Abstract
PURPOSE
Chronic lymphocytic leukemia (CLL) is uncommon in India. There are limited studies on CLL from the Indian subcontinent.
METHODS
This was a prospective study (2011-2017) of consecutively diagnosed patients with CLL at a single center. The diagnosis, prognosis, treatment indication, response criteria, and adverse events were recorded as per International Workshop on Chronic Lymphocytic Leukemia guidelines. Biosimilar rituximab dosing (375 mg/m2) was fixed for all cycles. Time to next treatment (TTNT) was defined as the time from front-line treatment initiation to next treatment or death from any cause. Overall survival (OS) was defined as the time from treatment initiation until death from any cause.
RESULTS
A total of 409 patients with CLL were enrolled over the study period. The median follow-up was 32 months (range, 2-135 months). The median age was 61 years, and 31.8% of patients with CLL were ≤ 55 years of age; 43.3% of patients had a cumulative illness rating scale score ≥ 3. Prognostic fluorescence in situ hybridization data were available in 53.3% of patients. Chlorambucil (94/180; 52.2%) and bendamustine + rituximab (BR; 57/180; 31.6%) were the most common regimens used up front. The overall response rates after front-line therapy were 74.4% and 91.2%, respectively. The TTNT was 33 months and not reached, respectively (P = .001). Grade 3/4 neutropenia and infections were seen in 52.6% and 38.5% of patients receiving BR. The median OS was not reached in both regimens (P = .25).
CONCLUSION
Indian patients with CLL are younger in chronological age but have higher morbidity burden. Treatment outcomes with biosimilar fixed-dose BR are comparable to those reported in the literature. Chlorambucil is still a valid option, given the economic burden of the disease and treatment.
INTRODUCTION
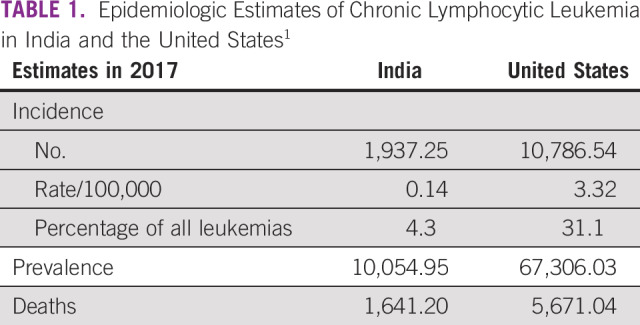
Population-wide studies have shown that chronic lymphocytic leukemia (CLL) shows remarkable geographic and ethnic variations. CLL is the most common leukemia in the United States (> 30% of all leukemia) and the least common leukemia in India (< 5% of all leukemia). The epidemiologic estimates of CLL in India are compared with that in the United States in Table 1.1 This lower incidence among Asians persists even for migrants and their descendants to the west.2,3 There are data to suggest that the clinical presentation of CLL may be different in the Asian population. This is seen in terms of the younger age of presentation, aggressive course, and shorter time to treatment.4 The exact reasons for the low prevalence of CLL in the Indian population are still unknown, and answers to these may exist in clinical-epidemiologic studies. There is paucity of CLL data from the Indian subcontinent, with scarce data on epidemiology, clinical presentation, and treatment outcomes.5 It is even more relevant in the era when most countries with universal health coverage are moving to chemotherapy-free regimens. Although chlorambucil monotherapy is considered palliative in these countries,6 it is often the only therapy affordable to most patients in the low to middle sociodemographic index (SDI) countries.7 This study sheds light on the demographics, clinical presentation, treatment options, and outcomes in India.
TABLE 1.
Epidemiologic Estimates of Chronic Lymphocytic Leukemia in India and the United States1
CONTEXT
Key Objective
In this prospective study, we report what is, to the best of our knowledge, the largest data set of chronic lymphocytic leukemia (CLL) from the Indian subcontinent.
Knowledge Generated
Indian patients with CLL are younger in chronological age but have higher morbidity burden. Treatment outcomes with biosimilar fixed-dose rituximab + bendamustine are comparable to those reported in the literature. Chlorambucil is still a valid option in regions with limited resources.
Relevance
These CLL data from a resource-limited setting highlight the differences in demographics and clinical presentation but show that treatment outcomes using the options available are comparable to the clinical trial and real-world data from regions without resource limitations.
