To the Editor:
Sickle cell disease (SCD) is characterized by vaso-occlusion and chronic hemolysis that impact the pulmonary and systemic circulation and leads to substantial morbidity and early mortality (1). An elevated tricuspid regurgitation velocity (TRV) is used to estimate pulmonary systolic pressure and as a screening modality for pulmonary hypertension (PH). A TRV ≥ 2.5 m/s identifies patients at higher risk of having PH (25% based on a mean pulmonary artery pressure of 25 mm Hg) and predicts a 3- to 10-fold-greater risk for mortality in SCD (2). Patients with SCD with an elevated TRV have: 1) elevated markers of intravascular hemolysis and endothelial dysfunction in the pulmonary and systemic microcirculation; and 2) either postcapillary or precapillary PH (2, 3). TRV elevations may be related to high cardiac output and episodic vaso-occlusive events and require confirmation by measuring plasma brain natriuretic peptide levels, assessing symptoms, such as exercise capacity, and confirmation by right-heart catheterization. Causes of PH in SCD include primary pulmonary vascular disease, PH secondary to left-sided heart failure with preserved or reduced ejection fraction, or secondary to chronic thromboembolic PH. Based on the multiple etiologies for PH in SCD, it has been classified as group 5 (unclear/multifactorial) by the Fifth World Symposium on PH (4).
Patients with SCD have a fourfold greater risk of venous thromboembolic events (VTEs) compared with individuals without SCD (5). In the Cooperative Study of SCD, the incidence of VTEs and pulmonary embolism (PE) was reported to be 5.2 and 3.6 events per 1,000 person-years, respectively (6). The hypercoagulable state in SCD is due to the pleotropic effects of intravascular hemolysis (ADP-, heme-, and oxyhemoglobin-mediated activation), platelet activation, endothelium dysfunction, and inflammation (1). The contribution of VTEs to high TRV values and PH in SCD has not been studied in large cohorts screened for PH. In this study, we assessed the possible association of PE with PH in two independent SCD cohorts using measured TRV values.
Methods
Derivation cohort from the Walk–Pulmonary Hypertension and SCD with Sildenafil Therapy screening study
The Treatment of Pulmonary Hypertension and SCD with Sildenafil Therapy (Walk-PHaSST) study was designed to assemble a large cohort of patients with SCD to screen their eligibly for sildenafil in a randomized clinical trial. This study recruited 720 adolescents and adults at 10 clinical centers in the United States and United Kingdom between 2007 and 2009. The University of Illinois at Chicago (UIC) was a participating site and we excluded 52 patients from UIC. Each study participant was comprehensively evaluated by collecting clinical, laboratory, and echocardiography data, as well as self-reported history of PE (7).
Validation cohort at the UIC
The UIC registry is a longitudinal cohort of patients with SCD recruited during a clinic visit. Between 2010 and 2016, this cohort has recruited and phenotyped 395 patients with SCD. Of these, 157 have had steady-state transthoracic echocardiograms performed as part of routine care and were included in this analysis. A history of PE was determined by a query for International Classification of Diseases 9th and 10th Revisions diagnostic codes and manually confirmed by reviewing the electronic medical charts.
Potential confounders, including age, sex, severe hemoglobin genotype, reticulocyte count, and serum creatinine, were defined among those variables considered plausible confounders, and included in the multivariate analyses.
