Abstract
Cancer in the United States accounts for $600 billion in health care costs, lost work time and productivity, reduced quality of life, and premature mortality. The future of oncology delivery must mend disconnects to equitably improve patient outcomes while constraining costs and burden on patients, caregivers, and care teams. Embedding learning health systems into oncology can connect care, engaging patients and providers in fully interoperable data systems that remotely monitor patients; generate predictive and prescriptive analytics to facilitate appropriate, timely referrals; and extend the reach of clinicians beyond clinic walls. Incorporating functional learning systems into the future of oncology and follow-up care requires coordinated national attention to 4 synergistic strategies: (1) galvanize and shape public discourse to develop and adopt these systems, (2) demonstrate their value, (3) test and evaluate their use, and (4) reform policy to incentivize and regulate their use.
INTRODUCTION
Organizational systems depend on reliable, secure, and sustainable communication structures to protect their core internal functions. Disconnects within those structures can present crucial points of vulnerability that—without repair—can lead to inefficiencies and expenses in the more benign case or to catastrophic failure in the worst.1 Health care is no exception. Many sectors of the economy take full advantage of technology and information systems to enhance, connect, and secure their communication and operational structures; however, health care lags behind and needs modernization.2-4 Legislation, including the Health Information Technology for Economic and Clinical Health Act5 in 2009, Medicare Access and CHIP Reauthorization Act6 of 2015, and 21st Century Cures Act7 in 2016, addressed the disconnects in information flow in health care by: (1) providing incentives for the meaningful use of health information technology to improve patient and system outcomes, (2) shifting Medicare payment policies to reimburse for value over volume, (3) accelerating the pace of biomedical discovery through data sharing, (4) enhancing the usability of systems to optimize workflows and empower care teams, and (5) improving interoperability between systems and preventing information blocking. These enabling components of legislation provide the groundwork for systemic change to mend structural and functional disconnects in health care.
A more robust data ecosystem—a learning and communication system in health care—is needed. A prima facie example is embodied within the 21st Century Cures Act: The Cancer Moonshot, which seeks to accelerate progress to accomplish in 5 years what would otherwise take 10, in part by sharing data and creating a “learning oncology ecosystem.” As envisioned by the National Cancer Institute (NCI), a data ecosystem would combine data from research and clinical care, integrating evidence-based interventions throughout the clinical care system, engaging patients and clinicians in data collection, and returning data back to the oncology community for quality improvement and discovery.8 This interoperable infrastructure is needed to meet demands for services as an aging population brings more cancer diagnoses and long-term survivors than can be handled by the insufficient supply of clinicians.9-11
To underscore this point, the national cancer program is best conceptualized as a “system of systems”12 that must interact across multiple levels of health care and public health to be effective. For example, public health programs and policies have reduced the smoking rate for adults in the United States and decreased lung cancers, and the implementation of screening guidelines has led to earlier detection and decreases in breast, cervical, prostate, and colorectal cancer mortality.13 However, evidence-based programs are inequitably implemented and create disparities in population outcomes. Although 70% of smokers visit the health care system, relatively few receive cessation counseling and follow-up.14 Similarly, failure to reliably recommend and follow up on results of cancer screening can lead to increased presentations of late-stage disease,15 a burden borne disproportionately by vulnerable populations.16 Disconnects during and after cancer care obstruct lines of communication leading to a lack of: (1) identification of patients for clinical trials; (2) patient engagement, patient-provider shared decision making, and patient adherence to care; (3) mitigation of acute, chronic, and late treatment effects interfering with function and quality of life; (4) detection of recurrences or subsequent cancers; (5) care coordination and efficiencies leading to waste or preventable expenses for emergency services or hospitalization; (6) connection