Abstract
Background
Older adults with visual impairments are at increased risk of negative health outcomes. Here, we investigate the association between visual impairment and frailty.
Methods
Cross-sectional and longitudinal relationships between visual impairment (distance visual acuity) and frailty (frailty phenotype criteria) were examined using data from the National Health and Nutrition Examination Survey (NHANES, 1999–2002, ≥60 years) and the Women’s Health and Aging Studies (WHAS III). Imbalance of potential confounders, particularly age, was addressed using propensity score-based adjustment. Multinomial logistic regression determined the odds of prefrailty and frailty at baseline in NHANES and ordinal logistic regression examined the odds of baseline and incident frailty over 3 years in WHAS III after adjustment for confounders and probability weighting (survey weights × inverse propensity scores).
Results
In NHANES (n = 2,639, 9% vision impairment), participants with visual impairment were more likely to be prefrail (odds ratio [OR] = 3.2; 95% confidence interval [CI]: 1.9–5.3) and frail (OR = 3.7; 95% CI: 1.5–9.2) than those without visual impairment. In WHAS III (n = 796, 26% mild, 37% moderate/severe vision impairment), participants with mild and moderate/severe vision impairment were more likely to be frail (OR = 2.0; 95% CI: 1.5–2.5; OR = 5.5; 95% CI: 4.2–7.2, respectively). A one-line worse visual acuity (0.1 logMAR increase) was associated with greater odds of frailty (OR = 1.5; 95% CI: 1.4–1.7). Of those non-frail at baseline (n = 549), moderate/severe visual impairment and one-line worse visual acuity was associated with greater odds of incident frailty (OR = 3.5; 95% CI: 1.4–8.4; OR = 1.3; 95% CI: 1.1–1.5, respectively) over 3 years.
Conclusions
Visual impairment may be an important, yet understudied risk factor for frailty.
Keywords: Visual impairment, Frailty, Sensory, Risk factors
Visual impairment (VI) is common among older adults and negatively affects many aspects of daily functioning; however, the impact of VI goes beyond day-to-day vision-related tasks (1). VI affects multiple domains of functioning and health, and older adults with vision loss have worse physical and cognitive functioning (2–4). Vision loss is associated with greater risk of disability (5), comorbidity (6), and mortality (7). These data suggest that older adults with VI are more vulnerable to negative health outcomes than those without VI.
Frailty is a geriatric syndrome defined as an increased vulnerability to adverse outcomes following stressors (8,9) and is hypothesized to result from multisystem dysregulation and decreased physical reserve. Identifying frail older adults is important, as frail individuals are at increased risk for adverse health outcomes, including falls, disability, hospitalization, and mortality (8,10–12).
Few studies have examined the association between VI and physiological frailty. The Beaver Dam Eye Study found that worse visual acuity and contrast sensitivity were associated with worse frailty index scores (13). Self-reported VI was also associated with increased risk of incident prefrailty and frailty, using the frailty phenotype, over a 4-year period (14). Most recently, data from the Progetto Veneto Anziani observation study found that low vision, among other factors, were associated with increased risk of worsening frailty (15). These studies have begun to establish a relationship between VI and frailty but are limited by including only cross-sectional analyses or using subjective assessments of vision, shown to be a poor indicator of objective visual functioning (16).
This study builds on prior studies and tests the hypotheses that older adults with VIs are more likely to be frail and are at increased risk of becoming frail than those without VI. We analyzed data from two cohort studies, the National Health and Nutrition Examination Survey (NHANES), and the Women’s Health and Aging Study I and II merged cohort (WHAS III). This work is novel by adding to our the understanding of the vision–frailty relationship by (i) assessing vision using objective and clinically relevant measures; (ii) using the frailty phenotype, an assessment of frailty grounded in theory; (iii) controlling for potential confounders using rigorous methods—propensity score adjustment; (iv) assessing both cross-sectional and longitudinal relationships; and (v) enhancing scientific rigor by analyzing data from two cohort studies of older adults.
Methods
Study Populations
NHANES
Data used were from the 1999–2002 cycles of the NHANES (17). These years were chosen based on availability of data on vision and the five frailty criteria (12). Our study sample was restricted to adults aged at least 60 years eligible to have walking speed data collected (a frailty criterion; n = 2,781), of which 5.1% (n = 142) were missing either frailty data or vision data, resulting in an analytic sample of 2,639. This study was approved by the NCHS Research Ethics Review Board.
WHAS III
WHAS I and II are longitudinal cohort studies of community-dwelling older women randomly sampled from the Medicare beneficiaries in Baltimore City and County. WHAS I included women aged at least 65 years screened to represent the one-third most disabled of the population (18,19). WHAS II included women aged 70–79 years screened to represent the two-thirds least disabled of the population (18–20). This study was approved by the Johns Hopkins IRB and each participant gave informed, written consent.
