ABSTRACT
Cells must interpret a complex milieu of extracellular cues to modulate intracellular signaling events linked to proliferation, differentiation, migration and other cellular processes. Integrins are heterodimeric transmembrane proteins that link the extracellular matrix (ECM) to the cytoskeleton and control intracellular signaling events. A great deal is known about the structural and functional properties for most integrins; however, the adhesion and signaling pathways controlled by αvβ8 integrin, which was discovered nearly 30 years ago, have only recently been characterized. αvβ8 integrin is a receptor for ECM-bound forms of latent transforming growth factor β (TGFβ) proteins and promotes the activation of TGFβ signaling pathways. Studies of the brain, lung and immune system reveal that the αvβ8 integrin–TGFβ axis mediates cell–cell contact and communication within complex multicellular structures. Perturbing components of this axis results in aberrant cell–cell adhesion and signaling leading to the initiation of various pathologies, including neurodegeneration, fibrosis and cancer. As discussed in this Review, understanding the functions for αvβ8 integrin, its ECM ligands and intracellular effector proteins is not only an important topic in cell biology, but may lead to new therapeutic strategies to treat human pathologies related to integrin dysfunction.
KEY WORDS: Extracellular matrix, Cancer, Angiogenesis, Microenvironment, Pathophysiology
Summary: αvβ8 integrin is essential for normal organ development and physiology and integrin dysfunction is linked to multiple human diseases.
Introduction
Cells must interpret a complex milieu of extracellular matrix (ECM) cues to modulate intracellular signaling events linked to proliferation, differentiation and migration. Precise control of these events promotes normal tissue development and physiology, and these events are often deregulated in diseases ranging from neurodegeneration to autoimmunity and cancer. Cells interact with each other as well as with ECM cues in the microenvironment via various adhesion receptors. The major receptors used by metazoan cells to interact with the ECM are integrins, which are heterodimeric transmembrane proteins formed from an α and β subunit that can engage with the ECM and modulate intracellular signaling functions (Hynes, 2002). In vertebrates, there are 24 integrin-encoding genes; 16 genes encoding integrin α subunits and eight genes encoding integrin β subunits. These integrin subunits can dimerize in various combinations to yield at least 24 distinct functional heterodimers that often display cell type-specific expression patterns and regulate distinct adhesion and signaling pathways.
In this Review, I will summarize adhesion and signaling functions for a specific integrin, αvβ8, in development, physiology and disease. αvβ8 integrin is a member of the αv subfamily of integrins, which comprises αvβ1, αvβ3, αvβ5 and αvβ6 (Weis and Cheresh, 2011). These integrins bind with high specificity to RGD peptide, a motif that is present in many ECM proteins, including vitronectin and collagen IV, as well as latent TGFβ1 and latent TGFβ3 (Hynes et al., 2002). The cDNA sequence encoding β8 integrin (Itgb8) was first isolated from mammalian tissues and reported in 1991 (Moyle et al., 1991). Biochemical and cell-based experiments showed that β8 integrin dimerizes exclusively with the αv integrin (Nishimura et al., 1994). Based on primary amino acid sequence, β8 integrin is predicted to be 81 kDa in size; however, modifications at one or more of seven possible N-glycosylation sites in the extracellular domain results in an apparent molecular mass of 100 kDa on SDS polyacrylamide gels. Itgb8 gene expression patterns were initially found to be quite restrictive, with mRNA detected mainly in the brain and kidney (Moyle et al., 1991). β8 protein expression analysis, however, shows detectable levels in most organs, with the exception of adipose tissue and blood (https://www.proteinatlas.org/ENSG00000105855-ITGB8/tissue). The mouse Itgb8 gene has been mapped to chromosome 12, whereas human ITGB8 is located on chromosome 7.
The objective of this Review is to summarize the current knowledge about αvβ8 integrin and its essential roles in development and pathophysiology. A particular emphasis will be placed on αvβ8 integrin-mediated activation of TGFβ signaling pathways during central nervous system (CNS) development. Intracellular proteins that bind to the β8 integrin cytoplasmic tail and activate signaling events will also be discussed. In addition, I will also summarize three-dimensional structural studies of αvβ8 integrin that reveal unexpected mechanisms of inside-out activation and ECM engagement, and highlight abnormal functions for αvβ8 integrin in diseases that range from immune disorders to cancer. Finally, future experimental priorities as well as potential strategies for therapeutically targeting components of the αvβ8 integrin–TGFβ adhesion and signaling axis to treat human diseases are highlighted.
Integrin αvβ8 activation of latent TGFβ ECM protein ligands
Early studies with cultured neuronal cells and in vitro ECM adhesion assays showed that αvβ8 integrin can bind to vitronectin, fibronectin and various other ECM ligands containing common RGD sequences (Ozawa et al., 2016; Venstrom and Reichardt, 1995). However, various in vitro biochemical reports and genetic studies have revealed that in vivo the major ECM ligands for αvβ8 integrin are latent TGFβ proteins (Worthington et al., 2011). The three mammalian TGFβ-encoding genes (TGFB1, TGFB2 and TGFB3) give rise to proteins that are deposited into the ECM as inactive latent complexes, with the bioactive cytokine non-covalently linked to latency-associated peptide (LAP). During TGFβ activation, structural rearrangement of the latent TGFβ complex leads to cytokine engagement with TGFβ receptor and activation of intracellular signaling events including those mediated by Smad transcription factors (Shi et al., 2011). Although there are several possible factors, including reactive oxygen species, that promote the activation of latent TGFβ (Barcellos-Hoff et al., 1994), integrin-dependent adhesion is a major regulatory mechanism. Knock-in strategies have been used to mutate the RGD sites within latent TGFβ1 to RGE, which abrogates integrin binding (Yang et al., 2007). These RGE mutant mice develop vascular phenotypes identical to those that develop in TGFβ1-null mice, highlighting the in vivo significance of integrin-dependent TGFβ activation. Biochemical studies have also shown that αvβ8 integrin can bind directly to the RGD sequence within the inhibitory LAP domain of latent TGFβ1 and latent TGFβ3 (Cambier et al., 2005).
In most integrin heterodimers, the cytoplasmic domain of the β-integrin subunit is involved in a molecular ‘handshake’ with the cytoplasmic tail of the adjacent α-integrin. These intracellular interactions maintain the extracellular region in an inactive state that is not engaged with ECM ligands (Ginsberg, 2014). This α–β juxtamembrane interaction is disrupted by the recruitment of FERM-domain-containing proteins, including talins and kindlins, to the cytoplasmic tail of the β-integrin subunit (Calderwood et al., 1999; Harburger et al., 2009; Zhang et al., 2008). However, the β8 integrin cytoplasmic tail is divergent from other integrins and does not contain NPXY motifs that interact with FERM-domain-containing proteins, suggesting that there must be distinct mechanisms that regulate αvβ8 integrin affinity for ECM ligands. Protein structure data reveal that the extracellular region of αvβ8 integrin is found mainly in an ‘extended-closed’ and, thus, partially active conformation, rather than the mixture of bent (inactive) and extended (active) conformations that is common in other integrins (Wang et al., 2017). In addition, the affinity of αvβ8 integrin for latent TGFβ1 is activated only 2- to 3-fold by Mn2+, whereas Mn2+ activates other integrins and enhances their interactions with ECM ligands by up to 50-fold (Wang et al., 2017). The αvβ8 integrin headpiece is not stabilized in an open conformation by Mn2+ but has lower overall affinity for latent TGFβ1, as compared to αvβ6 integrin (Wang et al., 2017). Although αvβ8 integrin is detected in an extended-closed structural conformation, subtle changes in the β8 integrin head regions involving the βI domain lead to enhanced engagement with latent TGFβ. This structural switch likely depends on interactions with cytoskeletal adaptor proteins, particularly the Band 4.1 proteins (McCarty et al., 2005a). Cryo-electron microscopy (EM) experiments largely support the crystal structure data, indicating that αvβ8 integrin on the cell surface is present mainly in an extended and partially active conformation, which allows for constitutive interactions with ECM ligands (Cormier et al., 2018). Although key contributions by the cytoskeleton are not addressed in the cryo-EM analyses, these results do reveal that association of αvβ8 integrin with latent TGFβ results in cytokine activation without integrin disengagement from the complex (Campbell et al., 2020). However, it should be noted that X-ray structural analyses of latent TGFβ reveal a highly dynamic molecule, with some conformations lacking integrin binding potentials (Hinck et al., 2016). Hence, it will be important to analyze complexes of αvβ8 integrin and latent TGFβ using crystallography methods to confirm the conclusions of the cryo-EM studies.
The various structural and biochemical data detailed above support a model in which αvβ8 integrin is present on the cell surface in a constitutively active conformation that engages with ECM ligands independently of inside-out activation pathways used by most other integrins, including αvβ6. Although αvβ6 integrin can also promote TGFβ activation (Munger et al., 1999), there are various mechanistic distinctions underlying how αvβ8 and αvβ6 engage with the latent complex and promote TGFβ activation. First, αvβ6 integrin is present on the cell surface mainly in a bent-closed conformation (Dong et al., 2017). Interactions with cytoskeletal adaptors, such as talins and kindlins, via NPXY motifs in the β6 cytoplasmic tail promote inside-out αvβ6 integrin activation and enhanced ligand engagement. In contrast, αvβ8 integrin has a divergent cytoplasmic domain (see signaling section below) and as detailed above is present in extended-closed conformation (Wang et al., 2017; Wang et al., 2019). Second, the β6 integrin cytoplasmic tail is absolutely required for TGFβ activation, whereas TGFβ activation can occur in the absence of the β8 cytoplasmic tail (Mu et al., 2002). Third, αvβ6 binding to latent TGFβ promotes cytokine activation but involves subsequent disengagement from the latent complex (Dong et al., 2014), which is distinct from the continuous engagement mechanism reported for αvβ8 integrin (Campbell et al., 2020). Finally, αvβ8 and αvβ6 integrins show largely non-overlapping cell expression patterns. Integrin αvβ6 is largely absent in glial cells of the CNS and is not expressed at significant levels in circulating immune cell types that utilize αvβ8 integrin for latent TGFβ activation. Along these lines, combined deletion of β6 and β8 integrins (Itgb6−/−;Itgb8−/−) phenocopy developmental pathologies seen in Itgb8−/− mice (Aluwihare et al., 2009).