METHODS
This was a prospective single-center study conducted from 2011-2017 at a large academic institute in North India. This study was approved by the institutional ethics committee. All consecutively diagnosed patients with CLL registered in the adult hematology clinic were enrolled in the study after informed consent. The diagnosis of CLL required ≥ 5 × 109/L clonal B lymphocytes in peripheral blood and was confirmed on multiparameter flow cytometry as per International Workshop on Chronic Lymphocytic Leukemia (iwCLL) guidelines.8 Patent demographics, clinical presentation, clinical stage, prognostic parameters (β2 microglobulin [β2m], lymphocyte doubling time [LDT], CD38 status, and fluorescence in situ hybridization [FISH] test) were recorded at diagnosis. Asymptomatic patients were kept on observation. Treatment was initiated as per the iwCLL criteria. The choice of treatment was at the discretion of the treating physician in consultation with the patient as per his/her financial and supportive care status. In general, fit patients with financial resources were offered bendamustine-rituximab (BR), unfit patients with financial resources were offered rituximab-chlorambucil (R-Clb), and patients without financial resources were offered chlorambucil ± prednisolone (Clb ± P)–based therapies. Biosimilar rituximab was dosed at 375 mg/m2 for all cycles 1-6, rather than escalating to 500 mg/m2 from cycle 2-6. Bendamustine was dosed at 90 mg/m2 on days 1 and 2 in a 28-day cycle. Chlorambucil dosing was 10 mg/m2 with/without prednisolone 60 mg/m2 for 5 days in a 28-day cycle. Fludarabine-cyclophosphamide-rituximab (FCR) was offered to very few fit patients with financial resources and good supportive care, and ibrutinib was beginning to be offered in patients with deletion 17p and financial resources toward the end of the study period. Patients with autoimmune cytopenias were treated with single-agent immunosuppressive therapy with prednisolone or rituximab. No routine anti-infective prophylaxis was given with any treatment. Vaccination, granulocyte colony-stimulating factor, and immunoglobulin use were at the discretion of the treating physician and as clinically indicated. Patients were followed up to monitor cytopenias as per iwCLL criteria8 and infections as per Common Terminology Criteria for Adverse Events version 4.0 criteria. The response was assessed at 2 months after completion of the treatment as per iwCLL criteria.8 This was mainly done clinically, with minimal investigations of hemogram, chest radiograph, and abdomen ultrasound. Computed tomography (CT) imaging and bone marrow examination were not done in all patients. Hence, responses were documented as unconfirmed complete response (CRu). Time to next treatment (TTNT) was defined as the time from front-line treatment initiation to next treatment or death from any cause. TTNT2 was defined as the time from second-line treatment initiation to subsequent next treatment or death from any cause. Progression-free survival (PFS) could not be calculated in most patients because of the lack of availability of absolute lymphocyte counts on each follow-up visit after treatment. Overall survival (OS) was defined as the time from treatment initiation until death from any cause.
Statistical Analysis
Descriptive statistics were calculated for all variables. Differences in proportions were assessed using the χ2 or Fisher exact test. Differences in means were tested using a Mann-Whitney U test or t test. The probability of survival was estimated using Kaplan-Meier analysis and log-rank test. A P value ≤ .05 was taken for statistical significance. Statistical analysis was performed with SPSS statistical software version 20.0 (SPSS, Chicago, IL).
