Abstract
Deficits in emotion perception (the ability to infer others’ emotions accurately) can occur as a result of neurodegeneration. It remains unclear how different neurodegenerative diseases affect different forms of emotion perception. The present study compares performance on a dynamic tracking task of emotion perception (where participants track the changing valence of a film character’s emotions) with performance on an emotion category labeling task (where participants label specific emotions portrayed by film characters) across seven diagnostic groups (N = 178) including Alzheimer’s disease (AD), behavioral variant frontotemporal dementia (bvFTD), semantic variant primary progressive aphasia (svPPA), non-fluent variant primary progressive aphasia (nfvPPA), progressive supranuclear palsy (PSP), corticobasal syndrome and healthy controls. Consistent with hypotheses, compared to controls, the bvFTD group was impaired on both tasks. The svPPA group was impaired on the emotion labeling task, whereas the nfvPPA, PSP and AD groups were impaired on the dynamic tracking task. Smaller volumes in bilateral frontal and left insular regions were associated with worse labeling, whereas smaller volumes in bilateral medial frontal, temporal and right insular regions were associated with worse tracking. Findings suggest labeling and tracking facets of emotion perception are differentially affected across neurodegenerative diseases due to their unique neuroanatomical correlates.
Keywords: cognitive empathy, emotion recognition, dementia, lesion, empathic accuracy
Introduction
Neurodegenerative diseases can cause profound impairments in empathy that are devastating for patients and their families (Hsieh et al., 2013; Brown et al., 2018, 2020). Empathy encompasses the ability to know, feel and respond to what others are feeling (Levenson and Ruef, 1992; Levenson et al., 2017). Among these components of empathy, knowing or accurately perceiving what another person is feeling is particularly important for successful social interactions (Ickes et al., 1990). Although research on emotion perception in neurodegenerative disease has burgeoned in the last two decades, there are major gaps in our understanding of how different facets of emotion perception are affected in various neurodegenerative diseases and the neural correlates of specific emotion perception deficits.
Measuring facets of emotion perception in neurodegenerative disease
In neurodegenerative disease, empathy has been primarily measured using informant reports, which are susceptible to reporter bias (Furnham and Henderson, 1982; Wadley et al., 2003). Studies examining more objective task-based measures of emotion perception have typically used emotion labeling tasks in which a single emotional judgment (e.g. happy, sad) is made based on a photograph of a static facial expression (Rosen et al., 2004; Werner et al., 2007). Although widely used, static emotion labeling tasks are limited in terms of ecological validity. Emotion recognition in the real-world often requires integrating multiple types of information (visual, auditory) from multiple bodily regions (face, posture and position), which are dynamically changing and occur in interpersonal contexts.
These real-world conditions can be realized to some extent using tasks that require identifying particular emotions portrayed by characters in films (e.g. happy, sad; Goodkind et al., 2015). However, performance on film-based emotion labeling tasks may be influenced by semantic and word knowledge (e.g. knowing the term ‘happy’ is associated with a smile). Moreover, these film-based emotion labeling tasks still fall short in some aspects of ecological validity. For example, during real-world social interactions, emotions can change quickly, requiring emotional judgments to be updated frequently.
To improve upon these limitations, we modified a dynamic tracking task originally developed to assess empathic accuracy in healthy individuals (Levenson and Ruef, 1992). Patients use a rating dial to track the moment-to-moment changes in an expressive film character’s emotional valence (positive–negative–neutral; Brown et al., 2018). In a small sample of individuals with diverse neurodegenerative diseases, we found that worse performance on this dynamic emotional valence tracking task was associated with orbitofrontal cortex atrophy (Goodkind et al., 2012). However, to our knowledge, no studies have examined diagnostic differences in emotional valence tracking abilities or compared neural correlates of emotion tracking to the neural correlates of emotion labeling.
Emotion perception impairments in neurodegenerative diseases
Neurodegenerative diseases affect different large-scale brain networks, resulting in different patterns of neurodegeneration and domains of primary impairment. Alzheimer’s disease (AD) typically features episodic memory impairment with medial temporal, posterior cingulate, precuneus and lateral temporoparietal atrophy. Behavioral variant frontotemporal dementia (bvFTD) features behavioral and emotional impairments with frontoinsular, frontopolar, anterior cingulate and striatal atrophy; semantic variant primary progressive aphasia (svPPA) features impaired word and object comprehension with left-predominant temporal pole and subgenual cingulate atrophy. Non-fluent variant primary progressive aphasia (nfvPPA) features effortful, nonfluent production of speech with left frontal operculum, dorsal anterior insula, and precentral gyrus atrophy. Corticobasal syndrome (CBS) features sensorimotor impairment and cortical sensory loss with frontoparietal, primary motor and sensory, and dorsal insula atrophy. Progressive supranuclear palsy (PSP) features eye movement, gait control and cognitive impairments with brainstem, subcortical, frontal and parietal cortical atrophy (Brun and Gustafson, 1976; Seeley et al., 2009; Boeve, 2011; Gorno-Tempini et al., 2011; Brown et al., 2017).
Given that emotion perception is thought to depend on several, complex widespread neural networks (Singer and Lamm, 2009; Marsh, 2018), it is not surprising that emotion perception can decline in a number of neurodegenerative diseases. Neural correlates of informant reports of empathy as well as performance on picture- and film-based emotion labeling tasks have been widely studied (e.g. Rankin et al., 2006; Kumfor et al., 2013a; Rosen et al., 2002). Such research has implicated anterior temporal regions and the orbitofrontal cortex in empathy impairments, particularly in frontotemporal dementia (FTD) spectrum syndromes that include bvFTD, svPPA and nfvPPA (Rosen et al., 2002; Rankin et al., 2006; Viskontas et al., 2007; Kipps et al., 2009; Eslinger et al., 2011). In addition, the insula and other limbic regions such as the amygdala are sometimes implicated (Rosen et al., 2002; Kipps et al., 2009; Seeley, 2010; Hsieh et al., 2012; Cerami et al., 2014). There are a number of inconsistencies in the literature, however, perhaps due to small sample sizes and lack of heterogeneous patterns of atrophy in samples (e.g. studies tend to examine only one or two syndromes rather than utilizing transdiagnostic samples). The neural correlates of emotion tracking tasks of emotion perception may differ from those of emotion labeling, leading these facets of emotion perception to decline differently in specific neurodegenerative diseases.
Numerous laboratory studies have found impairment in empathic functioning in patients with AD and bvFTD (Allender and Kaszniak, 1989; Cadieux and Greve, 1997; Keane et al., 2002; Wittenberg et al., 2008). Findings in AD have been less consistent (Bozeat et al., 2000; Lavenu and Pasquier, 2004) than those for bvFTD (where empathic deficits are a core feature of the disease; Baez et al., 2014; Oliver et al., 2014; Rascovsky et al., 2011). Given the impairments in empathy that are characteristic of bvFTD and the salience network regions critical for socioemotional processes that are targeted by the disease (e.g. frontoinsular and orbitofrontal cortices; Seeley et al., 2007, 2009), patients with bvFTD may have impairments in all forms of emotion perception. In contrast, patients with AD may perform worse with more cognitively demanding forms of empathy (Bozeat et al., 2000; Gregory et al., 2002; Sturm et al., 2013; Kumfor et al., 2014). The ability to accurately track another person’s changing emotions over time may depend on a network necessary for social working memory that overlaps with default mode network regions (e.g. precuneus and medial prefrontal regions) known to degenerate in AD (Greicius et al., 2004; Zaki et al., 2009; Meyer and Lieberman, 2012). Thus, we hypothesize that individuals with AD may have more difficulty tracking others’ dynamically changing emotional valence compared to labeling a particular emotion.
