Abstract
Rationale: Bronchoscopic lung volume reduction with Zephyr Valves improves lung function, exercise tolerance, and quality of life of patients with hyperinflated emphysema and little to no collateral ventilation.
Objectives: Post hoc analysis of patient-reported outcomes (PROs), including multidimensional measures of dyspnea, activity, and quality of life, in the LIBERATE (Lung Function Improvement after Bronchoscopic Lung Volume Reduction with Pulmonx Endobronchial Valves used in Treatment of Emphysema) study are reported.
Methods: A total of 190 patients with severe heterogeneous emphysema and little to no collateral ventilation in the target lobe were randomized 2:1 to the Zephyr Valve or standard of care. Changes in PROs at 12 months in the two groups were compared: dyspnea with the Transitional Dyspnea Index (TDI), focal score; the Chronic Obstructive Pulmonary Disease Assessment Test (CAT; breathlessness on hill/stairs); Borg; the EXAcerbations of Chronic pulmonary disease Tool–PRO, dyspnea domain; activity with the TDI, magnitude of task/effort/functional impairment, CAT (limited activities), and the St. George’s Respiratory Questionnaire (SGRQ), activity domain; and psychosocial status with the SGRQ, impacts domain, and CAT (confidence and energy).
Results: At 12 months, patients using the Zephyr Valve achieved statistically significant and clinically meaningful improvements in the SGRQ; CAT; and the TDI, focal score, compared with standard of care. Improvements in the SGRQ were driven by the impacts and activity domains (P < 0.05 and P < 0.001, respectively). Reduction in CAT was through improvements in breathlessness (P < 0.05), energy level (P < 0.05), activities (P < 0.001), and increased confidence when leaving home (P < 0.05). The TDI measures of effort, task, and functional impairment were uniformly improved (P < 0.001). The EXAcerbations of Chronic Pulmonary Disease Tool (EXACT)–PRO, dyspnea domain, was significantly improved in the Zephyr Valve group. Improvements correlated with changes in residual volume and residual volume/TLC ratio.
Conclusions: Patients with severe hyperinflated emphysema achieving lung volume reductions with Zephyr Valves experience improvements in multidimensional scores for breathlessness, activity, and psychosocial parameters out to at least 12 months.
Clinical trial registered with www.clinicaltrials.gov (NCT01796392).
Keywords: chronic obstructive pulmonary disease, severe emphysema, interventional bronchoscopy, patient-reported outcomes, Zephyr Valve
Breathlessness is a common and disabling symptom in patients with chronic obstructive pulmonary disease (COPD) (1) and is defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” as interpreted by the individual (2). Progression of COPD is associated with worsening breathlessness accompanied by a downward spiral of symptom-induced inactivity (3), muscle deconditioning and weakness (4), and significant negative impact on health-related quality of life and survival (5–8).
The emphysema-hyperinflated phenotype is characterized by a heightened sense of dyspnea and exercise intolerance caused by mechanical constraints imposed on ventilation at rest and during activity (9). The physiological model of patient-reported shortness of breath in COPD presented by Jolley and Moxham (10) describes the effects of daily activities on increasing respiratory muscle load-capacity imbalance, neural respiratory drive, and neuromechanical dissociation, which are magnified in severe emphysema and hyperinflation. Hyperinflation is a key contributor to an individual’s perception of breathlessness and to exercise limitation (11, 12), and is a predictor not only of risk of exacerbation (13) but of all-cause mortality (14, 15), prompting targeted strategies to reduce hyperinflation.
In selected individuals, lung volume reduction surgery has been shown to improve lung function, exercise capacity, dyspnea, and quality of life, and to extend survival (16, 17). However, only a small number of patients with COPD are eligible for lung volume reduction surgery, periprocedural morbidity and mortality is relatively high, and only a few hundred procedures are performed annually in the United States. Recent efforts have been focused on the development of less invasive approaches. Bronchoscopic lung volume reduction using the Zephyr Endobronchial Valve (Zephyr Valve; Pulmonx Corporation) has been shown in multiple randomized controlled trials (RCTs) to reduce hyperinflation and to improve lung function, exercise tolerance, dyspnea, and overall quality of life in individuals with heterogeneous (18–21) or homogeneous (22) emphysema and little to no interlobar collateral ventilation.
COPD is a multifaceted condition and assessment of airflow limitation alone does not adequately reflect the burden of the disease. FEV1 has been shown to correlate poorly with patient-reported outcomes (PROs), including dyspnea, activity, and health status (23, 24). Furthermore, there is a significant disparity between subjects' perception of disease severity and the degree of severity indicated by a unidimensional breathlessness scale (25), which may lead to undertreatment. Therefore, to quantify symptoms, the most efficient and objective way is to assess symptom severity, activity limitation, and psychosocial status using multidimensional patient-centric measures (26). In doing so, a better estimate of treatment effects may be gained.
The LIBERATE (Lung Function Improvement after Bronchoscopic Lung Volume Reduction with Pulmonx Endobronchial Valves used in Treatment of Emphysema) study is the largest multicenter prospective RCT of the Zephyr Valve in patients with severe heterogeneous emphysema, hyperinflation, and little to no interlobar collateral ventilation assessed using the Chartis System (Pulmonx Corp.). Multiple patient-centric assessments were included in the study to allow an in-depth evaluation of the impact of reduced hyperinflation on dyspnea and symptoms that are most meaningful to patients with COPD. Published PROs have so far focused on the modified Medical Research Council (mMRC) Dyspnea Scale and the St. George’s Respiratory Questionnaire (SGRQ) total score. The importance of PROs in a comprehensive evaluation of response to treatment is recognized and recommended in recent guideline updates (27).
We present the PROs of multidimensional measures of 1) dyspnea, from the Transitional Dyspnea Index (TDI), focal score; the COPD Assessment Test (CAT; breathlessness on hill/stairs); Borg; and the EXAcerbations of Chronic Pulmonary Disease Tool [EXACT]–PRO, dyspnea domain; 2) activity, from the TDI, magnitude of task/effort/functional impairment; CAT (limited activities); and the SGRQ, activity domain; and 3) psychosocial status, from the SGRQ, impacts domain, and CAT (confidence and energy) at 12 months after their bronchoscopic lung volume reductions with Zephyr Valves beyond the mMRC Dyspnea Scale and the SGRQ, total score, that were previously reported in the LIBERATE trial (21). Evaluation of these symptom metrics in this study population before and after endobronchial valve implantation has not been published.