RESULTS
Clinical Presentation of CLL
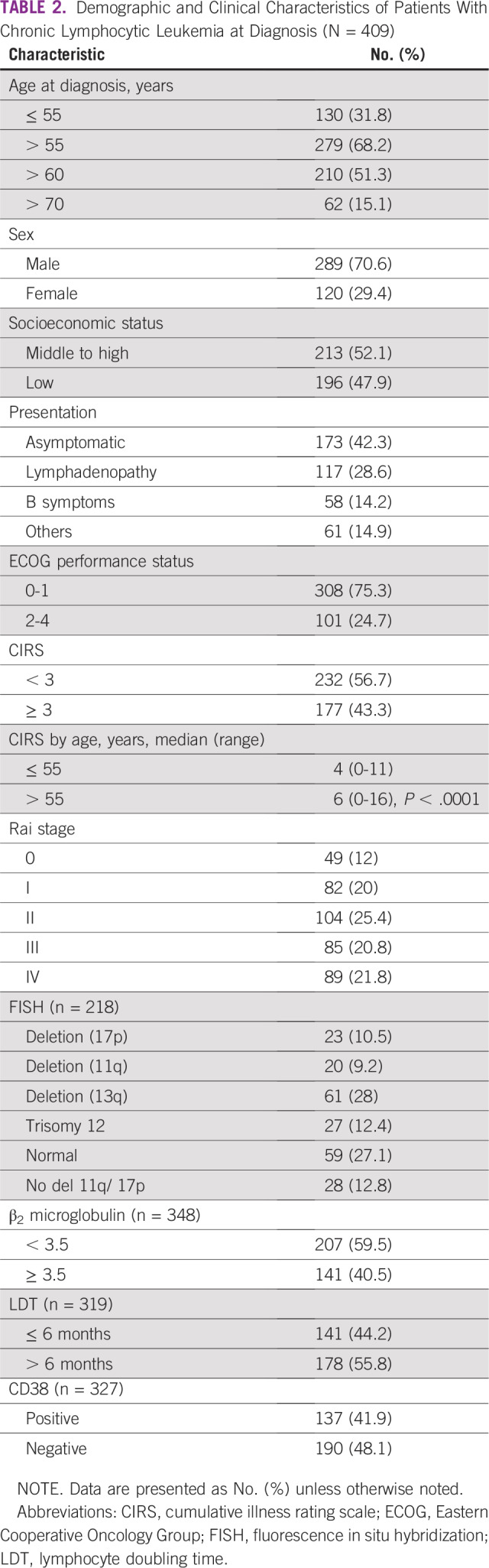
A total of 409 patients diagnosed with CLL were enrolled in the study period. The median follow-up was 32 months (range, 2-135 months). The median age of the patients with CLL at diagnosis was 61 years (range, 31-87 years). There was male preponderance, with male-to-female ratio of 2.4:1. The prevalence of young CLL (age ≤ 55 years) was 31.8% of the total study population. Our center is a tertiary-level referral center in north India; the majority of patients were from Punjab (42.8%), followed by Haryana (24.4%), Chandigarh (11%), and Himachal Pradesh (9.5%). The remainder of the patients (12.3%) belonged to the states of Uttar-Pradesh, Jammu and Kashmir, Uttarakhand, West-Bengal, Bihar, Gujarat, and Rajasthan. Most of the patients were in office jobs (28.1%), followed by homemakers (23.7%) and farmers (19.5%); the rest were either retired or in other occupations (23.7%). Approximately half (47.9%) of the patients were of low socioeconomic status. The majority (42.3%) of the patients were asymptomatic (incidentally detected lymphocytosis) at presentation; 28.6% patients were aware of lymphadenopathy at presentation. The rest of the patients (14.9%) presented with pneumonia or other infections. B symptoms were present in 14.2% of the patients. Two patients had concurrent diffuse large B-cell lymphoma at diagnosis. A quarter (24.7%) of the patients had an Eastern Cooperative Oncology Group performance status of 2-4. The cumulative illness rating scale (CIRS), which is a measure of the comorbidity burden, was ≥ 3 in 43.3% of patients. There was an even distribution of patients with CLL as per Rai stage, with 20%, 25.4%, 20.8%, and 21.8% belonging to Rai stage I, II, III, and IV, respectively. Relatively fewer patients presented with Rai stage 0 (12%). All the prognostic information was not available in all patients. Approximately 40% of patients had worse prognostic markers in the form of increased β2m, LDT < 6 months, or a positive CD38 by flow. FISH data were available in 53.3% of patients. Deletion 17p and 11q were present in 10.5% and 9.2% of patients, respectively. Immunoglobulin heavy chain gene (IgHV) mutation status was unavailable. The demographic and clinical characteristics of the patients are summarized in Table 2.
TABLE 2.
