Abstract
Background
Age-related hearing loss (impairment in hearing sensitivity and/or higher-order auditory processing) and cognitive decline are common co-occurring impairments in elderly adults. Their relation in the process of aging remains insufficiently understood. We aim to assess the temporal relations of decline in hearing sensitivity, higher-order auditory processing, and cognition in middle-aged adults.
Methods
This study included 1,274 Beaver Dam Offspring Study participants who participated in three examinations (baseline, 5-year, and 10-year follow-up). We assessed hearing sensitivity through pure-tone audiometry (PTA, averaged thresholds of 0.5, 1, 2, 4 kHz of the better ear), higher-order auditory processing as word recognition in competing message (WRCM) using the Northwestern University 6 word list in the better ear, and cognition through trail-making test performance (TMT). Linear mixed-effects models and linear regression models were used to determine associations over time and to what extent these measures influence each other over time.
Results
The longitudinal decline between all functions was associated with the strongest relationships between PTA and WRCM. The effect of baseline PTA on WRCM 10 years later (standardized ß = –.30) was almost twice as big as the effect of baseline WRCM on PTA 10 years later (standardized ß = –.18). The effect of baseline WRCM on TMT 10 years later and vice versa were small (standardized ß = –.05). No directional relationship between PTA and TMT was identified (standardized ß ≤ .02).
Conclusions
While hearing sensitivity might affect higher-order auditory processing, associations between hearing and cognition appear bidirectional and weak in midlife. We need to be cautious before inferring causal effects of hearing on cognition.
Keywords: Central auditory processing, Peripheral hearing, Cognitive decline, Pure-tone audiometry, Speech-in-noise
Age-related hearing loss and cognitive decline are co-occurring disabling conditions in aging (1) and have considerable consequences for quality of life and public health (2,3). Associations between hearing loss and cognitive decline or dementia have been observed in prospective studies (4–11). Still, the underlying mechanisms are insufficiently understood.
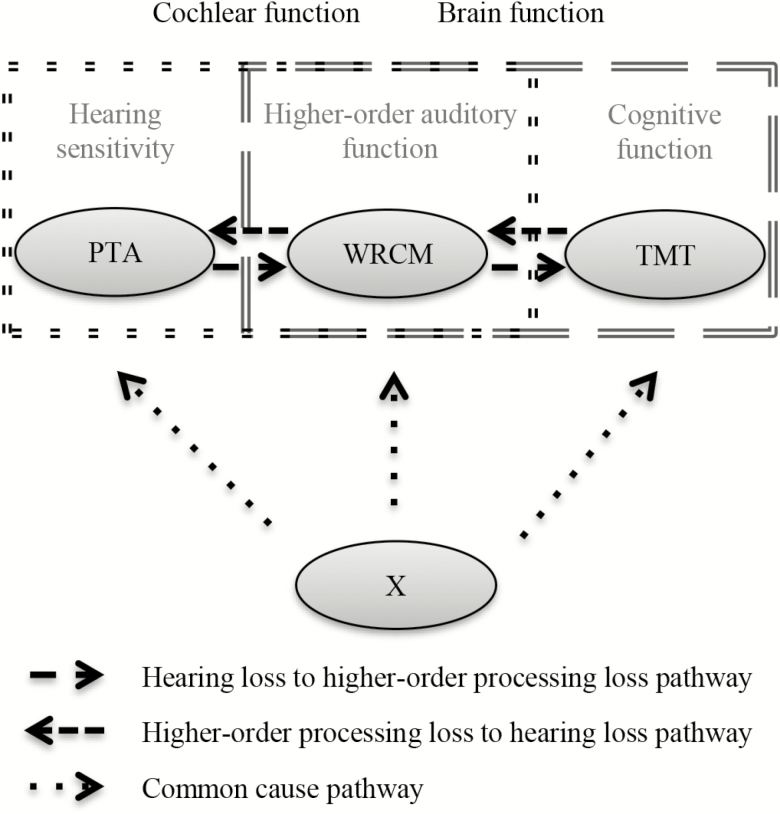
Four competing theories about the association between sensory and cognitive decline exist. (i) The common cause hypothesis (12) suggests a common underlying factor that drives age-related decline in both systems. (ii) The sensory deprivation hypothesis (12), and (iii) the information degradation hypothesis (13) assume that sensory decline precedes cognitive decline. Importantly, according to the sensory deprivation hypothesis only long, chronic sensory deprivation induces cognitive decline (12), whereas the information degradation hypothesis suggests immediate, potentially remediable effects (13). In contrast, (iv) the cognitive load on perception hypothesis (12,13) claims that age-related cognitive decline precedes or drives sensory decline. These theories are not mutually exclusive. Multiple processes are likely involved (14) and the decline in one pathway could affect the other (15). It is not known to what extent each of these mechanisms is involved in declining hearing and cognition.