αvβ8 integrin in CNS development
The brain is the most vascularized organ in the mammalian body with its complex network of blood vessels that interact with neurons and glia in multicellular complexes termed neurovascular units (Paredes et al., 2018). Growth factors, ECM proteins and adhesion receptors regulate communication between cells of the neurovascular unit in a coordinated manner to promote normal development and physiology (Fig. 1) (McCarty, 2009). Depletion of Itgav, the gene encoding the αv integrin subunit, in mice reveals essential roles for αv-containing integrins in neurovascular development (Bader et al., 1998). Embryos of αv integrin-null mice, which lack all five αv-containing heterodimers in all cells, display abnormal cerebral blood vessel morphogenesis and intracerebral hemorrhage (Bader et al., 1998; McCarty et al., 2002). Although the whole-body ablation of Itgb8 leads to very similar brain angiogenesis defects (Zhu et al., 2002), genetic deletion of any of the other four αv-associated β-subunits individually or in various combinations does not yield CNS phenotypes (Graus-Porta et al., 2001; Hodivala-Dilke et al., 1999; Huang et al., 2000; Munger et al., 1999). In germinal matrices, which are neurogenic regions of the developing brain, expression of Itgb8 in perivascular glial cells is activated by G protein-coupled receptor (GPCR) signaling via the Ric8 guanine nucleotide exchange factor (GEF) and the kinase p38α (also known as MAPK14) (Ma et al., 2017; Santhosh and Huang, 2015; Santhosh et al., 2019). Given that similar neurogenic regions in premature human neonates are prone to vascular insult leading to germinal matrix hemorrhage and/or intraventricular hemorrhage (Ballabh, 2010), it will be intriguing to determine whether expression of αvβ8 integrin or its regulatory components are deregulated and contribute to disease pathogenesis.
Fig. 1.
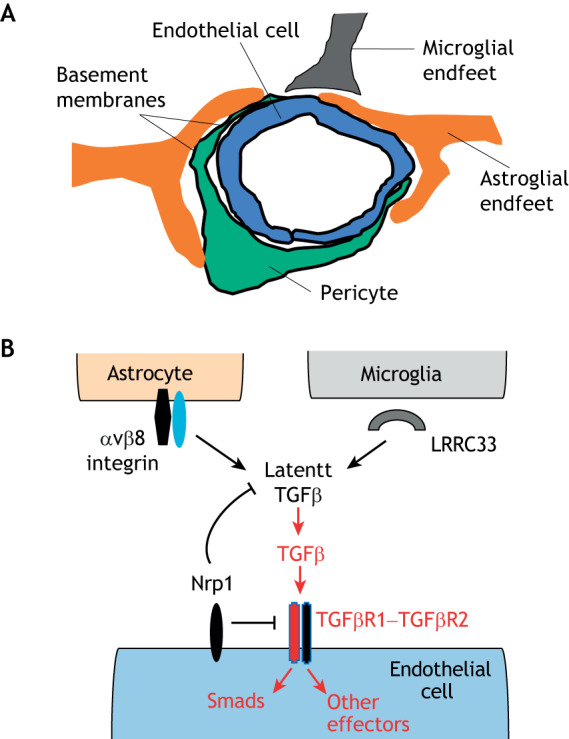
Integrin αvβ8 regulates neurovascular development via activation of latent TGFβs. (A) Schematic illustration of the multicellular composition of a brain neurovascular unit, comprised of vascular endothelial cells and pericytes that are juxtaposed with astrocyte and microglial end feet. There are at least two basement membranes (highlighted by dark black lines) within the neurovascular unit, highlighting the importance of cell–ECM contact and communication in neurovascular biology. (B) αvβ8 integrin expressed in perivascular astroglial cells cooperates with microglial cell-expressed and secreted LRRC33 to bind to latent TGFβs embedded within the ECM. Interactions between integrin and LRRC33 induce structural rearrangements within the latent TGFβ complex, leading to paracrine activation of TGFβ receptor signaling in vascular endothelial cells. TGFβ receptor signaling via Smad transcription factors and other effectors leads to changes in gene expression programs that regulate angiogenesis during CNS development. Nrp1, which is expressed in brain endothelial cells, suppresses TGFβ signaling by serving as a counter-receptor for αvβ8 integrin, thereby blocking latent TGFβ activation. Genetic or pharmacological disruption of integrin engagement of TGFβ receptor signaling leads to severe CNS angiogenesis pathologies. The TGFβ receptor complex consists of a heterodimer of type 1 TGFβ receptor (indicated in red) and type 2 TGFβ receptor (indicated in black).
In addition to brain vascular phenotypes, αv and β8 integrin knockout-mice develop a cleft palate, leading to early neonatal death due to the inability to feed (Bader et al., 1998). To study integrin functions selectively in the CNS, Cre-lox strategies were used to selectively ablate the αv gene (Itgav) in mice neural progenitor cells (McCarty et al., 2005b). Conditional knockout animals develop CNS-specific vascular phenotypes; however, they do not develop a cleft palate and many survive for several post-natal months. Interestingly, all CNS-specific αv conditional mutants display progressive neurological phenotypes, including seizures and ataxia, and die by 8 months of age (McCarty et al., 2005b). Similar brain and retinal phenotypes have been reported for the CNS-specific conditional β8 integrin mutants (Arnold et al., 2012; Hirota et al., 2011; Proctor et al., 2005), suggesting that the neurological impairments that develop in αv mutants are largely due to loss of function of αvβ8 integrin. In αv and β8 knockout mice, the severe brain vascular pathologies that are so apparent in embryonic and neonatal periods mostly resolve by adulthood (Mobley et al., 2009). Resolution of the neurovascular pathologies occurs within a developmental period (three to four post-natal weeks) when CNS blood vessels transition from an angiogenic to a quiescent status (Ma and Huang, 2015), suggesting that αvβ8 integrin provides essential roles in modulating these active phases of CNS blood vessel development.
Indeed, selective deletion of Itgav or Itgb8 in adult CNS astroglial cells does not lead to acute vascular pathologies (unpublished data, McCarty group). Recent data suggest that defective TGFβ-regulated fatty acid metabolism in brain vascular endothelial cells contribute to the adult-onset neurological deficits (Tiwary et al., 2018). In addition, the progressive neurological deficits in mice lacking β8 integrin in the CNS have been linked to defective TGFβ receptor signaling in microglial cells (Arnold et al., 2019). Hence, the progressive neurodegeneration phenotypes in adult Itgb8-mutant mice may be due to defective TGFβ signaling in multiple cell types of the brain.
TGFβ proteins activate multiple cytoplasmic pathways primarily through their transmembrane receptors, which contain serine/threonine kinase activities (Massague, 2008). Selective deletion of the type 2 TGFβ receptor gene Tgfbr2 in endothelial cells using the Alk1-Cre knock-in mouse model (Park et al., 2008) leads to defective brain angiogenesis and intracerebral hemorrhage (Nguyen et al., 2011), which is similar to phenotypes seen in αv and β8 integrin mutant mice. In addition, tamoxifen-inducible deletion of Tgfbr2 in angiogenic endothelial cells results in defective vascular development, including aberrant brain and retinal angiogenesis and hemorrhage (Allinson et al., 2012). In support of the mouse genetic results detailed above, single nucleotide polymorphisms in the human β8 integrin gene (ITGB8) that alter protein expression and function have been identified in patients with brain vascular malformations (Su et al., 2010) and spontaneous brain hemorrhage (Dardiotis et al., 2017); these are also linked to defects in the TGFβ receptor signaling pathway (Cunha et al., 2017).
Neuropilin 1 (Nrp1) is a transmembrane protein that was first discovered as a receptor for secreted semaphorins, as well as vascular endothelial growth factor-A (Tata et al., 2015). Nrp1 has also been reported to act a receptor for TGFβ proteins in cultured cell types, including vascular endothelial cells (Glinka and Prud'homme, 2008). Genetic ablation of Nrp1 in endothelial cells leads to CNS neurovascular phenotypes that are similar to those observed in αvβ8 integrin and TGFβ knockouts (Gerhardt et al., 2004; Gu et al., 2003). Based on these data, the functional links between Nrp1, αvβ8 integrin and latent TGFβ activation and signaling were further analyzed by using genetically engineered mouse models and in vitro co-culture assays. Two groups independently reported that Nrp1 suppresses canonical TGFβ receptor signaling in endothelial cells of the brain (Aspalter et al., 2015; Hirota et al., 2015). Nrp1 expressed in the cerebral endothelium also blocks latent TGFβ activation in the ECM by serving as a counter-receptor in trans for αvβ8 integrin expressed in perivascular astroglial cells (Hirota et al., 2015). Similar vascular pathologies in Nrp1-deficient mice have been reported in the developing retina (Mack et al., 2016). Hence, a precise balance of TGFβ signaling in endothelial cells is essential for the control of sprouting angiogenesis during CNS development. Recently, a new and important component in the αvβ8–TGFβ pathway has been identified. Leucine-rich repeat containing protein 33 (LRRC33, also known as NRROS), an extracellular protein secreted into the extracellular microenvironment by brain microglia is essential for latent TGFβ activation, possibly by cooperating with αvβ8 integrin in nearby astrocytes (Qin et al., 2018). Hence, astrocytes and microglia coordinately bind to latent TGFβs in the brain ECM to mediate their activation and signaling in the brain vasculature (Fig. 1).