Demographic and Clinical Characteristics of Patients With Chronic Lymphocytic Leukemia at Diagnosis (N = 409)
Treatment Outcomes
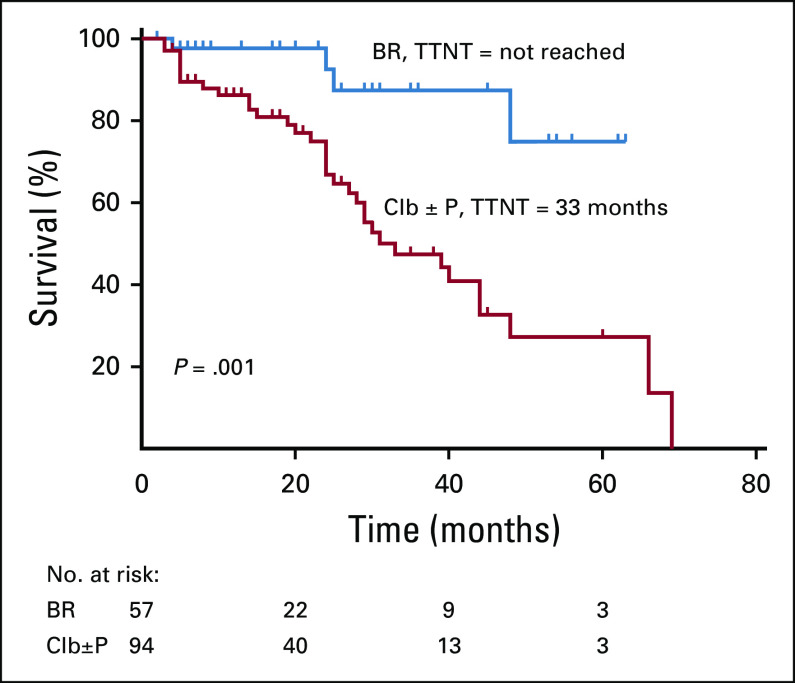
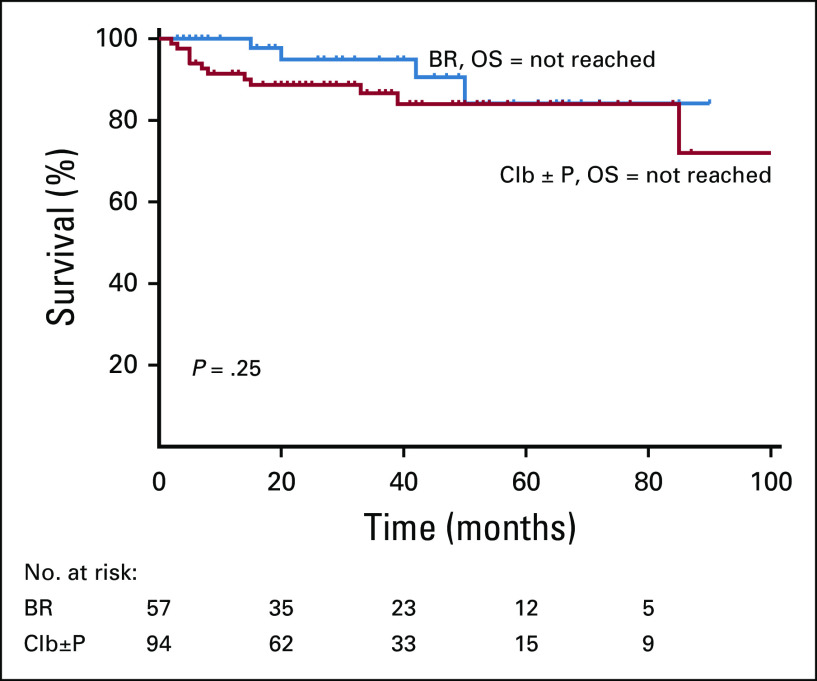
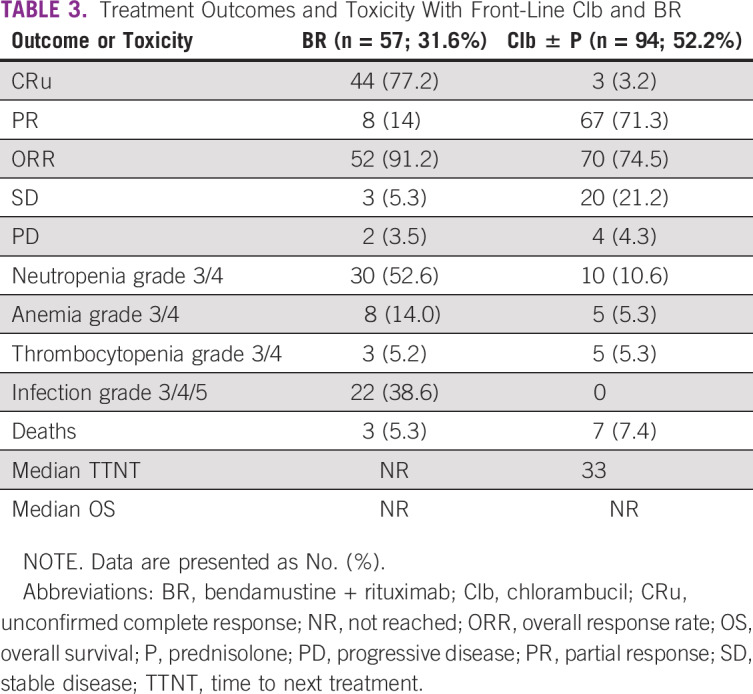
A total of 70 patients (17.1%) were lost to follow-up after registration. Of the rest, 199/339 (58.7%) patients required treatment as per iwCLL criteria, and 140 (41.3%) patients remained on observation. Out of these, 19 patients were treated for autoimmune cytopenias, and the remaining 180 patients were treated with chemoimmunotherapy for CLL. Most patients in our center received Clb ± P (52.2%)–based treatment. BR was the second most common regimen (31.6%). Other patients received FCR (3.3%), R-Clb (3.8%), CVP/CHOP (rituximab, cyclophosphamide, vincristine, prednisolone, hydroxydaunorubicin, oncovin; 4.4%), ibrutinib (n = 4), R-CHOP (n = 3), or R plus high-dose methylprednisolone (n = 1). A total of 77.2% of the patients attained CRu with BR therapy. Most patients attained partial response (71.3%) with Clb-based therapy. Severe (grade 3-4) neutropenia and consequently severe infections were seen in 52.6% and 38.5% of patients in the BR arm. Chlorambucil was well tolerated, with a lesser incidence of severe cytopenias and infections. The median TTNT was significantly different between BR and Clb ± P (not reached v 33 months; P = .001; Fig 1). The TTNT was not reached for FCR and R-Clb treatments as well in small patient numbers. The median OS was not reached in both BR and Clb ± P groups (P = .25; Fig 2; Table 3). Only 1 patient developed Richter’s transformation after front-line BR and went on to receive matched sibling allogeneic transplantation after 2 more lines of therapy.
