, an increasing body of research addresses the effects of PM on the immune system, and thereby involve a range of diseases, from cardiovascular diseases, airway diseases, especially regarding Th2 driven asthma/lung damage and skin eczemas, and Th17 driven autoimmunity [[107], [108], [109]]. They are discussed in several sections of this review. Both direct immunological inflammatory effects of airborne particles and indirect exacerbation of particle-induced damage have been reported, with oxidative stress and induction of a range of cytokines, which affect the lung epithelium appearing to be an important mechanism [[110], [111], [112]]. Moreover, insoluble material in the lungs as such can cause oxidative burst in inflammatory cells, e.g., macrophages or neutrophils [111]. However, whether or not AHR signaling is somehow involved in such immune effects, for instance by its regulation of cytokines, is not always clear or has not been explicitly analyzed or even considered. It is noteworthy that after the Seveso-Incident in 1976 – i.e., upon primarily TCDD exposure - the incidence of chronic obstructive pulmonary diseases (COPD) was doubled [113]; in general, PAH-bearing particles are considered causative for COPD [114], suggesting a link between AHR-agonists on air pollution particles with this disease.
A recent study reported, that persistent free radicals associated with combustion-generated particles may activate AHR and induce Th17 related cytokines (IL-1ß, IL-22, IL-33, keratinocyte-derived chemokine) leading to Th17 polarization and IL-17-dependent pulmonary inflammation [115]. The authors concluded that the ROS-mediated effects on Th17 polarization depends at least in part on activation of AHR. The mechanism of ROS-mediated activation of AHR is unclear. Previously, we have shown that the expression of AHR is regulated through a distal NF-κB-binding element located on the promoter of the human AHR gene [116]. Based on this finding, it is possible that ROS induces the expression of AHR via NF-κB signaling. We could also show that ambient urban PM2.5 collected in Sacramento (CA, USA) triggers polarization of Th17 cells mediated by activation of dendritic cells (DC) [117]. We found that PAH-containing PM2.5 directly activate AHR and induce inflammatory markers associated with an enhanced activation of DC responsible for differentiation of naive T cells towards a Th17-like phenotype. The important role of DC mediating elevated IL-17 responses and allergic responses has been confirmed in mice after treatment with vehicular ultrafine PM [118]. The deletion of AHR in CD11c+ cells protected against PM-mediated exacerbation of the allergic responses and PM-mediated IL-17 increase, underlining the key role of DC and AHR. Interestingly, the impact of AHR activation modulating the function of DC during development in mice suggested long-lasting consequences on immune function through environmental exposure in early life [119]. The findings of Joshua Mezrich's group [120] revealed that PAHs are a critical component in PM promoting Th17-polarization and secretion of IL-17A from T cells in vitro. More recently, studies by the same group showed that the PM matrix and chemical components other than PAHs in the organic fraction are also contributing to the observed IL-17 responses in vivo and the associated adverse health effects, including autoimmune diseases [107,121,122].
The skin offers another major interface to air pollution, and studies have looked at the immunological response in skin. Activation of AHR by either diesel exhaust particles or 7,12-dimethylbenz[a]anthracene induced expression of artemin, epidermal hyper-innervation and inflammation in mice. AHR activation and artemin expression positively correlated in the epidermis of patients with atopic dermatitis, an inflammatory condition of the skin, and induced both inflammatory genes, and genes related to skin innervation, leading to hypersensitivity to pruritus [18]. Inhaled PM, and thus the POPs and PAHs adsorbed on them, ultimately reach the gut and indeed can adversely affect the immune system there. Feeding PAH-containing fine air particles to mice resulted in gut inflammation and dysbiosis [123]. PM, POPs and PAHs can be obesogenic or exacerbate obesogenicity via their inflammatory potential in an AHR-dependent manner, which may be used for preventive approaches [[124], [125], [126], [127]]. Future studies are needed to clearly identify the most critical components of PM and their inflammatory potential and effectiveness to induce chronic inflammatory diseases.
5. Impact of chemicals and PM on the lung, ROS and inflammation mediated effects, and possible role of AHR
The lung is at the interface with both the internal and external environment and is susceptible to perturbation by air pollution and chemical exposures both systemically or by inhalation. Further, the lung is a high oxygen environment resulting in susceptibility to oxidant stress. Fortunately, the respiratory system has developed a number of defense mechanisms to resist chemical and particulate injury including abundant innate immune cells (alveolar macrophages) to remove particulates, a mucociliary escalator to remove detritus and metabolizing and antioxidant systems to resist chemical stressors. There is abundant evidence that the lung also contains numerous cells in which the AHR system plays a major role in regulating lung function, particularly in relation to environmental exposures. Lung cells with well studied AHR responses include a number of cell types that also have CYP monooxygenase activity, such as macrophages [129,130] and conducting airway and alveolar epithelial cells [[131], [132], [133]], particularly Club cells and alveolar type II cells. TRAP contains abundant PAHs in both the vapor and particulate phases. A series of studies using laboratory-generated combustion PM that was PAH rich vs. a similar PM that was not PAH rich found that PAH-rich PM was more impactful on lung antioxidant enzymes and caused more injury in the lung [134]. Further, PAH-rich particles alone were capable of stimulating AHR activity and an increase of CYP1A1 and CYP1B1 expression in vivo [133]. This PAH-rich PM exposure system is useful for teasing out the effects of carbonaceous PM associated with combustion generated PAHs, approximating near roadway exhaust, and this type of exposure disrupts postnatal lung development [135]. Work in 7 day old neonatal rats has shown that PAH-rich PM can activate the AHR in both the airways and the alveoli [133,134]. Further, neonates are more susceptible to inhaled PAH-rich PM lung toxicity than adults [134] and this exposure changes how the lung grows [136]. Other studies of combustion-generated soot have found that particles activate both AHR and NRF2 as well as inflammation related genes (see prior discussion in this manuscript of these responses and their interaction with AHR) [[137], [138], [139], [140]].
The AHR is now drawing much attention in the field of chronic lung diseases. It is becoming clear that AHR amplifies the adverse effect of tobacco smoke on ciliated cells, Club cells, and alveolar macrophages in mediating the pathogenesis of COPD as well as lung cancers [141,142]. Tobacco smoke and PM therein contain dioxins and PAHs that activate the AHR and mediate at least part of their toxicity through AHR signaling. Many PAHs are ligands of the AHR and are known to irritate the developing lung, predisposing to infections, reduced lung function, and increasing asthma. PAHs, including BaP, PCBs and dioxin-like compounds in tobacco smoke have the potential to induce inflammation and exacerbate chronic bronchitis, asthma, COPD, and lung cancer [143,144]. Furthermore, Kim et al. (2004) demonstrated that AHR promotes induction of CYP1A1 and CYP1B1, particularly among smokers [145]. Recent studies show that exposure to tobacco products, such as electronic cigarette aerosol, also activates the AHR pathway and induces CYP1A1, thus confirming the critical role of AHR to mediate toxicity of smoke from traditional tobacco cigarettes as well as from electronic cigarettes [146]. Lin et al. (2003) have shown that expression of AHR among human lung cancer patients is more intense in adenocarcinoma than in squamous cell carcinomas or matched normal cells [147]. As for potential biomarkers, obvious candidates are inflammatory markers, CYP1A1, and, as discussed below, lung-specific genes, such as cyclin O and mucin 5AC. The AHR-dependent up-regulation of inflammatory genes and mucin 5AC production by tobacco smoke has been shown in lung epithelial cells [148]. Together these studies show that the AHR is critical for pulmonary responses induced by tobacco smoke and PM and it will be important to define how the AHR amplifies the adverse effects and thereby promotes pathogenesis of chronic lung diseases.
Recently, it became evident that the AHR is involved in normal physiological processes of the lung and may affect lung growth and development. AHR regulates Muc5AC expression [[148], [149], [150]] and disordered AHR can affect alveolar growth [151]. Additionally, cyclin O, a factor required for multiciliogenesis, has been identified as a direct target of AHR in mouse tracheal epithelial cells [152]. There have been studies of hyperoxia related bronchopulmonary dysplasia. In this model, the genome-wide transcriptional profile in newborn C57BL6 mice shows that AHR has a critical regulatory role in lung development [153], which is correlated by studies of human fetal lung and hyperoxia [154] lending credence to the concept that AHR is involved in lung growth, development and response to injury. Studies of hyperoxia in AHR dysfunctional mice found that a decrease in pulmonary AHR activation in newborn mice increased hyperoxia-induced alveolar simplification and lung inflammation [151], supporting the hypothesis that a lack of AHR signaling changes normal lung development. While developmental roles for AHR have been most studied in animal models of bronchopulmonary dysplasia, it has been increasingly recognized to play a crucial role in normal physiological homeostasis of the lung [155], including ciliogenesis [152,156], mucin production [149], immune function [141] and traffic PM-mediated airways hyperresponsiveness [118]. AHR has a well-established role in regulation of inflammation in the lung, and DC and macrophages are key players in this response [141]. AHR in the lung promotes induction of CYP1A1 and CYP1B1, particularly among smokers or after PM exposure in human alveolar type I and II cells, Club cells and ciliated columnar epithelial cells lining airways. Furthermore, AHR-dependent regulation of antioxidant enzymes, such as NQO1, SOD, GSTs and UGTs, have been described above, indicating that deficiency or changes in AHR activity may lead to complex changes in enzymes of oxidative stress in the lung [157]. Whereas, activation of AHR by high doses of TCDD leads to significant induction of CYP1A1 and increased levels of ROS and inflammatory markers in the lung [158], low-dose and transient activation of AHR (e.g. natural and endogenous AHR ligands) may maintain the expression of antioxidative genes to protect from cell damage and tissue injury. While it is obvious that there are complex mechanisms involved in the activation of the AHR signaling pathway in the lung, the role of AHR implicated in molecular mechanisms of lung development and remodeling are only little understood.
6. Impact of environmental pollutants and PM on the cardiovascular system: ROS and inflammation mediated effects, and possible role of AHR
The following chapter focuses on the role of AHR mediating the effects of environmental pollutants including ambient PM leading to adverse health effects of the cardiovascular system. The correlation between exposure to POPs and the development of cardiovascular diseases (CVD) in humans, including acute myocardial infarction, has been described in several studies [[159], [160], [161]]. Epidemiologic studies also showed that occupational exposure to high doses of dioxin is associated with ischemic heart disease [162]. Further, a study examining U.S. Army veterans exposed to Agent Orange, a defoliant contaminated with dioxin, found a higher incidence of hypertension, stroke, and coronary heart disease in Vietnam and Korean veterans [163,164]. Another study including 12 cohorts also suggested that dioxin exposure is associated with mortality from ischemic heart disease [165]. These studies strongly suggest that exposure to POPs, such as dioxins, increase the risk of CVD.
