The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing respiratory disease rapidly led to a global pandemic threatening to overwhelm healthcare infrastructure and to require rationing of existing resources. After its identification in China in December 2019, this new virus, causing a disease termed Coronavirus Disease 2019 (COVID-2019), spread to other countries in the region before creating a worldwide health crisis. By early March 2020, more than 100,000 people had tested positive for the disease with nearly 4,000 dying (1). Per initial reports, the virus causes a spectrum of symptoms ranging from intermittent fevers and cough to profound respiratory failure and cardiogenic shock. The early clinical experience with this emerging pathogen indicates that approximately 15–30% of hospitalized patients develop acute respiratory distress syndrome (ARDS). According to one study, 12% of admitted patients progress to requiring mechanical ventilation, with 3% needing extracorporeal membrane oxygenation (ECMO) support (2).
There is no vaccine or targeted antiviral therapy currently available to reduce the burden of disease for patients with COVID-19. Care for these patients remains largely supportive with a therapeutic goal of maintaining homeostasis and reducing complications of mechanical ventilation. For patients with refractory hypoxemia, the World Health Organization guidelines recommend transferring patients to an ECMO center for consideration of advanced support (3). ECMO has previously been used successfully during pandemics of severe respiratory illness and may play an important role as salvage therapy in the COVID-19 pandemic (4, 5).
Based on current practice patterns, the role of ECMO in supporting patients during a pandemic depends on the severity of the pandemic. In a mild pandemic, hospitals may see a surge of 20% over usual volume. In a severe pandemic, the role of ECMO becomes vanishingly small as the burden of disease vastly surpasses available resources. At this juncture, the focus of patient care transitions from patient-centric to population-centric. The overarching goal of population-centric care is to limit total mortality to achieve “the greatest good for the greatest number” (6). In the scenario of a severe pandemic, in which resources are limited and staff are overburdened, intensive critical care services, such as ECMO, may be de-prioritized to redirect care toward numerous patients with a higher likelihood of survival.
ECMO is a resource-intensive therapy requiring consideration before its deployment. In the setting of a severe pandemic, with strain on both personnel and material resources, use of ECMO demands additional deliberation. Based on the existing clinical experience, the specific role of ECMO in the COVID-19 pandemic is unclear. As case numbers increase, so, too, will the number of patients with severe disease, with an anticipated increase in the call for use of ECMO to support patients with COVID-19–associated ARDS. Despite the fact that ECMO use in severe ARDS continues to grow (7), there are no ECMO-specific guidelines or references for centers on preparation for pandemics of severe respiratory illness. We suggest the following framework for ECMO centers to consider during a natural disaster/pandemic.
Guidelines for ECMO Initiation
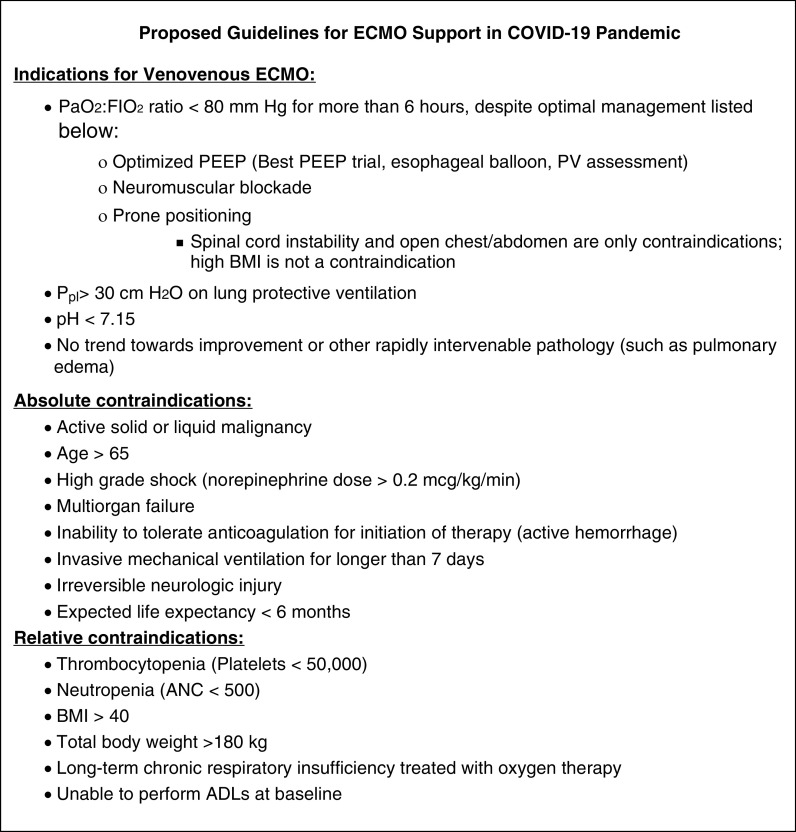
