Abstract
Preclinical studies are important in identifying the underlying mechanisms contributing to frailty. Frailty studies have mainly focused on male rodents with little directed at female rodents. Therefore, the purposes of this study were to identify the onset and prevalence of frailty across the life span in female mice, and to determine if frailty predicts mortality. Female C57BL/6 (n = 27) mice starting at 17 months of age were assessed across the life span using a frailty phenotype, which included body weight, walking speed, strength, endurance, and physical activity. The onset of frailty occurred at approximately 17 months (1/27 mice), with the prevalence of frailty increasing thereafter. At 17 months, 11.1% of the mice were pre-frail and by 26 months peaked at 36.9%. The percentage of frail mice progressively increased up to 66.7% at 32 months. Non-frail mice lived to 29 months whereas frail/pre-frail mice lived only to 26 months (p = .04). In closing, using a mouse frailty phenotype, we are able to identify that the prevalence of frailty in female mice increases across the life span and accurately predicts mortality. Together, this frailty phenotype has the potential to yield information about the underlying mechanisms contributing to frailty.
Keywords: Aging, Body weight, Mortality, Muscle, Physical function
Frailty is a complex phenotype seen in aging, which is associated with low physiologic reserves, decreased resilience, and resistance to stressors, causing vulnerability to adverse outcomes (1–4). Because the population of 60 year olds and older is expected to be 2 billion by 2050 and the prevalence of frailty increases with age, frailty will become an increasing challenge (5). Early identification of individuals at risk of developing frailty, even at a pre-frail status, has the potential to make a significant impact on age-related disability, dependence, and overall quality of life. Several lines of evidence attest that frailty can be prevented, or at least delayed, by the establishment of timely and appropriate interventions (6,7). Thus, intervening at the pre-frail stage by targeting key biological pathways may have important implications in either reversing pre-frailty or halting the subsequent development of frailty.
The investigation of frailty in preclinical models (naturally aging mice) using frailty assessment tools is an important step in the understanding of key biological pathways underlying the onset of frailty and increased prevalence of frailty across the life span. To foster translation of biological features of frailty, human frailty indexes and phenotypes have been reverse translated into mice (8–12). For instance, frailty is modeled as a clinical frailty index in mice by tracking the accumulation of age-specific deficits (9). The accumulation of deficits increases with age similar to what occurs in humans (10). Frailty is also modeled as a frailty phenotype using performance measures such as grip strength, walking speed, endurance, and activity levels (8,11,13). This phenotype identifies non-frail, pre-frail, and frail mice similar to the human frailty phenotype established by Fried and colleagues (3).
Recently, the frailty assessment tools for mice (ie, mouse frailty index and phenotype) were revised by the original research groups. Whitehead and colleagues (14) modified several of the original 31 measurements (9) because they were invasive and difficult to integrate into a longitudinal, life span research study. The updated frailty index includes clinical observations of several biological systems (ie, musculoskeletal, integument, ocular, nasal systems, and respiratory) and signs of discomfort, body weight, and temperature. On the other hand, the original mouse frailty phenotype (8) was recently modified with the inclusion of body weight and an established endurance test (ie, time to fatigue on a motorized treadmill) (12). Using this updated frailty phenotype, Baumann and colleagues (12) identified the onset and prevalence of frailty, and overall health and mortality risk in a cohort of male mice across the life span (14–37 months of age). The onset of frailty occurred at 17 months of age (100% survival rate) with the prevalence of frailty increasing across the life span; in that, nearly every mouse was frail by 32 months of age (52% survival rate). Because high body weight has potential to impact health status (12,15), the authors recommend heavy mice late in adulthood be considered positive for the frailty criterion of body weight.
Over the past 3 years, these mice frailty assessment tools have been successfully used by various research teams (11,13,16). The majority of the reported studies have used male mice with only a few studies investigating frailty in female mice. With this disparity in mind, in the current study we set out to determine the onset, prevalence, and mortality risk in female mice across the life span.
Methods
Animals and experimental design
Female C57BL/6 mice (n = 29) were purchased from Jackson Laboratory (Bar Harbor, ME) at 5 months of age. Mice were supplied with food and water ad libitum, housed under a 12-hour light to dark cycle at 20–23°C in specific pathogen-free facilities, and allowed to age until death. Of the initial cohort, two mice were killed at 19 months of age due to non-age-related causes, leaving a total of 27 mice for this study. All animal procedures were in accordance with the standards set by the institutional animal care and use committees at the University of Minnesota and Boston University.
Testing was initiated at 17 months of age and continued every 3 months (20, 23, . . . 32, or death) making this study a repeated measures research design, which allowed us to determine the life span of each individual mouse. On the basis of the National Institute of Aging, 17 months of age represents a 90% survival rate for female C57BL/6 mice and therefore any death prior to 17 months would be due to non-age-related conditions (malocclusion, hydrocephalus, or over-grooming). At every testing period (ie, 3-month interval), mice performed the assessments over a 1-week period (12). All procedures followed the same protocol for each testing period, and to ensure user reliability, all assessments were completed by the same investigators.
Body weight and body fat percentage
Body weights were obtained on an electronic scale (CS200; OHAUS, Parsippany, NJ), whereas body fat percentage was evaluated using a Lunar PIXImus densitometer (GE Lunar Corporation, Madison, WI). Briefly, a phantom mouse was first used as a calibration standard for quality control prior to each testing day. Mice were then anesthetized with isoflurane, placed on an adhesive specimen tray, and scanned with the skull excluded and tail included.
