Abstract
DNA methylation (DNAm) algorithms of biological age provide a robust estimate of an individual’s chronological age and can predict their risk of age-related disease and mortality. This study reviewed the evidence that environmental, lifestyle and health factors are associated with the Horvath and Hannum epigenetic clocks. A systematic search identified 61 studies. Chronological age was correlated with DNAm age in blood (median .83, range .13–.99). In a meta-analysis body mass index (BMI) was associated with increased DNAm age (Hannum β: 0.07, 95% CI 0.04 to 0.10; Horvath β: 0.06, 95% CI 0.02 to 0.10), but there was no association with smoking (Hannum β: 0.12, 95% CI −0.50 to 0.73; Horvath β:0.18, 95% CI −0.10 to 0.46). DNAm age was positively associated with frailty (three studies, n = 3,093), and education was negatively associated with the Hannum estimate of DNAm age specifically (four studies, n = 13,955). For most other exposures, findings were too inconsistent to draw conclusions. In conclusion, BMI was positively associated with biological aging measured using DNAm, with some evidence that frailty also increased aging. More research is needed to provide conclusive evidence regarding other exposures. This field of research has the potential to provide further insights into how to promote slower biological aging and ultimately prolong healthy life.
Keywords: Biomarker, Epigenetics, Epigenetic clock, Frailty, BMI
Aging is an inevitable process that eventually culminates in death. It is characterized by the progressive loss of function at the cellular, tissue and organ level, and can be observed by the general decline in physical and cognitive performance (1). Aging is also the most important risk factor for many human diseases such as diabetes, cancer, cardiovascular diseases, and dementia (2). Aging and related diseases are a global public health challenge (3).
Chronological age is, however, not a perfect measure of the aging process. The speed at which an individual ages relative to their chronological age varies, as does their risk of age-related diseases (4). Identifying ways to prolong healthy life perhaps through reducing the rate of aging, as well as reducing the occurrence of age-related disease, requires the development of human biomarkers of aging.
Epigenetic processes are reversible chemical and structural alterations to the genome that can lead to long-term changes in gene activity and consequently phenotype, without altering the underlying DNA sequence (5,6). DNA methylation (DNAm) is the most commonly studied epigenetic modification and typically involves the addition of a methyl group to a cytosine-guanine dinucleotide (termed “CpG” site). DNAm is influenced by both genetic and environmental factors, with an individual’s DNA methylome reflecting the cumulative exposure to external stimuli over their lifetime (7). As cells age, the epigenetic patterns also age and appear to be markers of the overall health and lifespan of the cell. There is a global decline in DNAm (7), as well as increased variability (8,9). However, a number of site-specific patterns of DNAm have also been observed that may play a role in the aging processes (10,11). These age-related DNAm changes have lead researchers to develop new biomarkers of biological age.
Although not the first studies of this kind (12), in 2013 two important studies were published that identified an algorithm based on the DNAm status at a set of specific CpG sites that were shown to vary with age (13,14). These algorithms, referred to as the Hannum clock and Horvath clock respectively, provide a robust estimate of a person’s chronological age, and an accurate predictor of a person’s functional capability (15). The difference between an individual’s DNAm age calculated using these clocks and their chronological age has been shown to be predictive of their overall health and longevity (16–18). An older DNAm age compared to chronological age is termed accelerated epigenetic aging, and has been associated with a number of age-related conditions, diseases and mortality risk (15,18). The pan tissue clock by Horvath has two unique properties: (a) it appears to apply to all nucleated cells in the body and (b) it applies to the entire age spectrum: from children to centenarians (15). The blood-based clock by Hannum was constructed in adults and hence leads to a substantial offset in children. The Hannum clock exhibits stronger correlations with blood cell counts than the Horvath clock that may be beneficial when it comes to predicting lifespan (16,18).
