Abstract
Assessing thromboembolic risk is crucial for proper management of patients with atrial fibrillation. Left atrial volume is a promising predictor of cardiac thrombosis. To determine whether left atrial volume can predict left atrial appendage thrombus in patients with atrial fibrillation, we conducted a prospective study of 73 patients. Left atrial and ventricular volumes were evaluated by cardiac computed tomography with retrospective electrocardiographic gating and then indexed to body surface area. Left atrial appendage thrombus was confirmed or excluded by cardiac computed tomography with delayed enhancement.
Seven patients (9.6%) had left atrial appendage thrombus; 66 (90.4%) did not. Those with thrombus had a significantly higher mean left atrial end-systolic volume index (139 ± 55 vs 101 ± 35 mL/m2; P =0.0097) and mean left atrial end-diastolic volume index (122 ± 45 vs 84 ± 34 mL/m2; P =0.0077). On multivariate logistic regression analysis, left atrial end-systolic volume index (per 10 mL/m2 increase) was significantly associated with left atrial appendage thrombus (odds ratio [OR]=1.24; 95% CI, 1.03–1.50; P =0.02); so too was the left atrial end-diastolic volume index (per 10 mL/m2 increase) (OR=1.29; 95% CI, 1.05–1.60; P =0.02).
These findings suggest that increased left atrial volume increases the risk of left atrial appendage thrombus. Therefore, patients with atrial fibrillation and an enlarged left atrium should be considered for cardiac computed tomography with delayed enhancement to confirm whether thrombus is present.
Keywords: Atrial appendage/diagnostic imaging, heart atria/diagnostic imaging, multidetector computed tomography/methods, risk assessment, thrombosis/diagnosis
Atrial fibrillation (AF) increases the risk of life-threatening and severely disabling thromboembolic events.1 Therefore, assessing and monitoring the risk of thromboembolism in patients with AF is crucial. Stroke risk is assessed by calculating a CHA2DS2-VASc score based on an established set of clinical parameters, a score of 0 corresponding to no risk and a score of 9 to highest annual risk.1 The left atrial appendage (LAA) is usually evaluated initially for the presence of thrombus by conventional, noninvasive transthoracic echocardiography2 or by more accurate, but invasive, transesophageal echocardiography (TEE).3
Technologic improvements in cardiac computed tomography (CT) have made it a viable, noninvasive alternative to TEE for assessing cardiac structures in patients with AF.3 However, cardiac CT often reveals areas of low-contrast enhancement that may or may not represent LAA thrombus. This may cause problems for patients with AF if additional TEE studies, with their associated risks, are needed to rule out thrombus.3 Cardiac CT with delayed enhancement (CTDE) may overcome this shortcoming.3
Left atrial (LA) enlargement is thought to be a structural precursor of AF.4 Therefore, accurate imaging to detect this abnormality could help to identify patients at risk for stroke. The association between LAA thrombus and cardiac CT–derived measurements such as LA and left ventricular (LV) volume5 or LV ejection fraction (LVEF)6 has not been fully investigated. Therefore, we used cardiac CTDE to determine whether LA volume can be used to predict the presence of LAA thrombus in patients with AF.
Patients and Methods
We prospectively enrolled 73 consecutive patients with a history of AF who were treated with warfarin for suspected coronary artery disease at our institution from December 2014 through July 2016 (Table I). Eight patients also had a history of pacemaker implantation; 7, coronary artery revascularization; 4, valvular replacement; and 1, untreated mitral valve stenosis. All 73 patients underwent cardiac CTDE by means of multidetector computed tomography (MDCT) with retrospective electrocardiographic gating to analyze cardiac volumes and to confirm or exclude LAA thrombus. This study was approved by an institutional ethics committee and conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent before being enrolled in the study.
Table I.