αvβ8 integrin and stem cell biology
In the subventricular zone of the post-natal rodent brain, neural stem and progenitor cells give rise to neuroblasts that migrate to the olfactory bulbs via the rostral migratory stream (Murase and Horwitz, 2004). Neuroblasts often use blood vessels and astrocytes that comprise ‘glial tubes’ as scaffolds and receive cues for directional migration (Bovetti et al., 2007; Whitman et al., 2009). Neural progenitors and neuroblasts express substantial levels of αvβ8 integrin, and ablation of Itgav or Itgb8 via Nestin-Cre in mice results in defective olfactory bulb development (Mobley and McCarty, 2011). Subventricular zone tissue explants cultured in ECM reveal that αvβ8 integrin is essential for migration of the neuroblast chain, with mutant cells displaying adhesion and migration defects. Neural stem cells in the human brain are largely restricted to the dentate gyrus although some neurogenesis occurs within the subventricular zone during development (Sanai et al., 2011). In support of its roles in neural stem cell migration, early work using cultured brain astrocytes revealed critical roles for αvβ8 integrin in cell migration and invasion (Milner et al., 1999, 2001). While essential roles for αvβ8 integrin in human neurogenesis have not been reported, there are recent data suggesting important functions for αvβ8 integrin in human-specific brain functions. For instance, quantitative RNA profiling of cultured organoids from humans and non-human primates have revealed gene signatures involved in human-specific brain evolution (Pollen et al., 2019), and, interestingly, ITGB8 is one gene that is highly expressed in human cerebral organoids compared with organoids from non-human primates. This suggests that αvβ8 integrin adhesion and signaling pathways promote structural complexity and/or higher order functions in the human brain.
Integrin αvβ8 also has crucial roles in other stem cell niches outside of the CNS. In the bone marrow, αvβ8 integrin in non-myelinating Schwann cells has been reported to regulate hematopoietic stem cell self-renewal and differentiation through the regulation of latent TGFβ activation and signaling (Yamazaki et al., 2011). Genetic ablation of TGFβ receptor signaling in hematopoietic stem cells, or ablation of αvβ8 integrin-expressing Schwann cells in the bone marrow results in diminished hematopoiesis (Yamazaki et al., 2011). A prior report has shown that αvβ8 integrin in myelinating Schwann cells is a receptor for fibrin in the ECM, and directly binds to the intermediate filament factor glial fibrillary acidic protein (GFAP) (Chernousov and Carey, 2003). However, genetic deletion of Itgav or Itgb8 in Schwann cells does not lead to defects in axonal myelination (Laura Feltri, personal communication). Finally, αvβ8 integrin has been reported to have essential roles in the differentiation of mesenchymal stem cells (MSCs) into chondrocytes, a cell type that is crucial for cartilage functions (LaPointe et al., 2013). In vitro differentiation of MSCs toward the chondrocyte lineage correlates with increased expression of ITGB8 mRNA, with RNAi-mediated silencing of ITGB8 expression resulting in suppressed chrondrocyte development (LaPointe et al., 2013).
αvβ8 integrin in immune system homeostasis
αvβ8 integrin-dependent regulation of TGFβ signaling is also important for the normal functions of many immune cells. These immune-cell-specific roles were first discovered more than a decade ago when studying the effects of Itgav deletion in hematopoietic stem cells (Lacy-Hulbert et al., 2007). Although the mutant mice did not display any defects in hematopoiesis, many adult mutants died prematurely and showed intestinal inflammation with spontaneous colorectal adenocarcinomas (Lacy-Hulbert et al., 2007). Concurrent experiments by another group that used immune-cell-specific Cre mouse models revealed that αvβ8 integrin in surveilling intestinal dendritic cells is essential for homeostasis of the gut epithelium through its role in the activation of latent TGFβs (Travis et al., 2007). Similar roles for αvβ8 integrin has subsequently been reported in the human intestinal tract (Fenton et al., 2017). Paracrine factors within the tissue microenvironment also have important roles in regulating Itgb8 expression and functions in dendritic cells (Boucard-Jourdin et al., 2016). In addition, epigenetic regulatory mechanisms can also impact Itgb8 gene expression. For example, the micro RNAs (miRs) mIR-19b-3p and mIR-106-5p suppress ITGB8 expression in intestinal epithelial cells, and these events drive the formation of colorectal carcinomas (Fang et al., 2011; Huang et al., 2017). Finally, important homeostatic roles for αvβ8 integrin have also been reported in the skin, where integrin-expressing dendritic cells (Mani et al., 2019) promote TGFβ activation and signaling in resident memory T cells (Hirai et al., 2019; Mohammed et al., 2016). TGFβs can also synergize with IL-1β to stimulate expression and secretion of the chemokine CCL20 (Brand et al., 2015), which is involved in T cell recruitment and activation. αvβ8 integrin expressed in regulatory T cells can also inhibit T cell-mediated inflammation via TGFβ activation and signaling (Worthington et al., 2015), whereas T cells that lack integrin expression are incapable of suppressing the inflammatory response. Glycoprotein A repetitions predomain (GARP; also known as LRRC32) is a transmembrane protein expressed in T regulatory cells that coordinates with αvβ8 integrin to activate latent TGFβs (Lienart et al., 2018). The influence of GARP in regulating immune inflammation is similar to roles for LRRC33 in facilitating integrin-mediated TGFβ activation in the CNS (Qin et al., 2018). In addition, integrin-mediated TGFβ activation is also linked to other immune responses, including Th17 cell induction in experimental autoimmune disorders (Araya et al., 2006; Melton et al., 2010) and intestinal monocyte control of immune tolerance (Kelly et al., 2018).
Integrin αvβ8 and organ fibrosis
Aberrant communication between epithelial cells with fibroblasts and immune cells in the lung, liver, kidney and other organs can lead to fibrosis, or pathological stromal cell hyperproliferation and abnormal ECM deposition (Kim et al., 2018). For example, interactions between pulmonary epithelial and mesenchymal fibroblasts within the epithelial-mesenchymal trophic unit (EMTU) of the lung play important roles in promoting normal organ development and physiology. Itgb8 expression in lung epithelial cells can be regulated at the transcriptional level by multiple pathways. In particular, a signaling cascade involving IL-1β, p38α kinase, and the transcription factors SP3 and AP-1 have been shown to induce ITGB8 expression in human epithelial cells (Markovics et al., 2010, 2011). Furthermore, by using ex vivo models of the EMTU, it has been shown that αvβ8 and αvβ6 integrins that are expressed in airway epithelial cells promote the activation of latent TGFβ proteins, leading to the recruitment of resident immune cells (Araya et al., 2006). Deregulation of integrin-mediated TGFβ activation in the EMTU results in lung fibrosis and leads to chronic obstructive pulmonary disease, as well as asthma (Araya et al., 2007; Kitamura et al., 2011; Minagawa et al., 2014). Liver fibrosis that results from colorectal carcinoma metastasis also involves αv integrin-dependent collagen deposition (Conti et al., 2008), which likely is triggered by latent TGFβ activation. TGFβ receptor signaling via Smads and other effectors stimulates synthesis of ECM proteins including collagens (McCarty, 2008). Furthermore, fibrotic pathologies in the kidney are promoted by αvβ8 integrin expressed by mesangial cells via activation of TGFβs (Lakhe-Reddy et al., 2014). Finally, interactions between αvβ8 integrin in mesangial cells and CD31 (also known as platelet endothelial cell adhesion molecule 1; PECAM1) in renal endothelial cells has been reported as a mechanism to balance latent TGFβ activation in the normal kidney. These interactions are altered during renal fibrosis, leading to hyperactivation of TGFβ signaling in the stroma (Khan et al., 2011).
Integrin αvβ8 as a viral receptor and mediator of membrane fusion
Various viruses utilize αvβ8 integrin as a receptor for cell entry and infection. For instance, the herpes simplex virus (HSV) gH–gL binary protein complex binds directly to the extracellular domain of αvβ8 integrin in epithelial and neuronal cell types (Gianni et al., 2015). This interaction with αvβ8 integrin leads to conformational changes in gH–gL, which initiate endocytosis of HSV through membrane fusion mechanisms that involve dynamin 2 (Gianni et al., 2013; Hutt-Fletcher and Chesnokova, 2010). Similar roles for the gH–gL complex have been reported for Epstein–Barr virus infection of epithelial cells and B cells (Hutt-Fletcher and Chesnokova, 2010). Similarly, the foot-and-mouth disease virus has also been reported to bind directly to αvβ8 integrin through the viral VP1 protein, which contains an RGD peptide sequence. Here, domain-swapping experiments reveal that entry of foot-and-mouth disease virus into cells involves the cytoplasmic domain of β8 integrin (Jackson et al., 2004), suggesting that this domain is important for the extracellular structure of integrin extracellular structure and/or intracellular downstream signaling pathways that are involved in viral infection.
Finally, genomic analyses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), which has caused the COVID-19 pandemic, reveals an RGD sequence in the viral spike (S) protein that is lacking in other coronaviruses (Sigrist et al., 2020). The RGD sequence is not within the well-characterized S-protein-binding domain for the angiotensin converting enzyme 2 (ACE2) receptor. While the functional significance of the RGD motif in the S protein remains to be determined experimentally, it is enticing to speculate that αvβ8 integrin may modulate binding and entry of SARS-CoV2, possibly by forming a cell surface complex with ACE2 in lung epithelial cells. Furthermore, there are reports of COVID-19 patients developing cerebral edema and severe neurological deficits (Mao et al., 2020), suggesting that SARS-CoV2 is causing encephalitis, possibly via RGD-dependent engagement with αvβ8 integrin.
Integrin αvβ8 functions in cancer initiation and progression
Abnormal regulation of αvβ8 integrin-mediated adhesion and downstream signaling pathways (see below) contribute to the initiation and progression of various cancers. Early work suggested that lung adenocarcinoma cells that express low levels of αvβ8 integrin generated more malignant tumors in xenograft models, and these events correlated with reduced activation of latent TGFβ and downstream signaling (Cambier et al., 2000). More recent studies in stratified epithelial tissues reveal that αvβ8 integrin-mediated TGFβ signaling suppresses basal epithelial cell growth via autocrine feedback pathways. Deregulation of these events in mice, either through genetic deletion of Itgav (McCarty et al., 2008) or Tgfbr2, the gene encoding TGFβ receptor type-2 (Guasch et al., 2007), leads to the initiation of squamous cell carcinoma. Furthermore, experiments using transgenic mouse models of squamous cell carcinoma (Savar et al., 2015), as well as human squamous cell carcinoma samples (Hsu et al., 2011), confirm that a reduced expression of αvβ8 integrin correlates with higher-grade tumors. However, in contrast to the data from squamous cell carcinomas, upregulation of β8 integrin expression in pancreatic cancer cells leads to more malignant disease, as well as to an enhanced resistance to chemotherapy (Jin et al., 2019). Hence, increased levels of αvβ8 integrin can have both pro-tumorigenic or anti-tumorigenic activities, depending on the type of cancer.