Since publication of these clocks, researchers have become increasingly interested in the variability in DNAm age between individuals, and in particular in the extent to which this could be impacted by environmental factors. It is known that DNAm patterns are influenced by external stimuli (19). Determining which environmental and lifestyle factors could influence DNAm age may thus help identify modifiable factors that could promote healthy aging. Over the last 5 years, there has been a considerable number of studies that have measured the epigenetic clock and investigated factors that were associated with age acceleration (AA) (15,20,21). A detailed review of possible mechanisms underlying the association between these factors and biological aging is beyond the scope of this review, but has been reviewed in detail recently (15). However, no systematic review has yet brought together this evidence to summarize the extent to which different environmental stimuli are associated with DNAm age.
The aim of this review is to identify and synthesize the evidence that in humans, environmental, health and lifestyle factors are associated with DNAm age, based on the two widely validated epigenetic clock measures.
Method
This systematic review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISM) guidelines (http://www.prisma-statement.org) (22). The protocol for the systematic review was registered with the International Prospective Register of Ongoing Systematic Reviews (PROSPERO) (23), registration number CRD42018108363.
Inclusion Criteria
This review considered all types of community-based, case-control or patient studies. We included studies that measured any type of “environmental” exposure and excluded studies if they focused on only ethnicity, age or sex. Studies were also excluded if they investigated these environmental exposures as outcomes predicted by the epigenetic clock (rather than the inverse), that meant excluding studies that measured the epigenetic clock at baseline and tracked disease incidence or mortality over time.
Epigenetic Clock
Studies met our eligibility criteria if they measured peripheral DNAm at the same time as the exposure(s) or prospectively, and if they used at least one of the widely validated and recognized epigenetic clock calculators by Horvath (14) or Hannum (13). We decided to focus on only these two epigenetic clocks, rather than including all epigenetic clocks (eg, (20,21,24–26)) for a number of reasons. These epigenetic clocks have been studied in a wide range of contexts and with regards to various exposures. It was thus highly likely that the scope and size of this review would already be large. Many of the other epigenetic clocks have only been assessed in a limited number of studies/exposures, meaning they could contribute little to this systematic review that aims to bring together all of the evidence. It would also be difficult to combine results across more than two different epigenetic clock measures, given the likely heterogeneity.
Horvath’s methylation age was based on DNAm measured at 353 CpGs (14), while Hannum’s methylation age was computed using a linear sum of methylation from 71 CpGs (13). Epigenetic AA, the discrepancy between an individual’s DNAm age and chronological age, is then frequently calculated. These measures have also been adapted to incorporate differences in cell counts when deriving the estimates (16,27). Studies eligible for inclusion measured at least one of the following:
AA-Horvath: residuals after regressing Horvath’s estimate of epigenetic age on chronological age.
AA-Hannum: residuals after regressing Hannum’s estimate of epigenetic age on chronological age.
Intrinsic epigenetic AA (IEAA): as AA-Horvath but is independent of blood cell counts.
Extrinsic epigenetic AA (EEAA): as AA-Hannum plus a weighted average of age-associated cell counts.
Search Strategy
Full details are provided in the Supplementary Methods. We conducted a systematic search of Embase, MEDLINE, Cochrane Central Register of Controlled Trials and PsychINFO to identify relevant articles. Studies published up until August 10, 2018 were eligible for inclusion. All steps were conducted independently by at least two authors.
Results
Search Results
The search yielded 483 citations articles; 117 were selected for full-text assessment and 61 studies were eligible for inclusion in this systematic review (Supplementary Figure S1 and Supplementary Results for full details).
Participants
The majority of studies involved adults (mean age between 18 and 65) and had sample sizes between 100 and 1,000. The smallest study only included 18 participants (28) and the largest 5,100 (29). Sixteen studies used a case-control design and most involved a subsample of participants from a larger prospective community-based cohort. Three studies used publicly available data (29–31), and one used registry data (28). A number of studies combined data from multiple cohorts (27,32), including one meta-analysis (33). Even when excluding the meta-analysis (33), multiple cohorts were used in more than one study; Women’s Health Initiative (WHI) Study (27,32,34–36), Lothian Birth Cohort 1936 (LBC1936) (37–39), Medical Research Council (MRC) National Survey of Health & Development (NSHD) (32,40,41), The Parkinson’s disease, Environment & Genes (PEG) study (27,32,42), Veterans Affairs Normative Aging Study (43,44), Adults in the Making Project (AIM) (45,46), Strong African American Healthy Adult Project (SHAPE) (45–48), Grady Trauma Project (GTP) (49,50), Family and Community Health Study (FACHS) (30,51), Invecchiare nel Chianti (INC) (32,36), and the Epidemiological Investigations of the Changes of Preventing, Recognizing Early and Optimally Treating Chronic Diseases in an Elderly Population (ESTHER) cohort (52,53).