Fewer studies have examined empathy in language variants of FTD (i.e. svPPA and nfvPPA). Behavioral and language FTD variants are often clustered into a single group (e.g. Werner et al., 2007). Studies examining language variants separately using caregiver report measures have yielded mixed results, with some studies suggesting impairment in svPPA or nfvPPA (Rosen et al., 2002, 2004; Rankin et al., 2005; Calabria et al., 2009; Hazelton et al., 2017) and others suggesting a lack of impairment (Eslinger et al., 2011; Russell et al., 2017). Only one study to our knowledge directly compared patients with svPPA, nfvPPA and bvFTD using a task-based measure of emotion perception; findings indicated that all FTD groups displayed impaired recognition of emotion in facial expressions (Kumfor et al., 2011). Interestingly, viewing higher intensity emotions improved performance for patients with bvFTD and nfvPPA, but not svPPA. Researchers concluded that patients with svPPA have a primary emotion processing impairment (Kumfor et al., 2011); however, given the language and semantic conceptual demands and word-knowledge requirements involved in labeling emotional facial expressions, it is possible that patients with svPPA performed poorly due to reduced semantic functioning. In line with this idea, one study found that three individuals with semantic dementia sorted photos of facial expressions by valence, but not by specific emotions (like anger and disgust; Lindquist et al., 2014). To understand empathic functioning in FTD syndromes more completely, research should utilize multiple emotion perception tasks that vary in semantic demands. We hypothesize that patients with svPPA will have difficulty labeling particular emotions but will be more capable of tracking emotional valence. In contrast, we hypothesize that patients with nfvPPA will have intact emotion labeling given their relatively spared semantic knowledge, but they may have more difficulty tracking emotions due to insula and inferior frontal atrophy potentially impacting emotion imitation and sharing (Gorno-Tempini et al., 2004; Shamay-Tsoory et al., 2009).
Although PSP and CBS are related to FTD due to similar cognitive behavioral symptoms and overlapping neuropathology (Kertesz and McMonagle, 2010), research considering empathy impairments in these diseases that significantly impact motor functioning has been quite rare. Patients with PSP have shown emotion recognition impairments for vocal sounds and facial expressions (Ghosh et al., 2009, 2012). PSP affects midbrain and dorsal and ventral medial prefrontal cortices (Brown et al., 2017), and the resulting dysexecutive deficits (e.g. reduced attention) may make tracking others’ emotions over time even more difficult (Olney et al., 2017). Regarding CBS, one study found empathy deficits based on caregiver reports (Southi et al., 2019). Another study found that patients with CBS were impaired in facial emotion recognition (Kumfor et al., 2014), suggesting that emotion labeling may be difficult for individuals with CBS. More research is needed to characterize impairments in empathy across these different neurodegenerative diseases.
Importantly, using objective measures of emotion labeling, existing research has not yet revealed consistent significant differences in emotion perception between patients with bvFTD and other FTD spectrum syndromes (e.g. Couto et al., 2013), which is surprising given the more extreme real-world emotion perception deficits observed in bvFTD. We suspect that emotion perception deficits in bvFTD may be more apparent due to deficits across facets of emotion perception, whereas patients with other syndromes can compensate with relatively spared functioning in the ability to either label or track emotions. We hypothesize that patients with bvFTD will be the only patient group with significant impairments relative to controls in both the ability to label and track emotions.
The present study
The present study compares two measures of emotion perception derived from basic affective science: (i) a film-based emotion labeling task where participants identify the specific emotions portrayed by characters engaged in social interactions in a series of film clips, and (ii) a dynamic tracking task where participants rate the changing emotional valence portrayed by a character in a film. To determine whether these two forms of emotion perception are differentially affected in neurodegenerative syndromes and have distinct neuroanatomical correlates, we examined differences in task performance across seven groups, including healthy controls (HCs), AD, bvFTD, svPPA, nfvPPA, PSP and CBS. In addition, we use a transdiagnostic whole-brain voxel based morphometry (VBM) approach to examine the neural correlates of performance on each task.
Methods
Participants
Participants were recruited from the Memory and Aging Center at the University of California, San Francisco (UCSF) for participation in an ongoing program of collaborative research. At UCSF, patients were assessed via neurological, neuropsychological and neuroimaging testing and diagnosed with current research criteria for AD (McKhann et al., 2011), bvFTD (bvFTD; Rascovsky et al., 2011), primary progressive aphasia of the semantic or nonfluent variant (Gorno-Tempini et al., 2011), CBS (Armstrong et al., 2013) or PSP (Boxer et al., 2017; Litvan et al., 1996). Of 214 participants who were recruited, 34 participants were excluded because they did not meet criteria for these diagnoses (e.g. a person receiving a diagnosis of mild cognitive impairment with mixed symptoms), and two participants were excluded due to a history of alcoholism and bipolar disorder. The resulting sample (N = 178) included 21 AD, 33 bvFTD, 31 svPPA, 22 nfvPPA, 22 CBS, 25 PSP and 24 HCs of a similar age range confirmed to have no psychiatric or neurological conditions.1
Procedure
All procedures were approved by the Committee for the Protection of Human Subjects at the Universities of California, San Francisco and Berkeley. Data were collected from 2013 to 2017. At UCSF, a clinician assessed disease severity using the Clinical Dementia Rating Scale and Sum of the Boxes scores were calculated for each participant (CDR-box; Morris, 1997) and a neuropsychologist assessed global cognitive functioning through the Mini-Mental State Examination (MMSE; Folstein et al., 1975). Participants came to the Berkeley Psychophysiology Laboratory at the University of California, Berkeley and completed a series of laboratory tasks designed to assess multiple aspects of emotional functioning (Levenson et al., 2008), including the two emotion perception tasks. For both tasks, participants were seated in a chair facing a 21-inch color computer monitor.2
Emotion Perception: emotion category labeling task
Participants viewed a series of 11 film clips (Goodkind et al., 2015) in which a character experiences one of 11 emotions (affection, amusement, anger, calmness, embarrassment, enthusiasm, disgust, fear, pride, sadness and shame). Each clip was 37 s in length and was preceded by a 30 s baseline during which an ‘X’ was presented on the screen. Clips were selected to be thematically simple enough for patients with neurodegenerative disease (Levenson et al., 2008). After watching each clip, participants were shown a photograph of the target character displaying a neutral expression (intended to cue memory). Participants were asked to identify the specific emotion the target character felt most strongly from a list of the 11 emotions depicted in the films. A total emotion recognition score was calculated by summing correct answers across all films,3 with a maximum score of 11 and minimum score of 0.
Emotion perception: dynamic valence tracking task
A rating dial (Ruef and Levenson, 2007) was placed near the dominant hand of the participant. The dial consisted of a small metal box with a knob and attached pointer that rotated across a 180° semi-circle anchored by the legends ‘very bad’ (depicted by a schematic frowning face) at the extreme left, ‘neutral’ (depicted by a schematic neutral face) in the middle, and ‘very good’ (depicted by a schematic smiling face) at the extreme right. The dial generated a voltage that reflected the dial position, and a computer sampled the voltage every 3 ms and computed the average dial position every second.
Participants were instructed to move the rating dial to indicate continuously how positive or negative they believed the target character in a film clip felt at each moment. Following instructions, the experimenter asked several questions to ensure that the participant understood the task and could move the dial. Participants viewed an 80 s film clip from a Disneyland commercial depicting an expressive woman having a conversation over dinner with a man. Throughout the clip, the woman’s emotions fluctuate between positive, neutral and negative valences. Time-lagged cross correlations were used to compute emotion perception: the agreement between a patient’s moment-to-moment ratings of the woman’s emotions and the averaged ratings from an expert panel of graduate students trained in the Facial Action and the emotional expressive behavior coding systems (Ekman and Friesen, 1978; Gross, 1996). To allow for differences in processing speed and dial movement speed, the maximum correlation coefficient was selected for lags between −10 and +10 s (Brown et al., 2018).
Statistical analyses
Behavioral analyses
We used ANOVA to examine diagnostic group differences in age, education, CDR-box, and MMSE, and performed a chi-square test for gender. Performance on each emotion perception task was standardized across all subjects (i.e. z-scores were calculated across the whole sample for each task). Diagnostic group differences in performance on the two empathy tasks were examined using repeated measures ANOVA with all post-hoc comparisons conducted using conservative Bonferroni corrections to account for multiple comparisons.