Baseline Characteristics of the 73 Patients
| Variable | Overall | LAA Thrombus (n=7) | No LAA Thrombus (n=66) | P Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (yr) | 60 ± 11 | 65 ± 18 | 59 ± 10 | 0.22 |
| Age ≥75 yr | 8 (11) | 3 (43) | 5 (8) | 0.03 |
| Male | 46 (63) | 4 (57) | 42 (64) | 0.74 |
| Body mass index (kg/m2) | 33 ± 9 | 30 ± 9 | 33 ± 9 | 0.39 |
| Hypertension | 68 (93) | 6 (86) | 62 (94) | 0.41 |
| Dyslipidemia | 55 (75) | 5 (71) | 50 (76) | 0.8 |
| Diabetes mellitus | 31 (42) | 2 (29) | 29 (44) | 0.43 |
| Current smoker | 5 (7) | 0 | 5 (8) | 0.45 |
| Family history of CAD | 27 (37) | 2 (29) | 25 (38) | 0.63 |
| PT-INR | 2.5 ± 0.7 | 2.3 ± 0.5 | 2.5 ± 0.7 | 0.42 |
| AF duration (yr) | 3.0 (1.0–6.0) | 4.0 (1.5–6.0) | 3.0 (1.0–6.0) | 0.91 |
| History of HF or LVEF <0.40 | 14 (19) | 0 | 14 (21) | 0.18 |
| History of stroke | 9 (12) | 1 (14) | 8 (12) | 0.87 |
| CHA2DS2-VASc score | 2.8 ± 1.6 | 2.7 ± 2.0 | 2.8 ± 1.5 | 0.95 |
| Persistent AF | 40 (55) | 5 (71) | 35 (53) | 0.65 |
| Drugs | ||||
| Antihypertensive | 65 (89) | 6 (86) | 59 (89) | 0.77 |
| Antidyslipidemic | 47 (64) | 5 (71) | 42 (64) | 0.68 |
| Antidiabetic | 26 (36) | 2 (29) | 24 (36) | 0.68 |
AF = atrial fibrillation; CAD = coronary artery disease; HF = heart failure; LAA = left atrial appendage; LVEF = left ventricular ejection fraction; PT-INR = international normalized ratio of prothrombin time
Data are expressed as mean ± SD, as number and percentage, or as median and interquartile range. P <0.05 (2-sided) was considered statistically significant for differences between groups.
Atrial Fibrillation Classification and CHA2DS2-VASc Score
Each patient's AF at baseline was classified as persistent or not persistent. Persistent AF was defined as recurrent AF lasting ≥7 days.7 The CHA2DS2-VASc score at baseline was calculated for each patient according to the following point system.8 Two points were assigned for a history of stroke or transient ischemic attack or age ≥75 years. One point was assigned for age 65 to 74 years; history of hypertension, diabetes, heart failure, and vascular disease (myocardial infarction, complex aortic plaque, or peripheral artery disease); and female sex.
Multidetector Computed Tomography
Multidetector computed tomography was performed with a 64-slice LightSpeed VCT scanner (GE Healthcare).9 The scanning parameters were as follows: collimation, 64 × 0.625 mm; table pitch adapted to heart rate, 0.18 to 0.24; rotation time, 350 ms; tube current-time product, 350 to 780 mAs; and tube voltage, 100 to 120 kV.
Before scanning, any patient with a persistently high heart rate of >60 beats/min was given β-blockers to achieve a target resting heart rate of <60 beats/min. Immediately before scanning, all patients were given sublingual nitroglycerin or nitroglycerin spray (0.4 mg). A test MDCT image acquisition was performed to determine how long to delay scanning after contrast enhancement. The image was obtained at the level of the ascending aorta after administration of 10 to 15 mL of contrast medium (Omnipaque™ 350; GE Healthcare) followed by 20 mL of normal saline. The delay (40 sec) was calculated by adding 5 sec to the time to peak enhancement in the ascending aorta.
Retrospective electrocardiographic (ECG)-gated cardiac CT with dose modulation was performed after intravenous administration of a 4-phase contrast bolus. First, 20 to 25 mL of contrast medium was administered at a rate of 5.5 to 5.7 mL/s, followed by 60 mL of contrast medium at 5.4 mL/s, 35 to 40 mL of contrast medium diluted 50% at 5.5 mL/s, and 35 to 40 mL of saline as a chaser bolus at 5 mL/s. Scanning for LAA thrombus was delayed 40 sec, without additional contrast, after the initial cardiac CT scan. The effective radiation dose in each phase of the cardiac CT study was estimated from the dose-length product.