Walking speed
Walking speed was evaluated using a rotarod (Rota-Rod R/S; LSi Letica, Cornella, Spain). Others have also used the rotarod test to assess endurance, balance, and coordination (8,17,18). Mice were first warmed up on the rotarod by walking at 4 rpm for 30 seconds, at which point rotarod speed increased 1 rpm every 8 seconds up to 40 rpm over a 5-minute period. Time, in seconds, was recorded when the mouse was unable to sustain the rotation speed of the rotarod. Each mouse performed three trials with a 10-minute rest period in between each trial. The best score of these trials was used as walking speed.
Strength
Strength was evaluated using a grip meter test (P/N760483; Coulbourn Instruments, Whitehall, PA). Mice were lowered over the top of a wire grid so that the front and hind paws gripped the grid. The tail of each mouse was then pulled back while keeping the torso of the mouse in a horizontal position. When the mouse was unable to maintain its grip, the trial was over and strength, in grams, was recorded. Each mouse performed two trials with a 10-minute rest period in-between each trial. The best score of these trials was used as peak grip strength.
Endurance
Endurance was evaluated using a time to fatigue test on a motorized treadmill (Exer 3/6 Treadmill; Columbus Instruments, Columbus, OH). After a brief warm-up (5 m/min for 5 minutes), mice remained on the treadmill and time to fatigue began with speed increasing 1 m/min every minute. Motivation was provided by tapping the mouse’s rear (19). Time to fatigue, in seconds, was recorded following the third time the mouse could no longer keep pace with the speed of the treadmill. Endurance was determined to be the total amount of time the mouse remained on the treadmill.
Physical activity
Physical activity was evaluated using the voluntary distance run on a running wheel (Model number: 80820F; Lafayette Instruments, Lafayette, IN). Mice were individually housed in wheel running cages for 4 days. The running distance, in revolutions, was recorded and converted to kilometer. The average distance ran per day (km/day) was used to score physical activity.
Frailty criteria
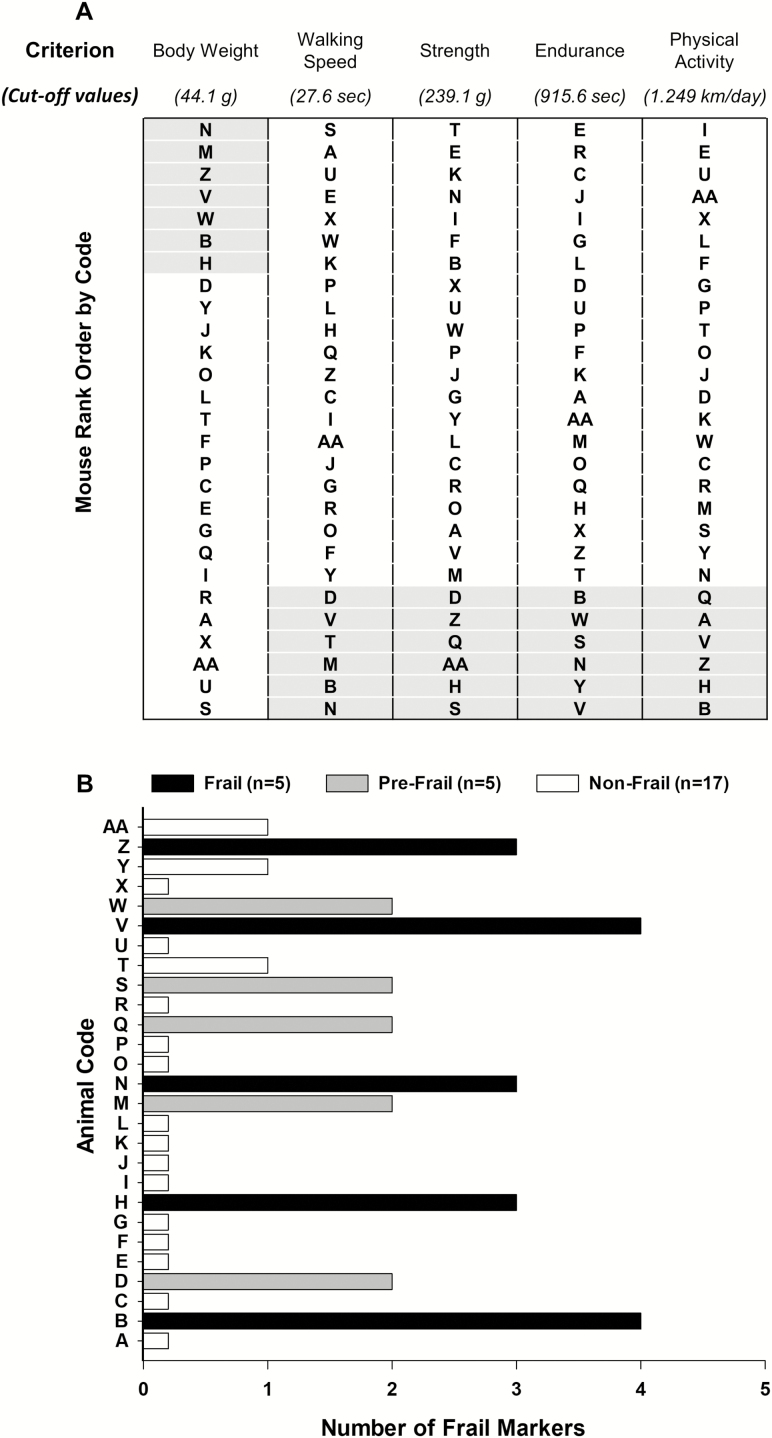
The frailty phenotype used in this study was previously described in detail (12). Briefly, mice that fell in the bottom 20% for walking speed, strength, endurance, or physical activity, or in the top 20% for body weight were considered positive for frailty (ie, for that given criterion). These criteria were used to identify frailty cut-off values at 20 months of age (Table 1). Mice with three or more positive frailty markers were identified as frail, mice with two positive markers were identified as pre-frail, and mice with one or no positive frailty marker were considered non-frail.