FIG 1.
Time to next treatment (TTNT) after first-line therapy. BR, bendamustine + rituximab; Clb, chlorambucil; P, prednisolone.
FIG 2.
Overall survival (OS) after first-line therapy. BR, bendamustine + rituximab; Clb, chlorambucil; P, prednisolone.
TABLE 3.
Treatment Outcomes and Toxicity With Front-Line Clb and BR
A total of 64 (35.5%) patients relapsed after first-line therapy. The most common second-line treatment options were BR (n = 17), Clb ± P (n = 13), and R-Clb (n = 12). Other treatment regimens included CVP (n = 7), CHOP/R-CHOP (n = 6), and ibrutinib (n = 3), and 1 patient each received FCR, lenalidomide, R-CVP, R-DHAP (dexamethasone, high-dose cytarabine, cisplatin), R-ESHAP (etoposide, methylprednisolone, high-dose cytarabine, cisplatin), or R-lenalidomide. These heterogeneous treatment regimens were not included for statistical analysis because of small patient numbers. The overall response rate (ORR) with BR and R-Clb–based therapy was similar at 87.5% and 88.8%, respectively. The incidence of grade 3/4 neutropenia was higher in the relapsed setting after BR (64.7%) compared with the front-line setting. The chlorambucil arm was still effective in achieving overall response at first relapse (76.9%). The TTNT2 was not statistically different between BR (42 months), R-Clb (not reached), and Clb groups (30 months; P = .2).