These analyses combined the WHAS I subset aged 70–79 years at baseline (n = 399) with the WHAS II cohort using weights based on the probability of selection into the cohort to create the WHAS III sample representing community-dwelling women across the full range of physical functioning (21–23). For WHAS I, follow-up examinations occurred every 6 months for 3 years (1992–1995); and for WHAS II, every 18 months for 9 years (1994–2003). Participants with missing data at baseline on frailty (n = 8) and vision (n = 27) and 6 missing sampling weights were excluded from cross-sectional analyses, resulting in a analytic baseline sample of 796 (WHAS I, n = 384; WHAS II, n = 412). Longitudinal analyses were further restricted to participants who were not frail at baseline (n = 549).
Vision
NHANES
Presenting visual acuity was assessed monocularly wearing habitual corrective lenses, if needed, using a chart in an auto-refractor (NIDEK ARK-760; Nidek Co Ltd., Tokyo, Japan) (24). If presenting visual acuity was less than 20/25, visual acuity was re-assessed after auto-refraction. Better-eye visual acuity was used in all analyses (25). Results were similar in analyses using worse-eye visual acuity. The visual acuity data are collected as discrete Snellen values, limiting the ability to examine visual acuity continuously. Presenting visual acuity was categorized as follows: (i) no VI (≥20/40) and (ii) VI (<20/40). Secondary analyses used better-eye best-corrected visual acuity and defined VI as less than 20/40 after refraction, which therefore categorizes individuals as having VI based on visual acuity impairments that cannot be corrected with spectacles (26).
WHAS III
WHAS I assessed presenting binocular visual acuity using a Goodlite Portable Eye Chart, wearing habitual correction if needed (18). WHAS II measured binocular presenting visual acuity at baseline using the Early Treatment Diabetic Retinopathy Study charts while participants wore habitual corrective lenses (27). Presenting visual acuity assessments from both cohorts were converted to logMAR units.
In WHAS III, there was a large number of participants with visual acuity equal to 20/40 (n = 502) and therefore using this cut point would have classified 58% as VI. However, in NHANES 87% of VI participants have visual acuity less than 20/40 but at least 20/60, precluding the use of similar classifications of VI in both studies. To enhance the comparability between studies, we defined VI in WHAS III as follows: (i) no VI (≥20/40), (ii) mild VI (<20/40 to ≥20/60), and (iii) moderate/severe VI (<20/60).
Frailty
We used the physical frailty phenotype, previously validated in older adults, where frailty is defined as meeting at least 3 criteria and prefrailty meeting 1 or 2 criteria (8,12).
NHANES
A modified version of the frailty phenotype was used (11,12).
Weakness: Reporting “some difficulty,” “much difficulty,” or “unable to do” when asked about difficulty lifting or carrying something as heavy as 10 pounds.
Poor endurance: Reporting “some difficulty” or “much difficulty” when asked about difficulty walking from one room to another.
Slowness: Slowest 20% based on time to complete 20 ft walk, adjusted for sex and standing height.
Low physical activity: Reporting “less active” when asked “compared with most men/women your age, would you say that you are more active, less active, or about the same.”
Shrinking: Unintentional weight loss of at least 10 lb or at least 5% over 1 year based on current weight, self-report of weight 1 year prior, and report if weight loss was intentional, or a body mass index (BMI) less than 18.5 kg/m2.
WHAS III
Similar data were collected in all visits of WHAS I and II, allowing for longitudinal analyses (21):
Weakness: Grip strength, assessed in the dominant hand using a dynamometer, with “weak” defined as the lowest 20% of the baseline population adjusted for BMI.
Poor endurance: Report of any of the following over the past month: low energy, felt unusually tired, or felt unusually weak.
Slowness: Slowest 20% of the baseline population on a 4-m walk, adjusted for height.
Low physical activity: Reduced energy expenditure based on the modified Minnesota Leisure Time Physical Activity Scale, which ascertains self-reported physical activity.
Shrinking: Unintentional weight loss defined as at least 10% unintentional weight loss since age 60 or BMI less than 18.5 kg/m2.
Other Covariates
In NHANES, age, sex, race, and years of education were assessed by self-report. BMI was calculated as body weight (kg)/(height [m])2. Smoking status was categorized (never or current/former) based on smoking at least 100 cigarettes. Diabetes was defined based on self-report of a physician diagnosis, or currently taking insulin or oral medication to lower blood sugar. Other comorbidities were assessed using self-report of a physician diagnosis of angina, arthritis, cancer, congestive heart failure, coronary heart disease, hypertension, liver disease, myocardial infarct, and stroke.
