Abstract
Nogo-B receptor (NgBR) is a type I receptor with a single transmembrane domain and specifically binds to ligand Nogo-B. A previous study demonstrated that NgBR was highly expressed in human breast invasive ductal carcinoma and promoted epithelial-mesenchymal transition in breast tumor cells. Our recent work found that NgBR expression was associated with a poor prognosis in human patients with hepatocellular carcinoma (HCC). Here, we elucidate that the increased expression of NgBR contributes toward the increased cell growth of human HCC cells both in vitro and in vivo. Cell viability and clonogenic survival analysis results demonstrated that knockdown of NgBR inhibits the cell growth in human HCC cells, which correlates with a reduction in the phosphorylation of Akt levels. Furthermore, overexpression of NgBR by the cotransfected pIRES-NgBR plasmid together with NgBR siRNA in human HCC cells can rescue impaired phosphorylation of Akt levels in NgBR knockdown human HCC cells. In addition, cell viability analyses showed that NgBR overexpression can rescue the cell growth inhibition presented in human HCC NgBR knockdown cells. Taken together, our results suggest that NgBR potentially acts as an oncogene in HCC by increasing Akt activity. Thus, NgBR may represent a new potential diagnostic and therapeutic target for the treatment of HCC.
Keywords: Akt signal pathway, cell growth, hepatocellular carcinoma, Nogo-B receptor
1 |. INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer and the second leading cause of cancer-related deaths worldwide.1–3 The incidence of HCC has increased in recent decades because of the increasing incidence of hepatitis C viral infection and other causes of cirrhosis.3 Despite improvements in clinical treatments, such as surgical resection, liver transplantation, and interventional therapy, the overall 5-year survival rate is still only around 5% and the long-term prognosis remains dismal.4 Because tumorigenesis and tumor progression in hepatic cells are caused by multiple genetic and molecular alterations, a single-molecule targeting therapy has yet to be discovered. Thus, the identification of target molecules that control the biological characteristics of HCC is important for the effective treatment of HCC.
Nogo-B is the member of the reticulon family of proteins and is found in most tissues.5,6 A previous study demonstrated that Nogo-B is highly expressed in caveolin-1–enriched microdomains of endothelial cells (EC) and the amino terminus (residues 1 to 200) of Nogo-B (AmNogo-B) serves as a chemoattractant for EC.7 The Nogo-B receptor (NgBR) was identified asa receptor specific for AmNogo-B by an expression-cloning approach.6,8 A previous study demonstrated that high-affinity binding of AmNogo-B to NgBR is sufficient for AmNogo-B–mediated chemotaxis and tube formation of EC.8 In addition, our previous work demonstrated that NgBR is necessary for in vivo angiogenesis in zebrafish via the Akt pathway.9 Otherwise, NgBR is highly expressed in human breast invasive ductal carcinoma and NgBR expression in breast tumor cells is highly related to the estrogen receptor and survival.10 A recent study also showed that NgBR promotes epithelial-mesenchymal transition in breast tumor cells.11 Furthermore, our recent studies demonstrated that NgBR expression was associated with a poor prognosis in human patients with HCC.12
However, the functions of NgBR in modulating the progression of HCC have not been investigated. The aim of this study was to investigate the biological functions and molecular mechanisms of NgBR in human HCC and to identify the target genes regulated by the NgBR.
2 |. MATERIALS AND METHODS
2.1 |. Cell lines and cell culture
Human HCC cell lines HepG2 and SMMC-7721 were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Normal liver cell line LO2 was obtained from KeyGen Biotech Co Ltd (Nanjing, China). All these cells were cultured in Dulbecco modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with antibiotics (1 × penicillin/streptomycin 100 U/mL; Gibco) and 10% fetal bovine serum (Gibco, Carlsbad, CA). Cells were incubated at 3700B0030C in a humidified atmosphere containing 5% CO2.
2.2 |. Antibodies
The NgBR rabbit monoclonal antibody (CloneID: EPR8668) was generated by Epitomics (Burlingame, CA) as a collaboration project. Rabbit polyclonal antibodies for the phosphorylation of AKT (Ser473), total Akt, β-actin, and all the secondary antibodies were purchased from Cell Signaling (Danvers, MA).
