Abstract
Context
The patterns of associations between glycated Hb (HbA1c) and mortality are still unclear.
Objective
To explore the extent to which ranges of HbA1c levels are associated with the risk of mortality among participants with and without diabetes.
Design, Setting, and Patients
This was a nationwide, community-based prospective cohort study. Included were 15,869 participants (median age 64 years) of the Health and Retirement Study, with available HbA1c data and without a history of cancer. Cox proportional hazards regression models were used to estimate hazard ratios with 95% CIs for mortality.
Results
A total of 2133 participants died during a median follow-up of 5.8 years. In participants with diabetes, those with an HbA1c level of 6.5% were at the lowest risk of all-cause mortality. When HbA1c level was <5.6% or >7.4%, the increased all-cause mortality risk became statistically significant as compared with an HbA1c level of 6.5%. As for participants without diabetes, those with an HbA1c level of 5.4% were at the lowest risk of all-cause mortality. When the HbA1c level was <5.0%, the increased all-cause mortality risk became statistically significant as compared with an HbA1c level of 5.4%. However, we did not observe a statistically significant elevated risk of all-cause mortality above an HbA1c level of 5.4%.
Conclusions
A U-shaped and reverse J-shaped association for all-cause mortality was found among participants with and without diabetes. The corresponding optimal ranges for overall survival are predicted to be 5.6% and 7.4% and 5.0% and 6.5%, respectively.
We observed a U-shaped association between HbA1c and all-cause mortality among participants with diabetes and an asymmetric reverse J-shaped association among those without diabetes.
Glycated Hb (HbA1c) represents average endogenous exposure to glucose for the prior 2 to 3 months (1). This biomarker has received much attention for its established relationship to type 2 diabetes and various vascular diseases and for the rising national and global prevalence of hyperglycemia and diabetes (2, 3).
In 2010, the American Diabetes Association (ADA) proposed the use of an HbA1c level of ≥6.5% as a threshold for the diagnosis of diabetes (4, 5). In fact, over the past few decades, researchers have been examining the associations of HbA1c with various health outcomes among individuals with and without diabetes. As a result, growing epidemiological evidence has linked higher HbA1c with adverse health outcomes, such as cardiovascular disease, blindness, kidney failure, and cancer, all of which may lead to premature death (6–9). However, concerns have been expressed regarding the optimal HbA1c ranges because hypoglycemia also may be hazardous and outweigh any potential benefit of glycemic control (10, 11). To date, a conclusive relationship has not been demonstrated between HbA1c and survival among different populations, and the true patterns of association are still an open issue, with some studies reporting nonlinear relationships (12, 13), whereas others suggest no such associations (14–16). This discrepancy has been a major obstacle to safely optimizing glycemic control, highlighting the need for further research to elucidate the safe glycemic range for survival.
In the current study, we investigated the range of HbA1c levels associated with all-cause mortality risk in participants with and without diabetes based on a sample of US adults aged ≥50 years from the Health and Retirement Study (HRS). We further studied HbA1c and mortality due to cardiovascular disease, cancer, and other causes.
Methods
Design, study setting, and participants
Initiated in 1992, the HRS is a longitudinal survey of a nationally representative sample of noninstitutionalized adults aged ≥50 years in the United States (17). Biomarker data were collected biennially on subsamples since 2006. For these analyses, we used four waves of the HRS covering 2006 to 2012. Respondents who met the following criteria were included in the study population: (1) completed an interview and had available data for HbA1c; (2) were without a history of cancer; (3) were aged ≥50 years; and (4) had reported their history of diabetes (yes/no). In total, 15,869 participants were eligible for our study. Specifically, 5407, 5168, 2723, and 2571 participants were included from the waves in 2006, 2008, 2010, and 2012, respectively. Diabetes cases (n = 3824) were defined as a self-reported history of diabetes (n = 3256) or no history of diagnosed diabetes but had elevated HbA1c levels ≥6.50% (n = 568). This definition provides the closest approximation of cases that could be classified as diabetes in clinical practice (2). We followed HRS participants from the date of the initial interview to the date of death, loss to follow-up, or last HRS interview (in the 2014 wave), whichever came first. All participants or their proxy respondents have provided written informed consent.
Assessment of HbA1c
In the HRS, HbA1c was measured by dried blood spots (DBSs). The HRS adjusted DBS values to levels consistent with National Health and Nutrition Examination Survey (NHANES), exploiting the fact that weighted NHANES and HRS samples are both population-based studies aiming to represent the noninstitutional U.S. population, and the population distributions should be the same. Specifically, the HRS determined the value of both assays at each percentile; and then transformed the DBS assays into the NHANES scale with adjustment for between-laboratory differences (18). It has been reported that the distribution of the DBS assays is similar to that in NHANES (19). The HbA1c results based on DBSs are highly correlated with the whole blood (r = 0.956) and have low within-assay imprecision and between-assay imprecision (1.4% and 2.2%, respectively (18, 19).
Assessment of deaths
Causes and time of death were determined by matching study records to the National Death Index and by exit interviews with proxy respondents. In our study, the primary outcome was all-cause mortality; the secondary outcomes were mortality due to cardiovascular disease, cancer, and other causes. If the causes of death were heart, circulatory, and blood conditions, it was classified as cardiovascular mortality. Cancer mortality was determined if the cause of death was recorded as cancer. Other mortality was defined as any deaths due to neither cardiovascular disease nor cancer.
