Abstract
Cardiac dysfunction in T2D is associated with excessive FA uptake, oxidation, and generation of toxic lipid species by the heart. It is not known whether decreasing lipid delivery to the heart can effect improvement in cardiac function in humans with T2D. Thus, our objective was to test the hypothesis that lowering lipid delivery to the heart would result in evidence of decreased “lipotoxicity,” improved cardiac function, and salutary effects on plasma biomarkers of cardiovascular risk. Thus, we performed a double-blind randomized placebo-controlled parallel design study of the effects of 12 weeks of fenofibrate-induced lipid lowering on cardiac function, inflammation, and oxidation biomarkers, and on the ratio of two plasma ceramides, Cer d18:1 (4E) (1OH, 3OH)/24:0 and Cer d18:1 (4E) (1OH, 3OH)/16:0 (i.e., “C24:0/C16:0”), which is associated with decreased risk of cardiac dysfunction and heart failure. Fenofibrate lowered plasma TG and cholesterol but did not improve heart systolic or diastolic function. Fenofibrate treatment lowered the plasma C24:0/C16:0 ceramide ratio and minimally altered oxidative stress markers but did not alter measures of inflammation. Overall, plasma TG lowering correlated with improvement of cardiac relaxation (diastolic function) as measured by tissue Doppler-derived parameter e′. Moreover, lowering the plasma C24:0/C16:0 ceramide ratio was correlated with worse diastolic function. These findings indicate that fenofibrate treatment per se is not sufficient to effect changes in cardiac function; however, decreases in plasma TG may be linked to improved diastolic function. In contrast, decreases in plasma C24:0/C16:0 are linked with worsening cardiac function.
Keywords: lipotoxicity, lipid treatments, diastolic function, systolic function
T2D is associated with an increased risk of heart failure in the absence of obstructive coronary heart disease (CHD), valvular abnormalities, alcoholism, or hypertension. Asymptomatic left ventricular (LV) diastolic dysfunction can be detected in up to 75% of people with T2D even in the absence of comorbidities (1, 2). Furthermore, systolic abnormalities including diminished mid-wall fractional shortening (FS) and peak systolic strain are present in up to 16% of asymptomatic patients (3–5). Unfortunately, asymptomatic cardiac dysfunction in patients with T2D can progress to clinical heart failure and “diabetic cardiomyopathy” (6, 7). There is a need to identify effective clinical measures that improve myocardial function or prevent progression to symptomatic heart failure in patients with T2D.
Studies of rodent models of T2D provide evidence that lipotoxicity is caused by increased plasma FFA and TG delivery to and oxidation/storage by the heart, which leads to oxidative stress and significantly contributes to risk of cardiac dysfunction (8–13). In addition to serving as an important cardiac energy source, FFAs are substrates for de novo biosynthesis of ceramides, complex lipids containing a sphingosine backbone coupled to fatty acyl chains (14). The supply of FFAs and a family of ceramide synthase enzymes with distinct but overlapping FFA substrate specificities and the supply of FFAs govern production of ceramide molecular species with differing acyl chain lengths. Perturbation of the distribution of ceramide molecular species promotes lipotoxicity in cultured cardiomyocytes (15). In the myocardium of T2D animal models and in models of lipid overload-induced cardiac function, total ceramides are elevated (11, 16–18). The observation that inhibition of de novo ceramide synthesis in these models improves cardiac function implicates this class of lipids as important mediators of lipotoxicity (18–20). Lipid-induced inflammation and oxidative stress have also been shown to contribute to LV dysfunction in rodent models (21–24).
Abnormalities of lipid metabolism in diabetes have also been associated with LV dysfunction in humans. In the Multiethnic Study of Atherosclerosis, incident heart failure in participants with diabetes was increased and was highest in diabetic subjects with hypertriglyceridemia (25). Increased plasma FFA concentrations in T2D upregulate myocardial FFA uptake, utilization, and oxidation, and steatosis (26–29). This metabolic pattern is associated with decreased efficiency, impaired energetics, and diastolic dysfunction (2, 26). Nonetheless, it has been difficult to causally link myocardial lipid overload to myocardial dysfunction in humans with diabetes. In the ACCORD lipid trial, the trend for lower rates of fatal or nonfatal congestive heart failure in the fenofibrate-treated versus placebo group was not significant (30), and cardiac function was not quantified as an outcome. More recently, the REDUCE-IT trial revealed lower hazard ratios for a composite cardiovascular end point in both diabetic and nondiabetic subjects, but myocardial function and heart failure were not reported as separate outcomes (31).
The purpose of this study was to test whether lowering lipid delivery to the heart improves cardiac function in human subjects with T2D. We employed a randomized, double-blinded trial design in which subjects were treated with fenofibrate or placebo. In addition to echocardiographic endpoints, we evaluated changes blood-based markers of ceramide metabolism, inflammation, and oxidative stress. We were particularly interested in the plasma ratio of Cer d18:1 (4E) (1OH, 3OH)/24:0 and Cer d18:1 (4E) (1OH, 3OH)/16:0 (i.e., C24:0/C16:0 for brevity). This biomarker is increased in patients with end-stage heart failure and improves following treatment with LV assist devices (20). Furthermore, this ratio is inversely associated with incident heart failure in community-based populations (32).
MATERIALS AND METHODS
We chose treatment with fenofibrate for this interventional trial because it has been shown to effectively lower plasma TG, even in subjects treated with statin medications for hypercholesterolemia (33, 34). We assessed the effect of adding fenofibrate to the existing medical regimens of subjects with T2D who were not already taking fibrates. There was protocol approval from the Institutional Review Board of Washington University School of Medicine. Informed consent was obtained from the human subjects or their representatives, and the study abides by the Declaration of Helsinki principles. All subjects underwent a history and physical exam, stress echocardiography, and phlebotomy for eligibility screening.
Subjects
Subjects were recruited from the St. Louis metropolitan area. In order to minimize possible confounding effects on cardiac function, subjects were excluded if they were <30 or >65 years old, smoked, performed >3.5 h/weekk rigorous cardiovascular exercise, had a blood pressure ≥140/90 mm Hg, atrial fibrillation, evidence of CHD on screening stress echocardiography, heart failure, hemoglobin A1c (HbA1c) >10%, recreational drug or severe alcohol use, history of liver disease, renal dysfunction (creatinine >1.5 mg/dl), use of anticoagulants, presence of paradoxical septal motion or more than mild valvular heart disease, or evidence for pericardial or infiltrative myocardial diseases. Women who were pregnant or lactating were also excluded. Subjects were instructed to continue on their usual medications, including lipid-lowering therapy throughout the study.
Randomization
Subjects were randomized (using randomization.com to create block randomization) in a blinded fashion to treatment with fenofibrate (160 mg/day) or identical-appearing placebo for 12 weeks. Subjects were instructed to continue their usual level of physical activity and an ATPIII diet consistent with American Diabetes Association recommendations. At the end of the intervention, 1H-magnetic resonance spectroscopy (1H-MRS) imaging of liver was repeated to assess the effects of intervention on systemic lipid exposure, and fasting blood and echocardiographic studies were repeated. Study physicians provided medical monitoring throughout the intervention to ensure safety of the participants, with interim monthly visits for history and pill counts to assess compliance. Study subjects recorded blood glucose concentrations daily, which were reviewed at monthly visits, and kept an acute event log, since TGs are acute phase reactants.