Age-related hearing loss typically comprises impaired cochlear and central processing function (1) making it difficult to determine mechanisms, particularly with behavioral measures. However, some test performances are more affected by altered cochlear function, others by changes in higher-order central processing. Decreased hearing sensitivity caused by cochlear defects can be measured by pure-tone audiometry (16). More complex tasks, for example, speech in competing message tasks are needed to measure higher-order processing abnormalities (7), which include changes in the auditory nerve (13).
Previous studies investigating hearing and cognition assessed different cognitive domains with various tests (10). Particularly, perceptual processing speed has shown to change throughout the whole adult lifespan in longitudinal studies (17) and should be a good marker of early cognitive change.
Several prospective studies assessed the association between hearing and cognition but had limitations. The majority of longitudinal research focused on older adults. Studies on early changes in midlife are scarce (18). Except for one (19), studies did not assess the temporality of events and compare the strengths of effects from hearing to cognition and vice versa. Finally, most studies investigated audiometrically assessed hearing while higher-order auditory processing has been neglected. Population-based studies on both aspects of hearing have been lacking.
To investigate the complex interplay of decline in different hearing functions and cognition (Figure 1), we conducted this study to determine the longitudinal associations of (i) hearing sensitivity and higher-order auditory processing, (ii) higher-order auditory processing and cognition, and (iii) hearing sensitivity and cognition, in middle-aged adults.
Figure 1.
Theoretical background of the association between hearing and cognition.
Methods
Study Population
This study involves participants of the Beaver Dam Offspring Study, a prospective cohort study of aging. The adult offspring of the population-based Epidemiology of Hearing Loss Study participants were eligible for the Beaver Dam Offspring Study. In the baseline examination (conducted in 2005–2008) 3,298 subjects (aged 21–84 years) participated (20). The 5-year follow-up (2010–2013) showed a participation rate greater than 80% (21) and the 10-year follow-up (2015–2017) a participation rate of 75% of baseline participants. Participation rate in the 10-year follow-up among those who participated in the 5-year follow-up was 86%. The study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin with written informed consent from all participants before each examination. Participants were included in these analyses if they were examined at all three examination waves. We excluded participants who were less than the age of 30 years at baseline, had probable cognitive impairment at baseline, reported the onset of hearing loss before the age of 20 years, or had ever undergone tympanoplasty, mastoidectomy, and/or stapedectomy.
Measurements
Examinations listed later were performed in all three waves. Each examination included tests of hearing, vision, olfaction, cognition, and numerous other measures, a blood draw, and medication intake, medical history, lifestyle, behavior, and hearing health history questionnaires (6). We asked participants about any ear surgeries, their hearing aid use, self-assessed hearing impairment, and the age of onset of the hearing loss, if any. Some participants opted to complete only the questionnaire.
Auditory Assessment
Audiometric testing was conducted in either a sound-treated booth or with insert earphones and followed American National Standards Institute standards for equipment (22,23).
Pure-tone air and bone conduction audiometry followed the American Speech-Language-Hearing Association guidelines (24). Pure-tone air conduction thresholds were obtained at 0.5, 1, 2, 3, 4, 6, and 8 kHz, and bone conduction thresholds at 0.5 and 2 kHz for both ears using clinical audiometers with TDH-50P earphones and ER-3A insert earphones (in cases of probable ear-canal collapse). When necessary, masking was done. Conductive hearing loss was defined as an air-bone gap of 15 dB or greater at 0.5 or 2 kHz. The pure-tone average (PTA) at 0.5, 1, 2, and 4 kHz in the better ear was used as a measure of hearing sensitivity. Higher thresholds indicate poorer hearing.
Word recognition in competing message (WRCM) was assessed with the Northwestern University Auditory Test Number 6 (25,26). Twenty-five words were presented by a single female voice to the better ear at 36 dB hearing level (HL) above the individual’s threshold at 2 kHz. If thresholds at 2 kHz were equal, the right ear was tested. The competing message (single male speaker) was added at a level 8 dB HL below the female speaker’s level in that same ear (26). We used the percentage of correctly repeated words to measure higher-order auditory processing. Higher scores indicate better performances.
Cognitive Assessment
The paper–pencil trail-making test (TMT) versions A (consecutive numbers are to be connected) and B (alternating consecutive numbers and letters are to be connected) were administered (27). Main outcome is completion time in seconds. Longer durations indicate poorer performance. Inability to complete the test in allotted 5 minutes resulted in a score of 301 seconds. The TMT measures attention, speed, and mental flexibility. TMT-B is considered more complex and makes greater demands on perceptual processes and motor speed than TMT-A (28). Therefore, we used TMT-B as a measure of cognitive function.
