Abstract
Rationale: Individuals eligible for lung cancer screening (LCS) by low-dose computed tomography (LDCT) are also at risk of chronic obstructive pulmonary disease (COPD) due to age and smoking exposure. Whether the LCS episode is useful for early detection of COPD is not well established.
Objectives: To explore associations between symptoms, comorbidities, spirometry, and emphysema in participants enrolled in the Lung Screen Uptake Trial.
Methods: This cross-sectional study was a prespecified analysis nested within Lung Screen Uptake Trial, which was a randomized study testing the impact of differing invitation materials on attendance of 60- to 75-year-old smokers and ex-smokers to a “lung health check” between November 2015 and July 2017. Participants with a smoking history ≥30 pack-years and who quit ≤15 years ago, or meeting a lung cancer risk of ≥1.51% via the Prostate Lung Colorectal Ovarian model or ≥2.5% via the Liverpool Lung Project model, were offered LDCT. COPD was defined and classified according to the GOLD (Global Initiative for Obstructive Lung Disease) criteria using prebronchodilator spirometry. Analyses included the use of descriptive statistics, chi-square tests to examine group differences, and univariable and multivariable logistic regression to explore associations between symptom prevalence, airflow limitation, and visually graded emphysema.
Results: A total of 560 of 986 individuals included in the analysis (57%) had prebronchodilator spirometry consistent with COPD; 67% did not have a prior history of COPD and were termed “undiagnosed.” Emphysema prevalence in those with known and “undiagnosed” COPD was 73% and 68%, respectively. A total of 32% of those with “undiagnosed COPD” had no emphysema on LDCT. Inhaler use and symptoms were more common in the “known” than the “undiagnosed” COPD group (63% vs. 33% with persistent cough [P < 0.001]; 73% vs. 33% with dyspnea [P < 0.001]). Comorbidities were common in all groups. Adjusted odds ratio (aOR) of respiratory symptoms were more significant for airflow obstruction (aOR GOLD 1 and 2, 1.57; confidence interval [CI], 1.14–2.17; aOR GOLD 3 and 4, 4.6; CI, 2.17–9.77) than emphysema (aOR mild, 1.12; CI, 0.81–1.55; aOR moderate, 1.33; CI, 0.85–2.09; aOR severe, 4.00; CI, 1.57–10.2).
Conclusions: There is high burden of “undiagnosed COPD” and emphysema in LCS participants. Adding spirometry findings to the LDCT enhances identification of individuals with COPD.
Clinical trial registered with www.clinicaltrials.gov (NCT02558101).
Keywords: lung cancer screening, low-dose computed tomography, emphysema, chronic obstructive pulmonary disease, case finding
Chronic obstructive pulmonary disease (COPD) is the third most common cause of death globally, after coronary heart disease (CHD) and stroke (1). The presence of emphysema, airflow limitation, increasing spirometric COPD severity, and exacerbation frequency are all associated with a greater risk of lung cancer (2, 3), though this effect is reduced after adjustment for smoking history and other confounders (4). Biologically, this association may be explained by a combination of chronic inflammation, impaired mucocilliary action, DNA damage and aberrant repair, and genetic susceptibility (5).
Lung cancer screening (LCS) using low-dose computed tomography (LDCT) significantly reduced the relative risk of lung cancer-specific and all-cause mortality by 20% and 6.7%, respectively, in the National Lung Screening Trial (NLST) (6). Both the Dutch–Belgian LCS trial and the Multicentric Italian Lung Detection study provide further evidence in a European population, in support of this finding (7, 8). Evidence suggests cohorts at higher risk for lung cancer may be more likely to benefit from LCS than those with lower risk (9), with a twofold increase in lung cancer prevalence, greater stage shift, and reduced overdiagnosis among those with airflow limitation compared with those without (9–11). However, those with more advanced COPD have been found not to have a significant reduction in lung cancer–specific mortality from LCS (9).
Age and smoking history are the strongest predictors of the development of lung cancer and COPD, enabling identification of a common population in which to carry out “case finding” for the two conditions. Indeed, LCS studies have reported COPD prevalence rates as high as 38% (12), almost fourfold higher than in the general population (13). Other studies have demonstrated a significant burden of undiagnosed COPD within primary care populations (13–16), though currently “screening” for asymptomatic COPD is not recommended by the U.S. Preventative Services Task Force (USPSTF) (17). Given that symptoms, exacerbation frequency, and comorbidities are important determinants of prognosis, and rate of decline of forced expiratory volume in 1 second (FEV1) is not currently modifiable with pharmacotherapy (18, 19), the GOLD (Global Initiative for Obstructive Lung Disease) guidance emphasizes the importance of the former parameters over spirometry values for guiding treatment decisions (20). Furthermore, comorbidities, such as CHD, hypertension, and osteoporosis, are frequent in COPD, and may be undertreated (18, 21). Optimization of these conditions may positively impact COPD outcomes. The GOLD 2019 report has recommended active COPD case finding for those with symptoms and/or risk factors (20), but it is unknown whether detecting airflow obstruction and emphysema in the LCS population confers any clinical benefit.
