Abstract
Background
Physical activity is a modifiable risk factor associated with health benefits. We hypothesized that a more active lifestyle in older adults is associated with a reduced risk of incident parkinsonism and a slower rate of its progression.
Methods
Total daily physical activity was recorded with an activity monitor in 889 community-dwelling older adults participating in the Rush Memory and Aging Project. Four parkinsonian signs were assessed with a modified motor portion of the Unified Parkinson’s Disease Rating Scale and summarized as a categorical measure and continuous global parkinsonian score. We used Cox models to determine whether physical activity was associated with incident parkinsonism and linear mixed-effects models to examine if physical activity was associated with the rate of progressive parkinsonism.
Results
During an average follow-up of 4 years, 233 of 682 (34%) participants, without parkinsonism, developed incident parkinsonism. In Cox models controlling for age, sex, and education, a higher level of physical activity was associated with a reduced risk of developing parkinsonism (hazard ratio = 0.79; 95% CI = 0.70–0.88, p < .001). This association was not attenuated when controlling for cognition, depressive symptoms, Apolipoprotein E ℇ4 allele, and chronic health conditions. In a linear mixed-effects model including all participants (N = 889) which controlled for age, sex, and education, a 1 SD total daily physical activity was associated with a 20% slower rate of progression of parkinsonism.
Conclusion
Older adults with a more active lifestyle have a reduced risk for parkinsonism and a slower rate of its progression.
Keywords: Parkinsonian disorders, Exercise, Activities of daily living, Risk factors, Aged 80 and older
In prior work late-life motor impairments such as parkinsonian signs including impaired gait and balance, bradykinesia, tremor, and rigidity, are common and by the age of 85 years may affect 50% or more of adults without a clinical diagnosis of Parkinson disease (PD) (1). These impairments are not “benign” as they are associated with an increased risk of death, disabilities, and incident cognitive impairment including mild cognitive impairment and Alzheimer’s disease (AD) dementia. Moreover, in a prior study of older adults, almost half without parkinsonism developed parkinsonism during 5 years of follow-up (1,2). Thus, identifying modifiable risk factors to slow or prevent the development of parkinsonism has potential to improve the quality of life in many older adults and facilitate the maintenance of independent living.
Physical activity is a volitional and modifiable behavior with a range of potential health benefits (3). Until recently, community-based studies have relied on self-reported measures of physical activity (2,4). Most self-report instruments have focused on physical exercise but have not concurrently assessed habitual physical activities which may play an important role in older adults (5). In prior work, we found that self-reported physical activity was not associated with incident parkinsonism when controlling for other clinical covariates (2). However, this finding may reflect the limitations of self-report when compared with objective metrics of motor performance.
Unobtrusive, wearable activity monitors are now available which can be employed to record all daily movement continuously over several days in the community-setting. These recordings can yield physical activity metrics for total activity free from recall bias and other sources of subjectivity and include both exercise and habitual activities (6). Examining these data are crucial for determining if a more active lifestyle is independently associated with incident parkinsonism and its rate of progression (7).
To fill this knowledge gap, we used data from nearly 900 community-dwelling older adults participating in the Rush Memory and Aging Project, a community-based cohort study of chronic conditions of aging. Annual clinical assessment of parkinsonism was based on 26 items from a modified version of the United Parkinson’s Disease Rating Scale as previously described (8,9). The total quantity of physical activity and its intensity was derived from multi-day recordings obtained with a wrist worn activity monitor. This monitor recorded all exercise and habitual physical activity continuously, 24 h/d, for up to 10 days as previously described (5,6).
Method
Participants
The Rush Memory and Aging Project (MAP) began to recruit older adults from retirement facilities, subsidized housings, and individual homes across Chicago Metropolitan area in 1997. Participants agree to annual clinical assessments and organ donation at the time of death. Actigraphy data collection was added to the study in 2005. The study employs rolling admissions so the analytic baseline for this study was the cycle at which an individual completed the first valid actigraphy assessment. The goal of these analyses was to examine the association of quantitative metrics of physical activity with incident parkinsonism and the annual rate of change in parkinsonism in older adults without a known cause for parkinsonism. Eligibility for these analyses included a valid baseline assessment of daily physical activity and an assessment of parkinsonism at the same cycle with at least one additional follow-up assessment of parkinsonism.
Since 2005, 1,214 participants had completed an assessment of both total daily physical activity and parkinsonism as described below. The primary analyses in this paper examine the association of physical activity with the risk and severity of parkinsonism over time. Therefore, we excluded 60 individuals with a known cause for parkinsonism at baseline including a clinical diagnosis of Parkinson’s disease for which they received dopaminergic medications (N = 21) or individuals who were receiving neuroleptic medications known to affect the severity of parkinsonism (N = 39) as their inclusion in these analyses would confound our findings. As our analyses focused on longitudinal outcomes, we excluded an additional 265 individuals who did not have one or more follow-up assessments of parkinsonism (45 participants died before their first follow-up, 92 individuals were lost in the follow-up, and 128 had missing parkinsonism data; Supplementary Figure e-1). This left 889 participants, including 682 without parkinsonism and 207 with parkinsonism at baseline, for these analyses.
Individuals included in these analyses (N = 889) and those excluded (N = 265) showed similar age at study entry, sex distribution, severity of parkinsonism, BMI, depressive symptoms, burden of vascular risk factors, and diseases. Individuals excluded from these analyses had lower levels of total daily physical activity and more years of education (Supplementary Table e-1).
Assessment of the Presence and Severity of Parkinsonism
Annual testing included a modified version of the United Parkinson’s Disease Rating Scale (UPDRS) administered by trained nurse clinicians (8,9). Twenty-six items were examined to assess four parkinsonian signs (parkinsonian gait, bradykinesia, rigidity, and tremor; Supplementary Methods and Table e-2). In prior work, we showed that assessments with this modified UPDRS have high inter-rater reliability and short-term stability among nurses and compared with a movement disorders specialist (8,9).
Categorical parkinsonism
A categorical measure of parkinsonism is necessary to identify incident cases of parkinsonism. An individual was categorized as having parkinsonism based on the clinical presence of two or more of the four parkinsonian signs assessed. Parkinsonism was not present if there were none or one of the four parkinsonian signs present. A parkinsonian sign was present if two or more of its included items showed mild abnormality. Further details about the construction and validation of these categories are in the Supplementary Methods.
