Abstract
Since the skin is one of the targets of the harmful effects of environmental insults, several studies have investigated the effects of outdoor stressors on cutaneous tissue. Ozone (O3), particulate matter (PM), and ultraviolet radiation (UV) have all been shown to induce skin damage through disruption of tissue redox homeostasis, resulting in the so called “OxInflammation” condition. However, few studies have explored whether these stressors can act synergistically in cutaneous tissues. In the present work, we evaluated whether O3, PM, and UV, which are the most common environmental skin insults, act synergistically in inducing skin damage, and whether this effect could be prevented through topical application of a cosmeceutical formulation mixture (CF Mix) containing 15% vitamin C (l-ascorbic acid), 1% vitamin E (α-tocopherol), and 0.5% ferulic acid. Human skin explants obtained from three different subjects were sequentially exposed to 200 mJ UV light, 0.25 ppm O3 for 2 h, and 30 min of diesel engine exhaust (DEE), alone or in combination for 4 days (time point D1 and D4). We observed a clear additive effect of O3 and DEE in combination with UV in increasing levels of several oxidative (4HNE, HO-1) and inflammatory (COX2, NF-κB) markers and loss of barrier-associated proteins, such as filaggrin and involucrin. Furthermore, daily topical pre-treatment with the CF Mix prevented upregulation of the inflammatory and oxidative markers and the loss of both involucrin and filaggrin. In conclusion, this study is the first to investigate the combined effects of three of the most harmful outdoor stressors on human skin and suggests that daily topical application may prevent pollution-induced skin damage.
Keywords: Pollution, Inflammation, Skin barrier, 4HNE, COX2
Graphical abstract
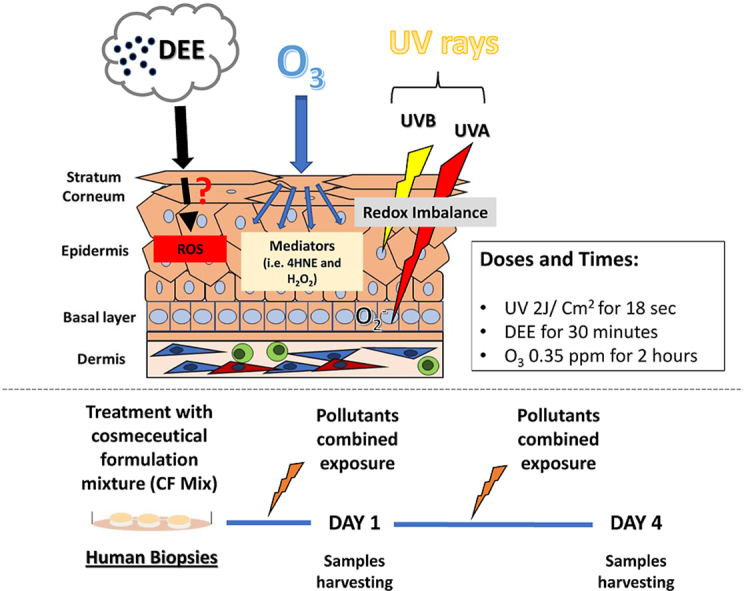
Mechanism of action of the different pollutants (Upper panel). Ex vivo human skin biopsies obtained from three different subjects were untreated or treated with a cosmeceutical formulation mixture containing 15% vitamin C, 1% vitamin E, and 0.5% ferulic acid. We exposed the biopsies to UV light alone and in combination with O3 and DEE and collected samples 1 day and 4 days after the exposure (Bottom panel).
Highlights
-
•
UV light in combination with O3 and DEE exposure synergistically increases levels of oxidative damage in human skin.
-
•
UV light, O3, and DEE result in an additive effect on cutaneous inflammatory markers and membrane barrier proteins.
-
•
Topical application of a cosmeceutical formulation mixture of CE Ferulic, vitamin E and C prevents pollution-induced damage.
1. Introduction
According to the World Health Organization (WHO), exposure to air pollution leads to approximately 4.2 million deaths each year (https://www.who.int/airpollution/en/) and impacts the function of multiple organs, including the heart, lungs, gut, and brain [[1], [2], [3], [4], [5], [6]]. However, recent literature has shown that cutaneous tissue is also susceptible to pollution. Indeed, development and exacerbation of a variety of skin conditions such as premature aging, psoriasis, acne, eczema, and atopic dermatitis are now linked to pollution exposure [[7], [8], [9], [10]]. Exposure of skin to UV light has been identified as the main risk factor contributing to the so-called “extrinsic skin aging” [11]. In addition, it has now been accepted that other pollutants, such as ozone (O3) and particulate matter (PM), can play key roles in pollution-induced skin damage and possibly exacerbate UV-induced skin conditions [7,8]. Previous studies have shown the ability of UV to act synergistically with PM by exciting polycyclic aromatic hydrocarbons (PAHs) in the core structure of particulates [12,13] and with O3 by increasing tissue peroxidation and decreasing cutaneous a-tocopherol [14].
Several reports have highlighted the mechanisms of action of single pollutants/stressors, [15]. For instance, O3, which is a small molecule and a strong oxidizing agent, directly acts on the surface of cutaneous tissue, disseminating its detrimental effects into the deeper epidermal layers through generation of a cascade of ozonation products. Although it is not a radical species per se, O3 is able to oxidize components of the cell membrane, mainly lipids, generating classical radical species, such as hydroxyl radicals that, in turn, drive the production of cytotoxic, nonradical species, including aldehydes. In addition to O3, public concern about the damaging effects of PM exposure on cutaneous tissues is escalating. Similar to O3, the mechanisms involved in PM-associated skin disorders also result from increased oxidative damage, due to PM composition [10,12].
