Abstract
Background:
Quadriceps dysfunction after anterior cruciate ligament (ACL) reconstruction is common and may affect return to sport due to resulting muscle atrophy and muscle weakness.
Purpose:
To systematically review the available literature regarding the impact of perioperative and postoperative interventions on quadriceps atrophy and loss of strength after ACL reconstruction.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
A systematic review was performed in accordance with the 2009 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using PubMed, CINAHL, Cochrane Central, and Embase. The quality of evidence was evaluated using the Modified Coleman Methodology Score to determine consensus scores. Eligible level 1 or level 2 studies included interventions of perioperative nerve block, intraoperative tourniquet use, postoperative nutritional supplementation, and postoperative blood flow restriction training. Additionally, the included studies quantified postoperative quadriceps measurements such as thigh circumference, quadriceps cross-sectional area (CSA), isokinetic quadriceps strength, and/or quadriceps electromyographic (EMG) testing.
Results:
In total, 15 studies met stated inclusion and exclusion criteria with the following intervention types: perioperative nerve block (n = 4), intraoperative tourniquet use (n = 5), postoperative nutritional supplementation (n = 3), and postoperative blood flow restriction (n = 3). Intraoperative tourniquet use resulted in decreased thigh circumference and detrimental EMG changes in quadriceps function in 3 of the 5 included studies. Perioperative femoral nerve blocks were associated with transient decreases in postoperative quadriceps strength, persisting up to 6 weeks after surgery, in 2 of the 4 studies. Postoperative blood flow restriction training augmented quadriceps size and function after ACL reconstruction in 2 of 3 studies. Postoperative nutritional supplementation was associated with increased quadriceps volume and strength in 1 of the 3 studies examined.
Conclusion:
The peri- and postoperative factors reviewed here may influence quadriceps atrophy and strength after ACL reconstruction. Our results tentatively indicated that blood flow restriction training may be beneficial to the quadriceps after ACL reconstruction and that intraoperative tourniquet use and nerve block administration may be detrimental; however, the strongest finding was that all of these interventions would benefit from further level 1 and 2 evidence studies, including multicenter, randomized controlled trials with extended follow-up, to definitively determine their impact on return to activity.
Keywords: ACL, anterior cruciate ligament reconstruction, tourniquet, nerve block, blood flow restriction, quadriceps
Approximately 100,000 anterior cruciate ligament (ACL) reconstructions are performed annually in the United States.7 The quadriceps muscles (rectus femoris, vastus lateralis, vastus medialis, and vastus intermedius) frequently atrophy after ACL reconstruction,12,20,28 and this negatively affects postoperative knee function and return to full activity. Atrophy and associated strength deficits in the postoperative knee may persist for 6 months after surgical intervention.32 Quadriceps atrophy and commensurate decreases in quadriceps strength may influence the ability to safely return to activities and sports.32
Muscle atrophy occurs rapidly after an ACL injury and is compounded by further atrophy during the early period of recovery after ACL reconstruction.35 Literature has demonstrated that leg immobilization results in rapid and significant loss of skeletal muscle mass34,35 in as few as 5 days of immobilization in healthy patients. The largest extent of postoperative atrophy is postulated to occur during the first 2 weeks of leg immobilization.34,35 The early period of ACL reconstruction recovery is also a critical period for graft protection.16 If the knee is not adequately immobilized in the immediate postoperative phase, tendon-to-bone healing may be impaired.16 Activity and weightbearing restrictions, especially in the first 6 weeks after the ACL reconstruction procedure, are intended to protect the new graft but may contribute to postoperative quadriceps atrophy.16
This systematic review assessed perioperative and postoperative factors influencing quadriceps atrophy and strength after ACL reconstruction. Atrophy was examined as an outcome variable because of its causal relationship with strength; additionally, researchers could safely measure atrophy during very early postoperative phases, when maximal strength testing is not feasible. We hypothesized that (1) perioperative nerve block administration and intraoperative tourniquet use would be associated with increased quadriceps atrophy and/or decreased strength postoperatively and (2) postoperative blood flow restriction training and postoperative supplement use would attenuate quadriceps atrophy and/or loss of strength postoperatively.
Methods
Literature Search
Search strategies were generated by a health sciences librarian and conducted according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines using PubMed, CINAHL (Cumulative Index of Nursing and Allied Health Literature), Cochrane Central, and Embase databases. Studies were queried for inclusion if they assessed a perioperative or postoperative intervention after ACL reconstruction. The Medical Subject Headings terms to identify ACL reconstruction included “Anterior Cruciate Ligament” OR “Anterior Cruciate Ligament Injuries” OR “Anterior Cruciate Ligament Reconstruction” AND “Quadriceps Muscle” OR “Thigh.” Additional keywords for intervention subtype were further included to assess for perioperative nerve block administration, perioperative tourniquet use, postoperative nutrition supplementation, and postoperative blood flow restriction training (Table 1).
Table 1.