Global parkinsonian score
The 26 UPDRS items were also summarized as a continuous global parkinsonian score which captures the overall severity of parkinsonian signs. This metric has been used extensively for longitudinal modeling of the progression of parkinsonism in older adults (2,10). The construction and validation of global parkinsonian score is included in the Supplementary Methods.
Assessment of Total Daily Physical Activity
All physical activities were recorded continuously 24 h/d for up to 10 days using an activity monitor worn on the nondominant wrist (Actical; Philips Healthcare, Andover, MA). Actical accelerometers generate a signal proportional to the magnitude and duration of detected motion. The analog signal is digitized, rectified, integrated across a 15-second span, and rounded to the nearest integer to create an activity count for each 15-second period with motion.
Total daily physical activity was the average sum of all recorded daily activity counts for all complete days of recordings as previously described (6,7). Prior studies have estimated activity counts recorded for different motor performances: walking at 2.5 mph is associated with 2354 activity counts/min or jogging at 4.5 mph is associated with 8640 activity counts/min, on average (11). Supplementary Figure e-2 shows a time series of all recorded activity counts for a single participant during 10 days (12). The intensity of daily physical activity may play a role in its potential health benefits (13). Intensity of total daily physical activity was calculated by dividing the total daily activity counts by total hours per day of nonzero epochs (activity counts per hour per day) (6,7).
Assessment of Other Clinical Covariates
Motor performance was assessed by 10 motor performance tests including: 1 and 2: pinch and grip strength, 3: the Purdue Pegboard, 4: rate of finger tapping, 5–8: duration and number of steps to walk eight feet and for turning 360°, and 9 and 10: duration of standing on one leg and on toes. These scores were scaled and averaged to obtain a previously published summary global motor score (14). Additional details about these performances and the construction of this composite metric are included in Supplementary Methods.
Trained technicians administered 19 cognitive tests as described previously from which a composite measure of global cognition was constructed. The individual tests included: Boston Diagnostic Aphasia Examination, immediate and delayed recall of the East Boston Story and Logical Story Memory A, Word List memory, Word List Recall, Word List Recognition, a 15-item word reading test, Bostin Naming Test, naming category exemplars (animal and fruits-vegetables) in 1-minute, Digit Span Forward and Backward, Digit Ordering, Symbol Digit Modalities Test, Number Comparison, Judgement of Line Orientation, and Standard Progressive Matrices (15,16). These 19 test scores were summarized in a global cognition score, as described previously and summarized in the Supplementary Methods (17).
Cognitive testing was scored by a computer and reviewed by a neuropsychologist to diagnose cognitive impairment. Then participants were evaluated by a physician who used all cognitive and clinical data to classify persons with respect to dementia, as previously described (18).
Depressive symptoms over the week before annual examination were assessed by 10-items version of the Center for Epidemiological Studies Depression (CES-D) scale (19). It has a range from 0 to 10, with higher scores denote more severe symptoms, and a score of 3 has been used as the cutoff score for depression (20). Self-reported questionnaires were used to assess late-life physical, social, and cognitive activities, as described previously and summarized in the Supplementary Methods (21).
Body mass index (BMI) was based on annual measured weight and height. Chronic health conditions included the sum of three self-reported vascular risk factors (hypertension, diabetes mellitus, and smoking) and sum of four self-reported vascular diseases (myocardial infarction, congestive heart failure, stroke, and claudication) (21).
Apolipoprotein E (APOE) gene is the best known genetic risk factor for late onset Alzheimer’s disease (AD). It has three common alleles, ℇ2, ℇ3, and ℇ4. Each individual has two alleles and the most common combination is ℇ3/ℇ3 (62%). Prior work has shown that 24% who are ℇ4 carriers (ℇ4/ℇ4 or ℇ3/ℇ4 or ℇ2/ℇ4) have about a twofold increased risk of AD dementia (22). APOE genotyping was performed at Polymorphic DNA Technologies (Alameda, CA) by investigators blinded to clinical and pathological data (22). We used these genotyping data to categorize individuals based on their APOE ℇ4 carrier status.
Statistical Analyses
Global parkinsonian scores were positively skewed, and these scores were square root transformed for these analyses, as we have done in prior publications (2). We used t-test to compare total daily physical activity between women and men. Pearson and Spearman correlation was used to assess the association between total daily physical activity and other clinical covariates. Including participants without parkinsonism at baseline, we used a discrete-time Cox proportional hazard model, adjusted for age, sex, and education to determine whether total daily physical activity predicts incident parkinsonism. We repeated this model replacing total daily physical activity with intensity of daily activity. Next, we added diverse clinical covariates to this model to identify potential confounders.
To determine whether total daily physical activity at baseline is associated with the rate of change in the severity of parkinsonism during the study, we used a linear mixed-effects model. To assess the association of total daily physical activity across the full spectrum of parkinsonism, we included all individuals both those with and without parkinsonism who had repeated continuous annual measures for the severity of parkinsonism. Model included terms were time in years since baseline (providing an estimate for rate of change in severity of parkinsonism), baseline total daily physical activity, and a second term examining the interaction between time and total daily physical activity as well as six additional terms for age, sex, education, and their interaction with time. We repeated this model replacing total daily physical activity with intensity of daily activity. The analyses were done using SAS version 9.4.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all study participants.
Results
The clinical characteristics of 889 subjects included in this study are summarized in Table 1. Total daily physical activity ranged from 0.2 × 105 to 13.6 × 105 activity counts per day (mean: 2.9 × 105; SD = 1.56 × 105). Total daily physical activity was inversely associated with age (r = −.27, p < .001), but not education (r = −0.03, p = .417), and women had higher levels of physical activity (p = .032). Intensity of daily physical activity ranged from 0.04 × 105 to 0.68 × 105 activity counts/h/d (mean: 0.29 × 105, SD = 0.10 × 105) and was positively correlated with total daily physical activity (r = .93, p < .001).
Table 1.