Volumetric Analysis
An experienced cardiologist (KO) blinded to the results of cardiac CTDE performed all volumetric analyses. Multidetector computed tomographic images were reconstructed at 5% intervals of the cardiac cycle. In each phase, the LA and LV volumes were delineated according to the atrial and ventricular contours and calculated by using the Simpson method for numerical integration on an Advantage Workstation 4.6 (GE Healthcare).10 The calculated volumes were then manually corrected for minor errors. The workstation software automatically calculated a time-volume curve. The maximal and minimal peaks on this curve were used to determine the end-diastolic and end-systolic volumes for both the LA and the LV. The workstation software also automatically calculated LV stroke volume and LVEF. The pulmonary vein confluences and LAA were excluded from volumetric analysis. Left atrial and LV volumes were divided by body surface area (calculated according to the DuBois formula11) to obtain the LA end-systolic volume index (LAESVI), LA end-diastolic volume index (LAEDVI), LV end-systolic volume index (LVESVI), and LV end-diastolic volume index (LVEDVI).
The reproducibility of LA volume readings was assessed by 2 experienced cardiologists (KO and RN) blinded to the original readings. One cardiologist (KO) read each of 73 cardiac CT studies twice to determine intraobserver variability. The other cardiologist (RN) read 45 of the cardiac CT studies read by KO to determine interobserver variability.
Left Atrial Appendage Thrombus Assessment
Two experienced cardiologists (MB and KO) visually inspected the cardiac CTDE images for filling defects suggestive of LAA thombus.9 A cardiac CTDE study was read as negative if it showed no filling defects in the LAA; as positive, if it showed a definite filling defect suggestive of thrombus.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median and interquartile range. Differences between groups were evaluated using the Student t test (for continuous variables) and the Pearson χ2 test or Fisher exact test (for categorical variables). Univariate analysis and stepwise multivariate logistic regression analysis were used to calculate the odds ratio (OR) for the relationship between the presence of LAA thrombus and either LAESVI (model 1) or LAEDVI (model 2). Each regression analysis was adjusted for persistent AF, CHA2DS2-VASc score, international normalized ratio of prothrombin time (PT-INR) at the time of cardiac CT, LVEF, and AF duration (years since diagnosis). Intra- and interobserver variability was evaluated by Pearson correlation coefficient analysis. P values <0.05 (2-sided) were considered statistically significant. All statistical analyses were done with SPSS version 23.0 for Windows (SPSS, an IBM company).
Results
Of the 73 patients enrolled, 7 (9.6%) had LAA thrombus, and 66 (90.4%) did not (Table I). Overall, the mean CHA2DS2-VASc score was 2.8 ± 1.6. Overall, 40 patients (55%) experienced AF during CT image acquisition; all 40 had persistent AF at baseline. Thirty-six patients achieved a heart rate <60 beats/min while receiving a β-blocker. The mean heart rate during image acquisition was 64 ± 12 beats/min, and it did not differ significantly between patients with and without LAA thrombus (61 ± 10 vs 64 ± 13 beats/min; P =0.56). Significantly more patients with LAA thrombus were ≥75 years of age (43% vs 8%; P =0.03). The mean PT-INR was 2.5 ± 0.7, and it was similar between those with and without LAA thrombus (2.3 ± 0.5 vs 2.5 ± 0.7; P =0.42). The mean effective radiation dose was 8.1 ± 2.8 mSv, and the dose-length product was 579 ± 203 mGy·cm.
Figure 1 shows representative cardiac CTDE images of an LAA thrombus in a 75-year-old woman. Patients with LAA thrombus had a significantly higher mean LAESVI (139 ± 55 vs 101 ± 35 mL/m2; P =0.0097) and higher mean LAEDVI (122 ± 45 vs 84 ± 34 mL/m2; P =0.0077) than those without thrombus (Table II). The 2 groups had similar LVESVI, LVEDVI, and LVEF measurements (Fig. 2). The intraobserver (r =0.93; P <0.001) and interobserver (r =0.94; P <0.001) correlation coefficients for LV volume measurements indicated little variability in reader assessments. After adjustment for persistent AF, CHA2DS2-VASc score, PT-INR, LVEF, and AF duration, multivariate logistic regression analysis revealed that LAESVI (per 10 mL/m2 increase) was significantly associated with the presence of LAA thrombus (OR=1.24; 95% CI, 1.03–1.50; P =0.02) (Table III, model 1). When LAEDVI was substituted for LAESVI, LAEDVI (per 10 mL/m2 increase) was also significantly associated with the presence of LAA thrombus (OR=1.29; 95% CI, 1.05–1.60; P =0.02) (Table III, model 2).