WHAS I and WHAS II collected data on age, race (black, white, or other), years of education, marital status (married, single, divorced, or widowed), current smoking status (yes or no), and physician diagnosis of diabetes by self-report at the baseline visit. BMI was calculated similar to methods used in NHANES. The total number of comorbid conditions was based on self-report at baseline for myocardial infarct, angina, congestive heart failure, other heart disease, hypertension, rheumatoid arthritis, stroke, cancer, hip fracture, Parkinson’s disease, lung disease, and hearing problems. Mini-Mental Status Examination and the 30-item Geriatric Depression Scales were conducted, with a cut-point of at least 14 indicating depressive symptoms (28).
Statistical Analysis
Chi-squared and t-tests were used to compare characteristics by VI status. The number and percentage of non-frail, prefrail, and frail individuals were compared by VI group within each cohort (as described earlier).
To address the imbalance of potential confounders, particularly age, between VI groups, propensity scores were estimated as the predicted probability of a participant being visually impaired using a logistic regression model that included variables hypothesized to be associated with frailty (29). In NHANES, the propensity model included age (cubic spline), sex, race, BMI, education, smoking status, diabetic status, marital status, and total number of comorbid conditions. In WHAS III, the propensity model included age (cubic spline), race, education, BMI, marriage status, smoking status, diabetic status, total number of comorbidities, Mini-Mental Status Examination scores, and depression. By comparing the distribution of the propensity scores across observed vision impaired groups, analyses were restricted to participants with overlapping propensity score ranges across groups (sample sizes shown in tables). All regression modeling to develop the propensity scores were weighted using study-specific survey weights (29).
Multinomial logistic regression was used to determine the relative odds of prefrailty/frailty at baseline in NHANES. In WHAS III, ordinal logistic regression analyses were used—where “prefrail/frail vs non-frail” and “frail vs prefrail/non-frail” are pooled, so that the inference of the resulting single odds ratio is for “greater vs lesser frailty status”—as there are few frail individuals without VI in this data set. In WHAS III, we estimated the relative odds of baseline frailty and incident frailty at year 3. NHANES models were adjusted for age (cubic spline), sex, race, smoking status, diabetic status, and total number of comorbid conditions. Taylor linearization was used for variance estimation (21). For WHAS III, models were adjusted for age (cubic spline), race, smoking status, diabetic status, and total number of comorbidities. Fitting for these models was inverse probability-weighted using the product of study survey weights and inverse propensity scores (survey weight × [1/PS]) (30). In addition, logistic regression was used to examine the cross-sectional relationship between logMAR score and frailty status in WHAS III. Sensitivity analyses were undertaken restricting NHANES participants to those aged at least 70 years—–the age range of the WHAS III sample—and using best-corrected visual acuity to define VI.
Analyses were conducted in SAS 9.2 (SAS Institute, Cary, NC) and STATA 15 (StataCorp, College Station, TX).
Results
Sample Characteristics
NHANES
Our primary NHANES analysis included 2,639 older adults aged at least 60 years (Table 1), of which 9% (n = 324) had VI. Compared with individuals without VI, those with VI were more likely to be older, less likely to be white, and had lower levels of education (p < .05 for all). In addition, 4% (n = 153) were classified as VI based on best-corrected visual acuity (Supplementary Table 1).
Table 1.
Participant Characteristics by Presenting Vision Impairment Status: National Health and Nutrition Examination Survey (NHANES)
| Overall n = 2,639 |
No visual impairmenta n = 2,315 (91%) |
Visual impairment n = 324 (9%) |
p-value | |
|---|---|---|---|---|
| Age, mean ± SD | 70.1 ± 7.2 | 69.6 ± 6.9 | 75.1 ± 9.0 | <.001 |
| Female, n (%) | 1,320 (55.6) | 1,161 (55.7) | 159 (55.6) | .975 |
| Race, n (%) | <.001 | |||
| Non-Hispanic White | 1,537 (82.6) | 1,374 (84.0) | 163 (69.6) | |
| Non-Hispanic Black | 437 (7.5) | 380 (7.2) | 57 (10.6) | |
| Mexican American | 512 (2.7) | 442 (2.6) | 70 (3.9) | |
| Other Hispanics and other race | 153 (7.2) | 119 (6.3) | 34 (16.2) | |
| Body mass index, n (%) (kg/m2) | .023 | |||
| Underweight (<18.5) | 39 (1.6) | 32 (1.5) | 7 (2.7) | |
| Normal (18.5–24.9) | 698 (27.5) | 589 (27.0) | 109 (32.8) | |
| Overweight (25–29.9) | 1,018 (37.4) | 892 (37.1) | 126 (40.6) | |
| Obese (≥30) | 838 (33.5) | 764 (34.4) | 74 (23.9) | |
| Education, n (%) | <.001 | |||
| Less than high school | 1,085 (29.9) | 908 (28.3) | 177 (45.2) | |
| High school or equivalent | 629 (29.6) | 556 (29.7) | 73 (28.5) | |
| More than high school | 920 (40.5) | 847 (42.0) | 73 (26.3) | |
| Current or former smokers, n (%) | 1,391 (53.2) | 1,234 (53.7) | 157 (48.1) | .173 |
| Diabetes, n (%) | 452 (14.8) | 378 (14.3) | 74 (19.1) | .071 |
| Total comorbiditiesb, n (%) | .059 | |||
| 0 comorbidities | 537 (20.0) | 478 (20.5) | 59 (15.3) | |
| 1–2 comorbidities | 1,602 (60.2) | 1,408 (60.3) | 194 (59.1) | |
| >2 comorbidities | 500 (19.8) | 429 (19.2) | 71 (25.6) |
Results reported as unweighted n (weighted %). Unadjusted p-values. Bold values denote statistical significance at p < .05 level.