Inclusion and Exclusion Criteria for Studies Assessing Postoperative Quadriceps Atrophy and Strength After Anterior Cruciate Ligament (ACL) Reconstruction
| Inclusion | Exclusion |
|---|---|
| Human studies English language Primary ACL reconstruction Revision ACL reconstruction Level 1 and 2 evidence Nutrition or blood flow restriction or supplement use or tourniquet intervention Quadriceps volume quantified after ACL reconstruction |
Non-English language Animal study Level 3 or 4 evidence Abstract Editorial Commentary Book chapter or review Systematic review Meta-analysis |
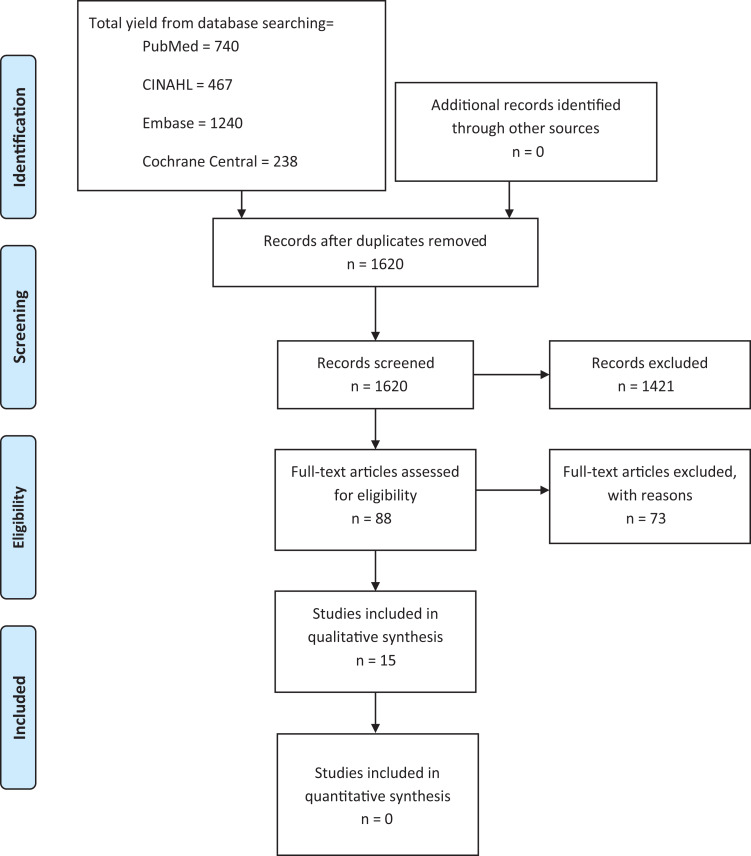
Our initial literature search yielded 1620 studies; they were evaluated by 2 orthopaedic research assistants (J.E.B., E.A.P.) under the supervision of 2 orthopaedic surgeons (K.R.D., R.W.W.), according to our inclusion and exclusion criteria (Table 1). In total, 88 full papers were reviewed, and 15 studies met inclusion criteria and were assessed qualitatively (Figure 1). The target interventions of the included studies assessed perioperative nerve block administration (n = 4),1,24,27,30 intraoperative tourniquet use (n = 5),2,3,11,17,25 postoperative supplement use (n = 3),6,19,33 and postoperative blood flow restriction training (n = 3).15,26,31 Specifically, 8 of the 15 studies assessed measures of quadriceps atrophy including cross-sectional area (CSA) (n = 3) and thigh circumference (n = 5) as an outcome variable.3,6,11,15,19,25,26,31 Results of primary and revision ACL reconstruction procedures were combined in these qualitative analyses, with primary versus revision status indicated if available.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of included and excluded studies examining postoperative quadriceps atrophy and strength following anterior cruciate ligament reconstruction.
Outcome Variables
The primary outcome variable of the present study was quadriceps atrophy after ACL reconstruction. For studies that used thigh circumference as a measure of gross atrophy, quadriceps atrophy was defined as a significant difference in mean thigh circumference between the treatment and control groups, often comparing both pre- and postoperative measurements.3,6,11,19,25 Studies that used magnetic resonance imaging (MRI) to measure quadriceps CSA defined atrophy in 1 of 3 ways: as a significant postoperative difference in the mean CSA ratio of injured to uninjured limbs between treatment and control groups; as a significant difference in the mean preoperative to postoperative size change (in centimeters) between treatment and control groups; or as a significant difference in the mean preoperative to postoperative size measurements (in percentage points) between treatment and control groups.15,26,31
Secondarily, the current study aimed to assess quadriceps strength and electromyographic (EMG) changes after ACL reconstruction. Strength outcomes after ACL reconstruction were quantified through use of isometric or isokinetic quadriceps strength measurements such as knee extensor torque and flexor torque.1,24,27,30 Additional muscle groups, including the hamstrings, hip flexors, abductors, and adductors, were assessed by Tyler et al.33 EMG changes after perioperative tourniquet use were further assessed in the postoperative period by 2 studies.3,17
Study Quality
The mean Modified Coleman Methodology Score (MCMS) (Appendix Tables A1 and A2) was assessed by 2 independent reviewers (J.E.B., E.A.P.). For the 15 included articles, the median MCMS was 64 (range, 43-71). The MCMS was similar among studies that assessed the impact on postoperative quadriceps volume and health after perioperative nerve block administration (62.8 ± 6.4),1,24,27,30 intraoperative tourniquet use (59.8 ± 11.0),2,3,11,17,25 postoperative supplement use (62.3 ± 3.8),6,19,33 and postoperative blood flow restriction training (58.3 ± 9.6)15,26,31 (between-groups P value = .9). One study2 received a score lower than 55 owing to small sample size and a lack of follow-up.
Results
Results were stratified by target intervention type (Table 2). In total, 7 studies included patients who underwent primary ACL reconstruction; 8 studies did not specify primary versus revision ACL procedures. Sample size (median, 161 participants; range, 84-247 participants) and participant age (median, 27.7 years; range, 26.3-28.5 years) were recorded. Mean body mass index (BMI) was indicated if assessed in the initial study (median, 71.5 kg/m2; range, 67.2-82.0 kg/m2), with average weights provided for studies that did not calculate BMI (median, 25.9 kg; range, 21.9-33.3 kg). Follow-up was at a median of 10 weeks (range, 1.9 days to 10.3 months) and was notably shorter for studies assessing the use of tourniquets and blood flow restriction training for ACL reconstruction recovery (Table 3). This limited the generalizability of the results for patients for whom return to sport is a priority, as that typically occurs around the 6- to 9-month period for uncomplicated primary reconstructions.