2.3 |. Transfection of siRNA and plasmid DNA
NgBR siRNA1 (S1 forward: GGAAAUACAUAGACCUACA, S1 reverse: UGUAGGUCUAUGUAUUUCC) and NgBR siRNA2 (S2 forward: GUAUGGAAAUAAACUUAUA, S2 reverse: UAUAAGUUUAUUUCCAUAC) oligonucleotides with 3′dTdT overhangs were synthesized by Shanghai GenePharma Co (Shanghai, China). Control small (or short) interfering RNA (siRNA) in experiments refers to an All-Star nonsilencing siRNA (forward sequence: GGGUAUCGACG AUUACAAAUU, reverse sequence: UUUGUAAUCGUCG AUACCCUG) synthesized by Shanghai GenePharma Co. Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA) was used for the transfection of siRNA according to the manufacturer’s instructions. Lipofectamine 2000 (Invitrogen) was used for the transfection of the NgBR expression plasmid pIRES-NgBR. The specificity of NgBR siRNA (siNgBR) and pIRES-NgBR has been confirmed in our previous publications.8,9
2.4 |. Quantitative real-time polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent according to the manual (TaKaRa Bio, Dalian, China) and cDNA was reverse-transcribed using the PrimeScript RT Reagent Kit (TaKaRa Bio) according to the manufacturer’s instructions. A real-time polymerase chain reaction (PCR) was performed using the QuantiTect SYBR Green PCR Kit (TaKaRa Bio) and was run on Stratagene MX3000P (Agilent, CA). The relative messenger RNA (mRNA) expression of each gene was normalized to β-actin RNA levels. The primers were synthesized by Invitrogen. The forward and reverse primers for NgBR are 5′-TGCCAGTTAGTAGCCCAGAAGCAA-3′ and 5′-TGATGTGCCAGGGAAGAAAGCCTA-3′, respectively. The forward and reverse primers for β-actin are 5′-TTCTACAATGAGCTGCGTGTGGCT-3′ and 5′-TAGCACAGCCTGGATAGCAACGTA-3′, respectively.
2.5 |. Western blot
Cells were harvested and lysed in an immune precipitation assay buffer (KeyGen Biotech Co Ltd) supplemented with 1 mM phenylmethylsulfonyl fluoride (KeyGen Biotech Co Ltd) and 1 mM of a phosphatase inhibitor cocktail (KeyGen Biotech Co Ltd). The protein concentration was determined using a BCA protein assay kit (KeyGen Biotech Co Ltd). Same amounts of protein samples were separated in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and then transferred to a polyvinylidene difluoride membrane (Pall Corporation, Port Washington, NY). The protein band intensities were evaluated using ECL Western blot analysis kit (Advansta, Menlo Park, CA) and were normalized to those of β-actin.
2.6 |. Clonogenic survival assay
Cells were seeded in triplicate into a 6-well culture dish (1000 cells per well). Cells were transfected with siNgBR- and/or NgBR-expressing vector pIRES-NgBR. Then, the cells were maintained for 10 days. The cell colonies were washed 3 times with phosphate-buffered saline buffer, fixed in methanol for 15 minutes, and stained with Crystal Violet (Sigma-Aldrich, St Louis, MO) for 15 minutes at room temperature. The plates were dried at room temperature and the colony numbers containing more than 50 cells were microscopically counted.
2.7 |. Cell viability assay
Cell viability was determined using the Cell Counting Kit-8 (CCK-8) assay according to the manufacturer’s instructions. The CCK-8 was purchased from Dojindo (Kumamoto, Japan). Cells were seeded in 96-well plates at 2000 cells per well. At 48 hours after seeding, cells were transfected with siRNA and/or vector. Then, after the cells were cultured for 0, 24, 48, and 72 hours, the medium was exchanged for 100 μL of RPMI-1640 and 10 μL of CCK-8 reagent was added. The cells were incubated for 2 hours at 37°C. The optical density was measured using an EnSpire 2300 Multilabel Reader (PerkinElmer, Waltham, MA) at 450 nm. Three replicates were prepared for each condition.
2.8 |. Tissue samples
The tissue samples containing 17 primary HCC tissues and their corresponding normal adjacent liver tissues were obtained from the Department of General Surgery of the Second Affiliated Hospital of Dalian Medical University (Dalian, China). We had previously obtained patient consent and approval from the Institute Research Ethics Committee of Dalian Medical University for the use of the clinical materials described in this study.
2.9 |. Animal studies
Male nu/nu mice (4 to 6 weeks old) were used and all animal experiments were conducted at the SPF Laboratory Animal Center at Dalian Medical University. Cholesterol-conjugated All Star nonsilencing siRNA and siNgBR for in vivo delivery were obtained from Shanghai GenePharma Co (Shanghai, China). SMMC-7721 cells (1 × 107 in 100 μL of phosphate-buffered saline) were inoculated subcutaneously into the flank of the nude mice. When the tumor diameters reached 4 mm × 5 mm, mice were randomly divided into 2 groups (n = 5/group): a nontargeting siRNA-injected group and an siNgBR-injected group. A 10 nmol indicated siRNA in 0.1 mL saline buffer was injected intratumorally twice a week for 16 days.12–14 Tumors were measured with a caliper every 4 days and the tumor volume was calculated using the formula V = 1/2 (width2 × length). All mice were killed by ether anesthesia and the total weight of the tumors in each mouse was measured.