Atherosclerosis is a chronic inflammatory condition and the primary cause of ischemic heart disease and stroke, leading to about 50% of all deaths in Western countries [166,167]. There is increasing evidence that exposure to air pollutants and ambient PM contribute to atherosclerotic cardiovascular disease (ASCVD) and the incidence of CVD [[168], [169], [170]]. Recent studies support the conclusion that exposure to air pollution causes an even higher acute risk for mortality from CVD than from pulmonary diseases [171]. Thus, the impact of air pollution on CVD represents a serious public health problem [172]. Air pollution and PM contain a complex mixture of compounds, such as PAHs and various metals (e.g. iron, nickel, copper), which may induce inflammatory responses [173,174]. Numerous studies implicate inflammatory pathways in early atherogenesis and the progression of the lesions [167]. A major source of ambient PM, especially in industrialized areas, is the combustion of fossil fuels by motor vehicles and industry, including power generation [6]. TRAP in urban areas is a main contributor to unhealthy ambient air quality, and is associated with increased cardiac and pulmonary mortality [175,176]. A recent study also showed a clearly stronger association of subclinical atherosclerosis with exposure to vehicular-specific PM as compared to the crustal source PM [177]. About 15% of the total U.S. population lives and works near major highways and thus have an increased risk to develop cardiovascular and pulmonary health problems [178]. The mechanisms by which PM, including TRAP emissions, is recognized on a cellular level and how chronic inflammatory responses lead to atherosclerosis are poorly understood. In recent years, PM generated by combustion from heavy-duty vehicles has been found to contain significant amounts of PAHs compared to light-duty vehicles and other PM sources [7,179]. AHR-dependent activation of CYP1A1 and inflammatory cytokines, including IL-8 and IL-1β, after exposure to PAH-containing PM has been shown in different cell types as well as in vivo [13,130,[180], [181], [182], [183], [184]]. Recent studies indicate that PAHs are major contributors to PM-mediated atherogenesis and AHR-induced toxicity [105,118,[185], [186], [187], [188]]. Despite evidence for a role of inflammatory mediators in atherosclerosis, less is known about how the expression of these inflammatory mediators are regulated through exposure to air pollutants. Recent studies implicate the interaction of PM and its components with AHR as a key event leading to elevated levels of pro-inflammatory cytokines, such as IL-1β, IL-8, monocyte chemotactic protein 1 (MCP-1; a.k.a. CCL2), CXCL2 and the promotion of Th17-immune responses [120,130,180]. Using an in vitro bioassay consisting of human macrophages, we found that activation of AHR leads to accumulation of lipids and foam cell formation [189]. Foam cell formation is an early indication of the development of atherosclerotic lesions. In the apolipoprotein E-deficient mouse model treatment with TCDD caused the progression of atherosclerosis, which was associated with induction of inflammatory markers and accumulation of foam cells [190]. Interestingly treatment with an inhibitor of CXCR2, a chemokine receptor for CXCL2 and IL-8, reduced the dioxin-induced and AHR-dependent progression of atherosclerotic lesions. The AHR-mediated foam cell formation in vivo was associated with increased expression of IL-1β and matrix metalloproteinases (MMP) in atherosclerotic plaques [190]. MMPs and IL-1β are critical players in atherogenesis and plaque disruption [191].
These data are in line with a previous study finding earlier and more severe atherosclerotic lesions in descending aortas associated with an increase in blood pressure and lipids after sub-chronic exposure to dioxin in apolipoprotein E-deficient mice [192]. These reports show that the atherogenic activity of environmental pollutants, such as dioxins and dioxin-like compounds contribute to the development of atherosclerosis through the induction of a vascular inflammatory response by activating the AHR-signaling pathway. There was also evidence that exposure to PM2.5 impairs the function of endothelial progenitor cells leading to vascular injury [193]. The authors concluded that pulmonary oxidative stress induced by PM2.5 exposure significantly contributed to the dysfunction of endothelial progenitor cells. These studies indicate that environmental pollutants, in particular ambient PM and their chemical components, may induce oxidative stress as well as inflammatory mediators leading to vascular injury and ASCVD.
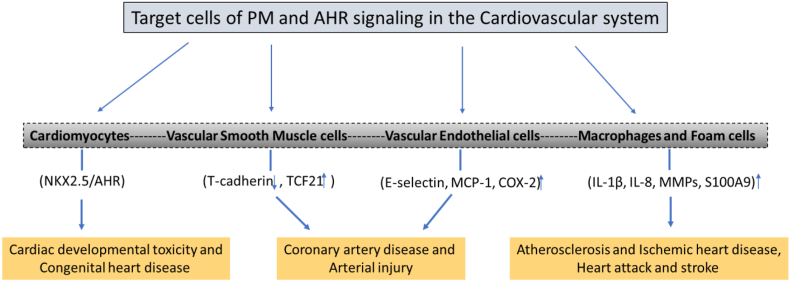
As shown in Fig. 3, activation or deficiency of AHR signaling can affect several cell types of the cardiovascular system, including cardiomyocytes [194], endothelial cells [21,180], and smooth muscle cells [[195], [196], [197]]. Consequently, the AHR may affect the function and development of the heart directly or target the cells, which are important to maintain function and homeostasis of the cardiovascular system. For instance, in vascular smooth muscle cells ligands of the AHR repressed T-cadherin expression [195]. T-cadherin is a highly expressed adhesion protein in cardiac and vascular tissues. In a different study with coronary artery smooth muscle cells, the interaction of transcription factor 21 (TCF21) and AHR has been described leading to the activation of pro-inflammatory gene expression [197]. Aung et al. (2011) investigated the effects of fine and ultrafine PM in human aortic endothelial cells [180]. The authors found that PM activated the AHR signaling pathway and induces the expression of inflammatory response genes, including E-selectin, CXCL2, MCP-1 and COX-2, in human aortic endothelial cells. Further, the importance of immune cells and inflammation in atherosclerosis is well established [198] and activation of AHR by POPs, including dioxins and PCBs, may affect the function and differentiation of macrophages, dendritic cells, and T lymphocytes [11]. Therefore, changes in inflammatory responses (e.g. expression of pro-inflammatory cytokines, MMPs, and S100 calcium-binding protein A9) induced by AHR ligands in immune cells have to be considered as an important step in the development of vascular lesions and ASCVD.
Fig. 3.
PM and AHR mediated effects on target cells and markers of the cardiovascular system and potential outcomes of CVD. NK2 homeobox 5 (NKX2.5); transcription factor 21 (TCF21), monocyte chemotactic protein 1 (MCP-1); matrix metalloproteinases (MMPs); S100 calcium-binding protein A9 (S100A9).
Lanham et al. (2014) reported that TCDD exposure in zebrafish leads to cardiovascular toxicity [199]. In this study, the authors found that a constitutively active AHR in cardiomyocytes of zebrafish would lead to cardiac malformations, loss of circulation, and pericardial edema. In earlier studies Rick Peterson's group [200] demonstrated that TCDD significantly reduced the development of the common cardinal vein in zebrafish embryos, resulting in alterations of vascular growth and remodeling in an AHR-dependent manner. More recently, Alvaro Puga's group [194] performed a similar study with mice using mouse strains with cardiomyocyte-specific AHR gene knockout. The results showed that AHR ablation protected haploinsufficient male mice from cardiac dysfunction. Interestingly, the effects were almost absent in female mice and suggested the interaction of AHR with the cardiomyocyte-specific NK2 homeobox 5 (NKX2.5) gene (Fig. 3). NKX2.5 is a homeobox-containing transcription factor and functions in heart formation and development. Furthermore, it has been shown that PM exposure may cause cardiac developmental toxicity in a zebrafish model [201]. Using the AHR antagonist CH223191 and the ROS scavenger N-acetyl-l-cysteine the authors concluded that PM and AHR-mediated generation of ROS contribute to DNA damage and apoptosis, causing cardiac developmental toxicity in zebrafish embryos. On the other hand, the organic fraction containing PAHs of PM2.5 extracts inhibited cardiac differentiation in an AHR-dependent using a mouse embryonic carcinoma stem cell model (186).
Future studies need to identify the mechanisms how and to what extent ROS and chronic inflammation contribute to cardiac developmental toxicity mediated by PM and AHR signaling.
7. AHR, ROS and inflammation: effects on skin
The epidermal barrier is essential to protect the underneath tissues and structures. Originating from epidermal stem cells in the basal layer, keratinocytes differentiate towards their way through the epidermal layers to the skin surface and finally transform to dead corneocytes. During this process of terminal differentiation, the plasma membrane of the keratinocytes is replaced by lipids, such as ceramides, fatty acids, and cholesterol, which interact with filaggrin, involucrin, loricrin and related proteins to build a compact lipid-protein complex called cornified envelope. In concert with other epidermal structures, e.g., tight junctions located in the stratum granulosum, the cornified envelope protects the skin against microbial insults, chemical exposure, ultraviolet radiation, mechanical stress, and dehydration. Disturbance of the epidermal barrier occurs in the majority of inflammatory skin diseases, including atopic dermatitis (AD), contact dermatitis and psoriasis [202,203]. As discussed above, cutaneous exposure to airborne PM and PAHs causes the formation of ROS, which may contribute to the development and/or aggravation of inflammatory skin diseases. Oxidative stress may either directly disturb keratinocyte differentiation and associated epidermal barrier function or stimulate MAPK signal transduction and downstream NF-κB and AP-1 transcription factors in keratinocytes to induce the expression of proinflammatory enzymes and cytokines, such as COX-2, tumor necrosis factor-α, and IL-4. COX-2, for instance, produces PGE2, which downregulates filaggrin expression in keratinocytes and thereby attenuates skin barrier function [58,204]. Likewise, IL-4, IL-13 and related Th2-derived cytokines are known to activate signal transducer and activator of transcription-6 and repress the expression of loricrin and involucrin in the epidermis from AD patients [205,206]. ROS originating from AHR-related processes may have also contributed to the degranulation of mast cells and the associated release of histamine and IL-4 observed in the nasal epithelium of human individuals challenged with house dust mite and diesel exhaust particles [207]. Another cytokine frequently present in chronic inflammatory skin diseases is IL-17, which also decreases filaggrin expression in keratinocytes [208] and is under transcriptional control of AHR [31,98].