Full details regarding the risk of bias assessment are given in the Supplementary Results and Supplementary Tables S1 and S2.
DNAm Age Outcome
Two studies used only saliva samples (54,55), with the remaining blood samples, but some used both blood and saliva or buccal epithelium (27,40,56–58). These samples were collected either at the same time as when the exposure was measured, or at a later time-point. Most studies measured DNAm using the Illumina Infinium HumanMethylation450K microarray (Illumina, San Diego, CA), but five studies used the newer Illumina Infinium MethylationEPIC BeadChip (28,54,59–61), and one the older 27K microarray (27). DNAm age was calculated using Horvath and/or Hannum’s epigenetic clock. Of the 61 studies, 48 reported the correlation between DNAm age and chronological age in their sample that was generally strong (range .13–.99, median .82), but not significant in one study (41) that included a very narrow age range (age SD 0.16).
Summary of Findings
The 61 studies included in this review examined a range of environmental, lifestyle and health exposures, and the findings are grouped according to common exposures in the referred tables and summaries below.
Socioeconomic Status, Education
Seven studies of 15,506 participants investigated the association between the level of education and DNAm age, and four reported no significant association (27,41,51,62) (Supplementary Table S3). However, four studies, including the largest study, reported significant associations specifically with the Hannum clock. The WHI study of older postmenopausal women found that education level was negatively associated with EEAA (36), that was replicated in a second smaller sample of WHI participants (n = 1,461) (27). Another two studies found a negative association between education and DNAm age, but only with the Hannum clock (59,63). Lower socioeconomic status trajectory in early life was associated with increased AA-Horvath (64), as were lower per capita income, increasing financial pressure (51) and high levels of economic hardship (48). Two studies of SEP and one socioeconomic status, reported no significant associations with DNAm age (40,58) and (63), respectively, although the largest study indicated that lower socioeconomic status was associated with higher DNAm age (59).
Smoking, Alcohol, Diet, Body Mass Index
Thirteen studies investigated the association between lifestyle choices such as smoking, alcohol consumption, and diet, or body mass index (BMI), with DNAm age (Table 1). The nine studies of smoking included a total of 16,844 participants and six of these reported no significant association (this included studies that had measured both Horvath and Hannum clocks and derivations) (27,36,51,53,62,65), with a seventh reporting no association in blood specifically (41). Two studies reported significant positive associations, with smoking increasing DNAm age (59,63). A proxy measure of smoking using genetic data was also associated with increased AA-Hannum (30).
Table 1.