Clinical Characteristics of Participants at Baseline (n = 889)
| Variable | Mean (SD) or n (%) |
|---|---|
| Age, y | 81.5 (7.39) |
| Female | 678 (76.3) |
| Caucasian | 856 (96.4) |
| Years of education | 14.9 (2.90) |
| Global cognition | 0.1 (0.62) |
| Global motor score (standardized metric) | 1.0 (0.23) |
| Body mass index (kg/m2) | 27.0 (5.22) |
| Self-report physical activity (h/week) | 3.5 (3.67) |
| Self-report cognitive activity (score 1–5) | 3.2 (0.63) |
| Self-report social activity (score 1–5) | 2.6 (0.61) |
| Depressive symptoms (score 0–10) | 1.0 (1.56) |
| APOE ℇ4 | 196 (23.6) |
| Number of 3 vascular risk factors (0–3) | 1.1 (0.81) |
| Hypertension | 500 (56.2) |
| Diabetes mellitus | 127 (14.3) |
| Smoking | 367 (41.5) |
| Number of 4 vascular diseases (0–4) | 0.4 (0.71) |
| Myocardial infarction | 101 (11.4) |
| Congestive heart failure | 43 (5.2) |
| Claudication | 95 (10.7) |
| Stroke | 101 (12.6) |
Notes: Global motor score is a composite measure summarizing 10 motor performance tests with higher scores showing stronger or faster motor performances (like grip strength or walking speed). Cognition is a composite measure summarizing 19 cognitive tests. Participants reported number of minutes of engagement in physical activities in the last 2 weeks (self-report physical activity) and rated frequency of involvement in social (self-report social activity) and cognitive (self-report cognitive activity) activities in the last 1 year (Supplementary Methods). Depressive symptoms were assessed by 10-items version of the Center for Epidemiological Studies Depression (CES-D) scale; higher scores denote more symptoms. Number of three vascular risk factors variable, ie, hypertension, diabetes mellitus, and smoking, has a range from 0 to 3 and higher scores indicate higher vascular risk burden. Likewise, number of four vascular diseases has a range from 0 to 4 and higher scores indicate greater vascular disease burden.
At baseline, diverse clinical covariates were related to both total daily physical activity and parkinsonism. Younger age, higher levels of social and physical activities, better cognition and motor performances, less depressive symptoms, and fewer vascular risk factors and diseases were associated with higher levels of total daily physical activities and less severe parkinsonism (Supplementary Table e-3). BMI was not related to total daily physical activity or the severity of parkinsonism, and education and ate-life cognitive activities were related to the parkinsonism score but not total daily physical activity (Supplementary Table e-3).
Total Daily Physical Activity and Incident Parkinsonism
To examine the association of physical activity with incident parkinsonism, we restricted our analyses to individuals without parkinsonism at baseline (n = 682/889, 77%). During an average of 4 years of follow-up (mean = 4.1, SD = 2.69), 233 of 682 (34%) participants developed parkinsonism. Average baseline level of total daily physical activity was higher in individuals who did not develop parkinsonism during this study (3.2 × 105 [SD = 1.57 × 105]) versus individuals who developed parkinsonism (2.8 × 105 [SD = 1.40 × 105]) (Supplementary Figure e-3).
Using a Cox proportional hazards model which controlled for age, sex, and education, baseline total daily physical activity was associated with incident parkinsonism (Table 2, Model A). One standard deviation total daily physical activity (similar to activity estimated for 18 minutes of jogging at 4.5 miles/h or 66 minutes of walking at 2.5 miles/h) was associated with a decrease in the risk of incident parkinsonism by 20%.
Table 2.
Total Daily Physical Activity and Incident Parkinsonism in Adults Without Parkinsonism at Baseline (n = 682)a
| Model Terms | Model A | Model B | Model C | Model D | Model E | Model F | Model G | Model H |
|---|---|---|---|---|---|---|---|---|
| Total daily physical activity | 0.79 (0.70–0.88), −0.24 (0.06, <0.001) |
0.86 (0.77–0.97), −0.15 (0.06, 0.012) |
0.81 (0.72–0.91), −0.21 (0.06, <0.001) |
0.79 (0.71–0.89), −0.23 (0.06, <0.001) |
0.80 (0.71–0.90), −0.23 (0.06, <0.001) |
0.79 (0.70–0.88), −0.24 (0.06, <0.001) |
0.85 (0.76–0.97), −0.16 (0.06, 0.011) |
0.92 (0.81–1.04), −0.08 (0.06, 0.188) |
| Global motor | 0.03 (0.01–0.08), −3.40 (0.46, <0.001) |
0.05 (0.02–0.15), −2.92 (0.51, <0.001) |
||||||
| Self-report physical activity | 0.95 (0.91–1.0), −0.05 (0.02, 0.038) |
0.95 (0.90–1.0), −0.05 (0.03, 0.059) |
0.98 (0.93–1.03), −0.02 (0.03, 0.443) |
|||||
| Self-report social activity | 0.73 (0.57–0.94), −0.32 (0.13, 0.014) |
0.82 (0.63–1.1), −0.19 (0.14, 0.161) |
0.92 (0.69–1.22), −0.09 (0.14, 0.553) |
|||||
| Self-report cognitive activity | 0.91 (0.72–1.2), −0.09 (0.12, 0.468) |
1.0 (0.81–1.4), 0.04 (0.13, 0.473) |
1.09 (0.83–1.43), 0.09 (0.14, 0.532) |
|||||
| Body mass index | 1.0 (1.0–1.1), 0.02 (0.01, 0.13) |
1.0 (1.0–1.1), 0.03 (0.02, 0.107) |
1.02 (0.99–1.05), 0.02 (0.02, 0.239) |
|||||
| Vascular risk factors | 1.2(1.0–1.5), 0.20 (0.09, 0.026) |
1.1 (0.95–1.4), 0.13 (0.10, 0.16) |
1.13 (0.93–1.37), 0.12 (0.10, 0.211) |
|||||
| Vascular diseases | 1.4 (1.1–1.6), 0.31 (0.10, 0.001) |
1.4 (1.1–1.7), 0.32 (0.10, 0.002) |
1.30 (1.05–1.61), 0.26 (0.11, 0.015) |
|||||
| Global cognition | 0.48 (0.37–0.63), −0.73 (0.14, <0.001) |
0.47 (0.34–0.67), −0.75 (0.17, <0.001) |
0.62 (0.43–0.88), −0.48 (0.18, 0.009) |
|||||
| Depressive symptoms | 1.1 (1.0–1.2), 0.09 (0.05, 0.053) | 1.1 (1.0–1.2), 0.09 (0.05, 0.053) |
1.13 (1.02–1.25), 0.12 (0.05, 0.021) |
|||||
| APOE ℇ4 | 0.68 (0.47–0.99), −0.38 (0.19, 0.047) |
0.78 (0.53–1.1), −0.25 (0.19, 0.193) |
0.76 (0.51–1.13), −0.27 (0.20, 0.174) |
|||||
| Dementia | 2.5(1.4–4.6), 0.91 (0.31, 0.003) | 0.97 (0.45–2.1), −0.03 (0.39, 0.946) |
1.31 (0.59–2.92), 0.27 (0.41, 0.504) |
aEach column shows the results of a single discrete time Cox proportional hazards model which controlled for age, sex, and education (not shown) and the clinical covariate shown in the left column. Each cell shows a hazard ratio (95% confidence interval), and a parameter estimate (standard error, p value) for the association of the covariate listed in the left column with incident parkinsonism.