between data systems for scheduling/quality reporting/billing/patient input; and (7) quality care leading to medical errors.17-19 These problems are exacerbated for the majority of cancer survivors who need complex care to manage multiple comorbid conditions20 and when care is coordinated across distance and health systems. Higher rates of cancer mortality in nonmetropolitan counties compared with urban or suburban counties21 are partially because rural areas lack services and patients must drive long distances for care.22 Meta-analyses reveal that patients with cancer living > 50 miles from a hospital present with more advanced stages of cancer, exhibit lower adherence to prescribed treatments, and have worse prognoses and quality of life than those closer to services.23
THE PROMISE OF SMART AND CONNECTED INFORMATION SYSTEMS
The good news is that disconnected care can be mitigated by enhancing capacities afforded through the modernization legislation and by solutions that create these systems of systems. To illustrate, a California health system used the tracking capacity of its electronic health record (EHR) to simultaneously trigger prompts for appointments to patients and their primary care teams. Population management dashboards allowed targeted outreach efforts for those who failed to engage. Integrating this preventive care facet into the operational system achieved a 6-fold increase in mammography and Pap smears and a 10-fold increase in colorectal cancer screening.24 Independent program evaluation confirmed that this systems-level approach led to equitable screening increases across the organization’s catchment area.25 Similarly, a systems-level intervention allowing patients to report symptoms electronically to their care teams produced a significant improvement in outcomes and quality of life26 and downstream survival.27
These interventions are encouraging, but they are insufficient to stave off the pending crisis in oncology care. The number of cancer survivors is projected to increase from 16.9 million28 to 26.1 million by 2040.29,30 As America ages, 73% of survivors will likely be ≥ 65 years in 2040 and have comorbid conditions and complex needs. Currently, US cancer costs are $600 billion in treatments12 and the additional costs to families in lost productivity, reduced functional capacity and quality of life, distress, and premature mortality. Transforming care models and enhancing capacities afforded through the modernization legislation can help address expanding costs of care and clinician shortages. Engaging in “business as usual” is not an option.11,18,31
The 2016 President’s Cancer Panel report, “Improving Cancer-Related Outcomes with Connected Health,”24 articulated a future vision for oncology care that can reduce burden on oncology care teams and costs by automating tasks and eliminating inefficiencies, extending the benefits of current evidence equitably, and improving patient and caregiver experiences, supporting them to engage proactively in their care. This report leveraged the Smart and Connected Health program, an NCI–National Science Foundation collaboration outlining an ecosystem to improve health care capacity through networking and information technology.32-34 The President’s Cancer Panel concluded “the time to act is now,” because breakthroughs in the smart and connected health ecosystem have expanded capabilities in prevention, early detection, treatment, and survivorship. However, for these to enable progress on the Cancer Moonshot goal will require: (1) improving connected health approaches, enabling effective teamwork in health care; (2) enhancing patient engagement; and (3) enriching clinical care by incorporating data from smartphones and wearable devices.
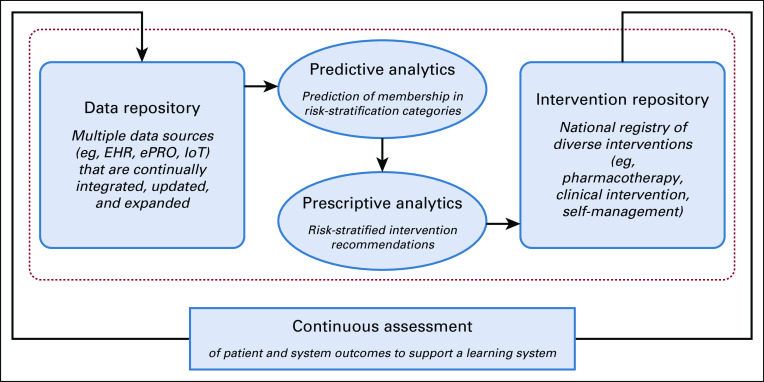
To accelerate progress, this article outlines a vision for embedding learning health systems into the future of oncology care by leveraging lessons learned from ongoing initiatives of the NCI, American Cancer Society (ACS), Oncology Nursing Society (ONS), and the Federal Communications Commission’s (FCC’s) Connect2Health Task Force. In this vision of a future that protects against structural and operational disconnects, patients and providers must be supported by fully interoperable data systems that allow clinical teams to extend their care beyond the physical constraints of offices and, where possible, to move from point-of-care to point-of-need.35 Such an expanded “thinking system”36 would use signals generated by a connected health care system to ensure that every patient remains connected with their caregivers and clinical teams through on-site care and remote monitoring, timely delivery of information, and “care” delivered in community and home settings via telehealth approaches wherever possible. In