Abstract
Excessive lipid deposition is a hallmark of NAFLD. Although much has been learned about the enzymes and metabolites involved in NAFLD, few studies have focused on the role of long noncoding RNAs (lncRNAs) in hepatic lipid accumulation. Here, using in vitro and in vivo models of NAFLD, we found that the lncRNA Gm15622 is highly expressed in the liver of obese mice fed a HFD and in murine liver (AML-12) cells treated with free fatty acids. Investigating the molecular mechanism in the liver-enriched expression of Gm15622 and its effects on lipid accumulation in hepatocytes and on NAFLD pathogenesis, we found that Gm15622 acts as a sponge for the microRNA miR-742-3p. This sponging activity increased the expression of the transcriptional regulator SREBP-1c and promoted lipid accumulation in the liver of the HFD mice and AML-12 cells. Moreover, further results indicated that metformin suppresses Gm15622 and alleviates NAFLD-associated lipid deposition in mice. In conclusion, we have identified an lncRNA Gm15622/miR-742-3p/SREBP-1c regulatory circuit associated with NAFLD in mice, a finding that significantly advances our insight into how lipid metabolism and accumulation are altered in this metabolic disorder. Our results also suggest that Gm15622 may be a potential therapeutic target for managing NAFLD.
Keywords: long noncoding ribonucleic acid, nonalcoholic fatty liver disease, micro-ribonucleic acid, sterol regulatory element-binding protein 1c, metformin, obesity, gene regulation, epigenetic regulation, fatty acid synthase, miR-742-3p
NAFLD is a metabolic syndrome with a widespread histological distribution, from simple hepatic steatosis to NASH with or without fibrosis. NASH may eventually develop into cirrhosis and hepatocellular carcinoma (1). A World Health Organization survey found that the number of obese people in the world has nearly tripled since 1975 (https://www.who.int/health-topics/obesity#tab=tab_1). With the rising rate of obesity and type 2 diabetes, NAFLD has become one of the most prominent liver diseases in the world, with an incidence rate of 25% (2). The highest incidence rate is in the Middle East and South America at between 30% and 35%, followed by Asia, where the incidence is between 25% and 30% (3). The major causes of NAFLD include the metabolic diseases associated with type 2 diabetes, genetic predisposition, environmental factors, and gender (3). Therefore, timely intervention of NAFLD is of critical importance for alleviating the public health burden.
Lipids obtained from the diet or synthesized by the liver and stored in adipose tissue are in an equilibrium state in terms of synthesis and energy utilization (4). Chylomicrons transport food lipids to the liver either via the low density lipoprotein receptor or in the form of FFAs that enter liver cells via CD36 (5). The de novo synthesis of lipids is mainly regulated by transcription factors, such as carbohydrate reactive element-binding protein and SREBP, which control the transcription levels of the rate-limiting enzyme, acetyl-CoA carboxylase, and FASN (6). Excessive lipids in the liver are transported out of the liver by ATP-binding cassette transporters. If this delicate balance is disrupted, lipid metabolism in the liver can be perturbed, leading to disease (7, 8). Therefore, the regulation of lipid homeostasis is crucial.
Recently, noncoding RNAs have been shown to be involved in the regulation of lipid homeostasis. Long noncoding RNAs (lncRNAs) and microRNAs (miRNAs) are potential diagnostic markers and targets for the treatment of NAFLD (9, 10). For example, lncRNA-ak012226 is related to lipid accumulation in NAFLD (11), while miR-150 deficiency can ameliorate hepatic steatosis and insulin resistance in NAFLD (12). Furthermore, the lncRNA NEAT1/miR-140 axis exacerbates NAFLD by interrupting AMPK/SREBP-1 signaling (13). Thus, targeting functional lncRNAs and/or miRNAs is an effective strategy to treat NAFLD.
Metformin is a first-line drug in the treatment of type 2 diabetes (14). In recent years, various studies have demonstrated that in addition to treating type 2 diabetes, it also has anti-cancer properties (15), can reverse pulmonary fibrosis, and can ameliorate NAFLD (16, 17). Although several studies have explored the molecular mechanism of metformin, its role in the pathological process of NAFLD needs to be further elucidated.
In this study, we found that Gm15622 was significantly upregulated in the liver of HFD-induced obese mice. Upregulated Gm15622 significantly increased lipid accumulation, whereas silencing Gm15622 significantly reduced lipid accumulation in AML-12 cells. Mechanistically, Gm15622 positively modulated the expression of SREBP-1c by acting as a miRNA sponge for miRNA-742-3p. We also found that metformin could target Gm15622 to regulate lipid accumulation in HFD mice. Therefore, our study provides new insights into the molecular function of the Gm15622/miR-742-3p/SREBP-1c signaling pathway in the pathogenesis of NAFLD and highlights the potential of Gm15622 as a new therapeutic target for NAFLD.
MATERIALS AND METHODS
Animals
All animal procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23, revised in 1996) and the approved regulations set by the Laboratory Animal Care Committee at Nanjing Normal University (permit 2090658, issued 20 April 2008). Twenty-four male C57BL/6J mice (6–8 weeks old) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, Jiangsu, China). The mice were housed in a room with a 12:12 h light/dark cycle and controlled temperature (22–24°C) and humidity (50–60%) with free access to water and food. After 1 week of quarantine, mice were randomly divided into two groups, 12 were given normal diet (ND; Research Diets, #1010039) and 12 were fed a HFD (Research Diets, #D12492). Eight weeks later, these mice were assigned to four groups (with six mice per group): ND with saline (ND + Saline), HFD with saline (HFD + Saline), HFD with 150 mg/kg/day metformin [HFD + met (150 mg/kg)], and HFD with 300 mg/kg/day metformin [HFD + met (300 mg/kg)] (18). Metformin (C4H11N5·HCl, HPLC ≥98%; Solarbio, Beijing, China) was dissolved in saline solution and given to mice every day by oral gavage for 9 weeks. Equal volumes of saline solution were given to control mice. Body weight was monitored every week.
Immunohistochemistry
After 9 weeks of metformin gavage, the mice were starved for 12 h. At the time of euthanization, the mice were anesthetized with an intraperitoneal injection of 20 μl/g bodyweight of tribromoethanol (250 mg/kg; duration approximately 40 min). Mouse liver tissues were harvested, immediately frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. A small piece of liver was submerged in 4% paraformaldehyde for H&E staining and Oil Red O staining (Service Biology, Wuhan, Hubei, China).