To contextualize the association of total daily physical activity with parkinsonism, we compared its parameter estimate (Table 2)with age’s (estimate = 0.081, SE = 0.012, p < .001): risk of parkinsonism in participants who were active one standard deviation more than average in total daily physical activity was equivalent to being 3 years younger.
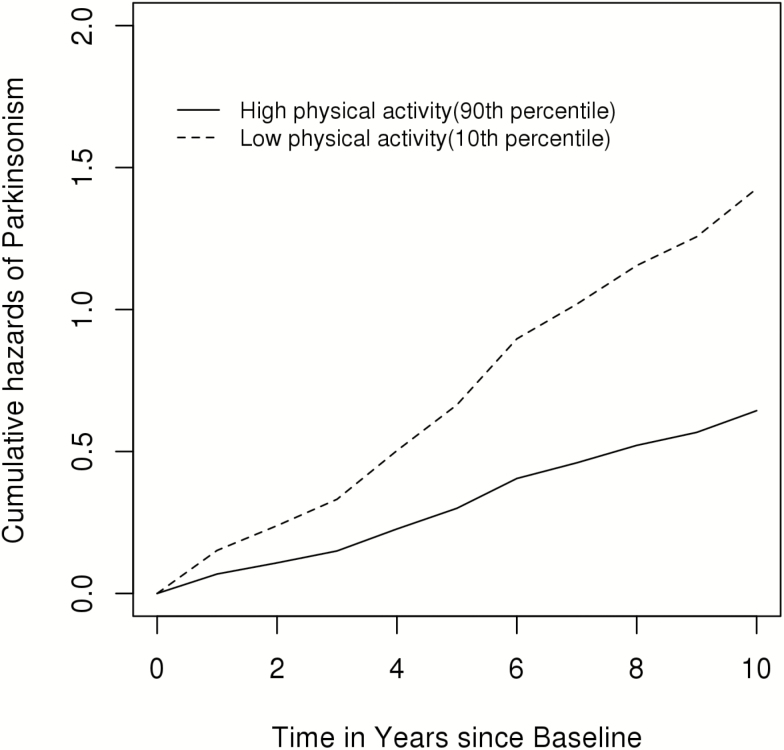
Figure 1 illustrates that a person with a high total daily physical activity (90th percentile; 4.9 × 105 activity count) had more than 50% decreased risk of developing parkinsonism when compared with an individual with low total daily activity (10th percentile; 1.3 × 105 activity count).
Figure 1.
Total daily physical activity and incident parkinsonism in participants without parkinsonism at baseline (n = 682). Cumulative hazard of the risk of parkinsonism in two average participants (women aged 82 with 15 years of education) with low (10th percentile; 1.3 × 105 activity count) vs high levels (90th percentile; 4.9 × 105 activity count) of total daily physical activity.
In a further analysis, we examined if the association of total daily physical activity with parkinsonism differed in men and women. We added a term for the interaction of sex with total daily physical activity to our core model, and the interaction term was not significant (p = .336), meaning that a higher level of total daily physical activity is associated with a lower risk for parkinsonism irrespective of individual’s sex.
Average baseline level of intensity of daily physical activity was higher in individuals who did not develop parkinsonism (0.31 × 105 [SD = 0.10 × 105]) during this study versus individuals who developed parkinsonism (0.28 × 105 [SD = 0.09 × 105]; Supplementary Figure e-4). We repeated the Cox model described above and found one standard deviation intensity of daily physical activity was associated with a decrease in the risk of incident parkinsonism by 35%.
Since motor performance vary and may affect the quantity of total daily physical activity and attenuate the association of total daily physical activity with parkinsonism, we controlled for global motor score which summarized 10 motor performances and was modestly associated with total daily physical activity (r = .33, p < .001) (23). Adding a term for global motor score together with total daily physical activity and demographic variables, the association of total daily physical activity with parkinsonism was attenuated by nearly 40% (Table 2, Model B).
Higher levels of diverse late-life activities may be associated with slower motor decline using other motor phenotype (21). Adding terms for self-reported physical, social, and cognitive activities to the core model (Table 2, Model A) did not attenuate the association of total daily physical activity with incident parkinsonism (Table 2, Model C).
Chronic health conditions might confound the association of total daily physical activity and incident parkinsonism (2). The association of total daily physical activity was not attenuated in models controlling for BMI, the sum of vascular risk factors and sum of vascular diseases (Table 2, Model D).
Cognition and depression are related to incident parkinsonism and APOE ℇ4 genotype is related to late-life motor and cognitive impairments in older adults (6,24,25). Adding terms for global cognition, depressive symptoms, and APOE ℇ4 status to the core model did not attenuate the association of total daily physical activity with parkinsonism (Table 2, Model E).
While individuals with dementia had a higher risk of developing parkinsonism, cognitive status did not attenuate the association between total daily physical activity and parkinsonism (Table 2, Model F). In a further model which included an interaction term, the association of total daily physical activity and incident parkinsonism did not vary in older adults with or without dementia (total daily physical activity × dementia: Est 0.430, SE = 0.391, p = .272).
In a single model which included all the nonmotor covariates together, the association of total daily physical activity was attenuated by 35% but remained associated with incident parkinsonism (Table 2, Model G).
In a final model, we added a term for global motor score together with all the other clinical covariates and the association of total daily physical activity was attenuated by about 60% and was no longer associated with incident parkinsonism (Table 2, Model H). Global motor score alone (Model B) and together with all the other covariates (Model H) most strongly attenuates the association between total daily physical activity and incident parkinsonism. These results suggest that motor performance may link the association of total daily physical activity with incident parkinsonism as observed in a previous study (2).