Although not completely confirmed, it has been hypothesized that ultrafine PMs can move through the skin through hair follicles or transdermally, generating oxidative stress not only at the surface level but also in the deeper cutaneous layers. In addition, PAHs, which are components of UFPs, can be absorbed through the skin and eventually damage the mitochondria, resulting in intracellular ROS production [16]. Besides anthropogenic pollution (such as PM and O3), living organisms are continuously exposed to UV light. Ultraviolet radiation (UVR) is a primary cause of extrinsic aging (photoaging). While UVB typically comprises less than 10% of UVR, it is a high energy component that is mainly absorbed by epidermal cells, while UVA, the more prevalent component, is weaker but penetrates into the dermis [17]. UVR absorbed from solar radiation can induce extensive skin damage through a variety of mechanisms, ranging from direct DNA damage to those resulting from oxidative stress caused by UVR-induced reactive oxygen species (ROS). Like high energy radiation, UVR induces ROS that are strong oxidizers capable of reacting with biomolecules on a nano to millisecond time scale. When UVR-induced ROS overwhelms the skin's natural defensive mechanisms, this results in DNA damage, lipid and protein peroxidation, and immune dysregulation.
As of today, only a few studies have investigated the possible additive effect of multiple pollutants on cutaneous tissues, although this paradigm represents the realistic, everyday urban environment. The present study aimed to investigate whether exposure to different pollutants, such as O3 and PM, could further exacerbate cutaneous OxInflammation induced by UV exposure. In addition, the possible protective effect of a commercially available cosmeceutical formulation mixture (CF Mix) containing 15% vitamin C (l-ascorbic acid), 1% vitamin E (α-tocopherol), and 0.5% ferulic acid was also evaluated under the several exposure conditions.
2. Results
2.1. Effect of combined stressors in skin oxidative damage before and after CF Mix topical application
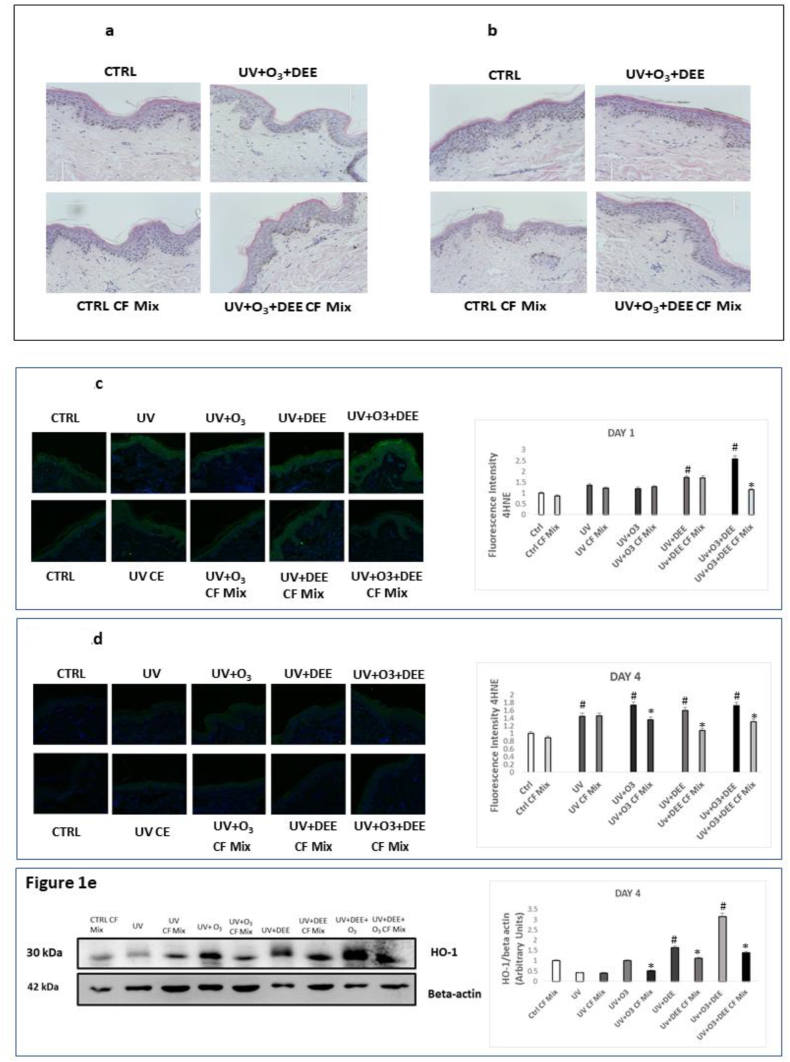
First, we wanted to determine whether the dosage/time of exposure altered skin structure. As shown in Fig. 1a and b, we did not observe any morphological alterations in tissues exposed to UV light alone or in combination with O3 and DEE, suggesting that the doses used are not overly aggressive and can represent real-life pollution exposure. Next, we wanted to determine whether exposure to the combination of outdoor stressors can have an additive effect in terms of cutaneous oxidative damage. As depicted in Figure 1c, 4-hydroxynonenal (4HNE) levels, a marker of lipid peroxidation [15], were clearly increased in response to individual UV light exposure and in combination with O3 and DEE at day 1. No additive effect of the pollutants was noticed at day 4 (Fig. 1d), confirming that the oxidation pathway is an early event of pollution-induced skin damage. Topical application of the CF Mix counteracted this effect, particularly at day 1, when the tissues were exposed to all three stressors (Fig. 1c). Interestingly, pre-treatment with the CF Mix still decreased 4HNE levels at day 4 as well (Fig. 1d).