Glioblastoma (GBM) is a highly aggressive primary tumor type with unique blood vessel pathologies, including uncontrolled microvascular proliferation, hemorrhage and edema (Lathia et al., 2015). Hyperactive TGFβ receptor signaling in GBM cells drives growth and survival (Penuelas et al., 2009). β8 promotes the perivascular growth of GBM cells, as well as their invasion along blood vessels through its role in the activation of latent TGFβs. Specifically, it was shown that GBM cell lines that express low levels of integrin β8 are less invasive, whereas cells that have high levels of integrin β8 give rise to tumor growth and perivascular invasion (Reyes et al., 2013a; Tchaicha et al., 2011) (Fig. 2). More recently, my group has discovered that αvβ8 integrin-expressing human GBM cells freshly sorted from patient tumors generate more malignant tumors in the mouse brain in comparison to GBM cells that express low or undetectable levels of αvβ8 integrin (Guerrero et al., 2017). In addition, αvβ8 integrin can functionally interact with Nrp1 in GBM cells to activate TGFβ signaling and promote cell cycle progression (Kwiatkowski et al., 2017). Similar pro-tumorigenic roles have been reported for αvβ8 integrin in GBM stem cells in promoting tumor recurrence following radiation and chemotherapy (Malric et al., 2019). In addition, miR-142-3p and miR-93 negatively regulate ITGB8 expression in glioma cells during brain tumor growth and invasion and thus might be a cause for their resistance to therapy (Li et al., 2018; Malekpour Afshar et al., 2017). Finally, in metastatic brain tumors there is a correlation between αvβ8 integrin expression and severity of metastatic lesions (Schittenhelm et al., 2013). In general, these various data indicate that increased expression of αvβ8 integrin leads to enhanced activation of TGFβ receptor signaling and these events collectively promote tumor cell growth and invasion.
Fig. 2.
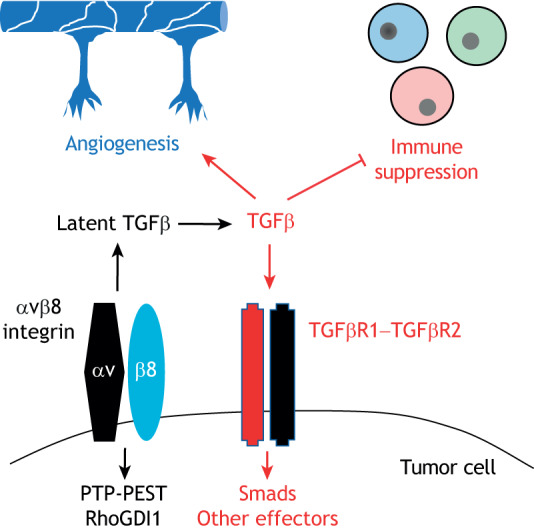
Integrin αvβ8 modulates cell–ECM adhesion and signaling events in the cancer microenvironment. αvβ8 integrin expressed in cancer cells (shown here is an example of the brain cancer GBM) mediates the activation of ECM-bound latent TGFβs in the tumor microenvironment. Active TGFβ subsequently engages with its receptors to coordinately activate paracrine angiogenic pathways in vascular endothelial cells and suppress T cell activation. In addition, TGFβ signaling in cancer cells activates intracellular signaling pathways involving PTP-PEST, RhoGDI1 and other effectors that promote growth and invasion in the tissue microenvironment.
Regulation of intracellular signaling effectors by αvβ8 integrin
Most if not all of the experimental evidence indicates that the primary biological function for αvβ8 integrin is the control of cell–cell communication in multiple tissues. However, αvβ8 integrin also regulates intracellular signaling pathways, although these pathways are distinct from other integrins (Fig. 3). As noted above, the amino acid sequence of the β8 integrin cytoplasmic tail is not homologous to that of other β subunits. Most integrin β subunits, including β1A, β2, β3A, β5 and β6, utilize NPXY motifs to interact directly with FERM domain proteins, such as talins and kindlins, which in turn controls cytoskeletal dynamics (Ginsberg, 2014), whereas β8 lacks these FERM-domain-binding motifs suggesting that αvβ8 integrin signaling involved the recruitment of distinct effector proteins.
Fig. 3.
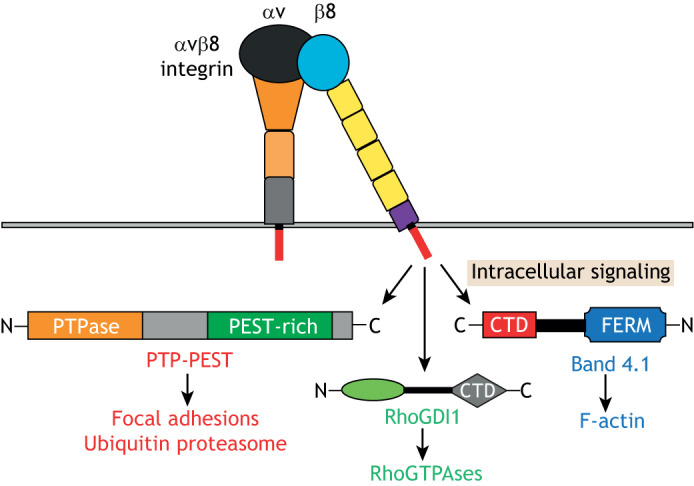
A summary of intracellular signaling pathways regulated by αvβ8 integrin. The cytoplasmic domain of β8 integrin can bind to various intracellular signaling effectors, including the non-receptor protein tyrosine phosphatase PTP-PEST, which subsequently regulates p130Cas activities in focal adhesions and VCP/p97 in the ubiquitin proteasome system. β8 integrin also binds directly to the Rho GTPase regulatory factor RhoGDI1, which controls the activation states of Rac1 and Cdc42 GTPase. Finally, the β8 integrin cytoplasmic domain can link to the actin cytoskeleton through interactions with Band 4.1 proteins.
The β8 integrin cytoplasmic domain has been shown to interact with RhoGDI1 (also known as ARHGDIA), a 25 kDa protein involved in regulation of Rho GTPase signaling (Lakhe-Reddy et al., 2006; McCarty et al., 2005a). There are at least three members of the RhoGDI family, and they function to extract active Rho GTPases from membranes to sequester them in the cytoplasm (Boulter et al., 2010; Garcia-Mata et al., 2011; Moissoglu and Schwartz, 2006). Thus, RhoGDIs act in concert with guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) to balance Rho activation. Integrin αvβ8 recruits RhoGDI1 to the leading edge of the cell in order to extract activated Rho proteins from the plasma membrane and sequester them in the cytoplasm (Lakhe-Reddy et al., 2006; Reyes et al., 2013b). Accordingly, silencing RhoGDI1 or uncoupling RhoGDI1–αvβ8 integrin associations by mutating integrin-recruitment sites in RhoGDI1 (see below) results in hyperactivation of the Rho family members Rac1 and Cdc42 with subsequent impairment of cell migration (Reyes et al., 2013b). Furthermore, the defective neurogenesis in αvβ8 integrin mutant mice have been linked to alterations in Rho GTPase activation (Lee et al., 2015). Moreover, during zebrafish brain development, interactions between αvβ8 integrin and βPix (also known as ARHGEF7), a scaffold protein with GEF activities that binds many proteins including the GAP Git1, have been identified (Liu et al., 2012). Mutations in zebrafish arhgef7 or itgb8 give rise to pathological angiogenesis and cerebral edema (Liu et al., 2012), although the exact mechanisms underlying these vascular pathologies remain unknown.
RhoGDIs are inactivated by phosphorylation on Y156 by the non-receptor tyrosine kinase Src; this diminishes their affinity for GDP-bound Rho proteins and induces the translocation of RhoGDI to the leading edge of the cell (DerMardirossian et al., 2006; Wu et al., 2009). My group has found that αvβ8 integrin binds to the non-receptor protein tyrosine phosphatase (PTP)-PEST (also known as PTPN12), and that this interaction promotes RhoGDI1 dephosphorylation, which results in its release from the membrane and sequestration of GDP-bound Rac1 and Cdc42 in the cytoplasm (Lee et al., 2015). PTP-PEST contains an N-terminal catalytic domain and proline, glutamate, serine and threonine (PEST)-rich sequences in the C-terminus. PTP-PEST is broadly expressed and has important roles in promoting cell adhesion and motility during development (Zheng and Lu, 2013). It has been recently shown that αvβ8 integrin signaling via PTP-PEST and the ubiquitin proteasome system component p97 (also known as VCP) is important for promoting GBM cell invasion by increasing focal adhesion turnover at the leading edge (Chen et al., 2018).
Similar to what is seen for other integrins, αvβ8 integrin serves as a link between the ECM and the cytoskeleton. Genetic screens have revealed that the β8 integrin cytoplasmic tail interacts with the FERM domain-containing protein Band 4.1B (also known as EPB41L3), which is important for the development of the CNS (McCarty et al., 2005a). Interestingly, β8 integrin binds directly to Band 4.1B through its C-terminal domain (CTD), but does not interact with the FERM domain of Band 4.1B (McCarty et al., 2005a). Mass spectrometry experiments also identified spinophilin (PPP1R9B) as a protein that binds to the β8 integrin cytoplasmic tail (Cheerathodi et al., 2016). Spinophilin is a 130 kDa cytoskeletal scaffolding protein that contains PDZ, protein phosphatase 1 (PP1) and actin-binding domains; it is highly expressed in the brain where it interacts with various transmembrane proteins (Carnero, 2012). Human GBM cells that lack spinophilin expression owing to RNAi-mediated gene silencing or CRISPR gene editing show increased numbers of filopodia in vitro, as well as enhanced invasive growth in the mouse brain, and all these pro-invasive properties have been shown to correlate with reduced levels of Rac1 activity (Cheerathodi et al., 2016).