such a system, data must be integrated into predictive and prescriptive analytics via machine learning/artificial intelligence and other analytic methods to support clinical decision making, enabling the right referral to the right resource at the right time, and to track referrals for evidence of completion and outcome. This approach must cover therapies and access to appropriate clinical trials, management of chronic or late effects, surveillance for recurrences and new cancers, and support for self-management, as needed. Algorithms would help triage care to the appropriate provider or to self-management materials, delivering care, information, and support outside of clinic walls as much as possible. This combination of extending the reach of clinicians and supporting self-management should help address clinician shortages while providing more equitable access to needed interventions. Such a system should be self-calibrating and error resistant, in the same ways that human factors specialists have engineered mission-critical operations in areas with low fault tolerance, such as aviation or autonomous navigation.37 Figure 1 offers a high-level schematic of how such a system could be constructed to support oncology care.
FIG 1.
The learning health care system for oncology care. EHR, electronic health record; ePRO, electronic patient-reported outcome; IoT, Internet of Things (e.g. implantable or wearable devices).
TRANSITIONING TO THE FUTURE: LEARNING SYSTEMS OF ONCOLOGY CARE
The vision presented in this article leverages two efforts to accelerate progress in taking learning oncology systems from concept to reality. In the first effort, The ACS and ONS Roundtable brought together a diverse group of > 40 stakeholder organizations to inform the development, testing, and implementation of digital tools for mitigating adverse effects of cancer in ways that are innovative, operationally tenable, and commercially viable. Participants in the 2018 meeting included content experts identified from cancer nonprofit organizations, technology companies, provider professional societies, health care systems, advocacy groups, research funders, and pharmaceutical companies. Solutions for catalyzing the development and implementation of learning oncology systems centered on four themes: (1) using shared stakeholder understanding to develop use cases for the digital systems to develop and test solutions; (2) creating and digitizing risk-stratified guidelines for symptom prevention or mitigation and function preservation; (3) identifying ways to use data to inform and foster patient-provider communication, patient-centered care, and patient activation and empowerment; and (4) implementing innovations in the design and funding of pilot systems that are needed to demonstrate return on investment of these systems.
In a second effort, the FCC’s Connect2Health Task Force, on behalf of the Linking and Amplifying User-Centered Networks through Connected Health (L.A.U.N.C.H.) collaborators (NCI, FCC Connect2Health Task Force, Amgen, University of California San Diego Design Lab, and UK Markey Cancer Center) convened a meeting in 2019 of senior thought leaders who were identified as experts in their relative disciplines from government, academia, the telecommunication and technology industry, health care systems, public health, biotechnology, design, and innovation sectors. The meeting gathered information and expert input related to the L.A.U.N.C.H. initiative’s goals of improving cancer outcomes in rural and underserved regions by codesigning cutting-edge cancer symptom management solutions enabled by broadband access and adoption. Participants endorsed L.A.U.N.C.H. goals of harnessing the power of collective, robust, cross-sector collaboration to address this problem and to empower communities in user-centered design methodologies to find sustainable, scalable methods for solving local health challenges. Major points of consideration to achieve L.A.U.N.C.H. goals included: developing robust demonstration projects in high cancer-burden areas, with Appalachia Kentucky representing the first use case; leveraging citizen-science evidence; ensuring a regulatory environment that can support an ecosystem of cross-sector collaborators through data sharing and accountability; enhancing stakeholder cooperation for achieving mutual goals; and recognizing that technology must be combined with meaningful community engagement including all stakeholders—patients/caregivers, families, and providers. In addition, the expert panel recommended further development of the unique value framework for this effort and that an evaluation approach using an iterative learning process would be more productive than relying on traditional evaluation methods (eg, randomized controlled trials).