In fact, several studies provided evidence for an involvement of AHR in the pathogenesis of AD [18,209], psoriasis [210], and vitiligo [211]. AD, for instance, results from a complex interplay between environmental and genetic factors and is characterized by skin barrier dysfunction, overexpression of proinflammatory cytokines and activation of Th2 cell-driven immune responses [212]. Interestingly, exposure to air pollution [3], in particular PAH-rich pollutants, such as tobacco smoke and TRAP [17,18,[213], [214], [215]], is positively associated with the development and worsening of AD in humans. In addition, a recent epidemiological study found a positive correlation between eczema in elderly women, exposure to TRAP and a single nucleotide polymorphism (rs2066853) in the AHR gene locus [216]. In contrast to these findings, exposure to dioxin-like AHR agonists seems to lower the risk of developing AD [[217], [218], [219]]. The underlying causes for this difference are quite enigmatic but may involve a ligand-specific modulation of proinflammatory and/or immunosuppressive responses. However, in contrast to an exposure to PAHs, TCDD treatment even accelerates keratinocyte differentiation and cornification in a ROS- and sirtuin 1-dependent manner [220,221]. On the other hand, these halogenated aromatic hydrocarbons also induce immunosuppressive regulatory T cells [31,99], which may alleviate the contribution of Th2 and Th17 lymphocytes to the pathogenesis of the disease. Even more surprisingly, PAH-rich crude coal tar is being used since decades to treat AD [209]. Topical application of the bacterial-derived AHR agonist tapinarof was also found to attenuate the clinical symptoms of AD [222]. Tapinarof as well as several other natural AHR agonists accelerate keratinocyte differentiation in vitro, which, at least in part, is triggered by an NRF2-mediated induction of antioxidative enzymes and the associated neutralization of oxidative stress [[223], [224], [225]]. However, the obvious discrepancy between PAHs as both risk factor and remedy for AD may be due to alterations in the microenvironment of healthy vs. inflamed skin and respective differences in skin-residing immune cell populations or epidermal barrier function at the time of exposure [103]. A recent study showed that the anti-inflammatory effect of medicinal coal tar is largely based on AHR-dependent induction of anti-microbial peptides [226] and, interestingly, tapinarof has antibiotic properties as well [227]. Hence, the beneficial effect of these AHR agonists may depend on a modulation of the skin microbiome, which significantly differs between AD patients and healthy individuals [228,229]. Lesional skin of AD patients exhibits a decreased microbial diversity together with an increased colonialization with Staphylococcus aureus [228]. Other bacteria, such as Cutibacterium acnes (formerly Propionibacterium acnes), are lower in abundance in AD as compared to healthy skin [229]. A small clinical study assessing the alterations of the skin microbiome in AD patients and healthy controls revealed that under topical coal tar treatment, the abundance of Staphylococcus aureus decreased while the Cutibacterium acnes abundance increased [226]. Interestingly, even though exhibiting proinflammatory properties when dominating in acne vulgaris [230], Cutibacterium acnes was recently shown to protect skin against oxidative stress by secreting antioxidant proteins, such as RoxP [231]. Hence, treatment of inflamed skin with certain AHR ligands partially restores the microbiome of healthy skin, which may be associated with a production of bacterial antioxidants and a neutralization of ROS, one of the driving forces in the pathogenesis of AD [209,212]. Further mechanistic studies, in particular considering the skin condition and the associated composition of the microbiome at the time of PM/PAH exposure, are needed to better understand the Janus-faced role of AHR in inflammatory skin diseases.
8. Conclusion and outlook
In industrialized countries, the prevalence of non-communicable diseases, including allergic, cardiovascular, respiratory and metabolic diseases, as well as cancer, is rapidly rising and poses not only a major threat to the health of the general population but also a tremendous economic challenge for health care systems. There is broad consensus that chronic exposure to air pollution, i.e. PM and associated PAHs, POPs and HMs, significantly contributes to this steep increase.
However, there are still many gaps in our knowledge how exactly particulate matter drives diseases. We here focus on the AHR as a sensor of PAHs and POPs adsorbed to particulate matter. Much data exist that show how AHR-activation by PAHs/POPs and the associated modulation of metabolic reactions, cytokine patterns and immune responses, results in oxidative stress, tissue damage and proinflammatory conditions in various target tissues, such as the lung, the skin, and the cardiovascular system. However, only recently, studies directly began to analyze the link “PM-PAH-AHR”, i.e. showed that PM adsorbed PAHs trigger AHR-dependent cellular changes. This development is welcome and will give important insights into the differing toxicity of PM whose PAH content changes depending on the geographical location or source of PM. Another important consideration regarding predictions of adverse health effects is the complexity of interactions of air pollution with AHR signaling in the target tissue and the potential pathophysiological responses. Both the properties of the particles and the context-dependent outcome of AHR-signaling in the particular tissues are relevant, as has been detailed in the above review. An interplay of these variables ultimately defines the toxicodynamic and toxicokinetic properties of the respective pollutants. Understanding this is necessary for proper risk assessment and for possible therapeutic interventions (notwithstanding the priority of reducing pollution). Accordingly, there is a clear and urgent need for further research aiming to unravel functional relevance of AHR for air pollution-associated adverse health effects and to assess its suitability as a target for molecular prevention.
We, the authors, are enthusiastic that the fast evolution of modern genome editing techniques, tailored experimental models, and Omics technologies, along with the ongoing developments in the areas of exposure assessment and gene-environment interaction studies will help to clarify these issues of global health relevance within the next years.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
The research of C.E. is in part funded through the Deutsche Forschungsgemeinschaft (DFG), grant ES103/9-1. The research of T.H.S. is supported by DFG grant HA7346/2-2. Research of C.F.A.V. reported in this publication is supported in part by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R21 ES030419 and R01 ES029126.
References
- 1.Wu W., Jin Y., Carlsten C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 2018;141(3):833–844. doi: 10.1016/j.jaci.2017.12.981. [DOI] [PubMed] [Google Scholar]
- 2.Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.H., Mortimer K., Perez-Padilla R., Rice M.B., Riojas-Rodriguez H., Sood A., Thurston G.D., To T., Vanker A., Wuebbles D.J. Air pollution and noncommunicable diseases: a review by the forum of international respiratory societies' environmental committee, Part 1: the damaging effects of air pollution. Chest. 2019;155(2):409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014;134(5):993–999. doi: 10.1016/j.jaci.2014.09.023. discussion 1000. [DOI] [PubMed] [Google Scholar]
- 4.Heusinkveld H.J., Wahle T., Campbell A., Westerink R.H.S., Tran L., Johnston H., Stone V., Cassee F.R., Schins R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. doi: 10.1016/j.neuro.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Lim C.C., Thurston G.D. Air pollution, oxidative stress, and diabetes: a life course epidemiologic perspective. Curr. Diabetes Rep. 2019;19(8):58. doi: 10.1007/s11892-019-1181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie M., Coons T.L., Hemann J.G., Dutton S.J., Milford J.B., Peel J.L., Miller S.L., Kim S.Y., Vedal S., Sheppard L., Hannigan M.P. Intra-urban spatial variability and uncertainty assessment of PM2.5 sources based on carbonaceous species. Atmos. Environ. 1994;60:305–315. doi: 10.1016/j.atmosenv.2012.06.036. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phuleria H.C., Geller M.D., Fine P.M., Sioutas C. Size-resolved emissions of organic tracers from light- and heavy-duty vehicles measured in a California roadway tunnel. Environ. Sci. Technol. 2006;40(13):4109–4118. doi: 10.1021/es052186d. [DOI] [PubMed] [Google Scholar]
- 8.Aristizabal B.H., Gonzalez C.M., Morales L., Abalos M., Abad E. Polychlorinated dibenzo-p-dioxin and dibenzofuran in urban air of an Andean city. Chemosphere. 2011;85(2):170–178. doi: 10.1016/j.chemosphere.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Ma J., Chen Z., Wu M., Feng J., Horii Y., Ohura T., Kannan K. Airborne PM2.5/PM10-associated chlorinated polycyclic aromatic hydrocarbons and their parent compounds in a suburban area in Shanghai, China. Environ. Sci. Technol. 2013;47(14):7615–7623. doi: 10.1021/es400338h. [DOI] [PubMed] [Google Scholar]
- 10.Nebert D.W. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothhammer V., Quintana F.J. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019;19(3):184–197. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]
- 12.Weng C.M., Wang C.H., Lee M.J., He J.R., Huang H.Y., Chao M.W., Chung K.F., Kuo H.P. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy. 2018;73(11):2192–2204. doi: 10.1111/all.13462. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto Y., Ide F., Kishi R., Akutagawa T., Sakai S., Nakamura M., Ishikawa T., Fujii-Kuriyama Y., Nakatsuru Y. Aryl hydrocarbon receptor plays a significant role in mediating airborne particulate-induced carcinogenesis in mice. Environ. Sci. Technol. 2007;41(10):3775–3780. doi: 10.1021/es062793g. [DOI] [PubMed] [Google Scholar]
- 14.Vogel C.F.A., Kado S.Y., Kobayashi R., Liu X., Wong P., Na K., Durbin T., Okamoto R.A., Kado N.Y. Inflammatory marker and aryl hydrocarbon receptor-dependent responses in human macrophages exposed to emissions from biodiesel fuels. Chemosphere. 2019;220:993–1002. doi: 10.1016/j.chemosphere.2018.12.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L., Guo W., Zeng D., Ma L., Lai X., Fang Q., Guo H., Zhang X. Heart rate variability mediates the association between polycyclic aromatic hydrocarbons exposure and atherosclerotic cardiovascular disease risk in coke oven workers. Chemosphere. 2019;228:166–173. doi: 10.1016/j.chemosphere.2019.04.101. [DOI] [PubMed] [Google Scholar]
- 16.Hu C., Hou J., Zhou Y., Sun H., Yin W., Zhang Y., Wang X., Wang G., Chen W., Yuan J. Association of polycyclic aromatic hydrocarbons exposure with atherosclerotic cardiovascular disease risk: a role of mean platelet volume or club cell secretory protein. Environ. Pollut. 2018;233:45–53. doi: 10.1016/j.envpol.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Hew K.M., Walker A.I., Kohli A., Garcia M., Syed A., McDonald-Hyman C., Noth E.M., Mann J.K., Pratt B., Balmes J., Hammond S.K., Eisen E.A., Nadeau K.C. Childhood exposure to ambient polycyclic aromatic hydrocarbons is linked to epigenetic modifications and impaired systemic immunity in T cells. Clin. Exp. Allergy. 2015;45(1):238–248. doi: 10.1111/cea.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidaka T., Ogawa E., Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Fujimura T., Aiba S., Nakayama K., Okuyama R., Yamamoto M. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017;18(1):64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 19.Karimi P., Peters K.O., Bidad K., Strickland P.T. Polycyclic aromatic hydrocarbons and childhood asthma. Eur. J. Epidemiol. 2015;30(2):91–101. doi: 10.1007/s10654-015-9988-6. [DOI] [PubMed] [Google Scholar]