Fig. 1.
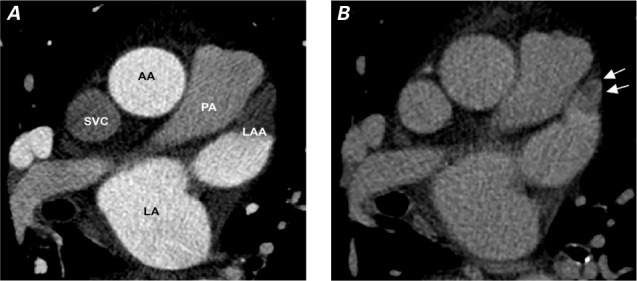
Representative cardiac computed tomograms from a 75-year-old woman with hypertension (CHA2DS2-VASc score, 4) show A) a single axial slice of the cardiac computed tomogram acquired initially without enhancement, showing evidence of a left atrial appendage (LAA) thrombus, and B) a definitive image of the LAA thrombus (arrows) acquired after a 40-sec delay for contrast enhancement. Volumetric analysis of the initial image revealed a large left atrial end-systolic volume of 159.3 mL (LAESVI, 97.9 mL/m2) and a large left atrial end-diastolic volume of 146.1 mL (LAEDVI, 89.8 mL/m2).
AA = ascending aorta; LA = left atrium; LAA = left atrial appendage; LAESVI = left atrial end-systolic volume index; LAEDVI = left atrial end-diastolic volume index; PA = pulmonary artery; SVC = superior vena cava
Table II.
Comparison of Volume Measurements Between Patients With and Without Left Atrial Appendage Thrombus
| Variable | Overall (n=73) | LAA Thrombus (n=7) | No LAA Thrombus (n=66) | P Value |
|---|---|---|---|---|
| LAESVI (mL/m2) | 104 ± 38 (95–113) | 139 ± 55 (88–191) | 101 ± 35 (92–109) | 0.0097 |
| LAEDVI (mL/m2) | 88 ± 37 (79–96) | 122 ± 45 (81–164) | 84 ± 34 (75–92) | 0.0077 |
| LVESVI (mL/m2) | 47 ± 21 (42–52) | 48 ± 18 (32–65) | 46 ± 22 (41–52) | 0.81 |
| LVEDVI (mL/m2) | 92 ± 25 (87–98) | 100 ± 29 (74–127) | 91 ± 24 (85–97) | 0.38 |
| LVEF | 0.51 ± 0.12 (0.48–0.54) | 0.52 ± 0.05 (0.48–0.57) | 0.51 ± 0.13 (0.48–0.54) | 0.78 |
LAA = left atrial appendage; LAEDVI = left atrial end-diastolic volume index; LAESVI = left atrial end-systolic volume index; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index
Data are presented as mean ± SD and 95% CI. P <0.05 (2-sided) was considered statistically significant for differences between groups.
Fig. 2.