Fig. 1.
Combined exposure to UV light, O3, and DEE does not alter tissue morphology but increases oxidative stress, which can be counteracted by topical application of a cosmeceutical formulation mixture. H&E staining of ex vivo human skin biopsies untreated or pre-treated with the cosmeceutical formulation mixture and then exposed to UV light alone or in combination with O3 and DEE for 1 day (a) and 4 days (b).Levels of 4-HNE, a marker of lipid peroxidation, in ex vivo human skin biopsies exposed to UV light alone or in combination with O3 and DEE and treated with the cosmeceuticals formulation mixture for 1 day (c) an 4 days (d). Green staining represents 4-HNE, and the blue staining (DAPI) for nuclei; original magnification 40x. Right panel represents the immunofluorescence (“c” and “d”) and immunoblotting (“e”) quantification performed by ImageJ. Data are expressed as arbitrary units (averages of three independent experiments), *p < 0.05 CF Mix vs pollutant, #p < 0.05 pollutants vs Ctrl by ANOVA) and β-actin was used as loading control (“e”). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To confirm the ability of the pollutants to affect tissue redox homeostasis, we also evaluated levels of hemeoxygenase-1 (HO-1), an enzyme involved in response to oxidative challenges [18]. As depicted in Fig. 1e, the combination exposure of all three stressors synergistically increased levels of HO-1 4 days after the challenges. We also observed that UV light in combination with either O3 or DEE increased levels of HO-1, compared to exposure to UV light alone. Furthermore, CF Mix topical application counteracted increase of HO-1 levels. Collectively, this data suggests that UV light, O3, and DEE can act synergistically to increase oxidative damage in the skin.
2.2. Pro-inflammatory responses activated by outdoor stressors are prevented by CF Mix topical application
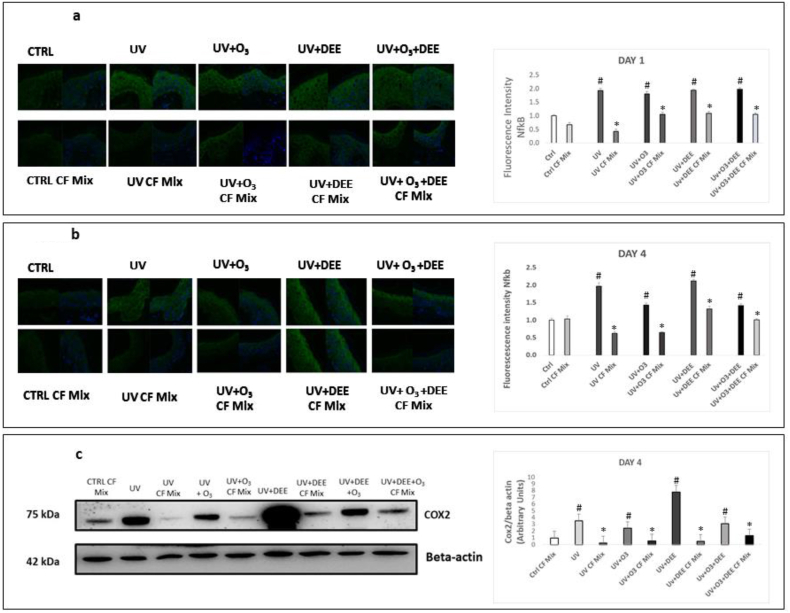
As a consequence of oxidative challenge, there is an activation of the redox-sensitive transcription factor NFκB [19], which is a key factor involved in the regulation of tissue inflammatory responses [20]. Activation of NFκB in keratinocytes upon exposure to UV, O3 and PM individually has been well-documented [[21], [22], [23], [24]]. We observed increased levels of NFκB in response to exposure to UV light individually and in combination with O3 and DEE after 24 h and 4 days of exposure, although we did not observe any additive effects of combined exposure (Fig. 2a and b). We also observed that topical application of the CF Mix prevented stressor-induced increases in NFκB levels after 24 h and 4 days of exposure (Fig. 2a and b). Since activation of NFκB results in the transcription of several inflammatory genes, including cyclooxygenase 2 (COX2) [25,26], we examined the levels of COX2 in tissues exposed to the pollutants. Similarly, the combination of pollutants increased COX2 levels after 4 days of exposure (Fig. 2c). In addition, we found that exposure to UV light and DEE in combination resulted in a dramatic increase in COX2 levels. We also observed that the CF Mix prevented stressor-mediated increases in COX2 levels (Fig. 2c).
Fig. 2.
