Abstract
PURPOSE
The coronavirus disease 2019 (COVID-19) pandemic has imposed a unique challenge to oncology patients and their treatment. There is no study related to the patients’ preference for systemic therapy during this pandemic. We have conducted a prospective study to analyze that aspect.
METHODS
All consecutive patients who visited during the lockdown period from April 1-10, 2020, for systemic chemotherapy were included in the study for a questionnaire-based survey to evaluate the willingness to continue chemotherapy during this pandemic and factors influencing the decisions.
RESULTS
A total of 302 patients were included (median age, 56 years; range, 21-77 years). Most common sites of cancer were breast (n = 114), lung (n = 44), ovary (n = 34), and colon (n = 20). Home address was within the city for 125 patients (42%), outside the city for 138 (46%), and outside the state for 37 (12%). Treatment was curative in 150 patients and palliative in 152. Educational status was primary and above for 231 patients and no formal schooling for 71. A total of 203 patients wanted to continue chemotherapy, 40 wanted to defer, and 56 wanted the physician to decide. Knowledge about COVID-19 strongly correlated with intent of treatment (P = .01), disease status (P = .02), knowledge about immunosuppression (P < .001), home location (P = .02), and education status (P = .003). The worry about catching SARS-CoV-2 was high in those with controlled disease (P = .06) and knowledge about immunosuppression (P = .02). Worry about disease progression was more with palliative intent (P < .001).
CONCLUSION
This study shows that oncology patients in our country are more worried about disease progression than the SARS-CoV-2 and wish to continue chemotherapy during this pandemic. The treatment guidelines in the COVID-19 scenario should incorporate patients’ perspectives.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread all over the world and has created havoc in every aspect of life. It has affected everything from health care to the economy. Countries are dealing with war-like situations to fight the COVID-19 pandemic. SARS-CoV-2 causes acute viral illness that mimics lower respiratory tract infection. In some patients, there is a higher mortality because of underlying comorbidities like diabetes; hypertension; heart disease; and others, including underlying malignancy. The treatment of cancer involves chemotherapy in both curative and palliative settings. Chemotherapy leads to a higher incidence of secondary infection as a result of myelosuppression, co-existing secondary immunodeficiency state because of underlying malignancy, and other medical complications that might require frequent hospital visits and/or admissions.
CONTEXT
Key Objective
In the COVID-19 pandemic, there are minimal data on patients’ perceptions and choices about the continuation of their anticancer therapy. Most of the guidelines have only focused on what clinicians, and not patients and their families, feel about anticancer treatment.
Knowledge Generated
Our study evaluated the aspects of patients’ willingness for systemic therapy, their major fears, and their priorities when it comes to selecting between cancer treatment and COVID-19. The majority of our patients wanted their cancer treatment to continue uninterrupted despite the fear of getting SARS-CoV-2 infection and logistic difficulties. The study also has shown that detailed counseling about the adverse events of systemic therapy is of utmost importance.
Relevance
In the era of patient-reported outcomes, strong data on patients’ perspectives should be taken into consideration while formulating guidelines and deciding anticancer treatment during the COVID-19 pandemic.
COVID-19 causes more complications in patients with cancer (39% v 8%).1 In the Italian population, 20% of patients who died as a result of COVID-19 had cancer.2 Treatment-related morbidity is more common in the real-world setting than in the setting of randomized controlled trials.3 Many oncology societies, such as ASCO and the European Society for Medical Oncology, have come up with guidelines on systemic chemotherapy during the SARS-CoV-2 pandemic.4,5 The majority of them have suggested reducing unnecessary hospital visits, delaying chemotherapy in selected palliative settings, delaying surgery for low-grade or indolent disease, and switching to oral agents whenever applicable.4-6 Almost all of these are based on expert opinion and available literature because it is not possible, as well as unethical, to generate level 1 evidence during a pandemic, keeping in mind the risk-benefit ratio of each anticancer treatment/intervention, with safety of the patient being the first priority.