Neuroimaging analyses
Because these data were accumulated over a long period of time, there was variation in the imaging equipment used. In total, 108 structural MRIs were acquired on a 3.0 T Siemens (Siemens, Iselin, NJ) TIM Trio scanner equipped with a 12-channel head coil using volumetric MPRAGE (160 sagittal slices; slice thickness, 1.0 mm; field of view (FOV), 256 × 230 mm; matrix, 256 × 230; voxel size, 1.0 mm × 1.0 mm × 1.0 mm; repetition time (TR), 2300 ms; TE, 2.98 ms; flip angle, 9°). A total of 29 structural MRIs were acquired on a 4 T Bruker MedSpec system with an 8-channel head coil controlled by a Siemens Trio console, using an MPRAGE sequence (192 sagittal slices; slice thickness, 1 mm; FOV, 256 mm × 224 mm; matrix, 256 × 224; voxel size, 1.0 mm × 1.0 mm × 1.0 mm; TR, 2840 ms; TE, 3 ms; flip angle, 7°). Ten structural MRIs were acquired on a 1.5 T Siemens Magnetom VISION system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil, using a magnetization prepared rapid gradient echo (MPRAGE) sequence [164 coronal slices; slice thickness, 1.5 mm; FOV, 256 mm × 256 mm; matrix, 256 × 256; voxel size, 1.0 mm × 1.5 mm × 1.0 mm; TR, 10 ms; echo time (TE), 4 ms; flip angle, 15°].
Participants underwent 3-T, 4-T or 1.5-T research-quality structural MRI. Table 1 displays the number of patients for each scanner type by diagnostic group. Scanner type did not differ significantly by diagnostic group, χ2 = 7.36, df = 6, P = 0.29. Structural neuroimaging analyses utilizing images collected across different modes of hardware can have robust effects (Abdulkadir et al., 2011) and are unlikely to cause artifacts at the level of strict statistical thresholds. Nonetheless, we covaried scanner type in all analyses. MRIs for HCs were included if acquired within 10 months of completing emotional assessments, and MRIs for patients were included if acquired within 3 months of completing emotional assessments. A total of 13 participants were excluded because they did not have structural MRIs within these time intervals. MRIs were visually inspected for excessive white matter hyperintensities, movement artifact and poor scan quality, and 18 scans were excluded based on these criteria. The final neuroimaging analyses were conducted using 147 MRIs (18 AD, 25 bvFTD, 18 nfvPPA, 27 svPPA, 18 CBS, 21 PSP, 20 HC).
Table 1.
Characteristics of participants by group
| Group | Controls | AD | bvFTD | nfvPPA | svPPA | CBS | PSP | All groups |
|---|---|---|---|---|---|---|---|---|
| n | 24 | 21 | 33 | 23 | 30 | 22 | 25 | 178 |
| Sex (% female) | 54.2 | 47.6 | 39.4 | 56.5 | 33.3 | 63.6 | 48 | 47.8 |
| Handedness (% right) | 95.8 | 85.7 | 87.9 | 95.7 | 96.7 | 90.9 | 72 | 89.3 |
| Age | 63.63 (10.56) | 60.43 (9.40) | 59.91 (8.76) | 68.3 (7.28) | 63.13 (5.84) | 66.91 (6.89) | 68.04 (6.38) | 64.11 (8.50) |
| Years of education | 17.08 (2.02) | 16.43 (3.52) | 15.52 (3.21) | 16.61 (3.84) | 16.37 (2.82) | 14.86 (4.31) | 17.04 (3.34) | 16.25 (3.34) |
| CDR-box | 0.02 (0.10) | 4.55 (1.67) | 7.21 (2.81) | 1.72 (1.55) | 3.75 (2.157) | 3.21 (2.05) | 5.00 (2.704) | 3.83 (3.05) |
| MMSE | 29.55 (0.60) | 21.68 (5.31) | 25.03 (4.26) | 25.38 (5.65) | 25.13 (3.62) | 24.00 (5.39) | 26.63 (3.44) | 25.42 (4.67) |
| Emotion labeling | 10.17 (0.82) | 8.14 (2.30) | 7.58 (3.12) | 8.22 (2.80) | 6.57 (2.13) | 8.50 (2.20) | 8.72 (2.03) | 8.18 (2.53) |
| Dynamic tracking | 0.84 (0.08) | 0.41 (0.36) | 0.36 (0.33) | 0.58 (0.31) | 0.60 (0.35) | 0.64 (0.27) | 0.47 (0.31) | 0.55 (0.32) |
| 1.5 T MRI | 0 | 2 | 3 | 0 | 2 | 1 | 2 | 108 |
| 3 T MRI | 17 | 9 | 16 | 17 | 20 | 15 | 14 | 10 |
| 4 T MRI | 3 | 7 | 6 | 1 | 5 | 2 | 5 | 29 |
Diagnostic groups differed significantly in age, F(6,171) = 4.87, P < 0.001, η2 = 0.14, levels of cognitive impairment (MMSE), F(6,159) = 6.63, P < 0.001, η2 = 0.20 and dementia severity (CDR-box), F(6,169) = 32.85, P < 0.001, η2 = 0.54.
Bonferroni corrected post-hoc comparisons of age differences suggest patients with bvFTD were significantly younger than patients with nfvPPA (Mdiff = 8.4, SE = 2.17, P = 0.003), PSP (Mdiff = 8.13, SE = 2.12, P = 0.004) and CBS (Mdiff = 7.00, SE = 2.20, P = 0.036). Patients with AD were significantly younger than patients with nfvPPA (Mdiff = 7.88, SE = 2.41, P = 0.028) and PSP (Mdiff = 7.61, SE = 2.36, P = 0.032).
Bonferroni corrected post-hoc comparisons of MMSE scores suggest patients with bvFTD (Mdiff = 4.51, SE = 1.19, P = 0.004), svPPA (Mdiff = 4.41, SE = 1.19, P = 0.006), nfvPPA (Mdiff = 4.17, SE = 1.30, P = 0.034), AD (Mdiff = 7.86, SE = 1.33, P < 0.001), and CBS (Mdiff = 5.54, SE = 1.33, P = 0.001) had greater cognitive impairment compared to controls. Patients with AD had significantly greater cognitive impairment than patients with PSP (Mdiff = 4.94, SE = 1.31, P = 0.005).
Bonferroni corrected post-hoc comparisons of CDR-box scores suggest patients with bvFTD (Mdiff = 7.19, SE = 0.57, P < 0.001), svPPA (Mdiff = 3.72, SE = 0.57, P < 0.001), PSP (Mdiff = 4.97, SE = 0.60, P < 0.001), AD (Mdiff = 4.53, SE = 0.63, P < 0.001) and CBS (Mdiff = 4.98, SE = 0.63, P < 0.001) had greater dementia severity scores than controls. Patients with bvFTD had significantly greater dementia severity compared to patients with svPPA (Mdiff = 3.46, SE = 0.53, P < 0.001), nfvPPA (Mdiff = 5.47, SE = 0.57, P < 0.001), PSP (Mdiff = 2.21, SE = 0.56, P = 0.002), AD (Mdiff = 2.66, SE = 0.60, P < 0.001) and CBS (Mdiff = 3.99, SE = 0.59, P < 0.0001). Patients with nfvPPA had significantly lower dementia severity compared to patients with PSP (Mdiff = 23.26, SE = 0.61, P < 0.001), svPPA (Mdiff = 2.01, SE = 0.58, P = 0.016), AD (Mdiff = 2.81, SE = 0.65, P < 0.001).
T1 images were pre-processed with statistical parametric mapping version 12 default parameters (http://www.fil.ion.ucl.ac.uk/spm/), including the light clean-up procedure in the morphological filtering step. T1 images were corrected for bias field and then segmented into gray matter, white matter and cerebrospinal fluid. Segmented images were visually inspected for adequate gray matter segmentation. Because T1 images were acquired from different scanners, images were spatially normalized into Montreal Neurological Institute space (Ashburner and Friston, 2005) and then smoothed with an 8-mm full-width at half-maximum Gaussian kernel. We adopted this approach based on similar studies in our field (e.g. Marchewka et al., 2014; Sturm et al., 2015). International Consortium for Brain Mapping default tissue probability priors (voxel size, 2.0 × 2.0 × 2.0 mm3) were used.