Topical application of a cosmeceuticals formulation mixture inhibits outdoor stressor-induced inflammation. Levels of NFκB in ex vivo human skin biopsies exposed to different combinations of pollutants (UV, DEE and O3) at day 1 (a) and day 4 (b) post-exposure was evaluated using immunofluorescence. Green staining represents NFκB, and the blue staining (DAPI) represents nuclei; Original magnification 40x. Quantification was performed using ImageJ (right panels). (c) Protein levels of COX2 were measured by immunoblotting. All Data are expressed as arbitrary units (averages of three different experiments), *p < 0.05 CF Mix vs pollutant, #p < 0.05 pollutants vs Ctrl by ANOVA and β-actin was used as loading control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.3. Effect of combined outdoor stressors exposure on skin barrier-associated proteins
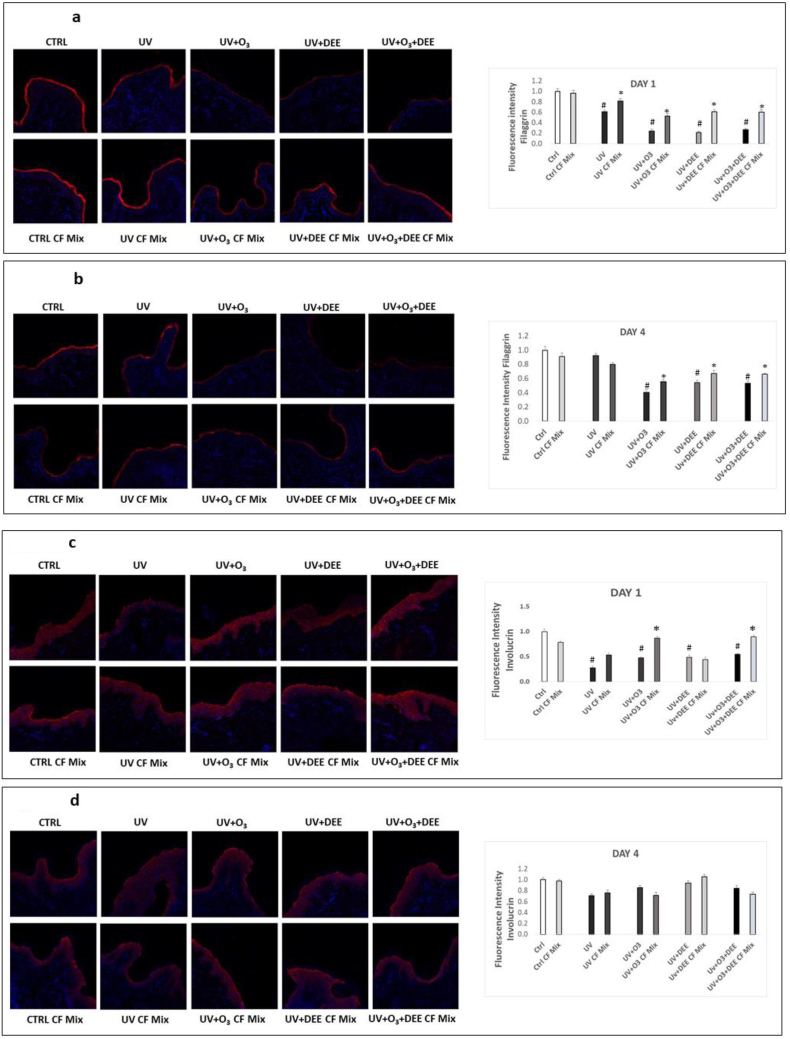
Since a defective skin barrier is a key feature of inflammatory skin disorders associated with pollutant exposure [27], we wanted to examine whether combined exposure to UV light, O3, and/or DEE affected the levels/localization of skin barrier-associated proteins. First, we examined effects of combined exposure on filaggrin, which is involved in facilitating keratin matrix formation [27]. We observed decreased filaggrin levels in response to the combined stressors exposure at day 1 (Fig. 3a) and 4 days (Fig. 3b). While pre-treatment with the CF Mix prevented this effect (Fig. 3a and b).
Fig. 3.
Combined exposure to outdoor stressors decreases levels of skin barrier-associated proteins, which is mitigated by topical application of a cosmeceutical formulation mixture. Levels of skin-barrier associated proteins filaggrin (a, b) and involucrin (d, e) were assessed using immunofluorescence on ex vivo human skin biopsies exposed to different combinations of pollutants for 1 day (a, c) and 4 days (b, d) and pretreated with the cosmeceutical formulation mixture. Red staining represents filaggrin (a, b) or involucrin (c,d), and the blue staining (DAPI) represents nuclei; original magnificent 40X. Quantification (right panels) of filaggrin and involucrin levels was performed using ImageJ. Data are expressed as arbitrary units (averages of three different experiments), *p < 0.05 CF Mix vs pollutant, #p < 0.05 pollutants vs Ctrl by ANOVA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we wanted to evaluate whether exposure to the combined stressors affected the expression of involucrin, a key protein involved in forming the cornified envelope of the stratum corneum [28]. We observed decreased involucrin levels in response to UV light individually and in combination with O3 and DEE after 1 day of exposure (Fig. 3c) and that pre-treatment with the CF Mix prevented this effect (Fig. 3c). After 4 days of exposure, we did not observe a significant effect on involucrin levels (Fig. 3d), suggesting that involucrin modulation is an early event under our experimental conditions.
2.4. Exposure to combined outdoor stressors increases aryl hydrocarbon receptor levels in the skin, which is prevented by CF Mix topical application
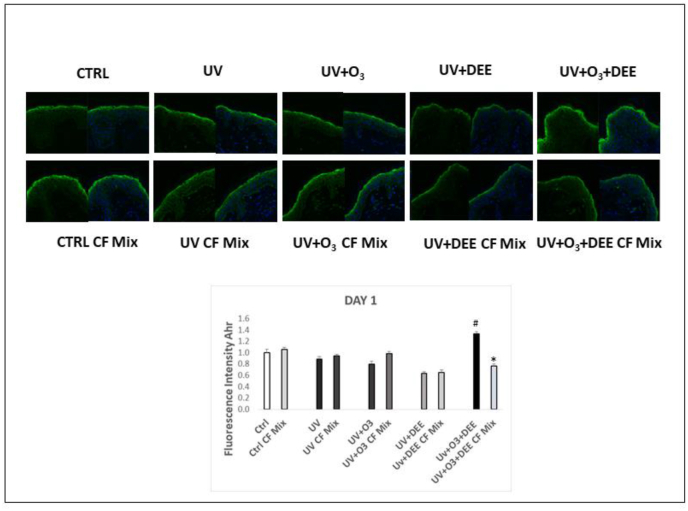
Previous work has shown the ability of PM to activate AhR [10,[29], [30], [31]]. Another study in keratinocytes has suggested that O3 can also affect AhR levels [32]. In order to understand whether combined pollution exposure can modulate AhR in a closer to life skin model, we measured AhR levels in our experimental conditions. We observed that exposure to the combination of UV, O3 and DEE increased AhR levels after 24 h of exposure and that pre-treatment with the CF Mix prevented this effect (Fig. 4). This data suggests that UV light, O3, and DEE act synergistically in increasing AhR levels in the skin, which can be also a consequence of the perturbed skin barrier that would then facilitate the pollutants skin interaction.