Most of these recommendations center around the health care providers. However, health care service is being allowed to run throughout the globe as an essential service, and a large number of patients are able to visit hospitals to continue their existing or planned treatment. There are no sufficient data on the patients’ perspectives about continuation of their treatment, associated risk of anticancer treatment, risk of contracting SARS-CoV-2 infection during hospital visits, existing knowledge of COVID-19, and caregiver stress and anxiety of caregiver. Many questions arise in patients’ minds: Should I continue chemotherapy? Can I get infected with SARS-CoV-2? Which one should I prioritize between my disease and the recommended precautions of social distancing and self-quarantine?
We have tried to answer these questions in this study using a detailed questionnaire-based survey. We conducted this study in our medical oncology department among consecutive patients with cancer who visited for their scheduled treatment. The main objective of this study was to gain the patients’ perspective with regard to their cancer care during this pandemic. To our knowledge, this study is the first of its kind from India, and possibly in the world, that evaluates this aspect.
METHODS
Patients
All patients age ≥ 18 years and who are actively undergoing systemic therapy for solid malignancies in the department of medical oncology were included from April 1 to 10, 2020, during the nationwide lockdown as a result of the COVID-19 pandemic. Verbal consent was obtained for all participants. It was a prospective observational study in all patients who fulfilled the aforementioned criteria. Because of the pandemic, a noninterventional survey-based observational study, and nonavailability of expedited institutional review board meeting, formal approval could not be obtained before initiating the study.
Aims and Objectives
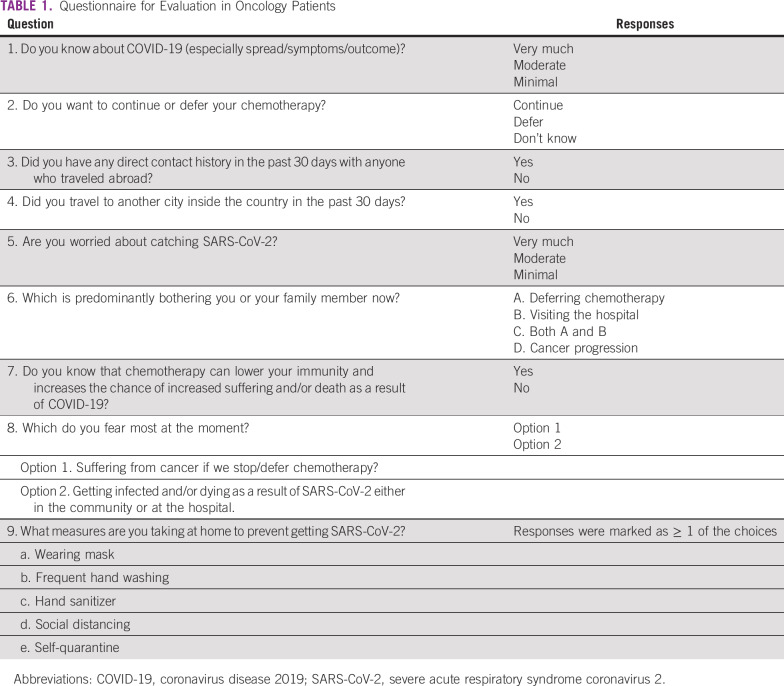
A survey questionnaire was prepared to understand the patients’ knowledge and understanding about the SARS-CoV-2 infection and the COVID-19 pandemic and their treatment-related factors, including the primary disease and the current viral outbreak. The primary objective of the study was to assess the mindset of patients about continuation of anticancer systemic therapy during this pandemic. The secondary objectives were to explore the variables associated with the responses. The primary end point was the proportion of patients who wish to continue or discontinue systemic therapy during the pandemic. The secondary end points were correlation of location, diagnosis, and intent of treatment with the responses; fear of catching SARS-CoV-2 infection vis-à-vis cancer progression owing to delayed/interrupted anticancer treatment because of the current countrywide lockdown; and analysis of steps taken by patients to prevent infection by SARS-CoV-2. The questionnaire (Table 1) included basic information, like age, sex, home proximity to the health care facility, diagnosis, intent of treatment, stage of treatment, and current state of the disease. For correlation analysis, diagnoses of breast, lung, and ovarian cancer were kept as individual variables, colon and rectal cancer were grouped together, and the rest of the diagnoses were grouped as other.
TABLE 1.