During a pandemic, criteria for initiating ECMO support must be clearly defined to reduce the burden on ECMO services and to enable bedside clinicians to appropriately guide expectations and patient management. Consensus guidelines regarding critical care during pandemics recommend having criteria derived a priori for critical care procedures that are objectively and universally applied rather than relying on clinical judgment applied on an individual basis (6). Preparing guidelines in advance eliminates subjectivity and inconsistency between individual providers, making allocation of intensive therapies more equitable and ethical. It is common practice for ECMO centers to institute specific guidelines and criteria for ECMO implementation. Guidelines should stress the importance of performing all other evidence-based interventions, such as lung-protective ventilation and prone positioning, before the consideration of ECMO. During emergencies, these criteria must be revised and adapted to triage current patient demand and prepare for anticipated needs. Given heightened demand for limited capacity, this policy must clearly define criteria for cessation of support to reduce futile care and enable allocation of limited resources. Patients receiving ECMO support require regular reevaluations to ensure that their recovery remains viable and to guide appropriate goals of care. During situations of mass critical care, it may be ethically permissible to withdraw ECMO to reallocate support to patients with a higher likelihood of benefit (6). Figure 1 details an example of ECMO guidelines for COVID-19. These protocols are subject to change as more information about the disease is obtained.
Figure 1.
Proposed indications and contraindications for extracorporeal membrane oxygenation (ECMO) support during the coronavirus disease 2019 (COVID-19) pandemic. ADLs = activities of daily living; ANC = absolute neutrophil count; BMI = body mass index; FiO2 = fraction of inspired oxygen; PaO2 = arterial oxygen tension; PEEP = positive end-expiratory pressure; Ppl = plateau pressure; PV = pressure–volume.
Allocation of Scarce Resources
We recommend that ECMO be incorporated into hospital plans for allocation of scarce resources under crisis standards of care. The group that convenes to develop this framework should be multidisciplinary and should include institutional experts in the following areas: emergency preparedness, disaster management, critical care medicine, palliative care medicine, ethics, and risk management. Once crisis standards of care are invoked, the plan for allocation of scarce resources will be activated. At this time, the decision to provide ECMO should be made by the triage team designated by the Hospital Incident Command System in conjunction with the ECMO service. The triage team will have a process of allocating resources that is based on ethical principles grounded in the following: attention to fairness, openness, transparency, and accountability in allocation of resources and protection of the rights of individuals with respect to privacy, confidentiality, and imposition of limitations on personal freedom (8). In particular, with ECMO, the goal will be to reserve this limited resource for those who would achieve the greatest benefit and limit prolonged runs in patients without recovery.
Equipment
During the hospital response preparation phase of a pandemic, it is important to determine current inventory and stockpile important equipment while recognizing that demand will be increased universally. Unlike other critical care equipment, such as mechanical ventilators, there is no regional or national stockpile of ECMO equipment available to assist hospitals in the face of a pandemic. Therefore, it is reasonable to plan for a 20–200% increase in ECMO equipment from baseline utilization (9). In the presence of a slow-onset pandemic, equipment targets can be made on the basis of a data-driven projected increase in patients. It is incumbent on each ECMO center to maintain essential equipment necessary for extracorporeal support, including vascular cannulas, circuit tubing, pump heads, and oxygenators. Advanced integrated systems would be anticipated to be costly and difficult to obtain in a pandemic, but more cost-effective approaches, such as implementing simple pump and oxygenator-only systems, can be considered. Preemptively expanding the number of ECMO circuits in the early phases of a pandemic is worthwhile as a means of expanding total capacity.