Biochemical analysis
Blood samples were collected from the retro-orbital plexus of mice and transferred into centrifuge tubes. After incubation overnight at 4°C, samples were centrifuged at 1,520 g for 10 min. Sera were then collected and stored at −80°C for subsequent analysis. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), TG, and total cholesterol (TC) levels in serum and in liver were measured using commercially available test kits (Jiancheng Institute of Biotechnology, Nanjing, Jiangsu, China). Simultaneously, protein concentrations in cell lysates were measured by the BCA Protein Assay method (Jiancheng Institute of Biotechnology).
Cell culture and treatment
AML-12 cells were cultured in a 1:1 mixture of DMEM and Ham’s F12 medium (Wisent, St. Bruno, Canada) supplemented with 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium (Cyagen, Guangzhou, Guangdong, China), 10% fetal bovine serum (Wisent), and 40 ng/ml dexamethasone (Sigma-Aldrich). For stimulation, AML-12 cells were co-incubated with 2 mM metformin (dissolved in DMSO) and 0.4 mM FFAs (an equimolar mixture of oleic acid and palmitic acid; reagent configuration: 57 mg of oleic acid and 51 mg of palmitic acid were placed in a 50 ml centrifuge tube, and 20 μl 10 M NaOH and 20 ml 12.5% BSA were added to the centrifuge tube to configure 10 mM of oleic acid and palmitic acid) for 24 h. In the control group, equal volumes of DMSO and BSA (Solarbio, #A8850; purity ≥99.0%) were added for 24 h. AML-12 cells were seeded at a density of 2.5 × 105 cells per well in 6-well plates or at 1 × 106 cells per 60 mm-diameter cell culture plate in a humidified atmosphere containing 5% CO2 at 37°C. Cell transfection experiments were performed using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. A mimic and an inhibitor of miR-742-3p were designed and synthesized by Ribobio (Guangzhou, Guangdong, China).
Cell viability assay
Cell viability was measured in triplicate using a commercially available kit [Cell Counting Kit-8 (CCK-8)] (Enogene, Nanjing, Jiangsu, China). AML-12 cells were seeded in 96-well plates at a density of 1×104 cells per well. The cells were incubated with different doses of metformin for 24 h or with 2 mM metformin for different times. Ten microliters of CCK-8 solution were then added to each well and incubated for 1 h. Cell viability was assessed by the absorbance of solutions detected at 450 nm using a microplate reader (Epoch, Biotech).
Quantitative real-time RT-PCR
Total RNA was extracted from liver tissues and AML-12 cells using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was generated from total RNA using a reverse transcription kit (Takara, Dalian, China). Gene expression was measured by qPCR (Lightcycler96, Roche, Basel, Switzerland) using a SYBR green kit (Yeasen, Shanghai, China). A complete list of primers used is shown in supplemental Table S1. 36B4 and U6 snRNAs were used to normalize mRNA and miRNA levels, respectively.
Western blotting
For protein analysis, harvested cells or liver tissue homogenates were suspended in RIPA buffer containing a protease inhibitor cocktail (Roche, Switzerland) and PMSF (Beyotime, China). Equal amounts of protein were loaded and separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were then incubated overnight using appropriate antibodies against SREBP-1c (Affinity Biosciences, Cincinnati, OH) and FASN (Proteintech, China). These experiments were performed in triplicate. Quantitation of protein was performed using the AlphaEascFC system (Alpha InnotechFluorChem 8800).
Luciferase reporter construction
A 3′-UTR region (838 nt) of the mouse Srebp-1c gene, which contains a putative target site for miR-742-3p, was identified from the NCBI database (https://www.ncbi.nlm.nih.gov/nuccore/NM_001313979.1) and amplified by PCR using primers listed in supplemental Table S1. The fragment was inserted between XhoI and NotI restriction enzyme sites, immediately downstream of the luciferase gene in the pmiR-RB-Report™ vector. At the same time, a Gm15622 sequence (1,464 nt) containing a putative target site for miR-742-3p was identified from the Mouse Genome Database (http://www.informatics.jax.org/marker/MGI:3783067). lncRNA Gm15622 is located on chromosome 23 in mouse genome (Chr13:93625382-93628633 bp, + strand) and has two exons. The full-length sequence was amplified by PCR using primers listed in supplemental Table S1. This fragment was inserted between XhoI and SgfI restriction enzyme sites, immediately downstream of the luciferase gene in the pmiR-RB-ReportTM vector. We also mutated 7 bp of the miR-742-3p binding site from TGGCTTT to ACCGAAA. WT and mutant inserts were confirmed by sequencing and transfected into AML-12 liver cells. After transfection, cells were lysed and supernatants were collected for analysis after centrifugation at 13,680 g for 2 min at 4°C. Relative luciferase activities (ratio of Renilla luciferase signal normalized to Firefly luciferase) were measured with commercially available test kits (E1910, Promega, Madison, WI) and examined on a Luminoscan Ascent (Thermo Fisher Scientific).
Statistical analysis
Data are presented as the mean ± SD from multiple samples. All experiments were repeated at least three times. Group differences were considered statistically significant at P < 0.05, as assessed by Student’s t-test, one-way or two-way ANOVA followed by Fisher’s least significant difference post hoc test using Origin 2017 (OriginLab Corporation).
RESULTS
lncRNA Gm15622 is highly expressed in mouse liver and is upregulated in the ob/ob, db/db, and fasted models
We examined the basal expression of Gm15622 in seven tissues of C57BL/6 male mice by RT-qPCR. Gm15622 was expressed in all tissues, but the highest expression was in the liver in our study (Fig. 1A). Different studies archived in the Expression Atlas database (https://www.ebi.ac.uk/gxa/genes/ensmusg00000085834?) also showed that Gm15622 was specifically expressed in the liver of C57BL/6 mice (Fig. 1B). We then examined the expression of Gm15622 in the ob/ob, db/db, and fasted models by RT-qPCR, and Gm15622 was upregulated in these models in our study (Fig. 1C–E). These results indicated that Gm15622 was a liver-enriched lncRNA and was upregulated in the liver of ob/ob, db/db, and fasted models.
Fig. 1.
Gm15622 is a liver-enriched lncRNA and is overexpressed in ob/ob, fasting, and db/db mice. A: The expression level of Gm15622 in seven tissues of male mice was detected by RT-qPCR in our study (four mice). B: Expression analysis of Gm15622 in C57BL/6 mouse tissues in 14 other publicly available datasets [data from Expression Atlas (https://www.ebi.ac.uk/gxa/genes/ensmusg00000085834)]. C–E: Gm15622 transcripts in the liver (8-week-old male C57BL/6 mice, diabetic db/db mice on a C57BKS background, and obese ob/ob mice on a C57BKS background were purchased from the Model Animal Research Center of Nanjing University) were measured by RT-qPCR in our study (n = 4 mice/group). **P < 0.01, ***P < 0.001.
