Abstract
Multi-component lipid emulsions, rather than soy-oil emulsions, prevent cholestasis by an unknown mechanism. Here, we quantified liver function, bile acid pools, and gut microbial and metabolite profiles in premature parenterally fed pigs given a soy-oil lipid emulsion, Intralipid (IL), a multi component lipid emulsion, SMOFlipid (SMOF), a novel emulsion with a modified fatty-acid composition [experimental emulsion (EXP)], or a control enteral diet (ENT) for 22 days. We assayed serum cholestasis markers, measured total bile acid levels in plasma, liver, and gut contents, and analyzed colonic bacterial 16S rRNA gene sequences and metabolomic profiles. Serum cholestasis markers (i.e., bilirubin, bile acids, and γ-glutamyl transferase) were highest in IL-fed pigs and normalized in those given SMOF, EXP, or ENT. Gut bile acid pools were lowest in the IL treatment and were increased in the SMOF and EXP treatments and comparable to ENT. Multiple bile acids, especially their conjugated forms, were higher in the colon contents of SMOF and EXP than in IL pigs. The colonic microbial communities of SMOF and EXP pigs had lower relative abundance of several gram-positive anaerobes, including Clostridrium XIVa, and higher abundance of Enterobacteriaceae than those of IL and ENT pigs. Differences in lipid and microbial-derived compounds were also observed in colon metabolite profiles. These results indicate that multi-component lipid emulsions prevent cholestasis and restore enterohepatic bile flow in association with gut microbial and metabolomic changes. We conclude that sustained bile flow induced by multi-component lipid emulsions likely exerts a dominant effect in reducing bile acid-sensitive gram-positive bacteria.
Keywords: liver, cholestasis, parenteral nutrition, sterols, fatty acids, metabolomics, microbiome, clostridium, Enterobacteriaceae
Infants born prematurely have increased metabolic needs for postnatal growth and development (1). The immaturity or surgical removal of the gastrointestinal tract due to congenital and acquired diseases requires that many premature infants require partial or exclusive nourishment via parenteral nutrition (PN). Exclusive PN for long periods of time is associated with complications that include cholestasis and liver injury, referred as PN-associated liver disease (PNALD), which can be as high as 50% in premature infants (2, 3). This condition often presents in infants secondary to intestinal resection and intestinal failure-associated liver disease (4). A key element in the pathogenesis of PNALD is cholestasis, or lack of bile flow from the liver into the duodenum leading to accumulation of bile acids and injury in the liver (2).
Development of cholestasis and subsequent liver injury in patients receiving PN has been linked to the lipid component of PN, in particular plant sources such as soybeans (5). Lipid emulsion formulations composed of fish oils have been shown to reduce cholestatic conditions associated with long-term PN (6–8). New generation lipid emulsions composed of a mixture of lipid sources, including soy oil, olive oil, fish oil, and/or medium-chain triglycerides (MCTs) have also been associated with lower rates of cholestasis and liver injury compared with pure soy oil-based emulsions in some, but not all, reported clinical studies (9–11). It is currently unclear how new generation lipid emulsions protect against cholestasis, but studies have implicated phytosterols, plant-derived compounds analogous to cholesterol in animals, abundant in oils sourced from plants. Infants given soybean-oil emulsions for prolonged periods (>1 month) accumulate phytosterols in plasma and liver tissue and this correlates with their progression to PNALD (12–14). Another proposed explanation is that the higher content of ω-3 FAs, such as the DHA and EPA, found in fish oils, and lower amounts of ω-6 FAs, such as linoleic acid (LA), rich in soy oil, may beneficially modulate immune responses and prevent inflammation-driven liver injury (15–17). In addition to the potential for improving the inflammatory state of the liver, increasing the DHA and AA content in parenteral lipid emulsions may be beneficial to growth and development of other organs, especially the brain. It has been shown that premature infants exhibit lower blood levels of DHA and AA postnatally and that these FA deficiencies correlate with increased rates of prematurity-related diseases (18).
Despite the many studies investigating various PN lipid emulsions in preterm infants, little is known about how prolonged total PN (TPN) impacts the developing gut microbiome and whether this is influenced by the type of lipid emulsion (19, 20). Bacteria in the intestine play a key role in the enterohepatic circulation of bile acids, namely to deconjugate bile salts and generate secondary bile acids (21). These processes have an important influence on bile acid profiles, transport, and their impact on host cell function mediated via the bile acid receptors, FXR and G protein-coupled bile acid receptor 1 (GPBAR1)/TGR5 (22, 23). Thus, we sought to determine how different lipid emulsions affect the gut microbiota community profiles.
In the current study, we sought to quantify liver function and track disease progression and correlate this with bile acid pool sizes and profiles of the gut microbiome and metabolome in neonatal preterm piglets administered TPN containing different lipid compositions. We tested three different lipid emulsions: soy-oil only, Intralipid (IL); soy, olive, and fish oils, SMOFlipid (SMOF); and another derived from the same three lipid sources but modified to contain higher DHA and AA, which we have called the experimental emulsion (EXP). We hypothesized that the multi-component lipid emulsions would protect against cholestasis and that the unique FA profiles of the different lipid emulsions would correlate with differences in the gut microbiome and metabolome.
MATERIALS AND METHODS
Animal care and study protocol
The study protocol was approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Mixed-breed (Duroc, Hampshire, Yorkshire, and Landrace) piglets were delivered six days preterm by cesarean section and housed in individual cages under a 12 h light-dark cycle, as described previously (24–27). Passive immunity was provided during the first 24 h via intravenous administration of maternal plasma (16 ml/kg). A total of 56 pigs were randomized to one of four groups: control enteral diet (ENT), TPN-IL, TPN-SMOF, or TPN-EXP. The number of piglets in each group at the end of the study was: ENT, 11 (three female); IL, 12 (four female); SMOF, 11 (five female); and EXP, 11 (four female). All piglets were implanted with jugular venous catheters, while piglets in the enteral formula group were also implanted with an orogastric Tygon feeding tube (6 French) to enable enteral formula feeding. Piglets in the TPN groups received a sham surgical procedure, but not the indwelling orogastric feeding tube. Ampicillin was administered intravenously (50 mg/kg) every other day throughout the study period to prevent catheter-related infections.
Nutrition and feeding protocol
All diets were isocaloric and isonitrogenous and, at full volume, consisted of 25 g/(kg·day) carbohydrates, 13 g/(kg·day) protein or amino acids, and 5 g/(kg·day) lipids as described previously (27) (Table 1). The detailed FA composition of the diets was described previously (27). The ENT used consisted of a commercially available ready-to-use preterm infant formula [Enfamil Premature (24 calorie); Mead Johnson Nutrition], which was fortified with added whey protein, lactose, electrolytes, and micronutrients to meet the nutritional needs of piglets and to match the intake levels in the TPN groups. DHA (DSM Nutritional Products Ltd.) was also added to the enteral formula to match the level in the SMOF group. Lipid emulsions were provided by Fresenius Kabi (Bad Homburg, Germany) and differed mainly by FA content, with SMOF, and EXP also having a slightly higher amount of vitamin E (all-rac-α-tocopherol). The EXP was similar to SMOF but was modified to increase DHA and AA and decrease EPA.
TABLE 1.
Daily dietary nutrient intake
| ENT | IL | SMOF | EXP | |
| Protein (g/kg) | ||||
| Free amino acid | — | 13 | 13 | 13 |
| Whey:casein (90:10) | 13 | — | — | — |
| Carbohydrate (g/kg) | ||||
| Glucose | — | 25 | 25 | 25 |
| Lactose/corn syrup solids | 25 | — | — | — |
| Fat/lipid (g/kg) | 5 | 5 | 5 | 5 |
| DHA (mg/kg) | 30.7 | 3.55 | 41.4 | 75.5 |
| AA (mg/kg) | 26.4 | 9.55 | 16.05 | 165 |
| EPA (mg/kg) | — | — | 100 | 13.8 |
| LA (mg/kg) | 1,216 | 2,567 | 1,311 | 1,289 |
| Vitamin E (IU/kg) | 7.5 | 4-5 | 7.5 | 7.5 |
Based on published or reported ingredient compositions except for FAs, which were based on FA analysis completed by LC-MS/MS as described in the Materials andMethods. ENT = commercial preterm infant formula [Enfamil Premature (24 calories); Mead Johnson Nutrition] containing the following sources of protein: 60% whey, 40% casein; carbohydrate: 60% corn syrup solids, 40% lactose; and fat: 40% MCT oil, 30% soy oil, 27% high oleic vegetable oil, 3% single-cell oil blend rich in DHA and AA. The infant formula was fortified with whey protein, lactose, and DHA to match the macronutrient level in the SMOF group. Electrolytes and micronutrients were also matched to the intake of the SMOF group.