Plasma/serum/urine measurements other than ceramides
Plasma insulin was measured using a sandwich electrochemiluminescence immunoassay and a Roche Cobas c601 analyzer, Basel, Switzerland). Plasma glucose levels were measured using enzymatic colorimetric assays on the Roche c501 analyzer (Roche Diagnostics, North America). After a 12 h fast, HDL, TG, and total cholesterol levels were measured from plasma using enzymatic colorimetric assays run on the Roche c501 analyzer. HbA1c was measured in whole blood samples using a turbidimetric inhibition immunoassay run on the Roche c501 analyzer. Glycated serum protein was measured using kits from Diazyme, and albumin was measured using kits from Roche Diagnostics, both on a Roche cobas c501. Serum FFAs were quantified using a WAKO colorimetric kit, and systemic inflammatory markers [high sensitivity C-reactive protein (hsCRP), TNF-α, and interleukin 6 (IL-6) were quantified using reagent kits from Roche Diagnostics]. Markers of oxidative stress (hexanoyl-lysine, propanoyl-lysine) were quantified in plasma and urine samples by LC-MS/MS (35–37).
Plasma ceramides
Plasma Cer d18:1 (4E) (1OH, 3OH)/24:0 and Cer d18:1 (4E) (1OH, 3OH)/16:0 were analyzed using an ABI 4000 QTRAP LC-MS/MS tandem quadrupole mass spectrometer with a linear ion trap interfaced to a Shimadzu high-performance LC system. Because the assay measured endogenous analytes in plasma, we compared the concentration-response of the analyte in a surrogate matrix (e.g., 5% BSA) to that of plasma samples. To determine recovery, the plasma or surrogate matrix was spiked with differing amounts of d4-C22:0 and d4-C24:0, extracted with chloroform/methanol and analyzed as above. Differences in matrix effects and extraction recoveries were compensated by internal standards. Details of the metabolomic analytical methods used were previously reported by our group (38).
Body composition and liver fat measurement
Subjects underwent baseline anthropomorphic and metabolic phenotyping with dual-energy X-ray absorptiometry analysis for determination of body composition (iDXA; GE Healthcare, Madison, WI). 1H-MRS for quantification of hepatic TG used 1.5 T Avanto or a 3 T Trio scanner, a PRESS sequence without water suppression and breath-hold techniques (39). Liver fat percentage was calculated as area under the lipid peak (methyl and methylene resonances) divided by the sum of area under water and lipid peaks, with correction for T2 relaxation.
Echocardiography
Cardiac structure and function were assessed using 2D-, Doppler, tissue Doppler, and strain echocardiography according to guidelines from the American Society of Echocardiography (40, 41). FS, a measure of systolic function, and tissue Doppler-derived e′, a measure of diastolic function, were primary outcome variables. Although our study was not powered to evaluate global longitudinal strain measures as endpoints, data using these approaches were analyzed in an exploratory fashion, as these newer techniques may be more sensitive to detect dysfunction (42). It should be noted that global longitudinal strain could be analyzed in only 27 subjects in the placebo group and 29 subjects in the fenofibrate group due to acoustic window limitations in some subjects.
Statistical analysis
SAS version 9.4 (SAS Inc, Cary, NC) was used for data analyses. The primary endpoint was change in systolic function as quantified by FS and diastolic function as quantified by e′. The secondary endpoint was change in plasma C24:0/C16:0 ceramide ratio. Other cardiac, inflammatory, and oxidative stress endpoints were tertiary endpoints. Two-sample t-tests were used for comparing continuous variables between groups and Chi-square tests were used for categorical variables. Satterthwaite analysis was performed if variables had unequal variances. For comparison of the proportion of subjects taking certain medications, two-sample Z test for proportions was used. One sample t-tests were used to compare means of the deltas of the endpoints. ANCOVA was used for comparison of the change in the endpoints between the groups after adjustment for predetermined baseline variables including age, sex, race, body fat percent, BMI, diastolic blood pressure, HbA1c, systolic blood pressure, fasting glucose, fasting TG, ethnicity, and hsCRP. Pearson correlation coefficients were determined between the change in TGs and the cardiac endpoints as well as among the Δ TGs, Δ liver fat, and Δ C24:0/C16:0 ceramide ratio. Shapiro-Wilk tests for normality of distribution were performed and appropriate data transformations were used when needed. Outlier values were determined using the rule that defines an outlier as a value larger than three times of interquartile range from the 75% percentile or smaller than three times of interquartile range from the 25% percentile, and outliers were removed from analysis as noted in the legends. Data are reported as mean ± SD. All statistical analyses were two-sided at significance level 0.05.
RESULTS
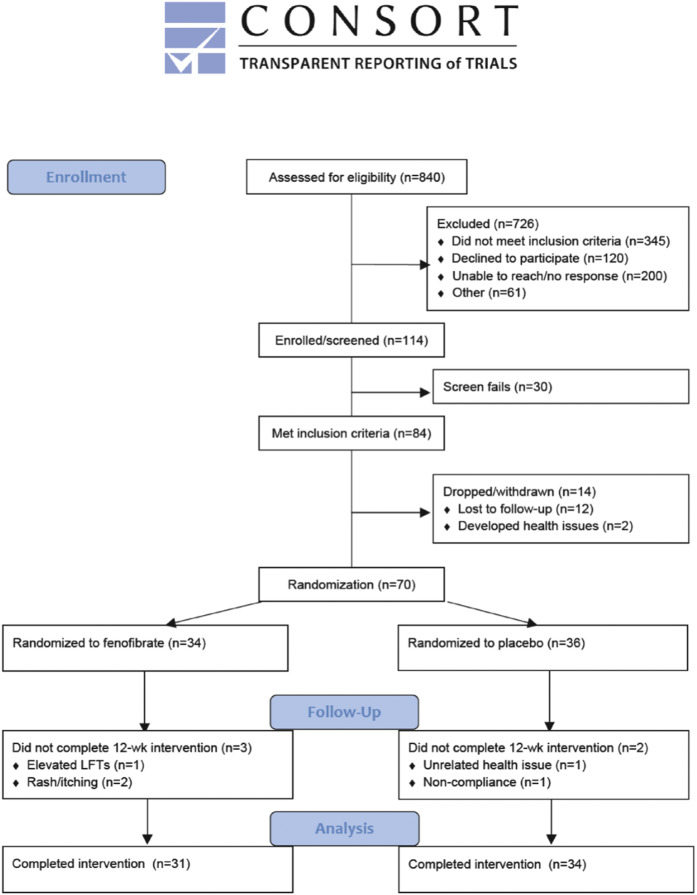
The CONSORT diagram (Fig. 1) outlines the recruitment, enrollment, screen failures, drop-outs, and those who finished the study. In short, of the 70 subjects randomized to fenofibrate (n = 34) or placebo (n = 36), 65 completed the study (31 in the fenofibrate group). Reasons for noncompletion included rash and pruritis (n = 2), elevated liver function tests (n = 1), unrelated health issue (n = 1), and noncompliance (n = 1).
Fig. 1.
A CONSORT diagram depicting enrollment flow, drop-outs, and reasons for drop out.