aNo visual impairment defined as presenting visual acuity ≥20/40 in better-seeing eye. Visual impairment defined as presenting visual acuity <20/40 in better-seeing eye.
bTotal comorbidities: angina, arthritis, cancer, congestive heart failure, coronary heart disease, hypertension, liver disease, myocardial infarct, and stroke.
WHAS III
The WHAS III analysis included 796 older adults aged 70–79 with vision and frailty data at baseline (WHAS I n = 384; WHAS II n = 412), of which 26% (n = 211) had mild VI and 37% (n = 291) had moderate/severe VI (Table 2). Similar to NHANES, mild and moderate/severe VI groups were older, less likely to be white, had fewer years of education, had more comorbidities, and lower proportions of individuals with normal BMI, compared with the group without VI. Of the 549 individuals who were not frail at baseline, 30% had mild and 21% had moderate/severe VI (Supplementary Table 2).
Table 2.
Participant Characteristics by Vision Impairment Status: Women’s Health and Aging Study (WHAS) III
| Overall n = 796 |
No Visual Impairmenta n = 294 (37%) |
Mild Visual Impairment n = 211 (26%) |
Moderate/severe Visual Impairment n = 291 (37%) |
p-value | |
|---|---|---|---|---|---|
| Age, mean ± SD | 74.2 ± 10.2 | 73.6 (10.6) | 74.6 (10.6) | 74.5 (9.3) | <.001 |
| Race, n (%) | <.001 | ||||
| Non-Hispanic White | 595 (76.4) | 234 (80.4) | 161 (76.6) | 200 (70.6) | |
| Non-Hispanic Black | 192 (22.5) | 57 (18.7) | 48 (21.9) | 87 (28.3) | |
| Other Hispanics and other race | 9 (1.1) | 3 (0.9) | 2 (1.5) | 4 (1.1) | |
| Body mass index, mean ± SD (kg/m2) | 27.7 (22.2) | 26.7 (20.8) | 28.4 (20.9) | 28.3 (24.0) | .001 |
| Body mass index, n (%) (kg/m2) | ` | <.001 | |||
| Underweight (<18.5) | 29 (4.2) | 9 (3.6) | 4 (1.6) | 16 (7.6) | |
| Normal (18.5–24.9) | 239 (31.6) | 112 (38.2) | 53 (26.7) | 74 (26.9) | |
| Overweight (25–29.9) | 273 (34.7) | 104 (34.7) | 80 (36.7) | 89 (32.8) | |
| Obese (≥30) | 231 (29.5) | 68 (23.5) | 72 (35.0) | 91 (32.7) | |
| Education, mean ± SD (years) | 11.4 ± 12.9 | 12.6 ± 13.3 | 11.2 ± 11.8 | 9.9 ± 11.2 | <.001 |
| Education, n (%) | <.001 | ||||
| Less than high school | 203 (23.5) | 35 (11.4) | 52 (24.8) | 116 (39.1) | |
| High school or equivalent | 360 (45.9) | 139 (48.4) | 100 (47.3) | 121 (41.2) | |
| More than high school | 231 (30.6) | 120 (40.3) | 58 (27.9) | 53 (19.8) | |
| Current smokers, n (%) | 99 (12.1) | 24 (7.6) | 28 (13.5) | 47 (16.9) | <.001 |
| Diabetes, n (%) | 122 (13.2) | 24 (6.9) | 26 (11.2) | 72 (23.5) | <.001 |
| Total comorbiditiesb, n (%) | <.001 | ||||
| 0 comorbidities | 48 (5.6) | 28 (8.6) | 12 (4.5) | 8 (2.5) | |
| 1–2 comorbidities | 168 (24.6) | 90 (33.0) | 49 (27.0) | 29 (10.9) | |
| >2 comorbidities | 580 (69.8) | 176 (58.4) | 105 (68.5) | 254 (86.7) |
Results reported as unweighted n (weighted %). Unadjusted p-values. Bold values denote statistical significance at p < .05 level.
aNo visual impairment defined as binocular presenting visual acuity ≥20/40. Mild visual impairment defined as binocular presenting visual acuity <20/40, but ≥20/60. Moderate/severe visual impairment defined as binocular presenting visual acuity <20/60.
bTotal comorbidities: myocardial infarct, angina, congestive heart failure, other heart disease, hypertension, rheumatoid arthritis, stroke, cancer, hip fracture, Parkinson’s disease, lung disease, and hearing problem.