All pigs were given TPN solutions continuously starting at a rate of 5 ml/(kg·h). Within the first six study days, the rate of TPN infusion for the IL, SMOF, and EXP groups was gradually increased to 10 ml/(kg·h) and maintained for the remaining days [22 days total, 240 ml/(kg·d)]. For the ENT group, the lipid emulsion provided during the first 6 days was SMOF, while enteral formula feeding every 3 h via orogastric tube began the day after surgery. Enteral feeding volumes were increased and the PN infusion volume was decreased proportionally, such that by day 7, PN was stopped and ENT piglets received the full feeding volume enterally [240 ml/(kg·day)]. Beginning on day 5, ENT pigs were trained to drink liquid formula from a stainless steel bowl. On day 10, orogastric feedings stopped, and bowl feeding transitioned to every 4 h.
Blood and tissue collection and analysis
Blood samples were collected after catheter placement during surgery (day 0) and on days 7, 14, and 22. Blood samples were divided to be analyzed for complete blood count and processed to yield serum and plasma. Whole blood cell counting analysis was done in EDTA-treated whole blood immediately. Serum and plasma were stored at −80°C until analysis. On day 22, all pigs were euthanized and total contents of the gallbladder were collected by needle aspiration, and livers were dissected and weighed. Luminal contents from the distal small intestine and proximal colon were sampled by flushing with sterile saline and collecting into sterile 5 ml tubes. Samples were either flash-frozen in liquid nitrogen or fixed in 10% paraformaldehyde for 24 h and then transferred to 70% ethanol before paraffin embedding and stored for later analysis. Complete blood count and serum chemistries were analyzed by the Comparative Pathology Laboratory at Baylor College of Medicine using commercially available kits. Plasma fibroblast growth factor 19 (FGF19) was measured using the Porcine FGF19 ELISA kit (RayBiotech). Fixed and paraffin-embedded sections of distal ileum were stained with Alcian blue and periodic acid-Schiff for measurement of villus height, crypt depth, and counting of goblet cells. Sections of liver tissue were stained with hematoxylin and eosin and then scored using eleven different metrics of liver injury and inflammation by a veterinary pathologist blinded to the treatment groups (scoring criteria are described in supplemental Table S1) as described previously (25).
Total bile acid analysis
Samples of plasma, liver tissue, and distal ileum tissue, as well as luminal contents from the small intestine and colon, were analyzed for total bile acids using an enzymatic colorimetric kit (BQ Kits). Total bile acid content within the liver tissue, gallbladder contents, distal ileum tissue, and small intestine and colon contents were extrapolated from the concentration in the tested sample and the overall mass of the organ or compartment as collected at the end of study.
Quantitative bile acid, sterol, and FA analysis by LC-MS/MS
Samples of gallbladder bile were analyzed for various conjugated and free bile acid concentrations by LC-MS/MS (Q-Exactive Orbitrap; Thermo Scientific) equipped with a HESI probe. The free and conjugated bile acid concentrations in samples were determined by reverse isotope dilution methodology using internal standards of d9-chenodeoxycholic acid (CDCA) and d9-glyco-CDCA (Cambridge Isotopes, Andover, MA). Sample preparation for bile acids was performed by solid-phase extraction (SPE) with 200 mg Bond Elut cartridges (Agilent Technologies). Briefly, SPE columns were preconditioned by passing 2 ml of methanol followed by 2 ml of LC-MS grade water and 2 ml of 100 mmol/l ammonium carbonate buffer at pH 9.3, after which the prepared plasma sample or tissue extract was loaded on to the column. The samples were prepared by mixing with an appropriate amount of internal standards (d9-CDCA and d9-glyco-CDCA; Cambridge Isotopes), 100 μl of 100 mmol/l ammonium carbonate buffer (pH 9.3), and 500 μl of LC-MS grade water. After sample loading on the SPE column, the columns were washed with 2 ml of LC-MS grade water followed by drying of the SPE columns under vacuum. The bile acids were eluted with 3 ml of methanol followed by drying and reconstitution with the mobile phase buffer for analysis by LC-MS. The chromatographic separation of free and conjugated bile acids was achieved using an Accela 1200 liquid chromatograph and a 150 × 4.6 mm, 2.7 u Ascentis Express C-18 (Sigma-Aldrich) analytical column maintained at 40°C. The mobile phase consisted of methanol (5 mM ammonium acetate and 0.012 formic acid) and water (5 mM ammonium acetate and 0.012 formic acid).
Samples of plasma, gallbladder bile, and liver were analyzed for cholesterol and phytosterol contents using high mass resolution LC-MS/MS (Thermo Q-Exactive Orbitrap) equipped with an atmospheric-pressure chemical ionization probe in positive ion mode. The concentrations of phytosterols in plasma were determined by reverse isotope dilution methodology after adding an appropriate amount of internal standards of corresponding phytosterols. Purified analytical grade (99%) 2H7-β-sitosterol-5-cholesten-24(RS)-ethyl-3β-ol-25,26,26,26,27,27,27-d7 and 2H3-campesterol-5-cholesten-24(RS)-methyl-d3-3β-ol (CDN Isotopes, Pointe-Claire, Quebec, Canada) and 2H6-cholesterol-2,2,3,4,4,6 (Cambridge Isotopes) were used as internal standards. Briefly, C18 SPE columns (200 mg Bond Elut cartridge; Agilent Technologies) were preconditioned with 2 ml of methanol followed by 2 ml of LC-MS grade water. Plasma samples were mixed with internal standards and then loaded on to the SPE columns; the columns were washed with 2 ml of LC-MS grade water; and the retained phytosterols were eluted by 3 ml of methanol. The eluted phytosterols in methanol were dried under vacuum and reconstituted in 100 μl of the mobile phase (80:20 acetonitrile:methanol). The chromatographic separation of phytosterols was achieved using an Accela 1200 liquid chromatograph and a 100 × 3.0 mm Kinetex, 2.6 u XB-C18 100A analytical column. The mobile phase consisted of acetonitrile:methanol (80:20) at an isocratic flow rate of 0.5 ml/min. Plasma oxyphytosterols were analyzed by GC-MS according to the procedure described previously (28). Samples of liver tissue were analyzed for FA contents using LC-MS/MS as described previously (29).
Liver gene expression
Quantitative real-time PCR was performed on samples of liver. Total RNA was isolated using TRIzol reagent (Invitrogen) and was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems). Real-time PCR was performed on a Bio-Rad CFX96 using PowerUp SYBR Green Master Mix (Applied Biosystems). Primers are given in supplemental Table S2. Target mRNA expression was quantified relative to β-actin and compared across samples using the 2−ΔΔCT method.
Sequencing of gut microbial communities
Samples of luminal contents from the proximal colon were flash-frozen in liquid nitrogen and stored at −80°C. Samples (∼100 mg) were extracted by bead beating using UltraClean microbead tubes (Mo Bio), and DNA was extracted using silica membrane spin-columns from a DNeasy blood and tissue kit (Qiagen), following the modified protocol described previously (30). DNA was quantified by measuring absorbance at 260 nm using a SpectraDrop micro-volume microplate and a SpectraMax plate reader (Molecular Devices). Amplification of the V4 region of the bacterial 16S rRNA gene was performed in duplicate 30 μl reactions, each with 30 ng DNA, 167 μM dNTPs, 500 nM of each forward and reverse primer, 1× Phusion HF buffer, and 0.6 units of Phusion high-fidelity polymerase (New England Biolabs). The primer combinations used incorporated dual barcodes to allow sample multiplexing as described previously (30). Reaction conditions were as follows: initial denaturing at 98°C for 30 s and then 26 cycles of 98°C for 10 s, 51°C for 20 s, and 72°C for 60 s. Duplicate reactions were pooled and cleaned using Agencourt AMPure XP magnetic beads (Beckman Coulter). Samples were combined at equal concentrations and sequenced in a single run using the 2 × 250 paired-end protocol on the MiSeq sequencing platform (Illumina).
Microbial community analyses
We received a total of 10,551,655 reads from the sequencer. Following demultiplexing and removal of reads with mismatched barcodes, we had a total of 9,264,408 reads. The average number of reads per sample was 210,548 (minimum = 107,749; maximum = 324,255). Sequence reads have been deposited with links to BioProject accession number PRJNA559479 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/). We utilized the mothur software package (v.1.39.0) to process the sequence reads (31, 32). We retained only sequence reads that had zero ambiguous bases, a maximum length of 260, and a maximum homopolymer length of 8. Reads were aligned to the V4 region of the SILVA database (v.123) and chimeras were removed with vsearch using the default parameters. Reads were then classified using the RDP training set (v.14), and observational taxonomic units (OTUs) were formed by clustering at the 0.03 level using opticlust (33). Subsequent analysis using the mothur-outputted biom file was performed in R with the phyloseq package (34). We first calculated observed OTU counts and the inverse of Simpson’s index to assess community richness and diversity, respectively. We then subsampled to the minimum sequence count and transformed the OTU abundances to relative proportions by dividing by the total count per sample. OTUs were taxonomically classified and combined together so that all subsequent analyses were done on the taxonomic groupings.