Gm15622 is elevated in the liver of HFD-fed obese mice
Mice fed the HFD for 18 weeks had a significantly increased body weight compared with the ND groups (Fig. 2A). Serum levels of TG, TC, ALT, and AST were also significantly increased in the HFD mice (Fig. 2B, C). The liver of HFD-fed mice was larger than normal and yellow, indicating the occurrence of lipid accumulation and liver steatosis. H&E and Oil Red O staining of liver sections also confirmed lipid accumulation (Fig. 2D). Indeed, the HFD significantly increased the hepatic levels of TG and TC (Fig. 2E). The HFD also increased expression of lncRNA Gm15622 and Gomafu (positive control). Correspondingly, the GEO database (https://www.ncbi.nlm.nih.gov/geoprofiles/106297548; GSE35961) also showed that Gm15622 was upregulated in the livers of HFD-fed C57BL/6 mice (Fig. 2F). Therefore, Gm15622 was elevated in the liver of HFD-fed mice.
Fig. 2.
Gm15622 levels are elevated in the liver of HFD-fed obese mice. A: Body weights were examined. Serum TG and TC (B) and (C) ALT and AST (C) were determined using commercial kits. D: Representative photo-images of mouse liver morphology (top, bars = 7 mm) and liver tissue sections stained with H&E (middle, bars = 100 μm) or Oil Red O (bottom, bars = 100 μm) to visualize lipid contents. E: TC and TG levels in the liver. F: RT-qPCR analysis of Gm15622 and lncRNA Gomafu (38) expression levels and expression analysis of Gm15622 in the liver of HFD mice [data source: GEO (GSE35961)]. Data are presented as the mean ± SD, n = 6 mice/group. *P < 0.05, **P < 0.01, ***P < 0.001.
Gm15622 regulates the expression of SREBP-1c and FASN in AML-12 cells
Gm15622 was liver-enriched and the HFD changed Gm15622 expression. We therefore suspected that Gm15622 might regulate genes involved in lipid synthesis. To explore the relationship between Gm15622 expression and genes involved in lipid synthesis, we constructed a Gm15622 overexpression plasmid and synthesized a Gm15622 interference sequence. They were transfected into AML-12 cells and 36 h later RT-qPCR and Western blotting were used to detect changes in mRNA and protein levels of lipid synthesis genes. After overexpression of Gm15622 for 36 h, SREBP-1c was upregulated at mRNA and protein levels, whereas FASN was upregulated only at the mRNA level (Fig. 3A–C). After siRNA knockdown of Gm15622, expression of SREBP-1c and FASN was decreased at mRNA and protein levels (Fig. 3D–F). Hence, Gm15622 can regulate the expression of SREBP-1c and FASN in vitro.
Fig. 3.
The expression of SREBP-1c and FASN in AML-12 cells is regulated by Gm15622. A: Efficiency of plasmid expression (pcDNA3.0-Gm15622) in AML-12 cells. B):AML-12 cells were transfected with a vector overexpressing Gm15622 or an empty vector. Thirty-six hours later, the mRNA levels of SREBP-1c and FASN were assessed by RT-qPCR. C: Protein levels of SREBP-1c (125 kDa) and FASN in AML-12 cells overexpressing Gm15622 were assessed by Western blotting (left) and are shown as densitometry determinations (right). D: Efficiency of si-Gm15622 RNA interference in AML-12 cells. E: mRNA levels of SREBP-1c and FASN in Gm15622-knockdown AML-12 cells. F: Protein levels of SREBP-1c (125 kDa) and FASN in Gm15622-knockdown AML-12 cells were assessed by Western blotting (left) and are shown as densitometry determinations (right). Experiments were repeated in triplicate and data are presented as the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
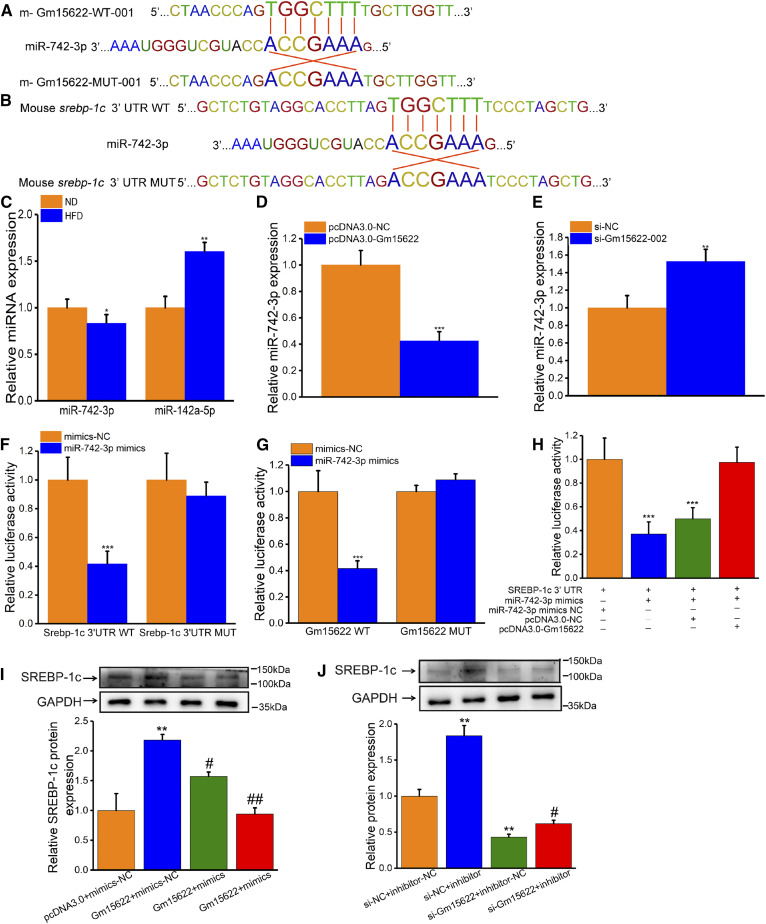
Gm15622 upregulates SREBP-1c expression by acting as a sponge of miR-742-3p
Using iLoc-LncRNA software (http://lin-group.cn/server/iLoc-LncRNA/pre.php), we predicted that Gm15622 was located in the cytoplasm. Using RNAhybrid 2.2, miRanda, and miRDB online database analysis, we found that the mouse Gm15622 sequence contains a miRNA-742-3p binding site (Fig. 4A). Surprisingly, the StarBase database (http://starbase.sysu.edu.cn/agoClipRNA.php?source=mRNA&flag=none&clade=mammal&genome=mouse&assembly=mm10&miRNA=all&clipNum=1&deNum=0&proNum=1&program=None&target=srebf1) also predicted that SREBP-1c is a target gene for miRNA-742-3p (Fig. 4B). These bioinformatic results indicated that Gm15622 might regulate the expression of SREBP-1c by interacting with miRNA-742-3p. Therefore, we examined the level of miR-742-3p in Gm15622-overexpression and Gm15622-knockdown AML-12 cells. The level of miRNA-742-3p was inversely correlated with the expression of Gm15622 or SREBP-1c (Fig. 4C–E). Next, we inserted the Gm15622 or SREBP-1c 3′-UTR sequence containing the miR-742-3p binding site and the Gm15622 or SREBP-1c 3′-UTR mutation site into the reporter plasmid downstream of the dual luciferase