Covariates
Participants’ sociodemographic information (age, education level, sex, and race), lifestyle (alcohol drinking and smoking status), and health conditions (history of cancer, diabetes, hypertension, heart disease, lung disease, mental health, psychiatric problems and stroke) were obtained. Anthropometric variables and biological indicators (C-reactive protein and total cholesterol) were also used. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Mental health was measured in terms of depression by using the eight-item version of the Center for Epidemiologic Studies–Depression scale (CES-D) (20). The HRS applied a measure termed “total recall” to reflect cognitive function. Specifically, a total of 20 points are derived from a memory task in which participants were asked to recall a list of unrelated nouns in the immediate trial (10 points) and the delayed trial (10 points) separately (21).
Statistical analysis
Nonnormally distributed data were described by medians and interquartile ranges (IQRs), and Mann-Whitney U tests were applied to test for differences. Normally distributed data were described by means and SDs, and differences were tested using unpaired t tests. Categorical data were described by frequencies and percentages, and the differences were analyzed by χ2 tests. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) with 95% CIs of all-cause and cause-specific mortality.
We applied Cox models with penalized splines using nonparametric smoothers to examine the potential nonlinear or irregular relationship of the hazard functions (22, 23). Flexible spline terms for HbA1c in fully adjusted Cox models with penalized splines were used for the participants with or without diabetes, with the corresponding degrees of freedom as 4 according to the Akaike information criterion.
The Cox models were adjusted for potential confounders that may be associated with both HbA1c and mortality. Three models with different adjustments were used: the first model was adjusted for age, sex, and race (white/Caucasian, black/African American, or other); the second model was further adjusted for other baseline characteristics and lifestyle, including BMI (continuous), education level (less than high school, general educational development, high-school graduate, some college or college, and above), smoking (never, ever, or current), and drinking (no or does not drink, 1 to 4 d/wk, or 5 to 7 d/wk); the third fully adjusted model was additionally adjusted for health conditions and biological indicators, including cognitive function scores (continuous), CES-D (continuous), hypertension (yes/no), heart disease (yes/no), stroke (yes/no), lung disease (yes/no), psychiatric problems (yes/no), C-reactive protein (continuous), and total cholesterol (continuous).
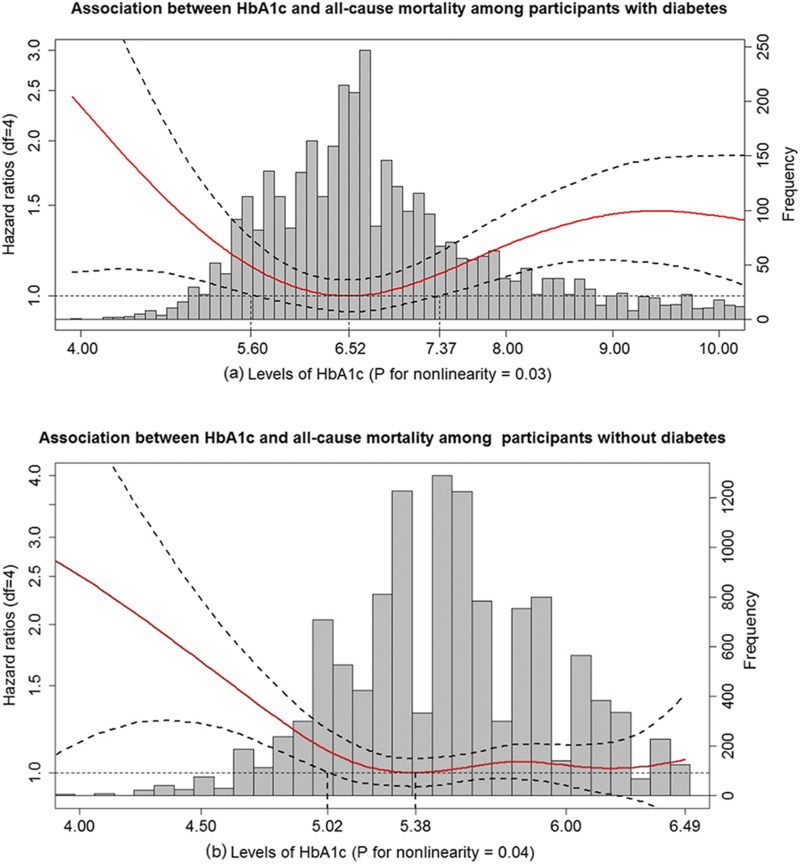
As for participants with diabetes, the increased all-cause mortality risk became statistically significant when HbA1c level was <5.60% or >7.37% as compared with an HbA1c level of 6.52% (nadir point) (as shown in Fig. 1). We subsequently stratified those with diabetes into low (≤5.60%), medium (>5.60 and <7.37%), and high (≥7.37%) groups based on the observed U-shaped curve. Individuals in the low group were further divided into two subgroups [denoted very low (<5.40%) and medium-low (5.40% to 5.60%)] using the median (5.40%) of the low group, whereas the subjects in the high group were also similarly divided into two subgroups [denoted very high (>8.43%) and medium-high (7.37% to 8.43%)] using the median (8.43%) of the high group. As a result, those with diabetes were grouped into five categories: very low (<5.40%), medium-low (5.40% to 5.60%), medium (>5.60 and <7.37%, reference group), medium-high (7.37% to 8.43%), and very high (>8.43%).
Figure 1.