Fig. 4.
Exposure to combined stressors increases aryl hydrocarbon receptor (AhR) levels in the skin, which is inhibited by topical application of a cosmeceutical formulation mixture. Levels of AhR in ex vivo human skin biopsies exposed to different combination of pollutants (UV, DEE and O3) at day 1. Green staining represents AhR, and the blue staining (DAPI) represents nuclei; Original magnification 40x. Fluorescence intensity levels were quantified using ImageJ (bottom panel). All Data are expressed as arbitrary units (averages of three different experiments), #p < 0.05 CF Mix vs Pollutant #p < 0.05 pollutants vs Ctrl by ANOVA). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Due to the lack of studies evaluating the effects of combined exposure to UV light, O3, and PM, the present work aimed to investigate whether these outdoor stressors can act synergistically in inducing skin damage. UV light is one of the strongest outdoor stressors; therefore, we focused our efforts in understanding the synergy between this outdoor stressor and the other 2 most toxic anthropogenic environmental air pollutants, PM and O3. We confirmed that the combination of outdoor stressors induced oxidative damage by measuring levels of 4HNE, a marker of lipid peroxidation, and HO-1, a defensive enzyme under the control of Nrf2, which is a redox sensitive transcription factor. As a consequence of altered redox homeostasis, we also assessed levels of inflammatory markers, NFκB and COX2. In response to exposure to the combination of stressors, we clearly noticed an additive effect on both inflammatory and oxidative markers, especially in tissues exposed to all three stressors.
We believe that these additive effects are due to different ways of interaction of the stressors with the skin. Although it has been suggested that all of these stressors are able to induce oxidative damage, this damage possibly derives from different pathways [15]. For instance, O3 does not penetrate the skin but does interact with the polyunsaturated fatty acids (PUFAs) and squalene found in the stratum corneum. Thus, it generates a cascade of bioactive molecules (among them 4HNE and H2O2) that affect the deeper layers of the tissue [33]. A recent study, using an ex-vivo approach, was able to mimic squalene oxidation by O3 and demonstrated the ability of the derived mono- and di-carbonyls to penetrate the skin epidermis [34]. This recent study confirms the idea that the skin is also a gateway for certain pollutants, as previously suggested by Weschler et al. [35].
More unclear is the mechanism by which particulates can affect cutaneous tissues. The toxicological properties of diesel-derived PM, especially for ultrafine particles (UFP) is mainly attributed to the presence of PAHs that are present in the particle structure, although their ability to penetrate healthy skin is still under debate. Filon et al. have suggested that only UFP can penetrate damaged skin and that the larger particles will interact with the outermost layer of the skin [36]. The theory that PM can enter the skin via the hair follicle is now less accepted, as this eventual passive penetration would be almost negligible [37]. Our group was able to show that, in a 3D in vitro model, several particles could reach the epidermis [21]. However, this study was limited by the fact that skin produced using this model consists of a thinner stratum corneum and an overall more permeable structure [21]. Kammer et al. demonstrated that PAHs topically applied to the skin of human volunteers were found in the deep stratum layers using the tape stripping technique [38]. On the other hand, the ability of PM to enter skin cells in vitro has been well documented, which results in localization to the mitochondria, thereby inducing damage and ROS production [16,39,40]. Therefore, it is possible that, although PM particles are not able to deeply penetrate skin, they can enter the stratum corneum and possibly induce a cascade of bioactive molecules, similar to the mechanism by which O3 induces skin damage, by reacting with lipids and squalene, which could result in the production of different end products. Moreover, the presence of transition metals in the particles could also be a source of oxidative damage [41,42].
In contrast, UV light can easily penetrate the epithelium (UVB), reach the dermis (UVA), and can be absorbed by proteins, lipids, and DNA, eventually inducing the production of ROS [11,43]. This was also confirmed in our study, where we observed increased levels of 4HNE and proinflammatory mediators in tissues exposed to UV, as proof of oxidative damage.
As a result of the aforementioned cascade of effects, exposure to these outdoor stressors also alters barrier function of the skin, amplifying the effects of further exposure while facilitating the interaction of pollutants with the tissue. It may be possible, that, although PM itself can barely penetrate the skin, in the presence of other pollutants, the effects of multiple stressors on the skin barrier could make the skin more accessible to further penetration by outdoor stressors and/or their derived bioactive products. In support of this idea, we observed that filaggrin and involucrin, which are important for differentiation and maintaining the proper skin barrier function [44], are decreased in response to combined exposure. In a fairly recent work, researchers demonstrated that PAH decreases levels decreases filaggrin levels in pig skin, making this effect a possible consequence of PM-induced oxidative damage [45]. In addition, Jin et al. observed that barrier disruption via tape-stripping resulted in increased penetration of PM in murine skin [40]. Thus, exposure to UV light, O3, and PM in combination, which reflects the everyday urban environment, could result in increased penetration of single pollutants and/or their resulting bioactive molecules, such as 4HNE.