Baseline characteristics
The characteristics of both groups are shown in Table 1. The two groups were very similar with no significant differences in age, sex, racial composition, anthropomorphic and metabolic characteristics, oxidative stress markers, or hemodynamic measurements. Only one inflammatory marker, hsCRP, was different, higher at baseline in the fenofibrate group. The placebo group had a slightly lower relative wall thickness than the fenofibrate group at baseline, but LV mass and volume and systolic (FS, ejection fraction, global longitudinal strain, tissue Doppler s′ average) and diastolic (e′ average, E/e′ septal) functional measurements were not significantly different (Table 1). The proportion of subjects in both groups who were taking statins, diabetes medications (including insulin), calcium channel blockers, diuretics, angiotensin enzyme-converting inhibitors/angiotensin receptor blockers, or β-blockers was not different (Table 2). Thus, recruitment and randomization led to entry of highly similar subjects in treatment and placebo groups. There were no differences in baseline medications between the groups (Table 3).
TABLE 1.
Baseline characteristics
| Normal Range | Placebo (n = 34) | Fenofibrate (n = 31) | P | |
| Age (years) | NA | 54.4 ± 8.6 | 54.5 ± 8.6 | 0.96 |
| Sex (percent women) | NA | 64.7 | 74.2 | 0.41 |
| Race (percent white) | NA | 76.5 | 77.4 | 0.62 |
| BMI (kg/m2) | 18.5–24.9a | 35.5 ± 5.9 | 36.9 ± 7.5 | 0.39 |
| Body fat (%) | Women, 10–31; men, 2–25b | 44.7 ± 8.1 | 46.0 ± 8.5 | 0.55 |
| Liver fat (%) | 5.56c | 13.76 ± 7.93 | 15.96 ± 10.08 | 0.38 |
| Total cholesterol (mmol/l) | <3.8d | 4.43 ± 1.06 | 4.69 ± 0.85 | 0.27 |
| LDL cholesterol (mmol/l) | <2.6d | 2.30 ± 0.98 | 2.56 ± 0.70 | 0.22 |
| HDL cholesterol (mmol/l) | NA | 1.14 ± 0.32 | 1.24 ± 0.34 | 0.24 |
| TGs (mmol/l) | NA | 2.30 ± 1.00 | 2.01 ± 1.34 | 0.32 |
| Fasting FFAs (mmol/l) | NA | 0.597 ± 0.233 | 0.622 ± 0.219 | 0.66 |
| Fasting insulin (pmol/l) | NA | 165.44 ± 124.19 | 206.86 ± 204.58 | 0.33 |
| Fasting glucose (mmol/l) | <5.5e | 7.54 ± 2.49 | 7.40 ± 1.59 | 0.79 |
| HbA1c percent (mmol/mol) | <5.7e | 7.0 ± 1.1 (52.93 ± 12.11) | 7.1 ± 1.1 (54.30 ± 11.55) | 0.64 |
| Glycated albumin (%) | NA | 15.65 ± 3.82 | 16.21 ± 3.25 | 0.53 |
| Ceramide 24:0 (μg/ml) | 0.735–3.50f | 2.48 ± 0.70 | 2.35 ± 0.61 | 0.46 |
| Ceramide 16:0 (μg/ml) | 0.0612–0.270f | 0.203 ± 0.043 | 0.214 ± 0.052 | 0.36 |
| Ceramide 24:0/C16:0 | 6.43–25.0f | 12.35 ± 3.04 | 11.21 ± 2.38 | 0.10 |
| hsCRP (mg/l) | ≤3a | 4.08 ± 2.80 | 6.58 ± 5.18g | 0.02 |
| TNF-α (pg/ml) | NA | 1.81 ± 0.66 | 1.79 ± 0.51 | 0.88 |
| IL-6 | NA | 4.39 ± 6.31 | 4.13 ± 1.88 | 0.82 |
| Plasma Nε-(hexanoyl)-lysine (ng/mg) | NA | 0.16 ± 0.04 | 0.16 ± 0.06 | 0.64 |
| Plasma Nε-(propanoyl)-lysine (ng/mg) | NA | 3.02 ± 0.67 | 3.18 ± 0.63 | 0.30 |
| Urinary Nε-(hexanoyl)-lysine (ng/mg) | NA | 45.24 ± 23.76 | 45.80 ± 25.07 | 0.93 |
| Urinary Nε-(propanoyl)-lysine (ng/mg) | NA | 233.4 ± 132.0 | 235.74 ± 202.41 | 0.96 |
Data shown are mean ± SD. NA, not available. Bold font indicates a significant P value.
Per the Centers for Disease Control (https://www.CDC.gov).
Per the American Council on Exercise (https://www.ACEfitness.org).
Per (45).
Per the American Heart Association (https://www.heart.org).
Per the American Diabetes Association (https://www.diabetes.org/a1c).
Per (32).
Outliers excluded: n = 2.
TABLE 2.
Baseline hemodynamic and cardiac measures
| Normal Range | Placebo (n = 34) | Fenofibrate (n = 31) | P | |
| Heart rate (bpm) | 60–100a | 71.9 ± 8.9 | 73.6 ± 10.0 | 0.47 |
| Systolic blood pressure (mmHg) | <120a | 127.2 ± 10.7 | 125.9 ± 11.9 | 0.65 |
| Diastolic blood pressure (mmHg) | <80a | 78.6 ± 7.5 | 75.3 ± 10.1 | 0.14 |
| Mean arterial pressure (mmHg) | <93a | 95.9 ± 7.9 | 94.3 ± 7.9 | 0.40 |
| LV mass (g) | Men, 96–200;b women, 66–150b | 145.9 ± 33.4 | 153.3 ± 39.2 | 0.42 |
| LV end-diastolic volume (ml) | Men, 106 ± 22;b women, 76 ± 15b | 88.7 ± 18.6 | 86.9 ± 21.0 | 0.72 |
| Relative wall thickness | Men, 0.24–0.42;b women, 0.22–0.42b | 0.43 ± 0.08 | 0.48 ± 0.10 | 0.04 |
| Ejection fraction (%) | Men, 62 ± 5;b women, 64 ± 5b | 68 ± 5 | 68 ± 6 | 0.96 |
| FS (%) | ≥18b | 38 ± 6 | 39 ± 7 | 0.61 |
| Global longitudinal strain (%)c | ≤ −21.2 ± 2.4b | −17.81 ± 2.1 | −17.8 ± 2.56 | 0.98 |
| s′ average (cm/s) | NA | 8.10 ± 1.73 | 7.82 ± 1.27 | 0.46 |
| e′ average (cm/s) | NA | 7.99 ± 1.78 | 8.30 ± 1.70 | 0.48 |
| E/e′ septal | <15b | 10.44 ± 2.83 | 10.16 ± 3.69 | 0.74 |
Data shown are mean ± SD or minimum–maximum (range of normal). Bold font indicates a significant P value.
Per the American Heart Association (https://www.heart.org)
Per the American Society of Echocardiography (https://www.asecho.org)
Placebo group, n = 27; fenofibrate group, n = 29 due to echocardiographic image limitations.
TABLE 3.