All animal maintenance and procedures were carried out in strict accordance with the recommendations established by the Animal Care and Ethics Committee of Dalian Medical University as well as the guidelines of the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Animal Care and Ethics Committee of Dalian Medical University.
2.10 |. Statistical analysis
Data are represented as mean ± standard deviation (SD). Analysis of variance, Student t test, and the Wilcoxon test were used to compare the values of the test and the control samples. A P < .05 defined statistical significance. SPSS 17.0 software was used for all statistical analyses.
3 |. RESULTS
3.1 |. NgBR expression is increased in human HCC cells and tissues
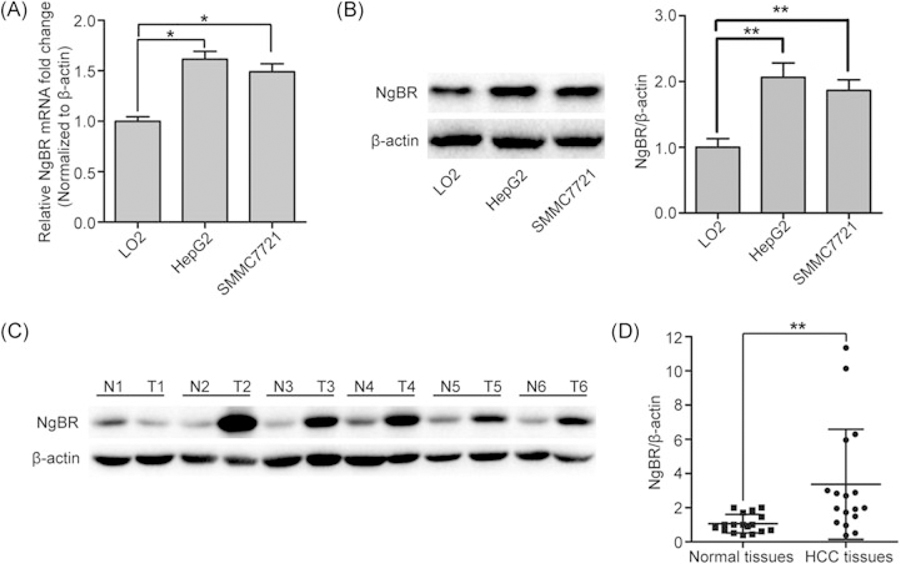
Total RNA was extracted from human HCC cell lines (HepG2 and SMMC-7721) and a normal liver cell line (LO2) to perform real-time PCR analyses. The mRNA expression levels of NgBR were significantly higher than those in the LO2 cells (Figure 1A). In addition, total protein was extracted from the LO2, HepG2, and SMMC-7721 cells, and the protein expression levels of NgBR were detected using Western blot analysis. The protein expression levels of NgBR were also higher in the HepG2 and SMMC-7721 cells compared with those in the LO2 cells (Figure 1B). To further confirm that NgBR was overexpressed in HCC tissues, the protein levels of NgBR in HCC and matching normal adjacent liver tissues were analyzed using Western blot. As shown in Figure 1C,D and Supporting Information Figure S1, the NgBR protein was overexpressed in HCC tissues, but not in the matched normal adjacent liver tissues.
FIGURE 1.