Furthermore, we conducted the Mini-Mental-State Examination (MMSE) in participants aged 50 years and older (29). Probable cognitive impairment was defined as an MMSE score of less than 24 and/or a history of diagnosed dementia.
Other Variables
We evaluated baseline covariates as potential confounders: age, sex, race, income, education, history of cardiovascular disease, smoking history, history of chemotherapy, years of musical training, occupational noise exposure, history of heavy drinking, regular exercise, loop diuretics intake, non-high-density lipoprotein cholesterol levels (N-HDL-C), high-sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), intima-media thickness, body mass index, hypertension, diabetes, and depression (Supplementary Material 1).
Statistical Analyses
We assessed the strength of associations of (i) hearing sensitivity (PTA) and higher-order auditory processing (WRCM), (ii) higher-order auditory processing and cognition (TMT), and (iii) hearing sensitivity and cognition over 10 years.
For each relationship, we used linear mixed-effects models to quantify the strength of the association using all data from baseline, 5-year, and 10-year follow-up. Each model included age (mean centered at baseline) as the timescale variable and covariates of baseline age in decades (to account for different baseline hazards of different age groups) and sex. The first model included WRCM as dependent variable and PTA and the interaction of PTA with age as independent variables. The second model included TMT as dependent variable and WRCM and the interaction of WRCM with age as independent variables and the third model included TMT as dependent variable and PTA and the interaction of PTA with age as independent variables. Each included term was allowed to vary over time, with the exception of age at baseline in decades and sex. A random intercept and a random slope were also included in each linear mixed-effects model (Equations 1–3 in Supplementary Material 2). We repeated the models including further potential confounding variables.
Next, we used multivariable linear regression models to quantify the strength of the association of each variable at baseline (hearing sensitivity/higher-order auditory processing/cognition) with each other variable at the 10-year follow-up time point. Each model was adjusted for age, sex, and dependent variable at baseline (performance in quartiles). We repeated the models including further potential confounding variables. The strengths of the standardized associations from each linear model were compared to gauge the directionality of effects.
Data Preparation and Confounding
We log-transformed and z-standardized (with baseline values) TMT. Ages of the oldest participants were reported as more than 75 at baseline, more than 80 at 5-year follow-up, and more than 84 at 10-year follow-up.