Some of the results have previously been reported in abstract form (28).
Methods
The LIBERATE study design (NCT 01796392) has been published previously (21). The multicenter study conducted under a U.S. Food and Drug Administration–approved Investigational Device Exemption for the Zephyr Valve was approved by the respective institutional review boards or ethics committees at each site, and all participating subjects were provided written informed consent. The study protocol is summarized in the online supplement.
Multiple discrete and validated self-administered questionnaires were completed at baseline and follow-up visits, and have not been previously reported (see Table E1 in the online supplement for timing of each assessment). These included: for dyspnea, the TDI, focal score; Borg scale of perceived exertion; EXACT-PRO diary, dyspnea domain; and the CAT question regarding breathlessness on hill/stairs; for activity, the TDI, magnitude of task, magnitude of effort, and functional impairment; the CAT questions regarding limited activities; and the SGRQ, activity domain; and, for psychosocial status, the SGRQ, impacts domain, and the CAT questions regarding confidence and energy. Neither the study participants nor the assessing physicians were blinded to the randomization. At follow-up assessments, study participants were not reminded of, and thus were not aware of, their baseline scores.
Study participants also maintained a daily diary in which they noted adherence with the pulmonary rehabilitation program, completed the EXACT-PRO questionnaire, and noted health status changes. The following question was administered as part of the daily diary: “Mark the scale to show the intensity of the emphysema symptoms you had today (scale of 0 = none to 10 = intolerable).” Although this question was not validated, it asks a global question of clinical interest using a visual analogue scale of 0 to 10 for rating the subject’s perception of emphysema symptom intensity every day. Days that were better or worse were determined by a follow-up score that was above (worse) or below (better) the baseline score.
Statistical Analysis
All data were collected prospectively; however, inferential statistics for the SGRQ domains, the TDI domains, the mMRC Dyspnea Scale, CAT individual questions, Borg, and EXACT-PROs were not prospectively specified in the study protocol and hence are considered post hoc. Statistical analyses were performed using SAS version 9.3 (SAS Institute). Descriptive statistics include means, standard deviations, and 95% confidence intervals. Change from baseline to 12 months and the percentage of subjects meeting a minimal clinically important difference (MCID; or “responder”) per group were reported using an MCID of greater than or equal to 15% increase for FEV1 (29), greater than or equal to 25 m increase for 6-minute-walk distance (6MWD) (30), greater than or equal to a 4-point reduction for the SGRQ (31), greater than or equal to a 1-point increase for the TDI (32), and greater than or equal to a 1-point reduction for mMRC Dyspnea Scale (33) and Borg (34). Changes from baseline were reported for EXACT-PRO, dyspnea domain, and individual CAT (35) questions only.
Missing data for the prospectively defined primary and secondary endpoints were imputed as baseline carried forward for deaths, by linear interpolation for intermittent missing values, by multiple imputation methods for truncated missing values. Cohen’s effect size was calculated as the difference in means divided by pooled standard deviation (referred to as Cohen’s D). Continuous variables were compared with the Wilcoxon rank sum (for nonparametric data) or an analysis of covariance with the respective baseline value as the covariate (for parametric data), and categorical variables were compared with the Fisher exact test.
Scatter plots and Pearson correlation coefficients were performed to assess the relationships between hyperinflation (residual volume [RV] and RV/TLC) and dyspnea (EXACT-PRO), lung function (FEV1), quality of life (the SGRQ, total score), and exercise capacity (6MWD). Statistical significance was determined at the P < 0.05 level. Poisson regression adjusted for each subject’s length of follow-up was used to analyze daily diary data for days that were better or worse from baseline.
Results
Subject Characteristics
A total of 190 patients with severe heterogeneous emphysema and hyperinflation with little or no collateral ventilation in the target lobe were enrolled with a 2 to 1 randomization (128 Zephyr Valve and 62 standard of care [SoC]). Baseline demographic and clinical characteristics data have previously been reported (21) and are provided for reference in Table E2. Except for a higher proportion of Global Initiative for Chronic Obstructive Lung Disease stage IV subjects in the SoC group, there were no differences between groups for the other variables. Twelve subjects did not complete the 12-month assessments: 9 in the Zephyr Valve group (2 withdrew consent, 2 withdrawn by investigators, and 5 died) and 3 in the SoC group (2 withdrawn by investigators and 1 died) (21).
Dyspnea Measures
Multiple measures of dyspnea showed statistically significant improvements in the Zephyr Valve group compared with SoC at 12 months (Table 1). The mean group differences for the change from baseline to 12 months (Δ Zephyr Valve–SoC) were −0.8 points for mMRC Dyspnea Scale (P < 0.001; previously reported [21]); 4.3 points for the TDI, focal score (P < 0.001); −0.9 points for Borg after the 6-minute-walk test (P < 0.001); −8.8 points for the EXACT-PRO, dyspnea domain (P = 0.002); and −0.6 points for the CAT, dyspnea question (P = 0.002).
Table 1.