Studies Investigating the Association Between Smoking, Alcohol Consumption, Diet or Body Mass Index and DNAm Age
| Ref | Study (Country) | n: Mean Age (SD) or Range, Sex, Other Information | Exposure | Sample; Platform | Epigenetic Clock | ↑ DNAm Age [r With Chronological Age] | Adjustments |
|---|---|---|---|---|---|---|---|
| (31) | Publically available data, 3 studies (United States) | Total 274. Further details not provided | BMI | Blood; 27K or 450K | AA-Horvath | ↑ BMI (r = .26, p = .012) in 1 study. NS other 2 studies (r = −.07 to −.18, p ≥ .10). [r = .88] | Age |
| (30) | EAS & FACHS studies (United States) | EAS 656: age 63 (15), % ♀ not stated, white. FACHS 180, age 49 (9), 62% ♀, black | DNAm proxies of smoking, alcohol | Blood; not stated | AA-Hannum |
Smoking (β: 8.73, p < .001). Alcohol, low and high levels of alcohol (nonlinear, p < .05). [r = .71] |
None |
| (53) | Esther cohort study (Germany) | 1477, 62 (6.5), 49.4% ♀ | Cotinine levels in serum, smoking | Blood; 450K | AA-Horvath, Hannum |
NS Cotine (β: 0.0011 & β: −0.0013, p > .79, Horvath & Hannum, respectively) NS Smoking (Horavth current β: −0.13, p = .88; former β: 0.10, p = .79; Hannum current β: −0.34, p = .43; former β: −0.51, p = .16). [r = .75, .78] |
Age, sex, BMI, alcohol, health |
| (27) | WHI cohort (United States) from a 9 cohort study | 1462 WHI: aged 50–80, 100% ♀. Different racial/ethnic groups. | Smoking (3 grps), alcohol (4 grps), BMI | Blood; 450K | IEAA EEAA |
NS Alcohol (eg, heavy IEAA β: −0.40, p = .69; EEAA β: −0.83, p = .45). NS Smoking (eg, current IEAA β: 0.38, p = .72; EEAA, β: 0.12, p = .93). NS BMI (IEAA β: 0.035, p = .10; EEAA β: 0.045, p = .09) [r = .65 to .93] |
Age, sex, cell % education, ethnicity |
| (51) | FACHS (United States) | 100: age 48.5 (9.2), 100 ♀, 94% African-American, 17.8% <12th grade education | Smoking, alcohol, diet, BMI | Blood; 450K | AA-Hannum |
NS Smoking (β: 0.04), NS Alcohol (β: −0.12), NS BMI (β: −0.08), NS Diet (β: 0.03) Exact p values not stated. [r = .82] |
Education, exercise, trauma, married |
| (35) | WHI (United States) | 43: age 61.5 (6.9), 16 year FU. Replication sample of 157, aged 50–79. 100% postmenopausal ♀. | BMI | Blood; 450K | AA-Horvath | ↑ BMI (β: 0.29, p = .001) & Waist circumference (β: 0.48, p = .03). NS in replication (β: 0.04, p = .56). [r = .89] | Age, cell %, ethnicity, smoking |
| (36) | WHI Study (United States) & InCHIANTI study (Italy). | 4,173: age 64 (7.1), 100% ♀; 402: age 71 (16), 56% ♀, all |
Smoking, alcohol, diet, BMI | Blood, serum; 450K. | IEAA, EEAA | ↓ Fish Intake EEAA (β: −2.92, p = .003), NS IEAA β: −0.38, p = .71). ↓ Poultry Intake IEAA (β: −3.30, p = .001), NS EEAA (β: −0.73, p = .46). ↓ Alcohol EEAA (β: −3.23, p = .001), NS IEAA β: −0.36, p = .72). ↑ BMI both (βs: 4.14 to 4.86, p < .001) NS Smoking (βs: 0.07 to 0.12, p > .90) |
Various |
| (66) | YFS, 25 year FU & V90+, 4 year FU (Finland) | YFS 183: age 19.2 (3.2), 60.7% ♀; 183: age 44.2 (3.2), 60.7% ♀. V90 + 119: aged 90 (0), %♀ not stated. | BMI and 25 year change in BMI | Blood; 450K | IEAA | ↓ BMI (β: −0.236, p = .002) & change in BMI in middle-aged adults (r = −.19, p = .009). NS young adults (r = −.11, p = .14) & nonagenarians (r = .12, p = .21). [r = −.14] | None |
| (41) | MRC-NSHD cohort; ALSPAC (replication) (United Kingdom) | 790: age 53, 100% ♀. Followed until age 60–64. 988: age 46.9 (4.5), 100% ♀. |