In addition to filaggrin and involucrin, we observed that AhR, which is involved in xenobiotic responses and also skin barrier function [31,46], is increased in response to combined exposure, suggesting that these stressors are indeed recognized as xenobiotics. This is the first study showing that exposure to all of these outdoor stressors can be correlated to AhR activation, which is another source of ROS and that can further amplify the induced damage. PM-induced activation of AhR has been well documented by the Krutman group [7,[47], [48], [49]] as being one of the main mechanisms of PM-induced damage and increased skin pigmentation [[49], [50], [51]]. On the other hand, besides an in vitro study on keratinocytes [32], the ability of O3 to induce AhR activation in skin explants has never been documented. Activation of AhR could be a consequence of the bioactive products derived from the oxidation of squalene or fatty acids in the stratum corneum that are then recognized as xenobiotic by AhR.
Finally, although skin health can be improved by diet [52], the daily use of topical applications to prevent pollution-induced skin damage is still strongly recommended. This has been confirmed by the use of specific combinations of a Cosmeceuticals formulation mixture that has been previously shown to also protect against single pollutants in pre-clinical and clinical studies [23,53,54]. The formulation that has been applied in this study is commercially available and composed of 15% ascorbic acid, 1% alpha tocopherol and 0.5% ferulic acid. Previous studies have shown the ability of this composition, not only to penetrate the skin, but also to have an additive effect, compared to protection mediated by single components [[55], [56], [57]]. The authors suggest that adding Ferulic acid to the formula, an hysroxycinnamic acid, probably protects L-ascorbic and a-tocopherol, by serving as a sacrificial substance [55]. In addition, Murray et al. demonstrated that this formulation was able to prevent UV-induced erythema, sunburn cells, p53 activation, and DNA damage [58]. Furthermore, the ability of this formula to prevent pollution-induced damage is likely due to the activation of skin defensive mechanisms, as a consequence of its demonstrated percutaneous absorption [55] and not to its UV absorption properties, as the same authors have shown its inability to act as a sunscreen [57].
Besides the current work, only a few studies have shown the possible interaction between different pollutants. For example, the Marrot group has demonstrated the “photo-pollution” effect of PAH and UVR in both 2D and 3D skin models [12,59]. In addition, our group was also able to demonstrate the ability of ozone in vivo to enhance the damaging cutaneous effects of UV by decreasing levels of endogenous antioxidant micronutrients, thus potentiating the inflammatory impact of UV [14]. In conclusion, the current study brings new insights on the consequences of skin exposure to multiple pollutants in combination and how daily topical application of specific cosmeceutical formulations can protect cutaneous tissues against outdoor stressors.
4. Materials and methods
4.1. Culture and exposure of ex vivo human biopsies
Healthy human skin was obtained from elective abdominoplasties, as approved by the IBC at NC State. Subcutaneous fat was trimmed from 12 mm punch biopsies, and biopsies were rinsed with PBS containing antibiotics/antimycotic. Next, biopsies were cultured in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in 5% CO2 with the upper part of the epidermis exposed to the outside environment.
The next day, media was changed, and an antioxidant mixture containing 15% vitamin C (l-ascorbic acid), 1% vitamin E (α-tocopherol), and 0.5% ferulic acid (CE Ferulic, SkinCeuticals Inc., New York, NY) was topically applied. After 24 h of pre-treatment, biopsies were exposed to 200 mJ UVA/UVB light alone as in Ref. [14], UV light and then 0.25 ppm of O3 for 2 h in an O3 chamber as in Ref. [60], UV light then 30 min of DEE, or UV light, O3, and then DEE. Samples were collected 24 h after the first exposure or continued to be exposed for four days. DEE was generated by a Kubota RTV-X900 diesel engine (3-cylinder, 4-cycle diesel with overhead valves, 1123 cc that has 24.8 HP at 3000 rpm).
4.2. Hematoxylin & Eosin staining
Tissues were briefly fixed in 10% neutral-buffered formalin and then embedded in paraffin. 4 μm thick sections were deparaffinized in xylene and then rehydrated in a series of alcohol gradients. Sections were then stained with Mayer's hematoxylin, washed with tap water, then immersed in 95% EtOH, 100% EtOH, and xylene, and mounted onto slides.
4.3. Immunofluorescence
Paraffin-embedded 4 μm sections of skin biopsies were deparaffinized in xylene and rehydrated in decreasing alcohol gradients. Antigen retrieval was achieved using heat-based epitope retrieval with sodium citrate buffer (Thermo Fisher Scientific, USA) (pH 6.0) at a sub-boiling temperature in a 500 W microwave for 10 min. After cooling, sections were washed in PBS, blocked with 5% BSA in PBS, and incubated with primary antibodies for 4HNE (dil. 1:500) (AB5605, Millipore), NFκB (dil. 1:500) (8242, Cell Signaling), AhR (dil. 1:500) (83,200, Cell Signaling), involucrin (dil. 1:50) (sc-21748, Santa Cruz), or filaggrin (dil. 1:50) (sc-66192) in 2% BSA in PBS. Sections were then washed in PBS and incubated with fluorochrome-conjugated secondary antibodies (dil. 1:1000) (Alexa Fluor 568 A11004 or Alexa Fluor 488 A11055) in 2% BSA in PBS at RT, and then washed with PBS. Nuclei were stained with DAPI (1,874,814, Invitrogen) in PBS, and sections were then washed with PBS. Sections were mounted using PermaFluor mounting media (ThermoFisher Scientific) and imaged on a Zeiss LSM10 microscope. Images were quantified using ImageJ.