While the recruitment of signaling effectors is essential for αvβ8 integrin-driven cell migration and invasion especially in cancer (Reyes et al., 2013a), it remains to be determined whether intracellular signaling is coupled to latent TGFβ engagement and signaling. Recent 3D structure data suggest that conversion from the extended-closed conformation to a more ECM ligand-engaged state requires coupling to the cytoskeleton (Wang et al., 2019), likely via inside-out mechanisms involving Band 4.1 proteins and/or other cytoplasmic effectors such as spinophilin, which provide links to the cytoskeleton. It is intriguing to speculate that PTP-PEST also promotes ECM interactions by regulating the phosphorylation status of the β8 cytoplasmic domain or via dephosphorylation of spinophilin and/or Band 4.1 proteins. Alternatively, αvβ8 integrin-mediated recruitment of intracellular signaling effectors may occur as a result of ECM ligand binding through classic outside-in signaling. It would be interesting to use genetic strategies to delete the β8 integrin cytoplasmic tail and determine whether this impacts latent TGFβ activation and signaling.
Conclusions and future directions
The importance of αvβ8 integrin in promoting cell–cell communication during the initiation and progression of several diseases, ranging from immune disorders to cancer, makes this integrin as well as its latent TGFβ ECM ligands attractive targets for possible therapeutic interventions. In particular, the specificity of αvβ8 integrin for RGDLXX(L/I) sequences within its ligands make it a suitable target for the use of function-blocking antibodies, as well as small-molecule inhibitors that block its adhesion to the ECM. Similar strategies have been employed to target the related αv-containing integrins αvβ3 and αvβ5 by using the cyclic RGD compound cilengitide as an anti-angiogenic agent or an anti-tumor cell growth agent, although these efforts have largely failed in clinical trials (Weller et al., 2016). However, strategies to target αvβ8 integrin may be more effective given its atypical extended-closed structure (Wang et al., 2017; 2019) and its unique mechanisms of latent TGFβ engagement and activation (Campbell et al., 2020). It will be interesting to determine whether neutralizing anti-β8 integrin antibodies or RGDLXX(L/I)-containing peptide mimetics could be effective in treating fibrosis. These agents might also serve as effective anti-viral strategies to block binding between αvβ8 integrin and HSV or FMDV proteins and subsequent membrane fusion events required for viral entry and infection.
It is worth keeping in mind that blocking αvβ8 integrin may have broad effects that are related to the subsequent inhibition of TGFβ activation and signaling. Immune checkpoint inhibitors have shown limited efficacy in many cancer clinical trials, including primary brain and pancreatic tumors, likely due to a suppression of immune cell activation by alternative pathways. TGFβ activation is one pathway that tumor cells utilize to suppress the immune system and evade blockade by immune-checkpoint inhibitors (Jiao et al., 2019) (Fig. 2). Therefore, blocking TGFβ signaling or the activation of latent TGFβ activation through αvβ8 integrin inhibition in cancer may be an effective strategy to augment the effects of immune-checkpoint inhibitors. Indeed, roles for αvβ8 integrin and TGFβ proteins in the suppression of T cells have recently been shown in various tumor types (Stockis et al., 2017; Takasaka et al., 2018). However, blocking αvβ8 integrin-mediated TGFβ activation in immune cells outside of the tumor microenvironment may lead to unwanted side effects, such as for example, the intestinal autoimmune phenotypes observed in the mice lacking αv or β8 integrin expression in immune cells. Additional strategies to inhibit αvβ8 integrin could involve blocking intracellular signaling through the β8 cytoplasmic tail. In this context, synthetic peptides and/or small molecules that block the activation of PTP-PEST and other signaling proteins could be an effective strategy for inhibiting tumor cell growth and invasion. It will also be critical to determine the relative importance of intracellular signaling pathways and whether they are coupled to ECM engagement, especially involving the latent TGFβ complex.
In summary, the past 15 years have seen major advances in our understanding of roles for αvβ8 integrin, its latent TGFβ ECM ligands and cytoplasmic effector proteins in development, physiology and disease. Important future directions will involve using advanced proteomic technologies to identify and characterize additional ECM protein ligands and intracellular signaling partners for αvβ8 integrin. Efforts to identify the ‘adhesome network’ for various other integrins have yielded novel findings concerning the dynamic nature of integrin adhesion and signaling (Geiger and Yamada, 2011). In addition, use of quantitative RNA sequencing strategies will be important to identify αvβ8 integrin-dependent gene expression profiles in single cells isolated from healthy and diseased tissues. Furthermore, it will be important to elucidate the molecular mechanisms that lead to conversion of the extended-closed αvβ8 extracellular domains into their fully active conformation, including the contributions from cytoplasmic effector proteins such as Band 4.1B and/or extracellular regulatory factors, such as LRRC33 or GARP. Additionally, it will be necessary to determine how the αvβ8 integrin–TGFβ pathway is functionally connected to other signaling networks, and how these events are linked to development and disease. The potential blockade of these pathways could result in effective therapies to benefit patients with diseases that are associated with αvβ8 integrin and/or TGFβ dysfunction.
Acknowledgements
I would like to thank the members of the McCarty laboratory for insightful comments on the manuscript. I tried to be as comprehensive in citing experimental studies, but I apologize to any colleagues if relevant publications were not cited.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
My work was supported, in part, by grants from the National Institutes of Health (R01NS087635, R21NS103841 and P50CA127001), the Cancer Prevention and Research Institute of Texas (RP180220), the Brockman Foundation, and the Terry L. Chandler Foundation. Deposited in PMC for release after 12 months.
References
- Allinson K. R., Lee H. S., Fruttiger M., McCarty J. H. and Arthur H. M. (2012). Endothelial expression of TGFβ type II receptor is required to maintain vascular integrity during postnatal development of the central nervous system. PLoS ONE 7, e39336 10.1371/journal.pone.0039336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluwihare P., Mu Z., Zhao Z., Yu D., Weinreb P. H., Horan G. S., Violette S. M. and Munger J. S. (2009). Mice that lack activity of αvβ6- and αvβ8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell Sci. 122, 227-232. 10.1242/jcs.035246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J., Cambier S., Morris A., Finkbeiner W. and Nishimura S. L. (2006). Integrin-mediated transforming growth factor-β activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am. J. Pathol. 169, 405-415. 10.2353/ajpath.2006.060049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya J., Cambier S., Markovics J. A., Wolters P., Jablons D., Hill A., Finkbeiner W., Jones K., Broaddus V. C., Sheppard D. et al. (2007). Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Invest. 117, 3551-3562. 10.1172/JCI32526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T. D., Ferrero G. M., Qiu H., Phan I. T., Akhurst R. J., Huang E. J. and Reichardt L. F. (2012). Defective retinal vascular endothelial cell development as a consequence of impaired integrin αVβ8-mediated activation of transforming growth factor-β. J. Neurosci. 32, 1197-1206. 10.1523/JNEUROSCI.5648-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T. D., Lizama C. O., Cautivo K. M., Santander N., Lin L., Qiu H., Huang E. J., Liu C., Mukouyama Y. S., Reichardt L. F. et al. (2019). Impaired αVβ8 and TGFβ signaling lead to microglial dysmaturation and neuromotor dysfunction. J. Exp. Med. 216, 900-915. 10.1084/jem.20181290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspalter I. M., Gordon E., Dubrac A., Ragab A., Narloch J., Vizan P., Geudens I., Collins R. T., Franco C. A., Abrahams C. L. et al. (2015). Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat. Commun. 6, 7264 10.1038/ncomms8264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B. L., Rayburn H., Crowley D. and Hynes R. O. (1998). Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507-519. 10.1016/S0092-8674(00)81618-9 [DOI] [PubMed] [Google Scholar]
- Ballabh P. (2010). Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr. Res. 67, 1-8. 10.1203/PDR.0b013e3181c1b176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Derynck R., Tsang M. L. and Weatherbee J. A. (1994). Transforming growth factor-beta activation in irradiated murine mammary gland. J. Clin. Invest. 93, 892-899. 10.1172/JCI117045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard-Jourdin M., Kugler D., Endale Ahanda M. L., This S., De Calisto J., Zhang A., Mora J. R., Stuart L. M., Savill J., Lacy-Hulbert A. et al. (2016). β8 integrin expression and activation of TGF-β by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J. Immunol. 197, 1968-1978. 10.4049/jimmunol.1600244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E., Garcia-Mata R., Guilluy C., Dubash A., Rossi G., Brennwald P. J. and Burridge K. (2010). Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat. Cell Biol. 12, 477-483. 10.1038/ncb2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S., Hsieh Y. C., Bovolin P., Perroteau I., Kazunori T. and Puche A. C. (2007). Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 27, 5976-5980. 10.1523/JNEUROSCI.0678-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand O. J., Somanath S., Moermans C., Yanagisawa H., Hashimoto M., Cambier S., Markovics J., Bondesson A. J., Hill A., Jablons D. et al. (2015). Transforming growth factor-β and interleukin-1β signaling pathways converge on the chemokine CCL20 promoter. J. Biol. Chem. 290, 14717-14728. 10.1074/jbc.M114.630368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood D. A., Zent R., Grant R., Rees D. J., Hynes R. O. and Ginsberg M. H. (1999). The Talin head domain binds to integrin β subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274, 28071-28074. 10.1074/jbc.274.40.28071 [DOI] [PubMed] [Google Scholar]