Table 1.
Frailty Criteria and Cut-off Values
| Human Frailty Phenotype (3) | Mouse Frailty Phenotype (12) | Cut-off Values |
|---|---|---|
| Low activity | Voluntary wheel running | Lower 20% (1.249 km/d) |
| Poor endurance | Treadmill fatigue test | Lower 20% (915.6 s) |
| Weakness | Grip meter | Lower 20% (239.1 g) |
| Slowness | Rotarod test | Lower 20% (27.6 s) |
| Lower body weight | Body weight | Upper 20% (44.1 g) |
The age of 20 months was selected for three reasons. First, in our initial cohort (n = 29) and that published by National Institute of Aging, this age represents >75% survival for female C57BL/6 mice, meaning it is near the maximal age before mice begin dying, making it an optimal age to predict frailty. In fact, from 20 to 26 months of age, approximately 30% of the mice in our cohort died. Second, 20 months for a mouse equate to approximately 60–70 human years (3,10), which correspond to the initial age bracket assessed by Fried and colleagues (3). Finally, because frailty is thought to be reversible, this age provides adequate time to implement possible life-changing interventions. We have previously demonstrated that the same reasoning can be applied to male C57BL/6 mice at 23 months of age (12).
To determine if the frailty criteria outlined earlier accurately predict mortality, survival curves were constructed on mice identified as frail, pre-frail, and non-frail at 20 months of age. The cut-off values obtained at 20 months (Table 1) were then used to quantify the onset and prevalence of frailty for all other age groups (ie, 17–32 months).
Statistical analysis
A one-way repeated measures analysis of variance (mouse × time) was used to test age-related changes in body weight, body fat, walking speed, strength, endurance, and physical activity. In the event of a significant analysis of variance, a Bonferroni correction was used. The relationships between body weight and body fat percentage were fit with a simple linear regression and the square of the correlation coefficient (R2) was calculated. Differences between frail/pre-frail and non-frail were analyzed with an independent t test. A Kaplan–Meier test was used to assess life span characteristics and for comparison between groups (ie, frail, pre-frail, and non-frail). An α level of <.05 was used for all analyses. Values are presented as mean ± SEM. Statistical analyses were performed with Sigma Plot 14.0 (Systat Software Inc., Point Richmond, CA) or SPSS 24.0 (IBM Corp, Armonk, NY) software.
Results
Mouse mortality and identifying frail mice
The survival curve of the 27 female mice that completed the study is depicted in Supplementary Figure 1. The first and last mouse died at 21.1 and 34.3 months, respectively, and the mean survival age was 28.1 ± 0.6 months. In order to identify frail mice cut-off values for each frailty criterion (20th percentile), mice were rank ordered for each criterion from best to worst with the exception of body weight (Figure 1A). Mice were identified to have a positive marker of frailty if the mice performed below the cut-off values for walking speed (27.6 seconds), strength (239.1 g), endurance (915.6 seconds), or physical activity (1.249 km/d; Table 1). Mice were considered positive for the body weight frailty marker if they were 44.1 g or greater (Table 1). Using these criteria, we identified 17 mice as non-frail, 5 mice as pre-frail, and 5 mice as frail at 20 months of age (Figure 1B).
Figure 1.
Frailty status at 20 months of age. The mice were coded A–Z and AA (n = 27) and rank ordered by performance (A). The cut-off values of each criterion (body weight, walking speed, strength, endurance, and physical activity) are shown in A. The gray-shaded areas identify the mice in the bottom 20% for performance and the top 20% for body weight. The summary of the criteria of number of frail markers for each mouse at 20 months of age (B). Frailty was defined if a mouse had three or more positive markers, whereas pre-frailty was designated if a mouse had two positive markers. Mouse B, H, N, V, and Z were identified as frail (black). Mouse D, M, Q, S, and W were identified as pre-frail (gray). The remaining mice (n = 17) were identified as non-frail (white).