Four Key Strategies to Facilitate the Development, Adoption, and Use of Learning Oncology Systems
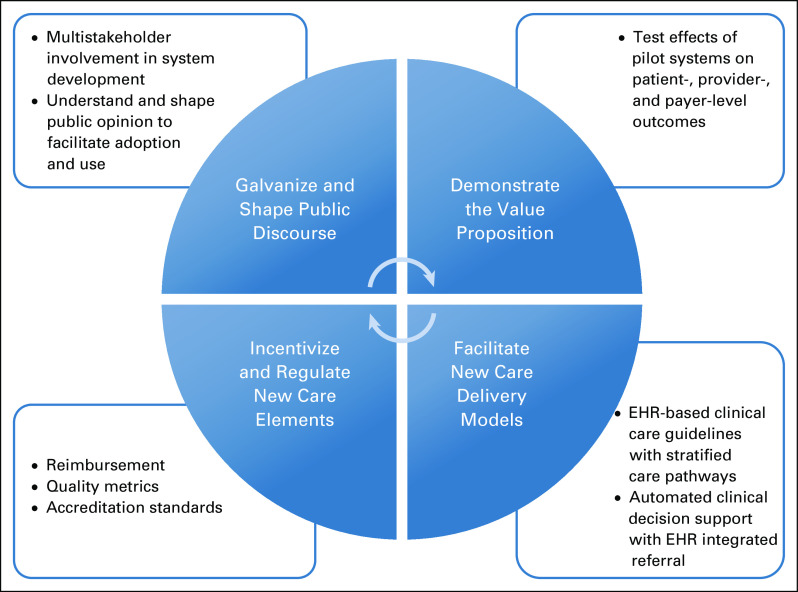
Lessons learned from the ACS/ONS Roundtable and from the L.A.U.N.C.H. initiative point to the need to pursue four simultaneous synergistic strategies to embed functional learning systems into the future of oncology and cancer follow-up care. These include (1) galvanizing and shaping public discourse to inform the development of these systems and facilitate their adoption, implementation, and sustainability; (2) demonstrating the value of learning systems to multiple oncology stakeholders; (3) facilitating new care-delivery models that use learning systems; and (4) incentivizing and regulating care, using policy that supports the use of these systems. As shown in Figure 2 and described here, these four strategies will require research, practice change, and policy reform.
FIG 2.
Four key strategies to facilitate the development, adoption, and use of learning health care systems for oncology care. EHR, electronic health record.
Galvanizing and shaping public discourse to inform the development of learning oncology systems and facilitate their adoption and use.
Designing an effective system for mending the disconnects in health care requires understanding the needs of its end users. Such a system will only be useful to the extent that it solves the challenges that disconnect care—for example, the fragmentation of care components and many transitions in cancer care.38 To identify both the challenges and solutions, providers and patients and their families should be involved as “end users” in the design of these learning systems. User-centered design approaches should be used that incorporate patient- and provider-identified priorities and use local knowledge to solve them. This stakeholder involvement will ensure systems are designed to be responsive to their needs and local conditions. Stakeholder involvement is also critical for shaping discourse about these systems in ways that facilitate their adoption and use. Implementation science approaches can be used to evaluate new systems of care and develop and test strategies for optimal integration of effective processes.39,40
To support a full spectrum of the care system from discovery to delivery, learning oncology systems must consider how best to collect and integrate the molecular, genomic, clinical, behavioral, physiologic, and environmental data needed to understand patient risks and needs and point to effective and timely interventions.41 These systems would point providers and patients toward appropriate clinical trials; care management for physical, psychosocial, or functional chronic and late effects of therapies; prevention strategies for recurrence, subsequent cancers, or late effects of therapy; and empowering and engaging patients in their care and self-management as needed. Creating systems that can achieve these goals will require partnerships with advocacy groups involved in patient data, safety, liquidity, interoperability, and ownership efforts; industry partners who provide or rely on these data for prescriptions to specific drugs or care pathways; and health care administrators, because these systems also must provide timely access to data on utilization, supply, and costs as well as quick and accurate reporting of quality metrics and other regulatory measures.