Smoking, Change in BMI | Buccal & Blood; 450K | AA-Horvath | ↑ BMI Buccal (β: 0.085, p = .02), NS Blood (β: 0.04, p = .42), ↑ Blood ALSPAC (β: 0.13, p = .001). ↓ Smoking Buccal (current β: −1.8, p = .001), NS Blood (β: −0.16, p = .30), NS ALSPAC (β: 0.17, p = .43). [r = .02]. | Cell % |
| (62) | GOCS (Chile) | 94: age 8.1–12, 100% ♀, low-middle income | Smoking, BMI, weight | Blood; 850K | AA-Horvath | NS Smoking (r = .01), NS BMI (r = .06), NS Weight (maternal r = .12 & birth r = .14), exact p not stated). [r = .34] | Cell %, birth maternal BMI, education |
| (63) | MCC study (Australia) | 2,818: age 27–76, 39% ♀, healthy controls matched to cancer cases | Smoking, alcohol, healthy eating index, BMI (3 categories) | Blood; 450K | AA-Horvath, AA-Hannum, IEAA, EEAA |
Smoking (EEAA β: 2.12, p < .001; IEAA β: 1.33, p = .02) Overweight & Obese (EEAA β: 0.69, p = .01; β: 1.24, p < .001). NS IEAA β: 0.52, p = .09; β: 0.29, p = .47) NS Alcohol (βs −0.01 to 0.47, p > .10) NS Healthy Eating (βs −0.01 to −0.02 p ≥ .10) [r = .73 to .76] |
Various |
| (65) | Family study, GOLDN (United States) | 830: age 48 (16), ~52% ♀, Caucasian. High-fat meal challenge after fasting. | Smoking, alcohol | Whole blood; 450K | IEAA, EEAA |
Alcohol drinkers >median IEAA (∆+9.0%, p = .01). NS EEAA (∆+4.1%, p = .23). NS Smokers (IEAA ∆0.2%, p = .89, EEAA ∆1.2%, p = .52). [r = .94, 0.91] |
Age, cell % smoking, drinking, lipid |
| (59) | GS-SFHS (Scotland) | 5100: age 48.5 (14.0), 61.6% ♀ | Smoking, BMI | Blood; 850K | IEAA, EEAA |
Smoking (IEAA β: 0.03, p = .03; EEAA β:0.06, p < .001). ↑ BMI (IEAA β: 0.09, p < .001; EEAA β: 0.06, p < .001). |
Age, sex |
Note: AA = epigenetic age acceleration; BMI = body mass index; EAS = Effects of ageing study; EEAA = extrinsic epigenetic age acceleration; FU = follow-up; GS-SFHS = Generation Scotland- Scottish Family Health Study; GOLDN = Genetics of Lipid Lowering Drugs and diet Network; GOCS = Longitudinal Growth & Obesity Cohort Study; IEAA = intrinsic epigenetic age acceleration; MCC = Melbourne Collaborative Cohort Study; MRC-NSHD = Medical Research Council National Survey of Health & Development; NS = not significant; SD = standard deviation; V90+ = Vitality 90+ (V90+); YFS = The Young Finns Study; WHI = Women’s Health Initiative study.
Six studies of 13,455 participants investigated alcohol consumption (27,30,36,51,63,65). Only two found a significant association with DNAm age (36,65), and a third found a significant association using a proxy measure of alcohol consumption (30). Interestingly, two studies found a nonlinear association, with both low and high levels of alcohol consumption associated with increased DNAm age (30,36).
Three studies of 7,493 participants examined diet and DNAm age (36,51,63). Two studies found no association (36,51,63) whereas one of the largest studies of older women (from WHI) found that fish and poultry intake were associated with lower EEAA and IEAA, respectively (36). It is important to note that self-reported dietary variables are difficult to assess accurately, and this latter study found a significant association measured specifically mean carotenoid levels (an index of vegetable intake).
Of the ten studies investigating BMI, six contributed a total of 1,760 participants (31,35,41,51,62,66) and there were four much larger studies (27,36,59,63). BMI was positively associated with DNAm age in five of the studies, including the three largest (36,59,63) but in Finnish middle-aged adults the association was in the reverse direction (66).