Analysis of colonic metabolomic profiles
Analysis of the metabolomic profiles of colon contents samples was performed in collaboration with Metabolon, Inc. (Durham, NC). A total of 44 samples (n = 11 per treatment) were analyzed. Proteins were removed from wet-mass equivalent samples by methanol precipitation and centrifugation, the remaining solvent was removed using a TurboVap (Zymark), and sample extracts were stored under nitrogen before further processing. Sample extracts were reconstituted and analyzed using a Waters ACQUITY UPLC system, a Q-Exactive mass spectrometer (Thermo Scientific), a heated ESI (HESI-II) source, and an Orbitrap mass analyzer. Analysis of each sample included both hydrophobic and hydrophilic reverse phase UPLC-MS/MS with positive ion mode ESI as well as reverse phase UPLC-MS/MS with negative ion mode ESI and hydrophilic interaction LC/UPLC-MS/MS with negative ion mode ESI. MS scan range was m/z 70–1,000. Compounds were identified by comparison of the raw data to Metabolon’s curated library of standards. For subsequent analysis, missing values were imputed with half the compound minimum, the generalized log transformation was applied, and values were mean-centered and scaled by the compound standard deviation (35).
Statistical methods
The primary endpoints in the study were measurements of liver function, bile acid pools, and colon contents metabolite and microbial profiles. Secondary endpoints were distal ileum morphometry and goblet cell counts, plasma FGF19, phytosterol contents, serum chemistries, whole blood cell counts, and gene expression in liver. All statistical analysis of results was performed using the R software program for statistics and graphical presentation. Pairwise Mann-Whitney U tests were used to determine significance of gene expression results, bacterial richness and diversity measurements, and relative abundance of specific bacterial genera and metabolites. Kruskal-Wallis rank sum tests were used to determine which of the top bacterial genera and which metabolites differed across the treatment groups. A Holm-Bonferonni correction was applied to all tests with multiple hypothesis. Significance was assessed at the α < 0.05 level.
RESULTS
Body and organ weights
During the course of the study, 11 pigs died prior to completing the study and were not included in the results. The number of pigs lost in each group was ENT (n = 2), IL (n = 3), SMOF (n = 3), EXP (n = 4). The causes of death were not confirmed but were presumed to be related to issues of prematurity, infection, or lipid overload syndrome. Overall pig body weights and body growth rate during the study did not differ across the four treatment groups (supplemental Table S3). However, the liver weights in all TPN groups were larger than the ENT group (40.3 g/kg body weight) and those in the IL group (59.5 g/kg body weight) were significantly larger than SMOF and EXP (49.7 and 47.7 g/kg body weight, respectively).
Serum cholestasis markers
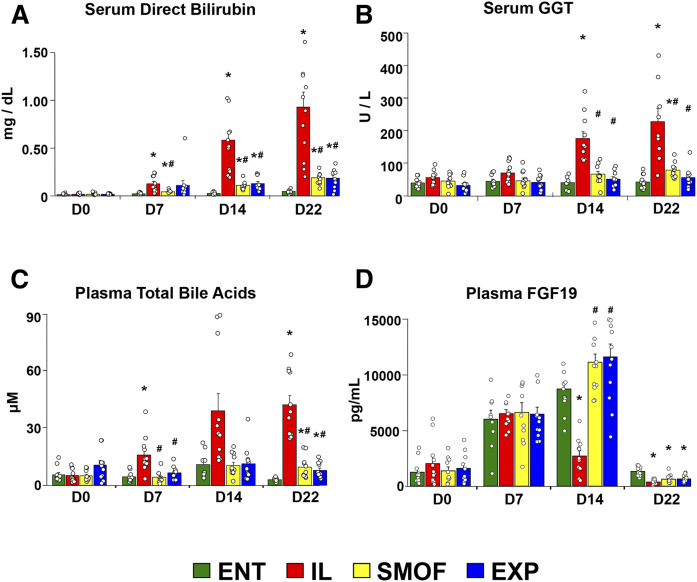
Liver function progressively worsened in TPN-fed pigs over the course of the study, as seen in Fig. 1. Serum direct bilirubin increased in all TPN groups compared with ENT; however, the increase within the IL group was significantly greater than that of the SMOF or EXP groups (Fig. 1A). Serum γ-glutamyl transferase (GGT) also increased over the study in the IL group, but not in the ENT, SMOF, or EXP groups (Fig. 1B). We also observed evidence of cholestasis due to TPN administration as plasma levels of total bile acids were elevated in all TPN-fed pigs compared with ENT, again with levels in the IL group higher than those in SMOF or EXP (Fig. 1C). Other liver enzymes and blood counts increased over the study from baseline but for the most part did not differ by group (supplemental Fig. S1).
Fig. 1.
Serum direct bilirubin and GGT, plasma bile acids, and FGF19. Mean levels of direct bilirubin (A) and GGT (B) measured at baseline and weekly until the end of the study in serum. Mean levels of total bile acids (C) and FGF19 (D) measured at baseline and weekly until the end of the study in plasma. Bars represent standard error. *P < 0.05 versus ENT, #P < 0.05 versus IL (Holm-adjusted pairwise t-tests).
Plasma FGF19
Plasma FGF19 concentrations increased in all groups over the first week of the study (Fig. 1D). On day 14, FGF19 concentrations were lower in IL versus all other groups. At the end of the study, plasma FGF19 was decreased in all groups to near what it was on day 0, with levels in the ENT group slightly higher than the TPN groups.
Liver histopathology
We observed a broad trend for higher values on most measures of liver injury and inflammation in all the TPN groups compared with ENT (supplemental Fig. S2A). Lobular inflammation was higher in IL compared with ENT groups (supplemental Table S4). The one notable exception was hepatocellular glycogenation, which was significantly higher in ENT pigs compared with SMOF. The most common features of injury were lobular inflammation, portal infiltrate, and ductulitis.
Liver FA content analysis by LC-MS
We analyzed the concentrations of a panel of 27 specific FAs in liver tissue samples (supplemental Table S5). Overall, the FAs with highest concentration were C16:0, C18:0, C18:1, C18:2, and C20:4, observed at one or two orders-of-magnitude higher concentration than all the others we analyzed. Of these, C18:1 (oleic acid) was most abundant and was significantly higher in all TPN groups compared with ENT. Hierarchical clustering of the entire panel revealed a group of FAs noticeably lower in IL pigs compared with ENT, yet also higher in SMOF and EXP. This group is exemplified by C22:6 (DHA) and C24:1. In general, MCTs were an order-of-magnitude lower in liver tissue compared with the longer FAs, either saturated or unsaturated. It was apparent that most of the TPN pigs had a higher overall amount of FAs in their liver, with the notable exception of saturated FAs and PUFAs being somewhat lower on average in SMOF pigs compared with IL and EXP, and consequently the total FA amount not reaching statistical significance in the comparison against ENT.
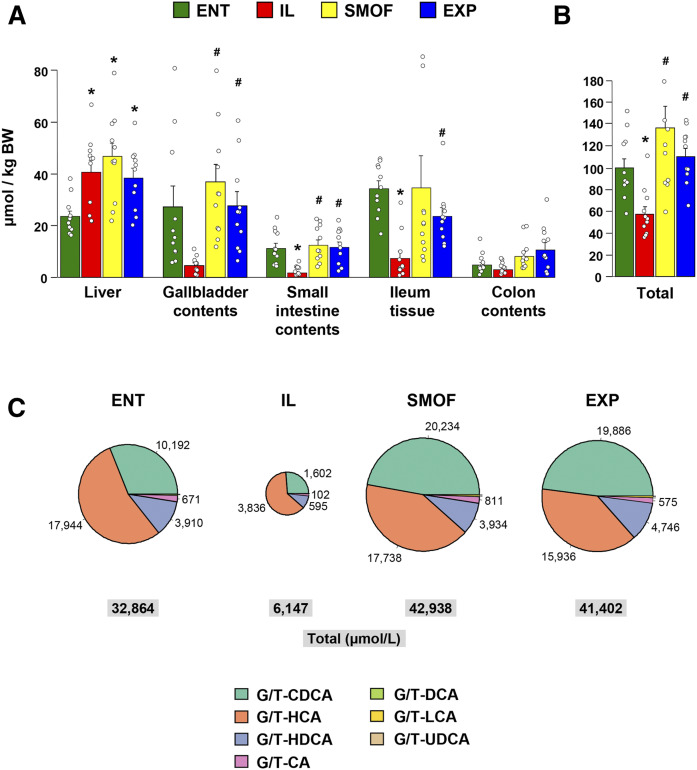
Bile acid pools
Similar to that observed in plasma, total bile acid levels in various body compartments at the end of the study differed according to treatment (Fig. 2A, supplemental Table S6). The bile acid content within the liver (per kilogram of body weight) was higher in all TPN groups compared with ENT. In gallbladder contents, small intestinal contents, and distal ileum tissue, the total bile acid contents were lowest in the IL group. The most notable difference between treatments was observed in markedly higher small intestinal contents and distal ileum tissue bile acid content in ENT, SMOF, and EXP compared with IL. Colon bile acid content was numerically higher in SMOF and EXP than in IL, but not statistically different. The total bile acid pool size (sum of total bile contents in liver, gallbladder contents, small intestinal contents, distal ileum tissue, and colon contents) was significantly lower in IL versus ENT, SMOF, and EXP (Fig. 2B).
Fig. 2.