Questionnaire for Evaluation in Oncology Patients
Inclusion and Exclusion Criteria
Ability to read, write, or comprehend spoken language was a prerequisite for entry into this study. The questionnaire was filled out by either the patient or a family member if the patient was unable to read. Patients were excluded if they were unable to comprehend spoken language, were in severe physical distress because of cancer- or therapy-related complications, had altered sensorium, were not receiving any active systemic therapy and oral targeted therapy, had no evidence of disease recurrence or progression, were planned only for best supportive care, and were being considered an inpatient admission for supportive care.
Statistical Analysis
Descriptive statistics were used for demographics and clinical characteristics. Baseline demographics, disease, and treatment status were correlated with survey answers. Pearson’s correlation coefficient was used to analyze the degree of correlation between variables. The χ2 or Fisher’s exact test was used to detect an association between categorical variables. The t test was applied to compare continuous variables between groups. SAS 9.4 software (SAS Institute, Cary, NC) was used for the statistical analysis.7
RESULTS
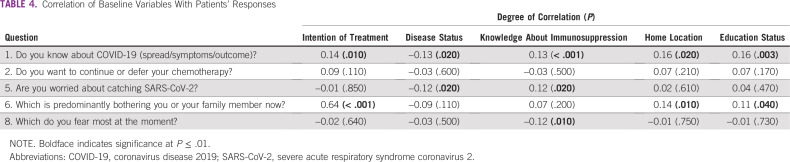
A total of 302 patients were enrolled in this study with a male:female ratio of 98:204 and median age of 56 years (range, 21-77 years). The baseline demographic features are listed in Table 2. The most common sites of primary cancer were breast in 114 patients (37.75%), lung in 44 (14.57%), ovary in 34 (11.26%), colon in 20 (6.62%), pancreas and periampulla in 16 (5.3%), gall bladder in 12 (3.97%), and stomach in 11 (3.64%; Table 2). The responses to various questions are listed in Table 3.
TABLE 2.
Baseline Demographic Characteristics
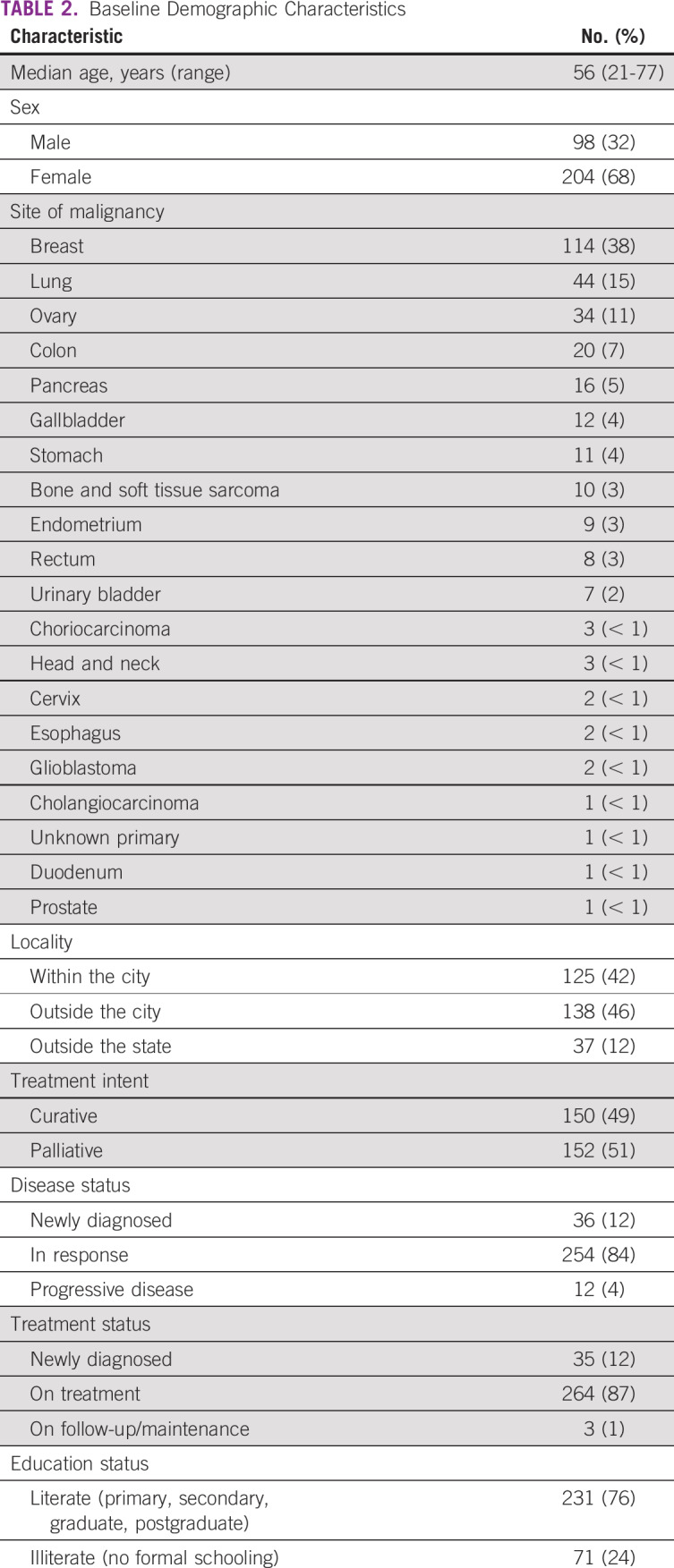
TABLE 3.