Dyspnea, activity, and psychosocial status outcomes: absolute change from baseline at 12 months
| Zephyr Valve (n = 128) |
SoC (n = 62) |
∆Zephyr-SoC | Effect Size | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Months | Change from Baseline | Baseline | 12 Months | Change from Baseline | ||||
| Dyspnea outcomes | |||||||||
| mMRC Dyspnea Scale*, points | 2.4 ± 0.97 | 1.9 ± 1.04 | −0.5 ± 1.17 | 2.2 ± 0.83 | 2.5 ± 0.95 | 0.3 ± 1.03 | −0.8† | 0.73 | <0.001‡ |
| TDI*§, focal score, points | — | — | 2.3 ± 5.25 | — | — | −2.0 ± 4.35 | 4.3† | 0.89 | <0.001‖ |
| Borg after 6MWT¶, points) | 4.45 ± 2.17 | 3.93 ± 2.18 | −0.45 ± 2.24 | 4.94 ± 2.28 | 5.36 ± 2.23 | 0.42 ± 2.37 | −0.87† | 0.38 | <0.001‡ |
| EXACT-PRO, dyspnea domain, points | 49.2 ± 13.32 | 43.5 ± 17.59 | −5.5 ± 18.6 | 48.1 ± 10.83 | 51.1 ± 14.66 | 3.3 ± 11.25 | −8.8† | 0.57 | 0.002‡ |
| CAT Q4**, breathlessness, points | 4.1 ± 1.11 | 3.5 ± 1.39 | −0.5 ± 1.56 | 4.1 ± 1.00 | 4.2 ± 1.03 | 0.1 ± 1.20 | −0.6† | 0.43 | 0.002‡ |
| Activity outcomes | |||||||||
| 6MWD*, m | 311.3 ± 81.3 | 323.9 ± 111.9 | 12.98 ± 81.54 | 301.9 ± 78.5 | 276.3 ± 93.5 | −26.33 ± 81.50 | 39.3† | 0.48 | 0.002‡ |
| TDI§, magnitude of task, points | — | — | 0.8 ± 1.8 | — | — | −0.6 ± 1.5 | 1.4† | 0.85 | <0.001‖ |
| TDI§, magnitude of effort, points | — | — | 0.8 ± 1.9 | — | — | −0.7 ± 1.5 | 1.5† | 0.86 | <0.001‖ |
| TDI§, functional impairment, points | — | — | 0.7 ± 1.8 | — | — | −0.7 ± 1.6 | 1.4† | 0.82 | <0.001‖ |
| CAT Q5††, activity, points | 3.3 ± 1.21 | 2.6 ± 1.47 | −0.7 ± 1.7 | 3.5 ± 1.05 | 3.5 ± 1.04 | 0.0 ± 1.3 | −0.7† | 0.46 | <0.001‡ |
| SGRQ, activity domain, points | 78.80 ± 13.73 | 66.26 ± 22.28 | −12.0 ± 21.46 | 78.35 ± 12.79 | 78.87 ± 14.31 | 0.70 ± 14.31 | −12.7† | 0.70 | <0.001‡ |
| Psychosocial status | |||||||||
| SGRQ, total*, points | 55.15 ± 14.09 | 47.35 ± 18.39 | −7.55 ± 15.71 | 53.10 ± 14.14 | 53.12 ± 15.41 | −0.50 ± 15.50 | −7.05† | 0.45 | 0.004‡ |
| SGRQ, impacts domain, points | 43.17 ± 17.79 | 33.17 ± 19.09 | −9.58 ± 19.00 | 39.40 ± 18.09 | 38.92 ± 19.41 | 0.14 ± 17.34 | −9.72† | 0.53 | 0.004‡ |
| CAT Q6‡‡, confidence, points | 2.0 ± 1.65 | 1.4 ± 1.49 | −0.6 ± 1.78 | 1.8 ± 1.64 | 1.8 ± 1.60 | 0.1 ± 1.81 | −0.7† | 0.39 | 0.024‡ |
| CAT Q8§§, energy, points | 3.0 ± 1.30 | 2.4 ± 1.36 | −0.6 ± 1.57 | 2.9 ± 1.32 | 2.9 ± 1.38 | 0.1 ± 1.67 | −0.7† | 0.43 | 0.014‡ |
Definition of abbreviations: 6MWD = 6-minute-walk distance; 6MWT = 6-minute-walk test; CAT Q = Chronic obstructive pulmonary disease Assessment Test question; EXACT-PRO = EXAcerbations of Chronic pulmonary disease Tool–Patient-Reported Outcome; mMRC = modified Medical Research Council; SGRQ = St. George’s Respiratory Questionnaire; SoC = standard of care; TDI = Transitional Dyspnea Index.
Values are mean ± standard deviation.
Minimal clinically important difference: Borg score, −1 point; CAT, −2 points; mMRC, −1 point; SGRQ, −4 points; and the TDI, +1 point.
Previously reported (21).
Favors the Zephyr Valve (intervention group).
P value from an analysis of covariance with factor of treatment groups and baseline value as covariate.
The TDI responses reflect participants’ perception of change from baseline at the time of assessment (12 mo).
P value from the Wilcoxon rank sum test.
Borg scale measured after completing the 6MWT was used for comparison owing to very low baseline pretest scores creating a ceiling effect on the analysis of change.
CAT Q4: “When I walk up a hill or one flight of stairs, I am not breathless.”
CAT Q5: “I am not limited doing any activities at home.”
CAT Q6: “I am confident leaving my home despite my condition.”
CAT Q8: “I have lots of energy.”
The absolute changes from baseline to 12 months for the SGRQ domains, the TDI domains, and the individual questions in CAT are provided in Figures E1–E3.
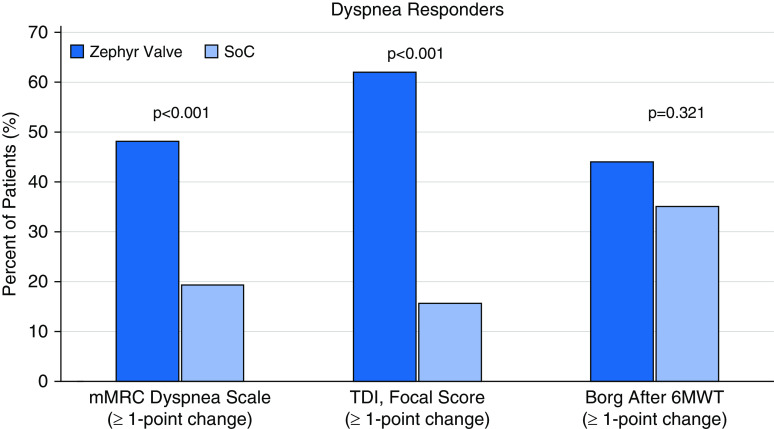
Dyspnea Responders
As shown in Figure 1, significantly more responders at 12 months were observed for the Zephyr Valve compared with SoC group patients for improvements in mMRC Dyspnea Scale of greater than or equal to a 1-point decrease (48.2% vs. 19.0%, respectively; P < 0.001) and for the TDI, focal score, of greater than or equal to a 1-point increase (61.9% vs. 15.8%, respectively; P < 0.001). Responders for Borg dyspnea score after the 6-minute-walk test favored the Zephyr Valve over SoC, but this was not statistically significant (≥1-point decrease; 44.1% vs. 35.1%, respectively; P = 0.321).