First, we examined structural differences between patient groups and the HC group to characterize neurodegeneration. Next, we conducted whole-brain VBM analyses to examine relationships between structural gray matter maps and (i) the emotion labeling task; and (ii) the dynamic tracking task. We included diagnosis (six variables for seven groups, parameterized using 1 for the diagnosis of interest and 0 for the remaining diagnoses), age, gender (male = 0, female = 1), handedness (right = 0, left = 1, ambidextrous = 2), disease severity (CDR-box), cognitive functioning (MMSE), MRI scanner field strength (two variables for the three field strengths, parameterized using 1 for the field strength of interest and 0 for the remaining field strengths) and total intracranial volume (the sum of gray matter, white matter and cerebrospinal fluid volume to control for individual differences in head size) as nuisance covariates. Images were overlaid with MRIcron (http://people.cas.sc.edu/rorden/mricron/index.html) on an MRI average brain based on the gray and white matter templates used for pre-processing.
Consistent with prior studies (e.g. Sturm et al., 2018), a priori significance was established at P < 0.005 uncorrected to visualize effects and with a cluster extent at PFWE < 0.05 to show regions significant with strict statistical thresholds. We reported clusters with a minimum size of 150 mm3. Using vlsm2 (Bates et al., 2003), we ran 5000 permutation analyses to derive a study-specific error distribution. The combined peak and extent thresholds were used to determine the one-tailed T threshold for multiple comparisons correction at PFWE < 0.05. Permutation analysis is a resampling approach to significance testing by which a test statistic is compared with the null distribution derived from the present study’s data set and is an accurate representation of Type 1 error at P < 0.05 across the entire brain (Hayasaka and Nichols, 2004).
De-identified behavioral data are available from the corresponding authors. Investigators can request imaging data from the UCSF Memory and Aging Center.
Results
Demographic and clinical data for each diagnostic group are detailed in Table 1. No significant differences were found between diagnostic groups in terms of gender, χ2 = 7.36, df = 6, P = 0.29, or years of education, F(6,171) = 1.46 P = .20, η2 = 0.05. However, diagnostic groups differed in age, F(6,171) = 4.87, P < 0.001, η2 = 0.14, levels of cognitive impairment (MMSE), F(6,159) = 6.63, P < 0.001, η2 = 0.20, and dementia severity (CDR), F(6,169) = 32.85, P < 0.001, η2 = 0.54. Thus, for our analyses comparing task performance across diagnostic groups, we examined whether age, cognitive impairment or dementia severity influenced performance on each emotion perception task. Performance on the emotion category labeling and emotional valence tracking task was correlated, r(178) = 0.39, P < 0.001.
Differences between and within diagnostic groups on the emotion perception tasks
A repeated measures ANOVA with the two emotion perception tasks as the repeated measure and diagnostic group as a between subjects factor revealed a significant interaction between emotion perception task and diagnostic group, F(6, 171) = 5.33, P < 0.001, partial η2 = 0.16 (see Figure 1).
Fig. 1.

Emotion perception task performance by diagnostic group. Error bars represent 95% confidence intervals. Blue asterisks reflect differences on the emotion category labeling task between group, red asterisks reflect differences on the dynamic valence tracking task and black asterisks reflect within group differences.
On the emotion category labeling task, Bonferroni corrected post-hoc comparisons between all diagnostic groups revealed that specific diagnostic groups performed significantly worse than controls, including bvFTD (Mdiff = −1.02, SE = 0.25, P = 0.001) and svPPA (Mdiff = −1.42, SE = 0.25, P < 0.001). Patients with svPPA also performed significantly worse than patients with PSP (Mdiff = −0.85, SE = 0.25, P = 0.018).
On the dynamic valence tracking task, Bonferroni corrected post-hoc comparisons between all diagnostic groups revealed that several diagnostic groups performed significantly worse than controls, including bvFTD (Mdiff = −1.51, SE = 0.24, P < 0.001), nfvPPA (Mdiff = −0.81, SE = 0.26, P = 0.047), AD (Mdiff = −1.33, SE = 0.27, P < 0.001), and PSP (Mdiff = −1.16, SE = 0.26, P < 0.001). Patients with bvFTD also performed significantly worse than patients with svPPA (Mdiff = 0.77, SE = 0.23, P = 0.016) and CBS (Mdiff = 0.88, SE = 0.25, P = 0.010).
Within diagnostic groups, Bonferroni corrected post-hoc comparisons revealed that performance was significantly worse on the dynamic tracking task compared to the emotion labeling task for individuals with bvFTD (Mdiff = 0.37, SE = 0.18, P = 0.043), PSP (Mdiff = 0.47, SE = 0.21, P = 0.024) and marginally for AD (Mdiff = 0.40, SE = 0.23, P = 0.074). Performance was significantly worse on the emotion labeling task compared to the dynamic tracking task for individuals with svPPA (Mdiff = −0.81, SE = 0.18, P < 0.001).
Next, we investigated the possible role of age, global cognitive functioning (MMSE), and dementia severity (CDR-box)—the three variables with significant diagnostic group differences—on emotion perception using repeated measures ANCOVAs. There were no significant interactions between emotion perception task and age, F(1, 176) = 0.31, P = 0.58, partial η2 = 0.002, emotion perception task and global cognitive functioning, F(6, 164) = 2.52, P = 0.11, partial η2 = 0.02, or emotion perception task and dementia severity, F(1, 174) = 2.08, P = 0.15, partial η2 = 0.01. The interaction between emotion perception task and diagnostic group remained significant after adjusting for these factors in the model, F(6, 156) = 4.11, P < 0.001, partial η2 = 0.14.
Neural correlates of the emotion perception tasks
The full patient sample (AD, bvFTD, nfvPPA, svPPA, CBS and PSP altogether) had widespread gray matter atrophy compared to the HC group (Figure 2).
Fig. 2.
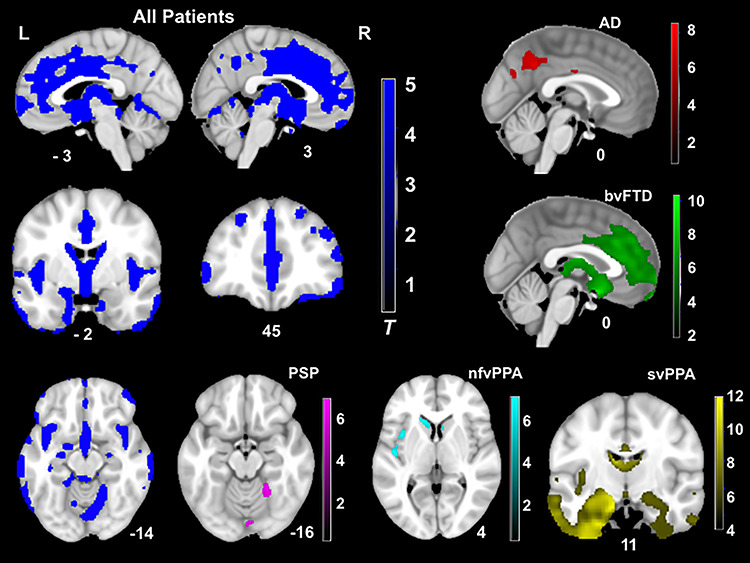
T-score maps of brain areas for which volume loss was greater than HCs, when controlling for age, gender, handedness, total intracranial volume and scanner field strength (PFWE < 0.05). Compared to controls, the patient group had smaller volume in the frontal (e.g. medial prefrontal and orbitofrontal cortex, anterior cingulate cortex and insula), striatum and temporal (e.g. caudate, putamen, amygdala and hippocampus) and parietal regions (e.g. precuneus). Thus, patients altogether showed a widespread pattern of neurodegeneration. The CBS group did not show significant volume loss compared to controls at this threshold.
For the emotion category labeling task, smaller gray matter volumes in several regions were associated with worse emotion recognition (P < 0.005, uncorrected). Bilateral regions included caudate, thalamus, inferior orbitofrontal cortex, inferior frontal triangularis and middle frontal gyrus. Left hemisphere regions included fusiform gyrus, supplementary motor area and posterior insula. Right hemisphere regions included the superior frontal gyrus and rectus. No clusters survived correction with family wise error (PFWE < 0.05). See Table 2 for T-scores and significance levels for all associated regions. Figure 3 displays the statistical maps.
Table 2.