Responses to Survey Questions
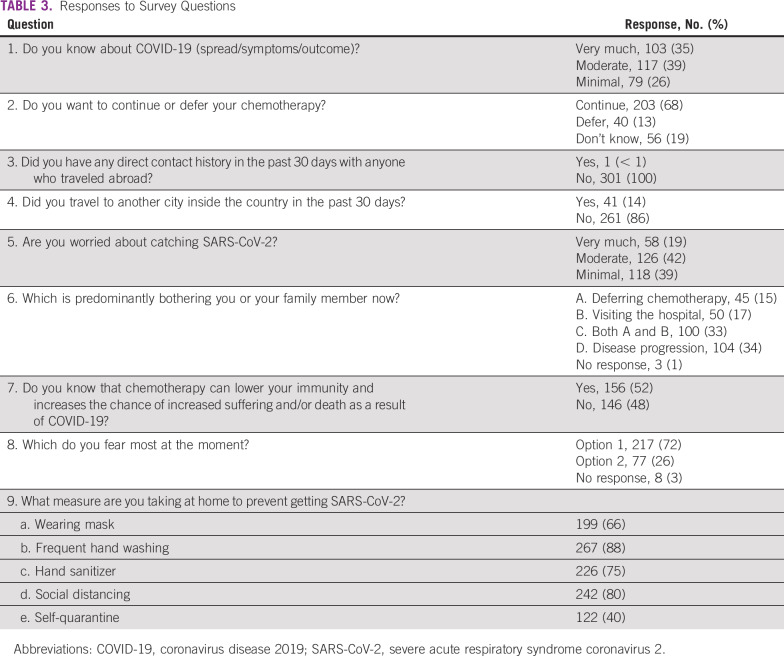
Knowledge about COVID-19 (question 1) strongly correlated with curative intent of disease (P = .01), disease status in ongoing response (P = .02), more knowledge about immunosuppression (question 7; P < .001), distant location of home address (P = .02), and higher education level (P = .003). The desire to continue chemotherapy (question 2) did not significantly correlate with any of the baseline parameters. Worry about catching SARS-CoV-2 (question 5) strongly correlated with palliative intent of treatment (P < .001) and higher knowledge about the immunosuppressive effects of chemotherapy (P = .02). The worry about disease progression versus contracting SARS-CoV-2 significantly correlated with intent of treatment (P < .001), distant home address (P = .01), and education status (P = .04). The fear of having COVID-19 more than cancer directly correlated with higher knowledge about immunosuppression (P = .01). The correlation coefficients and their significance with important baseline variables are listed in Table 4.
TABLE 4.
Correlation of Baseline Variables With Patients’ Responses
DISCUSSION
This study is the first of its kind in the setting of the SARS-CoV-2 pandemic to assess the mindset of patients with cancer toward various aspects of their anticancer treatment. Our study consisted of approximately 300 patients, of whom the majority was female. Because breast cancer is one of the most common malignancies in the urban population in India,8 this could account for the higher number of female patients in the study. The median age was 56 years, which is again likely a result of the high number of patients with breast cancer9 and relatively lower median age of all cancer types in India compared with western countries.