Demonstrating the value proposition of learning oncology systems is needed to make the business case for these systems to users, payers, and policy makers.
To begin developing a learning oncology system, health care delivery systems could launch a pilot program that evaluates system outcomes that demonstrate the desired return on investment (ROI) or value proposition at patient, provider, and payer levels. For example, a learning oncology system could allow patients to complete electronic patient-reported outcomes measures for remote surveillance of their symptoms/needs. Algorithms would alert clinicians to patients’ needs and connect patients experiencing problematic symptoms or functional problems to self-management materials or (when needed) appropriate referrals for timely and appropriate interventions. Such a pilot system could be evaluated to determine its effects on: (1) patient symptom burden and function, health outcomes, and patient satisfaction (patient level); (2) oncology clinic efficiency, including allowing for more new patient visits (provider level); (3) timely interventions/referrals for supportive care, downstream unplanned hospitalizations or emergency department visits, achievement of quality goals, including screening for recurrence and new primary cancers, and overall costs (payer level); and (4) reductions in variations in and disparities in care. For example, a recent pragmatic trial of an intervention including a real-time EHR-based registry to signal missed appointments or unmet care milestones, a navigator, and clinical feedback improved completion of treatment of all patients with breast and lung cancer and narrowed disparities between Black and White patients.42 Data from pilot tests of initial systems can be used to refine and improve the system and to engage stakeholders who may be initially resistant to changing from old practices.
Facilitating new care delivery models that use learning oncology systems will require building tools that aid care delivery through EHRs.
Digital tools must make care more effective and efficient rather than providing more “boxes to check” that reduce provider time with patients. Digital clinical care guidelines should be stratified by severity/patient need and inform patient-provider communication about decision making during care encounters. Fatigue management provides an example. Patients with low levels of fatigue could be provided with appropriate prevention and management information delivered electronically through a patient portal or online resource. Patients with clinically significant fatigue levels could be provided with timely access to services in their communities to the extent possible (eg, LIVESTRONG at the YMCA program) or to more intensive clinical evaluation and management, as needed. Clinical decision support tools built into the EHR could access patient-reported fatigue scores over time, flag when fatigue levels rise to significant levels, automatically provide electronic self-management support, and provide suggested stepped-care pathways for the clinician to discuss with the patient. Such discussions would happen virtually to the extent possible to limit the need for travel and office visits and preserve clinician time for the highest-acuity and new patients.
To make symptom management guidelines more actionable by a learning oncology system, current clinical care guidelines and assessment tools need to be digitized and integrated with EHRs. Expert consensus is needed to determine the algorithms that would guide appropriate stepped care interventions on the basis of levels of symptom burden/functional problem/need. Building digital tools into EHRs can facilitate quality care and bring more precision to how individuals affected by cancer are treated to meet their needs, values, and preferences.
Methods to incentivize and regulate care in ways that support the development and implementation of learning oncology systems are needed.
With the COVID-19 pandemic, care components critical to a learning oncology system, such as remote monitoring, telehealth, and care management, are rapidly being deployed and reimbursed by health care payers, under temporary relaxation of regulations. However, reimbursement is complicated and inconsistent, with numerous exceptions (ie, few standardized processes), and there is risk that progress in policies made during the COVID-19 crisis will regress once the crisis subsides. Furthermore, these technologies rely on broadband internet, which could exacerbate health disparities for patients who lack internet access. Research to demonstrate the value proposition of these components to payers and defining the best practices for their use can help in paving the way for their reimbursement. Where COVID-19 has spurred connections in data systems, the value proposition may be available from administrative databases comparing relevant outcomes, care efficiency, and costs in pre- versus post–COVID-19 periods. Quality metrics from the National Quality Forum, the ASCO Quality Oncology Practice Initiative, or the ONS would help benchmark and spur the development of learning oncology system components. Incorporating these care components into accreditation standards such as those from the American College of Surgeons’ Commission on Cancer would further help develop and support the implementation of these systems.