Table 2.
Demographics of Study Participants Undergoing ACL Reconstruction With Perioperative and Postoperative Interventionsa
| Intervention and Study Author (Year) | No. of Patients (Male, Female) | No. of Participants, Experimental, Control | Age, y, Median (Range) or Mean ± SD | BMI, kg/m2 or [Weight, kg]; Experimental, Control | Total No. of Patients (% Male) | Total No. of Participants, Experimental, Control | Overall Age, y, Mean ± SD | Overall BMI, kg/m2 or [Weight, kg]; Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Perioperative femoral nerve blockb | 247 (53.8) | 120, 127 | 26.3 ± 4.2 | 82.0 ± 1.0 [25.3 ± 2.2] |
||||
| Abdallah1 (2016) Magnussen24 (2017) Okoroha27 (2018) Runner30 (2018) |
100 (64, 36) 30 (11, 19) 44 (27, 16) 73 (31, 42) |
48, 52 14, 16 23, 21 35, 38 |
32.4 (28.9-35.9) 22.35 ± 9.25 25.9 ± 11.05 24.65 ± 0.6 |
81.3, 79.4 [27.6, 25.1] 82.7, 74.4 [25.0, 23.3] |
||||
| Intraoperative tourniquet use | 196 (76.0) | 102, 94 | 28.0 ± 4.4 | 72.2 ± 2.2 | ||||
| Kokki17 (2000) Appell2 (1993) Nicholas25 (2001) Faggal11 (2015) Arciero3 (1996) |
31 (22, 9) 14 (14, 0) 48 (29, 19) 63 (54, 9) 40 (30, 10) |
13, 18 14, 0 25, 23 30, 33 20, 20 |
31 (16-48) (25-38) 32.5 25.2 24 (18-37.5) |
72, 75 Not indicated 72.3, 76.7 Not indicated Not indicated |
||||
| Postoperative supplement use | 125 (68.0) | 62, 63 | 28.5 ± 4.6 | 70.7 [26.5 ± 2.3] |
||||
| Tyler33 (2004) Laboute19 (2013) Barker6 (2009) |
60 (33, 27) 45 (32, 13) 20 (20, 0) |
30, 30 22, 23 10, 10 |
30.4 24 (18-45) 33 |
70.7 [25, 25.6] [28.9, 29.7] |
||||
| Postoperative blood flow restriction training | 84 (55.6) | 42, 42 | 27.3 ± 3.3 | 67.2 ± 8.0 | ||||
| Takarada31 (2000) Iverson15 (2016) Ohta26 (2003) |
16 (8, 8) 24 (14, 10) 44 (25, 19) |
8, 8 12, 12 22, 22 |
22.7 27.4 (18-42) 29 (18-52) |
58.8, 62.2 76.9, 77.6 65, 63 |
||||
| Median (range) values for pooled interventions |
160.5 (84-247) | 82 (42-120) | 27.7 (26.3-28.5) | 71.5 (67.2-82.0) [25.9 (21.9-33.3)] |
||||
aACL, anterior cruciate ligament; BMI, body mass index.
bAll nerve block studies compared patients who had femoral nerve blocks versus patients with no nerve blocks or adductor canal blocks; femoral nerve blocks are considered the experimental group for this analysis.
Table 3.
Significant Changes in Postoperative Quadriceps Measures After ACL Reconstruction Stratified by Intervention Type; Pooled Quadriceps Measures per Intervention Typea
| Total Patients (Experimental, Control) | Follow-up, Median (Range) | Pooled Quadriceps Outcomes per Interventionb | Significant Postoperative Quadriceps Changes, Studies/Patients (% Experimental Group) |
|---|---|---|---|
| Perioperative femoral nerve blockc (4 studies) | |||
| 247 (120, 127) | 5.6 mo (0.2-10.3 mo) | Strength (–) | 2 studies/62 patients (51.7%) |
| Intraoperative tourniquet use (5 studies) | |||
| 196 (102, 94) | 6.8 wk (1 d to 6 mo) | EMG values (–) | 1 study/31 patients (60.8%) |
| Muscle structure (–) | 1 study/14 patients (100%) | ||
| Leg circumference (–) | 2 studies/55 patients (73.3%) | ||
| Postoperative supplement use (3 studies) | |||
| 125 (62, 63) | 3.3 mo (3 wk to 6 mo) | Leg circumference (+) | 1 study/22 patients (68.8%) |
| Postoperative blood flow restriction training (3 studies) | |||
| 84 (42, 42) | 6.8 wk (2-16 wk) | Extensor cross-sectional area (+) | 3 studies/30 patients (71.4%) |
| Strength (+) | 1 study/22 patients (100%) | ||
aACL, anterior cruciate ligament; EMG, electromyography.
bA minus symbol indicates that the outcome was associated with decreases in quadriceps measurements postoperatively. A plus symbol indicates that the outcome was associated with increases in quadriceps measurements postoperatively.
cAll nerve block studies compared patients who had femoral nerve blocks versus patients with no nerve blocks or adductor canal blocks; femoral nerve block denoted experimental group.
In total, 8 of the 15 studies assessed the primary endpoint of the study, quadriceps volume.3,6,11,15,19,25,26,31 Quadriceps atrophy was most commonly assessed by use of quadriceps CSA or thigh circumference (Table 3). Quadriceps atrophy is typically defined in the literature as a significant difference in preoperative versus postoperative measures of mean thigh circumference or mean quadriceps CSA.15,26,31 The 3 studies15,26,31 assessing CSA in this review were in the blood flow restriction training group, whereas 5 studies3,6,11,19,25 of the other interventions assessed thigh circumference. The remaining 7 studies assessed the secondary outcome variable, quadriceps strength1,24,27,30,33 and EMG or muscle molecular structure changes postoperatively2,17 (Table 3).