Onset and prevalence of frailty
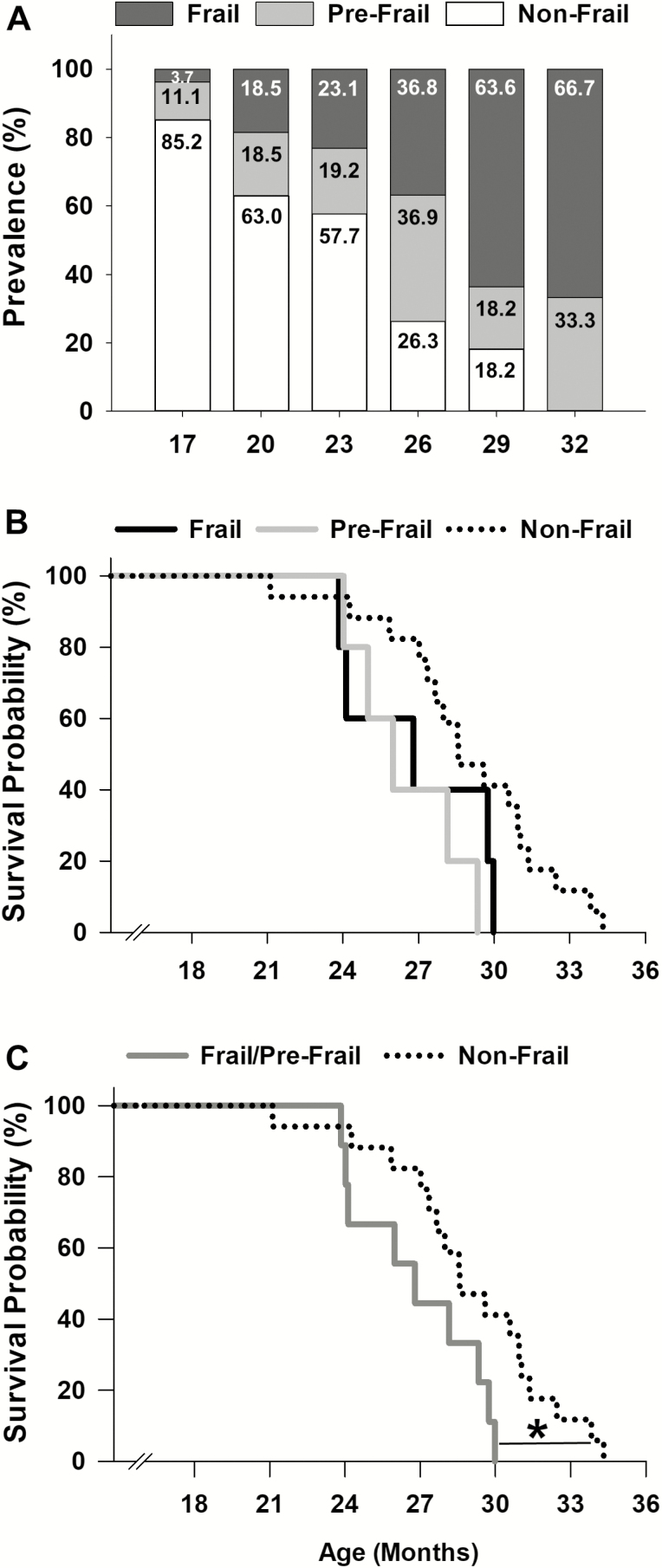
Cut-off values calculated at 20 months of age (Table 1, Figure 1A) were then used to determine the onset and prevalence of frailty for all other age brackets (Figure 2A). At 17 months of age, 11.1% of the mice were pre-frail, and by 26 months increased and peaked at 36.9%. Following 26 months, this percentage declined to 18.2% at 29 months. The onset of frailty occurred at approximately 17 months, and represented only 3.7% of the mouse cohort (1/27 mice). The percentage of frail mice progressively increased to 66.7% at 32 months. In contrast, the percentage of non-frail mice steadily declined from 85.2% at 17 months to 18.2% at 29 months. Beyond 29 months, no mice were identified as non-frail (Figure 2A).
Figure 2.
Frailty: onset, prevalence, and mortality. Prevalence of frailty using the cut-off values at 20 months of age was determined across the life span in female C57BL/6 mice (A). The onset of frailty was identified at 17 months of age (A). Kaplan–Meier survival curves for frail, pre-frail, and non-frail mice (B). Kaplan–Meier survival curves for frail/pre-frail and non-frail mice (C). * indicates p < .05 comparing frail/pre-frail (dark gray) to non-frail (black dot).
Predicting mortality of frail mice
Mice identified as frail, pre-frail, and non-frail at 20 months were assessed using a Kaplan–Meier survival analysis to determine if our frailty criteria accurately predict mortality (Figure 2B). The five frail mice had a mean survival time of 26.9 ± 1.3 months and a 60% probability of dying before 27 months. Four of the five pre-frail mice died before 29 months (80% probability) and had a mean survival age of 26.5 ± 1.0 months. The mean survival age of the non-frail mice (n = 17) was 29.0 ± 0.8 months. Although not significantly different (p = .06), non-frail mice tended to live longer than frail and pre-frail mice (Figure 2B). Therefore, we combined frail and pre-frail mice into one group and reanalyzed the data, and revealed that non-frail mice lived significantly longer than frail/pre-fail mice (p = .04) (Figure 2C).