Unadjusted Analyses
NHANES
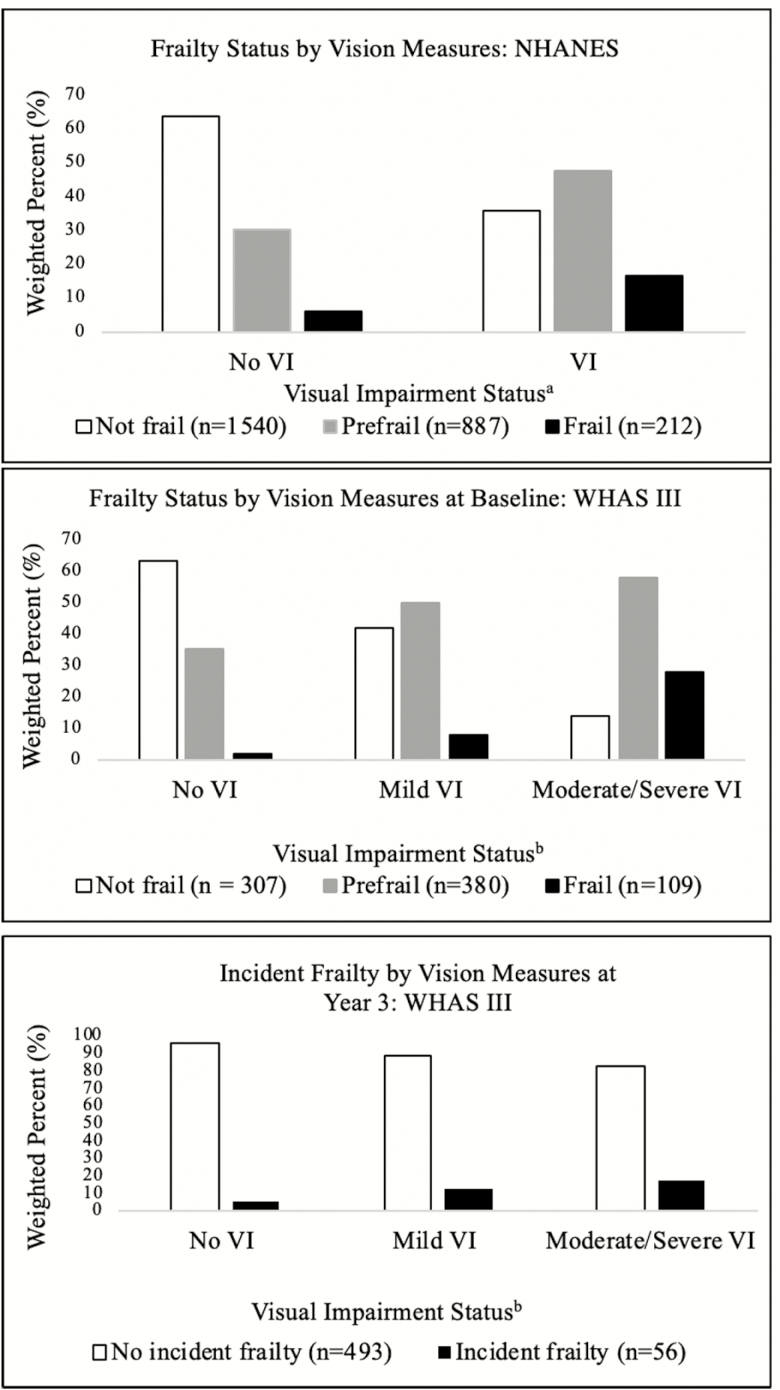
Overall, 32% of the population was prefrail and 7% was frail. Compared with the group without VI, those with VI had a larger proportion of prefrail and frail individuals (prefrail: 30% vs. 48% frail: 6% vs. 17%, respectively, p < .001; Figure 1). In secondary analyses using best-corrected visual acuity data, there was a significantly higher proportion of prefrail and frail individuals in the VI group compared with those without VI (56% vs. 31% prefrail, 15% vs. 7% frail, p < .001).
Figure 1.
Frailty Status by Vision Measures: National Health and Nutrition Examination Survey (NHANES) and Women’s Health and Aging Study (WHAS) III. aNo VI (visual impairment) defined as presenting visual acuity ≥20/40 in better-seeing eye. VI defined as presenting visual acuity <20/40 in better seeing-eye. bNo VI defined as binocular presenting visual acuity ≥20/40. Mild VI defined as binocular presenting visual acuity <20/40, but ≥20/60. Moderate/severe VI defined as binocular presenting visual acuity <20/60.