Baseline medications (numbers of patients taking the medications)
| Placebo (n = 34) | Fenofibrate (n = 31) | P | |
| Statin | 16 | 16 | 0.71 |
| Insulin | 7 | 6 | 0.90 |
| Metformin | 25 | 25 | 0.50 |
| Thiazolidinedione | 1 | 0 | 0.34 |
| Sulfonylurea | 10 | 8 | 0.75 |
| DPP-4 inhibitor | 3 | 4 | 0.60 |
| SGLT-2 inhibitor | 5 | 4 | 0.83 |
| GLP-1 agonist | 6 | 6 | 0.86 |
| Calcium channel blocker | 7 | 7 | 0.84 |
| Beta-blocker | 3 | 5 | 0.37 |
| ACE-inhibitor/ARB antagonist | 19 | 17 | 0.94 |
| Diuretics | 6 | 8 | 0.42 |
Effects of 12 weeks of fenofibrate/placebo intervention on anthropomorphic, metabolic, hemodynamic, and oxidative stress and inflammation markers
Changes in anthropomorphic and metabolic characteristics, inflammatory and oxidative stress markers, and hemodynamics from post-to-pre-intervention are shown in Table 4. Importantly, there were no significant differences in BMI or diabetes control between the fenofibrate and placebo groups during the intervention period, as measured by glycated albumin, fasting insulin, and fasting glucose. Similarly, it is important to note that there were no differences between the changes in the two groups’ hemodynamics (heart rate, blood pressure), because these variables can effect a change in cardiac function measurements.
TABLE 4.
Changes in metabolic, inflammatory, and cardiovascular measures
| Placebo (n = 34) | Fenofibrate (n = 31) | P | |
| Δ BMI (kg/m2) | 0.21 ± 0.93 | −1.46 ± 7.71 | 0.24 |
| Δ Liver fat (%) | 0.27 ± 3.40 | 1.71 ± 4.54 | 0.16 |
| Δ Total cholesterol (mmol/l) | 0.21 ± 0.69 | −0.28 ± 0.62 | 0.004 |
| Δ LDL cholesterol (mmol/l) | 0.12 ± 0.61 | −0.13 ± 0.53 | 0.08 |
| Δ HDL cholesterol (mmol/l) | 0.02 ± 0.17 | 0.05 ± 0.22 | 0.60 |
| Δ TGs (mmol/l) | 0.01 ± 0.67 | −0.31 ± 0.59 | 0.048 |
| Δ Fasting FFAs (mmol/l) | 0.01 ± 0.25 | 0.10 ± 0.25 | 0.14 |
| Δ Fasting insulin (pmol/l) | −5.65 ± 65.37 | −10.18 ± 67.71 | 0.78 |
| Δ Fasting glucose (mmol/l) | 0.11 ± 2.25 | 0.63 ± 1.82 | 0.31 |
| Δ Glycated albumin (%) | 0.20 ± 1.63 | 0.43 ± 2.33 | 0.66 |
| Δ C24:0 ceramide | 0.045 ± 0.64 | −0.41 ± 0.36 | 0.00073 |
| Δ C16:0 ceramide | 0.0029 ± 0.028 | −0.0061 ± 0.27 | 0.19 |
| Δ C24:0/C16:0 ceramide ratio | −0.04 ± 2.44 | −1.6 ± 1.66 | 0.0036 |
| Δ hsCRP (mg/l) | −0.03 ± 2.27 | −1.10 ± 2.94 | 0.11 |
| Δ TNF-α (pg/ml) | −0.04 ± 0.68 | 0.32 ± 0.97 | 0.09 |
| Δ IL-6 (pg/ml) | −1.01 ± 5.97 | 0.65 ± 3.52 | 0.17 |
| Δ Plasma Nε-(hexanoyl)-lysine (ng/ml) | −0.053 ± 0.035 | −0.059 ± 0.055 | 0.65 |
| Δ Plasma Nε-(propanoyl)-lysine (ng/ml) | −0.737 ± 0.685 | −1.124 ± 0.720 | 0.03 |
| Δ Urinary Nε-(hexanoyl)-lysine (ng/ml) | −7.10 ± 24.66 | −15.85 ± 23.85 | 0.65 |
| Δ Urinary Nε-(propanoyl)-lysine (ng/ml) | −44.73 ± 68.05 | −40.38 ± 76.31 | 0.81 |
| Δ Heart rate (bpm) | −1.59 ± 8.86 | −1.58 ± 10.02 | 1.000 |
| Δ Mean arterial pressure (mmHg) | 0.3 ± 8.3 | 3.4 ± 8.8 | 0.15 |
| Δ LV end-diastolic volume (ml) | −1.71 ± 10.87 | 0.52 ± 11.59 | 0.43 |
| Δ Relative wall thickness | −0.02 ± 0.05 | −0.02 ± 0.04 | 0.74 |
| Δ Ejection fraction (%) | 1.4 ± 4.8 | 3.1 ± 5.2 | 0.19 |
| Δ FS (%) | 0.030 ± 0.083 | 0.025 ± 0.090 | 0.80 |
| Δ Global longitudinal strain (%)a | −0.26 ± 2.02 | −0.23 ± 1.76 | 0.96 |
| Δ s′ average (cm/s) | 0.16 ± 1.81 | 0.68 ± 1.04 | 0.17 |
| Δ e′ average (cm/s) | 0.55 ± 1.58 | −0.02 ± 1.35 | 0.13 |
| Δ E/e′ septal | −0.21 ± 2.56 | −0.40 ± 3.13 | 0.79 |
Data are shown as Post-Pre, mean ± SD. Bold font indicates a significant P value. Outliers excluded: n = 1 in fenofibrate group for Δ TG; n = 1 in fenofibrate group for Δ insulin; n = 3 (2 placebo, 1 fenofibrate) for Δ urinary propanoyl-lysine. HOMA-IR, homeostatic model assessment of insulin resistance.
Placebo group, n = 27; fenofibrate group, n = 29 due to echocardiographic image limitations.
To evaluate evidence for oxidative stress, we focused on the products of the reaction of oxidized lipids with lysine residues in proteins, Nε-(hexanoyl)- and Nε-(propanoyl)-lysine, which have been shown to be stable markers of oxidative stress in plasma and urine of diabetic patients (43). There was a significant difference between the change (post-pre) in the plasma concentration of Nε-(propanoyl)-lysine between the fenofibrate and placebo groups, with the fenofibrate group having a greater decrease in the concentration of this marker (Table 4). However, there was no difference between the two treatment groups in the changes of the other oxidative stress markers analyzed. There were also no differences in the changes in well-established inflammatory markers, (TNF-α, IL-6, and hsCRP) between the groups.