NgBR expression in HCC cell lines and tissues. (A) A high mRNA level of NgBR was detected in human HCC cell lines (HepG2 and SMMC-7721) compared with a normal liver cell line (LO2). mRNA level of NgBR was analyzed using real-time polymerase chain reaction and normalized to the β-actin (*P < .05). (B) A high NgBR protein level was detected in HepG2 and SMMC-7721 compared wiyh LO2. Protein level was monitored using Western blot (left panel). Band intensities were quantified using Image Lab 5.0 software and were normalized to β-actin (right panel). The data are presented as the mean ± SD of 3 independent experiments (**P < .01). (C) Western blot analysis of NgBR protein expression in HCC tissues (T1 to T6) and matching normal adjacent tissues (N1 to N6). (D) Band intensities of 17 primary HCC tissues (T1 to T17) and their matching normal adjacent liver tissues (N1 to N17) were quantified using Image Lab 5.0 software and were normalized to β-actin (**P < .01). HCC, human hepatocellular carcinoma; mRNA, messenger RNA; NgBR, Nogo-B receptor
3.2 |. NgBR knockdown decreases the viability of human HCC cells in vitro
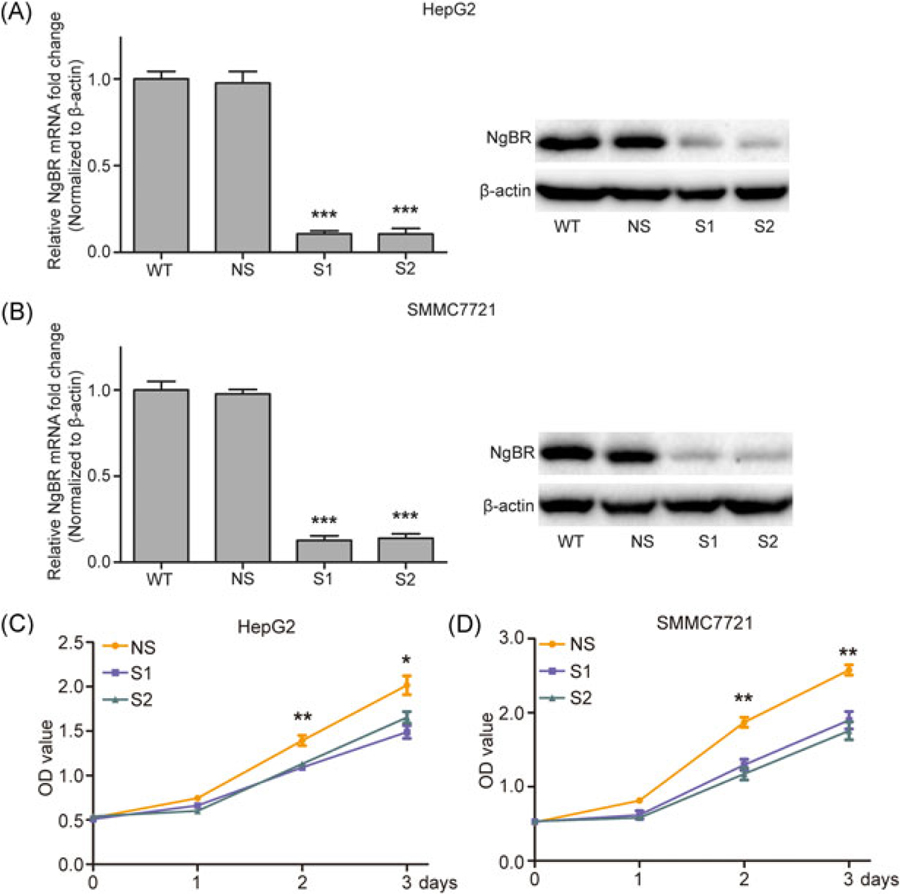
To investigate whether NgBR knockdown affects the growth of HCC cells, siNgBR was used to silence the expression of NgBR. The results demonstrate that both siNgBRs (S1, S2) can effectively reduce the mRNA and protein levels of NgBR (Figure 2A,B). The CCK-8 assay was used to analyze the viability of HepG2 and SMMC-7721 cells at 0, 24, 48, and 72 hours after transfection with siNgBRs. The results indicate that NgBR knockdown significantly decreases the viability of HepG2 and SMMC-7721 cells (Figure 2C,D).
FIGURE 2.