To evaluate confounding, age- and sex-adjusted models were computed for each potential individual confounder. Variables that were associated with either overall or change in performance of both measures of interest (PTA and WRCM; WRCM and TMT; PTA and TMT) were used as covariates. Resulting covariates for all models were income, education, regular exercise, hsCRP, IL-6, history of cardiovascular disease, smoking, intima-media thickness, occupational noise exposure, and loop diuretics intake. Further covariates for PTA and TMT models were body mass index, history of heavy drinking, depression, years of musical training, diabetes, and N-HDL-C, and for PTA and WRCM models chemotherapy.
Sensitivity Analyses
To evaluate if associations in nonhearing aid users and in participants without conductive hearing loss were consistent with effects in the whole cohort, models were repeated excluding hearing aid users (n = 53) and people with conductive loss (n = 83).
Statistical analyses were conducted using R, version 1.0.44 (30) with packages dplyr (31) and lmerTest (32).
Results
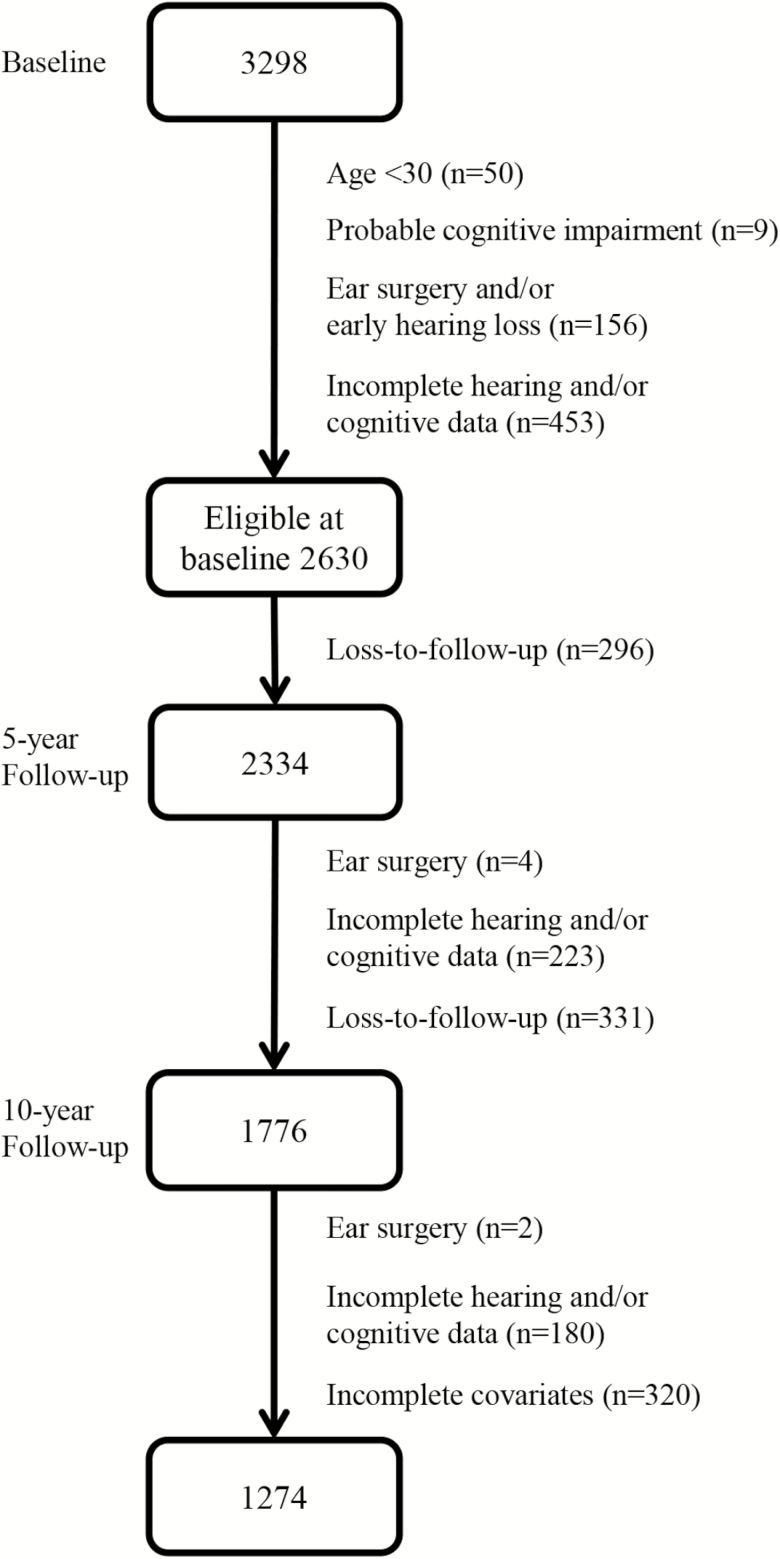
The analysis focused on 1,274 participants (for flow-chart of participant eligibility and inclusion see Figure 2). Most participants with incomplete hearing and cognitive data participated by questionnaire only and did not come for an examination. Differences in age, sex, PTA, WRCM, and TMT between the eligible at baseline sample and the analytic sample were minor and nonsignificant (Supplementary Material 3).
Figure 2.
Flow-chart of participant eligibility and inclusion.
Participants were mostly Caucasian (98%), 51% were women and they had a mean age of 49 (range 30–75) years at baseline (for descriptive statistics see Table 1). PTA decreased on average 0.6 dB per year (0.3 dB for 0.5 kHz, 0.4 dB for 1 kHz, 0.7 dB for 2 kHz, 1.1 dB for 4 kHz), WRCM 0.8% per year, and TMT 0.03 SD per year. The average follow-up time was 9.5 years.
Table 1.
Characteristics of the Analytic Sample (n = 1,274) at Baseline Assessment of the Beaver Dam Offspring Study (2005–2008)
| Age, yrs, M (SD) | 48.7 (8.4) |
| Sex, n (%) | |
| Women | 656 (51.5) |
| Men | 618 (48.5) |
| Income, n (%) | |
| $0–49k | 381 (29.9) |
| $50–99k | 648 (50.9) |
| $100k– | 245 (19.2) |
| Education, n (%) | |
| 12 years or less | 404 (31.7) |
| 13 years or more | 870 (68.3) |
| Smoking, n (%) | |
| Never | 698 (54.8) |
| Former | 380 (29.8) |
| Current | 196 (15.4) |
| CVD, n (%) | 75 (5.9) |
| Exercise at least once a week, n (%) | 762(59.8) |
| Occupational noise exposure, n (%) | 531 (41.7) |
| Loop diuretics intake, n (%) | 8 (0.6) |
| Heavy drinking, n (%) | 225 (17.7) |
| Depression, n (%) | 304 (23.9) |
| Diabetes, n (%) | 62 (4.9) |
| History of chemotherapy, n (%) | 15 (1.2) |
| Intima-media thickness, mm, M (SD) | 0.7 (0.1) |
| hsCRP, mg/L, M (SD) | 2.7 (4.6) |
| IL-6, pg/mL, M (SD) | 2.5 (5.4) |
| N-HDL-C, mg/dL, M (SD) | 154.5 (37.4) |
| Body mass index, M (SD) | 30.4 (6.5) |
| Years of musical training, M (SD) | 4.4 (8.9) |
| PTA, dBHL, M (SD) | 8.9 (8.5) |
| WRCM, %correct, M (SD) | 64.5 (13.9) |
| TMT B, s, M (SD) | 64.1 (25.0) |
Note: CVD = history of cardiovascular disease; dBHL = decibel hearing level; hsCRP = high-sensitivity C-reactive protein; IL-6 = interleukin-6; M = mean; N-HDL-C = non-high-density lipoprotein cholesterol; PTA = pure-tone average 0.5–4 kHz; SD = standard deviation; TMT = trail-making test; WRCM = word recognition in competing message.