Total bile acid levels in various body compartments and conjugated bile acid pools in gallbladder contents. Mean levels of total bile acids as measured in samples from different tissues and regions of the gut (A) as well as the summed total pool size (B). Bars represent standard error. *P < 0.05 versus ENT, #P < 0.05 versus IL (Holm-adjusted pairwise t-tests). C: Pie charts showing mean concentrations of conjugated bile acids (combined glyco- and tauro-conjugated) in gallbladder contents. Pies are sized in proportion to total concentration. Values are micromoles per liter. The legend shows bile acid species abbreviations; G/T, glyco-/tauro-conjugates; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; CA, cholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid.
Across all groups, gallbladder bile was composed primarily of conjugated primary bile acids (Fig. 2C, Table 2), with more glyco-conjugated than tauro-conjugated bile. The most abundant bile acid observed in the ENT and IL groups was glyco-hyocholic acid, while in the SMOF and EXP groups, it was glyco-CDCA. Comparing across the treatment groups, the concentrations of bile acids observed in IL pigs, both the glyco- and tauro-conjugated forms as well as the unconjugated forms, are several fold lower than those observed in the ENT, SMOF, or EXP pigs.
TABLE 2.
Bile acid concentrations in gallbladder contents of preterm piglets after 22 days
| ENT | IL | SMOF | EXP | |
| G-CDCA | 6,439 ± 1,249 | 1,107 ± 198a | 16,241 ± 3,380a,b | 15,803 ± 2,007a,b |
| G-HCA | 12,196 ± 1,584 | 3,401 ± 294a | 15,024 ± 2,632b | 11,644 ± 1,699b |
| G-HDCA | 2,625 ± 404 | 526 ± 91a | 3,288 ± 560b | 3,702 ± 569b |
| G-CA | 375 ± 86 | 55 ± 9a | 703 ± 209b | 442 ± 80b |
| G-DCA | 28 ± 12 | 2 ± 1 | 16 ± 6 | 12 ± 5 |
| G-LCA | 25 ± 6 | 3 ± 1a | 112 ± 37b | 120 ± 27a,b |
| G-UDCA | 1 ± 0 | 0.1 ± 0 | 6 ± 5 | 2 ± 1 |
| Total glyco-conjugated | 21,689 ± 3,050 | 5,093 ± 422a | 35,391 ± 6,386b | 31,725 ± 3,739b |
| T-CDCA | 3,753 ± 705 | 495 ± 115a | 3,993 ± 1,285 | 4,084 ± 729b |
| T-HCA | 5,748 ± 815 | 436 ± 85a | 2,714 ± 692a,b | 4,292 ± 935b |
| T-HDCA | 1,285 ± 263 | 70 ± 21a | 646 ± 147b | 1,044 ± 307b |
| T-CA | 297 ± 140 | 47 ± 10a | 108 ± 42a,b | 133 ± 43a,b |
| T-DCA | 60 ± 10 | 6 ± 2a | 37 ± 14 | 50 ± 19 |
| T-LCA | 33 ± 6 | 1 ± 0a | 50 ± 21 | 75 ± 21b |
| Total tauro-conjugated | 11,175 ± 1,743 | 1,055 ± 207a | 7,547 ± 2,137 | 9,677 ± 1,760b |
| CDCA | 0.5 ± 0 | 0.1 ± 0a | 0.7 ± 0b | 0.6 ± 0b |
| HCA | 22 ± 5 | 3 ± 1a | 42 ± 10b | 18 ± 3b |
| HDCA | 0.5 ± 0 | 0.7 ± 0 | 0.4 ± 0 | 0.2 ± 0 |
| Total unconjugated | 23 ± 5 | 4 ± 1a | 43 ± 10b | 19 ± 3b |
| All bile acids | 32,887 ± 4,680 | 6,151 ± 468a | 42,982 ± 8,448b | 41,421 ± 5,205b |
Data are presented in micromoles per liter. Values given are mean ± standard error of the mean. G-/T-, glyco-/tauro-conjugates; HCA, hyocholic acid; HDCA, hyodeoxycholic acid; CA, cholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; UDCA, ursodeoxycholic acid. T-UDCA, CA, DCA, LCA, and UDCA are not shown as they were below limit of detection.
P < 0.05 versus ENT, Holm-Bonferroni adjusted pairwise t-test.
P < 0.05 versus IL, Holm-Bonferroni adjusted pairwise t-test.
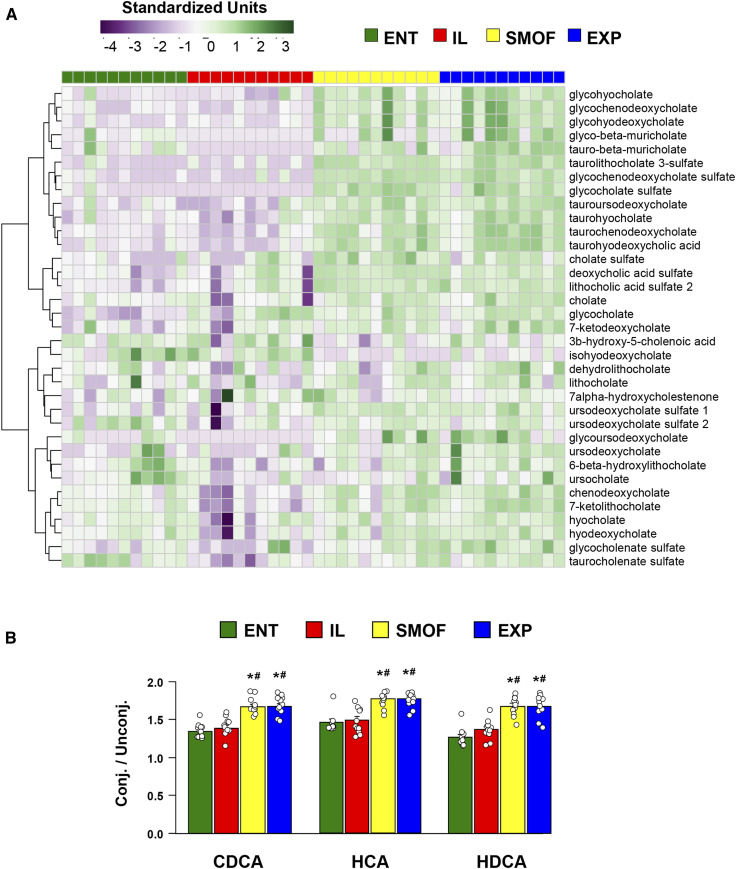
The bile profiles measured by the metabolomic analysis of colon contents reflect the trend seen in the total bile acid pool measurements, in that the levels of most bile acid species are higher in SMOF and EXP pigs as compared with ENT or IL (Fig. 3A). This trend appeared to hold strongest for both glyco- and tauro-conjugated primary and secondary bile acids, while unconjugated secondary bile acids were more evenly observed across groups. Notable exceptions, where levels were relatively higher in ENT and IL pigs versus SMOF or EXP, include the secondary bile acids 3b-hydroxy-5-cholenoic acid and isohyodeoxycholate.
Fig. 3.
Colonic contents bile acid profiles and relative ratios of conjugated to unconjugated bile acids. A: Heatmap showing profiles of bile acid species detected in samples from colon contents, with bile acid species arranged by hierarchical clustering. Values are relative standardized units (dimensionless). B: Relative ratios of total conjugated (both glyco- and tauro-conjugated) to unconjugated chenodeoxycholate (CDCA), hyocholate (HCA), and hyodeoxycholate (HDCA) found in colon contents samples. Values are ratios of relative standardized units (dimensionless). Bars represent standard error. *P < 0.05 versus ENT, #P < 0.05 versus IL (Holm-adjusted pairwise t-tests).
We also detected differences between treatment groups in the relative levels of conjugated and unconjugated bile acid species (Fig. 3B). The relative ratios of glyco- and tauro-conjugated to unconjugated chenodeoxycholate, hyocholate, and hyodeoxycholate were higher in SMOF and EXP compared with ENT and IL. We observed a higher relative ratio of cholate to ursocholate in SMOF versus ENT pigs, but no differences were seen for the ratios of chenodeoxycholate to lithocholate, hyocholate to hyodeoxycholate, or chenodeoxycholate to ursodeoxycholate (data not shown).
Phytosterols
Plasma phytosterols were higher in all TPN groups compared with ENT, with IL also significantly higher than SMOF and EXP (Table 3). Likewise, levels in liver tissue were highest in IL, followed by SMOF and EXP, then ENT. The variability in gallbladder contents was such that although SMOF and EXP pigs showed a higher average compared with ENT and IL, the difference was not significant. The levels of individual phytosterols showed similar trends, with β-sitosterol, the most abundant, being significantly higher in IL compared with SMOF, EXP, and ENT for both plasma and liver samples. We observed similar differences in plasma but not liver campesterol and stigmasterol. No differences were detected for cholesterol levels in any of the measured tissues. There was no treatment group difference in levels of oxidized cholesterol or oxidized phytosterol in plasma (supplemental Table S7). However, it is notable that the absolute concentrations of oxidized cholesterol and phytosterols were an order of magnitude lower (nanomolar) than the parent compounds that were present in the micromolar range.
TABLE 3.