gene for detection of luciferase activity. The luciferase assay revealed that transfection of miR-742-3p mimics reduced Gm15622 and SREBP-1c expression in the wild-type group compared with that in the negative control group, and that this inhibition was reversed when the binding site was mutated, indicating the same sequence-specific binding of miR-149-5p to the Gm15622 or SREBP-1c 3′-UTR (Fig. 4F, G). To further determine the relationship between Gm15622, miR-742-3p, and SREBP-1c, the SREBP-1c 3′-UTR plasmid was cotransfected with miR-742-3p and Gm15622 and analyzed by luciferase assays. Gm15622 could counteract the inhibition of SREBP-1c expression by miR-742-3p (Fig. 4H). Consistent with previous results, overexpression of Gm15622 increased the protein level of SREBP-1c in AML-12 cells, and this effect was counteracted by miR-742-3p mimics (Fig. 4I). Knockdown of Gm15622 decreased protein levels of SREBP-1c,which was reversed by miR-742-3p inhibitors (Fig. 4J). Therefore, these results demonstrated that Gm15622 upregulated the expression of SREBP-1c by acting as a sponge of miR-742-3p.
Fig. 4.
Gm15622 can upregulate SREBP-1c expression by acting as a sponge of miR-742-3p. A: CLIP-seq results show that SREBP-1c is a target gene of miR-742-3p (data from StarBase database). The positions of miR-742-3p binding sites on SREBP-1c are shown. B: Interactions between miR-742-3p and Gm15622 were predicted using RNAhybrid 2.2, miRanda, and miRDB. The positions of miR-742-3p binding sites on Gm15622 are shown. C: RT-qPCR analysis of miR-742-3p and miR-142a-5p (39) levels in the liver of HFD mice (n = 6 mice/group). D: Level of miR-742-3p in AML-12 cells overexpressing Gm15622. E: Level of miR-742-3p in Gm15622-knockdown AML-12 cells. F: The SREBP-1c 3′-UTR was cloned downstream of the luciferase gene (Luc-SREBP-1c-WT) and transfected into AML-12 cells with miR-742-3p mimic or negative control (NC). To avoid nonspecific binding, the miR-742-3p binding sites in SREBP-1c were mutated to generate Luc-SREBP-1c-MUT. Luciferase activity was detected 48 h after transfection (***P < 0.001 vs. NC). G: miR-742-3p mimics or NC were cotransfected with Luc-Gm15622-WT or MUT into AML-12 cells. Luciferase activity was detected 48 h after transfection (*P < 0.05 vs. NC). H: Luc-SREBP-1c-WT and miR-742-3p were cotransfected into AML-12 cells with Gm15622 plasmid or the pcDNA3.0 vector to verify the competing endogenous RNA activity of Gm15622. Luciferase activity was detected 48 h after transfection (***P < 0.001 vs. NC). I: AML-12 cells were transfected with different combinations of Gm15622 and miR-742-3p mimic. Western blot analysis was conducted to detect SREBP-1c expression. (**P < 0.01, pcDNA3.0-Gm15622 vs. pcDNA3.0; #P < 0.05, pcDNA3.0-Gm15622 + miR-742-3p mimic (50 nM) vs. pcDNA3.0-Gm15622; ##P < 0.01, pcDNA3.0-Gm15622 + miR-742-3p mimic (100 nM) vs. pcDNA3.0-Gm15622). J: AML-12 cells with or without si-Gm15622 were transfected with miR-742-3p inhibitor or inhibitor control. Western blot analysis was conducted to detect SREBP-1c expression (**P < 0.01, si-NC + inhibitor-NC vs. si-NC + inhibitor; ***P < 0.001, si-Gm15622 + inhibitor-NC vs. si-NC + inhibitor NC; ###P < 0.001, si-Gm15622 + inhibitor vs. si-NC + inhibitor).
Metformin reduces lipid accumulation and inhibits lipogenic gene expression in AML-12 cells
The expression levels of Gm15622, SREBP-1c, and FASN in AML-12 cells were detected by RT-qPCR after treatment with four lipid-lowering drugs (celastrol, metformin, berberine, and genipin). The four drugs reduced Gm15622, SREBP-1c, and FASN expression, with metformin having the strongest effect (Fig. 5A). We therefore explored how metformin regulates SREBP-1c and FASN. First, the cytotoxicity of metformin in AML-12 cells was evaluated to determine nontoxic metformin doses. CCK-8 assays revealed that metformin at a concentration of 2 mM or lower did not inhibit cell viability over 24 h (supplemental Fig. S1A, B). We therefore selected 2 mM metformin for 24 h as a safe concentration for AML-12 cells. We then examined expression level changes of Gm15622, miR-742-3p, SREBP-1c, and FASN after incubation of AML-12 cells with FFAs and 2 mM metformin. The level of Gm15622 and the expression of SREBP-1c and FASN at mRNA and protein levels were decreased, while the level of miR-742-3p was increased in AML-12 cells cotreated with FFAs and metformin (Fig. 5B–E). Correspondingly, Oil Red O staining showed that metformin can attenuate the increase in lipid accumulation caused by FFAs (Fig. 5F). Taken together, metformin may reduce lipid accumulation and expression of lipid synthetic genes in AML-12 cells by modulating Gm15622 or miR-742-3p.
Fig. 5.