Total Daily Physical Activity and Progression of Parkinsonism
To ensure that our results are not due to the cutoff employed for categorizing parkinsonism, we examined the association of total daily physical activity with the annual rate of change in the severity of parkinsonism using a previously validated continuous measure available in 889 participants (Supplementary Methods). Global parkinsonian scores ranged from 0 to 33.0, with an average of 7.1 (SD = 6.55), with a higher level indicating more severe parkinsonism.
We employed a linear mixed-effects model to examine association of total daily physical activity with the annual rate of change in the global parkinsonian score controlling for age, sex, and education and their interaction with time. During about 6 years of follow-up (mean = 5.8; SD = 2.96), on average, the severity of parkinsonism increased in the average participant by 0.4 (6% of the baseline level of parkinsonism)/year (Table 3, Model A). A higher level of total daily physical activity was associated with less severe parkinsonism at baseline (Table 3, Model A) and was also associated with a slower rate of progressive parkinsonism during the study (Table 3, Model A). Similar findings were observed when repeated these models using intensity of daily physical activity instead of the total quantity of total daily physical activity (Table 3, Model B).
Table 3.
Physical Activity and the Annual Rate of Change in Parkinsonisma
| Adults With and Without Parkinsonism at Baseline (n = 889) | Adults Without Parkinsonism at Baseline (n = 682) | |||
|---|---|---|---|---|
| Model Terms | Model A | Model B | Model C | Model D |
| Annual rate of change in parkinsonism (Time) | 0.048 (0.008, < 0.001) | 0.101 (0.024, <0.001) | 0.067 (0.009, <0.001) | 0.154 (0.027, <0.001) |
| Age | 0.073 (0.005, < 0.001) | 0.066 (0.005, <0.001) | 0.068 (0.005, <0.001) | 0.065 (0.005, <0.001) |
| Sex | −0.145 (0.087, 0.094) | −0.172 (0.085, 0.044) | −0.251 (0.080, 0.002) | −0.264 (0.080, 0.001) |
| Education | −0.050 (0.013, < 0.001) | −0.052 (0.013, <0.001) | −0.040 (0.012, <0.001) | −0.041 (0.012, <0.001) |
| Total daily physical activity | −0.115 (0.024, < 0.001) | −0.024 (0.023, 0.297) | ||
| Intensity of daily physical activity | −2.829 (0.381, <0.001) | −0.995 (0.364, 0.006) | ||
| Age × Time | 0.004 (0.001, < 0.001) | 0.004 (0.001, <0.001) | 0.006 (0.001, <0.001) | 0.006 (0.001, <0.001) |
| Sex × Time | 0.014 (0.016, 0.383) | 0.014 (0.016, 0.406) | 0.021 (0.018, 0.245) | 0.021 (0.018, 0.253) |
| Education × Time | 0.001 (0.002, 0.732) | 0.001 (0.002, 0.747) | −0.001 (0.003, 0.630) | −0.001 (0.003, 0.611) |
| Total daily physical activity × Time | −0.010 (0.005, 0.036) | −0.017 (0.005, <0.001) | ||
| Intensity of daily physical activity × Time | −0.180 (0.074, 0.015) | −0.295 (0.081, <0.001) | ||
aEach column shows a single linear mixed-effect model. In each model, the outcome is the global parkinsonian score; the terms included in each model are listed in the first column on the left. Each cell shows the estimates (standard error and p value) for either the cross-sectional association of the model terms’ with the level of parkinsonism at baseline or the association of the term with the annual rate of change in parkinsonism (Time).
In further analyses, we added an additional interaction term and found that the association of total daily physical activity or intensity of daily physical activity with the progression of parkinsonism was similar in men and women (data not shown).
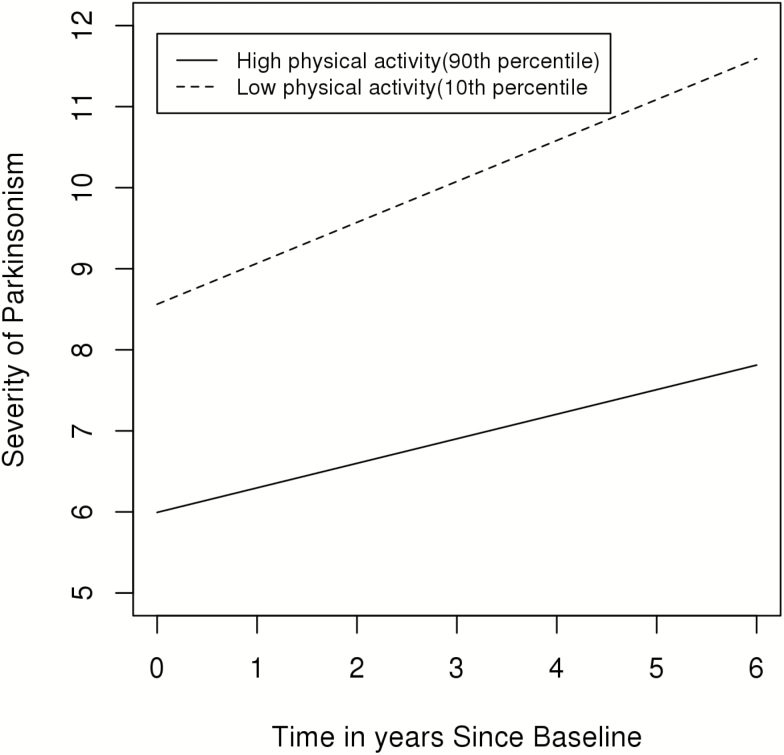
Figure 2 illustrates the trajectories of the progression of parkinsonism during the study for two similar average participants (82 years old females with 15 years of education) one had a high level (90th percentile; 4.7 × 105 activity count) versus the second who had a low level (10th percentile; 1.0 × 105 activity count) of total daily physical activity. The rate of progression was 40% slower in the individual with a higher level of baseline total daily physical activity.
Figure 2.
Total daily physical activity and the progression of parkinsonism (n = 889). A model-based figure contrasting the trajectory of the annual rate of change in parkinsonism for two average participants (women 82 years old with 15 years of education) with low (10th percentile; 1.0 × 105 activity count) and high (90th percentile; 4.7 × 105 activity count) levels of total daily physical activity.