- 20.IARC Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 21.Eckers A., Jakob S., Heiss C., Haarmann-Stemmann T., Goy C., Brinkmann V., Cortese-Krott M.M., Sansone R., Esser C., Ale-Agha N., Altschmied J., Ventura N., Haendeler J. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 2016;6:19618. doi: 10.1038/srep19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita A., Torii K., Maeda A., Yamaguchi Y. Molecular basis of tobacco smoke-induced premature skin aging. J. Invest. Dermatol. Symp. Proc. 2009;14(1):53–55. doi: 10.1038/jidsymp.2009.13. [DOI] [PubMed] [Google Scholar]
- 23.Murray I.A., Patterson A.D., Perdew G.H. Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Canc. 2014;14(12):801–814. doi: 10.1038/nrc3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dere E., Lee A.W., Burgoon L.D., Zacharewski T.R. Differences in TCDD-elicited gene expression profiles in human HepG2, mouse Hepa1c1c7 and rat H4IIE hepatoma cells. BMC Genom. 2011;12:193. doi: 10.1186/1471-2164-12-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson E.A., McCulloch C., Koganti A., Goodwin S.B., Sutter T.R., Silkworth J.B. Divergent transcriptomic responses to aryl hydrocarbon receptor agonists between rat and human primary hepatocytes. Toxicol. Sci. 2009;112(1):257–272. doi: 10.1093/toxsci/kfp200. [DOI] [PubMed] [Google Scholar]
- 26.Farmahin R., Crump D., O'Brien J.M., Jones S.P., Kennedy S.W. Time-dependent transcriptomic and biochemical responses of 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) are explained by AHR activation time. Biochem. Pharmacol. 2016;115:134–143. doi: 10.1016/j.bcp.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Nault R., Forgacs A.L., Dere E., Zacharewski T.R. Comparisons of differential gene expression elicited by TCDD, PCB126, betaNF, or ICZ in mouse hepatoma Hepa1c1c7 cells and C57BL/6 mouse liver. Toxicol. Lett. 2013;223(1):52–59. doi: 10.1016/j.toxlet.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denison M.S., Faber S.C. And now for something completely different: diversity in ligand-dependent activation of Ah receptor responses. Curr. Opin. Toxicol. 2017;2:124–131. doi: 10.1016/j.cotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyot E., Chevallier A., Barouki R., Coumoul X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov. Today. 2013;18(9–10):479–486. doi: 10.1016/j.drudis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Matikainen T., Perez G.I., Jurisicova A., Pru J.K., Schlezinger J.J., Ryu H.Y., Laine J., Sakai T., Korsmeyer S.J., Casper R.F., Sherr D.H., Tilly J.L. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat. Genet. 2001;28(4):355–360. doi: 10.1038/ng575. [DOI] [PubMed] [Google Scholar]
- 31.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Farez M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 32.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae S., Pan X.C., Kim S.Y., Park K., Kim Y.H., Kim H., Hong Y.C. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ. Health Perspect. 2010;118(4):579–583. doi: 10.1289/ehp.0901077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puntarulo S., Cederbaum A.I. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic. Biol. Med. 1998;24(7–8):1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 35.Park J.Y., Shigenaga M.K., Ames B.N. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc. Natl. Acad. Sci. U. S. A. 1996;93(6):2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shertzer H.G., Nebert D.W., Puga A., Ary M., Sonntag D., Dixon K., Robinson L.J., Cianciolo E., Dalton T.P. Dioxin causes a sustained oxidative stress response in the mouse. Biochem. Biophys. Res. Commun. 1998;253(1):44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- 37.Albertolle M.E., Guengerich F.P. The relationships between cytochromes P450 and H2O2: production, reaction, and inhibition. J. Inorg. Biochem. 2018;186:228–234. doi: 10.1016/j.jinorgbio.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorsky L.D., Koop D.R., Coon M.J. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J. Biol. Chem. 1984;259(11):6812–6817. [PubMed] [Google Scholar]
- 39.Ingelman-Sundberg M., Johansson I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes P-450. J. Biol. Chem. 1984;259(10):6447–6458. [PubMed] [Google Scholar]
- 40.Kuthan H., Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur. J. Biochem. 1982;126(3):583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- 41.Kukielka E., Cederbaum A.I. NADPH- and NADH-dependent oxygen radical generation by rat liver nuclei in the presence of redox cycling agents and iron. Arch. Biochem. Biophys. 1990;283(2):326–333. doi: 10.1016/0003-9861(90)90650-n. [DOI] [PubMed] [Google Scholar]
- 42.Knerr S., Schaefer J., Both S., Mally A., Dekant W., Schrenk D. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced cytochrome P450s alter the formation of reactive oxygen species in liver cells. Mol. Nutr. Food Res. 2006;50(4–5):378–384. doi: 10.1002/mnfr.200500183. [DOI] [PubMed] [Google Scholar]
- 43.Ayres J.G., Borm P., Cassee F.R., Castranova V., Donaldson K., Ghio A., Harrison R.M., Hider R., Kelly F., Kooter I.M., Marano F., Maynard R.L., Mudway I., Nel A., Sioutas C., Smith S., Baeza-Squiban A., Cho A., Duggan S., Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential--a workshop report and consensus statement. Inhal. Toxicol. 2008;20(1):75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- 44.Weissenberg A., Sydlik U., Peuschel H., Schroeder P., Schneider M., Schins R.P., Abel J., Unfried K. Reactive oxygen species as mediators of membrane-dependent signaling induced by ultrafine particles. Free Radic. Biol. Med. 2010;49(4):597–605. doi: 10.1016/j.freeradbiomed.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Ovrevik J. Oxidative potential versus biological effects: a review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019;20(19) doi: 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penning T.M. Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2014;27(11):1901–1917. doi: 10.1021/tx500298n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burczynski M.E., Harvey R.G., Penning T.M. Expression and characterization of four recombinant human dihydrodiol dehydrogenase isoforms: oxidation of trans-7, 8-dihydroxy-7,8-dihydrobenzo[a]pyrene to the activated o-quinone metabolite benzo[a]pyrene-7,8-dione. Biochemistry. 1998;37(19):6781–6790. doi: 10.1021/bi972725u. [DOI] [PubMed] [Google Scholar]
- 48.Palackal N.T., Burczynski M.E., Harvey R.G., Penning T.M. The ubiquitous aldehyde reductase (AKR1A1) oxidizes proximate carcinogen trans-dihydrodiols to o-quinones: potential role in polycyclic aromatic hydrocarbon activation. Biochemistry. 2001;40(36):10901–10910. doi: 10.1021/bi010872t. [DOI] [PubMed] [Google Scholar]
- 49.Gelboin H.V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol. Rev. 1980;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- 50.Penning T.M., Ohnishi S.T., Ohnishi T., Harvey R.G. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem. Res. Toxicol. 1996;9(1):84–92. doi: 10.1021/tx950055s. [DOI] [PubMed] [Google Scholar]
- 51.Shou M., Harvey R.G., Penning T.M. Reactivity of benzo[a]pyrene-7,8-dione with DNA. Evidence for the formation of deoxyguanosine adducts. Carcinogenesis. 1993;14(3):475–482. doi: 10.1093/carcin/14.3.475. [DOI] [PubMed] [Google Scholar]
- 52.Shultz C.A., Quinn A.M., Park J.H., Harvey R.G., Bolton J.L., Maser E., Penning T.M. Specificity of human aldo-keto reductases, NAD(P)H:quinone oxidoreductase, and carbonyl reductases to redox-cycle polycyclic aromatic hydrocarbon diones and 4-hydroxyequilenin-o-quinone. Chem. Res. Toxicol. 2011;24(12):2153–2166. doi: 10.1021/tx200294c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burczynski M.E., Lin H.K., Penning T.M. Isoform-specific induction of a human aldo-keto reductase by polycyclic aromatic hydrocarbons (PAHs), electrophiles, and oxidative stress: implications for the alternative pathway of PAH activation catalyzed by human dihydrodiol dehydrogenase. Canc. Res. 1999;59(3):607–614. [PubMed] [Google Scholar]
- 54.Hockley S.L., Arlt V.M., Brewer D., Giddings I., Phillips D.H. Time- and concentration-dependent changes in gene expression induced by benzo(a)pyrene in two human cell lines, MCF-7 and HepG2. BMC Genom. 2006;7:260. doi: 10.1186/1471-2164-7-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamashita N., Kanno Y., Saito N., Terai K., Sanada N., Kizu R., Hiruta N., Park Y., Bujo H., Nemoto K. Aryl hydrocarbon receptor counteracts pharmacological efficacy of doxorubicin via enhanced AKR1C3 expression in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2019;516(3):693–698. doi: 10.1016/j.bbrc.2019.06.119. [DOI] [PubMed] [Google Scholar]
- 56.Smith J., Neupane R., McAmis W., Singh U., Chatterjee S., Raychoudhury S. Toxicity of polycyclic aromatic hydrocarbons involves NOX2 activation. Toxicol. Rep. 2019;6:1176–1181. doi: 10.1016/j.toxrep.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinel-Marie M.L., Sparfel L., Desmots S., Fardel O. Aryl hydrocarbon receptor-dependent induction of the NADPH oxidase subunit NCF1/p47 phox expression leading to priming of human macrophage oxidative burst. Free Radic. Biol. Med. 2009;47(6):825–834. doi: 10.1016/j.freeradbiomed.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Lee C.W., Lin Z.C., Hu S.C., Chiang Y.C., Hsu L.F., Lin Y.C., Lee I.T., Tsai M.H., Fang J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016;6:27995. doi: 10.1038/srep27995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amara N., Bachoual R., Desmard M., Golda S., Guichard C., Lanone S., Aubier M., Ogier-Denis E., Boczkowski J. Diesel exhaust particles induce matrix metalloprotease-1 in human lung epithelial cells via a NADP(H) oxidase/NOX4 redox-dependent mechanism. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293(1):L170–L181. doi: 10.1152/ajplung.00445.2006. [DOI] [PubMed] [Google Scholar]
- 60.Ryu Y.S., Kang K.A., Piao M.J., Ahn M.J., Yi J.M., Hyun Y.M., Kim S.H., Ko M.K., Park C.O., Hyun J.W. Particulate matter induces inflammatory cytokine production via activation of NFkappaB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox. Biol. 2019;21:101080. doi: 10.1016/j.redox.2018.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wada T., Sunaga H., Ohkawara R., Shimba S. Aryl hydrocarbon receptor modulates NADPH oxidase activity via direct transcriptional regulation of p40phox expression. Mol. Pharmacol. 2013;83(5):1133–1140. doi: 10.1124/mol.112.083303. [DOI] [PubMed] [Google Scholar]
- 62.Fisher A.B. Redox signaling across cell membranes. Antioxidants Redox Signal. 2009;11(6):1349–1356. doi: 10.1089/ars.2008.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohammadi-Bardbori A., Vikstrom Bergander L., Rannug U., Rannug A. NADPH oxidase-dependent mechanism explains how arsenic and other oxidants can activate aryl hydrocarbon receptor signaling. Chem. Res. Toxicol. 2015;28(12):2278–2286. doi: 10.1021/acs.chemrestox.5b00415. [DOI] [PubMed] [Google Scholar]