Metformin reduces lipid accumulation and inhibits lipogenic gene expression in AML-12 cells. A: Expression levels of Gm15622, SREBP-1c, and FASN were detected after treatment with four lipid-lowering drugs (2.5 μM celastrol, 2 mM metformin,10 μM berberine, and 20 μM genipin) (39–42). B: AML-12 cells were co-incubated with metformin (2 mM) and 0.4 mM FFAs (an equal molar mixture of oleic acid and palmitic acid) for 24 h. RT-qPCR analysis of Gm15622 expression levels. RT-qPCR analysis of miR-742-3p levels (C) and hepatic lipogenesis mRNA levels (D). E: Protein levels of lipogenic factors assessed by Western blotting (left) and densitometry determinations (right) are shown. F: Oil Red O staining was performed to assess intracellular lipid accumulation. Bars = 50 μm. Experiments were repeated in triplicate and data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. BSA; ###P < 0.001 vs. FFAs without metformin.
Metformin inhibits SREBP-1c and FASN expression in AML-12 cells by targeting Gm15622
To explore the specific molecular mechanism by which metformin reduces lipid accumulation through lncRNAs, we constructed the pcDNA3.0-Gm15622 overexpression plasmid and synthesized a Gm15622 interference sequence (siRNA). After Gm15622 was overexpressed in AML-12 cells for 36 h, cells were treated with metformin (2 mM) for 24 h. Gm15622 overexpression increased the expression of SREBP-1c and FASN at mRNA and protein levels, and decreased the level of miR-742-3p. Metformin treatment decreased the expression of SREBP-1c and FASN at mRNA and protein levels, but did not influence miR-742-3p levels after Gm15622 was overexpressed in AML-12 cells (Fig. 6A–C, supplemental Fig. S2A). Metformin (2 mM) treatment of Gm15622 knockout AML-12 cells for 24 h decreased the expression of SREBP-1c and FASN at mRNA and protein levels, and increased the level of miR-742-3p. Metformin treatment could decrease the expression of SREBP-1c and FASN at mRNA and protein levels, but did not influence miR-742-3p levels after Gm15622 was knocked out in AML-12 cells (Fig. 6D–F, supplemental Fig. S2B). These results confirmed that metformin inhibited SREBP-1c and FASN expression levels in vitro by targeting Gm15622.
Fig. 6.
Metformin inhibits SREBP-1c and FASN expression by targeting Gm15622 in AML-12 cells. A, B: Gm15622 was overexpressed for 36 h and then cells were treated with metformin (2 mM) for 24 h. RT-qPCR analysis of Gm15622 and miR-742-3p expression levels. C: Protein levels of SREBP-1c (125 kDa) and FASN were assessed by Western blotting and densitometry determinations are shown. D, E: Gm15622-knockout AML-12 cells were treated with metformin (2 mM) for 24 h. RT-qPCR analysis of Gm15622 and miR-742-3p expression levels. F: Protein levels of SREBP-1c (125 kDa) and FASN, assessed by Western blotting (left) and densitometry determinations (right), are shown. Experiments were repeated in triplicate and data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. pcDNA3.0+DMSO/si-NC + DMSO; ##P < 0.001 vs. si-Gm15622 without metformin; ###P < 0.001 vs. pcDNA3.0-Gm15622 without metformin.
Metformin reduces body weight gain, alleviates hyperlipidemia, and decreases lipid accumulation in the liver of HFD-fed obese mice
To explore the role of metformin in vivo, we next used mice that had been fed a HFD for 18 weeks and then gavaged with different concentrations of metformin. In the 9 weeks of metformin treatment, the obesity of control mice receiving saline gavage became more severe. In contrast, the average body weight gain of HFD-fed obese mice treated with metformin at 150 mg/kg/day was decreased, and a high dose of metformin (300 mg/kg/day) completely inhibited HFD-induced obesity (Fig. 7A). To determine the possible hypolipidemic effect of metformin on HFD-fed obese mice, the serum lipid profile was examined. As expected, feeding of a HFD for 18 weeks led to robust increases in serum TG and TC levels (Fig. 7B, C), while metformin administration efficiently inhibited such increases in a dose-dependent manner. ALT and AST are two biomarkers of liver injury, and we found that metformin antagonized the HFD-induced increase in serum levels of ALT and AST (Fig. 7D, E), indicating that metformin possessed a hepato-protective effect. Morphological observations showed that mice fed HFD had large livers of a yellow color, indicating lipid accumulation and hepatic steatosis, while metformin improved these phenotypes. H&E and Oil Red O staining of liver sections also confirmed that metformin inhibited HFD-induced severe hepatic fat accumulation (Fig. 7F).
Fig. 7.
Metformin reduces body weight gain, alleviates hyperlipidemia, and decreases lipid accumulation in the liver of HFD-fed obese mice. A: Mice were fed a HFD for 18 weeks and were then treated with metformin (150 or 300 mg/kg/day) for 9 weeks by gavage. Body weights were recorded weekly. B–E: After treatment with metformin for 9 weeks, mice were euthanized. TG, TC, ALT, and AST levels in the serum were assessed using commercial kits. F: Representative photo-images of mouse liver morphology (top, bars = 7 mm) and liver tissue sections stained with H&E (middle, bars = 100 μm) or Oil Red O (bottom, bars = 100 μm) to visualize lipid contents. To demonstrate more intuitively that metformin can improve lipid accumulation in the liver of NAFLD mice, the results in the ND group were reused from Fig. 2D. Data are presented as the mean ± SD, n = 6 mice/group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. ND; #P < 0.05 and ##P < 0.01 vs. HFD without metformin.