Figure 1.
Responders based on minimal clinically important difference of greater than or equal to a 1-point decrease for the mMRC Dyspnea Scale and Borg after a 6MWT, and greater than or equal to a 1-point increase for the TDI, focal score, from baseline to 12 months. Dark blue bars represent the Zephyr Valve group and the light blue bars represent the SoC group. 6MWT = 6-minute-walk test; mMRC = modified Medical Research Council; SoC = standard of care; TDI = Transitional Dyspnea Index.
Activity Measures
Multiple measures of activity levels assessed at 12 months showed statistically significant improvements in the Zephyr Valve group compared with SoC group (see Table 1). An improvement in the 6MWD of 39.3 m in favor of the Zephyr Valve group was reported previously (21).
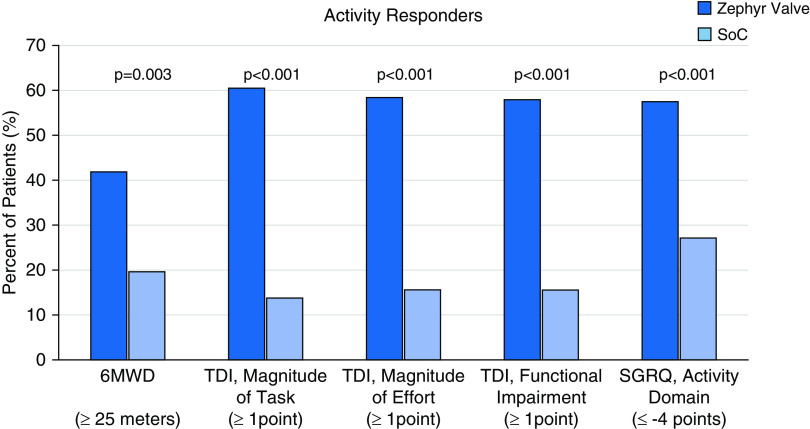
Activity Responders
Significantly more responders at 12 months were observed for the Zephyr Valve group compared with SoC for 6MWD (previously reported [21]; 41.8% vs. 19.6%, respectively; P = 0.003), the TDI, magnitude of task (60.5% vs. 13.8%, respectively; P < 0.001); the TDI, magnitude of effort (58.3% vs. 15.5%, respectively; P < 0.001); the TDI, functional impairment (57.9% vs. 15.5%, respectively; P < 0.001); and the SGRQ, activity domain (57.4% vs. 27.1%, respectively; P < 0.001) (Figure 2).
Figure 2.
Responders based on minimal clinically important difference of greater than or equal to a 25 m increase for 6MWD, greater than or equal to a 1-point increase for the TDI domain scores (magnitude of task, magnitude of effort, and functional impairment), and greater than or equal to a 4-point decrease for the SGRQ, activity domain. Dark blue bars represent the Zephyr Valve group and the light blue bars represent the SoC group. 6MWD = 6-minute-walk distance; SGRQ = St. George’s Respiratory Questionnaire; SoC = standard of care; TDI = Transitional Dyspnea Index.
Psychosocial Status
At 12 months, statistically significant improvements for the Zephyr Valve group over SoC group were demonstrated for absolute changes from baseline to 12 months for the SGRQ, total score (−7.1 points; P = 0.004; previously reported [21]); the SGRQ, impact score (−9.7 points; P = 0.004); and CAT questions regarding confidence (−0.7 points; P = 0.024) and energy (−0.7 points; P = 0.014) (see Table 1).
There were significantly more SGRQ responders at 12 months in the Zephyr Valve group compared with SoC, with 56.2% versus 30.2%, respectively, using an MCID of −4 points (previously reported in Reference 21). For the SGRQ, impacts domain, the responder rates were 63.2% versus 42.4% (Zephyr Valve vs. SoC, respectively; P = 0.01) using an MCID of −4 points.
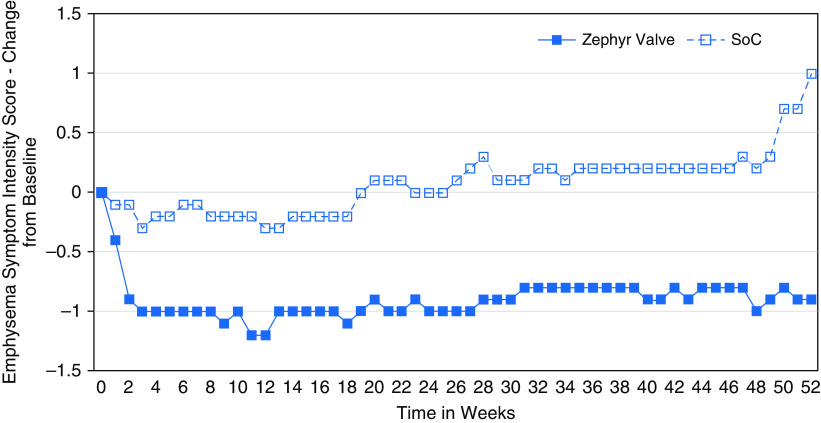
Daily Diary Scores
Compared with SoC, the Zephyr Valve group had significantly more days per year when their emphysema symptom intensity was better than baseline (≥1-point improvement) with 206 versus 102 days (Zephyr Valve vs. SoC, respectively; P < 0.001; Poisson regression adjusted for each subject’s length of follow-up) and significantly fewer days that were worse than baseline (≥1-point worsening) with 95 versus 122 days (Zephyr Valve vs. SoC, respectively; P < 0.001). Patients in the Zephyr Valve group experienced the same degree of emphysema symptom intensity as at baseline on fewer days compared with the SoC patients (64 days vs. 141 days, Zephyr Valve vs. SoC, respectively; P < 0.001). The weekly averages of the daily change from baseline in the emphysema symptom intensity over the 12 months is shown in Figure 3.
Figure 3.
Emphysema symptom intensity presented as weekly averages of the daily change from baseline values up to 12 months for the Zephyr Valve (blue closed squares) versus SoC (blue open squares) groups. SoC = standard of care.