HbA1c in relation to all-cause mortality among participants with and without diabetes. (a) Association between HbA1c and all-cause mortality among participants with diabetes. (b) Association between HbA1c and all-cause mortality among participants without diabetes. HR (solid red lines) and 95% CIs (dashed black lines) from Cox models with penalized splines. Multivariate analyses were adjusted for age, sex, race, BMI, education level, smoking, drinking, cognitive function scores, CES-D scores, hypertension, heart disease, stroke, lung disease, psychiatric problems, C-reactive protein, and total cholesterol. The levels of HbA1c associated with the lowest risk of all-cause mortality were used as references (6.52% for participants with diabetes; 5.38% for participants without diabetes). The bar graph indicates the distribution of HbA1c levels among participants with and without diabetes. df, degrees of freedom.
As for those without diabetes, our data showed that participants with an HbA1c <5.02% had a higher all-cause mortality risk as compared with those with an HbA1c level of 5.38% (nadir point) (as shown in Fig. 1). However, we did not observe a statistically significant elevated risk of all-cause mortality above an HbA1c level of 5.38%. Based on the reverse J-shaped curve, we first stratified those without diabetes into low (≤5.02%) and medium (>5.02). The low group was further divided into two subgroups [denoted very low (<4.88%) and low (4.88% to 5.02%)] based on the median (4.88%) of this category. The medium group was also divided into two subgroups [denoted medium-low (>5.02% and <5.38%) and medium-high (≥5.38%)] based on the nadir point (5.38%). We further stratified medium-high into two groups using the median (5.70%) of this category [denoted medium-high 1 (5.38% to 5.70%) and medium-high 2 (>5.70%)]. Finally, those without diabetes were divided into five groups: very low (<4.88%), low (4.88% to 5.02%), medium-low (>5.02% and <5.38%, reference group), medium-high 1 (5.38% to 5.70%), and medium-high 2 (>5.70%).
Supplemental analyses were also conducted based on prior relevant publications and possible clinical significance. Accordingly, five groups were generated for participants with diabetes: <6.00%, ≥6.00 and <6.50%, ≥6.50 and <7.50% (reference category, given the largest number of participants), ≥7.50 and <8.50%, and ≥8.50%. Separately, participants without diabetes were initially grouped into four categories mainly according to ADA recommendations (24): <5.00% (suspected hypoglycemia), ≥5.00 and <5.7% (normoglycemia; reference category), and 5.70% to 6.49% (prediabetes). The prediabetes category was further divided into two subgroups as for a previous study (12): ≥5.70 and <6.00% and 6.00% to 6.49% (denoted by low and high risk for adverse outcomes). In sensitivity analyses, we repeated the analysis with exclusion of those with a history of stroke, heart disease, and lung disease for all-cause mortality to test the robustness of the results. Individuals who died within the first 2 years of follow-up were also excluded. In addition, given the possibility of hemoglobinopathies, those with low HbA1c levels (<4.50%) were also excluded for sensitivity analyses.
We conducted subgroup analyses of the associations between HbA1c and mortality by age group (<65 years and ≥65 years), sex (men/women), race (white/Caucasian, black/African American, or other), and BMI (<25 and ≥25 kg/m2). We also explored the possible interactions between HbA1c and aforementioned characteristics with respect to all-cause mortality. Statistical significance of the interactions was assessed by adding a product term into the model.
We used STATA, version 13 (StataCorp, College Station, TX) to conduct the primary analyses. Cox models with penalized splines were conducted using R version 3.4.2 (R foundation for Statistical Computing). All P values were two-tailed, and the significance level was set at an α level of 0.05.
Results
Baseline characteristics
The age of the 15,869 participants ranged from 50 to 101 with a median age of 64 years at baseline. Of these participants, 57.7% were women. After a median follow-up of 5.8 years, 2,133 participants died (mortality rate: 13.4%); follow-up for vital status was completed for 14,913 (92%) participants. Distributions of different modes of deaths were shown in an online repository (25). Specifically, 703 deaths and 1430 deaths were identified for participants with and without diabetes, respectively. Those with diabetes had a higher proportion of cardiovascular mortality compared with participants without diabetes (34.28% vs 30.35%; P for χ2 test <0.05).
The median level of HbA1c was 6.70% and 5.49% for participants with and without diabetes, respectively. Baseline characteristics for each of these two populations are shown in Table 1. Compared with participants without diabetes, those with diabetes were more likely to be older, male, black/African American, to have higher BMI, less education, lower cognitive scores, higher levels of C-reactive protein, and lower levels of total cholesterol. They were also more likely to be ever smokers, reporting no drinking, and with a history of stroke, heart disease, lung disease, psychological problems, and hypertension.
Table 1.