Perioperative Nerve Block Administration
Table 4 displays the results of postoperative quadriceps volume and strength stratified by intervention type. In total, 4 studies used perioperative femoral nerve blocks (FNBs)—some comparing femoral and adductor canal blocks with no persistent significant differences—and examined quadriceps strength changes postoperatively.1,24,27,30Abdallah et al1 found that FNB caused significantly less quadriceps weakness 45 minutes after the block compared with adductor canal blocks (ACB), with no patient-reported neurological symptoms at 1 week after surgery for either block type. Magnussen et al24 found that patients who received FNB had lower patient-reported outcome scores and quadriceps strength up to 6 weeks postoperatively compared with patients who did not receive blocks; however, this difference resolved by the 6-month follow-up point. The remaining 2 studies found no significant quadriceps strength differences between patients receiving FNB versus ACB as well as patients receiving FNB versus liposomal bupivacaine, respectively.27,30 Interpretation of nerve block study results was limited by the wide range of study follow-up periods (Table 3), particularly if attempting to generalize the results to patients returning to sport at the 6- to 9-month mark.
Table 4.
Postoperative Quadriceps Volume and Strength After ACLR Stratified by Intervention Typea
| Study Author (Year) | Primary or Revision ACLR | Target Intervention | ACLR Graft Type | Outcome Measure | Follow-up Period After ACLR | Difference in Results |
|---|---|---|---|---|---|---|
| Perioperative femoral nerve block | ||||||
| Abdallah1 (2016) | Primary | Perioperative FNB vs ACB | BTB, n = 29 STG, n = 71 |
24-hour analgesic consumption, VAS scores, MVIC | 1 day | FNB group had significantly lower MVIC and a significantly larger percentage reduction at 45 minutes after block; no patients reported persistent weakness at 1 week. |
| Magnussen24 (2017) | Not indicated | Perioperative FNB vs no nerve block | STG all patients | KOOS, isokinetic quadriceps strength testing | 6 months | KOOS and quadriceps strength (limb symmetry) were lower for the FNB group at 6 weeks only. |
| Okoroha27 (2018) | Primary | Perioperative FNB vs liposomal bupivacaine | BTB, n = 31 STG, n = 12 |
Isokinetic quadriceps strength, functional testing | 9 months | No significant differences were seen between the block and control groups; 13% of the FNB patients had persistent motor/sensory complications at follow-up. |
| Runner30 (2018) | Primary | Perioperative FNB vs ACB | QTB, n = 58 BTB, n = 3 TAA, n = 10 QTA, n = 2 |
Analgesic consumption, time to straight-leg raise, isokinetic strength testing | 6 months | No significant differences were seen between the FNB and ACB groups for any measures. |
| Intraoperative tourniquet use | ||||||
| Kokki17 (2000) | Not indicated | 250 mm Hg vs 350 mm Hg tourniquet during ACLR | BTB all patients | Peroneal nerve MCV and SCV; EMG of vastus medialis | 3 weeks | No significant difference was observed between groups; both groups had significant, detrimental EMG/NCS changes postoperatively. |
| Appell2 (1993) | Not indicated | 400 mm Hg tourniquet during ACLR | STG all patients | Alterations in muscle structure of vastus lateralis biopsies | 1 day | Identifiable muscle damage was present at 15 minutes after tourniquet inflation and continued to worsen during surgery. |
| Nicholas25 (2001) | Primary | Tourniquet (300 mm Hg) vs no tourniquet during ACLR | BTB all patients | Thigh and calf circumference, isometric plantarflexion and dorsiflexion strength | 6 months | Significantly greater decrease in thigh girth occurred in the tourniquet group. |
| Faggal11 (2015) | Primary | Tourniquet (350 mm Hg) vs no tourniquet during ACLR | STG all patients | Pain; hemarthrosis; drainage; isokinetic hamstring and quadriceps strength; thigh and calf circumference | 6 months | Experimental group had significantly greater drainage, hemarthrosis, early pain, and smaller calf and thigh girth at 2 weeks. |
| Arciero3 (1996) | Primary | Tourniquet (269 mm Hg) vs no tourniquet for ACLR | BTB all patients | Thigh and calf girth, EMG, creatine phosphate levels, arthrometry, single-leg hop, Lysholm knee score, quadriceps and hamstring isokinetic testing | 1 year | No significant differences were noted. |
| Postoperative supplement use | ||||||
| Tyler33 (2004) | Not indicated | Creatine supplements after ACLR | BTB all patients | Isokinetic strength of quadriceps, hamstring, hip flexor, abductors, and adductors; isokinetic power of quadriceps, hamstring | 6 months | No significant differences were observed. |
| Laboute19 (2013) | Not indicated | Leucine supplements during 2- to 3-week period 200 days after ACLR | STG, n = 39 BTB, n = 5 |
Thigh perimeter, flexor and extensor isokinetic strength, single-leg testing, body fat percentage | 2-3 weeks (all patients 6-7 months after ACLR) | Experimental group had significantly larger thigh circumference 10 cm proximal to the patella. |
| Barker6 (2009) | Not indicated | Vitamin E and C supplements preoperatively until after ACLR | STG, n = 19 BTB, n = 1 |
Antioxidant levels, thigh circumference, muscle fiber circumference, muscle cytokine levels, single-leg power, single-leg isometric force | 3 months | Experimental group had no significant difference in outcome measures; patients with higher baseline vitamin C levels had significant positive correlation with muscle strength recovery. |
| Postoperative blood flow restriction training | ||||||
| Takarada31 (2000) | Not indicated | Blood flow restriction after ACLR | Not indicated | Knee extensor and flexor CSA on MRI | 2 weeks | Significantly less extensor CSA loss was observed in experimental group at POD 14. |
| Iverson15 (2016) | Primary | Blood flow restriction during exercises after ACLR | STG all patients | Quadriceps CSA on MRI | 2 weeks | No significant difference was observed at POD 14. |
| Ohta26 (2003) | Not indicated | Blood flow restriction during exercises after ACLR | STG all patients | Knee extensor and flexor torque; extensor, flexor, and adductor CSA on MRI; muscle fiber diameter | 4 months | Experimental group had significant increase in strength, larger extensor CSA. |
aACB, adductor canal block; ACLR, anterior cruciate ligament reconstruction; BTB, bone-tendon-bone graft; CSA, cross-sectional area; EMG, electromyography; FNB, femoral nerve block; KOOS, Knee injury and Osteoarthritis Outcome Score; MCV, motor conduction velocity; MRI, magnetic resonance imaging; MVIC, maximal voluntary isometric quadriceps contraction; NCS, nerve conduction study; POD, postoperative day; QTA, quadriceps tendon allograft; QTB, quadriceps tendon autograft; SCV, sensory conduction velocity; STG, semitendinosus and gracilis graft (hamstring graft); TAA, tibialis anterior allograft; VAS, visual analog scale.