DISCUSSION
This the largest real-world data set of CLL from India, to the best of our knowledge. The sex ratio is comparable to that reported in the literature. Our study has a higher proportion of younger (age ≤ 55 years) patients with CLL (31.8%) compared with other studies in the West (10%).9 The median age is also a decade earlier in Indian patients (61 years) compared with those reported in the literature from high-SDI countries (70 years).9 This could be due to a referral bias but has been observed for other hematologic malignancies from India as well.10,11 This assumes significance, given younger patients with CLL are likely to have worse prognostic factors and inferior OS compared with age- and sex-matched populations.12 More patients in our study presented with inferior health status as determined by CIRS ≥ 3 (43.3% v 11%) compared with the patients from high-SDI countries.13 This might be due to the late presentation to the clinic or poor fitness profile of Indian patients.14
Although Rai staging was available in all patients, basic prognostic laboratory information, including β2m, LDT, or CD38, was not available in a quarter of the patients. FISH data were available in 53.3% of patients, which is higher than would be available for other centers even in high-SDI countries15 (49.9%), because they were obtained as a part of a research project in this academic center. IgHV mutation testing is still not routine at our center, although this test is available at a few private laboratories and research centers.16
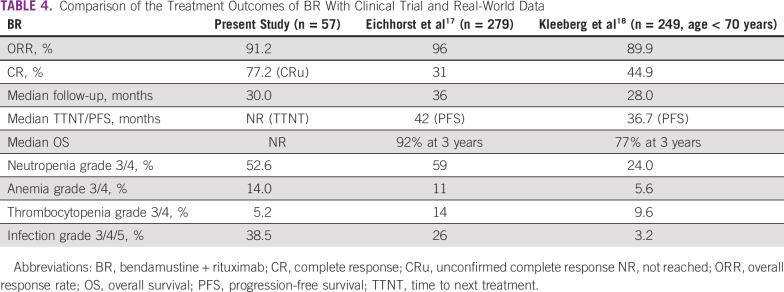
The overall response rates with BR were comparable to the outcomes reported from clinical trial17 and real-world data from Germany18 (Table 4). We had a higher unconfirmed CR rate compared with CR rates in clinical trials, as most patients did not get end-of-treatment CT imaging and bone marrow examination, which are desirable in clinical trials for documenting outcomes. The median TTNT was better documented in the patients than the PFS. Most patients did not have absolute lymphocyte counts on follow-up to document disease progression to calculate PFS. Also, TTNT is a better surrogate of treatment outcomes than PFS, as patients with CLL are treated only when symptomatic after relapse. The TTNT in our BR cohort was not reached, as compared with the PFS from trial data (42 months) and real-world data (30.6 months). The adverse event profile was comparable across the studies, except for higher rate of infections in our study group. Some differences in the frequency of cytopenias reported might be due to the different criteria used (National Cancer Institute Common Toxicity Criteria used in other studies v the iwCLL criteria used in our study). BR was still an effective treatment at first relapse, with a TTNT2 of 42 months ,compared with 31.3 months reported from a real-world European study.19
TABLE 4.
Comparison of the Treatment Outcomes of BR With Clinical Trial and Real-World Data
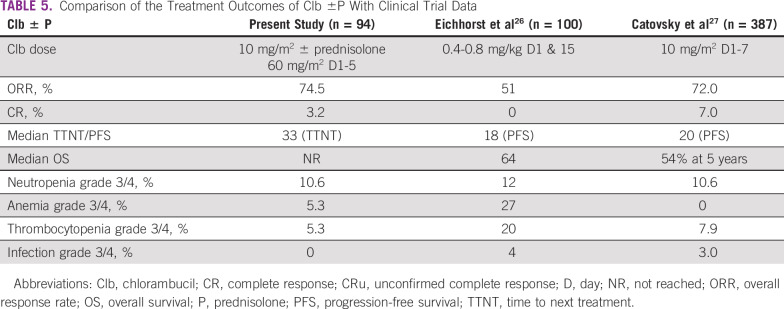
In today’s world, there is little role for chlorambucil monotherapy in high-SDI countries.20 Consequently, there are no recent real-world data on the same from these countries. However, this represents a major therapeutic option even to date in India, given the outpatient administration and meager costs, specifically in patients of low socioeconomic strata, which were half our study population. The outcomes with chlorambucil therapy, when compared with trials,18,19 showed a comparable response rate and a longer TTNT (Table 5). The adverse event profile was comparable and manageable compared with patients in the BR cohort. The median TTNT2 after Clb-based retreatments was still significant, as with first-line treatment, specifically when used in combination with rituximab. Retreatment at first relapse with the same agents used front line (BR- or Clb-based treatments) is still effective in a subset of patients who have a TTNT > 24 months after initial therapy.
TABLE 5.