Neural correlates of empathic accuracy from emotion labeling task. Smaller volume in bilateral inferior frontal, bilateral caudate, bilateral thalamus and left insula regions was associated with worse empathic accuracy from the emotion labeling task when controlling for diagnosis, age, sex, handedness, disease severity, cognitive functioning, scanner type and total intracranial volume. Results are shown at P < 0.005, uncorrected and cluster size >150mm3
| Anatomical region | Cluster volume mm3 | x | y | z | Maximum T-score |
|---|---|---|---|---|---|
| Left inferior frontal triangularis gyrus | 6669 | −46 | 36 | 15 | 4.32 |
| Left inferior frontal orbitofrontal cortex | a | ||||
| Right inferior frontal triangularis gyrus | 4836 | 51 | 30 | −9 | 3.69 |
| Right inferior frontal orbitofrontal cortex | a | ||||
| Right caudate | 1370 | 12 | 12 | 12 | 3.04 |
| Left middle frontal gyrus | 1357 | −33 | 38 | 34 | 4.38 |
| Left caudate | 1313 | −14 | 8 | 12 | 3.22 |
| Left middle temporal gyrus | 1171 | −64 | −27 | 3 | 4.00 |
| Right thalamus | 1144 | 8 | −16 | 9 | 3.84 |
| Left middle frontal gyrus | 702 | −26 | 9 | 54 | 3.51 |
| Left posterior insula | 604 | −42 | −10 | 4 | 3.02 |
| Left superior frontal gyrus | 476 | −24 | 52 | 24 | 3.35 |
| Right gyrus rectus | 392 | 8 | 39 | −30 | 3.17 |
| Left thalamus | 378 | −6 | −16 | 8 | 3.39 |
| Left supplementary motor area | 290 | −6 | 24 | 57 | 4.00 |
| Left inferior frontal triangularis gyrus | 263 | −48 | 21 | 26 | 3.35 |
| Left precentral gyrus | 243 | −45 | 9 | 40 | 3.13 |
| Right frontal pole | 233 | 28 | 57 | 12 | 3.01 |
| Right frontal pole | 189 | 14 | 58 | 30 | 2.97 |
| Right superior frontal gyrus | 169 | 26 | 3 | 60 | 3.32 |
Results considered significant at P < 0.005.
aSignifies that these regions were included in the cluster above.
Fig. 3.
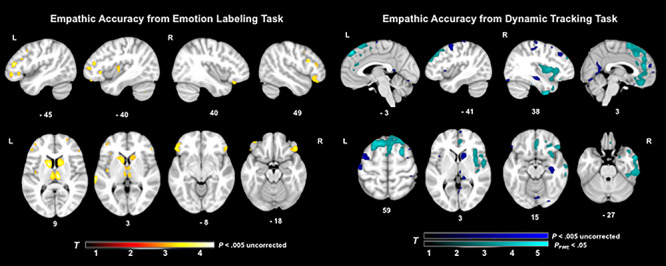
T-score maps of brain areas for which volume loss was associated with worse emotion perception on the emotion labeling film task and dynamic tracking task, when controlling for diagnosis, age, gender, handedness, disease severity, cognitive functioning, total intracranial volume and scanner field strength. Smaller volume (maximum T-score = 4.16) in bilateral inferior frontal, bilateral caudate, bilateral thalamus and left insula regions was associated with worse emotion labeling (P < 0.005, uncorrected). Smaller volume (maximum T-score = 5.22) in bilateral and predominantly right medial frontal, right anterior and middle temporal, right insular and bilateral cingulate and pre- and post-central gyri regions was associated with worse emotion perception (P < 0.005, uncorrected). Two large clusters in superior medial frontal and right anterior temporal and insular areas were also significant with family wise error correction (PFWE < 0.05) for the dynamic tracking task.
For the dynamic valence tracking task, smaller gray matter volumes in several bilateral and predominantly right hemisphere regions were associated with worse emotion perception. The two largest clusters included bilateral superior medial frontal gyrus, anterior cingulate cortex, supplementary motor area and the right middle temporal gyrus, inferior temporal gyrus, fusiform gyrus, middle temporal pole, amygdala, insula and inferior orbitofrontal cortex (PFWE < 0.05). At a less stringent statistical threshold, smaller gray matter volumes in several other regions were associated with worse emotion perception on the dynamic tracking task (P < 0.005, uncorrected). See Table 3 for T-scores and significance levels for all associated regions. Figure 3 displays the statistical maps for dynamic tracking.
Table 3.
Neural correlates of empathic accuracy on the dynamic tracking task. Smaller volume in bilateral and predominantly right medial frontal, anterior temporal, insular, pre- and post-central gyri regions was associated with worse empathic accuracy on the dynamic tracking task when controlling for diagnosis, age, sex, handedness, disease severity, cognitive functioning, scanner type and total intracranial volume. Results are shown at P < 0.005, uncorrected and cluster size >150mm3
| Anatomical Region | Cluster Volume mm3 | x | y | z | Maximum T-score |
|---|---|---|---|---|---|
| Superior medial frontal gyrus | 31334a | −6 | 45 | 48 | 5.23 |
| Anterior cingulate cortex | b | ||||
| Supplementary motor area | b | ||||
| Right middle temporal gyrus | 27459a | 57 | 3 | −28 | 4.38 |
| Right inferior temporal gyrus | b | ||||
| Right fusiform gyrus | b | ||||
| Right middle temporal pole | b | ||||
| Right amygdala | b | ||||
| Right insula | b | ||||
| Right inferior orbitofrontal cortex | b | ||||
| Right caudate | 2822 | 12 | 14 | 4 | 3.44 |
| Left precentral gyrus | 1698 | −34 | −4 | 66 | 3.77 |
| Gyrus rectus | 1677 | 4 | 36 | −20 | 3.62 |
| Right middle frontal gyrus | 1596 | 48 | 36 | 22 | 3.66 |
| Right precentral gyrus | 1536 | 52 | 10 | 33 | 3.64 |
| Right angular gyrus | 1110 | 57 | −57 | 34 | 3.51 |
| Right lingual gyrus | 1073 | 12 | −45 | −2 | 2.98 |
| Right middle frontal gyrus | 1046 | 40 | 33 | 39 | 3.81 |
| Right hippocampus | 1040 | 36 | −20 | −15 | 3.43 |
| Left middle frontal gyrus | 938 | −30 | 58 | 20 | 3.80 |
| Left superior parietal gyrus | 884 | −21 | −46 | 72 | 4.12 |
| Right supramarginal gyrus | 837 | 57 | −24 | 46 | 3.22 |
| Right post-central gyrus | b | ||||
| Left post-central gyrus | 810 | −50 | −18 | 60 | 3.25 |
| Right precentral gyrus | 766 | 44 | −21 | 66 | 3.98 |
| Right superior frontal gyrus | 631 | 16 | 57 | 38 | 4.15 |
| Left superior frontal gyrus | 601 | −18 | 4 | 70 | 3.63 |
| Right lingual gyrus | 520 | 33 | −87 | −18 | 3.01 |
| Right superior parietal gyrus | 469 | 15 | −54 | 69 | 3.42 |
| Vermis cerebellum | 452 | 4 | −78 | −14 | 3.05 |
| Right lingual gyrus | 449 | 15 | −33 | 0 | 3.11 |
| Right thalamus | b | ||||
| Right precentral gyrus | 439 | 36 | −22 | 54 | 2.94 |
| Left post-central gyrus | 435 | −36 | −42 | 68 | 3.54 |
| Right post-central gyrus | 381 | 32 | −48 | 70 | 3.25 |
| Right precentral gyrus | 365 | 28 | −14 | 72 | 3.42 |
| Right posterior middle temporal gyrus | 348 | 64 | −44 | −4 | 3.01 |
| Right superior occipital gyrus | 290 | 24 | −84 | 40 | 3.10 |
| Right superior medial frontal gyrus | 230 | 4 | 57 | 18 | 3.27 |
| Right putamen | 216 | 27 | 2 | −3 | 2.88 |
| Right supramarginal gyrus | 186 | 63 | −20 | 32 | 2.94 |
Results considered significant at P < 0.005, uncorrected.
aDenotes the cluster significant at PFWE < 0.05.
bSignifies that these regions were included in the cluster above.