Specific lipids were significantly affected by fenofibrate treatment compared with placebo
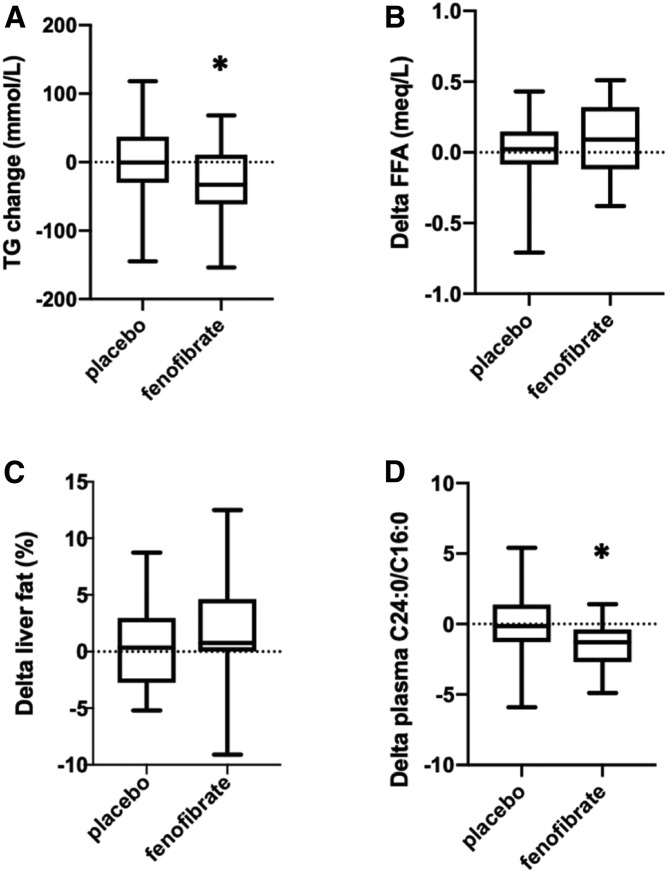
As expected, fenofibrate lowered plasma TG more than placebo (P < 0.05; Fig. 2, Table 4). This difference persisted after multivariable modeling (P = 0.04). This difference was also maintained after log transformation of the TG (P = 0.003) and in a multivariable model of delta log TG (P = 0.02). Consistent with the known effects of fenofibrate to enhance lipolysis of TG-rich lipoproteins, subjects receiving fenofibrate had significantly decreased cholesterol (P = 0.004) and a trend for lower LDL following treatment (Table 4). The change in plasma FFAs from post- to pre-intervention, however, was not different between the groups (Fig. 2) before or after multivariate adjustments.
Fig. 2.
Lipid alterations after placebo or fenofibrate intervention. A: Serum TGs decreased more after fenofibrate treatment compared with after placebo (*P < 0.05). B: No significant change in plasma FFAs. C: Lack of significant change in liver fat percentage between the fenofibrate and placebo groups. D: The ratio of the plasma C24:0/C16:0 ceramides decreased more after fenofibrate treatment than after placebo (*P < 0.004).
Lipid delivery to nonadipose tissues that exceeds the capacity for acute utilization in oxidative or biosynthetic pathways leads to ectopic steatosis. To determine whether the decreases in plasma TG had a measurable effect on the integrated lipid exposure of nonadipose tissues over the course of the 12 week treatment, we used proton MRS to quantify hepatic TG noninvasively. Liver TG was chosen as an endpoint rather than cardiac TG because the higher liver TGs facilitate sensitive and accurate assessments of systemic lipid exposure (44). There was no significant difference between the fenofibrate and placebo groups with respect to change in liver fat percentage, whether the measures were analyzed before or after log transformation (Fig. 2). There was also no difference in liver fat percentage or log delta-liver fat percentage after multivariate modeling. Because liver fat is typically a more sensitive measure of ectopic lipid accumulation than myocardial fat (45), it is unlikely that the fenofibrate and placebo groups would have demonstrated differences in myocardial TG.
Excess FAs are also incorporated into the de novo synthetic pathway for ceramides. While the ratio of C24:0 to C16:0 ceramide is inversely associated with incident heart failure (32), it is not known how this measure is impacted by TG lowering. Thus, we quantified the plasma C24:0 ceramide and C16:0 ceramide and calculated the C24:0/C16:0 ceramide ratio before and after treatment with fenofibrate. Fenofibrate treatment resulted in a significant lowering of the C24:0 ceramide (P = 0.00073) and the ceramide ratio (P = 0.004) compared with placebo treatment (Fig. 2, Table 4). After adjustment for the predetermined baseline characteristics, this difference in Δ C24:0/C16:0 ceramide ratio remained significant (P = 0.003). There were no significant correlations among delta C24:0/C16:0 ceramide ratio, delta plasma TG, or delta liver fat, with or without data transformation.
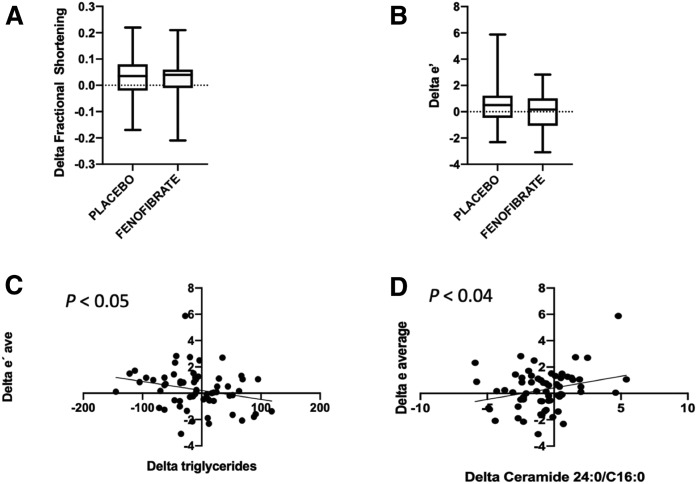
Diastolic relaxation was inversely related to change in plasma TG and directly related to change in plasma C24:0/C16:0 ceramide ratio
As shown in Fig. 3 and Table 4, there were no significant changes in systolic or diastolic functional measures after treatment with fenofibrate, as compared with placebo. Specifically, there was no change in the primary endpoints: FS or e′. However, there was a negative correlation between the Δ TGs and LV relaxation as measured by Δ e′ average (r = −0.25, P < 0.05). This suggests that TG lowering contributes to improved diastolic performance. On the other hand, change in LV relaxation, as measured by e′, correlated directly with change in plasma C24:0/C16:0 ceramide ratio (r = 0.26, P < 0.04). There was also a trend toward a direct correlation between change in systolic function, as measured by delta s′, with change in plasma C24:0/C16:0 ceramide ratio (r = 0.24, P = 0.056). These changes provide evidence that the plasma ceramide ratio may have utility as a plasma biomarker for cardiac function.
Fig. 3.
Relationships between cardiac function and metabolism changes. A: Change in FS after placebo or fenofibrate (P = NS); box and whiskers (minimum–maximum) plot. B: Change in the e′ average after placebo or fenofibrate (P = NS). C: Linear regression between Δ e′ and Δ TGs (r = 0.25, P < 0.05). D: Linear regression between Δ e′ and Δ C24:0/C16:0 ceramide ratio (r = 0.26, P <0.04).
DISCUSSION
This randomized clinical trial was designed to evaluate the effects of decreasing plasma TG on cardiac function and biomarkers of metabolic stress in patients with T2D. As expected, fenofibrate, which increases plasma TG clearance and activates PPARα, significantly decreased plasma TG. As compared with placebo, fenofibrate treatment did not alter the primary cardiac function endpoints, FS, or e′. Nonetheless, lowering of plasma TG across both groups did relate to improvement of LV relaxation, as measured by e′ average. This suggests that lowering systemic lipid exposure is beneficial for cardiac function.