As shown in Table 1, the calculated effect size for all parameters assessed ranged from 0.38 to 0.89 and in all cases were greater than the Cohen’s D of 0.2, which represents a small effect size.
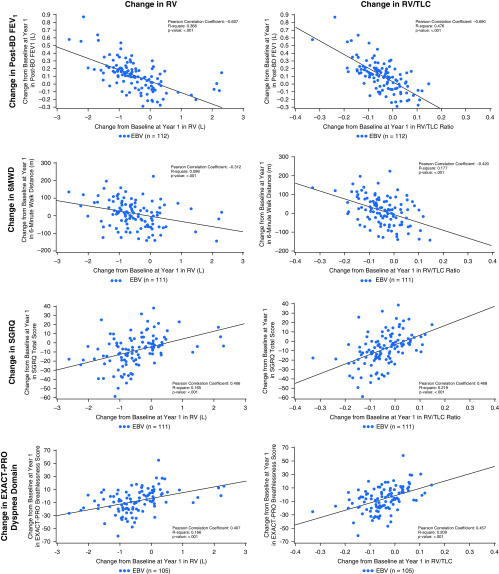
Relationships between Reduction in Hyperinflation and Effectiveness Assessed Using PRO Measures
Clinically meaningful improvement in static hyperinflation at 12 months favoring the Zephyr Valve group has been reported (21). Figure 4 shows that reductions in RV (L) and RV/TLC are significantly correlated with improvements in FEV1; 6MWD; the SGRQ, total score; and dyspnea (EXACT-PRO).
Figure 4.
Correlation between reduction in hyperinflation (RV) and RV/TLC ratio, and changes from baseline to 12 months, for postbronchodilator FEV1; 6MWD; the SGRQ; and EXACT-PRO, dyspnea domain. Data are for the Zephyr Valve group. 6MWD = 6-minute-walk distance; BD = bronchodilator; EBV = Zephyr endobronchial valve; EXACT-PRO = EXAcerbations of Chronic pulmonary disease Tool–Patient-Reported Outcome; FEV1 = forced expiratory volume in 1 second; RV = residual volume; SGRQ = St. George’s Respiratory Questionnaire; TLC = total lung capacity.
Discussion
This post hoc analysis of data from the LIBERATE multicenter RCT demonstrated statistically significant and clinically meaningful improvements in multiple patient-reported measures of dyspnea, activity, and psychosocial status favoring the Zephyr Valve over SoC at 12 months. The findings of this study expand on the results of RCTs evaluating the Zephyr Valve that have previously demonstrated physiological benefits and improvements in several measures of health-related quality of life, principally using the mMRC Dyspnea Scale and the SGRQ (18–22, 36). It is also consistent with a meta-analysis that demonstrated a correlation between lung volume reduction and clinical benefit (37). Of particular significance is the finding that the effect size based on Cohen’s D is moderate to large for each of the parameter assessed.
Dyspnea is usually the most burdensome symptom in patients with COPD and is frequently multifactorial in origin (38). Hyperinflation is a key factor driving exertional dyspnea in an individual with advanced emphysema (11). Multiple PROs demonstrated statistically significant improvements in the Zephyr Valve group compared with SoC at 12 months, notably the TDI, focal score, and the EXACT-PRO, dyspnea domain. The TDI demonstrates a high correlation between dyspnea and stage of disease severity (39), and measures aspects of daily living as they relate to the amount of breathlessness experienced (40). The EXACT-PRO offers a validated daily diary–based symptom assessment that helps to better characterize exacerbations (41) and reduce the recall bias affecting 60% of events that go unreported (42). Use of these questionnaires complement the information obtained from lung function measurements, helping to provide a more consistent description of an individual’s sensation of breathlessness in response to lung volume reduction treatment.
Most patients with COPD are relatively inactive, spending significantly less time standing and walking than persons without COPD (43). Dyspnea is the principal symptom limiting exercise in patients with advanced COPD, leading to activity avoidance (3, 43–45). It is important to break this cycle because reduced physical activity is a predictor of future risk of exacerbation, hospitalization, and early mortality (46, 47). Multiple measures of activity levels in patients treated with Zephyr Valves showed statistically significant improvements compared with SoC at 12 months, notably the TDI measures of effort, task, and functional impairment. These changes in activity levels can be very meaningful for patients. For example, a 3-point change in the TDI, focal score, implies a return to most work/leisure activities previously impacted, and 54.9% of patients treated with Zephyr Valve achieved this in LIBERATE.
The impact of COPD is not limited to the physical restrictions levied on the lifestyle of the patient. The experience of breathlessness “derives from interactions among multiple physiological psychological, social, and environmental factors, and may induce secondary physiological and behavioural responses” (2). Breathlessness is associated with panic, anxiety, and depression (48). The inability to engage in activities of daily living can be one of the most distressing symptoms for patients with COPD. The opportunity to improve quality of life and permit resumption, even in part, of familiar activities is highly desirable (49). Our findings show that patients using the Zephyr Valve had improvements in psychosocial status, demonstrated by statistically significant improvements for patients using the Zephyr Valve over SoC in the SGRQ, impacts domain, and in CAT questions on patient confidence and energy.
Patients frequently report good and bad days with significant fluctuations over time (50–53). The quantitative improvement in all three aspects of breathlessness, activity levels, and psychosocial well-being measured at specified time points is also reflected in the daily diary assessment of patients, with those using the Zephyr Valve experiencing significantly more days that were better and fewer days that were worse over 12 months.
The improvements in multidimensional PROs were correlated with reduction in hyperinflation and elaborate on the clinical benefits observed in individuals undergoing this treatment (37). Utilization of varied multidimensional questionnaires and of a daily diary assessment tool, as demonstrated in this study, afford a more comprehensive evaluation of the impact of the Zephyr Valve that is personalized to the symptoms of the individual and supports a phenotype-based management strategy as advocated by the Global Initiative for Chronic Obstructive Lung Disease guidelines (38). These data add to the growing evidence base for the Zephyr Valve accomplishing and maintaining improvements that are not limited to lung function and objective exercise assessment but, importantly, to disease-specific symptoms and health-related quality of life, which permits patients the ability to reengage in their activities of daily living.