Intraoperative Tourniquet Use
There were 3 studies that included intraoperative tourniquet use and measured thigh circumference changes postoperatively.3,11,25 Of these studies, 2 articles demonstrated that tourniquet use resulted in significantly smaller circumference of the postoperative thigh, with Kokki et al17 following patients for 3 weeks and Nicholas et al25 following patients for 6 months. Arciero et al3 found no difference in postoperative quadriceps volume after intraoperative tourniquet use, following patients for 1 year. There were 2 studies that assessed quadriceps EMG changes postoperatively after perioperative tourniquet use.3,17 Kokki et al found significant negative EMG changes after tourniquet use, whereas Arciero et al did not find a significant difference in EMG readings between the control and experimental group. Appell et al2 examined muscle structure changes in tourniquet patients via biopsy after 15, 30, 60, and 90 minutes of inflation, demonstrating significant detrimental changes in muscle cell and fiber composition. Finally, 3 studies using intraoperative tourniquets examined quadriceps strength changes postoperatively and found no significant differences in postoperative quadriceps strength after use of a tourniquet.3,11,25
Postoperative Supplement Use
We identified 2 studies that examined the influence of supplement use on thigh circumference changes after ACL reconstruction.6,19 Laboute et al19 found significantly larger thigh circumference in the operative leg of patients taking a postoperative leucine supplement; the investigators chose leucine because of in vivo evidence that it positively affects muscle fiber synthesis. Patients in that study were administered 1320 mg of leucine divided into 3 daily doses, taken during an average of 2.7 weeks, approximately 6 months after ACL reconstruction. Thigh circumference was measured at the beginning and the end of the 2- to 3-week training period.19 Barker et al6 found no significant difference in thigh circumference between patients taking preoperative and postoperative vitamin E and C supplements versus patients without a supplementation regimen. The experimental participants took 200 international units of vitamin E and 500 mg of vitamin C supplements twice daily, beginning 2 weeks before surgery and ending 3 months after surgery. Thigh circumference was measured preoperatively and at the first postoperative visit. Via a similar approach, Tyler et al33 determined no quadriceps strength benefits from postoperative creatine supplementation. The literature suggests that leucine supplementation may provide a mitigative effect in the setting of postoperative quadriceps atrophy after ACL reconstruction.6 However, given the limited number of studies meeting the stated inclusion and exclusion criteria regarding amino acid supplementation and vitamin supplementation, further studies are warranted to provide definitive evidence regarding their influence on quadriceps atrophy after ACL reconstruction.6,19
Postoperative Blood Flow Restriction Training
We identified 3 studies that used MRI to assess the effect of postoperative blood flow restriction training on quadriceps CSA changes postoperatively.15,26,31 Of these, Takarada et al31 and Ohta et al26 found significantly larger quadriceps CSA in patients undergoing blood flow restriction training sessions, whereas Iverson et al15 found no difference in the quadriceps CSA between patients undergoing blood flow restriction training compared with the control group. Ohta et al26 assessed postoperative blood flow restriction training by examining quadriceps strength changes postoperatively and found a significant increase in quadriceps strength after blood flow restriction training. The blood flow restriction training studies followed patients for an average of 6.8 weeks after surgery (range, 2-16 weeks).15,26,31
Discussion
This systematic review assessed the influence of perioperative and postoperative interventions on quadriceps atrophy and strength for patients undergoing ACL reconstruction. Postsurgical atrophy is a “second hit” for patients, as the quadriceps begins to rapidly atrophy after the initial ACL injury. Results from the current systematic review suggest that each of the primary interventions investigated may influence postoperative quadriceps atrophy and strength. However, overarching findings of this study suggest that large randomized controlled trials (RCTs) with adequate follow-up periods are necessary to further determine the risk-benefit ratio of each intervention, with longer follow-up periods particularly relevant for patients who wish to return to sport. ACL reconstruction is a frequently performed procedure with no cross-institutional standardized perioperative or postoperative set of interventions. This lends itself to the consideration that a large, multicenter RCT may be both feasible and effective at determining which interventions to avoid and which to use to positively affect quadriceps strength and attenuate postoperative atrophy.