Association of PTA and WRCM
Better PTA was associated with better WRCM performance and slower decline in the linear mixed-effects model. WRCM significantly differed by –0.36% per dB difference in PTA (95% CI: –0.42, –0.29; standardized ß = −.22). WRCM decline significantly accelerated 0.03% per year per dB difference in PTA (95% CI: –0.03, –0.02; standardized ß = −.28; Table 2).
Table 2.
Longitudinal Associations of PTA, WRCM, TMT Over 10-Year Follow-up
| % Change in WRCM (95% CI) | SD Change in TMT (95% CI) | SD Change in TMT (95% CI) | |||
|---|---|---|---|---|---|
| [Standardized effect]a | [Standardized effect]a,b | [Standardized effect]a,c | |||
| Determinant | Determinant | Determinant | |||
| Age, yr | –0.37 (–0.47, –0.27) [ß = –.21] | Age, yr | 0.03 (0.02, 0.04) [ß = .27] | Age, yr | 0.007 (0.001, 0.01) [ß = .06] |
| PTA, dB | –0.36 (–0.42, –0.29) [ß = –.22] | WRCM, % | –0.002 (–0.003, 0.0003) [ß = –.02] | PTA, dB | 0.005 (0.001, 0.01) [ß = .05] |
| PTA × age, dB/yr | –0.03 (–0.03, –0.02) [ß = –.28] | WRCM × age, %/yr | –0.0003 (–0.0004, –0.0001) [ß = –.14] | PTA × age, dB/yr | 0.001 (0.0002, 0.001) [ß = .09] |
Note: DB = decibel; PTA = pure-tone average 0.5–4 kHz; SD = standard deviation; TMT = trail-making test; WRCM = word recognition in competing message.
In linear regression models, the significant effect of PTA baseline on WRCM at 10-year follow-up (standardized ß = –.30) was almost twice as big as the significant effect of WRCM baseline on PTA at 10-year follow-up (standardized ß = –.18; Figure 3). Effect sizes were not attenuated with adjustment for confounding (Table 3).
Figure 3.
Strengths of temporal effects between pure-tone audiometry, word recognition in competing message, and trail-making test.
Table 3.
Relationship Between Each Measure at Baseline (PTA/WRCM/TMT) With Each Other Measure at the 10-Year Follow-up
| Outcome Change (95% confidence interval) per one unit increase [standardized effect] | ||||||
|---|---|---|---|---|---|---|
| PTA at 10-year follow-up, dB | WRCM at 10-year follow-up, % | TMT at 10-year follow-up, SD | ||||
| Determinant | Age-sex adjusted | Fully adjusted | Age-sex adjusted | Fully adjusted | Age-sex adjusted | Fully adjusted |
| PTA at baseline, dB | –0.61a (–0.71, –0.52) |
–0.61a,b (–0.71, –0.51) [ß = –.30] |
0.005a (–0.001, 0.011) |
0.002a,b,c (–0.004, 0.008) [ß = .02] |
||
| WRCM at baseline, % | –0.15a (–0.17, –0.12) |
–0.15a,b (–0.17, –0.12) [ß = –.18] |
–0.005a (–0.01, –0.002) |
–0.004a,b,d (–0.01, –0.001) [ß = –.05] |
||
| TMT at baseline, SD | 0.17a (–0.22, 0.56) |
0.13a,b,c (–0.27, 0.54) [ß = .01] |
–1.20a (–2.03, –0.37) |
–0.93a,b,d (–1.78, –0.07) [ß = –.05] |
Note: DB = decibel; PTA = pure-tone average 0.5–4 kHz; SD = standard deviation; TMT = trail-making test; WRCM = word recognition in competing message.
Association of WRCM and TMT
Better WRCM was associated with slower TMT decline in the linear mixed-effects model. TMT performance differed –0.002 SD per 1% WRCM performance difference (95% CI: –0.003, 0.0003; standardized ß = –.02). TMT performance decline significantly accelerated 0.0003 SD per year per 1% WRCM performance difference (95% CI: –0.0004, –0.0001; standardized ß = –.14; Table 2).