Next, we repeated the linear model described above and added the same covariates described above for the Cox models to determine which covariates attenuated the association of total daily physical activity with progressive parkinsonism. The association of higher levels of total daily physical activity with slower progressive parkinsonism was unchanged when adding terms for self-reported late-life activities (physical, social, and cognitive), chronic health conditions (BMI, vascular risk factors, and diseases), depressive symptoms, APOE ℇ4 and dementia status (Table 4, Models C–G). Addition of the global motor score to the core mixed model attenuated the association between higher levels of total daily physical activity with slower parkinsonism progression (Table 4, Models B and H). The effects of these covariates on the linear mixed-effect models were similar to the results obtained above for the Cox models. In contrast, global cognition attenuated the association in the mixed models (Table 4, Models E and H), but not in the Cox models (Table 2, Models E and H). So, these results suggest that motor performance and probably cognition may link the association of total daily physical activity with the development and progression of parkinsonism, as observed in a previous study (2).
Table 4.
The Association of Physical Activity, Other Clinical Covariates and the Annual Rate of Change in Parkinsonism (n = 889)a
| Term | Model A | Model B | Model C | Model D | Model E | Model F | Model G | Model H |
|---|---|---|---|---|---|---|---|---|
| Total daily physical activity × Time | −0.010 (0.005, 0.036) |
−0.007 (0.005, 0.124) |
−0.102 (0.005, 0.030) |
−0.012 (0.005, 0.016) |
−0.006 (0.005, 0.202) |
−0.010 (0.005, 0.029) |
−0.007 (0.005, 0.154) |
−0.004 (0.005, 0.387) |
| Global motor × Time | −0.055 (0.036, 0.119) |
−0.047 (0.040, 0.235) |
||||||
| Self-report physical activity × Time | 0.001 (0.002, 0.768) |
−0.000 (0.002, 0.895) |
−0.000 (0.002, 0.978) |
|||||
| Self-report social activity × Time | −0.006 (0.012, 0.641) |
−0.005 (0.012, 0.708) |
−0.001 (0.013, 0.966) |
|||||
| Self-report cognitive activity × Time | −0.025 (0.012, 0.035) |
−0.204 (0.013, 0.103) |
−0.022 (0.013, 0.090) |
|||||
| Body mass index × Time | −0.002 (0.001, 0.270) |
−0.002 (0.001, 0.255) |
−0.001 (0.002, 0.329) |
|||||
| Vascular risk factors × Time | 0.001 (0.001, 0.895) |
0.002 (0.009, 0.793) |
0.003 (0.009, 0.740) |
|||||
| Vascular diseases × Time | −0.001 (0.010, 0.602) |
−0.004 (0.010, 0.676) |
−0.004 (0.005, 0.399) |
|||||
| Global cognition × Time | −0.041 (0.014, 0.002) |
−0.047 (0.016, 0.004) |
−0.039 (0.017, 0.022) |
|||||
| Depressive symptoms × Time | 0.002 (0.004, 0.708) |
0.003 (0.005, 0.508) |
0.004 (0.005, 0.399) |
|||||
| Any E4 × Time | 0.000 (0.016, 0.988) |
0.010 (0.016, 0.551) |
0.013 (0.017, 0.436) |
|||||
| Dementia × Time | −0.003 (0.037, 0.938) |
−0.063 (0.045, 0.160) |
−0.063 (0.045, 0.166) |
aEach column shows the results of a single mixed-effect model which examines the association of total daily physical activity with the annual rate of change in parkinsonism alone (Model A) or when terms for other clinical covariates are also included (Models B–H). The cells in each column shows the estimate (standard error and p value) and shows only the model terms which highlight the association of total daily physical activity with the annual rate of change in parkinsonism alone and when terms for other clinical covariates listed on the left are included in the model. Each model (A–H) included an additional eight terms which are not shown including a term for Time (the annual rate of change in parkinsonism) and six terms which controlled for age, sex, education, and their interaction with time and the cross-sectional association of the clinical covariate shown in the left column with baseline parkinsonism (not shown).
In a sensitivity analysis, we repeated the previous model (Table 3, Model C) excluding individuals with parkinsonism at baseline (N = 207), and including only individuals without parkinsonism (N = 682). Our results showed that the average participant showed progression of parkinsonism by about 3% of the baseline level of parkinsonism/y (Table 3, Model C), and higher levels of total daily physical activity (Table 3, Model C) or its intensity (Table 3, Model D) was associated with slower rate of parkinsonism progression.
While no participants had a clinical diagnosis of PD or were receiving neuroleptic medications at baseline, 55 were diagnosed with PD or received neuroleptic medications during an average of 6 years of follow-up. In a further sensitivity analysis we excluded these 55 participants and repeated our primary models (Table 2, Model A; Table 3, Model A). A higher level of total daily physical activity remained associated with a lower risk for the parkinsonism and associated with a slower rate of parkinsonism progression (data not shown).
Discussion
In a study of nearly 900 community-dwelling older adults without parkinsonism, a more active lifestyle, based on metrics derived from continuous recordings of physical activity with an activity monitor, was associated with a reduced risk of developing parkinsonism and a slower rate of its annual progression. An active lifestyle remained associated with incident parkinsonism when controlling for individual differences in diverse health covariates. These results lend support for public health efforts to promote a more active lifestyle to prevent or slow the progression of late-life motor impairment in older adults. Yet, further work is needed to determine the mechanisms underlying the potential motor benefits of a more active lifestyle.
World population is aging. In 2015, 8.5% of the world population were older than 65 and by 2050, that figure is estimated to rise to 16.7% for the world and to more than 20% for the United States (26). Parkinsonism is common among elderly people, up to 50% or more of adults older than 85 years old (1,27). Furthermore, parkinsonism is associated with a more rapid rate of cognitive decline (28), increased risk of dementia (29), disability (1), and death (1,30). Since the cause of parkinsonism in many older adults is unclear, treatments are lacking for most cases. In the absence of specific therapies, identifying modifiable risk factors that may decrease the burden of parkinsonism in our aging population is therefore a health priority.