- Cambier S., Mu D. Z., O'Connell D., Boylen K., Travis W., Liu W. H., Broaddus V. C. and Nishimura S. L. (2000). A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 60, 7084-7093. [PubMed] [Google Scholar]
- Cambier S., Gline S., Mu D., Collins R., Araya J., Dolganov G., Einheber S., Boudreau N. and Nishimura S. L. (2005). Integrin αvβ8-mediated activation of transforming growth factor-β by perivascular astrocytes: an angiogenic control switch. Am. J. Pathol. 166, 1883-1894. 10.1016/S0002-9440(10)62497-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. G., Cormier A., Ito S., Seed R. I., Bondesson A. J., Lou J., Marks J. D., Baron J. L., Cheng Y. and Nishimura S. L. (2020). Cryo-EM reveals integrin-mediated TGF-beta activation without release from latent TGF-beta. Cell 180, 490-501.e16. 10.1016/j.cell.2019.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A. (2012). Spinophilin: a new tumor suppressor at 17q21. Curr. Mol. Med. 12, 528-535. 10.2174/156652412800619987 [DOI] [PubMed] [Google Scholar]
- Cheerathodi M., Avci N. G., Guerrero P. A., Tang L. K., Popp J., Morales J. E., Chen Z., Carnero A., Lang F. F., Ballif B. A. et al. (2016). The cytoskeletal adapter protein spinophilin regulates invadopodia dynamics and tumor cell invasion in glioblastoma. Mol. Cancer Res. 14, 1277-1287. 10.1158/1541-7786.MCR-16-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Morales J. E., Guerrero P. A., Sun H. and McCarty J. H. (2018). PTPN12/PTP-PEST regulates phosphorylation-dependent ubiquitination and stability of focal adhesion substrates in invasive glioblastoma cells. Cancer Res. 78, 3809-3822. 10.1158/0008-5472.CAN-18-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernousov M. A. and Carey D. J. (2003). αVβ8 integrin is a Schwann cell receptor for fibrin. Exp. Cell Res. 291, 514-524. 10.1016/S0014-4827(03)00409-9 [DOI] [PubMed] [Google Scholar]
- Conti J. A., Kendall T. J., Bateman A., Armstrong T. A., Papa-Adams A., Xu Q., Packham G., Primrose J. N., Benyon R. C. and Iredale J. P. (2008). The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumor growth and survival via alphav integrin ligation. Clin. Cancer Res. 14, 6405-6413. 10.1158/1078-0432.CCR-08-0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier A., Campbell M. G., Ito S., Wu S., Lou J., Marks J., Baron J. L., Nishimura S. L. and Cheng Y. (2018). Cryo-EM structure of the αvβ8 integrin reveals a mechanism for stabilizing integrin extension. Nat. Struct. Mol. Biol. 25, 698-704. 10.1038/s41594-018-0093-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha S. I., Magnusson P. U., Dejana E. and Lampugnani M. G. (2017). Deregulated TGF-β/BMP signaling in vascular malformations. Circ. Res. 121, 981-999. 10.1161/CIRCRESAHA.117.309930 [DOI] [PubMed] [Google Scholar]
- Dardiotis E., Siokas V., Zafeiridis T., Paterakis K., Tsivgoulis G., Dardioti M., Grigoriadis S., Simeonidou C., Deretzi G., Zintzaras E. et al. (2017). Integrins AV and B8 gene polymorphisms and risk for intracerebral hemorrhage in greek and polish populations. Neuromolecular Med. 19, 69-80. 10.1007/s12017-016-8429-3 [DOI] [PubMed] [Google Scholar]
- DerMardirossian C., Rocklin G., Seo J. Y. and Bokoch G. M. (2006). Phosphorylation of RhoGDI by Src regulates Rho GTPase binding and cytosol-membrane cycling. Mol. Biol. Cell 17, 4760-4768. 10.1091/mbc.e06-06-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Hudson N. E., Lu C. and Springer T. A. (2014). Structural determinants of integrin β-subunit specificity for latent TGF-β. Nat. Struct. Mol. Biol. 21, 1091-1096. 10.1038/nsmb.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Zhao B., Iacob R. E., Zhu J., Koksal A. C., Lu C., Engen J. R. and Springer T. A. (2017). Force interacts with macromolecular structure in activation of TGF-beta. Nature 542, 55-59. 10.1038/nature21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Deng Z., Shatseva T., Yang J., Peng C., Du W. W., Yee A. J., Ang L. C., He C., Shan S. W. et al. (2011). MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene 30, 806-821. 10.1038/onc.2010.465 [DOI] [PubMed] [Google Scholar]
- Fenton T. M., Kelly A., Shuttleworth E. E., Smedley C., Atakilit A., Powrie F., Campbell S., Nishimura S. L., Sheppard D., Levison S. et al. (2017). Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin αvβ8. Mucosal. Immunol. 10, 624-634. 10.1038/mi.2016.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R., Boulter E. and Burridge K. (2011). The ‘invisible hand': regulation of RHO GTPases by RHOGDIs. Nat. Rev. Mol. Cell Biol. 12, 493-504. 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. and Yamada K. M. (2011). Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect Biol. 3, a005033 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H., Ruhrberg C., Abramsson A., Fujisawa H., Shima D. and Betsholtz C. (2004). Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev. Dyn. 231, 503-509. 10.1002/dvdy.20148 [DOI] [PubMed] [Google Scholar]
- Gianni T., Salvioli S., Chesnokova L. S., Hutt-Fletcher L. M. and Campadelli-Fiume G. (2013). αvβ6- and αvβ8-integrins serve as interchangeable receptors for HSV gH/gL to promote endocytosis and activation of membrane fusion. PLoS Pathog. 9, e1003806 10.1371/journal.ppat.1003806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni T., Massaro R. and Campadelli-Fiume G. (2015). Dissociation of HSV gL from gH by αvβ6- or αvβ8-integrin promotes gH activation and virus entry. Proc. Natl. Acad. Sci. USA 112, E3901-E3910. 10.1073/pnas.1506846112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H. (2014). Integrin activation. BMB Rep. 47, 655-659. 10.5483/BMBRep.2014.47.12.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y. and Prud'homme G. J. (2008). Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84, 302-310. 10.1189/jlb.0208090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J. C. and Müller U. (2001). β1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367-379. 10.1016/S0896-6273(01)00374-9 [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L. and Ginty D. D. (2003). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45-57. 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch G., Schober M., Pasolli H. A., Conn E. B., Polak L. and Fuchs E. (2007). Loss of TGFβ signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313-327. 10.1016/j.ccr.2007.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero P. A., Tchaicha J. H., Chen Z., Morales J. E., McCarty N., Wang Q., Sulman E. P., Fuller G., Lang F. F., Rao G. et al. (2017). Glioblastoma stem cells exploit the αvβ8 integrin-TGFβ1 signaling axis to drive tumor initiation and progression. Oncogene 36, 6568-6580. 10.1038/onc.2017.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger D. S., Bouaouina M. and Calderwood D. A. (2009). Kindlin-1 and −2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284, 11485-11497. 10.1074/jbc.M809233200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck A. P., Mueller T. D. and Springer T. A. (2016). Structural biology and evolution of the TGF-beta family. Cold Spring Harb. Perspect. Biol. 8, a022103 10.1101/cshperspect.a022103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T., Zenke Y., Yang Y., Bartholin L., Beura L. K., Masopust D. and Kaplan D. H. (2019). Keratinocyte-mediated activation of the cytokine TGF-β maintains skin recirculating memory CD8+ T cells. Immunity 50, 1249-1261.e5. 10.1016/j.immuni.2019.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S., Liu Q., Lee H. S., Hossain M. G., Lacy-Hulbert A. and McCarty J. H. (2011). The astrocyte-expressed integrin alphavbeta8 governs blood vessel sprouting in the developing retina. Development 138, 5157-5166. 10.1242/dev.069153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S., Clements T. P., Tang L. K., Morales J. E., Lee H. S., Oh S. P., Rivera G. M., Wagner D. S. and McCarty J. H. (2015). Neuropilin 1 balances beta8 integrin-activated TGFbeta signaling to control sprouting angiogenesis in the brain. Development 142, 4363-4373. 10.1242/dev.113746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke K. M., McHugh K. P., Tsakiris D. A., Rayburn H., Crowley D., Ullman-Culleré M., Ross F. P., Coller B. S., Teitelbaum S. and Hynes R. O. (1999). β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103, 229-238. 10.1172/JCI5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A., Esmaeli B., Hayek B., Hossain M. G., Shinder R., Lazar A. J. and McCarty J. H. (2011). Analysis of αv integrin protein expression in human eyelid and periorbital squamous cell carcinomas. J. Cutan. Pathol. 38, 570-575. 10.1111/j.1600-0560.2011.01687.x [DOI] [PubMed] [Google Scholar]
- Huang X., Griffiths M., Wu J., Farese R. V. Jr. and Sheppard D. (2000). Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol. Cell. Biol. 20, 755-759. 10.1128/MCB.20.3.755-759.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Cai J. L., Huang P. Z., Kang L., Huang M. J., Wang L. and Wang J. P. (2017). miR19b-3p promotes the growth and metastasis of colorectal cancer via directly targeting ITGB8. Am. J. Cancer Res. 7, 1996-2008. [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M. and Chesnokova L. S. (2010). Integrins as triggers of Epstein-Barr virus fusion and epithelial cell infection. Virulence 1, 395-398. 10.4161/viru.1.5.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Lively J. C., McCarty J. H., Taverna D., Francis S. E., Hodivala-Dilke K. and Xiao Q. (2002). The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb. Symp. Quant. Biol. 67, 143-153. 10.1101/sqb.2002.67.143 [DOI] [PubMed] [Google Scholar]
- Jackson T., Clark S., Berryman S., Burman A., Cambier S., Mu D., Nishimura S. and King A. M. (2004). Integrin alphavbeta8 functions as a receptor for foot-and-mouth disease virus: role of the beta-chain cytodomain in integrin-mediated infection. J. Virol. 78, 4533-4540. 10.1128/JVI.78.9.4533-4540.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S., Subudhi S. K., Aparicio A., Ge Z., Guan B., Miura Y. and Sharma P. (2019). Differences in tumor microenvironment dictate T Helper lineage polarization and response to immune checkpoint therapy. Cell 179, 1177-1190.e13. 10.1016/j.cell.2019.10.029 [DOI] [PubMed] [Google Scholar]