Comparison between frail/pre-frail and non-frail mice early in life
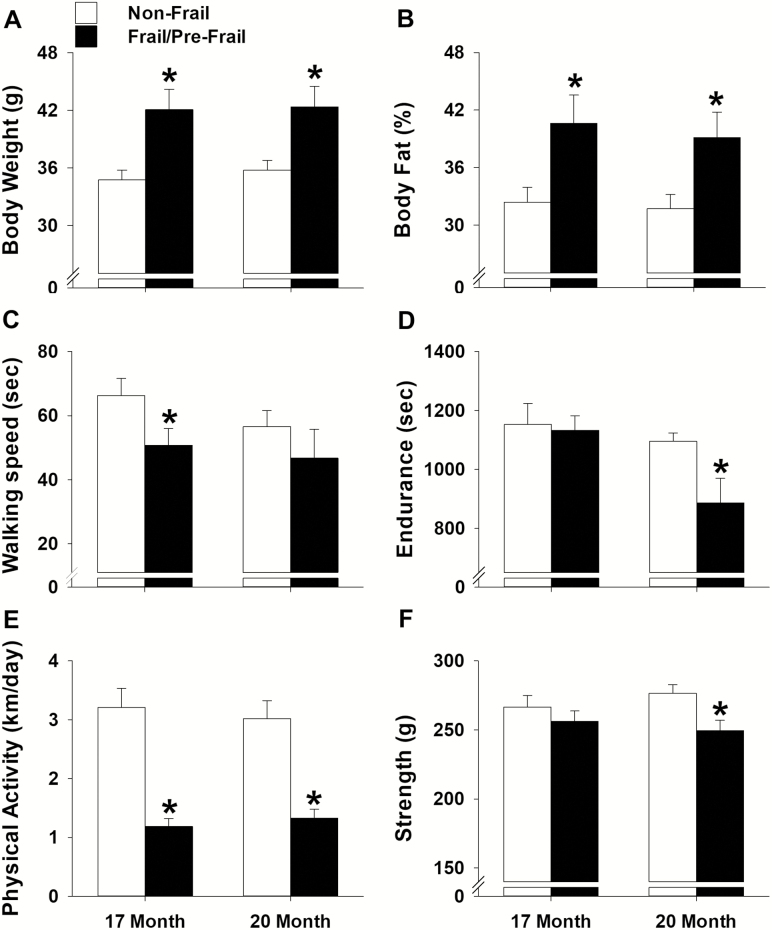
To assess if the mice identified as frail or pre-frail at 20 months presented morphological and/or functional differences from the non-frail mice earlier in life, the frail and pre-frail mice were again grouped together and compared to the non-frail mice at 17 and 20 months of age (Figure 3). Frail/pre-frail mice weighed 18%–21% more and possessed 23%–25% more body fat than the non-frail mice at 17 and 20 months of age (p ≤ .01; Figure 3A and B). Walking speed was also different between frail/pre-frail and non-frail mice earlier in life, with time spent on the rotarod being 30% less at 17 months (p = .05; Figure 3C). Endurance measured as treadmill time to fatigue was 24% less in frail/pre-frail mice when compared to non-frail mice at 20 months (p = .01; Figure 3D). Moreover, distance per day for voluntary wheel running was 56%–62% less in frail/pre-frail mice at 17 and 20 months of age (p ≤ .01; Figure 3E). In contrast to the robust difference in voluntary wheel running, strength determined by a grip test was only 10% less in frail/pre-frail mice when compared to non-frail mice at 20 months of age (p = .01; Figure 3F).
Figure 3.
Comparison between frail/pre-frail and non-frail mice early in life. Mice identified as frail/pre-frail (n = 10) and non-frail (n = 17) using the cut-off values determined at 20 months of age. An independent t test was performed to assess differences in body weight (A), % body fat (B), walking speed (C), endurance (D), physical activity (E), and strength (F) at 17 and 20 months of age. All values are expressed as mean ± standard error. * indicates p < .05 comparing frail/pre-frail (black) to non-frail (white).
Age-related changes
Because of the longitudinal research design used in this study, it was possible to evaluate age-related changes in the same mice across several testing periods. For this analysis, a subset of 11 mice was assessed using a repeated measures analysis of variance across 17–29 months of age, five consecutive testing periods.
Body weight and body fat percentage
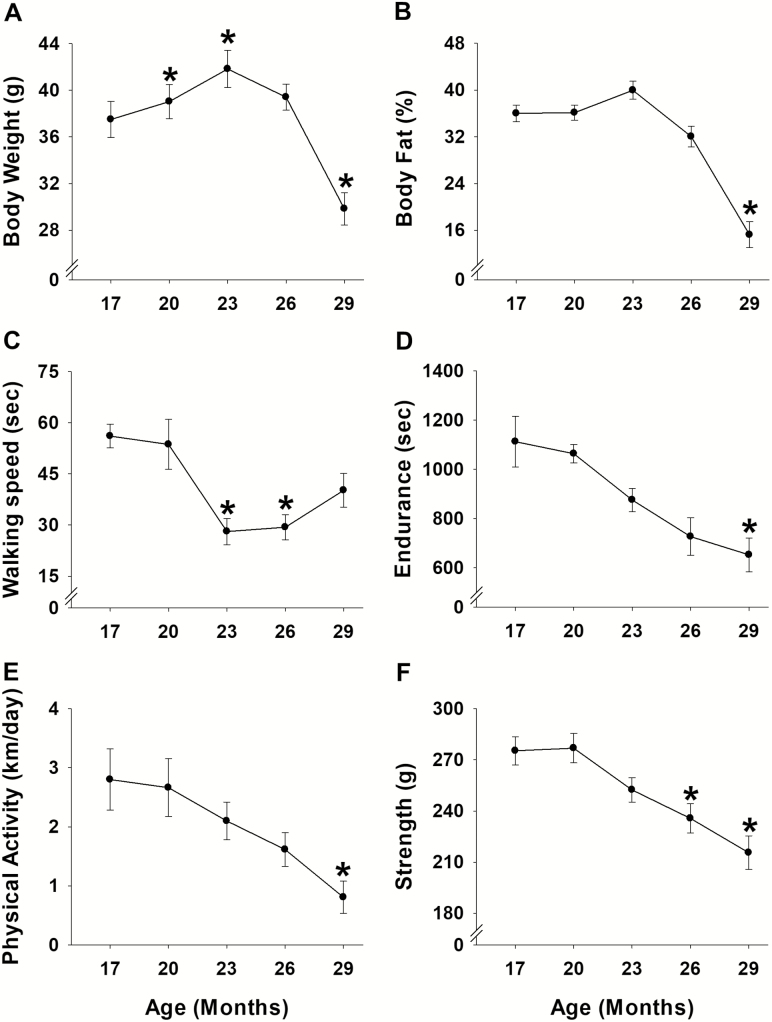
Body weight increased 4%–11% at 20 and 23 months (p ≤ .04), and returned to baseline at 26 months before decreasing 20% at 29 months (p = .05; Figure 4A). Body fat percentage also increased 11% (not significant, p = .06 at 23 months) before decreasing 20% at 29 months (p = .001; Figure 4B). Correlational analyses revealed that body weight and body fat percentage were related from 17 to 29 months of age (Supplementary Figure 2A–F), reaching a peak correlation of .89 (p < .001) at 29 months.