The strengths of the study include the use of standardized, validated, disease-specific, treatment-responsive instruments such as the SGRQ, CAT, and the TDI that provide a complement and refinement to previously published physiological data that is meaningful to the individual, and which is crucial to guide therapeutic management. Moreover, a daily diary assessment adds greater detail and context to what is a variable tapestry of symptoms from day to day. Furthermore, they provide an easy and cost-efficient way for acquiring data to characterize complex issues such as breathlessness and health-related quality of life in COPD that cannot be achieved by any one physiological correlate (26). Lastly, the presence of a SoC group out to 12 months affords more accurate measurement of the impact of the Zephyr Valve on these multidimensional metrics. Limitations of the study include the use of these qualitative tools that can be subject to recall bias (54) and could be further confounded due to the lack of blinding. Neither the study participants nor the assessing physicians were blinded to the randomization, which could introduce bias for self-reported outcomes. However, the strong correlation between reduction in hyperinflation as assessed by a reduction in RV and RV/TLC ratio, both of which are objective measures, and the changes from baseline to 12 months for postbronchodilator FEV1; 6MWD; the SGRQ score; and the EXACT-PRO, dyspnea domain, suggest that the results are robust. It should also be noted that the number of patients who did not complete the 12-month assessments was greater in those randomized to Zephyr Valves than in the SoC group, which could have biased the results toward benefit. Finally, a select group of patients with severe emphysema and hyperinflation with little or no collateral ventilation was treated, and the results cannot be generalized to other grades of airflow limitation.
Conclusions
Post hoc analysis of data from the LIBERATE study demonstrate that patients with severe emphysema and hyperinflation who achieve lung volume reductions following treatment with Zephyr Valves experience moderate to large improvements in multidimensional scores for breathlessness, activity, and psychosocial parameters that may permit reengagement in activities of daily living out to at least 12 months. The interruption of the downward spiral of symptom-induced inactivity, muscle deconditioning, and ensuing weakness allows patients to experience improved activity, feeling of well-being, more confidence, and a better quality of life. These results supplement the findings of published randomized controlled studies in patients with heterogeneous (18–21) and those with homogeneous (22) emphysema, in whom accompanying improvements in lung function, exercise capacity, dyspnea, and overall quality of life have consistently been shown.
Supplementary Material
Acknowledgments
The LIBERATE Study Group: Lewis Katz School of Medicine at Temple University, Philadelphia, PA: Gerard J. Criner, Francis Cordova, Parag Desai, Nathaniel Marchetti, Victor Kim, Kartik Shenoy, John Travaline, Jiji Thomas, and Lii-Yoong H. Criner; St. Joseph’s Hospital and Medical Center, Phoenix, AZ: Richard Sue, Shawn Wright, Aaron Thornburg, and Terry Thomas; University of Alabama at Birmingham UAB Lung Health Center, Birmingham, AL: Mark Dransfield, Surya Bhatt, James Michael Wells, and Necole Seabron-Harris; University of Louisville, Louisville, KY: Hiram Rivas-Perez, Umair Gauhar, Tanya A Wiese (now at Norton Healthcare, Louisville, KY), and Crissie Despirito; University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, Jessica Bon Field, Divay Chandra, Joseph Leader, Roy Semaan, and Christina Ledezma; Royal Brompton Hospital and Imperial College, London, UK: Pallav Shah, Samuel Kemp, Justin Garner, Arafa Aboelhassan, Karthi Srikanthan, Eric Tenda, Anita Abraham, and Cai Sim; Duke University Medical Center, Durham, NC: Momen Wahidi, Kamran Mahmood, Scott Shofer, and Kathleen Coles; Hospital das Clinicas de Porto Alegre, Porto Alegre, RS, Brazil: Hugo Goulart de Oliveira, Guilherme Augusto Oliveira, Betina Machado, Igor Benedetto, Fabio Svartman, Amarilio de Macedo Neto, Leonardo Schreiner, and Taiane Vieira; University of California, Davis, Sacramento, CA: Brian Morrissey, Ken Yoneda, Tina Tham, and Daniel Tompkins; Instituto do Coracao, Hospital das Clinicas, Faculdade de Medicina, Universidade de Sao Paulo, São Paulo, SP, Brazil: Paulo F. Guerreiro Cardoso, Rodrigo Athanazio, Felipe Nominando, Samia Rached, and Luciana Cassimiro; University of California, San Francisco, San Francisco, CA: Steven Hays, Eric Seeley, Pavan Shrestha, and Gabriela R. Dincheva; Beth Israel Deaconess Medical Center, Boston, MA: Adnan Majid, Daniel Alape-Moya, Mihir Parikh, Alichia Paton, and Alexis Agnew; Medical University of South Carolina, Charleston, SC: Nicholas Pastis, Jr., Charlie Strange, Tatsiana Beiko, Danielle Woodford, and Mary Blanton; Houston Methodist Hospital, Texas Medical Center, Houston, TX: Lisa Kopas, Timothy Connolly, Jose Fernando Santacruz, and Bhavin Shah; Orlando Regional Medical Center, Orlando, FL: Mark Vollenweider, Luis Herrera, Rumi Khan, and Kristine Sernulka; University of Southern California, Los Angeles, CA: P. Michael McFadden, Richard Barbers, and Michelle Hernandez; Cleveland Clinic Foundation, Cleveland, OH: Michael Machuzak, Francisco Almeida, Joseph Cicenia, Thomas Gildea, Atul Mehta, Sonali Sethi, and Yvonne Meli; Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles, Torrance, CA: David Hsia, Richard Casaburi, William Stringer, and Leticia Diaz; Stanford Hospital and Clinics, Stanford, CA: Arthur Sung, Meghan Ramsey, Ryan Van Wert, Karen Morris; University Hospital Bristol National Health Service Foundation Trust, Bristol, UK: Nabil Jarad, Tim Batchelor, Iara Sequeiros, and Katy Tucker; University Hospital of Wales, Cardiff, UK: Malgorzata Kornaszweska, Hazem Fallouh, Ramsey Sabit, Hatam Naase, Joseph George, Azin Salimian, and Helen Dyer; Southern Illinois University School of Medicine, Springfield, IL: Stephen Hazelrigg, Kristal Adams, Karen Bade; Palo Alto Medical Foundation, El Camino Hospital, Mountain View, CA: Ganesh Krishna, Bryan S. Benn, Michelle Canfield, Sharmila Vetri Villalan, and Travis Stewart; and University Medical Center Groningen, Groningen, the Netherlands: Dirk-Jan Slebos, Nick Ten Hacken, Karin Klooster, Jorine Hartman, and Sonja Augustijn.