In this study, we aimed to explore the clinical significance of COPD and emphysema detected at LCS. Specifically, to: 1) assess the prevalence of airflow limitation, respiratory symptoms, and radiological emphysema in an LCS population; 2) determine whether LCS participants with COPD differ from those with “undiagnosed COPD” in terms of symptoms, comorbidities, and inhaler use, and whether symptom prevalence differs in participants with and without emphysema; and 3) explore associations between airflow limitation, emphysema, and respiratory symptoms.
Methods
Study Design, Participants, and Setting
This was a nested, cross-sectional study using data from the UK-based randomized controlled LSUT (Lung Screen Uptake Trial), the design of which has been described previously (22). Briefly, individuals between 60 and 75 years of age, who were coded as current smokers within the past 5–7 years, were invited by their general practitioner for a “lung health check” (LHC) at one of two London hospitals. The primary aim of LSUT was to test differences in uptake of LCS between individuals randomly allocated to either “standard” or “targeted” invitation materials, which were designed to engage smokers from low-socioeconomic communities. Individuals attending the LHC were invited to participate in the study.
At the time of recruitment, emerging data supported that risk-based selection into screening may be preferable to the USPSTF-advocated age and smoking-based criteria (23, 24), due to enrichment of lung cancer in the selected population and a resultant optimized risk–benefit balance from LCS. In light of this evidence, both strategies were considered appropriate for selecting LCS participants. In the present study, those meeting the USPSTF criteria for LCS (i.e., ≥30 pack-years and quit ≤15 yr ago) (25), or a lung cancer risk of ≥1.51% in 6 years as determined by the Prostate Lung Colorectal Ovarian model (26) or ≥2.5% in 5 years as determined by the Liverpool Lung Project model (27), were offered LDCT. Exclusion criteria included lack of capacity to consent, physical status contraindicating LDCT, or a prior chest CT scan within the previous 12 months.
Data Collection
Self-reported demographics (age, sex, ethnicity, education level, and Index of Multiple Deprivation [IMD] score and rank), smoking, family and medical history, height, weight, and blood pressure were collected by a study practitioner at the LHC.
Symptoms
Participants were asked about current or recent history of respiratory symptoms. Study practitioners were instructed to read out and explain the symptoms and ask participants to state if they experienced these symptoms currently or in the previous 12 months. Specifically, participants were asked about dyspnea (explained as “shortness of breath”), persistent cough (“a cough lasting ≥6 wk”), and lower respiratory tract infection (“a chest infection with productive cough”). Participants who reported a history of persistent cough or dyspnea within the last 12 months were regarded as having respiratory symptoms. This composite outcome was used to try to capture those participants who, in the context of airflow obstruction, may have COPD, and may reasonably be expected to undergo further medical assessment to determine this. The modified Medical Research Council dyspnea score was also assessed and recorded by the study practitioner.
Comorbidities
Participants were asked about a known history of COPD, asthma, previous pneumonia, CHD, hypertension (including those controlled with antihypertensives), hypercholesterolemia, diabetes mellitus, and osteoporosis. Participants were labeled as “undiagnosed COPD” if they had evidence of airflow limitation and did not report a prior diagnosis of COPD, chronic bronchitis, or emphysema. Participants were also asked about inhaled therapy use and were given names of different classes of inhalers and commonly used examples.
Spirometry
Spirometry was measured in all participants using a Vitalograph Micro handheld spirometer during the LHC in accordance with the joint European Respiratory Society and American Thoracic Society (28) and British Thoracic Society recommendations (29). Additional bronchodilator was not given. As timing of usage was not confirmed, we perceived the spirometry measured at the LHC to be “prebronchodilator,” as this would be the case for the majority of participants. Participant ethnicity, age, and height were used to calculate predicted values, enabling absolute and percent predicted values to be recorded. Airflow limitation was classified in accordance with GOLD (20), meaning participants were defined as having COPD if FEV1:forced vital capacity (FVC) was <70%. Participants with COPD with FEV1 ≥80%, <80% but ≥50%, <50% but ≥30%, or <30% were classified as GOLD class I, II, III, or IV, respectively.
Emphysema
The scans were acquired using a standard low-dose protocol, and were single read by consultant thoracic radiologists with CT reporting expertise, and experience ranging from 5 to 28 years. Visual, semiquantitative assessment of emphysema grade, based on published precedent in COPD (30), was subjectively determined on a visual scale of none, mild, moderate or severe. A total of 5% of scans was second read by a second external radiologist for quality assurance purposes.
Sample Size and Statistical Analysis
The sample size of the LSUT cohort was based on the primary behavioral research question, and has been described in the published protocol (22). The present analysis was prespecified in the main study protocol. Participants who had never smoked or who had missing spirometry data were excluded. For the present study, 986 participants were divided into three groups: “no COPD” (FEV1:FVC ≥70%), “undiagnosed COPD” (FEV1:FVC<70% and no reported history of COPD), and “known COPD” (FEV1:FVC <70% with reported history of COPD).