- Jin S., Lee W. C., Aust D., Pilarsky C. and Cordes N. (2019). β8 integrin mediates pancreatic cancer cell radiochemoresistance. Mol. Cancer Res. 17, 2126-2138. 10.1158/1541-7786.MCR-18-1352 [DOI] [PubMed] [Google Scholar]
- Kelly A., Gunaltay S., McEntee C. P., Shuttleworth E. E., Smedley C., Houston S. A., Fenton T. M., Levison S., Mann E. R. and Travis M. A. (2018). Human monocytes and macrophages regulate immune tolerance via integrin αvβ8-mediated TGFβ activation. J. Exp. Med. 215, 2725-2736. 10.1084/jem.20171491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Lakhe-Reddy S., McCarty J. H., Sorenson C. M., Sheibani N., Reichardt L. F., Kim J. H., Wang B., Sedor J. R. and Schelling J. R. (2011). Mesangial cell integrin αvβ8 provides glomerular endothelial cell cytoprotection by sequestering TGF-β and regulating PECAM-1. Am. J. Pathol. 178, 609-620. 10.1016/j.ajpath.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. K., Sheppard D. and Chapman H. A. (2018). TGF-beta1 signaling and tissue fibrosis. Cold Spring Harb. Perspect Biol. 10, a022293 10.1101/cshperspect.a022293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Cambier S., Somanath S., Barker T., Minagawa S., Markovics J., Goodsell A., Publicover J., Reichardt L., Jablons D. et al. (2011). Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J. Clin. Invest. 121, 2863-2875. 10.1172/JCI45589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski S. C., Guerrero P. A., Hirota S., Chen Z., Morales J. E., Aghi M. and McCarty J. H. (2017). Neuropilin-1 modulates TGFβ signaling to drive glioblastoma growth and recurrence after anti-angiogenic therapy. PLoS ONE 12, e0185065 10.1371/journal.pone.0185065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A., Smith A. M., Tissire H., Barry M., Crowley D., Bronson R. T., Roes J. T., Savill J. S. and Hynes R. O. (2007). Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc. Natl. Acad. Sci. USA 104, 15823-15828. 10.1073/pnas.0707421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhe-Reddy S., Khan S., Konieczkowski M., Jarad G., Wu K. L., Reichardt L. F., Takai Y., Bruggeman L. A., Wang B., Sedor J. R. et al. (2006). β8 integrin binds Rho GDP dissociation inhibitor-1 and activates Rac1 to inhibit mesangial cell myofibroblast differentiation. J. Biol. Chem. 281, 19688-19699. 10.1074/jbc.M601110200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhe-Reddy S., Li V., Arnold T. D., Khan S. and Schelling J. R. (2014). Mesangial cell αvβ8-integrin regulates glomerular capillary integrity and repair. Am. J. Physiol. Renal. Physiol. 306, F1400-F1409. 10.1152/ajprenal.00624.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe V. L., Verpoorte A. and Stevens M. M. (2013). The changing integrin expression and a role for integrin β8 in the chondrogenic differentiation of mesenchymal stem cells. PLoS ONE 8, e82035 10.1371/journal.pone.0082035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia J. D., Mack S. C., Mulkearns-Hubert E. E., Valentim C. L. and Rich J. N. (2015). Cancer stem cells in glioblastoma. Genes Dev. 29, 1203-1217. 10.1101/gad.261982.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. S., Cheerathodi M., Chaki S. P., Reyes S. B., Zheng Y., Lu Z., Paidassi H., DerMardirossian C., Lacy-Hulbert A., Rivera G. M. et al. (2015). Protein tyrosine phosphatase-PEST and β8 integrin regulate spatiotemporal patterns of RhoGDI1 activation in migrating cells. Mol. Cell. Biol. 35, 1401-1413. 10.1128/MCB.00112-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yang H., Han K., Zhu D., Lun P. and Zhao Y. (2018). A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis by increasing the expression of miR-142-3p target ITGB8 in glioma. Biochem. Biophys. Res. Commun. 498, 254-261. 10.1016/j.bbrc.2018.01.076 [DOI] [PubMed] [Google Scholar]
- Lienart S., Merceron R., Vanderaa C., Lambert F., Colau D., Stockis J., van der Woning B., De Haard H., Saunders M., Coulie P. G. et al. (2018). Structural basis of latent TGF-β1 presentation and activation by GARP on human regulatory T cells. Science 362, 952-956. 10.1126/science.aau2909 [DOI] [PubMed] [Google Scholar]
- Liu J., Zeng L., Kennedy R. M., Gruenig N. M. and Childs S. J. (2012). βPix plays a dual role in cerebral vascular stability and angiogenesis, and interacts with integrin αvβ8. Dev. Biol. 363, 95-105. 10.1016/j.ydbio.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Ma S. and Huang Z. (2015). Neural regulation of CNS angiogenesis during development. Front. Biol. 10, 61-73. 10.1007/s11515-014-1331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Santhosh D., Kumar T. P. and Huang Z. (2017). A brain-region-specific neural pathway regulating germinal matrix angiogenesis. Dev. Cell 41, 366-381.e4. 10.1016/j.devcel.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack F., Baumert B. G., Schäfer N., Hattingen E., Scheffler B., Herrlinger U. and Glas M. (2016). Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat. Rev. 43, 83-91. 10.1016/j.ctrv.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Malekpour Afshar R., Mollaei H. R., Shokrizadeh M. and Iranpour M. (2017). Evaluation expression of microrna-93 and integrin Beta8 in different types of glioma tumors. Asian Pac. J. Cancer Prev. 18, 603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malric L., Monferran S., Delmas C., Arnauduc F., Dahan P., Boyrie S., Deshors P., Lubrano V., Da Mota D. F., Gilhodes J. et al. (2019). Inhibiting integrin beta8 to differentiate and radiosensitize glioblastoma-initiating cells. Mol. Cancer Res. 17, 384-397. 10.1158/1541-7786.MCR-18-0386 [DOI] [PubMed] [Google Scholar]
- Mani V., Bromley S. K., Aijo T., Mora-Buch R., Carrizosa E., Warner R. D., Hamze M., Sen D. R., Chasse A. Y., Lorant A. et al. (2019). Migratory DCs activate TGF-β to precondition naive CD8+ T cells for tissue-resident memory fate. Science 366 10.1126/science.aav5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. Published online April 10, 2020. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovics J. A., Araya J., Cambier S., Jablons D., Hill A., Wolters P. J. and Nishimura S. L. (2010). Transcription of the transforming growth factor β activating integrin β8 subunit is regulated by SP3, AP-1, and the p38 pathway. J. Biol. Chem. 285, 24695-24706. 10.1074/jbc.M110.113977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovics J. A., Araya J., Cambier S., Somanath S., Gline S., Jablons D., Hill A., Wolters P. J. and Nishimura S. L. (2011). Interleukin-1β induces increased transcriptional activation of the transforming growth factor-β-activating integrin subunit β8 through altering chromatin architecture. J. Biol. Chem. 286, 36864-36874. 10.1074/jbc.M111.276790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. (2008). TGFβ in cancer. Cell 134, 215-230. 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H. (2008). Alphav integrins lead the way for colorectal metastases. Clin. Cancer Res. 14, 6351-6353. 10.1158/1078-0432.CCR-08-1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H. (2009). Cell adhesion and signaling networks in brain neurovascular units. Curr. Opin Hematol. 16, 209-214. 10.1097/MOH.0b013e32832a07eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H., Monahan-Earley R. A., Brown L. F., Keller M., Gerhardt H., Rubin K., Shani M., Dvorak H. F., Wolburg H., Bader B. L. et al. (2002). Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol. Cell. Biol. 22, 7667-7677. 10.1128/MCB.22.21.7667-7677.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H., Barry M., Crowley D., Bronson R. T., Lacy-Hulbert A. and Hynes R. O. (2008). Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am. J. Pathol. 172, 1740-1747. 10.2353/ajpath.2008.070700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H., Cook A. A. and Hynes R. O. (2005a). An interaction between {alpha}v{beta}8 integrin and Band 4.1B via a highly conserved region of the Band 4.1 C-terminal domain. Proc. Natl. Acad. Sci. USA 102, 13479-13483. 10.1073/pnas.0506068102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. H., Lacy-Hulbert A., Charest A., Bronson R. T., Crowley D., Housman D., Savill J., Roes J. and Hynes R. O. (2005b). Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132, 165-176. 10.1242/dev.01551 [DOI] [PubMed] [Google Scholar]
- Melton A. C., Bailey-Bucktrout S. L., Travis M. A., Fife B. T., Bluestone J. A. and Sheppard D. (2010). Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 120, 4436-4444. 10.1172/JCI43786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R., Huang X., Wu J., Nishimura S., Pytela R., Sheppard D. and ffrench-Constant C. (1999). Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J. Cell Sci. 112, 4271-4279. [DOI] [PubMed] [Google Scholar]
- Milner R., Relvas J. B., Fawcett J. and ffrench-Constant C. (2001). Developmental regulation of alphav integrins produces functional changes in astrocyte behavior. Mol. Cell. Neurosci. 18, 108-118. 10.1006/mcne.2001.1003 [DOI] [PubMed] [Google Scholar]
- Minagawa S., Lou J., Seed R. I., Cormier A., Wu S., Cheng Y., Murray L., Tsui P., Connor J., Herbst R. et al. (2014). Selective targeting of TGF-beta activation to treat fibroinflammatory airway disease. Sci. Transl. Med. 6, a79 10.1126/scitranslmed.3008074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley A. K., Tchaicha J. H., Shin J., Hossain M. G. and McCarty J. H. (2009). Beta8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J. Cell Sci. 122, 1842-1851. 10.1242/jcs.043257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley A. K. and McCarty J. H. (2011). β8 integrin is essential for neuroblast migration in the rostral migratory stream. Glia 59, 1579-1587. 10.1002/glia.21199 [DOI] [PubMed] [Google Scholar]
- Mohammed J., Beura L. K., Bobr A., Astry B., Chicoine B., Kashem S. W., Welty N. E., Igyarto B. Z., Wijeyesinghe S., Thompson E. A. et al. (2016). Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-β. Nat. Immunol. 17, 414-421. 10.1038/ni.3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moissoglu K. and Schwartz M. A. (2006). Integrin signalling in directed cell migration. Biol. Cell 98, 547-555. 10.1042/BC20060025 [DOI] [PubMed] [Google Scholar]
- Moyle M., Napier M. A. and McLean J. W. (1991). Cloning and expression of a divergent integrin subunit beta 8. J. Biol. Chem. 266, 19650-19658. [PubMed] [Google Scholar]