4.4. Protein extraction and Western blotting
Skin explants were homogenized using a Qiagen TissueLyser in T-PER tissue protein extraction reagent (Thermo Scientific) containing Halt protease inhibitor cocktail (Thermo Scientific). Protein content was measured using Bradford assays. Equivalent amounts of proteins were loaded onto polyacrylamide SDS. Gels were electroblotted onto membranes and blocked in TBS containing 0.5% Tween 20 and 5% milk. Membranes were incubated with primary antibodies for COX2 (12,282, Cell Signaling) or HO-1 (PA00553, BioRad) in TBS-T with 1% non-fat milk (BioRad,USA), washed, and incubated with horseradish peroxidase-conjugated secondary antibodies (170–6515 or 170–6516, BioRad). Bound antibodies were detected by chemiluminescence (BioRad). ??-actin (A3854, Sigma) was used as loading control. Densitometry analysis was performed using Image J software.
4.5. Statistics
Statistical analyses were performed by using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla CA). For comparisons between groups, analysis of variance (ANOVA) followed by Bonferroni's post-hoc test was conducted. All data were expressed as means ± standard deviations (SD). p ≤ 0.05 was considered as significant in all cases.
Acknowledgements
The authors thank SkinCeuticals for research support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101481.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jiang S. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. Int. Arch. Occup. Environ. Health. 2016;89(6):911–921. doi: 10.1007/s00420-016-1129-3. [DOI] [PubMed] [Google Scholar]
- 2.Morakinyo O.M. Health outcomes of exposure to biological and chemical components of inhalable and respirable particulate matter. Int. J. Environ. Res. Publ. Health. 2016;13(6) doi: 10.3390/ijerph13060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aalapati S. Toxicity and bio-accumulation of inhaled cerium oxide nanoparticles in CD1 mice. Nanotoxicology. 2014;8(7):786–798. doi: 10.3109/17435390.2013.829877. [DOI] [PubMed] [Google Scholar]
- 4.Mills N.L. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am. J. Respir. Crit. Care Med. 2006;173(4):426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 5.Genc S. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012;2012 doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghorani-Azam A., Riahi-Zanjani B., Balali-Mood M. Effects of air pollution on human health and practical measures for prevention in Iran. J. Res. Med. Sci. 2016;21:65. doi: 10.4103/1735-1995.189646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krutmann J. The skin aging exposome. J. Dermatol. Sci. 2017;85(3):152–161. doi: 10.1016/j.jdermsci.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Puri P. Effects of air pollution on the skin: a review. Indian J. Dermatol. Venereol. Leprol. 2017;83(4):415–423. doi: 10.4103/0378-6323.199579. [DOI] [PubMed] [Google Scholar]
- 9.Fuks K.B., Woodby B., Valacchi G. Hautarzt; 2019. Skin Damage by Tropospheric Ozone. [Google Scholar]
- 10.Kim K.E., Cho D., Park H.J. Air pollution and skin diseases: adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 11.Drakaki E., Dessinioti C., Antoniou C.V. Air pollution and the skin. Front. Environ. Sci. 2014;2(11) [Google Scholar]
- 12.Marrot L. Pollution and sun exposure: a deleterious synergy. Mechanisms and opportunities for skin protection. Curr. Med. Chem. 2018;25(40):5469–5486. doi: 10.2174/0929867324666170918123907. [DOI] [PubMed] [Google Scholar]
- 13.Yu H. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light - a pathway leading to the generation of reactive oxygen species, lipid peroxidation, and dna damage. Int. J. Environ. Res. Publ. Health. 2006;3(4):348–354. doi: 10.3390/ijerph2006030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valacchi G. Ozone potentiates vitamin E depletion by ultraviolet radiation in the murine stratum corneum. FEBS Lett. 2000;466(1):165–168. doi: 10.1016/s0014-5793(99)01787-1. [DOI] [PubMed] [Google Scholar]
- 15.Pecorelli A. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors. 2019;45(4):536–547. doi: 10.1002/biof.1513. [DOI] [PubMed] [Google Scholar]
- 16.Li N. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valejo Coelho M.M., Matos T.R., Apetato M. The dark side of the light: mechanisms of photocarcinogenesis. Clin. Dermatol. 2016;34(5):563–570. doi: 10.1016/j.clindermatol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Araujo J.A., Zhang M., Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharmacol. 2012;3:119. doi: 10.3389/fphar.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byun M.S. Dual effect of oxidative stress on NF-kappakB activation in HeLa cells. Exp. Mol. Med. 2002;34(5):332–339. doi: 10.1038/emm.2002.47. [DOI] [PubMed] [Google Scholar]
- 20.Liu T. NF-kappaB signaling in inflammation. Signal. Transduct. Tatget. Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnani N.D. Skin damage mechanisms related to airborne particulate matter exposure. Toxicol. Sci. 2016;149(1):227–236. doi: 10.1093/toxsci/kfv230. [DOI] [PubMed] [Google Scholar]
- 22.Valacchi G. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med. 2004;36(5):673–681. doi: 10.1016/j.freeradbiomed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Valacchi G. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0131097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis D.A., Spandau D.F. UVB activation of NF-kappaB in normal human keratinocytes occurs via a unique mechanism. Arch. Dermatol. Res. 2007;299(2):93–101. doi: 10.1007/s00403-006-0729-2. [DOI] [PubMed] [Google Scholar]