Descriptive statistics were used to describe the demographic and clinical characteristics of participants and the prevalence of airflow limitation, emphysema, and symptoms in each group. Chi-square tests were used to determine group differences in symptom prevalence, comorbidities and inhaler use, and differences in symptom prevalence between those with and without emphysema. Univariable and multivariable logistic regression was used to explore associations between symptom prevalence, airflow limitation, and emphysema. Multivariable models were adjusted for age, smoking pack-years, reported history of osteoporosis and CHD, FEV1 % predicted, and grade of emphysema. Variables included in the final model were chosen if acknowledged to be a priori confounders for respiratory symptom prevalence. Sex, IMD quintile, and body mass index were not included in the final model, as they were believed not to confound the dependent outcome. The final model included only those who had completed an LDCT (n = 761). Due to the variability of current symptom prevalence within the composite outcome variable, a sensitivity analysis was performed testing the final model against an alternative outcome variable (current cough or breathlessness). Radiologist interobserver agreement was assessed using the weighted κ test (κw) with quadratic weights in the 5% of LDCT scans that were double read as part of the quality-assurance process. Missing values were excluded from the analyses (and were present for only one variable: IMD score).
Results
Of the 1,005 participants recruited to LSUT between November 2015 and July 2017, 19 were excluded because they had never smoked or due to missing data, and 986 participants were included in the present analysis. The majority of participants were white, had left school at or before the age of 15 years, were from the two most deprived IMD quintiles, and were current smokers with a heavy smoking history (Table 1).
Table 1.
Participant characteristics by chronic obstructive pulmonary disease group
| Variables | COPD Groups [n (%)] or [median (IQR)] |
||
|---|---|---|---|
| No COPD (n = 426) | Undiagnosed COPD (n = 377) | Known COPD (n = 183) | |
| Age, yr | 65 (62,68) | 66 (63,69) | 66 (63–70) |
| Female | 213 (50.0) | 142 (37.7) | 93 (50.8) |
| Ethnicity | |||
| White | 324 (76.1) | 320 (84.9) | 171 (93.4) |
| Black/African/Caribbean | 62 (14.5) | 31 (8.2) | 7 (3.8) |
| Other | 40 (9.2) | 24 (6.3) | 5 (2.7) |
| Highest level of education | |||
| Left school at or before age 15 yr | 207 (48.6) | 187 (49.6) | 121 (66.1) |
| Completed high school level or equivalent | 45 (10.6) | 41 (10.9) | 18 (9.8) |
| A-levels or equivalent | 44 (10.3) | 41 (10.9) | 11 (6.0) |
| Further education | 23 (5.4) | 15 (4.0) | 9 (4.9) |
| Bachelor’s degree | 51 (12.0) | 53 (14.1) | 15 (8.2) |
| Further higher degree | 46 (10.8) | 36 (9.6) | 4 (2.2) |
| Other/prefers to say | 10 (2.4) | 4 (1.1) | 5 (2.7) |
| Index of multiple deprivation quintile | |||
| 1 (most deprived) | 239 (56.1) | 199 (52.8) | 99 (54.1) |
| 2 | 136 (31.9) | 141 (37.4) | 60 (32.8) |
| 3 | 12 (2.8) | 6 (1.6) | 2 (1.1) |
| 4 | 1 (0.23) | 1 (0.3) | 0 (0) |
| 5 (least deprived) | 0 (0) | 0 (0) | 0 (0) |
| Missing | 38 (8.9) | 30 (12.0) | 22 (12.02) |
| Smoking history | |||
| Current | 288 (67.6) | 252 (66.8) | 121 (66.1) |
| Former smoker | 138 (32.3) | 125 (33.2) | 62 (33.9) |
| Years smoked, yr | 46 (41–50) | 47 (42–52) | 48 (45–54) |
| Average smoking intensity, cigarettes/d | 15 (10–20) | 15 (10–20) | 20 (15–25) |
| Lung function | |||
| FEV1, L | 2.29 (1.86–2.72) | 2.03 (1.64–2.54) | 1.57 (1.18–1.95) |
| FEV1 % predicted | 91 (79–103) | 79.0 (65–91) | 65 (50–80) |
| FVC, L | 2.97 (2.39–3.36) | 3.33 (2.68–4.01) | 2.75 (2.18–3.48) |
| FVC % predicted | 94 (81–107) | 100 (85–115) | 91 (73–108) |
| FEV1:FVC, % | 76.5 (73–80) | 64 (59–68) | 58 (50–64) |
| BMI, kg/m2 | 26.8 (23.6–30.1) | 25.2 (22.5–28.6) | 25.6 (22–29.1) |
| mMRC dyspnea score | |||
| 0-Breathless on strenuous exercise only | 310 (72.7) | 254 (67.4) | 68 (37.2) |
| 1-Slightly breathless, e.g., up hills | 103 (24.2) | 110 (29.2) | 88 (48.1) |
| 2-Slower than contemporaries | 11 (2.6) | 9 (2.4) | 21 (11.5) |
| 3-100 m exercise tolerance | 1 (0.2) | 4 (1.1) | 6 (3.3) |
| 4-Housebound | 1 (0.2) | 0 (0) | 0 (0) |
| LDCT performed | 317 (74.4) | 297 (78.8) | 147 (80.3) |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IQR = interquartile range; LDCT = low-dose computed tomography; LHC = lung health check; mMRC = modified Medical Research Council Dyspnea scale.