- 64.Wincent E., Bengtsson J., Mohammadi Bardbori A., Alsberg T., Luecke S., Rannug U., Rannug A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U. S. A. 2012;109(12):4479–4484. doi: 10.1073/pnas.1118467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anwar-Mohamed A., Elshenawy O.H., Soshilov A.A., Denison M.S., Chris Le X., Klotz L.O., El-Kadi A.O. Methylated pentavalent arsenic metabolites are bifunctional inducers, as they induce cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase through AhR- and Nrf2-dependent mechanisms. Free Radic. Biol. Med. 2014;67:171–187. doi: 10.1016/j.freeradbiomed.2013.10.810. [DOI] [PubMed] [Google Scholar]
- 66.Wu J.P., Chang L.W., Yao H.T., Chang H., Tsai H.T., Tsai M.H., Yeh T.K., Lin P. Involvement of oxidative stress and activation of aryl hydrocarbon receptor in elevation of CYP1A1 expression and activity in lung cells and tissues by arsenic: an in vitro and in vivo study. Toxicol. Sci. 2009;107(2):385–393. doi: 10.1093/toxsci/kfn239. [DOI] [PubMed] [Google Scholar]
- 67.Kubli S.P., Bassi C., Roux C., Wakeham A., Gobl C., Zhou W., Jafari S.M., Snow B., Jones L., Palomero L., Thu K.L., Cassetta L., Soong D., Berger T., Ramachandran P., Baniasadi S.P., Duncan G., Lindzen M., Yarden Y., Herranz C., Lazaro C., Chu M.F., Haight J., Tinto P., Silvester J., Cescon D.W., Petit A., Pettersson S., Pollard J.W., Mak T.W., Pujana M.A., Cappello P., Gorrini C. AhR controls redox homeostasis and shapes the tumor microenvironment in BRCA1-associated breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2019;116(9):3604–3613. doi: 10.1073/pnas.1815126116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freyberger A., Schnitzler R., Schiffmann D., Degen G.H. Prostaglandin-H-synthase competent cells derived from ram seminal vesicles: a tool for studying cooxidation of xenobiotics. Mol. Toxicol. 1987;1(4):503–512. [PubMed] [Google Scholar]
- 69.Eling T.E., Thompson D.C., Foureman G.L., Curtis J.F., Hughes M.F. Prostaglandin H synthase and xenobiotic oxidation. Annu. Rev. Pharmacol. Toxicol. 1990;30:1–45. doi: 10.1146/annurev.pa.30.040190.000245. [DOI] [PubMed] [Google Scholar]
- 70.Vogel C., Schuhmacher U.S., Degen G.H., Bolt H.M., Pineau T., Abel J. Modulation of prostaglandin H synthase-2 mRNA expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Arch. Biochem. Biophys. 1998;351(2):265–271. doi: 10.1006/abbi.1997.0555. [DOI] [PubMed] [Google Scholar]
- 71.Vogel C. Prostaglandin H synthases and their importance in chemical toxicity. Curr. Drug Metabol. 2000;1(4):391–404. doi: 10.2174/1389200003338884. [DOI] [PubMed] [Google Scholar]
- 72.Jones D.A., Carlton D.P., McIntyre T.M., Zimmerman G.A., Prescott S.M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J. Biol. Chem. 1993;268(12):9049–9054. [PubMed] [Google Scholar]
- 73.Seibert K., Masferrer J.L. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4(1):17–23. [PubMed] [Google Scholar]
- 74.Prescott S.M., Fitzpatrick F.A. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta. 2000;1470(2):M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 75.Vogel C.F., Li W., Sciullo E., Newman J., Hammock B., Reader J.R., Tuscano J., Matsumura F. Pathogenesis of aryl hydrocarbon receptor-mediated development of lymphoma is associated with increased cyclooxygenase-2 expression. Am. J. Pathol. 2007;171(5):1538–1548. doi: 10.2353/ajpath.2007.070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussain T., Gupta S., Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Canc. Lett. 2003;191(2):125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 77.Pathak S.K., Sharma R.A., Steward W.P., Mellon J.K., Griffiths T.R., Gescher A.J. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur. J. Canc. 2005;41(1):61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 78.Vogel C., Boerboom A.M., Baechle C., El-Bahay C., Kahl R., Degen G.H., Abel J. Regulation of prostaglandin endoperoxide H synthase-2 induction by dioxin in rat hepatocytes: possible c-Src-mediated pathway. Carcinogenesis. 2000;21(12):2267–2274. doi: 10.1093/carcin/21.12.2267. [DOI] [PubMed] [Google Scholar]
- 79.Fritsche E., Schafer C., Calles C., Bernsmann T., Bernshausen T., Wurm M., Hubenthal U., Cline J.E., Hajimiragha H., Schroeder P., Klotz L.O., Rannug A., Furst P., Hanenberg H., Abel J., Krutmann J. Lightning up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. U.S.A. 2007;104(21):8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munoz M., Sanchez A., Pilar Martinez M., Benedito S., Lopez-Oliva M.E., Garcia-Sacristan A., Hernandez M., Prieto D. COX-2 is involved in vascular oxidative stress and endothelial dysfunction of renal interlobar arteries from obese Zucker rats. Free Radic. Biol. Med. 2015;84:77–90. doi: 10.1016/j.freeradbiomed.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 81.Hernanz R., Briones A.M., Alonso M.J., Vila E., Salaices M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin relaxation impairment after LPS in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2004;287(1):H225–H234. doi: 10.1152/ajpheart.00548.2003. [DOI] [PubMed] [Google Scholar]
- 82.Kohle C., Bock K.W. Activation of coupled Ah receptor and Nrf2 gene batteries by dietary phytochemicals in relation to chemoprevention. Biochem. Pharmacol. 2006;72(7):795–805. doi: 10.1016/j.bcp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 83.Ma Q., Kinneer K., Bi Y., Chan J.Y., Kan Y.W. Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap 'n' collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem. J. 2004;377(Pt 1):205–213. doi: 10.1042/BJ20031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dohr O., Vogel C., Abel J. Different response of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-sensitive genes in human breast cancer MCF-7 and MDA-MB 231 cells. Arch. Biochem. Biophys. 1995;321(2):405–412. doi: 10.1006/abbi.1995.1411. [DOI] [PubMed] [Google Scholar]
- 85.Park E.Y., Rho H.M. The transcriptional activation of the human copper/zinc superoxide dismutase gene by 2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator sites, the antioxidant responsive element and xenobiotic responsive element. Mol. Cell. Biochem. 2002;240(1–2):47–55. doi: 10.1023/a:1020600509965. [DOI] [PubMed] [Google Scholar]
- 86.Jaiswal A.K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991;30(44):10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- 87.Miao W., Hu L., Scrivens P.J., Batist G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005;280(21):20340–20348. doi: 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- 88.Yeager R.L., Reisman S.A., Aleksunes L.M., Klaassen C.D. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicol. Sci. 2009;111(2):238–246. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuji G., Takahara M., Uchi H., Matsuda T., Chiba T., Takeuchi S., Yasukawa F., Moroi Y., Furue M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J. Invest. Dermatol. 2012;132(1):59–68. doi: 10.1038/jid.2011.194. [DOI] [PubMed] [Google Scholar]
- 90.Haarmann-Stemmann T., Abel J., Fritsche E., Krutmann J. The AhR-Nrf2 pathway in keratinocytes: on the road to chemoprevention? J. Invest. Dermatol. 2012;132(1):7–9. doi: 10.1038/jid.2011.359. [DOI] [PubMed] [Google Scholar]
- 91.Schafer M., Willrodt A.H., Kurinna S., Link A.S., Farwanah H., Geusau A., Gruber F., Sorg O., Huebner A.J., Roop D.R., Sandhoff K., Saurat J.H., Tschachler E., Schneider M.R., Langbein L., Bloch W., Beer H.D., Werner S. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014;6(4):442–457. doi: 10.1002/emmm.201303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panteleyev A.A., Bickers D.R. Dioxin-induced chloracne--reconstructing the cellular and molecular mechanisms of a classic environmental disease. Exp. Dermatol. 2006;15(9):705–730. doi: 10.1111/j.1600-0625.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 93.Sutter T.R., Guzman K., Dold K.M., Greenlee W.F. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991;254(5030):415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- 94.Greenlee W.F., Dold K.M., Osborne R. Actions of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on human epidermal keratinocytes in culture, in Vitro Cell. Dev. Biol. 1985;21(9):509–512. doi: 10.1007/BF02620843. [DOI] [PubMed] [Google Scholar]
- 95.Esser C. Dioxins and the immune system: mechanisms of interference. A meeting report. Int. Arch. Allergy Immunol. 1994;104(2):126–130. doi: 10.1159/000236719. [DOI] [PubMed] [Google Scholar]
- 96.Jeon M.S., Esser C. The murine IL-2 promoter contains distal regulatory elements responsive to the Ah receptor, a member of the evolutionarily conserved bHLH-PAS transcription factor family. J. Immunol. 2000;165(12):6975–6983. doi: 10.4049/jimmunol.165.12.6975. [DOI] [PubMed] [Google Scholar]
- 97.Esser C., Rannug A., Stockinger B. The aryl hydrocarbon receptor and immunity. Trends Immunol. 2009;9:447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 98.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 99.Funatake C.J., Marshall N.B., Steppan L.B., Mourich D.V., Kerkvliet N.I. Cutting edge: activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin generates a population of CD4+ CD25+ cells with characteristics of regulatory T cells. J. Immunol. 2005;175(7):4184–4188. doi: 10.4049/jimmunol.175.7.4184. [DOI] [PubMed] [Google Scholar]
- 100.Apetoh L., Quintana F.J., Pot C., Joller N., Xiao S., Kumar D., Burns E.J., Sherr D.H., Weiner H.L., Kuchroo V.K. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Esser C., Rannug A. The aryl hydrocarbon receptor (AhR) in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67(2):259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 102.Shinde R., McGaha T.L. The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol. 2018;39(12):1005–1020. doi: 10.1016/j.it.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haarmann-Stemmann T., Esser C., Krutmann J. The janus-faced role of aryl hydrocarbon receptor signaling in the skin: consequences for prevention and treatment of skin disorders. J. Invest. Dermatol. 2015;135(11):2572–2576. doi: 10.1038/jid.2015.285. [DOI] [PubMed] [Google Scholar]
- 104.Julliard W., Fechner J.H., Mezrich J.D. The aryl hydrocarbon receptor meets immunology: friend or foe? A little of both. Front. Immunol. 2014;5:458. doi: 10.3389/fimmu.2014.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrysik Z., Vondracek J., Marvanova S., Ciganek M., Neca J., Pencikova K., Mahadevan B., Topinka J., Baird W.M., Kozubik A., Machala M. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: the role of polycyclic aromatic hydrocarbons. Mutat. Res. 2011;714(1–2):53–62. doi: 10.1016/j.mrfmmm.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 106.Arrieta D.E., Ontiveros C.C., Li W.W., Garcia J.H., Denison M.S., McDonald J.D., Burchiel S.W., Washburn B.S. Aryl hydrocarbon receptor-mediated activity of particulate organic matter from the Paso del Norte airshed along the U.S.-Mexico border. Environ. Health Perspect. 2003;111(10):1299–1305. doi: 10.1289/ehp.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O'Driscoll C.A., Gallo M.E., Fechner J.H., Schauer J.J., Mezrich J.D. Real-world PM extracts differentially enhance Th17 differentiation and activate the aryl hydrocarbon receptor (AHR) Toxicology. 2019;414:14–26. doi: 10.1016/j.tox.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O'Driscoll C.A., Owens L.A., Hoffmann E.J., Gallo M.E., Afrazi A., Han M., Fechner J.H., Schauer J.J., Bradfield C.A., Mezrich J.D. Ambient urban dust particulate matter reduces pathologic T cells in the CNS and severity of EAE. Environ. Res. 2019;168:178–192. doi: 10.1016/j.envres.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Julliard W., Owens L.A., O'Driscoll C.A., Fechner J.H., Mezrich J.D. Environmental exposures-the missing link in immune responses after transplantation. Am. J. Transplant. 2016;16(5):1358–1364. doi: 10.1111/ajt.13660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riedl M.A., Nel A.E. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8(1):49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 111.Barnes P.J. Reactive oxygen species and airway inflammation. Free Radic. Biol. Med. 1990;9(3):235–243. doi: 10.1016/0891-5849(90)90034-g. [DOI] [PubMed] [Google Scholar]