WHAS III
At baseline, 48% of the population was prefrail and 14% was frail. There were significantly higher proportions of individuals with prefrailty and frailty in the mild and moderate/severe VI groups (prefrailty: 50%, 58% vs. 35%, frailty: 8%, 28% vs. 2%, respectively, p < .001) compared with those without VI (Figure 1). In the incident frailty analysis, there was a higher proportion of individuals with mild and moderate/severe VI who developed incident frailty over the 3-year follow-up period compared with individuals without VI (incident frailty: 12%, 17% vs. 5%, respectively; Figure 1).
Adjusted Analyses
NHANES
In cross-sectional, weighted, fully adjusted models, individuals with VIs were 3.2 times more likely to be prefrail (95% confidence interval [CI]: 1.9–5.3), and 3.7 times more likely to be frail (95% CI: 1.5–9.2), compared with those without VI (Table 3). In a secondary analysis limited to individuals at least 70 years of age, individuals with VIs were also more likely to be prefrail and frail (Supplementary Table 3). When VI was defined by best-corrected visual acuity less than 20/40, individuals with VI were more likely to be prefrail, but not frail (Table 3).
Table 3.
Multinomial Logistic Regression Models of the Cross-Sectional Relationship between Frailty and Vision Measures: National Health and Nutrition Examination Survey (NHANES)
| Presenting visual acuitya,b (n = 2,458) | Best corrected visual acuitya,c (n = 2,292) | |||||
|---|---|---|---|---|---|---|
| Odds Ratio (OR) | 95% Confidence Interval (CI) | p-value | Odds Ratio (OR) | 95% Confidence Interval (CI) | p-value | |
| Prefrail | ||||||
| No VI | Reference | — | — | Reference | — | — |
| VI | 3.15 | 1.89–5.26 | <.001 | 5.67 | 1.97–16.38 | .002 |
| Frail | ||||||
| No VI | Reference | — | — | Reference | — | — |
| VI | 3.66 | 1.46–9.19 | .007 | 2.36 | 0.71–7.83 | .153 |
Note: Analyses restricted to participants with overlapping propensity score ranges across vision impairment groups (sample sizes as shown for each cohort study), and regression models inverse probability weighted using the product of study survey weights and inverse propensity scores (survey weight × [1/PS]). Bold values denote statistical significance at p < .05 level.
aAdjusted for: age (cubic spline), sex, race, smoking status, diabetic status, total number of comorbidities.
bNo VI (visual impairment) defined as presenting visual acuity ≥20/40 in better-seeing eye. VI defined as presenting visual acuity <20/40 in better-seeing eye.
cNo VI defined as best corrected, better-seeing eye visual acuity ≥20/40. VI defined as best corrected, better-seeing eye visual acuity <20/40.
WHAS III
Similar to NHANES, WHAS III participants with mild VI had a 2.0-fold greater odds of being more frail (95% CI: 1.5–2.5), and those with moderate/severe VI had 5.5-fold greater odds of being more frail (95% CI: 4.2–7.2), in fully adjusted models (Table 4). In addition, for each one-line worse visual acuity (0.1 logMAR increase), there was a 1.5 greater odds of frailty (95% CI: 1.4–1.7).
Table 4.
Ordinal Logistic Regression Models of the Cross-Sectional and Longitudinal Relationships between Frailty and Vision Measures in Women’s Health and Aging Study (WHAS) III
| WHAS III baselinea (n = 634) |
WHAS III at year 3a (n = 444) |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p-value | Odds Ratio | 95% CI | p-value | |
| Frail | ||||||
| No VIb | Reference | — | — | Reference | — | — |
| Mild VI | 1.96 | 1.52–2.52 | <.001 | 2.20 | 0.89–5.44 | .088 |
| Moderate/severe VI | 5.52 | 4.24–7.19 | <.001 | 3.45 | 1.42–8.38 | .006 |
| Per 0.1 LogMAR | 1.52 | 1.44–1.69 | <.001 | 1.26 | 1.09–1.45 | <.001 |
Note: Analyses restricted to participants with overlapping propensity score ranges across vision impairment groups (sample sizes as shown for each cohort study), and regression models inverse probability weighted using the product of study survey weights (WHAS) and inverse propensity scores (survey weight × [1/PS]). Bold values denote statistical significance at p < .05 level. CI = confidence interval.
aAdjusted for: age (cubic spline), race, smoking status, diabetic status, total number of comorbidities.
bNo VI defined as binocular presenting visual acuity ≥20/40. Mild VI defined as binocular presenting visual acuity <20/40, but ≥20/60. Moderate/severe VI defined as binocular presenting visual acuity <20/60.
Among WHAS III participants who were not frail at baseline (Table 4), individuals with mild VI were more likely to progress toward incident frailty over 3 years (OR = 2.2; 95% CI: 0.9–5.4), though this was not statistically significant. However, individuals with moderate/severe VI had a 3.5 greater odds of progressing toward incident frailty (95% CI: 1.4–8.4). Each line increase in logMAR score was associated with a 1.3 greater odds of incident frailty progression (95% CI: 1.1–1.5).