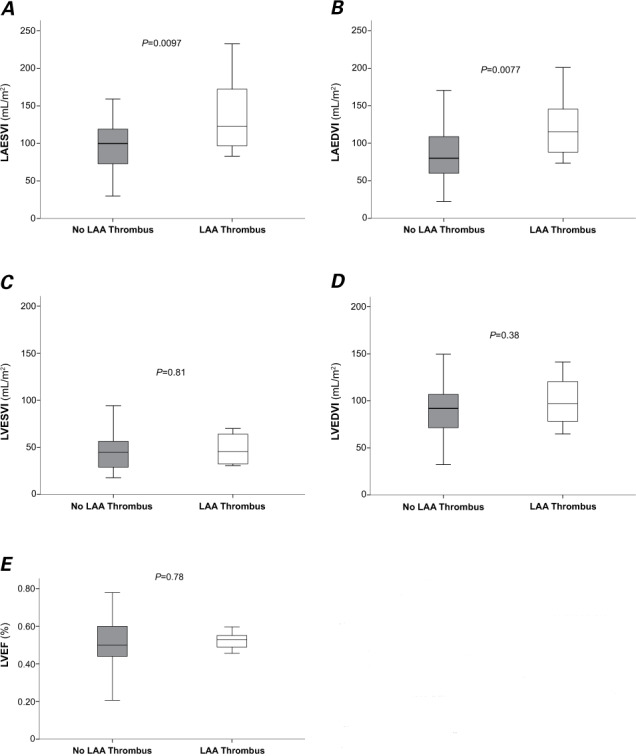
Box and whisker plots compare the estimated values for cardiac volumetric parameters derived from cardiac computed tomograms in patients with versus without left atrial appendage (LAA) thrombus: A) left atrial end-systolic volume index (LAESVI); B) left atrial end-diastolic volume index (LAEDVI); C) left ventricular end-systolic volume index (LVESVI); D) left ventricular end-diastolic volume index (LVEDVI); and E) left ventricular ejection fraction (LVEF). The line inside a box marks the median (50th percentile). The bottom and top of a box mark the interval between the 25th and 75th percentiles. Whiskers indicate the interval between the minimum and maximum values, excluding the 4 outlier values for each. P <0.05 (2-sided) was considered statistically significant.
Table III.
Univariate and Multivariate Analysis of Predictors of Left Atrial Appendage Thrombus
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Model 1* | Model 2** | |||||
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Persistent AF | 2.14 (0.39–11.86) | 0.38 | 1.47 (0.24–9.17) | 0.68 | 1.34 (0.20–8.91) | 0.76 |
| CHA2DS2-VASc score | 0.98 (0.59–1.62) | 0.94 | 0.97 (0.55–1.74) | 0.93 | 0.96 (0.54–1.71) | 0.88 |
| PT-INR | 0.61 (0.18–2.05) | 0.42 | 0.58 (0.16–2.02) | 0.39 | 0.56 (0.17–1.86) | 0.34 |
| LVEF | 1.01 (0.95–1.08) | 0.78 | 1.02 (0.94–1.10) | 0.65 | 1.02 (0.94–1.11) | 0.57 |
| AF duration (yr) | 0.99 (0.86–1.14) | 0.91 | 1.02 (0.88–1.18) | 0.80 | 1.02 (0.89–1.18) | 0.78 |
| LAESVI (per 10 mL/m2) | 1.24 (1.03–1.49) | 0.02 | 1.24 (1.03–1.50) | 0.02 | — | — |
| LAEDVI (per 10 mL/m2) | 1.29 (1.05–1.60) | 0.02 | — | — | 1.29 (1.05–1.60) | 0.02 |
AF = atrial fibrillation; LAA = left atrial appendage; LAEDVI = left atrial end-diastolic volume index; LAESVI = left atrial end-systolic volume index; LVEF = left ventricular ejection fraction; OR = odds ratio; PT-INR = international normalized ratio of prothrombin time
* Model 1 was adjusted for persistent AF, CHA2DS2-VASc score, PT-INR, LVEF, AF duration (yr), and LAESVI (per 10 mL/m2).
** Model 2 was adjusted for persistent AF, CHA2DS2-VASc score, PT-INR, LVEF, AF duration (yr), and LAEDVI (per 10 mL/m2).
P <0.05 (2-sided) was considered statistically significant.
Discussion
In this study, we found that increased LAESVI and LAEDVI measured by cardiac CT were independently associated with the presence of LAA thrombus detected by cardiac CTDE in patients with a history of AF and receiving anticoagulation therapy. This was true even after adjusting for CHA2DS2-VASc score, persistent AF, PT-INR, LVEF, and AF duration. In contrast, LV volume and LVEF were not associated with the presence of LAA thrombus. Although we contend that delayed-enhancement image acquisition should be included in all cardiac CT studies done in individuals with AF or a history of AF, the opportunity to do so is sometimes missed in clinical settings. A finding of LA enlargement on the initial cardiac CT could be a predictor of LAA thrombus, and additional delayed-enhancement imaging may confirm it.