Sterol content in the plasma, liver, and gallbladder contents of preterm piglets after 22 days
| ENT | IL | SMOF | EXP | |
| Plasma (μM) | ||||
| β-Sitosterol | 9.61 ± 1.06 | 60.0 ± 1.81a | 19.7 ± 1.97a,b | 21.0 ± 1.93a,b |
| Campesterol | 4.95 ± 0.31 | 10.4 ± 0.47a | 4.18 ± 0.48b | 3.54 ± 0.30b |
| Stigmasterol | 0.86 ± 0.06 | 13.5 ± 0.74a | 2.22 ± 0.23b | 2.27 ± 0.20b |
| Total phytosterols | 15.4 ± 1.36 | 84.0 ± 3.0a | 26.1 ± 2.64a,b | 26.8 ± 2.40a,b |
| Total phytosterol pool (μmol/kg body weight) | 0.99 ± 0.09 | 5.37 ± 0.19a | 1.67 ± 0.17a,b | 1.71 ± 0.15a,b |
| Cholesterol | 581 ± 25.6 | 625 ± 21.6 | 534 ± 25.9 | 515 ± 38.6b |
| Liver (nmol/g) | ||||
| β-Sitosterol | 17.0 ± 3.51 | 97.3 ± 25.3a | 31.6 ± 7.31b | 38.2 ± 7.91b |
| Campesterol | 11.9 ± 1.45 | 17.9 ± 4.04 | 10.8 ± 0.55 | 11.7 ± 1.01 |
| Stigmasterol | 9.97 ± 0.42 | 24.3 ± 5.71 | 10.3 ± 0.42 | 10.7 ± 0.33 |
| Total phytosterols | 39.1 ± 4.80 | 132 ± 35.6a | 53.1 ± 8.77b | 70.3 ± 10.7b |
| Total phytosterol pool (nmol/kg body weight) | 1.55 ± 0.18 | 8.52 ± 2.25a | 2.54 ± 0.32b | 2.89 ± 0.44b |
| Cholesterol | 1,514 ± 319 | 1,484 ± 319 | 1,427 ± 328 | 1,680 ± 294 |
| Gallbladder contents (μM) | ||||
| β-Sitosterol | 595 ± 56.6 | 598 ± 112 | 1173 ± 241 | 1139 ± 192 |
| Campesterol | 73.5 ± 18.6 | 50.1 ± 10.9 | 133 ± 35.3 | 125 ± 20.4 |
| Stigmasterol | 95.3 ± 24.0 | 133 ± 46.2 | 181 ± 37.4 | 173 ± 37.1 |
| Total phytosterols | 765 ± 72 | 801 ± 175 | 1488 ± 307 | 1439 ± 244 |
| Total phytosterol pool (µmol/kg body weight) | 584 ± 127 | 459 ± 113 | 1200 ± 252 | 955 ± 226 |
| Cholesterol | 3,327 ± 950 | 2,570 ± 653 | 7,326 ± 2236 | 7,225 ± 1424 |
Values given are mean ± standard error of the mean.
P < 0.05 versus ENT, Holm-Bonferroni adjusted pairwise t-test.
P < 0.05 versus IL, Holm-Bonferroni adjusted pairwise t-test.
Liver gene expression
We measured the expression of several liver genes related to bile acid synthesis, transport, and regulation, but did not detect any statistically significant differences across the treatment groups (supplemental Fig. S3). There were trends with biological relevance to the observed phenotypes, such as high relative expression of MDR1 in SMOF and EXP pigs, which pumps bile out of hepatocytes into bile ducts, and CYP7A1 in IL pigs, which is a crucial enzyme required for the synthesis of bile acids.
Intestinal histology
We observed a reduced villus height and crypt depth in all TPN groups compared with ENT pigs (supplemental Fig. S4). However, among the TPN groups, we found higher villus height in the EXP versus IL group, as well as greater crypt depth in both SMOF and EXP versus IL. In contrast, the number of goblet cells per unit of villus epithelium in TPN groups was nearly twice that of the ENT group, and crypt goblet cell counts were highest in IL pigs compared with all other groups (supplemental Fig. S4D). Goblet cells, which appear as cells containing large apical mucin granules stained deep blue, are visually more abundant per unit of epithelium in the TPN groups compared with ENT (supplemental Fig. S4E).
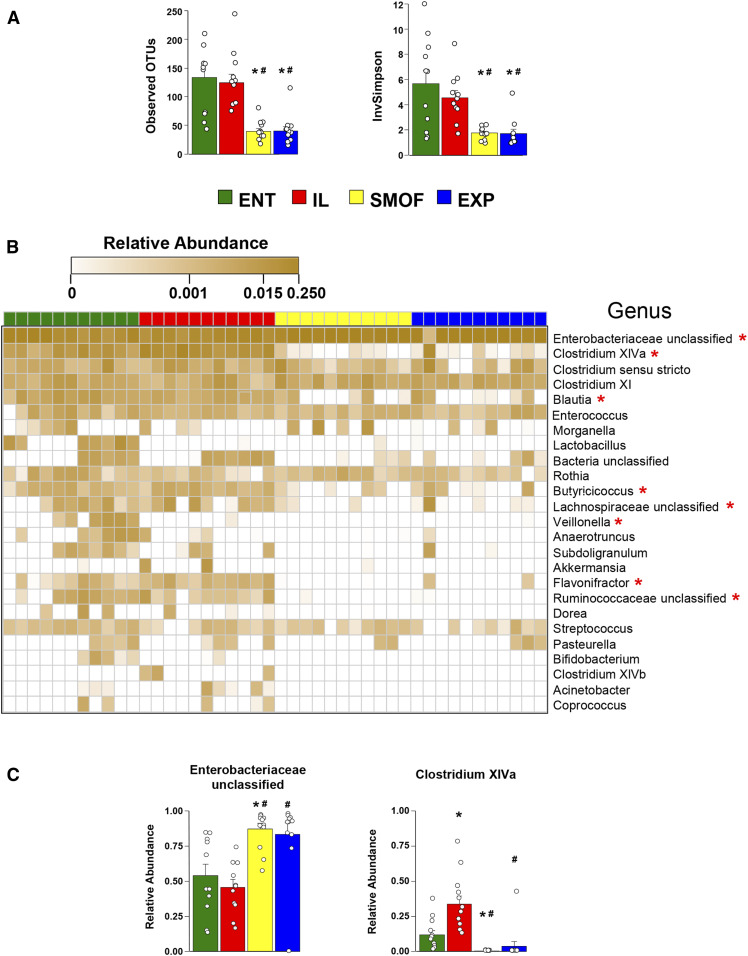
Gut microbiota
Upon comparing microbial communities in the colon, we observed greater richness and diversity in the ENT and IL groups compared with both SMOF and EXP (Fig. 4A). This was evident at the genus level (clustered at 97% sequence identity), with communities from SMOF and EXP pigs notably showing a lack of gram-positive anaerobes such as Clostridium cluster XIVa, Blautia, Butyricicoccus, Lachnospiraceae, Flavonifractor, and Ruminococcaceae (Fig. 4B). That the SMOF and EXP pigs harbor dramatically different communities compared with ENT and IL is exemplified by looking at the top two most abundant bacterial taxa in the dataset (Fig. 4C). In the majority of pigs from all treatments, the highest abundant bacteria are from the family Enterobacteriaceae. In ENT and IL pigs, these make up around half the community, while in SMOF and EXP pigs these comprise nearly 90% of the community. In ENT and IL pigs, the second most abundant bacteria are Clostridium cluster XIVa, making up around 25% of the community. In SMOF and EXP pigs, however, these Clostridium are only 0–1%. Some notable genera that comprised major portions of the community in most pigs and were nearly equal in relative abundance across groups include some Clostridium (sensu stricto as well as those from cluster XI), Enterococcus, Rothia, and Streptococcus.
Fig. 4.
Characterization of the colonic microbiota profile. (A) Mean OTU counts and the inverse of Simpson’s Diversity Index across the treatment groups. Bars represent standard error. *P < 0.05 versus ENT, #P < 0.05 versus IL (Holm-adjusted pairwise t-tests). B: Heatmap showing profiles of observed bacteria at the genus level (clustered at 97% sequence identity) and arrayed horizontally according to treatment group and vertically by decreasing overall abundance in the dataset. Values are sample-wise proportional abundances. *P < 0.05 (Holm-adjusted Kruskal-Wallis rank sum tests). C: Group-wise comparison of the top two most abundant taxa. Bars represent standard error. *P < 0.05 versus ENT, #P < 0.05 versus IL (Holm-adjusted pairwise t-tests).