Baseline Characteristics of Participants With and Without Diabetes
| Participants With Diabetes | Participants Without Diabetes | P Value | |
|---|---|---|---|
| No. of participants | 3824 | 12,045 | |
| HbA1c, median (IQR), % | 6.70 (6.14–7.58) | 5.49 (5.22–5.82) | <0.001 |
| Age, median (IQR), y | 65 (57–73) | 63 (56–72) | <0.001 |
| Female, n (%) | 2118 (55.39) | 7038 (58.43) | 0.001 |
| Race, n (%) | <0.001 | ||
| White/Caucasian | 2416 (63.40) | 9288 (77.25) | |
| Black/African American | 1000 (26.24) | 1913 (15.91) | |
| Other | 395 (10.36) | 822 (6.84) | |
| BMI, median (IQR), kg/m2 | 30.50 (26.60–35.00) | 27.10 (24.00–30.70) | <0.001 |
| Education level, n (%) | <0.001 | ||
| Less than high school | 1070 (27.98) | 2134 (17.72) | |
| GED | 225 (5.88) | 612 (5.08) | |
| High-school graduate | 1114 (29.13) | 3556 (29.53) | |
| Some college | 825 (21.57) | 2947 (24.47) | |
| College and above | 590 (15.43) | 2793 (23.19) | |
| C-reactive protein, median (IQR) | 2.58 (1.17–5.98) | 1.88 (0.89–4.09) | <0.001 |
| Total cholesterol, median (IQR) | 185.86 (159.39–216.47) | 200.43 (173.38–230.61) | <0.001 |
| Cognitive scores, median (IQR) | 14.00 (11.00–17.00) | 16.00 (13.00–18.00) | <0.001 |
| Hypertension, n (%) | 2867 (75.01) | 5858 (48.67) | <0.001 |
| Lung disease, n (%) | 394 (10.32) | 940 (7.81) | <0.001 |
| Heart disease, n (%) | 1113 (29.13) | 2145 (17.82) | <0.001 |
| Stroke, n (%) | 395 (10.33) | 661 (5.49) | <0.001 |
| Psychiatric problems, n (%) | 742 (19.43) | 1759 (14.62) | <0.001 |
| Smoker, n (%) | <0.001 | ||
| Never | 1618 (42.52) | 5234 (43.70) | |
| Ever | 1648 (43.31) | 4714 (39.36) | |
| Current | 539 (14.17) | 2030 (16.95) | |
| Alcohol drinker, n (%) | <0.001 | ||
| No | 2917 (76.38) | 7190 (59.75) | |
| 1–4 d/wk | 735 (19.25) | 3432 (28.52) | |
| 4–7 d/wk | 167 (4.37) | 1412 (11.73) |
Abbreviation: GED, general educational development.
HbA1c and all-cause mortality among participants with diabetes
As shown in the fully adjusted model, a U-shaped association for HbA1c with all-cause mortality was observed in participants with diabetes (P for nonlinearity = 0.03; Fig. 1). The minimum mortality was associated with HbA1c values of 6.52%. Compared with HbA1c level of 6.52%, HbA1c levels ranging from 5.60% to 7.37% did not significantly predict a higher mortality risk. To compare the ranges of HbA1c with mortality risk, we analyzed Cox proportional hazards models with the medium range of (5.60% to 7.37%) HbA1c as the reference group, after which a greater mortality risk was found in the very high group (>8.43%), with a fully adjusted HR of 1.40 (95% CI 1.09, 1.80). We also found an elevated risk of mortality (HR 1.66; 95% CI 1.19, 2.33) in the very low group (<5.40%) (Table 2). We have observed similar trends using the clinically defined categories (25).
Table 2.
Associations Between HbA1c and All-Cause Mortality According to the Cutoffs Deriving From the U-Shaped and Reverse J–Shaped Curves
| Cutoffs | HR (95% CI) for All-Cause Mortality | ||
|---|---|---|---|
| Participants With Diabetes | |||
| Model 1 | Model 2 | Model 3 | |
| Very low (<5.40%; n = 189) | 1.84 (1.34, 2.52) | 1.80 (1.31, 2.47) | 1.66 (1.19, 2.33) |
| Medium-low (5.40–5.60%; n = 215) | 1.02 (0.71, 1.48) | 1.04 (0.72, 1.50) | 1.07 (0.74, 1.55) |
| Medium (>5.60 and <7.37%; n = 2323) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Medium-high (7.37–8.43%; n = 550) | 1.22 (0.98, 1.52) | 1.19 (0.95, 1.48) | 1.25 (0.99, 1.58) |
| Very high (>8.43%; n = 547) | 1.49 (1.18, 1.88) | 1.44 (1.13, 1.82) | 1.40 (1.09, 1.80) |
| Participants Without Diabetes | |||
|---|---|---|---|
| Very low (<4.88%; n = 736) | 1.74 (1.36, 2.24) | 1.69 (1.31, 2.17) | 1.60 (1.21, 2.07) |
| Low (4.88–5.02%; n = 1002) | 1.27 (1.02, 1.60) | 1.29 (1.03, 1.62) | 1.30 (1.02, 1.65) |
| Medium-low (>5.02 and <5.38%; n = 2,992) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Medium-high 1 (5.38–5.70%; n = 3628) | 1.13 (0.97, 1.32) | 1.16 (1.00, 1.36) | 1.13 (0.96, 1.33) |
| Medium-high 2 (>5.70%; n = 3687) | 1.14 (0.99, 1.32) | 1.12 (0.96, 1.30) | 1.11 (0.95, 1.30) |
Model 1: adjusted for age, sex, and race. Model 2: further adjusted for BMI, education level, smoking, and drinking. Model 3: further adjusted for cognitive function scores, CES-D scores, hypertension, heart disease, stroke, lung disease, psychiatric problems, C-reactive protein, and total cholesterol. Model 3 was considered to be the fully adjusted model. Data in boldface indicate statistical significance at 5% level.
HbA1c and all-cause mortality among participants without diabetes
A reverse J-shaped association for HbA1c with all-cause mortality was observed in participants without diabetes (Fig. 1). The minimum mortality was associated with an HbA1c level of 5.38% (P for nonlinearity = 0.04) (Fig. 1). Specifically, compared with HbA1c level of 5.38%, HbA1c levels ranging from 5.02% to 6.49% were not significantly associated with a higher mortality risk. Compared with the reference range of HbA1c (medium-low, >5.02 and <5.38%), the predefined low group (HR 1.30; 95% CI 1.02, 1.65) and very low group (HR 1.60; 95% CI 1.21, 2.07) were associated with higher mortality risks (Table 2). In addition, our results yielded similar associations of HbA1c levels with all-cause mortality across clinically defined categories (25). Specifically, participants in the suspected hypoglycemia group (<5.00%) had a higher risk of all-cause mortality (HR 1.35; 95% CI 1.12, 1.63), compared with the normoglycemic group (≥5.00 and <5.7%).