Footnotes
Supported by Pulmonx Corporation, Redwood City, CA.
A complete list of the LIBERATE Study Group may be found before the beginning of the References.
Author Contributions: M.T.D. and J.L.G. are investigators; actively recruited and treated patients; and participated in acquisition, analysis, interpretation of the data, and development of the manuscript. G.J.C. is the principal investigator of the LIBERATE study; collaborated on design; advised on medical issues; actively recruited and treated patients; participated in acquisition, analysis, and interpretation of the data; and reviewed and approved the final manuscript. S.P.B., D.-J.S., K.K., F.C.S., P.L.S., N.T.M., R.D.S., S.W., H.R.-P., T.A.W., M.M.W., and H.G.d.O. are investigators; actively recruited and treated patients; participated in acquisition of data; provided revisions; and approved the final manuscript. B.A. managed the study database, performed all the statistical analyses, supported the interpretation of the statistics and their inclusion in the manuscript, and reviewed and approved the final manuscript. S.R. supported the analysis and interpretation of the data, and reviewed and approved the final manuscript. N.S.S. oversaw the trial operations, developed this post hoc analysis plan, supported the analysis and interpretation of the data, developed the data tables and figures, and reviewed and approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the LIBERATE Study Group, Lewis Katz, Gerard J. Criner, Gerard J. Criner, Francis Cordova, Parag Desai, Nathaniel Marchetti, Victor Kim, Kartik Shenoy, John Travaline, Jiji Thomas, Lii-Yoong H. Criner, Richard Sue, Shawn Wright, Aaron Thornburg, Terry Thomas, Mark Dransfield, Surya Bhatt, James Michael Wells, Necole Seabron-Harris, Hiram Rivas-Perez, Umair Gauhar, Tanya A. Wiese, Crissie Despirito, Frank Sciurba, Jessica Bon Field, Divay Chandra, Joseph Leader, Roy Semaan, Christina Ledezma, Pallav Shah, Samuel Kemp, Justin Garner, Arafa Aboelhassan, Karthi Srikanthan, Eric Tenda, Anita Abraham, Cai Sim, Momen Wahidi, Kamran Mahmood, Scott Shofer, Kathleen Coles, Hugo Goulart de Oliveira, Guilherme Augusto Oliveira, Betina Machado, Igor Benedetto, Fabio Svartman, Amarilio de Macedo Neto, Leonardo Schreiner, Taiane Vieira, Brian Morrissey, Ken Yoneda, Tina Tham, Daniel Tompkins, Paulo F. Guerreiro Cardoso, Rodrigo Athanazio, Felipe Nominando, Samia Rached, Luciana Cassimiro, Steven Hays, Eric Seeley, Pavan Shrestha, Gabriela R. Dincheva, Adnan Majid, Daniel Alape-Moya, Mihir Parikh, Alichia Paton, Alexis Agnew, Nicholas Pastis, Jr., Charlie Strange, Tatsiana Beiko, Danielle Woodford, Mary Blanton, Lisa Kopas, Timothy Connolly, Jose Fernando Santacruz, Bhavin Shah, Mark Vollenweider, Luis Herrera, Rumi Khan, Kristine Sernulka, P. Michael McFadden, Richard Barbers, Michelle Hernandez, Michael Machuzak, Francisco Almeida, Joseph Cicenia, Thomas Gildea, Atul Mehta, Sonali Sethi, Yvonne Meli, David Hsia, Richard Casaburi, William Stringer, Leticia Diaz, Arthur Sung, Meghan Ramsey, Ryan Van Wert, Karen Morris, Nabil Jarad, Tim Batchelor, Iara Sequeiros, Katy Tucker, Malgorzata Kornaszweska, Hazem Fallouh, Ramsey Sabit, Hatam Naase, Joseph George, Azin Salimian, Helen Dyer, Stephen Hazelrigg, Kristal Adams, Karen Bade, Ganesh Krishna, Bryan S. Benn, Michelle Canfield, Sharmila Vetri Villalan, Travis Stewart, Dirk-Ja n Slebos, Nick H. T. ten Hacken, Karin Klooster, Jorine Hartman, and Sonja Augustijn
References
- 1.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyspnea: mechanisms, assessment, and management. A consensus statement: American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 3.Schönhofer B, Ardes P, Geibel M, Köhler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997;10:2814–2819. doi: 10.1183/09031936.97.10122814. [DOI] [PubMed] [Google Scholar]
- 4.Casaburi R. Impacting patient-centred outcomes in COPD: deconditioning. Eur Respir Rev. 2006;15:42–46. [Google Scholar]
- 5.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(Suppl):S459–S471. doi: 10.1097/00005768-200106001-00016. [Discussion, pp. S493–S494] [DOI] [PubMed] [Google Scholar]
- 6.Yohannes AM, Baldwin RC, Connolly M. Mortality predictors in disabling chronic obstructive pulmonary disease in old age. Age Ageing. 2002;31:137–140. doi: 10.1093/ageing/31.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003;167:544–549. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 8.Yohannes AM, Raue PJ, Kanellopoulos D, McGovern A, Sirey JA, Kiosses DN, et al. Predictors of all-cause mortality in patients with severe COPD and major depression admitted to a rehabilitation hospital. Chest. 2016;149:467–473. doi: 10.1378/chest.15-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubé B-P, Guerder A, Morelot-Panzini C, Laveneziana P. The clinical relevance of the emphysema-hyperinflated phenotype in COPD. COPD Res Pract. 2016;2:1. [Google Scholar]
- 10.Jolley CJ, Moxham J. A physiological model of patient-reported breathlessness during daily activities in COPD. Eur Respir Rev. 2009;18:66–79. doi: 10.1183/09059180.00000809. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell DE, Banzett RB, Carrieri-Kohlman V, Casaburi R, Davenport PW, Gandevia SC, et al. Pathophysiology of dyspnea in chronic obstructive pulmonary disease: a roundtable. Proc Am Thorac Soc. 2007;4:145–168. doi: 10.1513/pats.200611-159CC. [DOI] [PubMed] [Google Scholar]
- 13.Zaman M, Mahmood S, Altayeh A. Low inspiratory capacity to total lung capacity ratio is a risk factor for chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2010;339:411–414. doi: 10.1097/MAJ.0b013e3181d6578c. [DOI] [PubMed] [Google Scholar]
- 14.Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 15.Tantucci C, Donati P, Nicosia F, Bertella E, Redolfi S, De Vecchi M, et al. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respir Med. 2008;102:613–619. doi: 10.1016/j.rmed.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 17.Criner GJ, Belt P, Sternberg AL, Mosenifar Z, Make BJ, Utz JP, et al. National Emphysema Treatment Trial Research Group. Effects of lung volume reduction surgery on gas exchange and breathing pattern during maximum exercise. Chest. 2009;135:1268–1279. doi: 10.1378/chest.08-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–1073. doi: 10.1016/S0140-6736(15)60001-0. [DOI] [PubMed] [Google Scholar]