There was an even distribution of patients from within the city and those from outside the city (41% and 45%, respectively), with a minority from outside the state (12%), which represents that those patients were not able to come to our clinics because of the countrywide lockdown and sealing of state borders to curb COVD-19 spread. The 4 most common cancer sites in this study were breast, lung, ovary, and colon, which is in accordance with the incidence of most common cancers in India.10,11 Among the common cancers, we did not have much head and neck cancer because it is treated by the radiation oncology team mostly in our institute and, hence, was excluded from our study.
The responses were noted with a disclaimer that stated that this survey has no correlation with the patients’ existing treatment plan. For the first question, which asked about the amount of information the patients have with regard to COVID-19, responses were almost evenly distributed among well informed, moderately informed, and minimally informed. This reflects the influence of various news and social media information as well as information read from web sites. Our population has a mixture of rural and urban patients, which may account for the even distribution of their perception of knowledge. The majority of the patients (68%) wanted their chemotherapy to be continued despite the pandemic, which reflects that patients are probably more worried about their cancer than SARS-CoV-2. Approximately 19% did not have an opinion and wanted to go with the decision of the treating physician. All but 1 patient had a travel history abroad in the preceding month. Similarly, 86% of patients did not have any domestic travel history in the preceding 30 days, which reflects the treatment compliance of our patients and that the majority of them do not travel during systemic chemotherapy. When asked about their degree of fear about catching COVID-19, most of the patients had either moderate or minimal fear (approximately 40%). This finding probably reflects the confidence in the steps patients were taking as an extra precaution to prevent infection because they were all undergoing chemotherapy, or it may reflect that the pandemic so far did not affect India as much, so the patients did not have much fear. Approximately two thirds of the patients were bothered about deferring chemotherapy, visiting hospitals, or both, and another third were bothered about cancer progression if therapy was hampered. Approximately 50% of patients actually knew that chemotherapy can cause immunosuppression, which can reflect poor prechemotherapy counseling or poor retention of given information. Approximately 70% of patients were worried about cancer progression if their chemotherapy was stopped. Fortunately, the majority of our patients were taking personal protective measures, including wearing masks (65%), washing their hands (88%), using hand sanitizer (75%), and practicing social distancing (80%). Approximately 40% of them were even practicing self-isolation, despite their COVID-19 status being unknown.
In a study by Gajra et al,12 patients’ pretreatment chemotherapy preference was evaluated from a clinical trial of adjuvant chemotherapy for breast cancer. Low chemotherapy preference was mostly associated with a poor adverse effect profile but did not have any correlation with survival. In our study, knowledge about COVD-19 correlated with curative intent of disease, which means that more patients who were receiving adjuvant or neoadjuvant therapy were reading or learning more about COVID-19. It also correlated with patients’ ongoing response to therapy, which might reflect greater curiosity to know more about COVID-19 and cancer treatment interaction. There was a strong correlation of education status with knowledge about COVID-19, for obvious reasons. Of note, the desire to continue chemotherapy did not vary on the basis of any of the baseline parameters, which highlights that irrespective of the disease, state of treatment, or education status, all our patients wanted chemotherapy to continue during the pandemic. In another study in patients with metastatic breast cancer, adverse effects proved to be a critical deciding factor for chemotherapy preference, followed by age, commute time, and relationship status.13 This shows that our patients did not bring time to commute or logistic difficulty into consideration while deciding about chemotherapy, even during a pandemic.
Another study found that 68% of patients wanted chemotherapy over supportive care.14 In our study, however, despite the viral epidemic, there was no correlation of intent of treatment with chemotherapy willingness (P = .11).
More patients who were receiving palliative chemotherapy worried more about disease progression than about SARS-CoV-2 infection, which likely is because they already know their poor prognosis and might be concerned about further deterioration of their disease status during the COVID-19 pandemic. In addition, patients with higher knowledge about immunosuppression with chemotherapy were more concerned about SARS-CoV-2 infection because they received an explanation about the immunosuppressive effects of chemotherapy and chances of complications as a result of COVID-19.