Discussion
Complementary findings in our analyses of two cohorts indicate that older adults with VI are more likely to be prefrail and frail than those without VI. The magnitude of these cross-sectional associations is striking. Longitudinal analyses using WHAS III data suggest that older adults with VI are more likely to progress toward frailty than those without VI, establishing temporality of this relationship.
In sensitivity analyses using best-corrected visual acuity data from NHANES, we also found an association between VI and prefrailty, although the relationship with frailty was no longer significant. However, only 4% of participants were categorized as VI based on best-corrected visual acuity. In our analyses restricting the NHANES sample to those 70 years and older, we observed an association between VI and prefrailty as well as frailty, but these results are attenuated from our primary analyses including participants 60 years and older. This sensitivity analysis was undertaken to better match the age range of NHANES and WHAS III—where participants were 70 years and older at baseline.
Results from this research mirror previous findings, although prior research is limited (13,14,31). Our study expands on this work by using objective assessments of VI and clinically meaningful criteria to define VI, as well as assessing frailty using the frailty phenotype—a theorized measure of multisystem dysregulation. The use of data from a nationally representative study sample (NHANES) allows for generalizability of our results to a broad population of older adults. In addition, we leveraged the available longitudinal data from WHAS III to further establish temporality in the relationship between VI and frailty onset. Our rigorous approach to control for potential confounders, which leveraged propensity scores, strengthens the case that observed associations reflect mechanisms related to VI.
Despite the strengths of our analyses, the limitations should be considered. First, we defined VI using different criteria in NHANES and WHAS III due to differences in the distribution of visual acuity between these data sets. Even when similar criteria were used to define VI, WHAS III had a strikingly higher proportion of individuals with VI. We can only speculate that these differences are due to variations in study population sampling, and we defined VI as mild or moderate/severe in WHAS III to enhance comparability. In addition, there are differences in the criteria used to define the frailty phenotype between WHAS III and NHANES, and the modifications made to the NHANES measurement of frailty may have conflated disability with frailty. However, given these differences in frailty assessment between the studies, the coherence of these findings is striking.
Research examining the vision–frailty relationship using objective measures of vision is limited, and more work is needed to understand the mechanism(s) underlying the relationship. We hypothesize three potential explanations of our results. First, the relationship between VI and frailty may be, in part, due to direct effects of VI on indicators of frailty, such as walking speed (2,3,32). Second, VI may be a determinant of frailty, where downstream consequences of VI lead to frail outcomes. For example, prior research has found that older adults with VIs have worse physical functioning and engage in less physical activity (33). We hypothesize that as a result of these physical functioning changes, overall fitness declines, and the risk of frailty increases. Future research should investigate these potential pathways to broaden our understanding of the vision-frailty relationship.
In summary, our results indicate that VI is an important yet understudied risk factor for frailty. The magnitude and coherence of these results across two cohorts, use of longitudinal data, and rigorous methods to account for age and other potential confounders, underscore the strength of these findings.
Funding
This work was supported by the Johns Hopkins University Older Americans Independence Center NIA P30AG021334 and NIA K01AG052640.
Supplementary Material
Acknowledgments
B.K.S. and M.L. (co-first authors) contributed to study design, data collection, analysis, and interpretation. J.T., V.V. contributed to data collection, analysis, and interpretation. K.B.R. contributed to study design, data analysis, and interpretation. All authors reviewed and contributed to writing.
Conflicts of interest
None.