COPD groups based on prebronchodilator spirometry at LHC appointment pre-LDCT: “no COPD” (FEV1:FVC at LHC ≥70%); “undiagnosed COPD” (FEV1:FVC at LHC <70% and no reported history of COPD); and “known COPD” (FEV1:FVC at LHC <70% and a reported history of COPD). Percent totals may not sum up due to rounding.
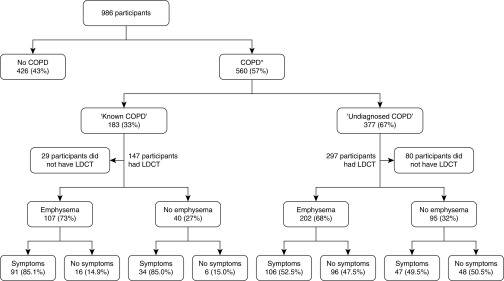
A total of 560 (57%) participants had prebronchodilator spirometry consistent with COPD. Of those, 183 (33%) reported a prior history of COPD, whereas 377 (67%) did not, and were thus labeled “undiagnosed COPD.” The proportion of participants noted to have emphysema on their LDCT was similar in those with known (n = 107, 73%) compared with “undiagnosed” COPD (n = 202, 68%); 135 (45%) of those without emphysema had prebronchodilator spirometry consistent with COPD. Symptoms were prevalent in 91 (85%) of those with known COPD compared with approximately half of those with “undiagnosed COPD,” irrespective of the presence of emphysema (Figure 1). A total of 190 of 377 participants (50.4%) with previously unknown airflow obstruction had persistent cough or dyspnea within the last 12 months (and this amounted to 19% of the entire cohort).
Figure 1.
Prevalence of respiratory symptoms (inclusive of those with a history of persistent cough or dyspnea during the 12 months preceding the lung health check [LHC]) in participants with a FEV1:FVC <70% on the pre–low-dose computed tomography (LDCT), prebronchodilator spirometry, and with or without emphysema detected at LDCT. *Termed “chronic obstructive pulmonary disease” (COPD) solely on the basis of LHC spirometry. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
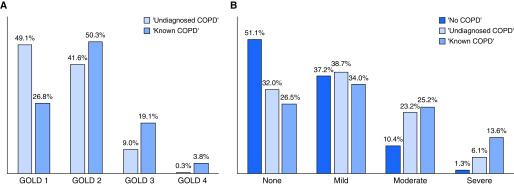
Totals of 342 (91%) and 141 (77%) participants had prebronchodilator spirometry consistent with GOLD class I or II in the “undiagnosed COPD” and known COPD groups, respectively (Figure 2A). Emphysema was prevalent in between one-half and three-quarters of participants, varying by COPD group (Figure 2B).
Figure 2.
Prevalence and grade of (A) airflow obstruction and (B) emphysema by chronic obstructive pulmonary disease (COPD) group. COPD groups: “no COPD” (FEV1:FVC ≥70%); “undiagnosed COPD” (FEV1:FVC <70% and no reported history of COPD); and “known COPD” (FEV1:FVC <70% and a reported history of COPD). FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; GOLD = Global Initiative for Obstructive Lung Diseases.
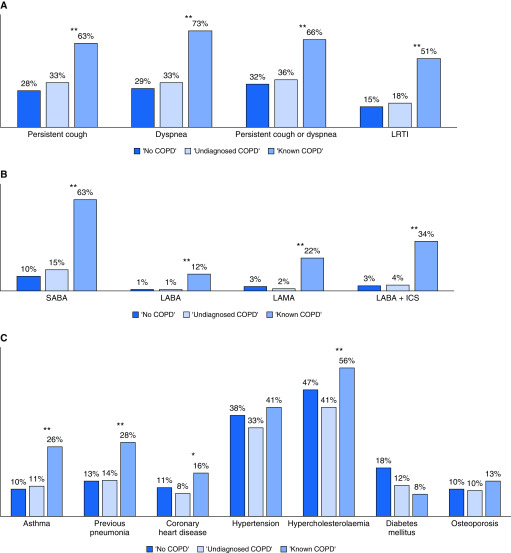
The prevalence of respiratory symptoms varied from 15% to 73% by symptom and group, whereas inhaler use varied from 10% to 63% by group for short-acting β-agonists. The prevalence of both symptom and inhaler use was significantly higher in those with known COPD compared with those with undiagnosed COPD (P < 0.001 for all comparisons; Figure 3A and 3B). Most comorbidities were similarly distributed across groups, whereas others were significantly more common in those with known COPD compared with undiagnosed COPD, including asthma (48 [26%] vs. 43 [11%], P < 0.001), prior pneumonia (56 [31%] vs. 51 [14%], P < 0.001), hypercholesterolemia (102 [56%] vs. 154 [41%], P < 0.001), and CHD (30 [16%] vs. 32 [8%], P = 0.005) (Figure 3E).