Prior studies in this cohort have suggested that self-reported late-life physical, cognitive, and social activity are related to incident parkinsonism and other motor constructs, but these associations were attenuated when other clinical covariates were included together in the same models (2). To circumvent the limitations of self-reported instruments, the current study employed activity monitors to obtain continuous recordings of all physical activity for up to ten days in the community setting. In contrast to our prior study, the current analyses found that an objective metric for total daily physical activity was independently associated with incident parkinsonism when controlling for other diverse clinical covariates.
Parkinsonism is one of several motor constructs employed by investigators to assess late-life motor impairment. Parkinsonism focuses on specific motor signs including impairment in gait, balance, rigidity, bradykinesia, and tremor whereas other constructs like sarcopenia focus on muscle strength and bulk (31). In fact, motor impairment in older adults affects diverse motor performances, including gait, balance and dexterity, muscle mass, and strength (32,33). Since movement is a volitional activity an individual with good motor performance may decide not to move and someone with poor performance may move a lot, which is why metrics of motor performance and the quantity of daily movement are only modestly related. So, the assessment of the quantity of total activity during daily living can only be obtained by testing in the community setting. If our conceptualization is correct, the current findings suggest that a more active lifestyle may decrease the progression of decline in other motor constructs such as sarcopenia or physical frailty (34,35).
The mechanism by which a more active lifestyle is linked with reduced parkinsonism in older adults is unclear. Parkinsonism in older adults is a heterogeneous group of disorders with diverse etiologies such as medications and age-related brain pathologies, including AD, PD, Lewy bodies (LBs), and cerebrovascular pathologies (36). White matter hyperintensity (WMH) burden observed in brain MRI imaging has been reported to be higher in people with mild parkinsonian signs than in people without (37). It is possible that physical activity is associated with a lower risk for parkinsonism through its effect on improving motor performances and/or cognition (2). This is supported in part by the fact that in models which examined other health covariates alone, the attenuation of the association of total daily physical activity and parkinsonism was greatest when we added a term for motor performance or global cognition alone (Table 2; Table 4, Model B) and total daily physical activity was no longer associated with incident parkinsonism when we added terms for motor performance and cognition to all the other covariates (Table 2, Model H). The interrelationship between the total daily physical activity and motor performance is complex and may include bidirectional causal and recursive pathways. Thus, it possible for the lower levels of physical activity to be due to lower levels of motor performance with the latter also driving the progression of parkinsonism. Careful intervention studies in human and studies in model organisms will be needed to elucidate these complex relationships. Another possibility is that higher levels of physical activity may modify the adverse effects of brain pathologies, reducing the severity of parkinsonism. Prior studies with other motor constructs suggest that higher levels of physical activity may provide motor reserve and modify the untoward effects of WMH and cerebrovascular pathologies (23). However, recent work in this cohort with several other risk factors suggest that some modifiable risk factors are unrelated to AD and related pathologies and affect clinical phenotypes altogether via as yet unidentified molecular mechanisms which do not have known pathological footprints or which have a more direct association with structural brain indices that promote better function (38). Indeed, studies have shown aged rats to have abnormal activation of oxidative and inflammatory processes, like renin–angiotensin system and RhoA/Rho Kinase pathway, making them susceptible for neurodegeneration (39,40). And, exercise like treadmill running could decrease markers of these neuroinflammatory and oxidative processes (39,40). Currently, studies are lacking which have examined the inter-relationship between total daily physical activity, brain pathologies and parkinsonism and are vital to fill some of these knowledge gaps about the potential mechanisms underlying the benefits of a more active lifestyle on parkinsonism in older adults.
The study has several strengths. We examined a large number of community-dwelling older adults who may be more representative of the general population than patients referred to neurological clinics, as in other studies. The follow-up rate exceeds 90%, and a uniform clinical procedure including a modified UPDRS and objective metrics of motor performance and total daily physical activity and a wide range of diverse clinical covariates were employed.
There are also a number of limitations. The cohort is selected, three quarters of the sample was comprised of women and 96% were Caucasian, underscoring the need to replicate our findings in a more general population. While we excluded cases with a history of PD who had received l-dopa, participants were not examined by a movement disorder specialist so it is possible that some may have had early PD. The relationship between the quantity of total daily physical activity, motor performance, and parkinsonism is complex and is likely to include both causal and recursive pathways which cannot be determined using observational data alone and will require intervention studies and studies in model organisms. And, although the qualitative clinical assessment of parkinsonian signs employed in this study is a robust predictor of adverse health outcomes, these signs lack specificity as many different etiologies may contribute to their development.
Funding
This work was supported by National Institute of Health (NIH; R01AG043379, R01AG47679, R01AG017917, R01 AG040039, R01 AG022018, R01NS078009, P30AG10161, P20 MD0068860, and R01AG15819). Dr. L.Y. is funded by NIH grants R01AG053446, RF1AG015819, R01AG017917, RF1AG036042, U01AG046152, R01AG054058, R01AG034374, R01AG033678, R01NS78009, R01AG47679, ROAG35472, R01DK099269, R01AG052488, R01AG050631, and RF1AG048056. Dr. D.A.B. receives funding from the National Institutes of Health. He serves on the Adjudication Committee for AD4833/TOMM40-301 Study by Takeda Pharmaceuticals, and the Data Monitoring Committee for ABBV-8E12 Study by AbbVie. Dr. A.S.B. receives support from NIH (R01AG043379, R01NS078009, R01AG56352, R01AG47976, R01AG017917, P30AG10161, P20 MD0068860, R01 AG040039, and R01 AG022018).
Authors’ Contributions
Dr. S.O.: statistical analyses (design, review, and critique), manuscript (writing the first draft, review, and critique); Dr. L.Y.: statistical analyses (design, execution, review, and critique), manuscript (review and critique); Dr. R.J.D.: manuscript (review and critique); Dr. D.A.B.: research project (conception, organization, and execution), statistical analyses (review and critique), manuscript (review and critique); Dr. A.S.B.: research project (conception, organization, and execution), statistical analyses (design, review, and critique), manuscript (writing the first draft, review, and critique).
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in older adults and its association with adverse health outcomes and neuropathology. J Gerontol A Biol Sci Med Sci. 2016;71:549–556. doi: 10.1093/gerona/glv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchman AS, Leurgans SE, Yu L, et al. . Incident parkinsonism in older adults without Parkinson disease. Neurology. 2016;87:1036–1044. doi: 10.1212/WNL.0000000000003059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Physical activity for everyone.https://health.gov/paguidelines/guidelines/. Accessed July 18, 2017.
