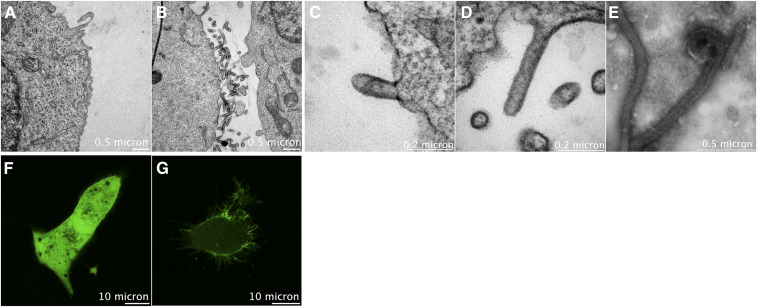
The Ebola virus (EBOV) causes a high rate of mortality as evidenced by the last outbreak in the Democratic Republic of the Congo. As of March, 2020, there have been more than 3,440 reported cases of EBOV with an ~66% fatality rate (1). EBOV is a lipid-enveloped virus that acquires its lipid envelope from the host cell plasma membrane (PM) of infected cells, the site of virus assembly and release. The virus matrix protein VP40 is known to coordinate the virus assembly at the PM through the formation of a large matrix of VP40 oligomers. VP40 expression in human cell lines is sufficient to form virus-like particles (VLPs) that are similar to infectious virions (average length ~2 μm and average diameter ~70–80 nm). Furthermore, it has been shown that VLPs bud in two different orientations that are VP40-dependent with more dominant horizontal orientation in the presence of the nucleocapsid (2). Phospholipids enriched at the inner PM leaflet, for example, PS and phosphatidylinositol-4,5-bisphosphate, attract VP40 to the PM and mediate viral matrix assembly (3, 4). VP40-mediated PS and PE exposure upon assembly on the outer viral membrane play another key role in viral entry through interactions with T cell immunoglobulin mucin domain-1 receptors, PS and PE-binding receptors on host cell membranes (5). The mechanism by which PS becomes exposed at the site of viral budding and on the outer viral lipid membrane are still under debate. In this study, we investigated additional properties of the PM required for efficient matrix assembly, particle emergence, elongation, and VLP budding. In the figure above, HEK293 cells expressing either empty vector or VP40 were collected 18 h posttransfection and processed for transmission electron microscopy imaging. Panel A shows the surface of cells expressing empty vector with few tubulations at the surface. However, VP40 expressing cells in B displayed large amount of VLPs on the cell surface. F and G are confocal images of HEK293 expressing either EGFP or EGFP-eVP40, respectively. Panel C shows an early budding stage of VP40 VLPs, while panel D shows a more advanced VLP elongation and budding stage at the surface of the cell prior to scission. Purified VLPs from cell culture medium are shown in panel E. In all the examples (B–E), cell lipids derived from the plasma membrane are critical to the respective states of viral assembly, budding, and spread of infection.
EQUIPMENT: FEI Tecnai T12 transmission electron microscope, Nikon Eclipse Ti Confocal inverted microscope
REAGENTS: Uracyl acetate
REFERENCES
- 1.World Health Organization. 2019. Ebola Virus disease: Democratic Republic of the Congo. External Situation Report 66, p 1–9. [Google Scholar]
- 2.Noda T., Ebihara H., Muramoto Y., Fujii K., Takada A., Sagara H., Kim J. H., Kida H., Feldmann H., and Kawaoka Y.. 2006. Assembly and budding of ebolavirus. PLoS Pathog. 2: e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adu-Gyamfi E., Johnson K. A., Fraser M. E., Scott J. L., Soni S. P., Jones K R., Digman M. A., Gratton E., Tessier C. R., and Stahelin R. V.. 2015. Host cell plasma membrane phosphatidylserine regulates the assembly and budding of ebola virus. J. Virol. 89: 9440–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson K. A., Taghon G. J. F., Scott J. L., and Stahelin R. V.. 2016. The ebola virus matrix protein, VP40, requires phosphatidylinositol 4,5-bisphosphate (PI(4,5)P 2) for extensive oligomerization at the plasma membrane and viral egress. Sci. Rep. 6: 19125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moller-Tank S., Kondratowicz A. S., Davey R. A., Rennert P. D., and Maury W.. 2013. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J. Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]