The clinical usefulness of cardiac CT in assessing and managing AF is established. It is used routinely to evaluate the location, size, and number of pulmonary veins before ablation for AF.12 It can also be used with high diagnostic accuracy to assess coronary artery anatomy and disease13 and to exclude LAA thrombus3 in patients with AF. However, delayed-enhancement imaging could dramatically increase diagnostic accuracy in detecting LAA thrombus even further beyond that of first-pass cardiac CT.14,15 Because some individuals with AF will still develop LAA thrombus despite anticoagulation therapy at recommended dosages,16–18 delayed-enhancement imaging should ideally be included whenever a patient with a history of AF undergoes cardiac CT.
Several studies that included echocardiography have revealed strong relationships between LA dilatation and LAA thrombus.19,20 Furthermore, LA enlargement is a potential predictor of stroke. In a study by Osranek and colleagues in patients with lone AF,21 LAESVI >32 mL/m2 was an independent risk factor for adverse events, and all cerebral infarctions occurred in patients with an LAESVI >32 mL/m2. Similarly, in a recent population-based prospective cohort study, echocardiographically confirmed LA enlargement (>45 mm) was independently associated with stroke incidence even after adjustment for CHA2DS2-VASc score and anticoagulation therapy (hazard ratio = 1.74; 95% CI, 1.25–2.42; P <0.01).22 In the Heinz Nixdorf Recall study, LA size measured by noncontrast CT was associated with major adverse cardiovascular events including stroke.23 In our study, the mean LAESVI in individuals with LAA thrombus was 139 mL/m2, significantly outside the normal range of 31.1 to 77.7 mL/m2 established by Lin and colleagues.24 Left atrial enlargement measured by cardiac CT was also independently associated with LAA thrombus, an established risk factor for stroke. Together, these findings suggest that patients shown to have an enlarged LA on cardiac CT should be considered at high risk for thromboembolism and therefore carefully managed.
Higher CHADS2 and CHA2DS2-VASc scores are potential risk factors for LAA thrombus.25,26 In our study, however, mean CHA2DS2-VASc scores did not differ significantly between patients with and without LAA thrombus (2.7 vs 2.8, P =0.95) (Table I). In contrast, age ≥75 years—one component of the CHA2DS2-VASc score—was significantly more prevalent in patients with LAA thrombus than in those without. Advanced age itself is an independent risk factor for LA enlargement and cardioembolic stroke,1 and this may explain why the mean LA volumes recorded in our study differed so much between groups.
Our study population was relatively younger than those in other studies (mean, 60 vs 66–69 yr), which may account for the lower CHA2DS2-VASc scores.25,26 Moreover, in our study, we detected thrombus in 3 patients (4.1%) who had CHA2DS2-VASc scores of 0 to 1. In a study of AF patients with low CHA2DS2-VASc scores,27 the investigators concluded that LA enlargement may be one of several factors associated with increased thromboembolic risk that are not measured or accounted for in the CHA2DS2-VASc score. If supported by future studies, this finding may warrant careful management of patients with AF who have an enlarged LA even if they have low CHA2DS2-VASc scores.
Study Limitations
Our study had several limitations. First, it was a prospective, single-center study that included only 73 patients with AF and suspected coronary artery disease. Studies in larger populations are warranted. Second, LAA thrombus was diagnosed by cardiac CTDE alone. Several studies that established the accuracy of cardiac CT in detecting LAA thrombus used TEE as a reference standard.3,9,16 Third, all cardiac CT images were acquired with retrospective ECG gating, which exposes patients to radiation throughout the cardiac cycle. Our current protocol could be improved by lowering tube voltage, adjusting tube current by weight, electrocardiographically controlling dose modulation, or prospectively acquiring sequential images.28 Prospectively gated CT image acquisition with systolic triggering may reduce the radiation exposure in patients with AF even during standard 64-row MDCT.29 However, the delayed-enhancement step in our protocol adds only <1 mSv to the total effective radiation dose, requires imaging of only the upper half of the heart, and is done prospectively and at low kilovoltage (typically 100 kVp). In the future, newer generations of dual-energy CT scanners may be used without ECG gating to differentiate between LAA thrombus and slow flow by measuring iodine concentrations in LAA filling defects.30 However, the clinical experience with dual-energy methods is limited, and additional clinical trials in a larger population are needed.