Comparison of the Treatment Outcomes of Clb ±P With Clinical Trial Data
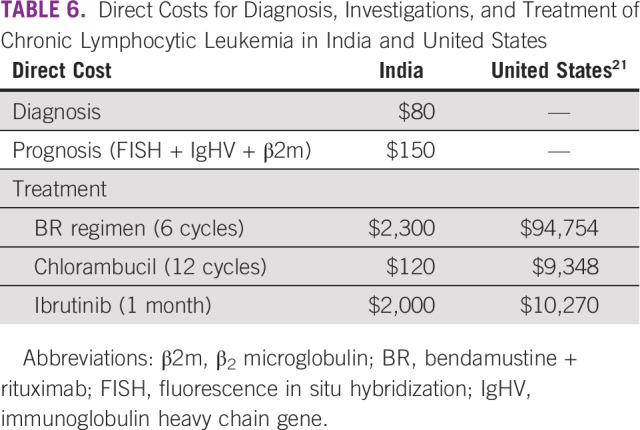
The per capita gross national income in India is approximately $2,000.21,22 Only a quarter of the population in India had some form of medical coverage until the year 2017.23 The cost of rituximab-based regimens is upward of $2,000 for 6 cycles (Table 6). This is why there was less use of rituximab-based regimens in our patients. However, with the launch of the Ayushman Bharat Pradhan Mantri Jan Arogya Yojana (AB-PMJAY) in 2018, which aims to publicly provide insurance coverage of $7,000 per family per year for up to 500 million people,24 the utilization rates of rituximab-based regimens are already up. A total of 45/80 (56.2%) patients have so far received rituximab-based regimens in the year 2018-2019 at our center.
TABLE 6.
Direct Costs for Diagnosis, Investigations, and Treatment of Chronic Lymphocytic Leukemia in India and United States
The outcomes of patients lost to follow-up are unknown. Prognostic information is unavailable for the majority of the patents. Data on dose and cycle adjustments were not documented in all patients. The CR was unconfirmed in the absence of a bone marrow examination. Minimal residual disease data were unavailable in this cohort of patients. TTNT rather than PFS data were available. A longer follow-up is required to study the overall survival outcomes. The data from this single-center study may not be entirely reflective of the practices or outcomes in all centers, given the heterogeneity in patients and the health care delivery systems, but they are likely representative of data from most tertiary referral centers in India.25
AUTHOR CONTRIBUTIONS
Conception and design: Deepesh P. Lad, Pankaj Malhotra, Neelam Varma, Subhash Varma
Administrative support: Subhash Varma
Provision of study material or patients: Deepesh P. Lad, Gaurav Prakash, Pankaj Malhotra, Sreejesh Sreedharanunni, Shano Naseem, Subhash Varma
Collection and assembly of data: V. Tejaswi, Nishant Jindal, Gaurav Prakash, Pankaj Malhotra, Arihant Jain, Sreejesh Sreedharanunni, Manupdesh Sachdeva, Shano Naseem, Neelam Varma, Subhash Varma
Data analysis and interpretation: V. Tejaswi, Nishant Jindal, Pankaj Malhotra, Alka Khadwal, Arihant Jain, Manupdesh Sachdeva, Shano Naseem, Neelam Varma, Subhash Varma
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1. Global Health Data Exchange: GBD results tool. http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2017-permalink/7370f9af81d6bc6eb855f6f4b122eacf.
- 2.Ruchlemer R, Polliack A. Geography, ethnicity and “roots” in chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54:1142–1150. doi: 10.3109/10428194.2012.740670. [DOI] [PubMed] [Google Scholar]
- 3.Pan JW, Cook LS, Schwartz SM, et al. Incidence of leukemia in Asian migrants to the United States and their descendants. Cancer Causes Control. 2002;13:791–795. doi: 10.1023/a:1020608328969. [DOI] [PubMed] [Google Scholar]
- 4.Gunawardana C, Austen B, Powell JE, et al. South Asian chronic lymphocytic leukaemia patients have more rapid disease progression in comparison to White patients. Br J Haematol. 2008;142:606–609. doi: 10.1111/j.1365-2141.2008.07226.x. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal N, Naithani R, Mahapatra M, et al. Chronic lymphocytic leukemia in India--A clinico-hematological profile. Hematology. 2007;12:229–233. doi: 10.1080/10245330701255064. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M. Chronic lymphocytic leukemia: 2020 update on diagnosis, risk stratification and treatment. Am J Hematol. 2019;94:1266–1287. doi: 10.1002/ajh.25595. [DOI] [PubMed] [Google Scholar]
- 7. Global Burden of Disease Collaborative Network: Global Burden of Disease Study 2015 (GBD 2015) Socio-Demographic Index (SDI) 1980–2015. Seattle, WA: Institute for Health Metrics and Evaluation, 2016. [Google Scholar]
- 8.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Cancer Institute, Surveillance, Epidemiology, and End Results Program: Cancer Stat Facts: Leukemia–Acute Myeloid Leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html.