CONCLUSION AND NEXT STEPS
Pursuing these four strategies to mend disconnects in cancer care will catalyze the embedding of learning oncology systems into the future of cancer care. However, this requires changing the “usual ways” research, heath care, public health, and health policy happen. Science must be adapted for real-world implementation—to be more flexible, pivot and adapt in real time to success/failures, demonstrate ROI to multiple stakeholders, and consider scalability from the beginning. This will require alternative methods to traditional randomized controlled trials; pragmatic trials and nonrandomized designs and rapid, iterative prototyping are necessary to test new models of care in real-world situations.43 With the large data sets generated by a learning oncology system deployed throughout a health care delivery system, Multiphase Optimization Strategy (MOST) and Sequential Multiple Assignment Randomized Trial (SMART) designs can help identify effective components for given patients and, when paired with large data sets from learning oncology systems, allow for very rapid-cycle testing of care delivery components and methods.44 This research may be funded by traditional sources (eg, National Institutes of Health) or by the Centers for Medicare and Medicaid Services or health insurance companies, private industry, or health care delivery systems.
Imbedding learning systems into oncology must occur in an evolving health care system that is struggling to innovate in multiple ways simultaneously, further complicating progress. Change management principles suggest that institutions begin to build a learning oncology system by connecting the bright spots in their organization,45 asking what is already going well (eg, telehealth programs? Stepped care pathways? Community outreach and engagement?). Clinical care may need robust linkages with components outside of the health care system (eg, companies that provide cloud-based EHR storage and analytics, genomic and biomarker testing companies, and companies that provide adjuncts to care, like health coaching or care planning). Facilitating these connections requires considering the challenges and the value proposition of connection at each level. For example, patients may use symptom tracker apps that need connection to the interventions and clinicians they need, or they will see no value in these data. Clinical systems must implement patient-reported outcomes in ways patients will complete and that generate data clinicians, patients, and administrators can use. Genomic testing and other clinical information must be integrated in patient records and connected to work flow. Technology companies often are not connected to how their data/products fit into the whole patient care trajectory or work flow. In short, EHRs must move from payment-driven systems to data ecosystems that capture the patient experience across the care trajectory to provide meaningful data to drive future efforts.
All of these parts must be incentivized to work together—the future will demonstrate whether that transformation will come by blunt force of necessity through capitated payments, or whether care components will be woven together by additional health care mergers and acquisitions or through grassroots efforts and successful lobbying of patients, families, or clinicians. Regardless, focused attention to pursuing the research, practice change, and policy reform strategies described herein will help US health care delivery systems innovate the future of oncology care and create smart, connected learning oncology systems that actually work in the service of better care, better outcomes, and better value.
The opinions expressed by the authors are their own, and this material should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
SUPPORT
Supported by Amgen and Select Medical’s ReVital Cancer Rehabilitation Program (American Cancer Society–Oncology Nursing Society Roundtable), and the National Cancer Institute, Federal Communications Commission, and Amgen (L.A.U.N.C.H.).