Effects of NgBR knockdown on the growth of HepG2 and SMMC7721 cells. (A,B) HepG2 and SMMC7721 cells were transfected with two siRNA targeting NgBR or they were left untreated; the levels of NgBR expression were determined by real-time polymerase chain reaction and Western blot assays. (C,D) Cell proliferation was quantified by the CCK-8 assay. Knockdown of NgBR decreases the growth of HepG2 and SMMC7721 cells. The data are means ± SD of 3 independent assays (*P < .05, **P < .01, ***P < .001). HCC, human hepatocellular carcinoma; NgBR, Nogo-B receptor; NS, nonsilencing siRNA; siRNA, small (or short) interfering RNA; S1, NgBR siRNA1; S2, NgBR siRNA2
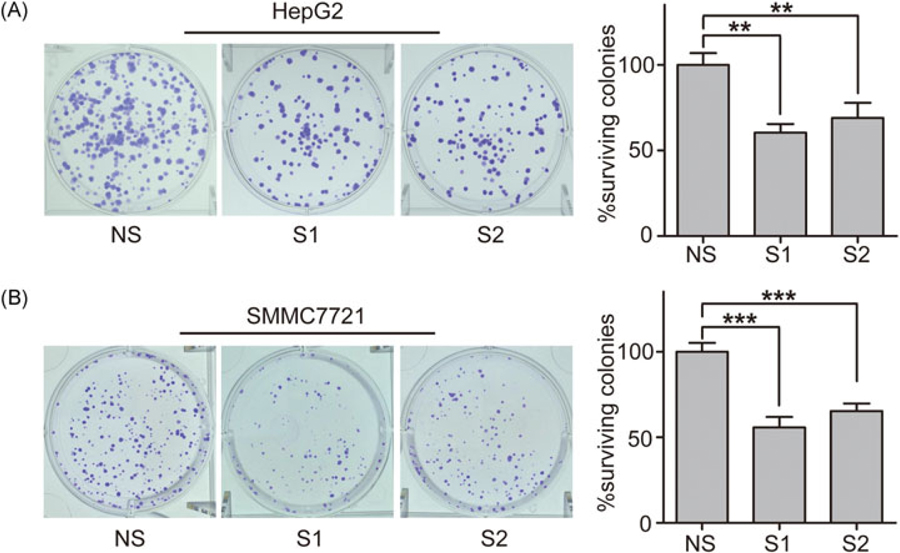
To confirm whether proliferation ability contributes toward the inhibitory effects of NgBR knockdown on the viability of HCC cells, a colony formation assay was used to analyze the clonogenicity of HepG2 and SMMC-7721 cells at 10 days after transfection with siNgBRs. The results demonstrated that NgBR knockdown significantly decreased HCC cell surviving colonies (Figure 3A,B).
FIGURE 3.
Knockdown of NgBR decreases the clonogenicity of HepG2 and SMMC7721 cells.(A,B) Clonogenic survival assay was used to measure the clonogenicity of HepG2 and SMMC7721 cells transfected with two siRNA targeting NgBR (left panel). The number of untreated cells is set as 100% and the results show the average percentage of surviving colonies. The data are presented as the mean ± SD of 3 independent experiments (right panel) (**P < .01, ***P < .001). NgBR, Nogo-B receptor; NS, nonsilencing siRNA; siRNA, small (or short) interfering RNA; S1, NgBR siRNA1; S2, NgBR siRNA2
3.3 |. NgBR knockdown inhibits human HCC cell growth via the phosphorylation of Akt
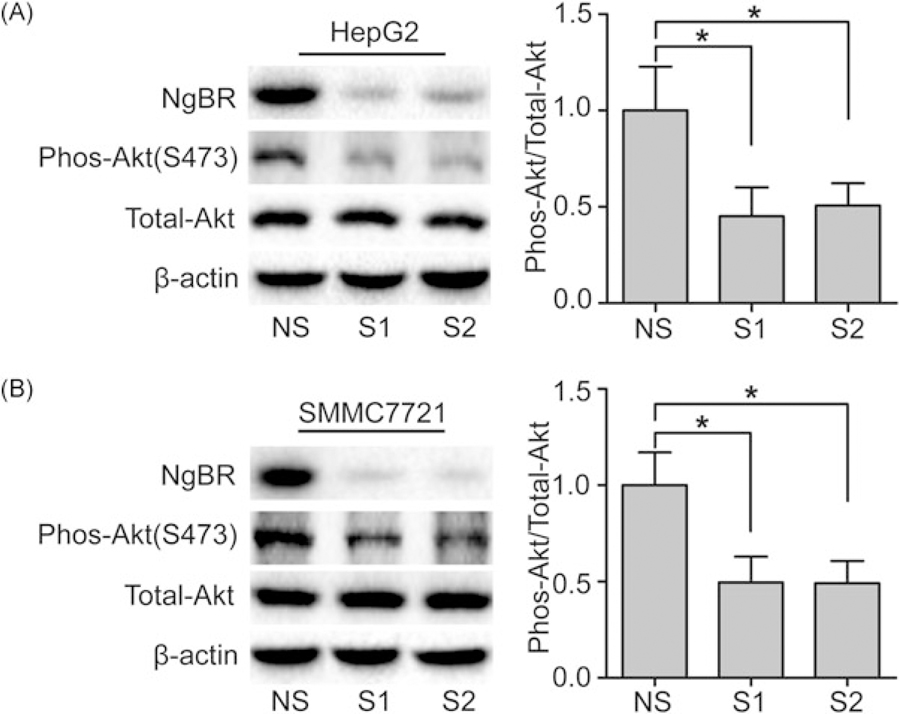