Discussion
The present study examined impairment in emotion perception across a variety of neurodegenerative diseases including AD, bvFTD, svPPA, nfvPPA, PSP and CBS compared to HCs. We compared performance on two tasks of emotion perception: an emotion category labeling task and a dynamic valence tracking task. Results suggest that for the emotion labeling task, patients with svPPA and bvFTD were most impaired, performing significantly worse on the task compared to controls. For the dynamic tracking task, however, a different pattern emerged with bvFTD, nfvPPA, AD and PSP showing impairment compared to controls. Thus, while most groups showed impairments in emotion perception compared to controls on one of the two tasks, patients with bvFTD were the only group that performed significantly worse than controls on both tasks. Interestingly, when examining performance on the two tasks within diagnostic categories, several groups performed relatively worse on the dynamic tracking task compared to the emotion labeling task, including bvFTD, PSP and marginally in AD. By contrast, for patients with svPPA, performance was significantly worse on the emotion labeling task.
These group comparisons highlight the value of using multiple tests of emotion perception to reveal differences between and within diagnostic groups. Patients with svPPA, but not patients with bvFTD, performed significantly worse than individuals with PSP on the emotion labeling task. Prior studies have used similar findings from emotion labeling tasks to argue that patients with svPPA have primary emotion processing deficits. However, our findings suggest that patients with svPPA performed significantly better when tracking the valence of others’ emotions, even when compared to individuals with bvFTD. As we suspected, svPPA patients’ emotion perception impairments appear to be more pronounced when emotion category labeling is required. This finding is consistent with the past research and predictions stemming from constructionist emotion theories, which suggest that the loss of semantic knowledge impairs perceptions of specific emotions but not perceptions of emotional valence (Lindquist et al., 2014). Findings also have implications for how caregivers might best convey emotional information to different patient groups. For example, our results suggest that patient groups who have difficulties recognizing fluctuating positive and negative affect (bvFTD, PSP and marginally in AD) may be better able to recognize specific sustained emotions, and research should examine whether caregivers could capitalize on patients’ remaining empathic strengths in order to better communicate emotion.
Neuroimaging results revealed similarities in the anatomical correlates of performance on each empathy task, including the bilateral middle frontal gyrus, right inferior frontal and orbitofrontal gyri, left supplementary motor area, right caudate and right thalamus. Prior research suggests that these regions are important for parsing incoming emotional information to discriminate and reason about others’ emotional states (Gray et al., 2002; Leslie et al., 2004; Johnston et al., 2005; Nummenmaa et al., 2006; Nummenmaa et al., 2008; Shamay-Tsoory, 2011; Kumfor et al., 2013b; Hua et al., 2018). Current results are also similar to those from prior studies (Kumfor and Piguet, 2012) and meta-analyses implicating inferior frontal cortex regions in emotion labeling (Dricu and Frühholz, 2016; Belyk et al., 2017).
Compared to emotion category labeling, dynamic valence tracking task performance was associated with a larger pattern of atrophy surviving correction for multiple comparisons, potentially reflecting a larger number of component processes or networks required. Prior studies with healthy participants revealed either no associations between tracking accuracy and regions such as the insula and anterior cingulate (Zaki et al., 2009; Mackes et al., 2018) or, in adolescents, a negative association (Kral et al., 2017). Such findings have led to suggestions that: (i) accurate judgments of emotions that do not include direct or implied displays of pain or disgust may not depend on insula and anterior cingulate regions (Zaki et al., 2009); and (ii) accurately tracking others emotions is related to cognitive empathy and mentalizing more so than affective empathy and emotion sharing (Mackes et al., 2018). However, the present results suggest that atrophy in insular and anterior cingulate regions is associated with impaired accuracy, even absent displays of pain or disgust. Given that our sample is older in age, and considering that emotion perception changes with age (Sze et al., 2012), additional research is needed to examine whether the neural correlates of accurate emotion perception change with age. The lateralized insula findings in the present study (i.e. smaller left posterior insula associated with labeling and smaller right insular atrophy associated with tracking) suggest that the tracking task recruits more attentional and affective-perceptual processes supported by the right insula (Stuss et al., 2001; Murphy et al., 2003), whereas emotion labeling recruits language and cognitive-evaluative processes supported by the left insula (Geschwind, 1970; Vigneau et al., 2006; Fan et al., 2011). This distinction may explain why patients with bvFTD, who are known to have insular atrophy, were impaired on both tasks.
Strength, limitations and future directions
In terms of strengths, the present study included two measures of emotion perception that closely reflect how emotions are recognized in the real-world. Both tasks present emotional information across multiple sensory modalities (e.g. visual auditory, multiple body regions including face and posture) and in interpersonal contexts, maximizing the ecological validity of the tasks. The dynamic tracking task provides an additional level of ecological validity by assessing the ability to track changing emotional valence over time. Our study is the first of its kind to compare two tasks of emotion perception across a large sample of patients with such a wide variety of neurodegenerative diseases.
Regarding limitations, the sample size for some diagnostic groups was small, which could inflate effect sizes, and differences in scanner field strength and head coils could lead to differences in data quality. Based on the current analyses, we cannot draw conclusions regarding whether the brain differences across groups explain behavioral differences among groups. Additionally, it is important to note that neuroimaging results for the emotion labeling task did not survive correction, potentially as a result of a lack of measurement granularity (i.e. our emotion labeling task can only result in 12 discrete values). In addition, our tasks did not allow separate consideration of the method (labeling vs tracking) and type (specific emotion category vs valence) of emotion perception. Additional tasks in which participants label emotional valence or track specific emotions could be developed to enable more fine-grained inferences regarding emotion perception.
Conclusions
Findings provide clear evidence that distinct facets of emotion perception deteriorate differentially across neurodegenerative diseases. Patients who have difficulties labeling others’ emotions might maintain the ability to track the changing valence of others’ emotions due to the different neural regions recruited for these tasks. Our results sound a cautionary note regarding the importance of measure selection when examining impairments in socioemotional functioning. Findings also provide new insights regarding the patterns of neurodegeneration associated with reduced ability to label and track others’ changing emotions, demonstrating common and task-specific neural correlates for two different types of emotion perception. Thus, findings advance our understanding of the behavioral and neurobiological basis of empathic dysfunction in neurodegenerative diseases. As the number of individuals affected by neurodegenerative disease continues to increase, research aimed at understanding the component processes of empathic impairments across a variety of neurodegenerative diseases will become increasingly important, and may prove useful for improving differential diagnosis, understanding the neural underpinnings of socioemotional functioning, and fostering more productive interactions between caregivers and patients.
Funding
Data collection was supported by a National Institute of Aging Program Project Grant awarded to Bruce L. Miller (P01AG019724) and the Larry L. Hillblom Network Grant for the Prevention of Age-Associated Cognitive Decline (2014-A-004-NET). Preparation of this manuscript was supported by a National Research Service Award from the National Institute on Aging awarded to C.L.B. (F31AG059378), a National Institute of Mental Health pre-doctoral fellowship awarded to A.Y.H. (5T32MH020006–20) and National Institute of Aging grants awarded to R.W.L. (R01AG041762) and V.E.S. (R01AG052496 R01AG057204).
Conflict of interest
None declared.
Acknowledgements
We would like to thank Scott Newton, Deepak Paul and all of the past and present members of the Berkeley Psychophysiology lab. We would also like to thank PPG and ADRC coordinators at UCSF.
Footnotes
The current sample has no overlap with the sample described in Goodkind et al. (2012). All participants in the current sample were also part of the sample described in Brown et al. (2018).
Participants were videotaped throughout the tasks and sensors were attached for physiological monitoring (e.g. heart rate, skin conductance); these data were not used for the present study.
All participants provided a response for each film clip.