AUTHOR CONTRIBUTIONS
Conception and design: Catherine M. Alfano, Deborah K. Mayer, Ellen Beckjord, David K. Ahern, Lisa K. Sheldon, Lisa M. Klesges, Eliah Aronoff-Spencer, Bradford W. Hesse
Financial support: Catherine M. Alfano
Administrative support: Catherine M. Alfano
Provision of study material or patients: Eliah Aronoff-Spencer
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Deborah K. Mayer
Stock and Other Ownership Interests: Carevive
Ellen Beckjord
Honoraria: Phillips
Travel, Accommodations, Expenses: Phillips
David K. Ahern
Employment: Abacus Health Solutions
Stock and Other Ownership Interests: Abacus Health Solutions
Patents, Royalties, Other Intellectual Property: Copyright for a Behavioral Health Comorbidity Management Program
Eliah Aronoff-Spencer
Research Funding: Dexcom (Inst), Tandem Diabetes Care (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vicente KJ. The Human Factor: Revolutionizing The Way People Live With Technology. ed 1. New York, NY: Taylor and Francis Books; 2003. [Google Scholar]
- 2.Cosgrove DM, Fisher M, Gabow P, et al. Ten strategies to lower costs, improve quality, and engage patients: The view from leading health system CEOs. Health Aff (Millwood) 2013;32:321–327. doi: 10.1377/hlthaff.2012.1074. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal D. Launching HITECH. N Engl J Med. 2010;362:382–385. doi: 10.1056/NEJMp0912825. [DOI] [PubMed] [Google Scholar]
- 4.Cutler DM, Davis K, Stremikis K. Why health reform will bend the cost curve. Issue Brief (Commonw Fund) 2009;72:1–16. [PubMed] [Google Scholar]
- 5. The American Recovery and Reinvestment Act of 2009 (ARRA), Public Law 111-5, 123 Stat 115 (February 17, 2009)
- 6. Medicare Access and CHIP Reauthorization Act, Public Law 114-10, (April 16, 2015)
- 7. 21st Century Cures Act, Public Law 114-255, 114th Cong, 2016.
- 8.National Cancer Institute NCI Cancer Research Data Ecosystem Infographic. https://www.cancer.gov/research/nci-role/bioinformatics/cancer-research-data-ecosystem-infographic
- 9.Erikson C, Salsberg E, Forte G, et al. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine . Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 11.Shulman LN, Sheldon LK, Benz EJ. The future of cancer care in the United States: Overcoming workforce capacity limitations. JAMA Oncol. 2020;6:327. doi: 10.1001/jamaoncol.2019.5358. [DOI] [PubMed] [Google Scholar]
- 12. National Academies of Sciences, Engineering, and Medicine: Guiding Cancer Control: A Path to Transformation. Washington, DC, National Academies Press, 2019. [PubMed] [Google Scholar]
- 13.American Cancer Society . Cancer Facts & Figures 2019. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 14.Fiore MC, Baker TB. Should clinicians encourage smoking cessation for every patient who smokes? JAMA. 2013;309:1032–1033. doi: 10.1001/jama.2013.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zapka J, Taplin SH, Price RA, et al. Factors in quality care--The case of follow-up to abnormal cancer screening tests--problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010:58–71. doi: 10.1093/jncimonographs/lgq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goding Sauer A, Siegel RL, Jemal A, et al. Current prevalence of major cancer risk factors and screening test use in the United States: Disparities by education and race/ethnicity. Cancer Epidemiol Biomarkers Prev. 2019;28:629–642. doi: 10.1158/1055-9965.EPI-18-1169. [DOI] [PubMed] [Google Scholar]
- 17. Hesse BW, Arora NK, Klein WM: Communication science: Connecting systems for health, in Hesse BW, Ahern DK, Beckjord E (eds): Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care. Boston, MA, Elsevier, 2016, pp 253-275. [Google Scholar]
- 18.Alfano CM, Leach CR, Smith TG, et al. Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J Clin. 2019;69:35–49. doi: 10.3322/caac.21548. [DOI] [PubMed] [Google Scholar]
- 19. Beckjord E, van Londen GJ, Rechis R: Survivorship, in Hesse BW, Ahern DK, Beckjord E (eds): Oncology Informatics: Using Health Information Technology to Improve Processes and Outcomes in Cancer Care. Elsevier, Cambridge, MA, 2016, pp 159-179. [Google Scholar]
- 20.Leach CR, Weaver KE, Aziz NM, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9:239–251. doi: 10.1007/s11764-014-0403-1. [DOI] [PubMed] [Google Scholar]