HbA1c and cause-specific mortality among participants with diabetes
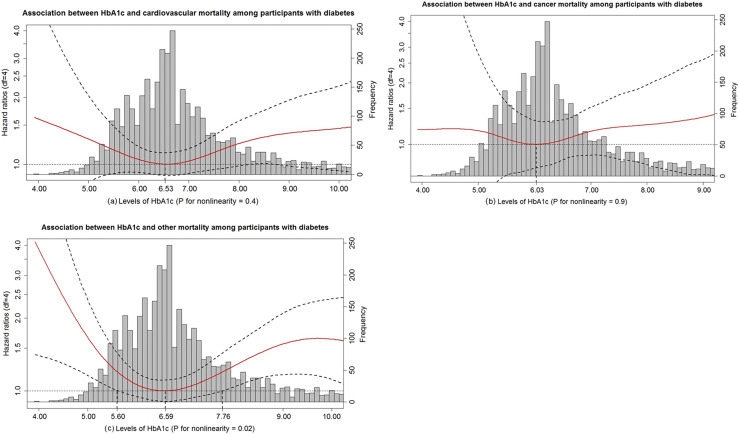
A U-shaped association between HbA1c and other mortality was observed in participants with diabetes, with the lowest risk of death other than cardiovascular and cancer associated with an HbA1c level of 6.59% (P for nonlinearity = 0.02) (Fig. 2). A similar but not statistically significant U-shaped pattern was also observed with respect to cancer and cardiovascular-related mortality (Fig. 3). This lack of statistical significance may be due to the limited sample size as reflected by the wide CIs.
Figure 2.
HbA1c in relation to cardiovascular, cancer, and other mortality among participants with diabetes. (a) Association between HbA1c and cardiovascular mortality among participants with diabetes. (b) Association between HbA1c and cancer mortality among participants with diabetes. (c) Association between HbA1c and other mortality among participants with diabetes. HR (solid red lines) and 95% CIs (dashed black lines) from Cox models with penalized splines. Multivariate analyses were adjusted for age, sex, race, BMI, education level, smoking, drinking, cognitive function scores, CES-D scores, hypertension, heart disease, stroke, lung disease, psychiatric problems, C-reactive protein, and total cholesterol. The levels of HbA1c associated with the lowest risk of cardiovascular, cancer, and other mortality were used as references (6.53%, 6.03%, and 6.59%, respectively). The bar chart indicates the distribution of HbA1c levels among participants with diabetes. df, degrees of freedom.
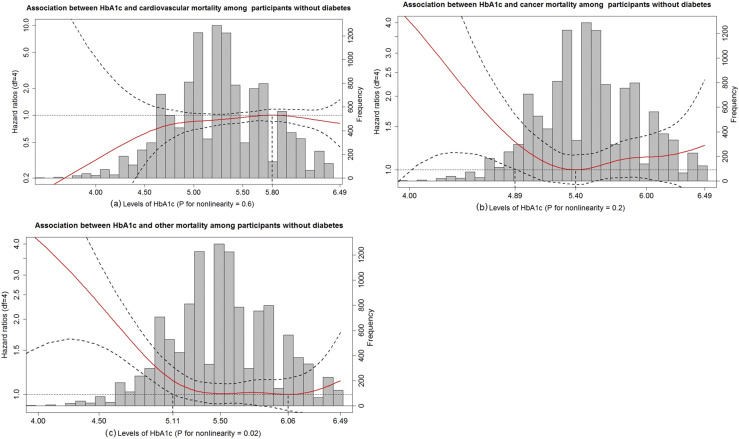
Figure 3.
HbA1c in relation to cardiovascular, cancer, and other mortality among participants without diabetes. (a) Association between HbA1c and cardiovascular mortality among participants without diabetes. (b) Association between HbA1c and cancer mortality among participants without diabetes. (c) Association between HbA1c and other mortality among participants without diabetes. HR (solid red lines) and 95% CIs (dashed black lines) from Cox models with penalized splines. Multivariate analyses were adjusted for age, sex, race, BMI, education level, smoking, drinking, cognitive function scores, CES-D scores, hypertension, heart disease, stroke, lung disease, psychiatric problems, C-reactive protein, and total cholesterol. The level of HbA1c associated with the highest risk of cardiovascular mortality (5.80%) were used as the reference. The levels of HbA1c associated with the lowest risk of cancer and other mortality were used as references (5.40% and 6.06%, respectively). The bar chart indicates the distribution of HbA1c levels among participants without diabetes. df, degrees of freedom.
HbA1c and cause-specific mortality among participants without diabetes
The patterns of associations of HbA1c with cancer and other mortality among those without diabetes appeared to be reverse J-shaped (Fig. 3), with a clear upturn in the risk of mortality when HbA1c levels were <4.89% and <5.11%, respectively. Notably, there was a nonsignificant increased risk of cardiovascular mortality below an HbA1c level of 5.80% among participants without diabetes. Results from penalized splines derived cutoffs and clinically defined categories yielded qualitatively similar results (25).