Figure 4.
Age-related changes for each criterion. Eleven mice were used to determine age-related changes in body weight (A), % body fat (B), walking speed (C), endurance (D), physical activity (E), and strength (F) using one-way repeated measures analysis of variance followed by Bonferroni post hoc. All values are expressed as mean ± standard error. * indicates p < .05 comparing to 17 months of age.
Functional characteristics
When compared to the initial baseline assessments performed at 17 months, age-related changes were observed for all functional characteristics (Figure 4). Walking speed determined by time on the rotarod was the first variable to experience an age-related change, decreasing 48%–50% at 23–26 months of age (p ≤ .01; Figure 4C). Strength measured by a grip test was the next variable to exhibit an age-related change, decreasing 14%–21% at 26–29 months (p ≤ .04; Figure 4F). Endurance assessed by time to fatigue test decreased 41% at 29 months (p < .03; Figure 4D). Physical activity determined by voluntary wheel running also experienced an age-related change at 29 months, decreasing 71% (p < .02; Figure 4E).
Discussion
In this study, we undertook a comprehensive approach to investigate a female cohort of mice across the life span and identified several important characteristics of frailty using a phenotype assessment tool. The frailty phenotype assessment tool included criteria of high body weight and impaired strength, walking speed, endurance, and physical activity. Using this approach, as previously demonstrated in male mice (12), it is possible to effectively characterize the onset and prevalence of frailty, and accurately predict mortality in female mice.
Frailty is a state of increased vulnerability to adverse health outcomes, a higher risk of disability, hospitalizations, and mortality (1–4). Two clinical assessment tools, the frailty index and the frailty phenotype, are frequently used to identify frail individuals. The frailty index conceptualizes frailty as a multidimensional clinical syndrome considering a wide array of outcome variables (10). It is defined as the number of deficits in an individual divided by the total number of deficits measured, and as such is an index of deficit accumulation. In contrast, the frailty phenotype denotes frailty functionally as represented by poor performance in three out of five criteria (ie, weight loss, exhaustion, weakness, slow walking speed, lack of activity) (3). These two clinical assessment tools were successfully reverse-translated for preclinical animal models with the overreaching goal to tease out the biological features associated with frailty status and diminished physical resilience (13,20).
Because frailty is prevalent in older women, the use of a female mouse model is relevant to age-related conditions that are pertinent to human frailty (21). To date, the number of studies investigating frailty in female mice has increased over the past 12 months. The majority of these studies used the frailty index score in various strains of mice (eg, 3xTg-AD, NIH Swiss, C57BL/6, CD-1) (22–25). The overall findings in most of the mouse strains indicate the frailty index score increases with age, suggesting that female mice experience age-associated health deficits (20,22,23).
In experimental aging research, the C57BL/6 is one of the most commonly used mouse strains (26). The C57BL/6 strain is popular because it has a well-defined short life span, the major physiological systems are well-documented, the cost is conservative, and there are similarities with human aging (27). In fact, initial studies with female C57BL/6 mice used a cross-sectional approach comparing various age groups (eg, 13 vs 30 months; 5, 19, and 28 months) (9,14). Recent studies report assessing frailty more than once in the same cohort of female C57BL/6 mice over a duration of 5–6 months (20,24). Specifically, these studies began assessment of frailty when the mice were 16 or 17 months of age and ended the testing when mice were 21 or 23 months of age (20,24). Regardless of the approach, longitudinal or cross-sectional, the frailty index score increased in the female C57BL/6 mice. In contrast to the frailty index score, investigations in female C57BL/6 mice using the frailty phenotype are limited; yet, this assessment tool is valuable. The evaluation of frailty across the adult life span (mice from 17 to 34.3 months of age, a duration of 17 months) in the same cohort of mice has the potential to yield significant information about the progression of frailty.
Here, we demonstrate that the prevalence of frailty in female C57BL/6 mice increased across the life span. Indeed, the prevalence of frailty (frail/pre-frail) increased from 42% to 72% between 23 and 26 months of age, when the survival rate was greater than 50%. An increased prevalence of frailty has recently been reported in female NIH Swiss mice using a modified accumulation deficit model (29 measures) (23). These studies validate that assessment tools for frailty accurately depict an increase in the prevalence of frailty across the life span in mice. Moreover, our data provide clarity to a previous report showing inconclusive results; in that, the prevalence of frailty did not increase when using the Valencia Score frailty phenotype (16). Collectively, these results are consistent with that seen in humans, where the prevalence of frailty in females increased from 3% to 11% at 65–75 years old to 60% at 80 years old (3).