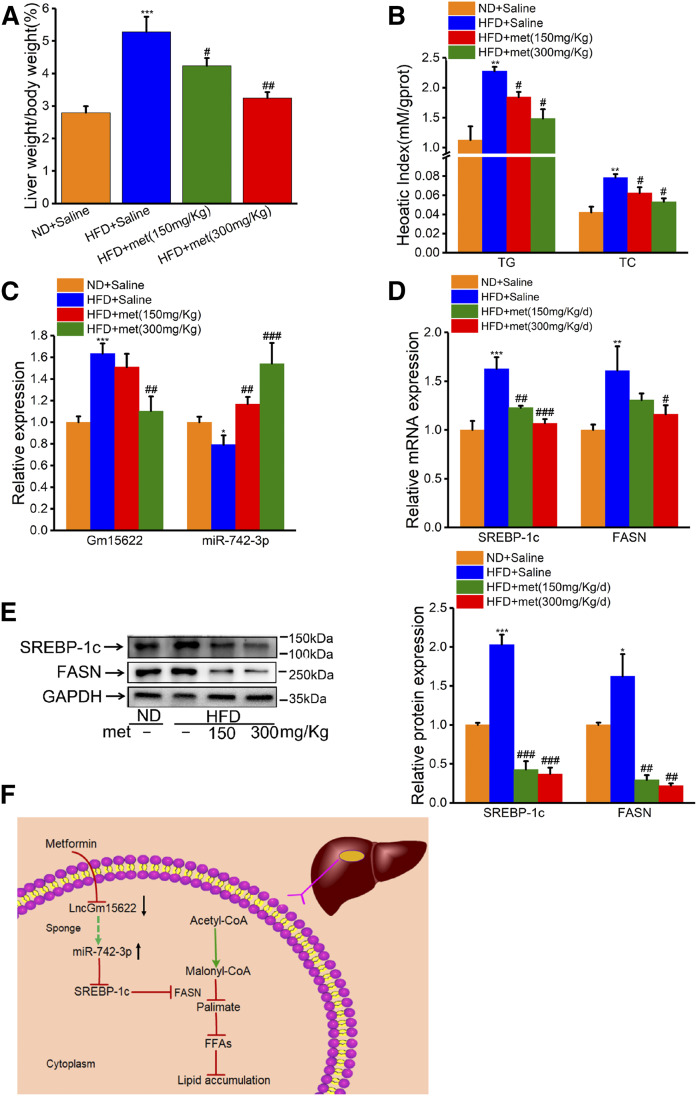
Metformin inhibits the expression of lipid synthesis genes in HFD-fed obese mice by inhibiting the expression of Gm15622
HFD significantly increased the ratio of liver weight to body weight, and the hepatic levels of TG and TC (Fig. 8A, B). These liver abnormalities were alleviated by metformin. HFD-fed mice had a robust increase in hepatic expression levels of Gm15622, but a substantial decrease in the level of miR-742-3p. Conversely, metformin reversed these changes in expression in a dose-dependent manner (Fig. 8C). To confirm the regulatory role of metformin in lipogenic gene expression, we examined protein levels in liver samples and found a trend similar to that of mRNA levels. Metformin inhibited HFD-feeding-induced upregulation of the hepatic protein levels of SREBP-1c and FASN in a dose-dependent manner (Fig. 8D, E). In conclusion, we found that metformin effectively antagonized HFD-induced hyperlipidemia and hepatic lipid accumulation in mice. These effects were achieved by regulating the Gm15622/miR-742-3p/SREBP-1c axis (Fig. 8F).
Fig. 8.
Metformin inhibits the expression of lipid synthesis genes in HFD-fed obese mice by inhibiting the expression of Gm15622. A: The ratio of liver weight to body weight. B: TG and TC levels in mouse liver. C, D: RT-qPCR analysis of Gm15622, miR-742-3p, SREBP-1c, and FASN levels. E: The protein levels of SREBP-1c (125 kDa) and FASN were assessed by Western blotting (left) and densitometry determinations (right). Data are presented as the mean ± SD, n = 6 mice/group. *P < 0.05, ***P < 0.001 vs. ND; ##P < 0.01 and ##P < 0.001 vs. HFD without metformin. F: A model illustrating the hypolipidemic effect of metformin through regulation of the Gm15622/miR-742-3p/SREBP-1c axis.
DISCUSSION
Excessive accumulation of lipids caused by unbalanced lipid metabolism in the liver is the main cause of NAFLD (8). NAFLD not only causes hepatocellular carcinoma but is also a component of metabolic syndrome together with obesity, type 2 diabetes, arteriosclerotic cardiovascular disease, and dyslipidemia (1, 2). Therefore, it imposes a huge clinical and economic burden on patients and society. Although the molecular mechanism of NAFLD has not been clearly elucidated, lncRNAs can be used as potential targets for the diagnosis and treatment of NAFLD (9, 19). In the present study, increased expression of the lncRNA Gm15622 in HFD-induced NAFLD was investigated. We found Gm15622 to be capable of sponging miR-742-3p, thereby increasing the amount of SREBP-1 protein, a transcription factor that regulates the expression of genes that control fatty acid lipid and cholesterol synthesis. Overexpression of Gm15622 can cause upregulation of SREBP-1c expression, promoting hepatic lipid accumulation. Conversely, Gm15622 siRNA knockdown can reduce SREBP-1c expression and inhibit lipid accumulation in the liver. Gain-of-function and loss-of-function experiments demonstrated that Gm15622 can regulate the miR-742-3p/SREBP-1c axis to improve lipid accumulation in vitro and in vivo. These findings support the rationale that Gm15622 acts as a novel biomarker and may serve as a therapeutic target for NAFLD.
lncRNAs are involved in the regulation of a variety of biological functions and changes in lncRNA expression can be related to the development of various diseases (20, 21). In 2016, Losko, Kotlinowski, and Jura (21) reviewed the lncRNAs that contribute to type 2 diabetes, cardiovascular disease, and obesity, as well as their mechanisms of action (22). Recent studies have shown that some lncRNAs, such as lncRNA-neat1 and lncRNA-MEG3, play important roles in the pathological process of NAFLD (23, 24). Although some lncRNAs have been identified as potential therapeutic targets in NAFLD, lipid accumulation is the most common cause of NAFLD (19, 25). Therefore, screening for lncRNAs related to lipid accumulation is crucial to prevent the occurrence of NAFLD. In this study, we focused on Gm15622, which promotes de novo synthesis of lipids by promoting the expression of SREBP-1c. Similarly, Liu et al. (26) found that knockdown of lncRNA-H19 can reduce liver fat production by directly regulating the miRNA-130a/PPARγ axis. In contrast, Hu et al. (27) found that lincRNA-DYNLRB2-2 knockdown inhibits lipid efflux by inhibiting the expression of ABCA1. Lipid accumulation in the liver can be caused by either increased production or decreased transport. To explore the function of Gm15622 and the potential of Gm15622 for treating NAFLD, we used siRNA to knockdown Gm15622, and we constructed a Gm15622 overexpression plasmid. Interestingly, knockdown of Gm15622 reduced the expression of SREBP-1c, while overexpression of Gm15622 had the opposite effect. By in vitro gain- and loss-of-function experiments, we determined that Gm15622 can positively regulate SREBP-1c expression in a transcription-dependent manner. Thus, we speculate that SREBP-1c is a downstream target of Gm15622 in NAFLD.