Subgroup and sensitivity analyses
Overall, we observed similar results in all subgroups (age, sex, race, and BMI) and no evidence of significant interactions (all interaction term P values >0.1). The results were broadly consistent after excluding participants with a history of stroke, heart disease, or lung disease at baseline for all-cause mortality among participants with diabetes (25). Exclusion of participants who were with low HbA1c levels (<4.50%) or who died within the first 2 years after the baseline survey did not materially change the results (25).
Discussion
The present cohort study was conducted in noninstitutionalized American adults without a history of cancer and aged ≥50 years. After adjusting for potential demographic, lifestyle, and biological confounding factors, we observed a U-shaped association between HbA1c and all-cause mortality among participants with diabetes and an asymmetric reverse J-shaped association among those without diabetes. We also observed a U-shaped association between HbA1c and other mortality in participants with diabetes. There were also trends toward U-shaped associations when restricting our data to cardiovascular and cancer mortality among this population, although a lack of statistical significance. The reverse J-shaped relationships in participants without diabetes were generally consistent in cancer and other mortality. However, our results suggested that the low HbA1c mortality seem not to apply to the cardiovascular death among this population without diabetes.
In recent years, safe ranges of HbA1c have been debated considering the fact that not only high but also low levels of HbA1c may predict adverse outcomes. Most observational studies investigating the association between HbA1c and mortality have categorized individuals into clinically relevant groups (13–15, 26–28), although these cutoffs are somewhat arbitrary, as the increase in mortality is not always continuous and does not start abruptly at a certain HbA1c level. Therefore, most of these observational studies have failed to accurately elucidate the precise ranges associated with low mortality risk. In our study, levels of HbA1c associated with the lowest risk of all-cause mortality appeared to be 5.60% to 7.37% and 5.02% to 6.49% for participants with and without diabetes, respectively. These patterns of associations indicate that moderate but not high or low levels of HbA1c are beneficial for overall survival among participants with diabetes. In contrast, moderate rather than low levels of HbA1c may result in more favorable health outcomes for individuals without diabetes. Cox models with penalized splines, using different curves based HbA1c categories, further confirmed the observed relationships.
Our findings are in general agreement with those of a large retrospective study of 27,965 patients with diabetes from the general population (aged ≥50 years) in the United Kingdom; the relationship between HbA1c and mortality was also generally U-shaped, with the best survival at an HbA1c level of ∼7.50% during 22 years of follow-up (29). In this study, the adjusted HR of all-cause mortality in the lowest HbA1c decile was 1.52 (95% CI 1.32, 1.76) and in the highest HbA1c decile was 1.79 (95% CI 1.56, 2.06). Similarly, in the post hoc analyses of the ACCORD trial (30), which included 10,251 participants with diabetes, the lowest risk of mortality in the control arm over 3.4 (median) years of follow-up was associated with HbA1c in the range of 7.00% to 8.00%.
Low HbA1c levels have been associated with increased all-cause mortality among participants without diabetes in several studies (12, 26, 31). For example, our findings are consistent with results from the German National Health Interview and Examination Survey 1998 (GNHIES98), in which low levels of HbA1c were linked to a high risk of all-cause mortality (12). However, this study did not evaluate different causes of death, thereby preventing further interpretation. In the current study, we identified reverse J-shaped associations of HbA1c with cancer-related and other mortality; however, this association was not found for cardiovascular mortality. Overall, these results suggest that cancer and other mortality but not cardiovascular mortality may have contributed to all-cause mortality among participants without diabetes. It is also of note that our findings showed no increased risk of any types of mortality related to prediabetic (5.70% to 6.40%) levels of HbA1c as proposed by the ADA. However, a few studies have reported that prediabetes status may predict a risk of mortality (31, 32). For example, in the Atherosclerosis Risk in Communities study, Aggarwal et al. (33) reported a higher risk of all-cause mortality for prediabetes, relative to a reference group with HbA1c levels between 5.00% and 5.70%. These inconclusive results may be explained in part by the clinical heterogeneity of the included population and by confounding factors associated with both low HbA1c and increased mortality. Another possible explanation may be differences in reference groups.
Mechanisms underlying the mortality risks of high and low HbA1c may be quite different. For instance, individuals with diabetes and high HbA1c may be unaware of their disease status or may adhere poorly to medical advice and self-management, leading to elevated risk of diabetes-related complications that are associated with a poor prognosis (34, 35). As for individuals without diabetes, low HbA1c may be a marker of poor health status such as malnutrition, unfavorable profiles of red blood cell–related factors, iron storage, and liver function or an early stage of chronic disease (31, 33, 36), all of which may be related to unfavorable clinical processes and result in increased risk of morbidity and mortality (37–39).
In contrast, the idea that low HbA1c levels are associated with mortality among participants with diabetes can at first seem counterintuitive. Many clinicians are now concerned about the detrimental effect of intensive therapy for individuals with diabetes, as the ADA and the Endocrine Society suggest that preventing hypoglycemia in individuals with diabetes may be more important than tight glycemic control (40). Our analyses also revealed that the rise in mortality rate was steeper and higher at the low end of the HbA1c distribution than at its high end among those with diabetes, further supporting this view. However, the cause of increased mortality for participants with diabetes with low HbA1c remains unclear. It has been suggested that hypoglycemia, weight gain, or drug interactions caused by different medications may contribute to excess mortality (30, 41).
Our findings revealed a nadir of all-cause mortality at HbA1c level of 6.52%, which is very close to the diagnostic criteria for diabetes proposed by the ADA (>6.50%) (4). Notably, this cutoff point of HbA1c of 6.50% was based on the links between HbA1c and microvascular disease, especially retinopathy (42), and was established under the presumption of a considerable linear increased risk of diabetes-related complications above this level of HbA1c. In fact, given that mortality is a more adverse health event than microvascular disease, a re-evaluation of the current cutoff for diabetes of 6.50% may be warranted taking into account mortality.