References
- Abdulkadir A., Mortamet B., Vemuri P., Jack C.R., Krueger G., Klöppel S. (2011). Effects of hardware heterogeneity on the performance of SVM Alzheimer’s disease classifier. NeuroImage, 58(3), 785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allender J., Kaszniak A.W. (1989). Processing of emotional cues in patients with dementia of the Alzheimer’s type. The International Journal of Neuroscience, 46(3–4), 147–55. [DOI] [PubMed] [Google Scholar]
- Armstrong M.J., Litvan I., Lang A.E., Bak T.H., Bhatia K.P., Borroni B., et al. (2013). Criteria for the diagnosis of corticobasal degeneration. Neurology, 80(5), 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. (2005). Unified segmentation. NeuroImage, 26(3), 839–51. [DOI] [PubMed] [Google Scholar]
- Baez S., Manes F., Huepe D., Torralva T., Fiorentino N., Richter F., et al. (2014). Primary empathy deficits in frontotemporal dementia. Frontiers in Aging Neuroscience, 6, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E., Wilson S.M., Saygin A.P., Dick F., Sereno M.I., Knight R.T., et al. (2003). Voxel-based lesion–symptom mapping. Nature Neuroscience, 6(5), 448–50. [DOI] [PubMed] [Google Scholar]
- Belyk M., Brown S., Lim J., Kotz S.A. (2017). Convergence of semantics and emotional expression within the IFG pars orbitalis. NeuroImage, 156, 240–8. [DOI] [PubMed] [Google Scholar]
- Boeve B.F. (2011). The multiple phenotypes of Corticobasal syndrome and Corticobasal degeneration: implications for further study. Journal of Molecular Neuroscience, 45(3), 350–3. [DOI] [PubMed] [Google Scholar]
- Boxer A.L., Yu J.-T., Golbe L.I., Litvan I., Lang A.E., Höglinger G.U. (2017). Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. The Lancet Neurology, 16(7), 552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S., Gregory C.A., Ralph M.A., Hodges J.R. (2000). Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? Journal of Neurology, Neurosurgery, and Psychiatry, 69(2), 178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.A., Hua A.Y., Trujillo A., Attygalle S., Binney R.J., Spina S., et al. (2017). Advancing functional dysconnectivity and atrophy in progressive supranuclear palsy. NeuroImage Clinical, 16, 564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Lwi S.J., Goodkind M.S., Rankin K.P., Merrilees J., Miller B.L., et al. (2018). Empathic accuracy deficits in patients with neurodegenerative disease: association with caregiver depression. American Journal of Geriatric Psychiatry, 26(4). doi: 10.1016/j.jagp.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.L., Wells J.L., Hua A.Y., Chen K.-H., Merrilees J., Miller B.L., et al. (2020). Emotion recognition and reactivity in persons with neurodegenerative disease are differentially associated with caregiver health. The Gerontologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A., Gustafson L. (1976). Distribution of cerebral degeneration in Alzheimer’s disease—a clinico-pathological study. Archiv für Psychiatrie und Nervenkrankheiten, 223(1), 15–33. [DOI] [PubMed] [Google Scholar]
- Cadieux N.L., Greve K.W. (1997). Emotion processing in Alzheimer’s disease. Journal of the International Neuropsychological Society, 3(5), 411–9. [PubMed] [Google Scholar]
- Calabria M., Cotelli M., Adenzato M., Zanetti O., Miniussi C. (2009). Empathy and emotion recognition in semantic dementia: a case report. Brain and Cognition, 70(3), 247–52. [DOI] [PubMed] [Google Scholar]
- Cerami C., Dodich A., Canessa N., Crespi C., Marcone A., Cortese F., et al. (2014). Neural correlates of empathic impairment in the behavioral variant of frontotemporal dementia. Alzheimers Dementia, 10(6), 827–34. [DOI] [PubMed] [Google Scholar]
- Couto B., Manes F., Montañés P., Matallana D., Reyes P., Velasquez M., et al. (2013). Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Frontiers in Human Neuroscience, 7, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dricu M., Frühholz S. (2016). Perceiving emotional expressions in others: activation likelihood estimation meta-analyses of explicit evaluation, passive perception and incidental perception of emotions. Neuroscience and Biobehavioral Reviews. doi: 10.1016/j.neubiorev.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. (1978). Facial Action Coding System: Investigator’s Guide, Mountain View, CA: Consulting Psychologists Press. [Google Scholar]
- Eslinger P.J., Moore P., Anderson C., Grossman M. (2011). Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 23(1), 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Duncan N.W., Greck M., Northoff G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 903–11. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. (1975). Mini-mental state: a practice method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–98. [DOI] [PubMed] [Google Scholar]
- Furnham A., Henderson M. (1982). The good, the bad and the mad: response bias in self-report measures. Personality and Individual Differences, 3(3), 311–20. [Google Scholar]
- Geschwind N. (1970). The Organization of Language and the brain. Science, 170(3961), 940–4. [DOI] [PubMed] [Google Scholar]
- Ghosh B.C., Calder A.J., Peers P.V., Lawrence A.D., Acosta-Cabronero J., Pereira J.M., et al. (2012). Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain, 135(7), 2089–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh B.C.P., Rowe J.B., Calder A.J., Hodges J.R., Bak T.H. (2009). Emotion recognition in progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry, 80(10), 1143–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M.S., Sollberger M., Gyurak A., Rosen H.J., Rankin K.P., Miller B., et al. (2012). Tracking emotional valence: the role of the orbitofrontal cortex. Human Brain Mapping, 33(4), 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M.S., Sturm V.E., Ascher E.A., Shdo S.M., Miller B.L., Rankin K.P., et al. (2015). Emotion recognition in frontotemporal dementia and Alzheimer’s disease: a new film-based assessment. Emotion (Washington, DC), 15(4), 416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., et al. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J., et al. (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.R., Braver T.S., Raichle M.E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 4115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C., Lough S., Stone V., Erzinclioglu S., Martin L., Baron-Cohen S., et al. (2002). Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain, 125(4), 752–64. [DOI] [PubMed] [Google Scholar]
- Greicius M., Srivastava G., Reiss A., Menon V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Science, 101(13), 4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (1996). Emotional Expressive Behavior (EEB) Coding System, Stanford, CA: Stanford University, Unpublished Manuscript. [Google Scholar]
- Hayasaka S., Nichols T.E. (2004). Combining voxel intensity and cluster extent with permutation test framework. NeuroImage, 23(1), 54–63. [DOI] [PubMed] [Google Scholar]
- Hazelton J.L., Irish M., Hodges J.R., Piguet O., Kumfor F. (2017). Cognitive and affective empathy disruption in non-fluent primary progressive aphasia syndromes. Brain Impairment, 18(1), 117–29. [Google Scholar]
- Hsieh S., Hornberger M., Piguet O., Hodges J.R. (2012). Brain correlates of musical and facial emotion recognition: evidence from the dementias. Neuropsychologia, 50(8), 1814–22. [DOI] [PubMed] [Google Scholar]
- Hsieh S., Irish M., Daveson N., Hodges J.R., Piguet O. (2013). When one loses empathy: its effect on carers of patients with dementia. Journal of Geriatric Psychiatry and Neurology, 26(3), 174–84. [DOI] [PubMed] [Google Scholar]
- Hua A.Y., Sible I.J., Perry D.C., Rankin K.P., Kramer J.H., Miller B.L., et al. (2018). Enhanced positive emotional reactivity undermines empathy in behavioral variant frontotemporal dementia. Frontiers in Neurology, 9, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes W., Stinson L., Bissonnette V., Garcia S. (1990). Naturalistic social cognition: empathic accuracy in mixed-sex dyads. Journal of Personality and Social Psychology, 59(4), 730–42. [Google Scholar]
- Johnston P.J., Stojanov W., Devir H., Schall U. (2005). Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. European Journal of Neuroscience, 22, 1221–32. [DOI] [PubMed] [Google Scholar]
- Keane J., Calder A.J., Hodges J.R., Young A.W. (2002). Face and emotion processing in frontal variant frontotemporal dementia. Neuropsychologia, 40(6), 655–65. [DOI] [PubMed] [Google Scholar]
- Kertesz A., McMonagle P. (2010). Behavior and cognition in corticobasal degeneration and progressive supranuclear palsy. Journal of the Neurological Sciences, 289(1–2), 138–43. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Nestor P.J., Acosta-Cabronero J., Arnold R., Hodges J.R. (2009). Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain, 132(3), 592–603. [DOI] [PubMed] [Google Scholar]