Another important characteristic of frailty is the time point within the life span at which frailty is first initiated (ie, onset of frailty). The significance of recognizing the onset of frailty lies in its potential to discover the biological mechanisms associated with the pre-frail status and to initiate timely interventions to prevent frailty. In this study, we estimate that the onset of frailty occurred approximately at 17 months of age. However, because we did not measure any time or age prior to 17 months, it is possible the onset of frailty may have occurred earlier in life. We suspect this is not the case as only 3.7% of our mouse cohort was considered frail at 17 months of age, which corresponds to 1 out of 27 mice, meaning at the most only 1 mouse was frail prior to this point. The difficulty in defining the precise year when frailty emerges in humans was reported recently in a study that examined the data from the Longitudinal Aging Study Amsterdam and the InCHIANTI Study (28). In order to capture the onset of frailty in both humans and in preclinical models, the research design must overcome this challenge.
Moreover, we were able to successfully predict that non-frail mice (29.0 months) live longer than frail/pre-frail mice (26.7 months). In fact, in close examination over a 6-month period (20–26 months of age), 44% of the frail/pre-frail mice died compared to only 12% of the non-frail mice. However, in order to identify mortality risk, it was necessary to combine pre-frail and frail mice at 20 months of age into one experimental group. Although we do not know the underlying mechanisms of why we cannot distinguish between frail and pre-frail female at this time point, our data still support that frail/pre-frail mice have impaired health and die earlier than non-frail mice. These findings suggest that a decline in specific physical activities such as walking speed, strength, activity level, and endurance can provide information about the underlying health status in mice.
Recently, there has been an increased awareness on the topics of resilience, frailty, and aging in both mice and humans. Resilience is defined as the ability of an organism to respond to stress (29,30). It is likely that frailty and resilience are closely related. The mouse frailty phenotype used in this study measured body weight, strength, and activity level, all measures that did not stress the mouse. In addition, time to fatigue (endurance) and walking speed were also analyzed, two assessments that evaluated the mouse’s ability to respond to added physical stresses, or stress that acutely disrupts normal physiological functions. With this in mind, the data suggest female mice with increased resilience have the potential to live longer. In contrast, female mice that performed poorly during the time to fatigue and walking speed tests early in life (frail/pre-frail status) exhibited signs of reduced resilience and typically died earlier in life. It is possible that reduced resiliency may play a causative role in the development of the frail/pre-frail status. Importantly, we suggest that the mouse frailty phenotype has the potential to test resiliency in adulthood and be an approach to test frailty interventions.
The underlying mechanisms contributing to the onset, increased prevalence of frailty across the life span, and risk of mortality in female mice are likely multifactorial. Currently, there are several proposed mechanisms such as low-grade inflammation, oxidative stress, cellular senescence, and DNA damage (31–33). This scenario is further complicated in aging females as they go through ovarian senescence, in which levels of estrogen decline (34–38). Estrogen status has been reported to influence many morphological and physiological measures (34,39–42). For instance, loss of estrogen (via ovariectomy) increases body weight and body fat percentage while reducing cage activities, voluntary wheel running distance, and skeletal muscle contractile function in female rodents (34,41,42). These observations are similar to those seen as women transition through menopause (43–45). Therefore, we suggest that an important factor contributing to frailty in females is estrogen status. Our data support this concept because around the time of ovarian senescence (~16–20 months of age in C57BL/6) (34,35), mice begin to experience an increase in body weight and a progressive reduction in overall function. Moreover, at this time we also observed that frail/pre-frail mice were different than non-frail mice, in particular when it came to body weight, body fat percentage, and voluntary wheel running distance. Although we cannot directly link estrogen status to our frailty phenotype, future studies should consider using a longitudinal life span design to determine how hormones, frailty (functional measures) status, and resilience interact.
In summary, here we show that the frailty phenotype is a useful assessment tool. This frailty phenotype is accurate in identifying the onset of frailty, the increasing prevalence of frailty, and the mortality risk across the life span of C57BL/6 female mice. Because the frailty phenotype is a well-validated tool, it may be beneficial in identifying mechanisms contributing to frailty and reduced resilience with age.
Funding
This work supported by the Travis Roy Endowed Professorship (to L.V.T.) and the National Institutes of Health (T32-AG029796 to L.V.T. and C.W.B., and T32-AR007612 to C.W.B.).
Supplementary Material
Acknowledgement
The authors thank Julimar Avila and Basya Pearlmutter for technical assistance.
Conflict of Interest
None declared.