We also evaluated secondary and tertiary outcomes related to biomarkers of cardiovascular risk and the pathogenesis of lipotoxicity. Fenofibrate treatment did not change liver steatosis or markers of inflammation, and only one of four markers of oxidative stress decreased significantly after fenofibrate treatment. On the other hand, we observed a significant decrease in plasma C24:0/C16:0 ceramide ratio after fenofibrate treatment as compared with placebo. Because the plasma ceramide ratio is inversely associated with incident CHD, heart failure, and death (32), and because a lower plasma ceramide ratio is associated with lower LV ejection fraction and diminished global circumferential strain (46), this finding is unanticipated, as TG lowering would be expected to improve cardiovascular risk (31). On the other hand, consistent with the hypothesis that decreasing C24:0/C16:0 ceramide ratios portend worse cardiovascular outcomes, across all subjects in the present study, decreases in this ratio related to a worsening LV relaxation, as measured by a decrease in e′ average.
The association between TG lowering and improved cardiac function, regardless of treatment group, correlates well with previous studies in animal models of T2D that show that decreasing excessive fat delivery to the heart, or exporting lipid from the heart, improves cardiac function (11, 13). It also correlates well with the finding that in humans with obesity (and some with T2D) who underwent gastric bypass-induced weight loss, plasma TG lowering was accompanied by lowered total myocardial FA utilization and oxidation as well as improved e′ average and E/e′ (47). In the present study, we did not find association of treatment group with improvement in cardiac function or measures of ectopic steatosis. Based on our calculations of the 95% confidence bounds, we can be 95% sure that fenofibrate does not cause more than a 0.5 (centimeters per second) increase in e′ average and a 0.06 unit increase on FS compared with placebo, which are relatively small changes. Our failure to detect improved cardiac function in subjects receiving fenofibrate may have related to duration of treatment (12 weeks) or sample size of the study (n = 65). Prior studies have shown variable effects on cardiovascular outcomes with the addition of fenofibrate to lower TGs in patients with diabetes or metabolic syndrome. For example, in the ACCORD lipid study, fenofibrate did not reduce CHD or heart failure events in patients with T2D (30), whereas the FIELD study showed that fenofibrate reduced cardiovascular disease events in women with T2D (48). These and other studies have focused on endpoints of hospitalization for CHD or heart failure and/or cardiovascular deaths, whereas our study focused on LV function as an outcome.
Ceramides are complex lipids with pleiotropic biological effects (49). Depending on fatty acyl chain lengths, these hydrophobic molecules differentially affect membrane structure and function as well as number of signaling pathways in cells (14, 49). Although the effects of each ceramide species is not completely understood, several lines of research now point to C16:0 as being involved in mitochondrial permeabilization and apoptosis (50) and linked to all-cause mortality in community-based cohort studies (32). In contrast, C24:0 ceramide appears to counteract the effects of C16:0 ceramide, preventing mitochondrial membrane permeabilization, and it is associated with decreased risk of CHD and all-cause mortality (32, 50). Likewise, the C24:0/C16:0 ceramide ratio is inversely associated with risk of incident CHD, heart failure, and all-cause mortality in meta-analyses (32). It is not known whether the plasma C24:0/C16:0 ceramide ratio is a reflection of changes in tissue lipid homeostasis or whether these lipids function directly in pathogenesis of cardiovascular disease. Fenofibrate is thought to decrease plasma TGs through activation of PPARs (51). Precisely how PPAR agonism affects the complex metabolic pathways that regulate levels of specific ceramide molecular species or the C24:0/C16:0 ratio is not known. Here, we provide evidence that fenofibrate treatment for TG lowering decreases the plasma C24:0 ceramide and the C24:0/C16:0 ceramide ratio, in contrast to treatment with statins or ezetimibe therapy, which lower both C24:0 and C16:0 ceramides but do not affect the ratio (52). This suggests that the major effect of fenofibrate treatment on the ceramide ratio occurs primarily through its effects on the very long-chain ceramide species. It is possible that fenofibrate-associated changes in the plasma ceramide profile may have offset some of the benefits of plasma TG lowering in some subjects. Longer-term studies in humans and mechanistic studies in animal models will be required to more fully evaluate the effects of fenofibrate on the ceramide ratio and its associations with cardiovascular risk.
Other factors that mitigate or amplify the lipotoxic response (steatosis, oxidative stress, and inflammation) were not broadly affected by fenofibrate treatment. Although steatosis has been linked with lipotoxic effects on the heart (53), some studies suggest that it may be more of a marker of excessive fat exposure rather than directly causative of lipotoxic effects (54, 55). In humans, positron emission tomography studies using FA tracers suggest that excessive fat oxidation and overall myocardial oxygen consumption may be more related to cardiac dysfunction than excessive fat storage (47). It is difficult to know the significance of the fenofibrate-associated decrease in one oxidative stress marker, plasma Nε-(propanoyl)-lysine, when other markers of oxidative stress were unchanged. Furthermore, the inflammatory markers examined were unchanged by fenofibrate therapy. These findings fit into a mixed picture of the effects of this drug on oxidative stress and inflammation markers in the literature that may reflect the limitations of these markers as much as different effects of the drug (56–59).
CONCLUSIONS
Decreasing plasma TG availability to the heart was associated with improved diastolic function in patients with T2D. In contrast, decreasing plasma C24:0/C16:0 ceramide ratio was associated with worsened diastolic function. Fenofibrate treatment did lower plasma TG, but it also lowered plasma C24:0/C16:0 ceramide ratio. Given the positive associations of plasma ceramide ratios with improved survival and lower incidence of CHD, this change may abate some of the benefit from plasma TG lowering and warrants further study.
Limitations
Although fenofibrate treatment (n = 31) versus placebo (n = 34) did not alter the primary cardiac function endpoints, there was a clear relationship between TG lowering and cardiac function across both groups (n = 65). Failure to detect improvement in cardiac function by treatment group, while still detecting a relationship between plasma TG and cardiac function, could relate, in part, to sample size. On the other hand, TG is more likely to represent a barometer of the lipid overload state, rather than a direct driver of lipotoxicity (54, 55). Differences in baseline hsCRP between treatment groups was noted, although we controlled for this in our analysis. The finding that fenofibrate treatment was associated with a decrease in plasma ceramide C24:0/C16:0 ratio does not automatically signify that fenofibrate is associated with worse cardiac outcomes; for it is not clear yet if low C24:0/C16:0 ceramide ratio is causally linked or simply associated with worse outcomes. Further mechanistic research and interventional studies are warranted. Finally, the findings of this study should not be extended to patients who do not fit the study’s entry criteria.
Data availability
The data for this study are in the tables and figures. Individual de-identified data are available upon request from Dr. Linda Peterson at lpeterso@wustl.edu and Ling Chen, MD, MSPH, PhD at lingchen@wustl.edu in accordance with all data sharing policies of the National Institutes of Health and Washington University School of Medicine. This trial was registered at clinicaltrials.gov as NCT01752842.
Acknowledgments
The authors thank Kristin O’Callaghan for her editorial assistance.
Footnotes
Abbreviations:
- CHD
- coronary heart disease
- FS
- fractional shortening
- HbA1c
- hemoglobin A1c
- 1H-MRS
- 1H-magnetic resonance spectroscopy
- hsCRP
- high sensitivity C-reactive protein
- IL-6
- interleukin 6
- LV
- left ventricular
This work was supported by National Institutes of Health Grants P20 HL113444, P30 DK020579, P30 DK056341, and UL1RR024992 (Clinical and Translational Science Awards, CTSA), R34 HL138253-01, and R21 HL145217-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A patent application for use of the ceramide biomarkers is pending (L.R.P., D.S.O., J.E.S.). D.S.O. is employed by and holds equity in Casma Therapeutics.