- Kral T.R.A., Solis E., Mumford J.A., Schuyler B.S., Flook L., Rifken K., et al. (2017). Neural correlates of empathic accuracy in adolescence. Social Cognitive and Affective Neuroscience, 12(11), 1701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Piguet O. (2012). Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychology Review, 22(3), 280–97. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Miller L., Lah S., Hsieh S., Savage S., Hodges J.R., et al. (2011). Are you really angry? The effect of intensity on facial emotion recognition in frontotemporal dementia. Social Neuroscience, 6(5–6), 502–14. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. (2013a). Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS One, 8(6), e67457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumfor F., Irish M., Hodges J.R., Piguet O. (2013b). The orbitofrontal cortex is involved in emotional enhancement of memory: evidence from the dementias. Brain, 136(10), 2992–3003. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Sapey-Triomphe L.-A., Leyton C.E., Burrell J.R., Hodges J.R., Piguet O. (2014). Degradation of emotion processing ability in corticobasal syndrome and Alzheimer’s disease. Brain, 137(11), 3061–72. [DOI] [PubMed] [Google Scholar]
- Lavenu I., Pasquier F. (2004). Perception of emotion on faces in frontotemporal dementia and Alzheimer’s disease: a longitudinal study. Dementia and Geriatric Cognitive Disorders, 19(1), 37–41. [DOI] [PubMed] [Google Scholar]
- Leslie K.R., Johnson-Frey S.H., Grafton S.T. (2004). Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage, 21(2), 601–7. [DOI] [PubMed] [Google Scholar]
- Levenson R.W., Ruef A.M. (1992). Empathy: a physiological substrate. Journal of Personality and Social Psychology, 63(2), 234–46. [PubMed] [Google Scholar]
- Levenson R.W., Ascher E., Goodkind M., McCarthy M., Sturm V., Werner K. (2008). Laboratory testing of emotion and frontal cortex In: Miller B., Goldenberg G., editors. Handbook of Clinical Neurology, Third Series, Vol. 88, New York, NY: Elsevier, pp. 489–98. [DOI] [PubMed] [Google Scholar]
- Levenson R.W., Lwi S.J., Brown C.L., Ford B.Q., Otero M.C., Verstaen A. (2017). Emotion In: Cacioppo G.G.B.J.T., Tassinary L.G., editors. Handbook of Psychophysiology, Cambridge: Cambridge University Press, pp. 444–64. [Google Scholar]
- Lindquist K.A., Gendron M., Barrett L.F., Dickerson B.C. (2014). Emotion perception, but not affect perception, is impaired with semantic memory loss. Emotion, 14(2), 375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R.C., et al. (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. [review] [88 refs]. Neurology, 47(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Mackes N.K., Golm D., O’Daly O.G., Sarkar S., Sonuga-Barke E.J.S., Fairchild G., et al. (2018). Tracking emotions in the brain – revisiting the empathic accuracy task. NeuroImage, 178, 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka A., Kherif F., Krueger G., Grabowska A., Frackowiak R., Draganski B. (2014). Influence of magnetic field strength and image registration strategy on voxel-based morphometry in a study of Alzheimer’s disease. Human Brain Mapping, 35(5), 1865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A. (2018). The neuroscience of empathy. Current Opinion in Behavioral Sciences, 19, 110–5. [Google Scholar]
- McKhann G., Knopman D.S., Chertkow H., Hymann B., Jack C.R., Kawas C., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement, 7(3), 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Lieberman M.D. (2012). Social working memory: neurocognitive networks and directions for future research. Frontiers in Psychology, 3, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.C. (1997). Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. International Psychogeriatrics, 9(Suppl 1), 173–176-178. [DOI] [PubMed] [Google Scholar]
- Murphy F.C., Nimmo-Smith I., Lawrence A.D. (2003). Functional neuroanatomy of emotions: a meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(3), 207–33. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hyönä J., Calvo M.G. (2006). Eye movement assessment of selective attentional capture by emotional pictures. Emotion, 6(2), 257–68. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hirvonen J., Parkkola R., Hietanen J.K. (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. NeuroImage, 43(3), 571–80. [DOI] [PubMed] [Google Scholar]
- Oliver L.D., Mitchell D.G.V., Dziobek I., Mackinley J., Coleman K., Rankin K.P., et al. (2014). Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia, 67, 14–36. [DOI] [PubMed] [Google Scholar]
- Olney N.T., Spina S., Miller B.L. (2017). Frontotemporal dementia. Neurologic Clinics, 35(2), 339–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin K.P., Kramer J.H., Miller B.L. (2005). Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cognitive and Behavioral Neurology, 18(1), 28–36. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. (2006). Structural anatomy of empathy in neurodegenerative disease. Brain, 129(11), 2945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain, 134(9), 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Perry R.J., Murphy J., Kramer J.H., Mychack P., Schuff N., et al. (2002). Emotion comprehension in the temporal variant of frontotemporal dementia. Brain, 125(10), 2286–95. [DOI] [PubMed] [Google Scholar]
- Rosen H.J., Pace-Savitsky K., Perry R.J., Kramer J.H., Miller B.L., Levenson R.W. (2004). Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dementia and Geriatric Cognitive Disorders, 17(4), 277–81. [DOI] [PubMed] [Google Scholar]
- Ruef A., Levenson R. (2007). Continuous Measurement of Emotion: The affect rating dial. In: Coan J.A., Allen J.J.B., editors. Handbook of Emotion Elicitation and Assessment. In: New York: Oxford University Press. [Google Scholar]
- Russell L.L., Gordon E., Bond R.L., Hardy C.J.D., Marshall C.R., Woollacott I.O.C., et al. (2017). Evaluating distinct components of empathic behavior in frontotemporal dementia. Alzheimer's & Dementia, 13(7), P470. [Google Scholar]
- Seeley W.W. (2010). Anterior insula degeneration in frontotemporal dementia. Brain Structure and Function, 214(5–6), 465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Crawford R.K., Zhou J., Miller B.L., Greicius M.D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62(1), 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. Neuroscientist, 17(1), 18–24. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–27. [DOI] [PubMed] [Google Scholar]
- Singer T., Lamm C. (2009). The social neuroscience of empathy. Annals of the New York Academy of Sciences, 1156(1), 81–96. [DOI] [PubMed] [Google Scholar]
- Southi N., Honan C.A., Hodges J.R., Piguet O., Kumfor F. (2019). Reduced capacity for empathy in corticobasal syndrome and its impact on carer burden. International Journal of Geriatric Psychiatry, 34(3), 497–503. [DOI] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Seeley W.W., Kramer J.H., Miller B.L., Rankin K.P. (2013). Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proceedings of the National Academy of Sciences, 110(24), 9944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Yokoyama J.S., Eckart J.A., Zakrzewski J., Rosen H.J., Miller B.L., et al. (2015). Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex, 64, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Sible I.J., Datta S., Hua A.Y., Perry D.C., Kramer J.H., et al. (2018). Resting parasympathetic dysfunction predicts prosocial helping deficits in behavioral variant frontotemporal dementia. Cortex, 109, 141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T., Gallup G.G., Alexander M.P. (2001). The frontal lobes are necessary for ‘theory of mind’. Brain, 124(2), 279–86. [DOI] [PubMed] [Google Scholar]
- Sze J.A., Goodkind M.S., Gyurak A., Levenson R.W. (2012). Aging and emotion recognition: not just a losing matter. Psychology and Aging, 27(4), 940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Duffau H., Crivello F., Houdé O., et al. (2006). Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed]
- Viskontas I.V., Possin K.L., Miller B.L. (2007). Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Annals of the New York Academy of Sciences, 1121(1), 528–45. [DOI] [PubMed] [Google Scholar]
- Wadley V.G., Harrell L.E., Marson D.C. (2003). Self- and informant report of financial abilities in patients with Alzheimer’s disease: reliable and valid? Journal of the American Geriatrics Society, 51(11), 1621–6. [DOI] [PubMed] [Google Scholar]
- Werner K.H., Roberts N.A., Rosen H.J., Dean D.L., Kramer J.H., Weiner M.W., et al. (2007). Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology, 69(2), 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg D., Possin K.L., Rascovsky K., Rankin K.P., Miller B.L., Kramer J.H. (2008). The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychology Review. doi: 10.1007/s11065-008-9056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America, 106(27), 11382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