- 25.Shi G. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am. J. Transl. Res. 2015;7(9):1612–1620. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995;270(52):31315–31320. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 27.Sandilands A. Filaggrin in the frontline: role in skin barrier function and disease. J. Cell Sci. 2009;122(Pt 9):1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egawa G., Kabashima K. Barrier dysfunction in the skin allergy. Allergol. Int. 2018;67(1):3–11. doi: 10.1016/j.alit.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Furue M. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014;306(9):769–779. doi: 10.1007/s00403-014-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuji G. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011;62(1):42–49. doi: 10.1016/j.jdermsci.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Hidaka T. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat. Immunol. 2017;18(1):64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F. Aryl hydrocarbon receptor is an ozone sensor in human skin. J. Invest. Dermatol. 2009;129(10):2396–2403. doi: 10.1038/jid.2009.85. [DOI] [PubMed] [Google Scholar]
- 33.Thiele J.J., Podda M., Packer L. Tropospheric ozone: an emerging environmental stress to skin. Biol. Chem. 1997;378(11):1299–1305. doi: 10.1515/bchm.1997.378.11.1299. [DOI] [PubMed] [Google Scholar]
- 34.Lakey P.S. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract. Sci. Rep. 2016;6:32916. doi: 10.1038/srep32916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weschler C.J. Transdermal uptake of diethyl phthalate and di(n-butyl) phthalate directly from air: experimental verification. Environ. Health Perspect. 2015;123(10):928–934. doi: 10.1289/ehp.1409151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larese Filon F. Nanoparticles skin absorption: new aspects for a safety profile evaluation. Regul. Toxicol. Pharmacol. 2015;72(2):310–322. doi: 10.1016/j.yrtph.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Patzelt A., Lademann J. Drug delivery to hair follicles. Expet Opin. Drug Deliv. 2013;10(6):787–797. doi: 10.1517/17425247.2013.776038. [DOI] [PubMed] [Google Scholar]
- 38.Kammer R., Tinnerberg H., Eriksson K. Evaluation of a tape-stripping technique for measuring dermal exposure to pyrene and benzo(a)pyrene. J. Environ. Monit. 2011;13(8):2165–2171. doi: 10.1039/c1em10245a. [DOI] [PubMed] [Google Scholar]
- 39.Park S.Y. Air pollution, autophagy, and skin aging: impact of particulate matter (PM10) on human dermal fibroblasts. Int. J. Mol. Sci. 2018;19(9) doi: 10.3390/ijms19092727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin S.P. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. J. Dermatol. Sci. 2018;91(2):175–183. doi: 10.1016/j.jdermsci.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 41.Romani A. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018;172:86–95. doi: 10.1016/j.mad.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Cervellati F. Proinflammatory properties and oxidative effects of atmospheric particle components in human keratinocytes. Chemosphere. 2019;240 doi: 10.1016/j.chemosphere.2019.124746. [DOI] [PubMed] [Google Scholar]
- 43.Valacchi G. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012;1271:75–81. doi: 10.1111/j.1749-6632.2012.06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias P.M. Stratum corneum defensive functions: an integrated view. J. Invest. Dermatol. 2005;125(2):183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 45.Pan T.L. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J. Dermatol. Sci. 2015;78(1):51–60. doi: 10.1016/j.jdermsci.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Haas K. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J. Invest. Dermatol. 2016;136(11):2260–2269. doi: 10.1016/j.jid.2016.06.627. [DOI] [PubMed] [Google Scholar]
- 47.Vogeley C. Role of the aryl hydrocarbon receptor in environmentally induced skin aging and skin carcinogenesis. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krutmann J. [Environmentally induced (extrinsic) skin aging] Hautarzt. 2016;67(2):99–102. doi: 10.1007/s00105-015-3750-6. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura M. Environment-induced lentigines: formation of solar lentigines beyond ultraviolet radiation. Exp. Dermatol. 2015;24(6):407–411. doi: 10.1111/exd.12690. [DOI] [PubMed] [Google Scholar]
- 50.Vierkotter A. Airborne particle exposure and extrinsic skin aging. J. Invest. Dermatol. 2010;130(12):2719–2726. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- 51.Li M. Epidemiological evidence that indoor air pollution from cooking with solid fuels accelerates skin aging in Chinese women. J. Dermatol. Sci. 2015;79(2):148–154. doi: 10.1016/j.jdermsci.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Woodby B. Skin health from the inside out. Annu. Rev. Food. Sci. Technol. 2020 doi: 10.1146/annurev-food-032519-051722. [DOI] [PubMed] [Google Scholar]
- 53.Valacchi G. Ozone-induced damage in 3D-Skin Model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016;82(3):209–212. doi: 10.1016/j.jdermsci.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 54.Valacchi G. Protective effects of topical vitamin C compound mixtures against ozone-induced damage in human skin. J. Invest. Dermatol. 2017;137(6):1373–1375. doi: 10.1016/j.jid.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 55.Lin F.H. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Invest. Dermatol. 2005;125(4):826–832. doi: 10.1111/j.0022-202X.2005.23768.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin J.Y. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003;48(6):866–874. doi: 10.1067/mjd.2003.425. [DOI] [PubMed] [Google Scholar]
- 57.Darr D. Topical vitamin C protects porcine skin from ultraviolet radiation-induced damage. Br. J. Dermatol. 1992;127(3):247–253. doi: 10.1111/j.1365-2133.1992.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 58.Murray J.C. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008;59(3):418–425. doi: 10.1016/j.jaad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Soeur J. Photo-pollution stress in skin: traces of pollutants (PAH and particulate matter) impair redox homeostasis in keratinocytes exposed to UVA1. J. Dermatol. Sci. 2017;86(2):162–169. doi: 10.1016/j.jdermsci.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Benedusi M. Circadian clock as possible protective mechanism to pollution induced keratinocytes damage. Mech. Ageing Dev. 2018;172:13–20. doi: 10.1016/j.mad.2017.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.