In linear regression models, the significant effects of WRCM baseline on TMT at 10-year follow-up and TMT baseline on WRCM at 10-year follow-up were equal (standardized ß = –.05; Figure 3). Effect sizes slightly decreased with adjustment for confounding (Table 3).
Association of PTA and TMT
Better PTA was associated with better TMT performance and slower decline in the linear mixed-effects model. TMT performance significantly differed 0.005 SD per dB difference in PTA (95% CI: 0.001, 0.01; standardized ß = .05). TMT decline significantly accelerated 0.001 SD per year per dB PTA difference (95% CI: 0.0002, 0.001; standardized ß = .09; Table 2).
In linear regression models, the nonsignificant effects of PTA baseline on TMT at 10-year follow-up and TMT baseline on PTA at 10-year follow-up were comparable but negligible (standardized ß = .02 and .01, respectively; Figure 3). Effect sizes decreased with adjustment for confounding (Table 3).
Sensitivity Analyses
Effect sizes remained the same, when excluding participants with conductive hearing loss (n = 83). In analyses excluding hearing aid users (n = 53), effects were slightly weaker in associations between PTA and WRCM and similar in the remaining (Supplementary Material 4).
Discussion
We found weak relationships between two measures of hearing and cognition in middle-aged adults over 10 years. There was no predominant pathway of effects going from hearing to cognitive decline and vice versa. The pathway from hearing to higher-order processing decline was more pronounced in associations between the two hearing tests.
Our results are consistent with longitudinal studies of hearing sensitivity, higher-order auditory processing, and cognition (7–10,33,34) and extend these to different aspects of hearing, middle-aged adults, and assessment of temporality of effects. We used two different hearing measures to investigate the complex relationship between auditory and cognitive processing. Decreased hearing sensitivity caused by cochlear defects can be measured by pure-tone audiometry (16) and reflects the most sensorineural processing measure. The more complex task of speech understanding in competing message was operationalized as a measure of higher-order auditory processing capability (7). To assess most upstream central processing, we used the cognitive test. Importantly, as previous research primarily focused on elderly adults with more advanced hearing loss (10), we extended findings to middle-aged adults facilitating our understanding of early development of age-related diseases.
Longitudinal relationships between the hearing functions were moderate. Effects between hearing and cognition were small and weakest for hearing sensitivity and cognition. This is consistent with results of a recent meta-analysis (r = –.09), which combined nine longitudinal studies on hearing sensitivity and different cognitive domains (10) including a majority of samples of elderly participants (average age 65 years and older). Such small effects imply that even if there was a causal effect of hearing on cognition, any potential benefit from amplification with hearing aids for restoring or preserving cognitive functions would be limited, and hearing aids do not restore normal hearing.
To assess the different possible mechanisms for the longitudinal associations, we compared the extent of effects going both directions—from hearing decline to higher-order central processing decline and vice versa. We found support for both pathways.
Effect of Hearing on Higher-Order Processing
Different theories propose causal effects of hearing loss on cognitive decline. The information degradation hypothesis (13) states that hearing loss increases cognitive load during auditory processing which negatively affects cognitive functioning. Correspondingly, elderly adults with hearing impairment recruit wider brain networks during perception (35). According to this hypothesis, effects of hearing on cognition are immediate and potentially remediable. In contrast, the sensory deprivation hypothesis (12) posits that perceptual decline causes permanent cognitive decline. Hearing impairment would alter brain structure and cause cognitive impairment (36). Animal studies indicate reorganization within the central auditory system after sensory deprivation (37). Correspondingly, our results indicate a strong effect of hearing sensitivity on auditory processing. However, the biological mechanism how potential reorganizations lead to impaired central auditory processing and how this could further cause detrimental brain changes and cognitive decline remains unknown. We found limited support for effects of hearing on cognition as measured by TMT in midlife. There were weak effects from higher-order auditory processing to cognition and none from hearing sensitivity to cognition. Consistently, previous studies report stronger effects of higher-order auditory function than of hearing sensitivity on cognition (11). Furthermore, associations between hearing sensitivity and cognition appear consistently small in nonimpaired populations. Effects are stronger when cohorts are older and/or more hearing impaired (10). A recent study found an effect of hearing sensitivity on cognition only in verbal/auditory tests but not nonauditory tests (including TMT) (19), which might reflect the task impurity problem.
Effect of Higher-Order Processing on Hearing
According to the cognitive load hypothesis (12,13) declining cognitive capacity places a cognitive load on perception, which is then poorer. Evidence for this hypothesis is scarce (14) and previous work questions that cognitive decline precedes sensory decline (19,38). Accordingly, we saw a very small effect from cognition as measured by TMT to higher-order auditory processing and the effect from higher-order auditory processing to hearing sensitivity, was only half as big as the opposite effect. Therefore, this mechanism might be present but not the most dominant one.