At the onset of a pandemic, programs typically will use equipment as per preexisting guidelines and practice patterns. ECMO coordinators should perform a daily inventory and order replacements to maintain adequate supplies and in anticipation of increasing demand. As the pandemic progresses or becomes more severe, ECMO protocols should be altered to be more selective in an effort to increase the likelihood of obtaining maximal benefit.
Capacity
Maximum capacity per center should be defined a priori. There are three main factors that usually determine the ECMO capacity of a hospital: ICU bed availability, registered nurse (RN)/ECMO specialist staffing, and number of ECMO circuits. During a crisis, ICU bed and staffing models should be optimized to care for the greatest number of patients possible. This may require a change to usual staffing models. An example may be to group all ECMO patients in a single geographical space to enable a higher patient-to-ECMO specialist/RN ratio. Another option is to create critical surge staffing protocols (10). In these protocols, staffing can be adjusted on the basis of the level of acuity such that stable ECMO patients can be supported by fewer providers than usual. The number of ECMO circuits available is the ultimate limiting factor in determining total patient support capacity. Device improvisation is an option to increase the number of ECMO machines available for use. Splicing an oxygenator to percutaneous ventricular assist devices is a viable option to provide oxygenation and ventilation support (11, 12). Depending on expertise and equipment available at individual centers, this may be a viable option for increasing ECMO capacity.
Collaboration with Other Local/Regional ECMO Centers
Data have shown that outcomes are better when patients with severe ARDS are transferred to ECMO centers (13). During a pandemic, ECMO centers maybe inundated with patients with severe ARDS transferred from community centers lacking these capabilities. To ensure access to ECMO centers for patients with severe ARDS from community centers, establishing a regional system to manage the surge in ECMO referral volume is vital. In the United Kingdom, the National Health Service has a standard operating procedure (SOP) dedicated to this exact scenario (14). There are several phases of this plan, including presurge, surge, escalation, and recovery phases. At the crux of this protocol are regular teleconferences and communication between ECMO centers, emphasizing capacity and an escalation–de-escalation plan. A similar concept can be instituted in nonnationalized healthcare settings as well. ECMO centers in geographical proximity should establish an SOP for ECMO during the pandemic. Important elements of this SOP include standardized criteria for ECMO initiation, a real-time reporting structure for ECMO capacity and volume at each center, and a system to facilitate transfers from the community to the available centers with capacity. Another important function of this collaboration can be to advise local community hospitals in implementing advanced respiratory failure interventions (including prone positioning) before consideration for transfer for ECMO to avoid unnecessary transfers and to conserve capacity.
Summary
ECMO will be increasingly used as a vital support modality during pandemics of severe respiratory illness. Whether they are dealing with COVID-19 or another novel virus causing severe disease, hospitals will need to have a formal plan to rapidly respond to expanding need for a limited resource. The plan will need to be scaled to the magnitude of the pandemic. Our suggested framework for ECMO use in pandemics has been adapted largely from the guidelines and consensus statements on the provision of mass critical care (Table 1). The overarching goal of ECMO use during a pandemic is to reduce mortality in patients with profound failure. Additional provisions must be made for the possibility of abandoning resource-intensive critical care therapies, such as ECMO, in the setting severe pandemics to focus care on less–resource-dependent therapies that may provide greater benefit to a greater number of patients. Establishing these guidelines and practice patterns is vital to supporting the overall mission of preserving the greatest number of quality life-years for the population as a whole while ensuring equitable care.
Table 1.