- 10.Yanamandra U, Sahu KK, Karunakaran P, et al. Adolescent and young adult chronic myeloid leukemia in real-world settings: Experience from a tertiary care institute in Northern India. J Adolesc Young Adult Oncol. 2019;8:94–97. doi: 10.1089/jayao.2018.0007. [DOI] [PubMed] [Google Scholar]
- 11.Yanamandra U, Saini N, Chauhan P, et al. AYA-myeloma: Real-world, single-center experience over last 5 years. J Adolesc Young Adult Oncol. 2018;7:120–124. doi: 10.1089/jayao.2017.0034. [DOI] [PubMed] [Google Scholar]
- 12.Parikh SA, Rabe KG, Kay NE, et al. Chronic lymphocytic leukemia in young (≤ 55 years) patients: A comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99:140–147. doi: 10.3324/haematol.2013.086066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goede V, Bahlo J, Robrecht S, et al: Cumulative illness rating scale (CIRS) provides prognostic information beyond the international prognostic index for chronic lymphocytic leukemia (CLL-IPI): An across-trial analysis by the GCLLSG. Presented at the 22nd Congress of the European Hematology Association, Madrid, Spain, June 22-25, 2017 (abstr P251) [Google Scholar]
- 14.Nampoothiri RV, Kasudhan KS, Patil AN, et al. Impact of frailty, melphalan pharmacokinetics, and pharmacogenetics on outcomes post autologous hematopoietic cell transplantation for multiple myeloma. Bone Marrow Transplant. 2019;54:2088–2095. doi: 10.1038/s41409-019-0631-0. [DOI] [PubMed] [Google Scholar]
- 15.Mato A, Nabhan C, Kay NE, et al. Real-world clinical experience in the Connect chronic lymphocytic leukaemia registry: A prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016;175:892–903. doi: 10.1111/bjh.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rani L, Gogia A, Singh V, et al. Comparative assessment of prognostic models in chronic lymphocytic leukemia: Evaluation in Indian cohort. Ann Hematol. 2019;98:437–443. doi: 10.1007/s00277-018-3525-0. [DOI] [PubMed] [Google Scholar]
- 17.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 18.Kleeberg UR, Linde H, Günther G, et al. Bendamustin-rituximab combination is a safe and effective, ambulatory treatment for elderly patients with chronic lymphocytic leukemia: Retrospective real-world analysis by age from a German registry and review of the literature. Anticancer Res. 2016;36:2827–2838. [PubMed] [Google Scholar]
- 19.Cuneo A, Follows G, Rigolin GM, et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study. Haematologica. 2018;103:1209–1217. doi: 10.3324/haematol.2018.189837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goede V, Eichhorst B, Fischer K, et al. Past, present and future role of chlorambucil in the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:1585–1592. doi: 10.3109/10428194.2014.963077. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Jain N, Ayer T, et al. Economic burden of chronic lymphocytic leukemia in the era of oral targeted therapies in the United States. J Clin Oncol. 2017;35:166–174. doi: 10.1200/JCO.2016.68.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Bank: Data: India. https://data.worldbank.org/country/india.
- 23. La Forgia G, Nagpal S: Government-sponsored health insurance in India: Are you covered? Washington, DC, The World Bank, 2012. [Google Scholar]
- 24.Ministry of Health and Family Welfare Operational Guidelines on Ayushman Bharat National Health Protection Mission. 2018 https://ayushmanbharatharyana.in/assets/pdfs/AB-NHPM%20Operational%20Guidelines%20June%202018.pdf
- 25.Philip C, George B, Ganapule A, et al. Acute myeloid leukaemia: Challenges and real world data from India. Br J Haematol. 2015;170:110–117. doi: 10.1111/bjh.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 27.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): A randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]