- 21.Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980-2014. JAMA. 2017;317:388–406. doi: 10.1001/jama.2016.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler SB, Basch E. Translating cancer surveillance data into effective public health interventions. JAMA. 2017;317:365–367. doi: 10.1001/jama.2016.20326. [DOI] [PubMed] [Google Scholar]
- 23.Ambroggi M, Biasini C, Del Giovane C, et al. Distance as a barrier to cancer diagnosis and treatment: Review of the literature. Oncologist. 2015;20:1378–1385. doi: 10.1634/theoncologist.2015-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.President’s Cancer Panel . Improving Cancer-Related Outcomes with Connected Health. Washington, DC: National Cancer Institute; 2016. [Google Scholar]
- 25.Rhoads KF, Patel MI, Ma Y, et al. How do integrated health care systems address racial and ethnic disparities in colon cancer? J Clin Oncol. 2015;33:854–860. doi: 10.1200/JCO.2014.56.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197–198. doi: 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Cancer Society . Cancer Treatment & Survivorship Facts & Figures 2019-2021. Atlanta, GA: American Cancer Society; 2019. [Google Scholar]
- 29.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “silver tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 31.Alfano CM, Mayer DK, Bhatia S, et al. Implementing personalized pathways for cancer follow-up care in the United States: Proceedings from an American Cancer Society-American Society of Clinical Oncology summit. CA Cancer J Clin. 2019;69:234–247. doi: 10.3322/caac.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.President’s Council of Advisors on Science and Technology . Designing a digital future: Federally funded research and development in networking and information technology. Washington, DC: Executive Office of the President of the United States; 2010. [Google Scholar]
- 33.President’s Council of Advisors on Science and Technology . Realizing the Full Potential of Health Information Technology to Improve Healthcare for Americans: The Path Forward. Washington, DC: Executive Office of the President of the United States; 2010. [Google Scholar]
- 34.President’s Council of Advisors on Science and Technology . Better Health Care and Lower Costs: Accelerating Improvement through Systems Engineering. Washington, DC: The White House; 2014. [Google Scholar]
- 35.Dentzer SE. Health Care Without Walls. Washington, DC: Network for Excellence in Health Innovation; 2018. [Google Scholar]
- 36.Randhawa GS, Xiao Y, Gorman PN. Designing a “thinking system” to reduce the human burden of care delivery. EGEMS (Wash DC) 2019;7:18. doi: 10.5334/egems.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tevaarwerk AJ, Klemp JR, van Londen GJ, et al. Moving beyond static survivorship care plans: A systems engineering approach to population health management for cancer survivors. Cancer. 2018;124:4292–4300. doi: 10.1002/cncr.31546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taplin SH, Clauser S, Rodgers AB, et al. Interfaces across the cancer continuum offer opportunities to improve the process of care. J Natl Cancer Inst Monogr. 2010;2010:104–110. doi: 10.1093/jncimonographs/lgq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: A new model for biomedical research. JAMA. 2016;315:1941–1942. doi: 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsey AT, Proctor EK, Chambers DA, et al. Designing for Accelerated Translation (DART) of emerging innovations in health. J Clin Transl Sci. 2019;3:53–58. doi: 10.1017/cts.2019.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. doi: 10.1016/j.jnma.2019.03.001. Cykert S, Eng E, Manning MA, et al: A multi-faceted intervention aimed at Black-White disparities in the treatment of early stage cancers: The ACCURE Pragmatic Quality Improvement trial. J Natl Med Assoc 10.1016/j.jnma.2019.03.001 [epub ahead of print on March 27, 2019] [DOI] [PubMed] [Google Scholar]
- 43.Peek CJ, Glasgow RE, Stange KC, et al. The 5 R’s: An emerging bold standard for conducting relevant research in a changing world. Ann Fam Med. 2014;12:447–455. doi: 10.1370/afm.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): New methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5) suppl:S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heath C, Heath D: Switch: How to Change Things When Change Is Hard (ed 1). New York, NY, Broadway Books, 2010. [Google Scholar]