Metabolome
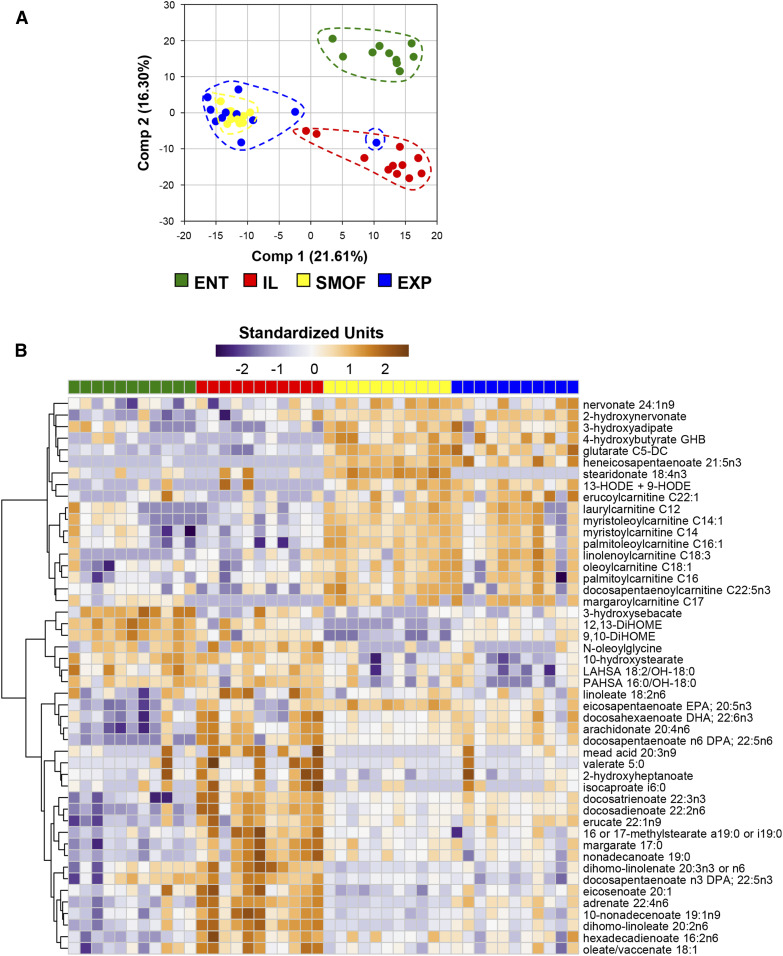
We identified 729 distinct chemical compounds via metabolomic analysis of samples from colon contents. Of these, 547 were significant (P < 0.05) when testing for group effects by ANOVA (supplemental Table S8). This large number of compounds allowed for distinct clustering of the different treatment groups using principal component analysis, with the ENT pigs clearly distinct from all TPN groups (Fig. 5A). Among the TPN groups, SMOF and EXP pigs mostly overlapped, with both being separate from the IL pigs.
Fig. 5.
Characterization of colonic luminal metabolite profiles. A: Principal components plot with samples from individual pigs represented by a single dot. B: Heatmap showing profiles of FAs, which were observed to be significant at P < 0.05 (Benjamini-Hochberg-adjusted ANOVA) and have a median fold difference of >2 between SMOF and IL groups. The individual FAs are arranged by hierarchical clustering. Values are relative standardized units (dimensionless).
As our primary intervention in this study was a change in the TPN lipid composition, we tested group-specific patterns among all lipid compounds, visualized as a heatmap of those compounds that were significantly different across groups (by ANOVA) while also exhibiting a median fold difference >2 between SMOF and IL (Fig. 5B). Several free FAs were notably higher in the IL group, while several carnitine derivatives were highest in the SMOF and EXP groups. In contrast, several short-chain FAs, such as valerate, caproate, and isocaproate, were all highest in IL-treated pigs. Steridonate, a derivative of α-linoleic acid, was enriched only in the SMOF pigs. The linoleic acid derivatives 9,10-DiHOME and 12,13-DiHOME were highest in ENT and lowest in SMOF. Among other classes of compounds, we observed that the tryptophan metabolite indolepropionate and the tyrosine metabolite tyramine, likely products of microbial metabolism, were notably increased in the ENT pigs compared with all TPN pigs (supplemental Table S8). Derivatives of benzoic acid were also found to differ by treatment group, with hippurate and 4-hydroxyhippurate being elevated in SMOF and EXP compared with ENT and IL, and 3-(4-hydroxyphenyl)propionate and 3-phenylpropionate being diminished.
DISCUSSION
TPN is a life-saving nutritional intervention in infants, yet prolonged TPN increases the risk cholestatic liver disease and this has been linked to parenteral lipid emulsions. The regulatory approval and increased use of new generation parenteral lipid emulsions has led to recent clinical trials showing that multi-component lipid emulsions (e.g., SMOF) lead to less cholestasis than soybean oil emulsions and recent clinical nutrition guidelines have recommended their use for infants (9, 11, 36–39). Consistent with clinical findings, the current study confirms our previous report in preterm pigs that new multi-component lipid emulsions (e.g., SMOF) prevent cholestasis compared soybean-oil-based emulsions (e.g., IL) (25). In the current study, we show new evidence that multi-component emulsions, regardless of relative levels of DHA, AA, and EPA, restore bile acid flow into the gut and differentially shape the gut microbiota and metabolome profile compared with soybean oil emulsions.
An important finding of this study was that both multi-component lipid emulsions (SMOF and EXP) increased bile acid pool sizes in the gut in association with reduced plasma markers of cholestasis, particularly total bile acids. Bile acid levels in small intestinal contents and in ileum tissue were markedly higher in ENT, SMOF, and EXP compared with IL pigs. Metabolomic profiling of colonic bile acids also showed increases, especially in the conjugated forms, of both primary pig bile acids, CDCA and hyocholic acid, and the secondary bile acid, hyodeoxycholic acid. Also increased in SMOF and EXP were sulfated forms of cholate and glycocholate, which increases their solubility, reduces their intestinal absorption, and thus promotes fecal excretion (40). It is worth noting that hyocholic acid (also known as γ-muricholic acid) (41) is a dominant bile acid is pigs but is also present in human infants (42–44). A similar study in term-born pigs showed a shift in the gallbladder bile profile from predominant hyocholic acid to CDCA in SMOF- versus IL-treated pigs (45). Another bile acid that was enriched in SMOF was isohyodeoxycholic acid, a 6β isomer of hyodeoxycholic acid, a secondary bile acid formed from hyocholic acid (46). This epimer likely results from bacterial modification in the colon and may thus reflect the unique microbial community in SMOF pigs. Another is 3b-hydroxy-5-cholenoic acid that is reported to originate from a unique fetal route of synthesis (47, 48). This bile acid has been detected both in meconium and amniotic fluid of preterm versus term infants at birth (48, 49), as well as older children with cholestasis associated with biliary atresia or hepatic ductular hypoplasia (50, 51). These findings suggest that the FA composition of the parenteral lipid emulsion results in increased bile flow into the gut and may alter endogenous bile acid synthesis pathways.
The changes in gut bile acid profiles were associated with remarkable differences in gut microbiota community structure. The higher relative ratios of conjugated to unconjugated bile acids in SMOF and EXP versus IL and ENT pigs was associated with the loss of several groups of gram-positive anaerobes as well as the shift toward a greater relative abundance of gram-negative Enterobacteriaceae. Conjugated bile acids are deconjugated in the gut by the bacterial bile salt hydrolase activity that is associated mostly with gram-positive species (52). Thus, the loss of several gram-positive microbes from the microbiota community would explain the greater relative abundance of conjugated bile acids in the colon, where normally 90% of bile acids exist in unconjugated forms (42). The most likely explanation for the differences in gut microbial communities among the TPN groups was the increased flow of bile and bile acids into the intestine. As mentioned above, most of the bacteria that were either missing or of lower relative abundance in SMOF and EXP compared with IL pigs were gram-positive bacteria, which are known to be more bile acid sensitive (53). This interpretation is supported by a study showing an increase in the relative abundance of bile-acid tolerant gram-negative Enterobacteriaceae in the small intestine of term-born piglets treated with SMOF compared to IL (54). Thus, both the SMOF and EXP emulsions maintained hepatic bile flow and the delivery of bile acids into the intestine and this led to loss of bile acid-sensitive gram-positive bacteria. The apparent paradox of gram-positive bacterias’ simultaneous sensitivity to bile as well as their preference for metabolizing bile through bile salt hydrolase may be explained in part by the lack of enteral substrate in the context of TPN. It has been shown that in species that rely on bile salt hydrolase for their resistance to bile toxicity, this resistance exhibits a reverse effect in the absence of nutrients (55). Thus, the absence of an abundant carbon source for these bacteria during prolonged TPN may actually render them more susceptible to the bactericidal effects of bile.
Another striking finding from our metabolomic analysis was the large changes in gut luminal metabolite profiles produced solely by changing the composition of parenteral lipid emulsion given intravenously. Many of the metabolites that were enriched in TPN-fed groups and among the IL, SMOF, and EXP were FAs and their metabolic products. Several FAs including palmitate, stearate, palmitoleate and their carnitine esters were 2- to 3-fold higher in TPN-fed versus ENT pigs. The finding of both the FAs and their carnitine esters suggest that they originated from lipids incorporated into host cells, likely hepatocytes or intestinal epithelial cells, and were then secreted in bile or sloughed into the gut lumen, respectively. Even during fasting or on a lipid-free diet, significant amounts of lipids are excreted in feces (56). This is consistent with our previous finding that the body tissue FA composition is strongly influenced by the FA composition of administered parenteral lipid emulsion (26). Likewise, in this study we observed changes in concentrations of FAs in liver tissue that correlated with treatment. Interestingly, we found a group of several FAs that seemed to be decreased in pigs receiving IL compared with ENT, but that seemed to be normalized in pigs receiving SMOF or EXP. We also note that based on comparing overall FA intake with liver tissue contents, it is clear that preterm piglets appear to preferentially oxidize MCTs for energy while storing longer chain FAs in hepatic lipid depots.