Conclusion
Increased LAESVI and LAEDVI are each independently associated with the presence of LAA thrombus detected by cardiac CTDE in patients with AF. Left atrial enlargement may provide a clue to the diagnosis of LAA thrombus in this patient population and warrants careful evaluation and interpretation. This may in turn help refine the thromboembolic risk stratification and management of patients with AF.
Acknowledgments
The authors thank Roxanne Terrazas and Sajad Hamal for participating in data collection and management.
References
- 1.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Tops LF, Schalij MJ, Bax JJ. Imaging and atrial fibrillation: the role of multimodality imaging in patient evaluation and management of atrial fibrillation. Eur Heart J. 2010;31(5):542–51. doi: 10.1093/eurheartj/ehq005. [DOI] [PubMed] [Google Scholar]
- 3.Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6(2):185–94. doi: 10.1161/CIRCIMAGING.112.000153. [DOI] [PubMed] [Google Scholar]
- 4.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47(12):2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 5.Walker JR, Abadi S, Solomonica A, Mutlak D, Aronson D, Agmon Y et al. Left-sided cardiac chamber evaluation using single-phase mid-diastolic coronary computed tomography angiography: derivation of normal values and comparison with conventional end-diastolic and end-systolic phases. Eur Radiol. 2016;26(10):3626–34. doi: 10.1007/s00330-016-4211-z. [DOI] [PubMed] [Google Scholar]
- 6.Asferg C, Usinger L, Kristensen TS, Abdulla J. Accuracy of multi-slice computed tomography for measurement of left ventricular ejection fraction compared with cardiac magnetic resonance imaging and two-dimensional transthoracic echocardiography: a systematic review and meta-analysis. Eur J Radiol. 2012;81(5):e757–62. doi: 10.1016/j.ejrad.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al.; Heart Rhythm Society Task Force on Catheter and Surgical Ablation 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9(4):632–96.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Chao TF, Lin YJ, Tsao HM, Tsai CF, Lin WS, Chang SL et al. CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol. 2011;58(23):2380–5. doi: 10.1016/j.jacc.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Budoff MJ, Shittu A, Hacioglu Y, Gang E, Li D, Bhatia H et al. Comparison of transesophageal echocardiography versus computed tomography for detection of left atrial appendage filling defect (thrombus) Am J Cardiol. 2014;113(1):173–7. doi: 10.1016/j.amjcard.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 10.Budoff MJ, Pagali SR, Hamirani YS, Chen A, Cheu G, Gao Y et al. Sex-specific biatrial volumetric measurements obtained with use of multidetector computed tomography in subjects with and without coronary artery disease. Tex Heart Inst J. 2014;41(3):286–92. doi: 10.14503/THIJ-12-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–13. [PubMed] [Google Scholar]
- 12.Shinbane JS, Girsky MJ, Chau A, Mao S, Budoff MJ. Three-dimensional computed tomography imaging of left atrial anatomy for atrial fibrillation ablation. Clin Cardiol. 2005;28(2):100. doi: 10.1002/clc.4960280211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vorre MM, Abdulla J. Diagnostic accuracy and radiation dose of CT coronary angiography in atrial fibrillation: systematic review and meta-analysis. Radiology. 2013;267(2):376–86. doi: 10.1148/radiol.13121224. [DOI] [PubMed] [Google Scholar]
- 14.Lazoura O, Ismail TF, Pavitt C, Lindsay A, Sriharan M, Rubens M et al. A low-dose, dual-phase cardiovascular CT protocol to assess left atrial appendage anatomy and exclude thrombus prior to left atrial intervention. Int J Cardiovasc Imaging. 2016;32(2):347–54. doi: 10.1007/s10554-015-0776-x. [DOI] [PubMed] [Google Scholar]