- 112.De Grove K.C., Provoost S., Brusselle G.G., Joos G.F., Maes T. Insights in particulate matter-induced allergic airway inflammation: focus on the epithelium. Clin. Exp. Allergy. 2018;48(7):773–786. doi: 10.1111/cea.13178. [DOI] [PubMed] [Google Scholar]
- 113.Consonni D., Pesatori A.C., Zocchetti C., Sindaco R., D'Oro L.C., Rubagotti M., Bertazzi P.A. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am. J. Epidemiol. 2008;167(7):847–858. doi: 10.1093/aje/kwm371. [DOI] [PubMed] [Google Scholar]
- 114.Brunekreef B., Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur. Respir. J. 2005;26(2):309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- 115.Jaligama S., Patel V.S., Wang P., Sallam A., Harding J., Kelley M., Mancuso S.R., Dugas T.R., Cormier S.A. Radical containing combustion derived particulate matter enhance pulmonary Th17 inflammation via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 2018;15(1):20. doi: 10.1186/s12989-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vogel C.F., Khan E.M., Leung P.S., Gershwin M.E., Chang W.L., Wu D., Haarmann-Stemmann T., Hoffmann A., Denison M.S. Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-kappaB. J. Biol. Chem. 2014;289(3):1866–1875. doi: 10.1074/jbc.M113.505578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Castaneda A.R., Pinkerton K.E., Bein K.J., Magana-Mendez A., Yang H.T., Ashwood P., Vogel C.F.A. Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicol. Lett. 2018;292:85–96. doi: 10.1016/j.toxlet.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xia M., Viera-Hutchins L., Garcia-Lloret M., Noval Rivas M., Wise P., McGhee S.A., Chatila Z.K., Daher N., Sioutas C., Chatila T.A. Vehicular exhaust particles promote allergic airway inflammation through an aryl hydrocarbon receptor-notch signaling cascade. J. Allergy Clin. Immunol. 2015;136(2):441–453. doi: 10.1016/j.jaci.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meyers J.L., Winans B., Kelsaw E., Murthy A., Gerber S., Lawrence B.P. Environmental cues received during development shape dendritic cell responses later in life. PloS One. 2018;13(11) doi: 10.1371/journal.pone.0207007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Voorhis M., Knopp S., Julliard W., Fechner J.H., Zhang X., Schauer J.J., Mezrich J.D. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PloS One. 2013;8(12) doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O'Driscoll C.A., Gallo M.E., Hoffmann E.J., Fechner J.H., Schauer J.J., Bradfield C.A., Mezrich J.D. Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PloS One. 2018;13(12) doi: 10.1371/journal.pone.0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O'Driscoll C.A., Owens L.A., Gallo M.E., Hoffmann E.J., Afrazi A., Han M., Fechner J.H., Schauer J.J., Bradfield C.A., Mezrich J.D. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part. Fibre Toxicol. 2018;15(1):35. doi: 10.1186/s12989-018-0271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kish L., Hotte N., Kaplan G.G., Vincent R., Tso R., Ganzle M., Rioux K.P., Thiesen A., Barkema H.W., Wine E., Madsen K.L. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PloS One. 2013;8(4) doi: 10.1371/journal.pone.0062220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brulport A., Le Corre L., Chagnon M.C. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology. 2017;390:43–52. doi: 10.1016/j.tox.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 125.Moyer B.J., Rojas I.Y., Kerley-Hamilton J.S., Nemani K.V., Trask H.W., Ringelberg C.S., Gimi B., Demidenko E., Tomlinson C.R. Obesity and fatty liver are prevented by inhibition of the aryl hydrocarbon receptor in both female and male mice. Nutr. Res. 2017;44:38–50. doi: 10.1016/j.nutres.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L., Nichols R.G., Correll J., Murray I.A., Tanaka N., Smith P.B., Hubbard T.D., Sebastian A., Albert I., Hatzakis E., Gonzalez F.J., Perdew G.H., Patterson A.D. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ. Health Perspect. 2015;123(7):679–688. doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim M.J., Pelloux V., Guyot E., Tordjman J., Bui L.C., Chevallier A., Forest C., Benelli C., Clement K., Barouki R. Inflammatory pathway genes belong to major targets of persistent organic pollutants in adipose cells. Environ. Health Perspect. 2012;120(4):508–514. doi: 10.1289/ehp.1104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bleck B., Grunig G., Chiu A., Liu M., Gordon T., Kazeros A., Reibman J. MicroRNA-375 regulation of thymic stromal lymphopoietin by diesel exhaust particles and ambient particulate matter in human bronchial epithelial cells. J. Immunol. 2013;190(7):3757–3763. doi: 10.4049/jimmunol.1201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saint-Georges F., Abbas I., Billet S., Verdin A., Gosset P., Mulliez P., Shirali P., Garcon G. Gene expression induction of volatile organic compound and/or polycyclic aromatic hydrocarbon-metabolizing enzymes in isolated human alveolar macrophages in response to airborne particulate matter (PM2.5) Toxicology. 2008;244(2–3):220–230. doi: 10.1016/j.tox.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 130.Vogel C.F., Sciullo E., Wong P., Kuzmicky P., Kado N., Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ. Health Perspect. 2005;113(11):1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin J., Dinsdale D., White I.N. Characterization of Clara and type II cells isolated from rat lung by fluorescence-activated flow cytometry. Biochem. J. 1993;295(Pt 1):73–80. doi: 10.1042/bj2950073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oyama T., Sugio K., Uramoto H., Iwata T., Onitsuka T., Isse T., Nozoe T., Kagawa N., Yasumoto K., Kawamoto T. Increased cytochrome P450 and aryl hydrocarbon receptor in bronchial epithelium of heavy smokers with non-small cell lung carcinoma carries a poor prognosis. Front. Biosci. : J. Vis. Literacy. 2007;12:4497–4503. doi: 10.2741/2404. [DOI] [PubMed] [Google Scholar]
- 133.Chan J.K., Vogel C.F., Baek J., Kodani S.D., Uppal R.S., Bein K.J., Anderson D.S., Van Winkle L.S. Combustion derived ultrafine particles induce cytochrome P-450 expression in specific lung compartments in the developing neonatal and adult rat. Am. J. Physiol. Lung Cell Mol. Physiol. 2013;304(10):L665–L677. doi: 10.1152/ajplung.00370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chan J.K., Fanucchi M.V., Anderson D.S., Abid A.D., Wallis C.D., Dickinson D.A., Kumfer B.M., Kennedy I.M., Wexler A.S., Van Winkle L.S. Susceptibility to inhaled flame-generated ultrafine soot in neonatal and adult rat lungs. Toxicol. Sci. : Off. J. Soc. Toxicol. 2011;124(2):472–486. doi: 10.1093/toxsci/kfr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee D., Wallis C., Wexler A.S., Schelegle E.S., Van Winkle L.S., Plopper C.G., Fanucchi M.V., Kumfer B., Kennedy I.M., Chan J.K. Small particles disrupt postnatal airway development. J. Appl. Physiol. 2010;109(4):1115–1124. doi: 10.1152/japplphysiol.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee D., Wallis C., Van Winkle L.S., Wexler A.S. Disruption of tracheobronchial airway growth following postnatal exposure to ozone and ultrafine particles. Inhal. Toxicol. 2011;23(9):520–531. doi: 10.3109/08958378.2011.591447. [DOI] [PubMed] [Google Scholar]
- 137.Rouse R.L., Murphy G., Boudreaux M.J., Paulsen D.B., Penn A.L. Soot nanoparticles promote biotransformation, oxidative stress, and inflammation in murine lungs. Am. J. Respir. Cell Mol. Biol. 2008;39(2):198–207. doi: 10.1165/rcmb.2008-0057OC. [DOI] [PubMed] [Google Scholar]
- 138.Chan J.K., Charrier J.G., Kodani S.D., Vogel C.F., Kado S.Y., Anderson D.S., Anastasio C., Van Winkle L.S. Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part. Fibre Toxicol. 2013;10:34. doi: 10.1186/1743-8977-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li N., Alam J., Venkatesan M.I., Eiguren-Fernandez A., Schmitz D., Di Stefano E., Slaughter N., Killeen E., Wang X., Huang A., Wang M., Miguel A.H., Cho A., Sioutas C., Nel A.E. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004;173(5):3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Y.M., Zhong C.Y., Kennedy I.M., Leppert V.J., Pinkerton K.E. Oxidative stress and NFkappaB activation in the lungs of rats: a synergistic interaction between soot and iron particles. Toxicol. Appl. Pharmacol. 2003;190(2):157–169. doi: 10.1016/s0041-008x(03)00157-1. [DOI] [PubMed] [Google Scholar]
- 141.Beamer C.A., Shepherd D.M. Role of the aryl hydrocarbon receptor (AhR) in lung inflammation. Semin. Immunopathol. 2013;35(6):693–704. doi: 10.1007/s00281-013-0391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsay J.J., Tchou-Wong K.M., Greenberg A.K., Pass H., Rom W.N. Aryl hydrocarbon receptor and lung cancer. Anticancer Res. 2013;33(4):1247–1256. [PMC free article] [PubMed] [Google Scholar]
- 143.Baginski T.K., Dabbagh K., Satjawatcharaphong C., Swinney D.C. Cigarette smoke synergistically enhances respiratory mucin induction by proinflammatory stimuli. Am. J. Respir. Cell Mol. Biol. 2006;35(2):165–174. doi: 10.1165/rcmb.2005-0259OC. [DOI] [PubMed] [Google Scholar]
- 144.Marshall N.B., Kerkvliet N.I. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 2010;1183:25–37. doi: 10.1111/j.1749-6632.2009.05125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim J.H., Sherman M.E., Curriero F.C., Guengerich F.P., Strickland P.T., Sutter T.R. Expression of cytochromes P450 1A1 and 1B1 in human lung from smokers, non-smokers, and ex-smokers. Toxicol. Appl. Pharmacol. 2004;199(3):210–219. doi: 10.1016/j.taap.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 146.Moses E., Wang T., Corbett S., Jackson G.R., Drizik E., Perdomo C., Perdomo C., Kleerup E., Brooks D., O'Connor G., Dubinett S., Hayden P., Lenburg M.E., Spira A. Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol. Sci. 2017;155(1):248–257. doi: 10.1093/toxsci/kfw198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin P., Chang H., Tsai W.T., Wu M.H., Liao Y.S., Chen J.T., Su J.M. Overexpression of aryl hydrocarbon receptor in human lung carcinomas. Toxicol. Pathol. 2003;31(1):22–30. doi: 10.1080/01926230390173824. [DOI] [PubMed] [Google Scholar]