The Common Cause Effect
Besides both causal pathways being simultaneously present, common causes might induce decline in both systems. Several sensory functions have been related to cognitive function (6) and concurrent changes in multiple perceptual and cognitive domains suggest a systemic central nervous system pathology and common neurodegenerative etiology. We found pathways between hearing and cognition and vice versa were of equivalent magnitude. Furthermore, effects from hearing to cognition substantially decreased with adjustment for known confounders. Residual confounding might exist. Promising candidates for common causes of neurodegeneration may be cardiovascular abnormalities, metabolic dysregulation, and inflammation (36,39). We might still lack adequate, sensitive measures for these processes.
Limitations
Our middle-aged sample showed little longitudinal change in higher-order auditory and cognitive function, which might contribute to the weak effects between hearing and cognition. The inclusion of complete cases only and loss to follow-up might have further prompted this, given a potentially rather healthy sample. Yet, the eligible baseline sample and the analytic sample did not differ in relevant baseline characteristics. Longer follow-ups might be needed to see effects.
We could not replicate the significant small effect of PTA on TMT found in the linear mixed-effects model with less data points in the linear regression model, likely due to reduced power to detect this small effect.
Behavioral measures cannot completely distinguish sensory from higher-order auditory processing. Pure-tone audiometry relies on higher-order processing, for example, regarding behavioral responses. Higher-order auditory processing was tested with an adjusted hearing level. Still, hearing levels affect speech understanding, for example, through distorted signal (16). This task impurity might have induced overestimation of effects between hearing tests. In addition, different sensitivities in hearing and cognition measures might affect the assessment of temporality of effects. However, we controlled for baseline levels of outcome measures, which limits potential biases. Unfortunately, only one cognitive test (TMT) was collected at each visit, which limits generalizability to other cognitive functions and/or general cognitive ability.
Age-related hearing loss and cognitive decline have considerable consequences for quality of life and public health. Better understanding of mechanisms for their co-occurrence in the process of aging has potential to inform future research directed at prevention and treatment applications. To explore a common pathology a systematic, holistic investigation of neurotoxins, metabolic, vascular, and inflammation processes as well as more sensitive measures, for example, of microvascular pathology might advance the field.
Conclusion
Hearing sensitivity decline might affect higher-order auditory processing decline. Higher-order auditory processing had bidirectional relationships over time with cognition. Worse baseline hearing was associated with cognitive decline and worse baseline cognition was associated with hearing decline. However, effects were weak in middle-aged adults. We should be cautious in concluding causal effects, as underlying biological mechanisms remain fairly unclear. Improved hearing might have limited benefit for prevention or delay of cognitive decline.
Funding
This work was supported by the National Institute on Aging of the National Institutes of Health (grant number R01 AG021917 to K.J.C.); and an Unrestricted Grant from Research to Prevent Blindness, Inc. to the UW. Madison Department of Ophthalmology and Visual Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None of the authors have any conflicts.
Supplementary Material
References
- 1. Humes LE, Dubno JR, Gordon-Salant S, et al. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol.. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist.. 2003;43:661–668. doi: 10.1093/geront/43.5.661 [DOI] [PubMed] [Google Scholar]
- 3. Wilson RS, Boyle PA, Segawa E, et al. The influence of cognitive decline on well-being in old age. Psychol Aging. 2013;28:304–313. doi: 10.1037/a0031196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J Am Geriatr Soc.. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x [DOI] [PubMed] [Google Scholar]
- 5. Lin FR, Yaffe K, Xia J, et al. ; Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. 2016;64:1981–1987. doi: 10.1111/jgs.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gates GA, Beiser A, Rees TS, D’Agostino RB, Wolf PA. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J Am Geriatr Soc.. 2002;50:482–488. doi: 10.1046/j.1532-5415.2002.50114.x [DOI] [PubMed] [Google Scholar]
- 8. Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg.. 2011;137:390–395. doi: 10.1001/archoto.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gates GA, Cobb JL, Linn RT, Rees T, Wolf PA, D’Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg.. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010 [DOI] [PubMed] [Google Scholar]