- 15.Sawit ST, Garcia-Alvarez A, Suri B, Gaztanaga J, Fernandez-Friera L, Mirelis JG et al. Usefulness of cardiac computed tomographic delayed contrast enhancement of the left atrial appendage before pulmonary vein ablation. Am J Cardiol. 2012;109(5):677–84. doi: 10.1016/j.amjcard.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Dorenkamp M, Sohns C, Vollmann D, Luthje L, Seegers J, Wachter R et al. Detection of left atrial thrombus during routine diagnostic work-up prior to pulmonary vein isolation for atrial fibrillation: role of transesophageal echocardiography and multidetector computed tomography. Int J Cardiol. 2013;163(1):26–33. doi: 10.1016/j.ijcard.2011.06.124. [DOI] [PubMed] [Google Scholar]
- 17.Ono K, Iwama M, Kawasaki M, Tanaka R, Watanabe T, Onishi N et al. Motion of left atrial appendage as a determinant of thrombus formation in patients with a low CHADS2 score receiving warfarin for persistent nonvalvular atrial fibrillation. Cardiovasc Ultrasound. 2012;10:50. doi: 10.1186/1476-7120-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scherr D, Dalal D, Chilukuri K, Dong J, Spragg D, Henrikson CA et al. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20(4):379–84. doi: 10.1111/j.1540-8167.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 19.Ayirala S, Kumar S, O'Sullivan DM, Silverman DI. Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr. 2011;24(5):499–505. doi: 10.1016/j.echo.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Faustino A, Providencia R, Barra S, Paiva L, Trigo J, Botelho A et al. Which method of left atrium size quantification is the most accurate to recognize thromboembolic risk in patients with non-valvular atrial fibrillation? Cardiovasc Ultrasound. 2014;12:28. doi: 10.1186/1476-7120-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD, Jr, Kopecky SL et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26(23):2556–61. doi: 10.1093/eurheartj/ehi483. [DOI] [PubMed] [Google Scholar]
- 22.Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. 2016;6:31042. doi: 10.1038/srep31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahabadi AA, Geisel MH, Lehmann N, Lammerding C, Kalsch H, Bauer M et al. Association of computed tomography-derived left atrial size with major cardiovascular events in the general population: the Heinz Nixdorf Recall Study. Int J Cardiol. 2014;174(2):318–23. doi: 10.1016/j.ijcard.2014.04.068. [DOI] [PubMed] [Google Scholar]
- 24.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A et al. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1(6):782–6. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Providencia R, Botelho A, Trigo J, Quintal N, Nascimento J, Mota P et al. Possible refinement of clinical thromboembolism assessment in patients with atrial fibrillation using echocardiographic parameters. Europace. 2012;14(1):36–45. doi: 10.1093/europace/eur272. [DOI] [PubMed] [Google Scholar]
- 26.Willens HJ, Gomez-Marin O, Nelson K, DeNicco A, Moscucci M. Correlation of CHADS2 and CHA2DS2VASc scores with transesophageal echocardiography risk factors for thromboembolism in a multiethnic United States population with nonvalvular atrial fibrillation. J Am Soc Echocardiogr. 2013;26(2):175–84. doi: 10.1016/j.echo.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Szymanski FM, Lip GY, Filipiak KJ, Platek AE, Hrynkiewicz-Szymanska A, Opolski G. Stroke risk factors beyond the CHA2DS2VASc score: can we improve our identification of “high stroke risk” patients with atrial fibrillation? Am J Cardiol. 2015;116(11):1781–8. doi: 10.1016/j.amjcard.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Hausleiter J, Meyer T, Hadamitzky M, Huber E, Zankl M, Martinoff S et al. Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation. 2006;113(10):1305–10. doi: 10.1161/CIRCULATIONAHA.105.602490. [DOI] [PubMed] [Google Scholar]
- 29.Clayton B, Roobottom C, Morgan-Hughes G. CT coronary angiography in atrial fibrillation: a comparison of radiation dose and diagnostic confidence with retrospective gating vs prospective gating with systolic acquisition. Br J Radiol. 2015;88(1055):20150533. doi: 10.1259/bjr.20150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hur J, Kim YJ, Lee HJ, Nam JE, Hong YJ, Kim HY et al. Cardioembolic stroke: dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology. 2012;263(3):688–95. doi: 10.1148/radiol.12111691. [DOI] [PubMed] [Google Scholar]