Figure 3.
Prevalence of (A) reported respiratory symptoms within 12 months preceding the lung health check, (B) reported inhaler use, and (C) reported comorbidities, by chronic obstructive pulmonary disease (COPD) group. *P ≤ 0.05 and **P ≤ 0.001. COPD groups: “no COPD” (FEV1:FVC ≥70%); “undiagnosed COPD” (FEV1:FVC <70% and no reported history of COPD); and “known COPD” (FEV1:FVC <70% and a reported history of COPD). FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting antimuscarinic agent; LRTI = lower respiratory tract infection; SABA = short-acting β-agonist.
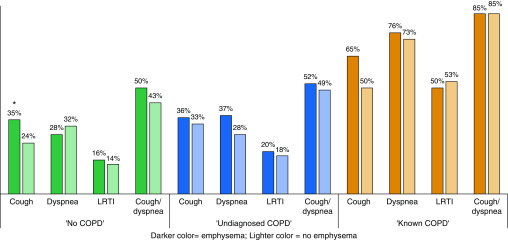
Respiratory symptoms did not vary by presence or absence of emphysema on LDCT. The only statistically significant difference was for persistent cough, which was higher in those with no airflow limitation, but with emphysema, on LDCT (n = 55, 35%) compared with those with no airflow limitation and no emphysema on LDCT (n = 24, 22%) (P = 0.03; Figure 4).
Figure 4.
Prevalence of reported respiratory symptoms within 12 months preceding the lung health check in those with and without emphysema by chronic obstructive pulmonary disease (COPD) group. COPD groups: “no COPD” (FEV1:FVC ≥70%); “undiagnosed COPD” (FEV1:FVC <70% and no reported history of COPD); and “known COPD” (FEV1:FVC <70% and a reported history of COPD). *P ≤ 0.05. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; LRTI = lower respiratory tract infection.
Variables not found to be significantly associated with symptom prevalence in univariable logistic regression analyses included age, sex, IMD quintile, body mass index, and smoking status. Unadjusted and adjusted (for age, pack-year smoking history, history of osteoporosis and CHD, emphysema, and airflow obstruction) odds ratios (ORs) for symptom prevalence are presented in Table 2. The association between respiratory symptoms and airflow limitation remained significant for participants with FEV1:FVC <70% and FEV1 ≥50% (adjusted OR [aOR], 1.74; confidence interval [CI], 1.57–2.17), and FEV1 <50% (aOR, 4.6; CI, 2.17–9.77). Conversely, the association between respiratory symptoms and grade of emphysema only retained significance for those with severe emphysema (aOR, 4.00; CI, 1.57–10.2).
Table 2.
Adjusted and unadjusted odds ratios for presence of persistent cough or dyspnea currently or within the past 12 months with airflow obstruction and emphysema in this cohort
| Unadjusted OR (CI) | P Value | Adjusted OR (CI) | P Value | |
|---|---|---|---|---|
| Age, yr | ||||
| 60–63 | 1 | 0.32 | 1 | 0.004 |
| 64–67 | 0.75 (0.55–1.02) | 0.54 (0.37–0.79) | ||
| 68–72 | 0.84 (0.59–1.19) | 0.55 (0.35–0.84) | ||
| 73–76 | 0.84 (0.55–1.29) | 0.53 (0.31–0.88) | ||
| Pack-year smoking history | ||||
| 0–20 | 1 | <0.001 | 1 | <0.001 |
| 21–40 | 1.54 (1.09–2.15) | 1.13 (0.73–1.76) | ||
| 41–60 | 2.02 (1.42–2.88) | 1.58 (1.00–2.49) | ||
| >60 | 4.64 (2.94–7.31) | 3.28 (1.87–5.77) | ||
| History of osteoporosis | ||||
| No | 1 | 0.045 | 1 | 0.021 |
| Yes | 1.53 (1.00–2.33) | 1.82 (1.08–3.07) | ||
| History of CHD | ||||
| Yes | 1 | <0.001 | 1 | 0.019 |
| No | 2.17 (1.41–3.35) | 1.85 (1.09–3.13) | ||
| Airflow obstruction | ||||
| FEV1:FVC ≥70% | 1 | <0.001 | 1 | <0.001 |
| FEV1:FVC <70% & FEV1 >50% | 1.74 (1.34–2.27) | 1.57 (1.14–2.17) | ||
| FEV1:FVC <70% & FEV1 <50% | 5.59 (3.03–10.3) | 4.60 (2.17–9.77) | ||
| Emphysema | ||||
| None | 1 | <0.001 | 1 | 0.011 |
| Mild | 1.12 (0.81–1.55) | 1.05 (0.74–1.48) | ||
| Moderate | 1.64 (1.09–2.48) | 1.33 (0.85–2.09) | ||
| Severe | 5.88 (2.41–14.4) | 4.00 (1.57–10.2) |
Definition of abbreviations: CHD = coronary heart disease; CI = confidence interval; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; OR = odds ratio.
Values given to two significant figures.