Two of the studies in the current review, Abdallah et al1 and Magnussen et al,24 found significant strength decreases when comparing FNB versus ACB, and FNB versus no nerve block, respectively. In both studies this weakness was transient, resolving within 1 to 6 weeks after surgery. In a double-blinded RCT, Lynch et al22 demonstrated equivalent results of ACB versus FNB for patients undergoing ACL reconstruction procedures, specifically regarding measures of pain control and thigh circumference at 7 days after surgery. Notably, that study did not assess the long-term effects of the blocks, an issue mirrored in our study results given the large range of follow-up time for nerve block studies. Bailey et al,5 in a prospective, randomized trial of FNB versus ACB, demonstrated decreased quadriceps deficits in the postoperative period when FNB was used, with persistent range of motion deficits detected at 6 months after surgery. Nerve blocks convey benefits for patient and surgeon, providing a nonopioid method of postoperative pain control. Nerve blocks also can be tailored to the site of autograft harvest when applicable, such as use of the saphenous block for bone–patellar tendon–bone grafts to target the infrapatellar branch of the saphenous nerve extending to the site of the patellar graft harvest.10,13 However, further research is warranted to determine whether the pain control benefit is offset by potential loss of quadriceps strength.
Intraoperative tourniquet use may have a detrimental impact on quadriceps function after ACL reconstruction, but the effects require further investigation. We reviewed 5 studies, 4 of which reported negative postoperative effects.2,11,17,25 Yet only 1 of these studies reported a negative postoperative impact (smaller thigh circumference) at a follow-up time when return to sport would be considered (6 months).25 Level 1 studies have supported decreased quadriceps strength at 3 months after surgery in patients who undergo total knee arthroplasty in association with tourniquet use.8 Immediate postoperative changes in CSA of the quadriceps and transient decreases in EMG measures (indicating decreased nerve function) were observed in the studies assessed. In one of the included studies, a double-blinded RCT by Nicholas et al,25 patients undergoing ACL reconstruction with a median tourniquet time of 85 minutes (range, 51-114 minutes) had additional deficits in thigh girth at 3 weeks after ACL reconstruction, with both the tourniquet and the nontourniquet groups demonstrating equivalent decreases in calf girth, dorsiflexion strength, and plantarflexion strength at this time point. At 6 months after surgery, there were no observable differences between groups. Kuo et al18 further supported these assertions in a systematic review and meta-analysis of tourniquet use after ACL reconstruction, suggesting equivalent strength outcomes postoperatively with the use of a tourniquet and observing clinically nonsignificant differences in thigh girth. The authors reported heterogeneous results pertaining to decreases in thigh girth across studies, with no differences in isokinetic quadriceps strength at 6 months after surgery between the tourniquet and nontourniquet groups. However, in the Kuo et al18 analysis, only the Arciero et al3 and Nicholas et al25 clinical trials provided detailed enough isokinetic strength data for meta-analysis, thus limiting the external generalizability of the results.
The present review did not assess the influence of tourniquet time on postoperative quadriceps function owing to incomplete information provided in the included studies. Literature is scarce regarding the impact of tourniquet time on postoperative quadriceps measures, especially for athletes wishing to return to sport. Patient-specific factors, including their preoperative fitness and conditioning level, may influence the effects of tourniquet use perioperatively. Additionally, surgeon preference may affect use patterns, as tourniquets provide better visualization and shorter surgical times during ACL reconstruction. These factors warrant further characterization of the risks versus benefits of tourniquet use.
In the studies included in this review, supplements were predominantly administered postoperatively, although 1 study began supplement administration 2 weeks before the ACL reconstruction procedure.6 The majority of these studies were conducted in the United States, where preoperative dietary regimens and patients’ nutritional status may differ from populations in other locales. Additionally, alternative supplement administration time points may influence postoperative quadriceps size and function, requiring further investigation. Interestingly, Tyler et al33 did not demonstrate significant changes in quadriceps size or function in the 12-week period after ACL reconstruction with creatine administration. These conclusions were supported by similar findings in an RCT assessing the influence of creatine supplementation during leg immobilization in young, healthy males.4 Additionally, Barker et al6 found no positive impact of vitamin administration on quadriceps health. Laboute et al19 found that leucine supplementation improved 1 quadriceps parameter (thigh circumference) when administered 6 months after surgery. This correlates with findings from an RCT by English et al,9 which showed that leucine partially attenuates loss of muscle mass and function during brief periods of bed rest. Further level 1 and 2 evidence would be necessary to support a finding of leucine-mediated attenuation of postoperative quadriceps atrophy.
Postoperative blood flow restriction training after ACL reconstruction is used to increase anabolic muscle stress without increasing knee joint stress.15 In this review, 2 of the 3 studies that examined postoperative blood flow restriction training reported increased quadriceps CSA,26,31 and 1 study26 reported increased quadriceps strength. It is worth noting that the follow-up periods in the studies analyzed in the present review (typically postoperative day 14) did not provide adequate follow-up to assess return to sport. Lipker et al21 also noted that short-term results of postoperative blood flow restriction training did not indicate significant changes, although longer term follow-up at 15 weeks did demonstrate measurable differences. Blood flow restriction training combined with low-resistance muscular training may promote substantive quadriceps benefits.21 Results from the current review suggest that postoperative blood flow restriction training may be associated with positive increases in quadriceps size and function after ACL reconstruction. Notably, heterogeneous populations and a paucity of prospective clinical studies limit the conclusions that may be drawn regarding the applicability of blood flow restriction training in patients undergoing ACL reconstruction. Blood flow restriction training does pose risks to participants,29 including cardiovascular threats during exercise, increased blood pressure, venous thromboembolism, increased reactive oxygen species, and the breakdown of striated muscle associated with metabolic demands and potential ischemia-reperfusion injury. Therefore, additional investigation assessing optimal use and risks of blood flow restriction after ACL reconstruction is needed.14
Conclusion
This review tentatively supported the hypotheses that intraoperative tourniquet use and nerve block administration may be detrimental to the quadriceps after ACL reconstruction and that blood flow restriction training may be beneficial. The postoperative supplement studies were too heterogeneous for early conclusions. This review clearly highlights the need for more level 1 and 2 evidence studies to investigate these interventions, potentially across multiple orthopaedic centers similar to the Multicenter Orthopaedic Outcomes Network (MOON), which has been able to longitudinally follow patients undergoing ACL reconstruction to analyze factors such as graft choice.23
Acknowledgment
The authors thank Jennifer DeBerg at the University of Iowa Hardin Library for the Health Sciences for her assistance in developing and executing the literature searches.