Results
Walk-PHaSST
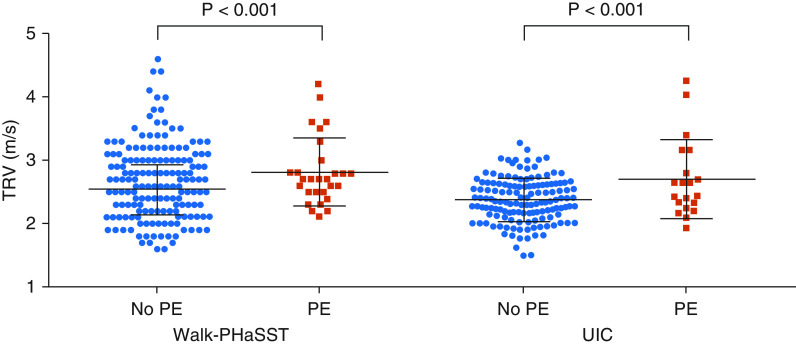
A total of 31 (4.6%) patients with SCD had a self-reported history of PE. Patients with a history of PE were older and had an average TRV 0.3 m/s higher compared with those without a PE history (Table 1). After adjusting for age, sex, severe genotype, reticulocyte count, creatinine, and LV lateral E/A (left ventricular early to late diastolic transmitral flow velocity); patients with PE history had an average 0.30 m/s (95% confidence interval [CI], 0.16–0.45; P < 0.001) higher TRV (Figure 1). Frequency of TRV ≥ 3.0 m/s was significantly higher in patients with versus without a history of PE (25% vs. 10%; P = 0.01). Frequency of NT-proBNP (N-terminal-pro hormone B-type natriuretic peptide) > 160 pg/ml was 30% in patients with a history of PE versus 23% in patients without PE (P = 0.49). A total of 55% of patients with a history of PE versus 5% of patients without a history of PE were on anticoagulation treatment at the time of study, supporting the accuracy of self-reported history. After adjusting for confounders, patients with anticoagulation treatment had, on average, 0.33 m/s (95% CI, 0.21–0.44; P < 0.001) -higher TRV compared with patients without history of anticoagulation treatment. Frequency of NT-proBNP > 160 pg/ml was 41% in patients with anticoagulation treatment versus 21% in patients without treatment (P = 0.004).
Table 1.
Clinical and laboratory findings by history of pulmonary embolism
| History of PE− | History of PE+ | |
|---|---|---|
| Walk-PHaSST | n = 637 | n = 31 |
| Age, yr | 36 (13.5) | 41 (12.5) |
| Female, n (%) | 342 (54) | 20 (65) |
| SS genotype, n (%) | 434 (76) | 18 (64) |
| Hemoglobin, mg/dl | 9.4 (1.9) | 9.8 (2.0) |
| Reticulocyte count, 109/L | 23.5 (12.9) | 19.8 (13.4) |
| White blood cell count, 109/L | 9.7 (3.8) | 9.7 (3.0) |
| Platelet count, 109/L | 354 (138) | 337 (95) |
| Creatinine, mg/dl | 0.87 (0.90) | 0.93 (0.54) |
| N-terminal-pro-BNP, pg/ml* | 65 (121) | 65 (145) |
| Left ventricular lateral E/A | 7.0 (2.8) | 6.5 (1.9) |
| TRV, m/s | 2.5 (0.40) | 2.8 (0.54) |
| UIC | n = 137 | n = 20 |
| Age, yr | 35 (13) | 34 (8.8) |
| Female, n (%) | 86 (64) | 14 (70) |
| SS genotype, n (%) | 115 (84) | 15 (75) |
| Hemoglobin, mg/dl | 9.1 (1.7) | 9.3 (2.0) |
| Reticulocyte count, 109/L | 32.0 (16.0) | 32.0 (14.0) |
| White blood cell count, 109/L | 9.8 (3.5) | 9.8 (2.6) |
| Platelet count, 109/L | 410 (150) | 410 (150) |
| Creatinine, mg/dl | 0.91 (0.68) | 0.90 (0.52) |
| N-terminal-pro-BNP, pg/ml* | 50 (16–169) | 87 (33–366) |
| Left ventricular lateral E/A | — | — |
| TRV, m/s | 2.4 (0.35) | 2.7 (0.62) |
Definition of abbreviations: BNP = brain natriuretic peptide; E/A = early to late diastolic transmitral flow velocity; PE = pulmonary embolism; PHaSST = Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy; SD = standard deviation; SS = hemoglobin SS; TRV = tricuspid regurgitation velocity; UIC = University of Illinois at Chicago.
Results are presented as mean (SD) unless otherwise specified.
Median (interquartile range).
Figure 1.
Mean (SD) tricuspid regurgitation velocity by history of pulmonary embolism (PE) in the Walk-PHaSST (Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy) and University of Illinois at Chicago cohorts. In each cohort, P values were calculated from a multivariate regression analysis adjusted for age, sex, severe genotype, reticulocyte count, creatinine, and LV lateral E/A (Walk-PHaSST). TRV = tricuspid regurgitation velocity; UIC = University of Illinois at Chicago.