In the sensitivity analysis, the findings were not significantly altered using current cough or breathlessness as the outcome variable (data not shown). Radiologist interobserver agreement in the 5% of LDCT scans that were double read as part of the quality assurance process was found to be “good” according to Landis and Koch (31) (κw = 0.65, P < 0.001).
Discussion
In this cross-sectional cohort study of 986 individuals attending an LHC, 57% had prebronchodilator spirometry consistent with COPD, 67% did not report a prior history of COPD (thus labeled “undiagnosed COPD”), and 32% of those with “undiagnosed COPD” had no evidence of emphysema on LDCT. Prevalence of symptoms, inhaler use, and certain comorbidities were significantly higher in those with known compared with undiagnosed COPD. The presence of airflow obstruction was more strongly associated with respiratory symptoms than emphysema. These results suggest that emphysema alone does not robustly identify participants with COPD.
The proportion of participants with spirometry consistent with COPD is higher in the present study (57%) than reported in NLST participants (10) (34%). Emphysema prevalence was higher than reported in other cohorts (32). These findings may be explained by the population recruited to this “real-world” study, where participants were invited for an National Health Service LHC rather than a clinical trial. The present study participants were older, more deprived, less educated, and more likely to be currently smoking when compared with NLST participants (6).
Whether case finding of individuals with airflow limitation or emphysema in the absence of respiratory symptoms is of value is controversial. Studies have reported increased exacerbation frequency in asymptomatic individuals with airflow limitation (13) and with radiological findings consistent with COPD (33). Few studies have evaluated the use of pharmacotherapy in asymptomatic COPD. One study in China demonstrated an improvement in annual post-bronchodilator FEV1 decline with tiotropium compared with placebo in patients with mild COPD (34), signaling possible benefit with pharmacotherapy for mild COPD, though more studies are needed. Presently, for those with minimal symptoms, simply lifestyle modifications, including smoking cessation, are recommended (18, 35).
We sought to explore what proportion of participants had “clinically significant” COPD (i.e., undiagnosed airflow obstruction plus respiratory symptoms). To our knowledge, no prior studies have reported COPD symptom prevalence in LCS participants, though findings consistent with ours have been reported in smokers without demonstrable airflow limitation (15). This group was previously termed “GOLD 0,” and although studies in this group are ongoing (36); this term is not used clinically, as it does not help predict who may later develop airflow obstruction (37). Our estimate that up to 19% of participants had potentially clinically significant undiagnosed COPD is an important consideration when devising LCS protocols and infrastructure.
The finding that those with and without emphysema were similar in terms of respiratory symptoms was surprising, as an association between all-cause mortality in smokers without airflow limitation and with emphysematous changes has been reported (38). This association, which was confirmed in the multivariable analysis, suggests that airflow limitation may be more pertinent for detecting “clinically significant” disease than LDCT-detected emphysema. Thus, measurement of spirometry in the LCS setting could enhance prognostication and education, all of which are not adequately achieved using the presence of emphysema on CT alone.
The integration of spirometry with symptom assessment in the LCS setting could enhance early detection of COPD and provide an opportunity to implement evidence-based smoking cessation interventions. Visual feedback of medical imaging may promote behavior change, such as smoking cessation (39), and research is underway to determine whether personalized feedback of LDCT-detected emphysema are effective at enhancing smoking cessation (40). It could also enable promotion of vaccination against respiratory infection, attention to diet, activity, symptom awareness, and initiation of appropriate pharmacotherapy. This needs to be balanced against risks associated with medication overuse, cost, primary care resource utilization, and impact on insurance eligibility. An added consideration is that a diagnosis of COPD may influence the overall benefit that an individual may stand to gain from LCS. It has been noted that having undiagnosed or early COPD confers a greater relative risk reduction in lung cancer–specific mortality than observed in NLST, whereas having more advanced COPD may be associated with no mortality benefit (9). As such, the shared decision-making discussion would benefit from spirometry readings to determine an individual’s specific potential benefits and harms of screening. Such issues have been highlighted in a recent official American Thoracic Society statement (41) as important factors to be raised in the LCS shared decision consultation.
The study was limited by possible selection bias, as it is possible that individuals with symptoms were more likely to attend an LHC appointment. The sample was predominantly of a white ethnic background, with a high current smoking rate and from a more socioeconomically deprived background than previous studies, and therefore likely to have higher rates of COPD and lung cancer than the wider LCS-eligible population.
Nevertheless, similar lung cancer prevalence and demographics have been described in other U.K. cohorts (42, 43), and, in light of emerging evidence advocating selection of LCS-eligible individuals based on lung cancer risk (23, 44), the findings reported here are generalizable to the desired population. We did not collect data on exacerbation history, which would enhance our classification of COPD severity. Many of the outcome measures were dependent on self-reported history and subject to recall bias. We acknowledge that some participants may have underreported a diagnosis of COPD or misreported COPD as asthma. We did not complete post-bronchodilator spirometry to classify COPD; several other studies, including NLST (10), have employed spirometry without additional bronchodilation as a metric, and GOLD has accepted this as a pragmatic approach to COPD case finding, directing people for additional testing to confirm the diagnosis (20). The present analysis could not account for unmeasured factors affecting symptom perception and prevalence, such as psychosocial influences and premorbid fitness. Emphysema was graded visually rather than quantitatively, and is subject to some interobserver bias, though “good” agreement between observers was detected on the 5% of scans read by two radiologists for quality assurance purposes. Despite these limitations, the findings presented here are novel with respect to the evaluation of associations between symptomatology, radiology, and physiological parameters in COPD, and highlight the potential benefits arising from incorporating spirometry into the assessment for LCS and the LHC approach adopted in LSUT.