Appendix
Table A1.
Coleman Methodology Score
| Part A |
| 1. Study size (10) |
| 2. Mean duration of follow-up (5) |
| 3. Number of surgical procedures (10) |
| 4. Type of study (15) |
| 5. Diagnostic certainty (5) |
| 6. Description of surgical procedure (5) |
| 7. Description of postoperative rehabilitation (10) |
| Part B |
| 1. Outcome measures (10) |
| 2. Outcome assessment (15) |
| 3. Selection process (15) |
| Total possible = 100 points |
Table A2.
Consensus Modified Coleman Methodology Score (MCMS) of Evidence Quality by Study
| Blood Flow Restriction | Supplement | Tourniquet | Nerve Block | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Takarada31 (2000) | Iverson15 (2016) | Ohta26 (2003) | Tyler33 (2004) | Laboute19 (2013) | Barker6 (2009) | Kokki17 (2000) | Appell2 (1993) | Nicholas25 (2001) | Faggal11 (2015) | Arciero3 (1996) | Abdallah1 (2016) | Magnussen24 (2017) | Okoroha27 (2018) | Runner30 (2018) | |
| MCMS part A | |||||||||||||||
| Study size—No. of patients | 0 | 0 | 4 | 4 | 4 | 0 | 0 | 0 | 4 | 4 | 4 | 7 | 0 | 4 | 7 |
| Mean follow-up | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Percentage of patients with follow-up | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 0 | 5 | 5 | 5 | 5 | 5 | 5 | 0 |
| No. of interventions per group | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Type of study | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 10 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Diagnostic certainty | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Description of surgical technique | 3 | 3 | 3 | 5 | 3 | 3 | 3 | 3 | 5 | 3 | 5 | 3 | 3 | 3 | 3 |
| Description of postoperative rehabilitation | 0 | 5 | 5 | 3 | 5 | 5 | 0 | 0 | 0 | 5 | 5 | 0 | 0 | 5 | 0 |
| MCMS Part B | |||||||||||||||
| 1. Outcome criteria | |||||||||||||||
| Outcome measures clearly defined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Timing of outcome assessment clear | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Use of outcome criteria with reported good reliability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Use of outcome with good sensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2. Procedure for reporting outcomes | |||||||||||||||
| Patients recruited | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Independent investigator | 0 | 2 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Written assessment | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Patient-centered data collected | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 3. Description of patient selection process | |||||||||||||||
| Selection criteria reported and unbiased | 0 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Recruitment rate reported and ≥80% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eligible patients not included in the study satisfactorily accounted for | 0 | 0 | 5 | 5 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 5 | 5 | 5 | 0 |
| Total MCMS a | 48 | 60 | 67 | 65 | 64 | 58 | 55 | 43 | 66 | 64 | 71 | 67 | 60 | 69 | 55 |
aMedian score, 64; range, 43-71.
Footnotes
Final revision submitted February 15, 2020; accepted March 3, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: K.R.D. has received educational support from Arthrex and Smith & Nephew, grant support from DJO, and hospitality payments from Stryker. R.W.W. has received educational support from Arthrex and Smith & Nephew, hospitality payments from Medical Device Business Systems and Smith & Nephew, and nonfinancial support from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abdallah FW, Whelan DB, Chan VW, et al. Adductor canal block provides noninferior analgesia and superior quadriceps strength compared with femoral nerve block in anterior cruciate ligament reconstruction. Anesthesiology. 2016;124(5):1053–1064. [DOI] [PubMed] [Google Scholar]
- 2. Appell HJ, Gloser S, Duarte JAR, Zellner A, Soares JMC. Skeletal muscle damage during tourniquet-induced ischaemia. Eur J Appl Physiol Occup Physiol. 1993;67(4):342–347. [DOI] [PubMed] [Google Scholar]
- 3. Arciero R, Scoville C, Hayda R, Snyder R. The effect of tourniquet use in anterior cruciate ligament reconstruction: a prospective, randomized study. Am J Sports Med. 1996;24(6):758-764. [DOI] [PubMed] [Google Scholar]
- 4. Backx EMP, Hangelbroek R, Snijders T, et al. Creatine loading does not preserve muscle mass or strength during leg immobilization in healthy, young males: a randomized controlled trial. Sports Med. 2017;47(8):1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bailey L, Griffin J, Elliott M, et al. Adductor canal nerve versus femoral nerve blockade for pain control and quadriceps function following anterior cruciate ligament reconstruction with patellar tendon autograft: a prospective randomized trial. Arthroscopy. 2019;35(3):921–929. [DOI] [PubMed] [Google Scholar]
- 6. Barker T, Leonard S, Hansen J, et al. Vitamin E and C supplementation does not ameliorate muscle dysfunction after anterior cruciate ligament surgery. Free Radic Biol Med. 2009;47(11):1611-1618. [DOI] [PubMed] [Google Scholar]
- 7. Csintalan RP, Inacio MCS, Funahashi TT. Incidence rate of anterior cruciate ligament reconstructions. Perm J. 2008;12(3):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dennis DA, Kittelson AJ, Yang CC, et al. Does tourniquet use in TKA affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res. 2016;474(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. English KL, Mettler JA, Ellison JB, et al. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr. 2016;103(2):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espelund M, Fomsgaard JS, Haraszuk J, Mathiesen O, Dahl JB. Analgesic efficacy of ultrasound-guided adductor canal blockade after arthroscopic anterior cruciate ligament reconstruction: a randomised controlled trial. Eur J Anaesthesiol. 2013;30(7):422–428. [DOI] [PubMed] [Google Scholar]