The current study has several strengths. This study performed Cox models with penalized splines to examine potential nonlinear associations between HbA1c and mortality. Our study would contribute to the literature by further elucidating the optimal ranges of HbA1c for mortality among people with and without diabetes. Second, the current study was conducted using a nationally, representative sample of US adults; the use of the National Death Index ensured complete information on death. We were also able to report on cause-specific mortality risks. Although the sample sizes were relatively low to provide stable estimates related to some causes of deaths, these data could help power future studies. Further evidence is needed to corroborate our findings in a larger sample.
Some limitations regarding the current study were as follows. First, HbA1c measurements may be affected by various clinical conditions such as anemia, chronic renal failure, and liver disease (12); thus, the current study should be interpreted with caution because the excess mortality observed in the low end of HbA1c distribution may be a reflection of poor health not fully captured even after extensive adjustment for comorbidities. A further limitation was that we used a single measurement of HbA1c at baseline; thus, we are not able to examine the link between HbA1c variability (a marker of glycemic control) and mortality. Moreover, we relied on self-report and HbA1c levels to define diabetes and nondiabetes; however, a substantial population of those with HbA1c levels <6.5% may have diabetes, prediabetes, or with postprandial hyperglycemia if their glycemic levels were evaluated precisely by oral glucose tolerance test. Thus, some misclassifications may have occurred. Second, we have no data on treatment of diabetes, and we cannot exclude gestational diabetes and type 1 diabetes because of the lack of this information. In addition, as this study was observational, we also cannot exclude the presence of residual confounding or reverse causality. Finally, as we only included middle-aged and older US adults, results may not necessarily be generalizable to other populations and age groups.
Conclusions
In summary, our findings suggested a U-shaped and reverse J-shaped association for all-cause mortality among participants with and without diabetes. Optimal ranges for overall survival are predicted by this study to be 5.60% to 7.37% and 5.02% to 6.49% for participants with and without diabetes, respectively.
Acknowledgments
Financial Support: This work was jointly supported by the National Key Research and Development Program of China (2018YFC2000400), the Construction of High-level University in Guangdong (C1034184, C1050008 and C1051007), the National Natural Sciences Foundation of China (81573207), and the National Institutes of Health (NIH/NIA P30-AG028716). The funders played no role in study design or implementation; data collection, management, analysis, and interpretation; manuscript preparation, review, or approval; or the decision to submit the manuscript for publication.
Author Contributions: F.-R.L. and X.-R.Z. designed the research and developed the analytical plan. F.-R.L. performed the statistical analyses and had primary responsibility for writing the manuscript. W.-F.Z. contributed to data cleaning. X.-B.W. and C.M. directed the study. X.G., V.B.K., Y.-B.L., M.-C.Z., G.-C.C., P.-L.C., M.-Y.Z., and X.-M.S. contributed to the acquisition, analysis, or interpretation of the data. F.-R.L., X.-R.Z., W.-F.Z., Z.-H.L., X.G., V.B.K., Y.-B.L., M.-C.Z., G.-C.C., P.-L.C., M.-Y.Z., A.K.A.K., X.-M.S., and C.M. critically reviewed the manuscript for important intellectual content. X.-B.W. and C.M. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ADA
American Diabetes Association
- BMI
body mass index
- CES-D
Center for Epidemiologic Studies–Depression scale
- DBS
dried blood spot
- HbA1c
glycated Hb
- HRS
Health and Retirement Study
- HR
hazard ratio
- IQR
interquartile range
- NHANES
National Health and Nutrition Examination Survey
References
- 1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. [DOI] [PubMed] [Google Scholar]
- 2. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160(8):517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. [DOI] [PubMed] [Google Scholar]
- 8. Joshu CE, Prizment AE, Dluzniewski PJ, Menke A, Folsom AR, Coresh J, Yeh HC, Brancati FL, Platz EA, Selvin E. Glycated hemoglobin and cancer incidence and mortality in the Atherosclerosis in Communities (ARIC) Study, 1990-2006. Int J Cancer. 2012;131(7):1667–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992;41(2):202–208. [DOI] [PubMed] [Google Scholar]
- 10. McLaren LA, Quinn TJ, McKay GA. Diabetes control in older people. BMJ. 2013;346(Apr 24):f2625. [DOI] [PubMed] [Google Scholar]
- 11. Rutter MK. Low HbA1c and mortality: causation and confounding. Diabetologia. 2012;55(9):2307–2311. [DOI] [PubMed] [Google Scholar]
- 12. Paprott R, Schaffrath Rosario A, Busch MA, Du Y, Thiele S, Scheidt-Nave C, Heidemann C. Association between hemoglobin A1c and all-cause mortality: results of the mortality follow-up of the German National Health Interview and Examination Survey 1998. Diabetes Care. 2015;38(2):249–256. [DOI] [PubMed] [Google Scholar]
- 13. Wan EYF, Fung CSC, Wong CKH, Chin WY, Lam CLK. Association of hemoglobin A1c levels with cardiovascular disease and mortality in Chinese patients with diabetes. J Am Coll Cardiol. 2016;67(4):456–458. [DOI] [PubMed] [Google Scholar]