Conclusions
This study has demonstrated the significant burden of COPD, respiratory symptoms, and comorbidities in a cohort of individuals attending an LHC. Half of those with undiagnosed COPD had respiratory symptoms, whereas one-third did not have emphysema. Airflow limitation was a stronger predictor for respiratory symptoms than emphysema. This emphasizes the importance and value of spirometry at LCS over and above detecting emphysema on LDCT. Further studies should evaluate the impact of early detection of airflow limitation and emphysema through LCS on behavior change and long-term COPD outcomes. To this end, we are expanding our studies to a larger cohort within the SUMMIT study (45).
Supplementary Material
Footnotes
Supported, as part of the Lung Screen Uptake Trial project, by a National Awareness and Early Diagnosis Initiative project grant awarded by Cancer Research UK (CRUK) and a consortium of funders (Department of Health [England]; Economic and Social Research Council; Health and Social Care R&D Division, Public Health Agency, Northern Ireland; National Institute for Social Care and Health Research, Wales; and the Scottish Government) (S.L.Q. and S.M.J); also supported by Wellcome Trust Senior Fellowship in Clinical Science WT107963AIA (S.M.J.) and by the Rosetrees Trust, the Roy Castle Lung Cancer Foundation, the Stoneygate Trust, the Welton Trust, the Garfield Weston Trust, and the University College London Hospital Charitable Foundation (S.M.J.). This work was undertaken at University College London Hospital/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centre’s funding scheme (N.N. and S.M.J.); also supported by CRUK Postdoctoral Fellowship C50664/A24460 (S.L.Q.).
Ethics Approvals and Registration: This study is part of the Lung Screen Uptake Trial, which was granted ethical approval by the City Road and Hampstead National Health Service Research Ethics Committee (reference no. 15/LO/1186) and was registered with clinicaltrials.gov (NCT02558101) and International Standard Registered Clinical/soCial sTudy no. ISRCTN21774741.
Author Contributions: M.R., S.L.Q., J.L.D., A.D., K.S., S.W.D., N.N., A.B., D.R.B., and S.M.J. were involved in the design and conduct of the study. M.R., S.L.Q., J.L.D., C.H., S.T., H.H., M.N.T., A.A., P.J.S., S.B., M.-J.S., and A.N. were involved in the data collection. M.R., S.L.Q., and S.W.D. carried out the data analyses. All authors were involved in the interpretation of the results and contributed to and approved the final manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Global Health Observatory (GHO) data—mortality and global health estimates. Geneva: WHO. 2018 [accessed 2018 Jun 25]. Available from: http://www.who.int/gho/mortality_burden_disease/en/
- 2.Carr LL, Jacobson S, Lynch DA, Foreman MG, Flenaugh EL, Hersh CP, et al. Features of COPD as predictors of lung cancer. Chest. 2018;153:1326–1335. doi: 10.1016/j.chest.2018.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 4.Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8:6–11. doi: 10.1097/JTO.0b013e318274a7dc. [DOI] [PubMed] [Google Scholar]
- 5.Seijo LM, Zulueta JJ. Understanding the links between lung cancer, COPD, and emphysema: a key to more effective treatment and screening. Oncology (Williston Park) 2017;31:93–102. [PubMed] [Google Scholar]
- 6.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 8.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30:1162–1169. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res. 2018;7:347–360. doi: 10.21037/tlcr.2018.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, et al. Airflow limitation and histology shift in the National Lung Screening Trial: the NLST-ACRIN cohort substudy. Am J Respir Crit Care Med. 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de-Torres JP, Casanova C, Marín JM, Zagaceta J, Alcaide AB, Seijo LM, et al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med. 2013;107:702–707. doi: 10.1016/j.rmed.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Mets OM, Buckens CFM, Zanen P, Isgum I, van Ginneken B, Prokop M, et al. Identification of chronic obstructive pulmonary disease in lung cancer screening computed tomographic scans. JAMA. 2011;306:1775–1781. doi: 10.1001/jama.2011.1531. [DOI] [PubMed] [Google Scholar]
- 13.Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 14.Hill K, Goldstein RS, Guyatt GH, Blouin M, Tan WC, Davis LL, et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182:673–678. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridevaux PO, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz J-M, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax. 2008;63:768–774. doi: 10.1136/thx.2007.093724. [DOI] [PubMed] [Google Scholar]
- 17.Siu AL, Bibbins-Domingo K, Grossman DC, Davidson KW, Epling JW, Jr, García FAR, et al. US Preventive Services Task Force (USPSTF) Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372–1377. doi: 10.1001/jama.2016.2638. [DOI] [PubMed] [Google Scholar]
- 18.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. 2017 [accessed 2018 Jun 25]. Available from: https://www.goldcopd.org.