References
- 1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.M255 [DOI] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 4. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States Washington, DC: United States Census Bureau, Economics and Statistics Administration; https://www.census.gov/library/publications/2014/demo/p25-1140.html. Assessed May 7, 2014 [Google Scholar]
- 6. Laosa O, Alonso C, Castro M, Rodriguez-Manas L. Pharmaceutical interventions for frailty and sarcopenia. Curr Pharm Des. 2014;20:3068–3082. doi: 10.2174/13816128113196660705. [DOI] [PubMed] [Google Scholar]
- 7. Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70:1045–1058. doi: 10.1093/gerona/glu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69:1485–1491. doi: 10.1093/gerona/glt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227. doi: 10.1093/gerona/glr193 [DOI] [PubMed] [Google Scholar]
- 10. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci. 2017;72:885–891. doi: 10.1093/gerona/glw337 [DOI] [PubMed] [Google Scholar]
- 12. Baumann CW, Kwak D, Thompson LV. Assessing onset, prevalence and survival in mice using a frailty phenotype. Aging (Albany NY). 2018;10:4042–4053. doi: 10.18632/aging.101692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seldeen KL, Lasky G, Leiker MM, Pang M, Personius KE, Troen BR. High intensity interval training improves physical performance and frailty in aged mice. J Gerontol A Biol Sci Med Sci. 2018;73:429–437. doi: 10.1093/gerona/glx120 [DOI] [PubMed] [Google Scholar]
- 14. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller RA, Harper JM, Galecki A, Burke DT. Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell. 2002;1:22–29. doi: 10.1046/j.1474-9728.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 16. Martinez de Toda I, Garrido A, Vida C, Gomez-Cabrera MC, Vina J, De la Fuente M. Frailty quantified by the “Valencia Score” as a potential predictor of lifespan in mice. J Gerontol A Biol Sci Med Sci. 2018;73:1323–1329. doi: 10.1093/gerona/gly064. [DOI] [PubMed] [Google Scholar]
- 17. Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12:155–162. doi: 10.1080/03610738608259454 [DOI] [PubMed] [Google Scholar]
- 18. Graber TG, Ferguson-Stegall L, Kim JH, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J Gerontol A Biol Sci Med Sci. 2013;68:1326–1336. doi: 10.1093/gerona/glt032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baumann CW, Kwak D, Ferrington DA, Thompson LV. Downhill exercise alters immunoproteasome content in mouse skeletal muscle. Cell Stress Chaperones. 2018;23:507–517. doi: 10.1007/s12192-017-0857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kane AE, Keller KM, Heinze-Milne S, Grandy SA, Howlett SE. A murine frailty index based on clinical and laboratory measurements: links between frailty and pro-inflammatory cytokines differ in a sex-specific manner. J Gerontol A Biol Sci Med Sci. 2019;74:275–282. doi: 10.1093/gerona/gly117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi: 10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane AE, Shin S, Wong AA, et al. Sex differences in healthspan predict lifespan in the 3xTg-AD mouse model of Alzheimer’s disease. Front Aging Neurosci. 2018;10:172. doi: 10.3389/fnagi.2018.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antoch MP, Wrobel M, Kuropatwinski KK, et al. Physiological frailty index (PFI): quantitative in-life estimate of individual biological age in mice. Aging (Albany NY). 2017;9:615–626. doi: 10.18632/aging.101206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keller K, Kane A, Heinze-Milne S, Grandy SA, Howlett SE. Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro-and anti-inflammatory cytokines in aging male and female C57BL/6 mice [ published online ahead of print September 25, 2018]. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Said SA, Isedowo R, Guerin C, et al. Effects of long-term dietary administration of estrogen receptor-beta agonist diarylpropionitrile on ovariectomized female ICR (CD-1) mice. Geroscience. 2018;40:393–403. doi: 10.1007/s11357-018-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Graber TG, Kim JH, Grange RW, McLoon LK, Thompson LV. C57BL/6 life span study: age-related declines in muscle power production and contractile velocity. Age (Dordr). 2015;37:9773. doi: 10.1007/s11357-015-9773-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12:8–21. doi: 10.1016/j.arr.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 28. Stenholm S, Ferrucci L, Vahtera J, et al. Natural course of frailty components in people who develop frailty syndrome: evidence from two cohort studies [ published online ahead of print August 01, 2018]. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71:1407–1414. doi: 10.1093/gerona/glw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadley EC, Kuchel GA, Newman AB; Workshop Speakers and Participants Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72:980–990. doi: 10.1093/gerona/glx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13(9B):3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Viña J, Borras C, Gomez-Cabrera MC. A free radical theory of frailty. Free Radic Biol Med. 2018;124:358–363. doi: 10.1016/j.freeradbiomed.2018.06.028 [DOI] [PubMed] [Google Scholar]
- 33. LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. doi: 10.1159/000382054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46:685–693. doi: 10.1016/j.exger.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parkening TA, Collins TJ, Smith ER. Plasma and pituitary concentrations of LH, FSH and prolactin in aged female C57BL/6 mice. J Reprod Fertil. 1980;58:377–386. doi: 10.1530/jrf.0.0580377. [DOI] [PubMed] [Google Scholar]
- 36. MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf). 1992;36:339–345. doi: 10.1111/j.1365-2265.1992.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 37. Burger HG, Dudley EC, Hopper JL, et al. The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab. 1995;80:3537–3545. doi: 10.1210/jcem.80.12.8530596 [DOI] [PubMed] [Google Scholar]
- 38. Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–636. doi: 10.1210/jcem-42-4-629 [DOI] [PubMed] [Google Scholar]
- 39. Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716 [DOI] [PubMed] [Google Scholar]
- 40. Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol (1985). 2006;100:548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 41. Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39:248–256. doi: 10.1249/01.mss.0000241649.15006.b8 [DOI] [PubMed] [Google Scholar]
- 42. Cabelka CA, Baumann CW, Collins BC, et al. Effects of ovarian hormones and estrogen receptor alpha on physical activity and skeletal muscle fatigue in female mice. Exp Gerontol. 2019;115:155–164. doi: 10.1016/j.exger.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. doi: 10.1093/gerona/glp082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karvonen-Gutierrez C, Kim C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition. Healthcare. 2016;4:1–16. doi: 10.3390/healthcare4030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond). 1993;84:95–98. doi: 10.1042/Cs0840095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.