lncRNAs located in the cytoplasm and nucleus have different mechanisms of action, which can be taken into account when investigating their functions (28). First, we used online iLoc-LncRNA software (http://lin-group.cn/server/iLoc-LncRNA/pre.php) to predict that Gm15622 is located in the cytoplasm, and the MGI database (http://www.informatics.jax.org/marker/MGI:3783067) to show that Gm15622 is an antisense lncRNA, indicating that Gm15622 may be involved in posttranscriptional regulation in the cytoplasm. Many studies have demonstrated that lncRNAs are involved in posttranscriptional regulation by acting as miRNA sponges to regulate the expression of miRNA targets (29, 30). We then used bioinformatics to predict that Gm15622 contains a target site for and can interact with miR-742-3p. In addition, SREBP-1c is a target gene of miR-742-3p. Finally, we found that overexpression of Gm15622 increased the expression of SREBP-1c, which was offset by overexpression of miR-742-3p. Similarly, knockdown of Gm15622 reduced the expression of SREBP-1c, which was reversed by down-regulation of miR-742-3p expression. Therefore, we propose that Gm15622 acts as a ceRNA in AML-12 cells to regulate SREBP-1c expression by sponging miR-742-3p, which may be a mechanism by which Gm15622 acts as a critical regulator in NAFLD.
Different pathological processes have been described for NAFLD (31). Therapeutic drugs for NAFLD are still in the development stage, and the most developed drugs, such as pioglitazone and GFT 505, are only in phase III clinical trials (32, 33). Metformin is the preferred drug for treating diabetes mellitus, but it can also affect the pathological process of some cancers (15, 16). For example, Chen et al. (34) found that metformin can be used as a new anticancer drug for oral squamous cell carcinoma, and Xia et al. (35) showed that metformin can reduce invasion and migration of cervix cancer cells by destroying the MALAT1/Mi-142-3p sponge. Metformin treatment also improves HFD-induced hepatic steatosis (17), and, in 2018, Min et al. (36) found that metformin combined with lactoferrin downregulated the expression of lipase (FASN, acetyl-CoA, and SREBP-1) in HFD mice. These studies illustrate the potential of metformin for NAFLD treatment and show that the molecular mechanism of metformin action in NAFLD warrants detailed elucidation. Metformin can affect the invasion and migration of cervical cancer by lncRNA interaction (35); therefore, metformin may play a role in NAFLD by targeting lncRNAs. In this study, we not only confirmed that metformin can reduce liver lipid accumulation and serum indicators (TG, TC, ALT, and AST) in HFD mice, but we also found that Gm15622 can be suppressed in NAFLD by metformin. These findings provide a new molecular mechanism for metformin in the treatment of NAFLD. The liver is the most metabolic organ and has partial immune effects (37) and Gm15622 plays an important role in these processes. The emergence of NAFLD-related lncRNAs as regulators of gene expression has greatly altered our understanding of the pathological mechanisms of NAFLD.
In summary, Gm15622 is an important molecule that affects lipid metabolism in the liver. These results not only illustrate how Gm15622 promotes lipid accumulation in the liver by targeting the miRNA-742-3p/srebp-1c axis, but also show that Gm15622 is a target for metformin treatment of NAFLD. Further studies are required in both animals and patients to elucidate the correlation between Gm15622 and NAFLD disease progression.
Supplementary Material
Acknowledgments
The authors thank Jeremy Allen, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- CCK-8
- Cell Counting Kit-8
- lncRNA
- long noncoding RNA
- ND
- normal diet
- TC
- total cholesterol
This work was supported by National Natural Science Foundation of China Grant 61771251, Key Project of Social Development in Jiangsu Province Grant BE2016773, National Natural Science Foundation of Jiangsu Grant BK20171443, the Qinglan Project of Jiangsu Province of China, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Achievements Incubation Project of Changzhou Institute of Innovation and Development of Nanjing Normal University Grant Z201801F06, and Nanjing Normal University Overseas Studies Grant NJNU-2017, and was sponsored by Nanjing University of Posts and Telecommunications Science Foundation (NUPTSF) Grant NY220041. The authors declare that they have no conflicts of interest with the contents of this article.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Bessone F., Razori M. V., and Roma M. G.. 2019. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 76: 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai W., Ye L., Liu A., Wen S. W., Deng J., Wu X., and Lai Z.. 2017. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore). 96: e8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z., Anstee Q. M., Marietti M., Hardy T., Henry L., Eslam M., George J., and Bugianesi E.. 2018. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen P., Leray V., Diez M., Serisier S., Le Bloc’h J., Siliart B., and Dumon H.. 2008. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. (Berl.). 92: 272–283. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy D. J., Kuchibhotla S. D., Guy E., Park Y. M., Nimako G., Vanegas D., Morton R. E., and Febbraio M.. 2009. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler. Thromb. Vasc. Biol. 29: 1481–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dentin R., Pegorier J. P., Benhamed F., Foufelle F., Ferre P., Fauveau V., Magnuson M. A., Girard J., and Postic C.. 2004. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 279: 20314–20326. [DOI] [PubMed] [Google Scholar]
- 7.Wlcek K., and Stieger B.. 2014. ATP-binding cassette transporters in liver. Biofactors. 40: 188–198. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q., Bengmark S., and Qu S.. 2010. The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD). Lipids Health Dis. 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Huang H., Xu C., Yu C., and Li Y.. 2017. Long non-coding RNA profiling in a non-alcoholic fatty liver disease rodent model: new insight into pathogenesis. Int. J. Mol. Sci. 18: E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo G., and Csak T.. 2016. Role of microRNAs in NAFLD/NASH. Dig. Dis. Sci. 61: 1314–1324. [DOI] [PubMed] [Google Scholar]
- 11.Chen X., Xu Y., Zhao D., Chen T., Gu C., Yu G., Chen K., Zhong Y., He J., Liu S., et al. 2018. LncRNA-AK012226 is involved in fat accumulation in db/db mice fatty liver and non-alcoholic fatty liver disease cell model. Front. Pharmacol. 9: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuge B., and Li G.. 2017. MiR-150 deficiency ameliorated hepatosteatosis and insulin resistance in nonalcoholic fatty liver disease via targeting CASP8 and FADD-like apoptosis regulator. Biochem. Biophys. Res. Commun. 494: 687–692. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y., Song Y., Liu C., and Geng J.. 2019. LncRNA NEAT1-MicroRNA-140 axis exacerbates nonalcoholic fatty liver through interrupting AMPK/SREBP-1 signaling. Biochem. Biophys. Res. Commun. 516: 584–590. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Rangel E., and Inzucchi S. E.. 2017. Metformin: clinical use in type 2 diabetes. Diabetologia. 60: 1586–1593. [DOI] [PubMed] [Google Scholar]