- 14. Sakurai M, Saitoh S, Miura K, Nakagawa H, Ohnishi H, Akasaka H, Kadota A, Kita Y, Hayakawa T, Ohkubo T, Okayama A, Okamura T, Ueshima H; NIPPON DATA90 Research Group. HbA1c and the risks for all-cause and cardiovascular mortality in the general Japanese population: NIPPON DATA90. Diabetes Care. 2013;36(11):3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palta P, Huang ES, Kalyani RR, Golden SH, Yeh HC. Hemoglobin A1c and mortality in older adults with and without diabetes: results from the National Health and Nutrition Examination Surveys (1988-2011). Diabetes Care. 2017;40(4):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schöttker B, Rathmann W, Herder C, Thorand B, Wilsgaard T, Njølstad I, Siganos G, Mathiesen EB, Saum KU, Peasey A, Feskens E, Boffetta P, Trichopoulou A, Kuulasmaa K, Kee F, Brenner H; CHANCES group. HbA1c levels in non-diabetic older adults - No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med. 2016;14(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crimmins E, Faul J, Kim JK, Weir D. Documentation of Biomarkers in the 2010 and 2012 Health and Retirement Study. Ann Arbor, MI: Survey Research Center, University of Michigan; 2015. [Google Scholar]
- 19. Crimmins E, Kim JK, McCreath H, Seeman T. Results from the Health and Retirement Study Biomarker Validation Project. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2013. [Google Scholar]
- 20. Domingue BW, Liu H, Okbay A, Belsky DW. Genetic heterogeneity in depressive symptoms following the death of a spouse: polygenic score analysis of the U.S. Health and Retirement Study. Am J Psychiatry. 2017;174(10):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morin RT, Midlarsky E. Depressive symptoms and cognitive functioning among older adults with cancer. Aging Ment Health. 2018;22(11):1465–1470. [DOI] [PubMed] [Google Scholar]
- 22. Eisen EA, Agalliu I, Thurston SW, Coull BA, Checkoway H. Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med. 2004;61(10):854–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malloy EJ, Spiegelman D, Eisen EA. Comparing measures of model selection for penalized splines in Cox models. Comput Stat Data Anal. 2009;53(7):2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li F-R, Zhang X-R, Zhong W-F, Li Z-H, Gao X, Byers Kraus V, Lv Y-B, Zou M-C, Chen G-C, Chen P-L, Zhang M-Y, Kuol Akech Kur A, Shi X-M, Wu X-B, Mao C Data from: Glycated hemoglobin and all-cause and cause-specific mortality among adults with and without diabetes. Figshare 2019. Accessed 23 February 2019. 10.6084/m9.figshare.7670936. [DOI] [PMC free article] [PubMed]
- 26. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith SA. Etiology. Higher “normal” glycated hemoglobin levels were associated with increased risk for diabetes, CVD, stroke, and mortality in adults. Ann Intern Med. 2010;153(2):JC1–JC13. [DOI] [PubMed] [Google Scholar]
- 28. Bancks MP, Odegaard AO, Pankow JS, Koh WP, Yuan JM, Gross MD, Pereira MA. Glycated hemoglobin and all-cause and cause-specific mortality in Singaporean Chinese without diagnosed diabetes: the Singapore Chinese Health Study. Diabetes Care. 2014;37(12):3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375(9713):481–489. [DOI] [PubMed] [Google Scholar]
- 30. Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER; Action to Control Cardiovascular Risk in Diabetes Investigators. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33(5):983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carson AP, Fox CS, McGuire DK, Levitan EB, Laclaustra M, Mann DM, Muntner P. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(6):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfister R, Sharp SJ, Luben R, Khaw KT, Wareham NJ. No evidence of an increased mortality risk associated with low levels of glycated haemoglobin in a non-diabetic UK population. Diabetologia. 2011;54(8):2025–2032. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal V, Schneider AL, Selvin E. Low hemoglobin A(1c) in nondiabetic adults: an elevated risk state? Diabetes Care. 2012;35(10):2055–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fabbian F, De Giorgi A, Monesi M, Pala M, Tiseo R, Misurati E, Parisi C, Volpi R, Graziani R, Mikhailidis DP, Manfredini R. All-cause mortality and estimated renal function in type 2 diabetes mellitus outpatients: Is there a relationship with the equation used? Diab Vasc Dis Res. 2015;12(1):46–52. [DOI] [PubMed] [Google Scholar]
- 35. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Christman AL, Lazo M, Clark JM, Selvin E. Low glycated hemoglobin and liver disease in the U.S. population. Diabetes Care. 2011;34(12):2548–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011;50(4):635–641. [DOI] [PubMed] [Google Scholar]
- 38. Abril-Ulloa V, Flores-Mateo G, Solà-Alberich R, Manuel-y-Keenoy B, Arija V. Ferritin levels and risk of metabolic syndrome: meta-analysis of observational studies. BMC Public Health. 2014;14(1):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lioudaki E, Ganotakis ES, Mikhailidis DP. Liver enzymes: potential cardiovascular risk markers? Curr Pharm Des. 2011;17(33):3632–3643. [DOI] [PubMed] [Google Scholar]
- 40. Slomski A. Avoiding hypoglycemia at all costs is crucial for some with diabetes. JAMA. 2013;309(24):2536–2537. [DOI] [PubMed] [Google Scholar]
- 41. Winterstein AG, Heckbert SR, Schambelan M. Intensive glucose lowering and cardiovascular outcomes. N Engl J Med. 2011;364(23):2263–2264, author reply 2264. [DOI] [PubMed] [Google Scholar]
- 42. Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med. 2012;157(3):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]