- 148.Wong P.S., Vogel C.F., Kokosinski K., Matsumura F. Arylhydrocarbon receptor activation in NCI-H441 cells and C57BL/6 mice: possible mechanisms for lung dysfunction. Am. J. Respir. Cell Mol. Biol. 2010;42(2):210–217. doi: 10.1165/rcmb.2008-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiba T., Uchi H., Tsuji G., Gondo H., Moroi Y., Furue M. Arylhydrocarbon receptor (AhR) activation in airway epithelial cells induces MUC5AC via reactive oxygen species (ROS) production. Pulm. Pharmacol. Therapeut. 2011;24(1):133–140. doi: 10.1016/j.pupt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 150.Lee Y.C., Oslund K.L., Thai P., Velichko S., Fujisawa T., Duong T., Denison M.S., Wu R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced MUC5AC expression: aryl hydrocarbon receptor-independent/EGFR/ERK/p38-dependent SP1-based transcription. Am. J. Respir. Cell Mol. Biol. 2011;45(2):270–276. doi: 10.1165/rcmb.2010-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shivanna B., Zhang S., Patel A., Jiang W., Wang L., Welty S.E., Moorthy B. Omeprazole attenuates pulmonary aryl hydrocarbon receptor activation and potentiates hyperoxia-induced developmental lung injury in newborn mice. Toxicol. Sci. : Off. J. Soc. Toxicol. 2015;148(1):276–287. doi: 10.1093/toxsci/kfv183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Villa M., Crotta S., Dingwell K.S., Hirst E.M., Gialitakis M., Ahlfors H., Smith J.C., Stockinger B., Wack A. The aryl hydrocarbon receptor controls cyclin O to promote epithelial multiciliogenesis. Nat. Commun. 2016;7:12652. doi: 10.1038/ncomms12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhattacharya S., Zhou Z., Yee M., Chu C.Y., Lopez A.M., Lunger V.A., Solleti S.K., Resseguie E., Buczynski B., Mariani T.J., O'Reilly M.A. The genome-wide transcriptional response to neonatal hyperoxia identifies Ahr as a key regulator. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307(7):L516–L523. doi: 10.1152/ajplung.00200.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shivanna B., Maity S., Zhang S., Patel A., Jiang W., Wang L., Welty S.E., Belmont J., Coarfa C., Moorthy B. Gene expression profiling identifies cell proliferation and inflammation as the predominant pathways regulated by aryl hydrocarbon receptor in primary human fetal lung cells exposed to hyperoxia. Toxicol. Sci. : Off. J. Soc. Toxicol. 2016;152(1):155–168. doi: 10.1093/toxsci/kfw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chiba T., Uchi H., Yasukawa F., Furue M. Role of the arylhydrocarbon receptor in lung disease. Int. Arch. Allergy Immunol. 2011;155(Suppl 1):129–134. doi: 10.1159/000327499. [DOI] [PubMed] [Google Scholar]
- 156.Toskala E., Smiley-Jewell S.M., Wong V.J., King D., Plopper C.G. Temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice, American journal of physiology. Lung Cell. Mol. Phys. 2005;289(3):L454–L459. doi: 10.1152/ajplung.00036.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan P.C., Hill G.D., Kissling G.E., Nyska A. Toxicity and carcinogenicity studies of 4-methylimidazole in F344/N rats and B6C3F1 mice. Arch. Toxicol. 2008;82(1):45–53. doi: 10.1007/s00204-007-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cruzan G., Harkema J.R., Hosako H., Wasil J.M., Murray F.J. Evaluation of the mode of action of mouse lung tumors induced by 4-methylimidazole, Regulatory toxicology and pharmacology. RTP (Regul. Toxicol. Pharmacol.) 2015;73(2):501–508. doi: 10.1016/j.yrtph.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 159.Goncharov A., Haase R.F., Santiago-Rivera A., Morse G., Akwesasne Task Force on the E., McCaffrey R.J., Rej R., Carpenter D.O. High serum PCBs are associated with elevation of serum lipids and cardiovascular disease in a Native American population. Environ. Res. 2008;106(2):226–239. doi: 10.1016/j.envres.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sergeev A.V., Carpenter D.O. Hospitalization rates for coronary heart disease in relation to residence near areas contaminated with persistent organic pollutants and other pollutants. Environ. Health Perspect. 2005;113(6):756–761. doi: 10.1289/ehp.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sergeev A.V., Carpenter D.O. Exposure to persistent organic pollutants increases hospitalization rates for myocardial infarction with comorbid hypertension. Prim. Prev. Insights. 2010;2:1–9. doi: 10.4137/PPRI.S4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Flesch-Janys D., Berger J., Gurn P., Manz A., Nagel S., Waltsgott H., Dwyer J.H. Exposure to polychlorinated dioxins and furans (PCDD/F) and mortality in a cohort of workers from a herbicide-producing plant in Hamburg, Federal Republic of Germany. Am. J. Epidemiol. 1995;142(11):1165–1175. doi: 10.1093/oxfordjournals.aje.a117575. [DOI] [PubMed] [Google Scholar]
- 163.Kang H.K., Dalager N.A., Needham L.L., Patterson D.G., Jr., Lees P.S., Yates K., Matanoski G.M. Health status of army chemical corps Vietnam veterans who sprayed defoliant in Vietnam. Am. J. Ind. Med. 2006;49(11):875–884. doi: 10.1002/ajim.20385. [DOI] [PubMed] [Google Scholar]
- 164.Kim J.S., Lim H.S., Cho S.I., Cheong H.K., Lim M.K. Impact of agent Orange exposure among Korean Vietnam veterans. Ind. Health. 2003;41(3):149–157. doi: 10.2486/indhealth.41.149. [DOI] [PubMed] [Google Scholar]
- 165.Humblet O., Birnbaum L., Rimm E., Mittleman M.A., Hauser R. Dioxins and cardiovascular disease mortality. Environ. Health Perspect. 2008;116(11):1443–1448. doi: 10.1289/ehp.11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lusis A.J. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 168.Brook R.D., Rajagopalan S., Pope C.A., 3rd, Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A., Peters A., Siscovick D., Smith S.C., Jr., Whitsel L., Kaufman J.D. Metabolism, Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. E. American Heart Association Council on, C.o.t.K.i.C.D. Prevention, P.A. Council on Nutrition. [DOI] [PubMed] [Google Scholar]
- 169.Pope C.A., 3rd, Burnett R.T., Thurston G.D., Thun M.J., Calle E.E., Krewski D., Godleski J.J. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 170.Kunzli N., Jerrett M., Garcia-Esteban R., Basagana X., Beckermann B., Gilliland F., Medina M., Peters J., Hodis H.N., Mack W.J. Ambient air pollution and the progression of atherosclerosis in adults. PloS One. 2010;5(2) doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jerrett M., Burnett R.T., Beckerman B.S., Turner M.C., Krewski D., Thurston G., Martin R.V., van Donkelaar A., Hughes E., Shi Y., Gapstur S.M., Thun M.J., Pope C.A., 3rd Spatial analysis of air pollution and mortality in California. Am. J. Respir. Crit. Care Med. 2013;188(5):593–599. doi: 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mateen F.J., Brook R.D. Air pollution as an emerging global risk factor for stroke. J. Am. Med. Assoc. 2011;305(12):1240–1241. doi: 10.1001/jama.2011.352. [DOI] [PubMed] [Google Scholar]
- 173.Ruckerl R., Greven S., Ljungman P., Aalto P., Antoniades C., Bellander T., Berglind N., Chrysohoou C., Forastiere F., Jacquemin B., von Klot S., Koenig W., Kuchenhoff H., Lanki T., Pekkanen J., Perucci C.A., Schneider A., Sunyer J., Peters A., Group A.S. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ. Health Perspect. 2007;115(7):1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Grunig G., Marsh L.M., Esmaeil N., Jackson K., Gordon T., Reibman J., Kwapiszewska G., Park S.H. Perspective: ambient air pollution: inflammatory response and effects on the lung's vasculature. Pulm. Circ. 2014;4(1):25–35. doi: 10.1086/674902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brugge D., Durant J.L., Rioux C. Near-highway pollutants in motor vehicle exhaust: a review of epidemiologic evidence of cardiac and pulmonary health risks. Environ. Health. 2007;6:23. doi: 10.1186/1476-069X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hoffmann B., Moebus S., Stang A., Beck E.M., Dragano N., Mohlenkamp S., Schmermund A., Memmesheimer M., Mann K., Erbel R., Jockel K.H., Heinz Nixdorf R.S.I.G. Residence close to high traffic and prevalence of coronary heart disease. Eur. Heart J. 2006;27(22):2696–2702. doi: 10.1093/eurheartj/ehl278. [DOI] [PubMed] [Google Scholar]
- 177.Aguilera I., Dratva J., Caviezel S., Burdet L., de Groot E., Ducret-Stich R.E., Eeftens M., Keidel D., Meier R., Perez L., Rothe T., Schaffner E., Schmit-Trucksass A., Tsai M.Y., Schindler C., Kunzli N., Probst-Hensch N. Particulate matter and subclinical atherosclerosis: associations between different particle sizes and sources with carotid intima-media thickness in the SAPALDIA study. Environ. Health Perspect. 2016;124(11):1700–1706. doi: 10.1289/EHP161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Boothe V.L., Shendell D.G. Potential health effects associated with residential proximity to freeways and primary roads: review of scientific literature, 1999-2006. J. Environ. Health. 2008;70(8):55–56. 33-41. [PubMed] [Google Scholar]
- 179.Ban-Weiss G.A., Lunden M.M., Kirchstetter T.W., Harley R.A. Size-resolved particle number and volume emission factors for on-road gasoline and diesel motor vehicles. J. Aerosol Sci. 2010;41(1):5–12. [Google Scholar]
- 180.Aung H.H., Lame M.W., Gohil K., He G., Denison M.S., Rutledge J.C., Wilson D.W. Comparative gene responses to collected ambient particles in vitro: endothelial responses. Physiol. Genom. 2011;43(15):917–929. doi: 10.1152/physiolgenomics.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ferecatu I., Borot M.C., Bossard C., Leroux M., Boggetto N., Marano F., Baeza-Squiban A., Andreau K. Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 2010;7:18. doi: 10.1186/1743-8977-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salcido-Neyoy M.E., Sanchez-Perez Y., Osornio-Vargas A.R., Gonsebatt M.E., Melendez-Zajgla J., Morales-Barcenas R., Petrosyan P., Molina-Servin E.D., Vega E., Manzano-Leon N., Garcia-Cuellar C.M. Induction of c-Jun by air particulate matter (PM(1)(0)) of Mexico city: participation of polycyclic aromatic hydrocarbons. Environ. Pollut. 2015;203:175–182. doi: 10.1016/j.envpol.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 183.Lauer F.T., Mitchell L.A., Bedrick E., McDonald J.D., Lee W.Y., Li W.W., Olvera H., Amaya M.A., Berwick M., Gonzales M., Currey R., Pingitore N.E., Jr., Burchiel S.W. Temporal-spatial analysis of U.S.-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2009;238(1):1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gualtieri M., Ovrevik J., Mollerup S., Asare N., Longhin E., Dahlman H.J., Camatini M., Holme J.A. Airborne urban particles (Milan winter-PM2.5) cause mitotic arrest and cell death: effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res. 2011;713(1–2):18–31. doi: 10.1016/j.mrfmmm.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 185.Keebaugh A.J., Sioutas C., Pakbin P., Schauer J.J., Mendez L.B., Kleinman M.T. Is atherosclerotic disease associated with organic components of ambient fine particles? Sci. Total Environ. 2015;533:69–75. doi: 10.1016/j.scitotenv.2015.06.048. [DOI] [PubMed] [Google Scholar]
- 186.Korashy H.M., El-Kadi A.O. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab. Rev. 2006;38(3):411–450. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 187.Puga A. Perspectives on the potential involvement of the AH receptor-dioxin axis in cardiovascular disease. Toxicol. Sci. 2011;120(2):256–261. doi: 10.1093/toxsci/kfq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim S.Y., Sheppard L., Kaufman J.D., Bergen S., Szpiro A.A., Larson T.V., Adar S.D., Diez Roux A.V., Polak J.F., Vedal S. Individual-level concentrations of fine particulate matter chemical components and subclinical atherosclerosis: a cross-sectional analysis based on 2 advanced exposure prediction models in the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2014;180(7):718–728. doi: 10.1093/aje/kwu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vogel C.F., Sciullo E., Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc. Toxicol. 2004;4(4):363–373. doi: 10.1385/ct:4:4:363. [DOI] [PubMed] [Google Scholar]