Our results confirm previous evidence that SMOF (25) and, in this study, EXP both prevent cholestasis and increase gut bile flow. A current theory to explain the protective effect of multicomponent emulsions is linked to phytosterols, which are present in lower concentrations in SMOF versus IL and have been shown to antagonize FXR and disrupt bile acid signaling (25, 57). Our results show that both SMOF and EXP emulsions markedly reduced the plasma concentration and liver concentration of phytosterols and nearly doubled biliary phytosterol content. Because an important route of phytosterol excretion is via hepatic ABCG5/G8 transporters, these shifts in phytosterol pool sizes reflect higher rates of bile flow in SMOF and EXP consistent with prevention of cholestasis. We did not find evidence of increased hepatic ABCG5/G8 expression or FXR activation in SMOF or EXP pigs, although there was a trend for lower CYP7A1 and increased MDR1. In contrast, we found evidence of increased gut FXR activation resulting from increased bile flow based on higher plasma FGF19 concentrations in SMOF and EXP versus IL pigs. FGF19 acts as a negative feedback signal for hepatic bile acid synthesis via CYP7A1 (58, 59). Thus, the mechanism to explain the prevention of cholestasis by SMOF and EXP warrants further study. However, the current results are consistent with the idea that reduced intravenous loads and hepatic accumulation of phytosterols are associated with reduced cholestasis.
In summary, multi-component lipid emulsion prevented cholestasis and restored enterohepatic bile flow, and this was associated with unique gut microbial and metabolomic profiles. Our results confirm that multi-component lipid emulsions prevent cholestasis and that further enrichment of DHA and AA and lowering EPA did not diminish the protection against cholestasis. This does not preclude the possibility that enrichment of DHA and/or lowering of LA in SMOF and EXP is protective against cholestasis. Furthermore, we postulate that phytosterols are involved, as both SMOF and EXP have lower phytosterol content than IL. We postulate that the limited systemic accumulation of phytosterols with multi-component emulsions was explained not only by the reduced parenteral load but also by sustained hepatic excretion through normal bile flow into the intestine. This increase in bile flow into the intestine resulted in loss of many bile-sensitive gram-positive bacteria and a relative increase in Enterobacteriaceae. Our current results provide further rationale to explore possible mechanistic links between the gut microbiome and the protective effects of multi-component lipid emulsions against PN-associated cholestasis.
Data availability
Microbial sequence reads have been deposited with links to BioProject accession number PRJNA559479 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/). A complete list of all metabolites from the colonic metabolomic analysis are available on request from Douglas Burrin, USDA-ARS Children’s Nutrition Research Center, E-mail: doug.burrin@usda.gov.
Supplementary Material
Acknowledgments
The authors wish to thank the veterinarians and animal care attendants from Baylor College of Medicine’s Center for Comparative Medicine for their assistance with animal housing and care in support of this study.
Footnotes
Abbreviations:
- CDCA
- chenodeoxycholic acid
- ENT
- enteral diet
- EXP
- experimental emulsion (novel emulsion with a modified fatty-acid composition)
- FGF19
- fibroblast growth factor 19
- GGT
- γ-glutamyl transferase
- IL
- Intralipid
- MCT
- medium-chain triglyceride
- OTU
- observational taxonomic unit
- PN
- parenteral nutrition
- SMOF
- SMOFlipid
- SPE
- solid-phase extraction
- TPN
- total parenteral nutrition
This work was supported in part by federal funds from the USDA Agricultural Research Service under Cooperative Agreement Number 3092-51000-060-01, and grants from the University of Copenhagen, Fresenius Kabi, the Whitlock Foundation, National Institutes of Health Grant DK-094616 (D.B.), and the Texas Medical Center Digestive Diseases Center (National Institutes of Health Grant P30 DK-56338). L.C. was supported by training fellowships from National Institutes of Health Grant T32-GM088129 and the Gulf Coast Consortia, and National Library of Medicine Training Program in Biomedical Informatics Grant T15-LM007093. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Embleton N. D., and Simmer K.. 2014. Practice of parenteral nutrition in VLBW and ELBW infants. World Rev. Nutr. Diet. 110: 177–189. [DOI] [PubMed] [Google Scholar]
- 2.Carter B. A., and Shulman R. J.. 2007. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat. Clin. Pract. Gastroenterol. Hepatol. 4: 277–287. [DOI] [PubMed] [Google Scholar]
- 3.Christensen R. D., Henry E., Wiedmeier S. E., Burnett J., and Lambert D. K.. 2007. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J. Perinatol. 27: 284–290. [DOI] [PubMed] [Google Scholar]
- 4.Lauriti G., Zani A., Aufieri R., Cananzi M., Chiesa P. L., Eaton S., and Pierro A.. 2014. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: a systematic review. JPEN J. Parenter. Enteral Nutr. 38: 70–85. [DOI] [PubMed] [Google Scholar]
- 5.Colomb V., Jobert-Giraud A., Lacaille F., Goulet O., Fournet J. C., and Ricour C.. 2000. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J. Parenter. Enteral Nutr. 24: 345–350. [DOI] [PubMed] [Google Scholar]
- 6.Gura K. M., Duggan C. P., Collier S. B., Jennings R. W., Folkman J., Bistrian B. R., and Puder M.. 2006. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 118: e197–e201. [DOI] [PubMed] [Google Scholar]
- 7.Puder M., Valim C., Meisel J. A., Le H. D., de Meijer V. E., Robinson E. M., Zhou J., Duggan C., and Gura K. M.. 2009. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann. Surg. 250: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandivada P., Fell G. L., Mitchell P. D., Potemkin A. K., O’Loughlin A. A., Gura K. M., and Puder M.. 2017. Long-term fish oil lipid emulsion use in children with intestinal failure-associated liver disease. JPEN J. Parenter. Enteral Nutr. 41: 930–937. [DOI] [PubMed] [Google Scholar]
- 9.Diamond I. R., Grant R. C., Pencharz P. B., de Silva N., Feldman B. M., Fitzgerald P., Sigalet D., Dicken B., Turner J., Marchand V., et al. 2017. Preventing the progression of intestinal failure-associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. JPEN J. Parenter. Enteral Nutr. 41: 866–877. [DOI] [PubMed] [Google Scholar]
- 10.Repa A., Binder C., Thanhaeuser M., Kreissl A., Pablik E., Huber-Dangl M., Berger A., and Haiden N.. 2018. A mixed lipid emulsion for prevention of parenteral nutrition associated cholestasis in extremely low birth weight infants: a randomized clinical trial. J. Pediatr. 194: 87–93.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hojsak I., Colomb V., Braegger C., Bronsky J., Campoy C., Domellof M., Embleton N., Fidler Mis N., Hulst J. M., Indrio F., et al. 2016. ESPGHAN committee on nutrition position paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J. Pediatr. Gastroenterol. Nutr. 62: 776–792. [DOI] [PubMed] [Google Scholar]
- 12.Carter B. A., Taylor O. A., Prendergast D. R., Zimmerman T. L., Von Furstenberg R., Moore D. D., and Karpen S. J.. 2007. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr. Res. 62: 301–306. [DOI] [PubMed] [Google Scholar]
- 13.Clayton P. T., Bowron A., Mills K. A., Massoud A., Casteels M., and Milla P. J.. 1993. Phytosterolemia in children with parenteral nutrition-associated cholestatic liver disease. Gastroenterology. 105: 1806–1813. [DOI] [PubMed] [Google Scholar]
- 14.Mutanen A., Nissinen M. J., Lohi J., Heikkila P., Gylling H., and Pakarinen M. P.. 2014. Serum plant sterols, cholestanol, and cholesterol precursors associate with histological liver injury in pediatric onset intestinal failure. Am. J. Clin. Nutr. 100: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 15.Alwayn I. P., Gura K., Nose V., Zausche B., Javid P., Garza J., Verbesey J., Voss S., Ollero M., Andersson C., et al. 2005. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr. Res. 57: 445–452. [DOI] [PubMed] [Google Scholar]
- 16.Baker M. A., Cho B. S., Anez-Bustillos L., Dao D. T., Pan A., O’Loughlin A. A., Lans Z. M., Mitchell P. D., Nose V., Gura K. M., et al. 2019. Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added alpha-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury. Am. J. Clin. Nutr. 109: 1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanten G. J., and Calder P. C.. 2007. Immune modulation by parenteral lipid emulsions. Am. J. Clin. Nutr. 85: 1171–1184. [DOI] [PubMed] [Google Scholar]
- 18.Martin C. R. 2014. Fatty acid requirements in preterm infants and their role in health and disease. Clin. Perinatol. 41: 363–382. [DOI] [PubMed] [Google Scholar]