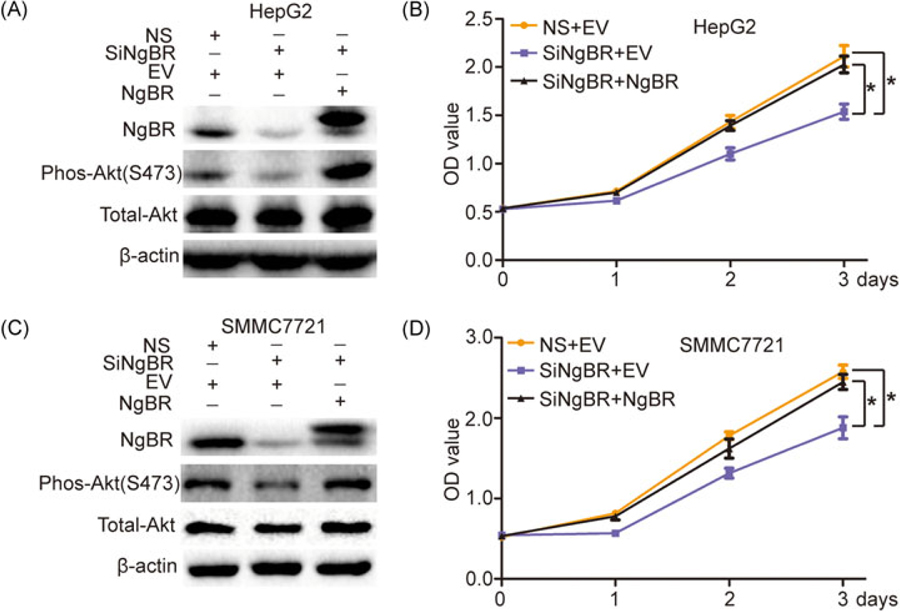
The phosphatidylinositol-3-kinase (PI3K)/Akt pathway is one of the core intracellular signaling pathways in the stimulation of growth factors.15 Activation of Akt plays an important role in cell survival and proliferation.16,17 To determine whether NgBR knockdown inhibits human HCC cell growth via the Akt signal pathway, we used Western blot analysis to detect the phosphorylation levels of Akt in HepG2 and SMMC-7721 cells. The results showed that NgBR knockdown in HepG2 and SMMC-7721 cells by siRNA significantly decreased the phosphorylation of Akt level, whereas the total Akt remained unchanged (Figure 4A,B). Furthermore, overexpression of NgBR by a cotransfected pIRES-NgBR plasmid together with siNgBR in HepG2 and SMMC-7721 cells could rescue impaired phosphorylation of Akt levels in NgBR knockdown HepG2 and SMMC-7721 cells (Figure 5A,C). Meanwhile, a CCK-8 assay showed that NgBR overexpression could rescue the cell growth inhibition observed in HepG2 and SMMC-7721 NgBR knockdown cells (Figure 5B,D). Collectively, our results demonstrate that NgBR regulates human HCC cell growth by regulating Akt phosphorylation in human HCC cells.
FIGURE 4.
NgBR knockdown inhibits the phosphorylation of Akt HepG2 and SMMC7721 cells. (A,B) Knockdown of NgBR decreases the phos-Akt levels in HepG2 and SMMC7721 cells. Phos-Akt levels were detected by Western blot assay (left panel). The expression of each protein was quantified as the densitometry value analyzed by Image Lab 5.0 software and is normalized to total Akt (right panel). The data are presented as the mean ± SD of 3 separate experiments (*P < .05). NgBR, Nogo-B receptor; NS, nonsilencing siRNA; siRNA, small (or short) interfering RNA; S1, NgBR siRNA1; S2, NgBR siRNA2
FIGURE 5.