- 10. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144:115–126. doi: 10.1001/jamaoto.2017.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan J, Sun Y, Sang S, Pham JH, Kong W. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep.. 2018;8:2137. doi: 10.1038/s41598-018-20496-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging.. 1994;9:339–355. doi: 10.1037/0882-7974.9.3.339 [DOI] [PubMed] [Google Scholar]
- 13. Schneider BA, Pichora-Fuller MK. Implications of Perceptual Deterioration for Cognitive Aging Research. In: Craik FIM, Salthouse TA, eds. The Handbook of Aging and Cognition (2nd Ed.). Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2000:155–219. [Google Scholar]
- 14. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev.. 2015;23(Pt B):154–166. doi: 10.1016/j.arr.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 15. Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol. 2017;2:69–79. doi: 10.1002/lio2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jerger J, Chmiel R, Wilson N, Luchi R. Hearing impairment in older adults: new concepts. J Am Geriatr Soc.. 1995;43:928–935. doi: 10.1111/j.1532-5415.1995.tb05539.x [DOI] [PubMed] [Google Scholar]
- 17. Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci.. 2004;5:87–96. doi: 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- 18. Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d [DOI] [PubMed] [Google Scholar]
- 19. Armstrong NM, An Y, Ferrucci L, Deal JA, Lin FR, Resnick SM. Temporal sequence of hearing impairment and cognition in the Baltimore Longitudinal Study of aging. Journals Gerontol Ser A. 2018. doi: 10.1093/gerona/gly268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors: the Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg.. 2011;137:432–439. doi: 10.1001/archoto.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer ME, Cruickshanks KJ, Nondahl DM, et al. Dichotic digits test performance across the ages: results from two large epidemiologic cohort studies. Ear Hear.. 2017;38:314–320. doi: 10.1097/AUD.0000000000000386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. American National Standards Institute. Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. New York, NY: ANSI, S3.1; 1999. [Google Scholar]
- 23. American National Standards Institute. Specification for Audiometers. New York, NY: ANSI, S3.6; 2010. [Google Scholar]
- 24. American Speech-Language-Hearing Association (ASHA). Guidelines for manual pure-tone threshold audiometry. ASHA. 1978;20:297–301. [PubMed] [Google Scholar]
- 25. Wilson RH, Zizz CA, Shanks JE, Causey GD. Normative data in quiet, broadband noise, and competing message for Northwestern University Auditory Test No. 6 by a female speaker. J Speech Hear Disord. 1990;55:771–778. doi: 10.1044/jshd.5504.771 [DOI] [PubMed] [Google Scholar]
- 26. Wiley TL, Cruickshanks KJ, Nondahl DM, Tweed TS, Klein R, Klein BE. Aging and word recognition in competing message. J Am Acad Audiol.. 1998;9:191–198. [PubMed] [Google Scholar]
- 27. Reitan RM. Trail Making Test Manual for Administration and Scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 28. Strauss E, Sherman EMS, Spreen O.Attention. In: Strauss E, Sherman EMS, Spreen O, eds. A Compendium of Neuropsychological Tests. 3rd ed New York, NY: Oxford University Press; 2006; pp. 546–677. [Google Scholar]
- 29. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 30. R Core Team. R: a Language and Environment for Statistical Computing. 2015. [Google Scholar]
- 31. Wickham H, Francois R.. dplyr: a Grammar of Data Manipulation. 2015. [Google Scholar]
- 32. Kuznetsova A, Brockhoff PB, Christensen RHB.. lmerTest: tests in Linear Mixed Effects Models. 2015. [Google Scholar]
- 33. Pronk M, Deeg DJ, Festen JM, et al. Decline in older persons’ ability to recognize speech in noise: the influence of demographic, health-related, environmental, and cognitive factors. Ear Hear.. 2013;34:722–732. doi: 10.1097/AUD.0b013e3182994eee [DOI] [PubMed] [Google Scholar]
- 34. Dubno JR, Lee FS, Matthews LJ, Ahlstrom JB, Horwitz AR, Mills JH. Longitudinal changes in speech recognition in older persons. J Acoust Soc Am.. 2008;123:462–475. doi: 10.1121/1.2817362 [DOI] [PubMed] [Google Scholar]
- 35. Erb J, Obleser J. Upregulation of cognitive control networks in older adults’ speech comprehension. Front Syst Neurosci.. 2013;7:116. doi: 10.3389/fnsys.2013.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whitson HE, Cronin-Golomb A, Cruickshanks KJ, et al. American geriatrics society and national institute on aging bench-to-bedside conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc.. 2018;66:2052–2058. doi: 10.1111/jgs.15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lesicko AM, Llano DA. Impact of peripheral hearing loss on top-down auditory processing. Hear Res.. 2017;343:4–13. doi: 10.1016/j.heares.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Humes LE, Busey TA, Craig J, Kewley-Port D. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten Percept Psychophys. 2013;75:508–524. doi: 10.3758/s13414-012-0406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menardo J, Tang Y, Ladrech S, et al. Oxidative stress, inflammation, and autophagic stress as the key mechanisms of premature age-related hearing loss in SAMP8 mouse Cochlea. Antioxid Redox Signal.. 2012;16:263–274. doi: 10.1089/ars.2011.4037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.