- 190.Wu D., Nishimura N., Kuo V., Fiehn O., Shahbaz S., Van Winkle L., Matsumura F., Vogel C.F. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 2011;31(6):1260–1267. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gough P.J., Gomez I.G., Wille P.T., Raines E.W. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J. Clin. Invest. 2006;116(1):59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dalton T.P., Kerzee J.K., Wang B., Miller M., Dieter M.Z., Lorenz J.N., Shertzer H.G., Nerbert D.W., Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc. Toxicol. 2001;1(4):285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 193.Haberzettl P., Conklin D.J., Abplanalp W.T., Bhatnagar A., O'Toole T.E. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2018;38(1):131–142. doi: 10.1161/ATVBAHA.117.309971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Q., Fan Y., Kurita H., Jiang M., Koch S., Rao M.B., Rubinstein J., Puga A. Aryl hydrocarbon receptor ablation in cardiomyocytes protects male mice from heart dysfunction induced by NKX2.5 haploinsufficiency. Toxicol. Sci. 2017;160(1):74–82. doi: 10.1093/toxsci/kfx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Niermann T., Schmutz S., Erne P., Resink T. Aryl hydrocarbon receptor ligands repress T-cadherin expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2003;300(4):943–949. doi: 10.1016/s0006-291x(02)02970-4. [DOI] [PubMed] [Google Scholar]
- 196.Karyala S., Guo J., Sartor M., Medvedovic M., Kann S., Puga A., Ryan P., Tomlinson C.R. Different global gene expression profiles in benzo[a]pyrene- and dioxin-treated vascular smooth muscle cells of AHR-knockout and wild-type mice. Cardiovasc. Toxicol. 2004;4(1):47–73. doi: 10.1385/ct:4:1:47. [DOI] [PubMed] [Google Scholar]
- 197.Kim J.B., Pjanic M., Nguyen T., Miller C.L., Iyer D., Liu B., Wang T., Sazonova O., Carcamo-Orive I., Matic L.P., Maegdefessel L., Hedin U., Quertermous T. TCF21 and the environmental sensor aryl-hydrocarbon receptor cooperate to activate a pro-inflammatory gene expression program in coronary artery smooth muscle cells, PLoS Genet. 2017;13(5) doi: 10.1371/journal.pgen.1006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lanham K.A., Plavicki J., Peterson R.E., Heideman W. Cardiac myocyte-specific AHR activation phenocopies TCDD-induced toxicity in zebrafish. Toxicol. Sci. 2014;141(1):141–154. doi: 10.1093/toxsci/kfu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bello S.M., Heideman W., Peterson R.E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol. Sci. 2004;78(2):258–266. doi: 10.1093/toxsci/kfh065. [DOI] [PubMed] [Google Scholar]
- 201.Ren F., Ji C., Huang Y., Aniagu S., Jiang Y., Chen T. AHR-mediated ROS production contributes to the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Sci. Total Environ. 2019:135097. doi: 10.1016/j.scitotenv.2019.135097. [DOI] [PubMed] [Google Scholar]
- 202.Schmuth M., Blunder S., Dubrac S., Gruber R., Moosbrugger-Martinz V. Epidermal barrier in hereditary ichthyoses, atopic dermatitis, and psoriasis. J. Dtsch Dermatol. Ges. 2015;13(11):1119–1123. doi: 10.1111/ddg.12827. [DOI] [PubMed] [Google Scholar]
- 203.Proksch E., Brasch J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin. Dermatol. 2012;30(3):335–344. doi: 10.1016/j.clindermatol.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 204.Acheva A., Schettino G., Prise K.M. Pro-inflammatory signaling in a 3D organotypic skin model after low LET irradiation-NF-kappaB, COX-2 activation, and impact on cell differentiation. Front. Immunol. 2017;8:82. doi: 10.3389/fimmu.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Howell M.D., Kim B.E., Gao P., Grant A.V., Boguniewicz M., DeBenedetto A., Schneider L., Beck L.A., Barnes K.C., Leung D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009;124(3 Suppl 2):R7–R12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 206.Kim B.E., Leung D.Y., Boguniewicz M., Howell M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008;126(3):332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Diaz-Sanchez D., Penichet-Garcia M., Saxon A. Diesel exhaust particles directly induce activated mast cells to degranulate and increase histamine levels and symptom severity. J. Allergy Clin. Immunol. 2000;106(6):1140–1146. doi: 10.1067/mai.2000.111144. [DOI] [PubMed] [Google Scholar]
- 208.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Selvakumar T.A., McPherson T., Taylor S., Ogg G.S. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 2012;21(2):104–110. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 209.van den Bogaard E.H., Bergboer J.G., Vonk-Bergers M., van Vlijmen-Willems I.M., Hato S.V., van der Valk P.G., Schroder J.M., Joosten I., Zeeuwen P.L., Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Invest. 2013;123(2):917–927. doi: 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.DiMeglio P., Duarte J.H., Ahlfors H., Owens N.D., Li Y., Villanova F., Tosi I., Hirota K., Nestle F.O., Mrowietz U., Gilchrist M.J., Stockinger B. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40(6):989–1001. doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schallreuter K.U., Salem M.A., Gibbons N.C., Maitland D.J., Marsch E., Elwary S.M., Healey A.R. Blunted epidermal L-tryptophan metabolism in vitiligo affects immune response and ROS scavenging by Fenton chemistry, part 2: epidermal H2O2/ONOO(-)-mediated stress in vitiligo hampers indoleamine 2,3-dioxygenase and aryl hydrocarbon receptor-mediated immune response signaling. Faseb. J. 2012;26(6):2471–2485. doi: 10.1096/fj.11-201897. [DOI] [PubMed] [Google Scholar]
- 212.Stefanovic N., Flohr C., Irvine A.D. The exposome in atopic dermatitis. Allergy. 2019 doi: 10.1111/all.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yi O., Kwon H.J., Kim H., Ha M., Hong S.J., Hong Y.C., Leem J.H., Sakong J., Lee C.G., Kim S.Y., Kang D. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ. Res. 2012;113:40–45. doi: 10.1016/j.envres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 214.Lee C.H., Chuang H.Y., Hong C.H., Huang S.K., Chang Y.C., Ko Y.C., Yu H.S. Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br. J. Dermatol. 2011;164(3):483–489. doi: 10.1111/j.1365-2133.2010.10116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee Y.L., Su H.J., Sheu H.M., Yu H.S., Guo Y.L. Traffic-related air pollution, climate, and prevalence of eczema in Taiwanese school children. J. Invest. Dermatol. 2008;128(10):2412–2420. doi: 10.1038/jid.2008.110. [DOI] [PubMed] [Google Scholar]
- 216.Schnass W., Huls A., Vierkotter A., Kramer U., Krutmann J., Schikowski T. Traffic-related air pollution and eczema in the elderly: findings from the SALIA cohort. Int. J. Hyg Environ. Health. 2018;221(6):861–867. doi: 10.1016/j.ijheh.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 217.Grandjean P., Poulsen L.K., Heilmann C., Steuerwald U., Weihe P. Allergy and sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environ. Health Perspect. 2010;118(10):1429–1433. doi: 10.1289/ehp.1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nakamoto M., Arisawa K., Uemura H., Katsuura S., Takami H., Sawachika F., Yamaguchi M., Juta T., Sakai T., Toda E., Mori K., Hasegawa M., Tanto M., Shima M., Sumiyoshi Y., Morinaga K., Kodama K., Suzuki T., Nagai M., Satoh H. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int. Arch. Occup. Environ. Health. 2013;86(8):849–859. doi: 10.1007/s00420-012-0819-8. [DOI] [PubMed] [Google Scholar]
- 219.Ye M., Warner M., Mocarelli P., Brambilla P., Eskenazi B. Prenatal exposure to TCDD and atopic conditions in the Seveso second generation: a prospective cohort study. Environ. Health. 2018;17(1):22. doi: 10.1186/s12940-018-0365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kennedy L.H., Sutter C.H., Leon Carrion S., Tran Q.T., Bodreddigari S., Kensicki E., Mohney R.P., Sutter T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013;132(1):235–249. doi: 10.1093/toxsci/kfs325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sutter C.H., Olesen K.M., Bhuju J., Guo Z., Sutter T.R. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J. Invest. Dermatol. 2019;139(4):818–826. doi: 10.1016/j.jid.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Peppers J., Paller A.S., Maeda-Chubachi T., Wu S., Robbins K., Gallagher K., Kraus J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019;80(1):89–98 e3. doi: 10.1016/j.jaad.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 223.Furue M., Hashimoto-Hachiya A., Tsuji G. Antioxidative phytochemicals accelerate epidermal terminal differentiation via the AHR-OVOL1 pathway: implications for atopic dermatitis. Acta Derm. Venereol. 2018;98(10):918–923. doi: 10.2340/00015555-3003. [DOI] [PubMed] [Google Scholar]
- 224.Smith S.H., Jayawickreme C., Rickard D.J., Nicodeme E., Bui T., Simmons C., Coquery C.M., Neil J., Pryor W.M., Mayhew D., Rajpal D.K., Creech K., Furst S., Lee J., Wu D., Rastinejad F., Willson T.M., Viviani F., Morris D.C., Moore J.T., Cote-Sierra J. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Invest. Dermatol. 2017;137(10):2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 225.Furue M., Hashimoto-Hachiya A., Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019;20(21) doi: 10.3390/ijms20215424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Smits J.P.H., Ederveen T.H.A., Rikken G., van den Brink N.J.M., van Vlijmen-Willems I., Boekhorst J., Kamsteeg M., Schalkwijk J., van Hijum S., Zeeuwen P., van den Bogaard E.H. Targeting the cutaneous microbiota in atopic dermatitis by coal tar via AHR-dependent induction of antimicrobial peptides. J. Invest. Dermatol. 2019 doi: 10.1016/j.jid.2019.06.142. [DOI] [PubMed] [Google Scholar]
- 227.Richardson W.H., Schmidt T.M., Nealson K.H. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl. Environ. Microbiol. 1988;54(6):1602–1605. doi: 10.1128/aem.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Paller A.S., Kong H.H., Seed P., Naik S., Scharschmidt T.C., Gallo R.L., Luger T., Irvine A.D. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;143(1):26–35. doi: 10.1016/j.jaci.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fyhrquist N., Muirhead G., Prast-Nielsen S., Jeanmougin M., Olah P., Skoog T., Jules-Clement G., Feld M., Barrientos-Somarribas M., Sinkko H., van den Bogaard E.H., Zeeuwen P., Rikken G., Schalkwijk J., Niehues H., Daubener W., Eller S.K., Alexander H., Pennino D., Suomela S., Tessas I., Lybeck E., Baran A.M., Darban H., Gangwar R.S., Gerstel U., Jahn K., Karisola P., Yan L., Hansmann B., Katayama S., Meller S., Bylesjo M., Hupe P., Levi-Schaffer F., Greco D., Ranki A., Schroder J.M., Barker J., Kere J., Tsoka S., Lauerma A., Soumelis V., Nestle F.O., Homey B., Andersson B., Alenius H. Microbe-host interplay in atopic dermatitis and psoriasis. Nat. Commun. 2019;10(1):4703. doi: 10.1038/s41467-019-12253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kim J., Ochoa M.T., Krutzik S.R., Takeuchi O., Uematsu S., Legaspi A.J., Brightbill H.D., Holland D., Cunliffe W.J., Akira S., Sieling P.A., Godowski P.J., Modlin R.L. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 2002;169(3):1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Andersson T., Erturk Bergdahl G., Saleh K., Magnusdottir H., Stodkilde K., Andersen C.B.F., Lundqvist K., Jensen A., Bruggemann H., Lood R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019;9(1):3596. doi: 10.1038/s41598-019-40471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]