- 19.Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–2335. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 20.Kemp SV, Slebos DJ, Kirk A, Kornaszewska M, Carron K, Ek L, et al. A multicenter RCT of Zephyr(R) endobronchial valve treatment in heterogeneous emphysema (TRANSFORM) Am J Respir Crit Care Med. 2017;196:1535–1543. doi: 10.1164/rccm.201707-1327OC. [DOI] [PubMed] [Google Scholar]
- 21.Criner GJ, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T, et al. LIBERATE Study Group. A multicenter randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE) Am J Respir Crit Care Med. 2018;198:1151–1164. doi: 10.1164/rccm.201803-0590OC. [DOI] [PubMed] [Google Scholar]
- 22.Valipour A, Slebos DJ, Herth F, Darwiche K, Wagner M, Ficker JH, et al. IMPACT Study Team. Endobronchial valve therapy in patients with homogeneous emphysema: results from the IMPACT study. Am J Respir Crit Care Med. 2016;194:1073–1082. doi: 10.1164/rccm.201607-1383OC. [DOI] [PubMed] [Google Scholar]
- 23.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. American Thoracic Society; European Respiratory Society Task Force on outcomes of COPD. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 24.Agusti A, Calverley PMA, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators Characterisation of COPD heterogeneity in the ECLIPSE cohort Respir Res 20101112220831787 [Google Scholar]
- 25.Rennard S, Decramer M, Calverley PM, Pride NB, Soriano JB, Vermeire PA, et al. Impact of COPD in North America and Europe in 2000: subjects’ perspective of confronting COPD International Survey. Eur Respir J. 2002;20:799–805. doi: 10.1183/09031936.02.03242002. [DOI] [PubMed] [Google Scholar]
- 26.van der Molen T, Miravitlles M, Kocks JW. COPD management: role of symptom assessment in routine clinical practice. Int J Chron Obstruct Pulmon Dis. 2013;8:461–471. doi: 10.2147/COPD.S49392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 28.Dransfield MT, Bhatt SP, Slebos D-J, Klooster K, Sciurba FC, Shah PL. Impact of Zephyr endobronchial valves on patient reported outcomes [abstract] Am J Respir Crit Care Med. 2019;199:A7309. [Google Scholar]
- 29.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 30.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, et al. National Emphysema Treatment Trial (NETT) Research Group. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37:784–790. doi: 10.1183/09031936.00063810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 32.Mahler DA, Witek TJ., Jr The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2:99–103. doi: 10.1081/copd-200050666. [DOI] [PubMed] [Google Scholar]
- 33.de Torres JP, Pinto-Plata V, Ingenito E, Bagley P, Gray A, Berger R, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121:1092–1098. doi: 10.1378/chest.121.4.1092. [DOI] [PubMed] [Google Scholar]
- 34.Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, Borg scale, and visual analog scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 35.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 36.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, et al. VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 37.van Geffen WH, Slebos DJ, Herth FJ, Kemp SV, Weder W, Shah PL. Surgical and endoscopic interventions that reduce lung volume for emphysema: a systemic review and meta-analysis. Lancet Respir Med. 2019;7:313–324. doi: 10.1016/S2213-2600(18)30431-4. [DOI] [PubMed] [Google Scholar]
- 38.Global Initiative for Chronic Obstructive Lung Disease, Inc. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2019 report. 2019 [accessed 2018 Nov 8]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf.
- 39.Mahler DA, Ward J, Waterman LA, McCusker C, ZuWallack R, Baird JC. Patient-reported dyspnea in COPD reliability and association with stage of disease. Chest. 2009;136:1473–1479. doi: 10.1378/chest.09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea: contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85:751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 41.Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13:965–975. doi: 10.1111/j.1524-4733.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 42.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. 2008;177:396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 43.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 44.Barnett M. Chronic obstructive pulmonary disease: a phenomenological study of patients’ experiences. J Clin Nurs. 2005;14:805–812. doi: 10.1111/j.1365-2702.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 45.Cooper CB. Airflow obstruction and exercise. Respir Med. 2009;103:325–334. doi: 10.1016/j.rmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 46.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31:667–677. doi: 10.1183/09031936.00125707. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell DE. Impacting patient-centred outcomes in COPD: breathlessness and exercise tolerance. Eur Respir Rev. 2006;15:37–41. [Google Scholar]
- 50.Gilbert C, Martin M, Hareendran A, Bushnell D, Patrick D, Schünemann H.Capturing individual variation in the experience of symptoms reported by patients with COPD [abstract] Am J Respir Crit Care Med 2007175A15–A1004.17633759 [Google Scholar]
- 51.Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin. 2009;25:2043–2048. doi: 10.1185/03007990903103006. [DOI] [PubMed] [Google Scholar]
- 52.Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37:264–272. doi: 10.1183/09031936.00051110. [DOI] [PubMed] [Google Scholar]
- 53.Espinosa de los Monteros MJ, Peña C, Soto Hurtado EJ, Jareño J, Miravitlles M. Variability of respiratory symptoms in severe COPD. Arch Bronconeumol. 2012;48:3–7. doi: 10.1016/j.arbres.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. doi: 10.2147/JMDH.S104807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.