UIC
A history of PE was observed in 12.7% of patients. Patients with a history of PE had an average TRV that was 0.3 m/s higher than in those without a history of PE (Table 1). After similar adjustments as in the Walk-PHaSST cohort, patients with a PE history had an average TRV that was 0.34 m/s (95% CI, 0.31–0.59; P < 0.001) greater than in patients without a PE history (Figure 1). Frequency of TRV ≥ 3.0 m/s was significantly higher in patients with versus without a history of PE (25% vs. 6%; P = 0.02). Frequency of NT‐proBNP > 160 pg/ml was 45% in patients with a history of PE versus 26% in patients without PE (P = 0.2).
Discussion
Our results support an independent, strong relationship between elevated TRV and PE in SCD. Venous thromboembolism is a serious comorbidity in patients with SCD that leads to a threefold-higher risk for mortality (6). It is estimated that patients with SCD have a sixfold or greater risk for PE compared with the general population (6). We observed a combined prevalence of PE by medical history in 6.2% of patients with SCD.
In the general population, it is estimated that up to 9.1% of acute PE events lead to chronic thromboembolic PH (8). Pulmonary emboli may transform into fibrotic lesions under conditions of increased inflammation, by defective angiogenesis from abnormal calcium homeostasis, or abnormal circulating fibrinogen or phospholipids impairing thrombus resolution (8). The inability to resolve the thrombotic lesion leads to vascular wall remodeling with pathologic features that overlap with what is observed in primary PH. In two independent SCD cohorts, we demonstrate that the TRV is approximately 10% higher in patients with versus without a history of PE. To our knowledge, there is only one other single-center study indicating a relationship between elevated TRV and VTEs in patients with SCD (9). In that study, non–catheter-related VTEs were associated with a 1.7-fold-greater risk for TRV ≥ 2.5 m/s.
Transthoracic echocardiograms were ordered as part of routine clinical care in the UIC cohort, and there may have been selection bias. Future studies measuring pulmonary vascular resistance by right-heart catheterization may help elucidate the relationship between PE and increased TRV.
Altogether, these findings strongly suggest an important role of VTEs in SCD pulmonary hypertension. Screening of patients with SCD with a high TRV value for VTEs could help us identify patients at greater risk for chronic thromboembolic PH and eligible for available therapies, such as anticoagulation, surgical pulmonary artery endarterectomy, and the soluble guanylate cyclase stimulator, riociguat (10).
Supplementary Material
Footnotes
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127:750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gladwin MT. Cardiovascular complications in patients with sickle cell disease. Hematology (Am Soc Hematol Educ Program) 2017;2017:423–430. doi: 10.1182/asheducation-2017.1.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthi A, Machado RF, Jison ML, Taveira-Dasilva AM, Rubin LJ, Hunter L, et al. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. doi: 10.1164/rccm.200610-1498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(suppl)(25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Noubiap JJ, Temgoua MN, Tankeu R, Tochie JN, Wonkam A, Bigna JJ. Sickle cell disease, sickle trait and the risk for venous thromboembolism: a systematic review and meta-analysis. Thromb J. 2018;16:27. doi: 10.1186/s12959-018-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naik RP, Streiff MB, Haywood C, Jr, Segal JB, Lanzkron S. Venous thromboembolism incidence in the Cooperative Study of Sickle Cell Disease. J Thromb Haemost. 2014;12:2010–2016. doi: 10.1111/jth.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gladwin MT, Barst RJ, Gibbs JS, Hildesheim M, Sachdev V, Nouraie M, et al. walk-PHaSST Investigators and Patients. Patients. Risk factors for death in 632 patients with sickle cell disease in the United States and United Kingdom. PLoS One. 2014;9:e99489. doi: 10.1371/journal.pone.0099489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkens H, Konstantinides S, Lang IM, Bunck AC, Gerges M, Gerhardt F, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272S:69–78. doi: 10.1016/j.ijcard.2018.08.079. [DOI] [PubMed] [Google Scholar]
- 9.Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: a serious and under-recognized complication. Am J Med. 2013;126:443–449. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.