- 11. Faggal M, Ayad K, Awad AW. Rehabilitation outcomes after torniquet versus no torniquet use during anterior cruciate ligament reconstruction. Physiotherapy. 2015;101(suppl 1):e370-e371. [Google Scholar]
- 12. Grapar Zargi T, Drobnic M, Vauhnik R, Koder J, Kacin A. Factors predicting quadriceps femoris muscle atrophy during the first 12 weeks following anterior cruciate ligament reconstruction. Knee. 2017;24(2):319–328. [DOI] [PubMed] [Google Scholar]
- 13. Höher J, Kersten D, Bouillon B, Neugebauer E, Tiling T. Local and intra-articular infiltration of bupivacaine before surgery: effect on postoperative pain after anterior cruciate ligament reconstruction. Arthroscopy. 1997;13(2):210–217. [DOI] [PubMed] [Google Scholar]
- 14. Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. 2017;51(13):1003. [DOI] [PubMed] [Google Scholar]
- 15. Iversen E, Rostad V, Larmo A. Intermittent blood flow restriction does not reduce atrophy following anterior cruciate ligament reconstruction. J Sport Health Sci. 2016;5(1):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kokki H, Vaatainen U, Miettinen H, et al. Tourniquet-induced ENMG changes in arthroscopic anterior cruciate ligament reconstruction: a comparison of low and high-pressure tourniquet systems. Ann Chir Gynaecol. 2000;89(4):313–317. [PubMed] [Google Scholar]
- 18. Kuo L-T, Yu P-A, Chen C-L, Hsu W-H, Chi C-C. Tourniquet use in arthroscopic anterior cruciate ligament reconstruction: a systematic review and meta-analysis of randomised controlled trials. BMC Musculoskelet Disord. 2017;18(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laboute E, France J, Trouve P, et al. Rehabilitation and leucine supplementation as possible contributors to an athlete’s muscle strength in the reathletization phase following anterior cruciate ligament surgery. Ann Phys Rehabil Med. 2013;56(2):102-112. [DOI] [PubMed] [Google Scholar]
- 20. Lepley AS, Grooms DR, Burland JP, et al. Quadriceps muscle function following anterior cruciate ligament reconstruction: systemic differences in neural and morphological characteristics. Exp Brain Res. 2019;237(5):1267–1278. [DOI] [PubMed] [Google Scholar]
- 21. Lipker LA, Persinger CR, Michalko BS, Durall CJ. Blood flow restriction therapy versus standard care for reducing quadriceps atrophy after anterior cruciate ligament reconstruction. J Sport Rehabil. 2019;28(8):897–901. [DOI] [PubMed] [Google Scholar]
- 22. Lynch JR, Okoroha KR, Lizzio V, et al. Adductor canal block versus femoral nerve block for pain control after anterior cruciate ligament reconstruction: a prospective randomized trial. Am J Sports Med. 2018;47(2):355–363. [DOI] [PubMed] [Google Scholar]
- 23. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magnussen R, Pottkotter K, Stasi S, et al. Femoral nerve block after anterior cruciate ligament reconstruction. J Knee Surg. 2017;30(4):323-328. [DOI] [PubMed] [Google Scholar]
- 25. Nicholas S, Tyler T, McHugh M, Gleim G. The effect on leg strength of tourniquet use during anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. 2001;17(6):603-607. [DOI] [PubMed] [Google Scholar]
- 26. Ohta H, Kurosawa H, Ikeda H, et al. Low-load resistance muscular training with moderate restriction of blood flow after anterior cruciate ligament reconstruction. Acta Orthop Scand. 2003;74(1):62–68. [DOI] [PubMed] [Google Scholar]
- 27. Okoroha KR, Khalil L, Jung EK, et al. Single-shot femoral nerve block does not cause long-term strength and functional deficits following anterior cruciate ligament reconstruction. Arthroscopy. 2018;34(1):205–212. [DOI] [PubMed] [Google Scholar]
- 28. Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424. [DOI] [PubMed] [Google Scholar]
- 29. Patterson SD, Hughes L, Warmington S, et al. Blood flow restriction exercise position stand: considerations of methodology, application, and safety. Front Physiol. 2019;10:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Runner R, Boden S, Godfrey W, et al. Quadriceps strength deficits after a femoral nerve block versus adductor canal block for anterior cruciate ligament reconstruction: a prospective, single-blinded, randomized trial. Orthop J Sports Med. 2018;6(9):2325967118797990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc. 2000;32(12):2035–2039. [DOI] [PubMed] [Google Scholar]
- 32. Thomas AC, Wojtys EM, Brandon C, Palmieri-Smith RM. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J Sci Med Sport. 2016;19(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tyler T, Nicholas S, Hershman E, et al. The effect of creatine supplementation on strength recovery after anterior cruciate ligament (ACL) reconstruction: a randomized, placebo-controlled, double-blind trial. Am J Sports Med. 2004;32(2):383-388. [DOI] [PubMed] [Google Scholar]
- 34. Wall BT, Dirks ML, Snijders T, et al. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol (Oxf). 2014;210(3):600–611. [DOI] [PubMed] [Google Scholar]
- 35. Wall BT, Morton JP, van Loon LJ. Strategies to maintain skeletal muscle mass in the injured athlete: nutritional considerations and exercise mimetics. Eur J Sport Sci. 2015;15(1):53–62. [DOI] [PubMed] [Google Scholar]