NgBR overexpression rescues cell growth inhibition through rescuing the changes in the phos-Akt protein level induced by siNgBR in HepG2 and SMMC7721 cells.(A,C) Overexpression of NgBR rescued the changes in the phos-Akt protein level induced by siNgBR in HepG2 and SMMC7721 cells. The HepG2 and SMMC7721 cells were cotransfected with siNgBR or NS as well as pIRES-NgBR or the pIRES empty vector (EV). Expressions of phos-Akt and total-Akt proteins were then examined using Western blot analysis. (B,D) Overexpression of NgBR rescued the cell growth inhibition induced by siNgBR in HepG2 and SMMC7721 cells. Cell proliferation was quantified by the CCK-8 assay. The data are means ± SD of 3 independent assays (*P < .05). CCK-8, cell counting kit-8; EV, pIRES empty vector; NgBR, Nogo-B receptor; NS, nonsilencing siRNA; siNgBR, NgBR siRNA; siRNA, small (or short) interfering RNA
3.4 |. Knockdown of NgBR decreases the growth of hepatocelluar carcinoma in vivo
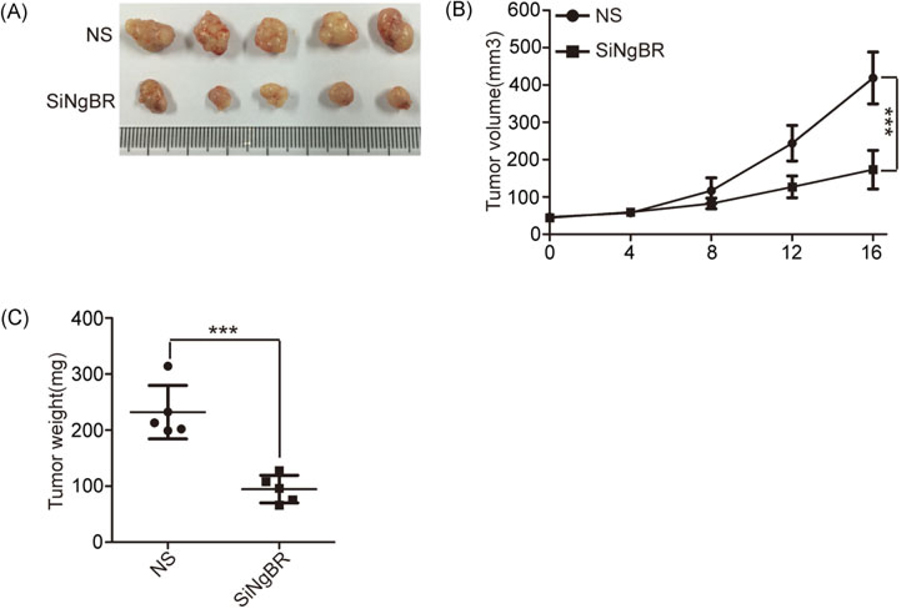
To further examine the in vivo roles of NgBR in HCC growth, we injected the SMMC-7721 cells into nude mice to grow tumor xenografts. As shown in Figure 6A–C, the results show that both the size and the weight of tumor xenografts were decreased in the siNgBR-injected group. Collectively, our data strongly suggest that NgBR knockdown reduces the growth of HCC in vivo.
FIGURE 6.
NgBR deficiency reduces the growth of hepatocellular carcinoma in vivo. (A) Knockdown of NgBR decreases the tumor size. The representative images of SMMC-7721 xenograft tumors injected intratumorally with nonsilencing control siRNA (NS) or NgBR siRNA (siNgBR). (B) Knockdown of NgBR decreases the tumor volumes. Tumor volumes of different tumor and treatment groups were calculated as described in methods. The data are presented as the mean ± SD of 3 independent experiments (***P < .001). (C) Knockdown of NgBR decreases the tumor weights. The data are presented as the mean ± SD of 3 independent experiments (***P < .001)
4 |. DISCUSSION
Growing evidence indicates that HCC is one of the most common cancers worldwide and is a major public health problem. Curative interventions, such as transplantation, resection, and thermal ablation, can be used for patients whose tumors or liver function fulfill the defined criteria.18 Due to the diagnosis at an advanced stage and high resistance to conventional systemic therapy, patients with HCC have a poor survival rate.19–21 However, the genetic and molecular events contributing toward the initiation and progression of HCC are still unclear. Thus, there is an urgent need to explore additional novel molecular markers in tumor progression for the effective treatment of HCC. NgBR has recently been identified as a receptor specific for AmNogo-B by an expression cloning approach. Importantly, a recent study demonstrated that NgBR is highly expressed in human breast invasive ductal carcinoma and the expression of NgBR is essential in promoting estrogen receptor (ER)-positive tumor cell proliferation via survivin induction in breast cancer.10 Another study showed that that NgBR is involved in the transition of breast epithelial cells to mesenchymal stem cells, which is one of the major mechanisms involved in breast cancer metastasis.11
We recently reported that NgBR promotes the chemoresistance of human HCC via the ubiquitination of p53 protein.12 However, the function and mechanisms of NgBR in HCC tumorigenesis are still unclear. In this study, a close association between NgBR and human HCC cell proliferation was observed. Our results demonstrated that knockdown of NgBR could significantly decrease cell viability and clonogenicity in vitro as well as the size and weight of tumor xenografts in vivo. Thus, the expression of NgBR might serve a critical function in the development of human HCC and may be a potential therapeutic target for human HCC.