To summarize, this study evaluated how patients feel about the status of their systemic treatment during the current COVID-19 pandemic. Disease state and intention of treatment affected their judgment, and in general, the majority of patients were more concerned about their disease than about the virus. In this era of patient-reported outcomes, patient mindset about treatment should be incorporated into informed decision-making processes.
In conclusion, this study shows that many patients are afraid of their treatment being delayed and more worried about cancer progression than SARS-CoV-2 infection and that there are gaps in patient understanding of the interplay of chemotherapy and immunosuppression. Therefore, the current consensus guidelines about holding and/or delaying or postponing anticancer treatment in our oncology patients need to be discussed in detail and to be customized according to disease status, stage, and intention of treatment. On the other hand, patients and their family members need repeated counseling and reassurance during each visit, whenever feasible.
ACKNOWLEDGMENT
We thank all our patients who showed the courage to come to the hospital in such difficult times and who agreed to respond to our survey.
AUTHOR CONTRIBUTIONS
Conception and design: Joydeep Ghosh, Sandip Ganguly, Deepak Dabkara, Bivas Biswas
Provision of study material or patients: Joydeep Ghosh, Sandip Ganguly, Bivas Biswas
Collection and assembly of data: Joydeep Ghosh, Sandip Ganguly, Debapriya Mondal, Prashant Pandey, Bivas Biswas
Data analysis and interpretation: Joydeep Ghosh, Sandip Ganguly, Deepak Dabkara, Bivas Biswas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1001/jama.2020.4683. Onder G, Rezza G, Brusaferro S: Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323:1775-1776, 2020. [DOI] [PubMed]
- 3.Prince RM, Powis M, Zer A, et al. Hospitalisations and emergency department visits in cancer patients receiving systemic therapy: Systematic review and meta-analysis. Eur J Cancer Care (Engl) 2019;28:e12909. doi: 10.1111/ecc.12909. [DOI] [PubMed] [Google Scholar]
- 4. American Society of Clinical Oncology: COVID-19 provider & practice information, 2020. https://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19.
- 5. doi: 10.1016/j.annonc.2020.03.286. Cortiula F, Pettke A, Bartoletti M, et al: Managing COVID-19 in the oncology clinic and avoiding the distraction effect. Ann Oncol 31:553-555, 2020. [DOI] [PMC free article] [PubMed]
- 6. National Institute for Health and Care Excellence: COVID-19 rapid guideline: Delivery of systemic anticancer treatments, 2020. https://www.nice.org.uk/guidance/ng161/chapter/7-Modifications-to-usual-service#Interim%20NHS%20England%20treatment%20regimens. [PubMed]
- 7. SAS Institute: SAS University Edition. https://www.sas.com/en_in/software/university-edition/download-software.html#mac-os-x.
- 8.Malvia S, Bagadi SA, Dubey US, et al. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol. 2017;13:289–295. doi: 10.1111/ajco.12661. [DOI] [PubMed] [Google Scholar]
- 9.Manoharan N, Nair O, Shukla NK, et al. Descriptive epidemiology of female breast cancer in Delhi, India. Asian Pac J Cancer Prev. 2017;18:1015–1018. doi: 10.22034/APJCP.2017.18.4.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh M, Prasad CP, Singh TD, et al. Cancer research in India: Challenges & opportunities. Indian J Med Res. 2018;148:362–365. doi: 10.4103/ijmr.IJMR_1711_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 12.Gajra A, McCall L, Muss HB, et al. The preference to receive chemotherapy and cancer-related outcomes in older adults with breast cancer CALGB 49907 (Alliance) J Geriatr Oncol. 2018;9:221–227. doi: 10.1016/j.jgo.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.3389/fonc.2018.00535. Spaich S, Kinder J, Hetjens S, et al: Patient preferences regarding chemotherapy in metastatic breast cancer—a conjoint analysis for common taxanes. Front Oncol 8:535, 2018. [DOI] [PMC free article] [PubMed]
- 14.Koedoot CG, de Haan RJ, Stiggelbout AM, et al. Palliative chemotherapy or best supportive care? A prospective study explaining patients’ treatment preference and choice. Br J Cancer. 2003;89:2219–2226. doi: 10.1038/sj.bjc.6601445. [DOI] [PMC free article] [PubMed] [Google Scholar]