- 19.Cahova M., Bratova M., and Wohl P.. 2017. Parenteral nutrition-associated liver disease: the role of the gut microbiota. Nutrients. 9: E987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierre J. F. 2017. Gastrointestinal immune and microbiome changes during parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 312: G246–G256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridlon J. M., Harris S. C., Bhowmik S., Kang D. J., and Hylemon P. B.. 2016. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 7: 22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann A. F., and Hagey L. R.. 2014. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J. Lipid Res. 55: 1553–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson P. A., and Karpen S. J.. 2015. Intestinal transport and metabolism of bile acids. J. Lipid Res. 56: 1085–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng K., Stoll B., Chacko S., Saenz de Pipaon M., Lauridsen C., Gray M., Squires E. J., Marini J., Zamora I. J., Olutoye O. O., et al. 2016. Vitamin E in new-generation lipid emulsions protects against parenteral nutrition-associated liver disease in parenteral nutrition-fed preterm pigs. JPEN J. Parenter. Enteral Nutr. 40: 656–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlaardingerbroek H., Ng K., Stoll B., Benight N., Chacko S., Kluijtmans L. A., Kulik W., Squires E. J., Olutoye O., Schady D., et al. 2014. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J. Lipid Res. 55: 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthrie G., Kulkarni M., Vlaardingerbroek H., Stoll B., Ng K., Martin C., Belmont J., Hadsell D., Heird W., Newgard C. B., et al. 2016. Multi-omic profiles of hepatic metabolism in TPN-fed preterm pigs administered new generation lipid emulsions. J. Lipid Res. 57: 1696–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina T. L., Stoll B., Mohammad M., Mohila C. A., Call L., Cui L., Guthrie G., Kunichoff D., Lin S., Welch-Jernigan R., et al. 2020. New generation lipid emulsions increase brain DHA and improve body composition, but not short-term neurodevelopment in parenterally-fed preterm piglets. Brain Behav. Immun. 85: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husche C., Weingartner O., Pettersson H., Vanmierlo T., Bohm M., Laufs U., and Lutjohann D.. 2011. Validation of an isotope dilution gas chromatography-mass spectrometry method for analysis of 7-oxygenated campesterol and sitosterol in human serum. Chem. Phys. Lipids. 164: 425–431. [DOI] [PubMed] [Google Scholar]
- 29.Mohammad M. A., Sunehag A. L., and Haymond M. W.. 2014. De novo synthesis of milk triglycerides in humans. Am. J. Physiol. Endocrinol. Metab. 306: E838–E847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6: 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozich J. J., Westcott S. L., Baxter N. T., Highlander S. K., and Schloss P. D.. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79: 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J., et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westcott S. L., and Schloss P. D.. 2017. OptiClust, an improved method for assigning amplicon-based sequence data to operational taxonomic units. MSphere. 2: e00073-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMurdie P. J., and Holmes S.. 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 8: e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Berg R. A., Hoefsloot H. C., Westerhuis J. A., Smilde A. K., and van der Werf M. J.. 2006. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 7: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira-da-Silva L., Nobrega S., Rosa M. L., Alves M., Pita A., Virella D., Papoila A. L., Serelha M., Cordeiro-Ferreira G., and Koletzko B.. 2017. Parenteral nutrition-associated cholestasis and triglyceridemia in surgical term and near-term neonates: A pilot randomized controlled trial of two mixed intravenous lipid emulsions. Clin. Nutr. ESPEN. 22: 7–12. [DOI] [PubMed] [Google Scholar]
- 37.Lam C. K. L., Church P. C., Haliburton B., Chambers K., Martincevic I., Vresk L., Courtney-Martin G., Bandsma R., Avitzur Y., Wales P. C., et al. 2018. Long-term exposure of children to a mixed lipid emulsion is less hepatotoxic than soybean-based lipid emulsion. J. Pediatr. Gastroenterol. Nutr. 66: 501–504. [DOI] [PubMed] [Google Scholar]
- 38.Lapillonne A., Fidler Mis N., Goulet O., van den Akker C. H. P., Wu J., and Koletzko B.; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition . 2018. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: lipids. Clin. Nutr. 37: 2324–2336. [DOI] [PubMed] [Google Scholar]
- 39.Pichler J., Simchowitz V., Macdonald S., and Hill S.. 2014. Comparison of liver function with two new/mixed intravenous lipid emulsions in children with intestinal failure. Eur. J. Clin. Nutr. 68: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 40.Alnouti Y. 2009. Bile acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol. Sci. 108: 225–246. [DOI] [PubMed] [Google Scholar]
- 41.Lundell K., and Wikvall K.. 2008. Species-specific and age-dependent bile acid composition: aspects on CYP8B and CYP4A subfamilies in bile acid biosynthesis. Curr. Drug Metab. 9: 323–331. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton J. P., Xie G., Raufman J. P., Hogan S., Griffin T. L., Packard C. A., Chatfield D. A., Hagey L. R., Steinbach J. H., and Hofmann A. F.. 2007. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G256–G263. [DOI] [PubMed] [Google Scholar]
- 43.Nishiura H., Kimura A., Yamato Y., Aoki K., Inokuchi T., Kurosawa T., and Matsuishi T.. 2010. Developmental pattern of urinary bile acid profile in preterm infants. Pediatr. Int. 52: 44–50. [DOI] [PubMed] [Google Scholar]
- 44.Zhou K., Wang J., Xie G., Zhou Y., Yan W., Pan W., Che Y., Zhang T., Wong L., Kwee S., et al. 2015. Distinct plasma bile acid profiles of biliary atresia and neonatal hepatitis syndrome. J. Proteome Res. 14: 4844–4850. [DOI] [PubMed] [Google Scholar]
- 45.Lavallee C. M., Lim D. W., Wizzard P. R., Mazurak V. C., Mi S., Curtis J. M., Willing B. P., Yap J. Y., Wales P. W., and Turner J. M.. 2019. Impact of clinical use of parenteral lipid emulsions on bile acid metabolism and composition in neonatal piglets. JPEN J. Parenter. Enteral Nutr. 43: 668–676. [DOI] [PubMed] [Google Scholar]
- 46.Eyssen H. J., De Pauw G., and Van Eldere J.. 1999. Formation of hyodeoxycholic acid from muricholic acid and hyocholic acid by an unidentified gram-positive rod termed HDCA-1 isolated from rat intestinal microflora. Appl. Environ. Microbiol. 65: 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Back P., and Ross K.. 1973. Identification of 3 beta-hydroxy-5-cholenoic acid in human meconium. Hoppe Seylers Z. Physiol. Chem. 354: 83–89. [DOI] [PubMed] [Google Scholar]
- 48.Back P., and Walter K.. 1980. Developmental pattern of bile acid metabolism as revealed by bile acid analysis of meconium. Gastroenterology. 78: 671–676. [PubMed] [Google Scholar]
- 49.Délèze G., Paumgartner G., Karlaganis G., Giger W., Reinhard M., and Sidiropoulos D.. 1978. Bile acid pattern in human amniotic fluid. Eur. J. Clin. Invest. 8: 41–45. [DOI] [PubMed] [Google Scholar]
- 50.Hernanz A., Codoceo R., Jara P., and Diaz C.. 1985. Unusual serum bile acid pattern in children with the syndrome of hepatic ductular hypoplasia. Clin. Chim. Acta. 145: 289–296. [DOI] [PubMed] [Google Scholar]
- 51.Minder E. I., Karlaganis G., and Paumgartner G.. 1979. Radioimmunological determination of serum 3 beta-hydroxy-5-cholenoic acid in normal subjects and patients with liver disease. J. Lipid Res. 20: 986–993. [PubMed] [Google Scholar]
- 52.Begley M., Hill C., and Gahan C. G.. 2006. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 72: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Begley M., Gahan C. G., and Hill C.. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29: 625–651. [DOI] [PubMed] [Google Scholar]
- 54.Lavallee C. M., MacPherson J. A. R., Zhou M., Gao Y., Wizzard P. R., Wales P. W., Turner J. M., and Willing B. P.. 2017. Lipid emulsion formulation of parenteral nutrition affects intestinal microbiota and host responses in neonatal piglets. JPEN J. Parenter. Enteral Nutr. 41: 1301–1309. [DOI] [PubMed] [Google Scholar]
- 55.De Smet I., Van Hoorde L., Vande Woestyne M., Christiaens H., and Verstraete W.. 1995. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 79: 292–301. [DOI] [PubMed] [Google Scholar]
- 56.Waldram R. 1975. Mechanisms of lipid loss from the small intestinal mucosa. Gut. 16: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Kasmi K. C., Anderson A. L., Devereaux M. W., Vue P. M., Zhang W., Setchell K. D., Karpen S. J., and Sokol R. J.. 2013. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci. Transl. Med. 5: 206ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt J. A., Luo G., Billin A. N., Bisi J., McNeill Y. Y., Kozarsky K. F., Donahee M., Wang D. Y., Mansfield T. A., Kliewer S. A., et al. 2003. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 17: 1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C. L., McDonald J. G., Luo G., Jones S. A., Goodwin B., Richardson J. A., et al. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2: 217–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Microbial sequence reads have been deposited with links to BioProject accession number PRJNA559479 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/). A complete list of all metabolites from the colonic metabolomic analysis are available on request from Douglas Burrin, USDA-ARS Children’s Nutrition Research Center, E-mail: doug.burrin@usda.gov.