- Mu D., Cambier S., Fjellbirkeland L., Baron J. L., Munger J. S., Kawakatsu H., Sheppard D., Broaddus V. C. and Nishimura S. L. (2002). The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157, 493-507. 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J. S., Huang X., Kawakatsu H., Griffiths M. J., Dalton S. L., Wu J., Pittet J. F., Kaminski N., Garat C., Matthay M. A. et al. (1999). The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319-328. 10.1016/S0092-8674(00)80545-0 [DOI] [PubMed] [Google Scholar]
- Murase S. and Horwitz A. F. (2004). Directions in cell migration along the rostral migratory stream: the pathway for migration in the brain. Curr. Top. Dev. Biol. 61, 135-152. 10.1016/S0070-2153(04)61006-4 [DOI] [PubMed] [Google Scholar]
- Nguyen H. L., Lee Y. J., Shin J., Lee E., Park S. O., McCarty J. H. and Oh S. P. (2011). TGF-β signaling in endothelial cells, but not neuroepithelial cells, is essential for cerebral vascular development. Lab. Invest. 91, 1554-1563. 10.1038/labinvest.2011.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S. L., Sheppard D. and Pytela R. (1994). Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J. Biol. Chem. 269, 28708-28715. [PubMed] [Google Scholar]
- Ozawa A., Sato Y., Imabayashi T., Uemura T., Takagi J. and Sekiguchi K. (2016). Molecular basis of the ligand binding specificity of αvβ8 Integrin. J. Biol. Chem. 291, 11551-11565. 10.1074/jbc.M116.719138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes I., Himmels P. and Ruiz de Almodovar C. (2018). Neurovascular communication during CNS development. Dev. Cell 45, 10-32. 10.1016/j.devcel.2018.01.023 [DOI] [PubMed] [Google Scholar]
- Park S. O., Lee Y. J., Seki T., Hong K. H., Fliess N., Jiang Z., Park A., Wu X., Kaartinen V., Roman B. L. et al. (2008). ALK5- and TGFBR2-independent role of ALK1 in the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Blood 111, 633-642. 10.1182/blood-2007-08-107359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas S., Anido J., Prieto-Sanchez R. M., Folch G., Barba I., Cuartas I., Garcia-Dorado D., Poca M. A., Sahuquillo J., Baselga J. et al. (2009). TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell 15, 315-327. 10.1016/j.ccr.2009.02.011 [DOI] [PubMed] [Google Scholar]
- Pollen A. A., Bhaduri A., Andrews M. G., Nowakowski T. J., Meyerson O. S., Mostajo-Radji M. A., Di Lullo E., Alvarado B., Bedolli M., Dougherty M. L. et al. (2019). Establishing cerebral organoids as models of human-specific brain evolution. Cell 176, 743-756.e17. 10.1016/j.cell.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor J. M., Zang K., Wang D., Wang R. and Reichardt L. F. (2005). Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J. Neurosci. 25, 9940-9948. 10.1523/JNEUROSCI.3467-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Garrison B. S., Ma W., Wang R., Jiang A., Li J., Mistry M., Bronson R. T., Santoro D., Franco C. et al. (2018). A milieu molecule for TGF-β required for microglia function in the nervous system. Cell 174, 156-171.e16. 10.1016/j.cell.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S. B., Narayanan A. S., Lee H. S., Tchaicha J. H., Aldape K. D., Lang F. F., Tolias K. F. and McCarty J. H. (2013a). αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion. Mol. Biol. Cell 24, 474-482. 10.1091/mbc.e12-07-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S. B., Narayanan A. S., Lee H. S., Tchaicha J. H., Aldape K. D., Lang F. F., Tolias K. F. and McCarty J. H. (2013b). αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion. Mol. Biol. Cell 24, 474-482. 10.1091/mbc.e12-07-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R. A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M. S., Huang E., Garcia-Verdugo J.-M. et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382-386. 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh D. and Huang Z. (2015). Regulation of the nascent brain vascular network by neural progenitors. Mech. Dev. 138, 37-42. 10.1016/j.mod.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhosh D., Sherman J., Chowdhury S. and Huang Z. (2019). Harnessing region-specific neurovascular signaling to promote germinal matrix vessel maturation and hemorrhage prevention. Dis. Model. Mech. 12, dmm041228 10.1242/dmm.041228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savar A., Acin S., Gonzalez C. L., El-Sawy T., Mejia O., Li Z., Esmaeli B., Lacy-Hulbert A., El-Naggar A. K., McCarty J. H. et al. (2015). Loss of epithelial p53 and αv integrin cooperate through Akt to induce squamous cell carcinoma yet prevent remodeling of the tumor microenvironment. Oncogene 34, 516-524. 10.1038/onc.2013.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm J., Klein A., Tatagiba M. S., Meyermann R., Fend F., Goodman S. L. and Sipos B. (2013). Comparing the expression of integrins alphavbeta3, alphavbeta5, alphavbeta6, alphavbeta8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors. Int. J. Clin. Exp. Pathol. 6, 2719-2732. [PMC free article] [PubMed] [Google Scholar]
- Shi M., Zhu J., Wang R., Chen X., Mi L., Walz T. and Springer T. A. (2011). Latent TGF-β structure and activation. Nature 474, 343-349. 10.1038/nature10152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist C. J., Bridge A. and Le Mercier P. (2020). A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res. 177, 104759 10.1016/j.antiviral.2020.104759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockis J., Lienart S., Colau D., Collignon A., Nishimura S. L., Sheppard D., Coulie P. G. and Lucas S. (2017). Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin αVβ8. Proc. Natl. Acad. Sci. USA 114, E10161-E10168. 10.1073/pnas.1710680114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Kim H., Pawlikowska L., Kitamura H., Shen F., Cambier S., Markovics J., Lawton M. T., Sidney S., Bollen A. W. et al. (2010). Reduced expression of integrin alphavbeta8 is associated with brain arteriovenous malformation pathogenesis. Am. J. Pathol. 176, 1018-1027. 10.2353/ajpath.2010.090453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaka N., Seed R. I., Cormier A., Bondesson A. J., Lou J., Elattma A., Ito S., Yanagisawa H., Hashimoto M., Ma R. et al. (2018). Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight 3 10.1172/jci.insight.122591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata M., Ruhrberg C. and Fantin A. (2015). Vascularisation of the central nervous system. Mech. Dev. 138, 26-36. 10.1016/j.mod.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchaicha J. H., Reyes S. B., Shin J., Hossain M. G., Lang F. F. and McCarty J. H. (2011). Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by beta8 integrin. Cancer Res. 71, 6371-6381. 10.1158/0008-5472.CAN-11-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwary S., Morales J. E., Kwiatkowski S. C., Lang F. F., Rao G. and McCarty J. H. (2018). Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci. Rep. 8, 8267 10.1038/s41598-018-26636-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis M. A., Reizis B., Melton A. C., Masteller E., Tang Q., Proctor J. M., Wang Y., Bernstein X., Huang X., Reichardt L. F. et al. (2007). Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361-365. 10.1038/nature06110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venstrom K. and Reichardt L. (1995). Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol. Biol. Cell 6, 419-431. 10.1091/mbc.6.4.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Dong X., Zhao B., Li J., Lu C. and Springer T. A. (2017). Atypical interactions of integrin αVβ8 with pro-TGF-β1. Proc. Natl. Acad. Sci. USA 114, E4168-E4174. 10.1073/pnas.1705129114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Su Y., Iacob R. E., Engen J. R. and Springer T. A. (2019). General structural features that regulate integrin affinity revealed by atypical αVβ8. Nat. Commun. 10, 5481 10.1038/s41467-019-13248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S. M. and Cheresh D. A. (2011). alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect Med. 1, a006478 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Nabors L. B., Gorlia T., Leske H., Rushing E., Bady P., Hicking C., Perry J., Hong Y. K., Roth P. et al. (2016). Cilengitide in newly diagnosed glioblastoma: biomarker expression and outcome. Oncotarget 7, 15018-15032. 10.18632/oncotarget.7588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman M. C., Fan W., Rela L., Rodriguez-Gil D. J. and Greer C. A. (2009). Blood vessels form a migratory scaffold in the rostral migratory stream. J. Comp. Neurol. 516, 94-104. 10.1002/cne.22093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington J. J., Klementowicz J. E. and Travis M. A. (2011). TGFβ: a sleeping giant awoken by integrins. Trends Biochem. Sci. 36, 47-54. 10.1016/j.tibs.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Worthington J. J., Kelly A., Smedley C., Bauche D., Campbell S., Marie J. C. and Travis M. A. (2015). Integrin αvβ8-mediated TGF-β activation by effector regulatory T cells is essential for suppression of T-cell-mediated inflammation. Immunity 42, 903-915. 10.1016/j.immuni.2015.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Moissoglu K., Wang H., Wang X., Frierson H. F., Schwartz M. A. and Theodorescu D. (2009). Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc. Natl. Acad. Sci. USA 106, 5807-5812. 10.1073/pnas.0810094106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Ema H., Karlsson G., Yamaguchi T., Miyoshi H., Shioda S., Taketo M. M., Karlsson S., Iwama A. and Nakauchi H. (2011). Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146-1158. 10.1016/j.cell.2011.09.053 [DOI] [PubMed] [Google Scholar]
- Yang Z., Mu Z., Dabovic B., Jurukovski V., Yu D., Sung J., Xiong X. and Munger J. S. (2007). Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J. Cell Biol. 176, 787-793. 10.1083/jcb.200611044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Jiang G., Cai Y., Monkley S. J., Critchley D. R. and Sheetz M. P. (2008). Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10, 1062-1068. 10.1038/ncb1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. and Lu Z. (2013). Regulation of tumor cell migration by protein tyrosine phosphatase (PTP)-proline-, glutamate-, serine-,and threonine-rich sequence (PEST). Chin J. Cancer 32, 75-83. 10.5732/cjc.012.10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Motejlek K., Wang D., Zang K., Schmidt A. and Reichardt L. F. (2002). beta8 integrins are required for vascular morphogenesis in mouse embryos. Development 129, 2891-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]