REFERENCES
- 1.Boyer J. K., Thanigaraj S., Schechtman K. B., and Perez J. E.. 2004. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am. J. Cardiol. 93: 870–875. [DOI] [PubMed] [Google Scholar]
- 2.Diamant M., Lamb H. J., Groeneveld Y., Endert E. L., Smith J. W., Bax J. J., Romijn J. A., de Roos A., and Radder J. K.. 2003. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J. Am. Coll. Cardiol. 42: 328–335. [DOI] [PubMed] [Google Scholar]
- 3.Andersen N. H., Poulsen S. H., Helleberg K., Ivarsen P., Knudsen S. T., and Mogensen C. E.. 2003. Impact of essential hypertension and diabetes mellitus on left ventricular systolic and diastolic performance. Eur. J. Echocardiogr. 4: 306–312. [DOI] [PubMed] [Google Scholar]
- 4.Ernande L., Rietzschel E. R., Bergerot C., De Buyzere M. L., Schnell F., Groisne L., Ovize M., Croisille P., Moulin P., Gillebert T. C., et al. 2010. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J. Am. Soc. Echocardiogr. 23: 1266–1272. [DOI] [PubMed] [Google Scholar]
- 5.Fang Z. Y., Schull-Meade R., Downey M., Prins J., and Marwick T. H.. 2005. Determinants of subclinical diabetic heart disease. Diabetologia. 48: 394–402. [DOI] [PubMed] [Google Scholar]
- 6.Schannwell C. M., Schneppenheim M., Perings S., Plehn G., and Strauer B. E.. 2002. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 98: 33–39. [DOI] [PubMed] [Google Scholar]
- 7.Zarich S. W., and Nesto R. W.. 1989. Diabetic cardiomyopathy. Am. Heart J. 118: 1000–1012. [DOI] [PubMed] [Google Scholar]
- 8.Taegtmeyer H., Golfman L., Sharma S., Razeghi P., and van Arsdall M.. 2004. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann. N. Y. Acad. Sci. 1015: 202–213. [DOI] [PubMed] [Google Scholar]
- 9.Young M. E., McNulty P., and Taegtmeyer H.. 2002. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 105: 1861–1870. [DOI] [PubMed] [Google Scholar]
- 10.Belke D. D., Larsen T. S., Gibbs E. M., and Severson D. L.. 2000. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 279: E1104–E1113. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., and Unger R. H.. 2000. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA. 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., et al. 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen L. B., Bartels E. D., and Bollano E.. 2002. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J. Biol. Chem. 277: 27014–27020. [DOI] [PubMed] [Google Scholar]
- 14.Park W. J., Park J. W., Merrill A. H., Storch J., Pewzner-Jung Y., and Futerman A. H.. 2014. Hepatic fatty acid uptake is regulated by the sphingolipid acyl chain length. Biochim. Biophys. Acta. 1841: 1754–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law B. A., Liao X., Moore K .S., Southard A., Roddy P., Ji R., Szulc Z., Bielawska A., Schulze P. C., and Cowart L. A.. 2018. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 32: 1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haus J. M., Kashyap S. R., Kasumov T., Zhang R., Kelly K. R., Defronzo R. A., and Kirwan J. P.. 2009. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 58: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., and Schaffer J. E.. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo S. B., Baicu C. F., Van Laer A., Geng T., Kasiganesan H., Zile M. R., and Cowart L. A.. 2012. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122: 3919–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji R., Akashi H., Drosatos K., Liao X., Jiang H., Kennel P. J., Brunjes D. L., Castillero E., Zhang X., Deng L. Y., et al. 2017. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight. 2: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigert C., Brodbeck K., Staiger H., Kausch C., Machicao F., Häring H. U., and Schleicher E. D.. 2004. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J. Biol. Chem. 279: 23942–23952. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen M. T., Satoh H., Favelyukis S., Babendure J. L., Imamura T., Sbodio J. I., Zalevsky J., Dahiyat B. I., Chi N. W., and Olefsky J. M.. 2005. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 280: 35361–35371. [DOI] [PubMed] [Google Scholar]
- 23.Boudina S., and Abel E. D.. 2007. Diabetic cardiomyopathy revisited. Circulation. 115: 3213–3223. [DOI] [PubMed] [Google Scholar]
- 24.Schilling J. D., Machkovech H. M., Kim A. H., Schwendener R., Schwedwener R., and Schaffer J. E.. 2012. Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 303: H1366–H1373. [Erratum. 2013. Am. J. Physiol. Heart Circ. Physiol. 304: H632.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebong I. A., Goff D. C., Rodriguez C. J., Chen H., Sibley C. T., and Bertoni A. G.. 2013. Association of lipids with incident heart failure among adults with and without diabetes mellitus: Multiethnic Study of Atherosclerosis. Circ. Heart Fail. 6: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGill J. B., Peterson L. R., Herrero P., Saeed I. M., Recklein C., Coggan A. R., Demoss A. J., Schechtman K. B., Dence C. S., and Gropler R. J.. 2011. Potentiation of abnormalities in myocardial metabolism with the development of diabetes in women with obesity and insulin resistance. J. Nucl. Cardiol. 18: 421–429, quiz 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGavock J. M., Lingvay I., Zib I., Tillery T., Salas N., Unger R., Levine B. D., Raskin P., Victor R. G., and Szczepaniak L. S.. 2007. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S., Adrogue J. V., Golfman L., Uray I., Lemm J., Youker K., Noon G. P., Frazier O. H., and Taegtmeyer H.. 2004. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 18: 1692–1700. [DOI] [PubMed] [Google Scholar]
- 29.Alavaikko M., Elfving R., Hirvonen J., and Jarvi J.. 1973. Triglycerides, cholesterol, and phospholipids in normal heart papillary muscle and in patients suffering from diabetes, cholelithiasis, hypertension, and coronary atheroma. J. Clin. Pathol. 26: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ACCORD Study Group, Ginsberg H. N., Elam M. B., Lovato L. C., Crouse J. R., Leiter L. A., Linz P., Friedewald W. T., Buse J. B., Gerstein H. C., et al. 2010. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362: 1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt D. L., Steg P. G., Miller M., Brinton E. A., Jacobson T. A., Ketchum S. B., Doyle R. T., Juliano R. A., Jiao L., Granowitz C., et al.; REDUCE-IT Investigators . 2019. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380: 11–22. [DOI] [PubMed] [Google Scholar]
- 32.Peterson L. R., Xanthakis V., Duncan M. S., Gross S., Friedrich N., Völzke H., Felix S. B., Jiang H., Sidhu R., Nauck M., et al. 2018. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart Assoc. 7: e007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May H. T., Anderson J. L., Pearson R. R., Jensen J. R., Horne B. D., Lavasani F., Yannicelli H. D., and Muhlestein J. B.. 2008. Comparison of effects of simvastatin alone versus fenofibrate alone versus simvastatin plus fenofibrate on lipoprotein subparticle profiles in diabetic patients with mixed dyslipidemia (from the Diabetes and Combined Lipid Therapy Regimen study). Am. J. Cardiol. 101: 486–489. [DOI] [PubMed] [Google Scholar]