- 19.Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186:975–981. doi: 10.1164/rccm.201207-1299OC. [DOI] [PubMed] [Google Scholar]
- 20.Global Initiative for Chronic Obstructive Lung Disease. GOLD reports for personal use. 2019 [accessed 2018 Jun 20]. Available from: https://goldcopd.org/gold-reports/
- 21.Barr RG, Celli BR, Mannino DM, Petty T, Rennard SI, Sciurba FC, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122:348–355. doi: 10.1016/j.amjmed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quaife SL, Ruparel M, Beeken RJ, McEwen A, Isitt J, Nolan G, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and ‘hard-to-reach’ patients. BMC Cancer. 2016;16:281. doi: 10.1186/s12885-016-2316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tammemägi MC. Application of risk prediction models to lung cancer screening: a review. J Thorac Imaging. 2015;30:88–100. doi: 10.1097/RTI.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Preventive Services Task Force. Final recommendation statement. AHRQ Pub. no. 13-05196-EF-3. September 24 2014 [accessed 2014 Nov 28]. Available from: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening.
- 26.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 29.Bellamy D, Booker R, Connellan S, Halpin D, Jennings J, Wiltshire L. Spirometry in practice: a practical guide of using spirometry in primary care, 2nd ed. London: British Thoracic Society (BTS) COPD Consortium; 2005. p. 3.
- 30.Gietema HA, Müller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol. 2011;18:661–671. doi: 10.1016/j.acra.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 32.Steiger David, Filopei Jason, Siddiqi Mohammad, Yip Rowena, Yankelevitz David, Henschke Claudia. Evidence of emphysema in a cohort of participants without symptoms undergoing low dose chest CT screening for lung cancer [abstract] Am J Respir Crit Care Med. 2017;195:A5177. [Google Scholar]
- 33.Jairam PM, van der Graaf Y, Lammers J-WJ, Mali WPTM, de Jong PA PROVIDI Study group. Incidental findings on chest CT imaging are associated with increased COPD exacerbations and mortality. Thorax. 2015;70:725–731. doi: 10.1136/thoraxjnl-2014-206160. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med. 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 35.Warnier MJ, van Riet EES, Rutten FH, De Bruin ML, Sachs APE. Smoking cessation strategies in patients with COPD. Eur Respir J. 2013;41:727–734. doi: 10.1183/09031936.00014012. [DOI] [PubMed] [Google Scholar]
- 36.Vrbica Ž, Labor M, Gudelj I, Labor S, Jurić I, Plavec D MARKO study group. Early detection of COPD patients in GOLD 0 population: an observational non-interventional cohort study—MARKO study. BMC Pulm Med. 2017;17:36. doi: 10.1186/s12890-017-0378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vestbo J, Lange P. Can GOLD stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166:329–332. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]
- 38.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollands GJ, Hankins M, Marteau TM. Visual feedback of individuals’ medical imaging results for changing health behaviour. Cochrane Database Syst Rev. 2010;(1):CD007434. doi: 10.1002/14651858.CD007434.pub2. [DOI] [PubMed] [Google Scholar]
- 40. ISRCTN. ISRCTN63825779: Yorkshire Enhanced Stop Smoking (YESS). September 25 2018 [updated 2020 Jan 15; accessed 2019 Jul 11]. Available from http://www.isrctn.com/ISRCTN63825779.
- 41.Rivera MP, Tanner NT, Silvestri GA, Detterbeck FC, Tammemägi MC, Young RP, et al. American Thoracic Society Assembly on Thoracic Oncology. Incorporating coexisting chronic illness into decisions about patient selection for lung cancer screening: an official American Thoracic Society research statement Am J Respir Crit Care Med 2018198e3–e13. [DOI] [PubMed] [Google Scholar]
- 42.Crosbie PA, Balata H, Evison M, Atack M, Bayliss-Brideaux V, Colligan D, et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax. 2018;74:405–409. doi: 10.1136/thoraxjnl-2017-211377. [DOI] [PubMed] [Google Scholar]
- 43.Arvanitis R, Jones L, Gaynor E, Gardner K, Hubbert C, Timoney M, et al. Proactive approaches to individuals at high risk of lung cancer: Accelerate, Coordinate, Evaluate (ACE) Programme. February 2018 [accessed 2018 May 23]. Available from: https://www.cancerresearchuk.org/sites/default/files/ace_proactive_lung_report_with_ economic_evaluation_final_version_1.1a.pdf.
- 44.ten Haaf K, Tammemägi MC, Bondy SJ, van der Aalst CM, Gu S, McGregor SE, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLoS Med. 2017;14:e1002225. doi: 10.1371/journal.pmed.1002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. ClinicalTrials.gov. The SUMMIT study: a cancer screening study (SUMMIT). May 2, 2019 [updated 2019 May 2; accessed 2020 Jan 24]. Available from: https://www.clinicaltrials.gov/ct2/show/nct03934866.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.