- 15.Coyle C., Cafferty F. H., Vale C., and Langley R. E.. 2016. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann. Oncol. 27: 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamad N., Malik S., Suchal K., Vasisht S., Tomar A., Arava S., Arya D. S., and Bhatia J.. 2018. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: pharmacological effects and molecular mechanisms. Biomed. Pharmacother. 97: 1544–1553. [DOI] [PubMed] [Google Scholar]
- 17.Rouabhia S., Milic N., and Abenavoli L.. 2014. Metformin in the treatment of non-alcoholic fatty liver disease: safety, efficacy and mechanism. Expert Rev. Gastroenterol. Hepatol. 8: 343–349. [DOI] [PubMed] [Google Scholar]
- 18.Kim H. J., Zhang X. H., Park E. Y., Shin K. H., Choi S. H., Chun B. G., and Kim D. H.. 2013. Metformin decreases meal size and number and increases c-Fos expression in the nucleus tractus solitarius of obese mice. Physiol. Behav. 110–111: 213–220. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y., Cai J., She Z., and Li H.. 2018. Insights into the epidemiology, pathogenesis, and therapeutics of nonalcoholic fatty liver diseases. Adv. Sci. (Weinh.). 6: 1801585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao A. K. D. M., Rajkumar T., and Mani S.. 2017. Perspectives of long non-coding RNAs in cancer. Mol. Biol. Rep. 44: 203–218. [DOI] [PubMed] [Google Scholar]
- 21.Losko M., Kotlinowski J., and Jura J.. 2016. Long noncoding RNAs in metabolic syndrome related disorders. Mediators Inflamm. 2016: 5365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehra M., and Chauhan R.. 2017. Long noncoding RNAs as a key player in hepatocellular carcinoma. Biomark. Cancer. 9: 1179299X17737301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang-Fu N., Cheng J. S., Wang Y., Li Z. W., and Wang S. H.. 2018. Neat1 regulates oxidized low-density lipoprotein-induced inflammation and lipid uptake in macrophages via paraspeckle formation. Mol. Med. Rep. 17: 3092–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P., Huang F. Z., Liu H. Z., Zhang T. Y., Yang M. S., and Sun C. Z.. 2019. LncRNA MEG3 functions as a ceRNA in regulating hepatic lipogenesis by competitively binding to miR-21 with LRP6. Metabolism. 94: 1–8. [DOI] [PubMed] [Google Scholar]
- 25.Gao B., Zhang X., Huang Y., Yang Z., Zhang Y., Zhang W., Gao Z. H., and Xue D.. 2017. Coding and non-coding gene regulatory networks underlie the immune response in liver cirrhosis. PLoS One. 12: e0174142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J., Tang T., Wang G. D., and Liu B.. 2019. LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARgamma axis in non-alcoholic fatty liver disease. Biosci. Rep. 39: BSR20181722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y. W., Yang J. Y., Ma X., Chen Z. P., Hu Y. R., Zhao J. Y., Li S. F., Qiu Y. R., Lu J. B., Wang Y. C., et al. 2014. A lincRNA-DYNLRB2–2/GPR119/GLP-1R/ABCA1-dependent signal transduction pathway is essential for the regulation of cholesterol homeostasis. J. Lipid Res. 55: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L. L. 2016. Linking long noncoding RNA localization and function. Trends Biochem. Sci. 41: 761–772. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Zhang Y. H., Deng Q., Li Y., Huang T., Zhou S., and Cai Y. D.. 2017. Cancer-related triplets of mRNA-lncRNA-miRNA revealed by integrative network in uterine corpus endometrial carcinoma. BioMed Res. Int. 2017: 3859582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan C. N., Ma L., and Liu N.. 2018. Systematic analysis of lncRNA-miRNA-mRNA competing endogenous RNA network identifies four-lncRNA signature as a prognostic biomarker for breast cancer. J. Transl. Med. 16: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobbina E., and Akhlaghi F.. 2017. Non-alcoholic fatty liver disease (NAFLD) - pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev. 49: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanyal A. J., Chalasani N., Kowdley K. V., McCullough A., Diehl A. M., Bass N. M., Neuschwander-Tetri B. A., Lavine J. E., Tonascia J., Unalp A., et al. 2010. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratziu V., Harrison S. A., Francque S., Bedossa P., Lehert P., Serfaty L., Romero-Gomez M., Boursier J., Abdelmalek M., Caldwell S., et al.; GOLDEN-505 Investigator Study Group . 2016. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 150: 1147–1159.e5. [DOI] [PubMed] [Google Scholar]
- 34.Chen C. H., Tsai H. T., Chuang H. C., Shiu L. Y., Su L. J., Chiu T. J., Luo S. D., Fang F. M., Huang C. C., and Chien C. Y.. 2017. Metformin disrupts malignant behavior of oral squamous cell carcinoma via a novel signaling involving late SV40 factor/Aurora-A. Sci. Rep. 7: 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia C., Liang S., He Z., Zhu X., Chen R., and Chen J.. 2018. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur. J. Pharmacol. 830: 59–67. [DOI] [PubMed] [Google Scholar]
- 36.Min Q. Q., Qin L. Q., Sun Z. Z., Zuo W. T., Zhao L., and Xu J. Y.. 2018. Effects of metformin combined with lactoferrin on lipid accumulation and metabolism in mice fed with high-fat diet. Nutrients. 10: E1628 10.3390/nu10111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarasenko T. N., and McGuire P. J.. 2017. The liver is a metabolic and immunologic organ: a reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol. Genet. Metab. 121: 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan C., Li J., Feng S., Li Y., and Tan L.. 2018. Long noncoding RNA Gomafu upregulates Foxo1 expression to promote hepatic insulin resistance by sponging miR-139-5p. Cell Death Dis. 9: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong H., Chen K., Feng M., Shao W., Wu J., Chen K., Liang T., and Liu C.. 2018. Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 285: 501–517. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y., Geng C., Liu X., Li M., Gao M., Liu X., Fang F., and Chang Y.. 2016. Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol. Metab. 6: 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Massey S., Story D., and Li L.. 2018. Metformin: an old drug with new applications. Int. J. Mol. Sci. 19: E2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao L., Cang Z., Sun H., Nie X., Wang N., and Lu Y.. 2017. Berberine improves glucogenesis and lipid metabolism in nonalcoholic fatty liver disease. BMC Endocr. Disord. 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.