- 34.Muhlestein J. B., May H. T., Jensen J. R., Horne B. D., Lanman R. B., Lavasani F., Wolfert R. L., Pearson R. R., Yannicelli H. D., and Anderson J. L.. 2006. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J. Am. Coll. Cardiol. 48: 396–401. [DOI] [PubMed] [Google Scholar]
- 35.Kato Y., and Osawa T.. 2010. Detection of lipid-lysine amide-type adduct as a marker of PUFA oxidation and its applications. Arch. Biochem. Biophys. 501: 182–187. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Il’yasova D., Sztaray J., Young S. P., Wang F., and Millington D. S.. 2010. Quantification of the oxidative damage biomarker 2,3-dinor-8-isoprostaglandin-F(2alpha) in human urine using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 399: 302–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato Y., Yoshida A., Naito M., Kawai Y., Tsuji K., Kitamura M., Kitamoto N., and Osawa T.. 2004. Identification and quantification of N(epsilon)-(hexanoyl)lysine in human urine by liquid chromatography/tandem mass spectrometry. Free Radic. Biol. Med. 37: 1864–1874. [DOI] [PubMed] [Google Scholar]
- 38.Jiang H., Hsu F. F., Farmer M. S., Peterson L. R., Schaffer J. E., Ory D. S., and Jiang X.. 2013. Development and validation of LC-MS/MS method for determination of very long acyl chain (C22:0 and C24:0) ceramides in human plasma. Anal. Bioanal. Chem. 405: 7357–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schonfeld G., Patterson B. W., Yablonskiy D. A., Tanoli T. S., Averna M., Elias N., Yue P., and Ackerman J.. 2003. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J. Lipid Res. 44: 470–478. [DOI] [PubMed] [Google Scholar]
- 40.Lang R. M., Bierig M., Devereux R. B., Flachskampf F. A., Foster E., Pellikka P. A., Picard M. H., Roman M. J., Seward J., Shanewise J. S., et al. 2005. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 41.Nagueh S. F., Appleton C. P., Gillebert T. C., Marino P. N., Oh J. K., Smiseth O. A., Waggoner A. D., Flachskampf F. A., Pellikka P. A., and Evangelisa A.. 2009. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 10: 165–193. [DOI] [PubMed] [Google Scholar]
- 42.Galderisi M., Cosyns B., Edvardsen T., Cardim N., Delgado V., Di Salvo G., Donal E., Sade L. E., Ernande L., Garbi M., et al.; 2016–2018 EACVI Scientific Documents Committee . 2017. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 18: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 43.Hisaka S., Kato Y., Kitamoto N., Yoshida A., Kubushiro Y., Naito M., and Osawa T.. 2009. Chemical and immunochemical identification of propanoyllysine derived from oxidized n-3 polyunsaturated fatty acid. Free Radic. Biol. Med. 46: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 44.Airhart S., Cade W. T., Jiang H., Coggan A. R., Racette S. B., Korenblat K., Spearie C. A., Waller S., O’Connor R., Bashir A., et al. 2016. A diet rich in medium-chain fatty acids improves systolic function and alters the lipidomic profile in patients with type 2 diabetes: a pilot study. J. Clin. Endocrinol. Metab. 101: 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikhalkova D., Holman S. R., Jiang H., Saghir M., Novak E., Coggan A. R., O’Connor R., Bashir A., Jamal A., Ory D. S., et al. 2018. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity (Silver Spring). 26: 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nwabuo C. C., Duncan M., Xanthakis V., Peterson L. R., Mitchell G. F., McManus D., Cheng S., and Vasan R. S.. 2019. Association of circulating ceramides with cardiac structure and function in the community: the Framingham Heart Study. J. Am. Heart Assoc. 8: e013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin C. H., Kurup S., Herrero P., Schechtman K. B., Eagon J. C., Klein S., Davila-Roman V. G., Stein R. I., Dorn G. W. II, Gropler R. J., et al. 2011. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring). 19: 1804–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.d’Emden M. C., Jenkins A. J., Li L., Zannino D., Mann K. P., Best J. D., Stuckey B. G., Park K., Saltevo J., and Keech A. C.; FIELD Study Investigators . 2014. Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 57: 2296–2303. [DOI] [PubMed] [Google Scholar]
- 49.Brice S. E., and Cowart L. A.. 2011. Sphingolipid metabolism and analysis in metabolic disease. In Sphingolipids and Metabolic Diseases. L. A. Cowart, editor. Landes Bioscience/Springer Science+Business Media, LLC, Berlin/Heidelberg. 1–17. [DOI] [PubMed] [Google Scholar]
- 50.Stiban J., and Perera M.. 2015. Very long chain ceramides interfere with C16-ceramide-induced channel formation: A plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim. Biophys. Acta. 1848: 561–567. [DOI] [PubMed] [Google Scholar]
- 51.Staels B., Dallongeville J., Auwerx J., Schoonjans K., Leitersdorf E., and Fruchart J. C.. 1998. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 98: 2088–2093. [DOI] [PubMed] [Google Scholar]
- 52.Tarasov K., Ekroos K., Suoniemi M., Kauhanen D., Sylvänne T., Hurme R., Gouni-Berthold I., Berthold H. K., Kleber M. E., Laaksonen R., et al. 2014. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99: E45–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szczepaniak L. S., Dobbins R. L., Metzger G. J., Sartoni-D’Ambrosia G., Arbique D., Vongpatanasin W., Unger R., and Victor R. G.. 2003. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn. Reson. Med. 49: 417–423. [DOI] [PubMed] [Google Scholar]
- 54.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V. Jr., Ory D. S., and Schaffer J. E.. 2003. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L., Shi X., Bharadwaj K. G., Ikeda S., Yamashita H., Yagyu H., Schaffer J. E., Yu Y. H., and Goldberg I. J.. 2009. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J. Biol. Chem. 284: 36312–36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walker A. E., Kaplon R. E., Lucking S. M., Russell-Nowlan M. J., Eckel R. H., and Seals D. R.. 2012. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 60: 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimura J., Dewa Y., Muguruma M., Kuroiwa Y., Yasuno H., Shima T., Jin M., Takahashi M., Umemura T., and Mitsumori K.. 2007. Effect of fenofibrate on oxidative DNA damage and on gene expression related to cell proliferation and apoptosis in rats. Toxicol. Sci. 97: 44–54. [DOI] [PubMed] [Google Scholar]
- 58.Mulvey C. K., Ferguson J. F., Tabita-Martinez J., Kong S., Shah R. Y., Patel P. N., Master S. R., Usman M. H., Propert K. J., Shah R., et al. 2012. Peroxisome proliferator-activated receptor-α agonism with fenofibrate does not suppress inflammatory responses to evoked endotoxemia. J. Am. Heart Assoc. 1: e002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belfort R., Berria R., Cornell J., and Cusi K.. 2010. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J. Clin. Endocrinol. Metab. 95: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are in the tables and figures. Individual de-identified data are available upon request from Dr. Linda Peterson at lpeterso@wustl.edu and Ling Chen, MD, MSPH, PhD at lingchen@wustl.edu in accordance with all data sharing policies of the National Institutes of Health and Washington University School of Medicine. This trial was registered at clinicaltrials.gov as NCT01752842.