Tiered approach to extracorporeal membrane oxygenation response during a pandemic
| Mild Surge: Focus Is on Increasing Capacity |
|---|
| • Develop criteria specific to pandemic for initiation and cessation of ECMO |
| • Stockpile necessary equipment |
| • Collocation/regionalization of ECMO patients |
| • Staffing protocols that allow ECMO specialist/RN to care for more patients on the basis of acuity |
| • Increase capacity by acquisition of more pumps and/or device improvisation with percutaneous VADs with oxygenators |
• Collaboration with other local/regional ECMO centers:
|
| Moderate Surge: Focus on Allocation of Scarce Resources |
|---|
| • Incorporate ECMO initiation decision into HICS framework for allocation of scarce resources |
| Major Surge: Scarce Resources May Not Be Offered |
|---|
| • National or regional guidance may mandate that ECMO no longer be offered in order to focus care on less–resource-intensive therapies that can provide a greater benefit to a greater number of patients |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; HICS = Hospital Incident Command System; RN = registered nurse; VAD = ventricular assist device.
Supplementary Material
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report – 49 2020 Mar 9[accessed 2020 Mar 15]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200309-sitrep-49-covid-19.pdf?sfvrsn=70dabe61_4
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. 2020 [accessed 2020 Mar 7]. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 4.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 5.Al-Dorzi HM, Aldawood AS, Khan R, Baharoon S, Alchin JD, Matroud AA, et al. The critical care response to a hospital outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: an observational study. Ann Intensive Care. 2016;6:101. doi: 10.1186/s13613-016-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biddison LD, Berkowitz KA, Courtney B, De Jong CM, Devereaux AV, Kissoon N, et al. Task Force for Mass Critical Care; Task Force for Mass Critical Care. Ethical considerations: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(Suppl):e145S–e155S. doi: 10.1378/chest.14-0742. [DOI] [PubMed] [Google Scholar]
- 7.Rush B, Wiskar K, Berger L, Griesdale D. Trends in extracorporeal membrane oxygenation for the treatment of acute respiratory distress syndrome in the United States. J Intensive Care Med. 2017;32:535–539. doi: 10.1177/0885066616631956. [DOI] [PubMed] [Google Scholar]
- 8.Leider JP, DeBruin D, Reynolds N, Koch A, Seaberg J. Ethical guidance for disaster response, specifically around crisis standards of care: a systematic review. Am J Public Health. 2017;107:e1–e9. doi: 10.2105/AJPH.2017.303882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hick JL, Einav S, Hanfling D, Kissoon N, Dichter JR, Devereaux AV, et al. Task Force for Mass Critical Care; Task Force for Mass Critical Care. Surge capacity principles: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(Suppl):e1S–e16S. doi: 10.1378/chest.14-0733. [DOI] [PubMed] [Google Scholar]
- 10.Salna M, Chicotka S, Biscotti M, III, Agerstrand C, Liou P, Ginsburg M, et al. Management of surge in extracorporeal membrane oxygenation transport. Ann Thorac Surg. 2018;105:528–534. doi: 10.1016/j.athoracsur.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Herlihy JP, Loyalka P, Jayaraman G, Kar B, Gregoric ID. Extracorporeal membrane oxygenation using the TandemHeart System's catheters. Tex Heart Inst J. 2009;36:337–341. [PMC free article] [PubMed] [Google Scholar]
- 12.Betit P, Matte GS, Howe R, Iudiciani P, Barrett C, Thiagarajan R, et al. The addition of a membrane oxygenator to a ventricular assist device in a patient with acute respiratory distress syndrome. J Extra Corpor Technol. 2011;43:264–266. [PMC free article] [PubMed] [Google Scholar]
- 13.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 14.NHS England. Management of surge and escalation in critical care services: standard operating procedure for adult respiratory extra corporeal membrane oxygenation. 2017 [accessed 2020 Mar 7]. Available from: https://www.england.nhs.uk/publication/management-of-surge-and-escalation-in-critical-care-services-standard-operating-procedure-for-adult-respiratory-extra-corporeal-membrane-oxygenation/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.