- 4. Santos D, Mahoney JR, Allali G, Verghese J. Physical activity in older adults with mild parkinsonian signs: a cohort study. J Gerontol A Biol Sci Med Sci. 2017;73(12):1682–1687. doi: 10.1093/gerona/glx133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6 [DOI] [PubMed] [Google Scholar]
- 6. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. doi: 10.1212/WNL.0b013e3182535d35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dawe RJ, Leurgans SE, Yang J, et al. . Association between quantitative gait and balance measures and total daily physical activity in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2018;73:636–642. doi: 10.1093/gerona/glx167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses’ ratings of parkinsonian signs with a modified unified Parkinson’s Disease Rating Scale. Neurology. 1997;49:1580–1587. doi:10.1212/wnl.49.6.1580 [DOI] [PubMed] [Google Scholar]
- 9. Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1999;54:M191–M196. doi:10.1093/gerona/54.4.m191 [DOI] [PubMed] [Google Scholar]
- 10. Buchman AS, Yu L, Wilson RS, Shulman JM, Boyle PA, Bennett DA. Harm avoidance is associated with progression of parkinsonism in community-dwelling older adults: a prospective cohort study. BMC Geriatr. 2014;14:54. doi: 10.1186/1471-2318-14-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77:64–80. doi: 10.1080/02701367.2006.10599333 [DOI] [PubMed] [Google Scholar]
- 12. Buchman AS, Wilson RS, Yu L, James BD, Boyle PA, Bennett DA. Total daily activity declines more rapidly with increasing age in older adults. Arch Gerontol Geriatr. 2014;58:74–79. doi: 10.1016/j.archger.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chastin SFM, De Craemer M, De Cocker K, et al. . How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med. 2019;53:370–376. doi: 10.1136/bjsports-2017-097563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med. 2011;9:42. doi: 10.1186/1741-7015-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J Gerontol A Biol Sci Med Sci. 2014;69:1536–1544. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson RS, Capuano AW, Bennett DA, Schneider JA, Boyle PA. Temporal course of neurodegenerative effects on cognition in old age. Neuropsychology 2016;30(5):591–599. doi: 10.1037/neu0000282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson RS, Beckett LA, Barnes LL, et al. . Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. doi:10.1037/0882-7974.17.2.179 [PubMed] [Google Scholar]
- 18. Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161–S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- 20. Wilson RS, Boyle PA, Capuano AW, et al. . Late-life depression is not associated with dementia-related pathology. Neuropsychology. 2016;30(2):135–142. doi: 10.1037/neu0000223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch Intern Med. 2009;169:1139–1146. doi: 10.1001/archinternmed.2009.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oveisgharan S, Buchman AS, Yu L, et al. . APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90:e2127–e2134. doi: 10.1212/WNL.0000000000005677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleischman DA, Yang J, Arfanakis K, et al. . Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology. 2015;84:1294–1300. doi: 10.1212/WNL.0000000000001417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23:63–69. doi:10.1097/wad.obo13e31818877b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilson RS, Nag S, Boyle PA, et al. . Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry. 2013;70:1320–1328. doi: 10.1001/jamapsychiatry.2013.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan H, Goodkind D, Kowal P.. U.S. Census Bureau, International Population Reports, P95/16-1, An Aging World: 2015. Washington, DC: U.S. Government Publishing Office; 2016. [Google Scholar]
- 27. Louis ED, Luchsinger JA, Tang MX, Mayeux R. Parkinsonian signs in older people: prevalence and associations with smoking and coffee. Neurology. 2003;61:24–28. doi:10.1212/01.wnl.0000072330.07328.d6 [DOI] [PubMed] [Google Scholar]
- 28. Lerche S, Brockmann K, Pilotto A, et al. . Prospective longitudinal course of cognition in older subjects with mild parkinsonian signs. Alzheimers Res Ther. 2016;8:42. doi: 10.1186/s13195-016-0209-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Louis ED, Tang MX, Schupf N. Mild parkinsonian signs are associated with increased risk of dementia in a prospective, population-based study of elders. Mov Disord. 2010;25:172–178. doi: 10.1002/mds.22943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bennett DA, Beckett LA, Murray AM, et al. . Prevalence of parkinsonian signs and associated mortality in a community population of older people. NEJM. 1996;334:71–76. doi:10.1056/nejm199601113340202 [DOI] [PubMed] [Google Scholar]
- 31. Rosenberg IH. Epidemiologic and methodologic problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19–21, 1988. Am J Clin Nutr. 1989;50:1121–1235. [PubMed] [Google Scholar]
- 32. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Louis ED, Bennett DA. Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord. 2007;22:1681–1688. doi:10.1002/mds.21433 [DOI] [PubMed] [Google Scholar]
- 34. Mijnarends DM, Koster A, Schols JM, et al. . Physical activity and incidence of sarcopenia: the population-based AGES-Reykjavik study. Age Ageing. 2016;45:614–620. doi: 10.1093/ageing/afw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing. PLoS One. 2017;12:e0170878. doi: 10.1371/journal.pone.0170878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buchman AS, Shulman JM, Nag S, et al. . Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–266. doi: 10.1002/ana.22588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louis ED, Brickman AM, DeCarli C, et al. . Quantitative brain measurements in community-dwelling elderly persons with mild parkinsonian signs. Arch Neurol. 2008;65:1649–1654. doi: 10.1001/archneurol.2008.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Negash S, Wilson RS, Leurgans SE, et al. . Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr Alzheimer Res. 2013;10:844–851. doi:10.2174/15672050113109990157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Munoz A, Correa CL, Lopez-Lopez A, Costa-Besada MA, Diaz-Ruiz C, Labandeira-Garcia JL. Physical exercise improves aging-related changes in angiotensin, IGF-1, SIRT 1, SIRT3 and VEGF in the substantia nigra. J Gerontol A Biol Sci Med Sci. 2018;73(12):1594–1601. doi: 10.1093/gerona/gly072 [DOI] [PubMed] [Google Scholar]
- 40. Muñoz A, Corrêa CL, Villar-Cheda B, Costa-Besada MA, Labandeira-Garcia JL. Aging-related increase in Rho kinase activity in the Nigral region is counteracted by physical exercise. J Gerontol A Biol Sci Med Sci. 2016;71:1254–1257. doi: 10.1093/gerona/glv179 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.