References
- 1. Ho VWT, Chen C, Merchant RA. Cumulative effect of visual impairment, multimorbidity, and frailty on intrinsic capacity in community-dwelling older adults. J Aging Health. 2019:089826431984781. doi: 10.1177/0898264319847818. [DOI] [PubMed] [Google Scholar]
- 2. Swenor BK, Simonsick EM, Ferrucci L, Newman AB, Rubin S, Wilson V; Health, Aging and Body Composition Study Visual impairment and incident mobility limitations: the health, aging and body composition study. J Am Geriatr Soc. 2015;63:46–54. doi: 10.1111/jgs.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swenor BK, Muñoz B, West SK. A longitudinal study of the association between visual impairment and mobility performance in older adults: the salisbury eye evaluation study. Am J Epidemiol. 2014;179:313–322. doi: 10.1093/aje/kwt257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ong SY, Ikram MK, Haaland BA, et al. Myopia and cognitive dysfunction: the singapore malay eye study. Invest Ophthalmol Vis Sci. 2013;54:799–803. doi: 10.1167/iovs.12-10460 [DOI] [PubMed] [Google Scholar]
- 5. Lam BL, Christ SL, Zheng DD, et al. Longitudinal relationships among visual acuity and tasks of everyday life: the Salisbury Eye Evaluation study. Invest Ophthalmol Vis Sci. 2013;54:193–200. doi: 10.1167/iovs.12-10542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crews JE, Jones GC, Kim JH. Double jeopardy: the effects of comorbid conditions among older people with vision loss. J Vis Impair Blind. 2006;100(Suppl):824–848. http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2007-03002-002&site=ehost-live. Accessed June 7, 2018. [Google Scholar]
- 7. Christ SL, Zheng DD, Swenor BK, et al. Longitudinal relationships among visual acuity, daily functional status, and mortality: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2014;132:1400–1406. doi: 10.1001/jamaophthalmol.2014.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- 9. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 11. Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. 2009;122:664–671.e2. doi: 10.1016/j.amjmed.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 13. Klein BE, Klein R, Knudtson MD, Lee KE. Relationship of measures of frailty to visual function: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2003;101:191–196; discussion 196. [PMC free article] [PubMed] [Google Scholar]
- 14. Liljas AEM, Carvalho LA, Papachristou E, et al. Self-reported vision impairment and incident prefrailty and frailty in English community-dwelling older adults: findings from a 4-year follow-up study. J Epidemiol Community Health. 2017;71:1053–1058. doi: 10.1136/jech-2017-209207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trevisan C, Veronese N, Maggi S, et al. Factors influencing transitions between frailty states in elderly adults: The Progetto Veneto Anziani Longitudinal Study. J Am Geriatr Soc. 2017;65:179–184. doi: 10.1111/jgs.14515 [DOI] [PubMed] [Google Scholar]
- 16. El-Gasim M, Munoz B, West SK, Scott AW. Discrepancies in the concordance of self-reported vision status and visual acuity in the Salisbury Eye Evaluation Study. Ophthalmology. 2012;119:106–111. doi: 10.1016/j.ophtha.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. CDC NCHS. https://wwwn.cdc.gov/nchs/nhanes/NhanesCitation.aspx. Accessed November 6, 2017.
- 18. Guralnik J, Fried L, Simonsick E, Lafferty M.. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability.; 1995. https://books.google.com/books?hl=en&lr=&id=9eJ174um3VIC&oi=fnd&pg=PP7&ots=e6gQfaWHYQ&sig=vCpohCoe4x1SjvYvWFEOo9wS9ew. Accessed October 16, 2018. [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 20. Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: a physiological approach. Cardiovascular Health Study Research Group. J Clin Epidemiol. 1994;47:747–760. doi:10.1016/0895-4356(94)90172-4 [DOI] [PubMed] [Google Scholar]
- 21. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262 [DOI] [PubMed] [Google Scholar]
- 22. Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43 [DOI] [PubMed] [Google Scholar]
- 23. Fried L, Kasper J, Guralnik J, Simonsick E. The Women’s Health and Aging Study: An Introduction 1995. https://scholar.google.com/scholar?cluster=9561995746359004239&hl=en&as_sdt=20000005&sciodt=0,21. Accessed June 7, 2018.
- 24. Centers for Disease Control and Prevention (CDC). Vision Procedures Manual 2008. https://wwwn.cdc.gov/nchs/data/nhanes/2007–2008/manuals/manual_vi.pdf. Accessed November 15, 2017.
- 25. Scilley K, Jackson GR, Cideciyan AV, Maguire MG, Jacobson SG, Owsley C. Early age-related maculopathy and self-reported visual difficulty in daily life. Ophthalmology. 2002;109:1235–1242. doi: 10.1016/s0161-6420(02)01060-6 [DOI] [PubMed] [Google Scholar]
- 26. Ophthalmology AA of. Eye Health Statistics 2015. https://www.aao.org/newsroom/eye-health-statistics. Accessed July 6, 2018.
- 27. Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. Am J Ophthalmol. 1982;94(1):91–96. doi: 10.1016/0002-9394(82)90197-0 [DOI] [PubMed] [Google Scholar]
- 28. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 29. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Heal Serv Outcomes Res Methodol. 2001;2(3/4):169–188. doi:10.1023/A:1020363010465 [Google Scholar]
- 30. DuGoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. doi: 10.1111/1475-6773.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng TP, Feng L, Nyunt MS, Larbi A, Yap KB. Frailty in older persons: multisystem risk factors and the Frailty Risk Index (FRI). J Am Med Dir Assoc. 2014;15:635–642. doi: 10.1016/j.jamda.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 32. Swenor BK, Muñoz B, West SK. Does visual impairment affect mobility over time? The Salisbury Eye Evaluation Study. Invest Ophthalmol Vis Sci. 2013;54:7683–7690. doi: 10.1167/iovs.13-12869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Landingham SW, Willis JR, Vitale S, Ramulu PY. Visual field loss and accelerometer-measured physical activity in the United States. Ophthalmology. 2012;119:2486–2492. doi: 10.1016/j.ophtha.2012.06.034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.