It has been reported that the PI3K/Akt pathway plays a crucial role in tumorigenesis22 and is one of the core intracellular signaling pathways in the stimulation of growth factors.15 Once phosphorylated and activated, Akt phosphorylates downstream signaling effectors to regulate a wide range of cellular events, such as protein synthesis, cell proliferation, cell survival, migration, and angiogenesis.23,24 Importantly, previous studies have demonstrated that abnormal activation of the PI3K/Akt pathway frequently occurs in HCC and is associated with a poor prognosis.25 In this study, we found a robust loss of phosphorylated Akt in NgBR knockdown human HCC cells (HepG2 and SMMC-7721) compared with their control cells. Furthermore, we demonstrated that overexpression of NgBR in human HCC cells can rescue impaired phosphorylation of Akt levels. Interestingly, we also clearly demonstrated that altered phosphorylation of Akt levels was triggered by NgBR, which is consistent with alterations in cell viability in human HCC cells.
5 |. CONCLUSION
The current study showed that NgBR was highly expressed in HCC cell lines and in tissues of patients with HCC, which promoted human HCC cell growth by increasing Akt phosphorylation in human HCC cells. All these results indicate that NgBR could be a novel potential therapeutic target for the treatment of HCC.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by funds from the National Natural Science Foundation of China (81471755, 21405153, 31770893) and the Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-B03-2014). B Zhao is grateful for the support from the “Hundred Talents Program” of CAS. This workstudy was supported in part by start-up funds from the Medical College of Wisconsin (MCW), NIH R01HL108938, and We Care Fund to Q Miao.
Funding information
National Natural Science Foundation of China, Grant/Award Numbers: 81471755, 31770893, 21405153; clinical capability construction project for liaoning provincial hospitals, Grant/Award Number: LNCCC-B03-2014; Medical College of Wisconsin, Grant/Award Number: NIH R01HL108938
Footnotes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin 2012;62:394–399. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- 4.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. [DOI] [PubMed] [Google Scholar]
- 5.Huber AB, Weinmann O, Brösamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci 2002;22:3553–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephson A, Trifunovski A, Widmer HR, Widenfalk J, Olson L, Spenger C. Nogo-receptor gene activity: cellular localization and developmental regulation of mRNA in mice and humans. J Comp Neurol 2002;453:292–304. [DOI] [PubMed] [Google Scholar]
- 7.Acevedo L, Yu J, Erdjument-Bromage H, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med 2004;10:382–388. [DOI] [PubMed] [Google Scholar]
- 8.Miao RQ, Gao Y, Harrison KD, et al. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci USA. 2006;103:10997–11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao B, Chun C, Liu Z, et al. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116: 5423–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Zhao B, North P, Kong A, Huang J, Miao QR. Expression of NgBR is highly associated with estrogen receptor alpha and survivin in breast cancer. PLoS One. 2013;8:e78083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Xu B, Hu W, et al. Comprehensive proteome quantification reveals NgBR as a new regulator for epithelial-mesenchymal transition of breast tumor cells. J Proteomics. 2015;112:38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C, Zhao B, Long F, et al. Nogo-B receptor promotes the chemoresistance of human hepatocellular carcinoma via the ubiquitination of p53 protein. Oncotarget. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao Y, Wang J, Qin Y, et al. Ku80 cooperates with CBP to promote COX-2 expression and tumor growth. Oncotarget. 2015;6(10):8046–8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b- 3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Jia L, Ma H, Li Y, Ma Z, Zhao Y. Axl gene knockdown inhibits the metastasis properties of hepatocellular carcinoma via PI3K/Akt-PAK1 signal pathway. Tumour Biol 2014;35: 3809–3817. [DOI] [PubMed] [Google Scholar]
- 16.Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81: 727–736. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Fang Z, Lin H, et al. Depletion of Cks1 and Cks2 expression compromises cell proliferation and enhance chemotherapy-induced apoptosis in HepG2 cells. Oncol Rep 2016;35:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. [DOI] [PubMed] [Google Scholar]
- 19.Llovet J, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329–338. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698–711. [DOI] [PubMed] [Google Scholar]
- 21.Tong SW, Yang YX, Hu HD, et al. Proteomic investigation of 5-fluorouracil resistance in a human hepatocellular carcinoma cell line. J Cell Biochem 2012;113:1671–1680. [DOI] [PubMed] [Google Scholar]
- 22.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 2014;46:372–383. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009;8:627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol 2011;7: 1149–1167. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Han L, Zhang J, Xia Q. Down-regulated NRSN2 promotes cell proliferation and survival through PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Dig Dis Sci 2015;60:3011–3018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.