Physical Activity, Frailty
The three studies of physical activity, involving 7,493 participants, found no association with DNAm age (36,51,63). However, a small study of older individuals found a negative association with step count (38) (Table 2). Four studies looked at different measures of physical function and performance (38,39,41,67), and two found a negative association between grip strength and DNAm age (39,58). The other measures of performance, such as balance and time to rise from a chair, were only associated with DNAm age in individual studies. Three studies assessed frailty in a total of 3,092 participants and all reported that it was associated with increased DNAm age (two using AA-Horvath and the third with EEAA) (37,52,68).
Table 2.
Studies Investigating the Association Between Physical Activity or Frailty and DNAm Age
| Ref | Study (Country) | n: Mean Age (SD) or Range, Sex, Other Information | Exposure | Sample; Platform | Epigenetic Clock | ↑ DNAm Age [r With Chronological Age] | Adjustments |
|---|---|---|---|---|---|---|---|
| (39) | LBC1936 (United Kingdom) | Year 1: 920, age 69.5 (0.83), 49.8% ♀. Year 6: 273, age 76.2 (0.68), 48.4% ♀ | Walking speed, FEV & grip strength | Blood; 450K | AA-Horvath | ↓ Grip strength (β: −0.05, p = .01) & FEV (β: −0.06, p = .006). NS Walking Speed (β: 0.03, p = .45) | Age, sex, cell %, height & smoking |
| (52) | Two subsamples from the Esther cohort (Germany) | 969: age 62.1 (6.5), 50% ♀; 864: age 63 (6.7), 54.5% ♀ | Frailty index | Blood; 450K | AA-Horvath | Frailty, overall and in both samples (β: 0.20 to 0.286, p < .001). | Age, sex, cell %, smoking, alcohol, cancer history |
| (51) | FACHS cohort (United States) | 100: age 48.5 (9.2), 100 ♀, 94% African-American, 17.8% <12th grade education. | Physical exercise | Blood (wave 5); 450K | AA-Hannum | NS Physical Exercise (β: −0.07, p > .05). [r = .82] | Education, BMI, smoking, alcohol, diet, trauma |
| (68) | Louisiana Healthy Aging Study (United States) | 262: age 86 (10), 60.7% ♀, all Caucasian. 206 deceased after 4.4 years | Frailty index | Blood; 450K | IEAA | Frailty (β: 0.20, p = .001), but NS after adjusting for age (effect size not provided, p > .05). [r = .63] | Age, cell % |
| (36) | WHI study (United States) & InCHIANTI study (Italy). | 4,173: age 64 (7.1), 100% ♀; 402: age 71 (16), 56% ♀, all |
Physical activity | Blood, serum; 450K. | IEAA, EEAA | NS Physical Activity. (EEAA β −1.70, p = .09; IEAA β −1.06, p = .91). | Numerous factors |
| (41) | MRC-NSHD cohort; ALSPAC (replication) (United Kingdom) | 790: age 53, 100% ♀. Followed until age 60–64. 988: age 46.9 (4.5), 100% ♀. |
Change in grip strength, standing balance time, chair rise speed | Buccal & Blood (n = 152); 450K | AA-Horvath | ↓ Grip strength, blood NSHD 0.42 kg/y, p = .03), NS buccal (β: −0.07 kg/y, p = .35) & NS ALSPAC (β: −0.03, p = .50). NS all others (βs −0.002 to 0.09, p > .10). [r = .02], NS] |
Cell %, height, BMI, education, SEP (NSHD also smoking) |
| (67) | The Dunedin Study (New Zealand) | 818: aged 38 years, 48% ♀ (n = 743 with longitudinal data) | Physical function | Blood; 450K | AA-Horvath, AA- Hannum | ↓ Balance, Coordination, Physical limitations, Hannum (r = −.05 to −.09, p < .05); NS Horvath (r = −.07 to 0, p > .06). NS Grip Strength (r = −.05 to 0, p > .15) | Sex |
| (63) | MCC study (Australia) | 2,818: age 27–76, 39% ♀, healthy controls matched to cancer cases | Physical activity | Blood; 450K | AA-Horvath, AA- Hannum, IEAA, EEAA | NS Physical Activity (AA-Horvath β: 0.10, p = .45; AA-Hannum β: 0.22, p = .07; IEAA β: 0.07, p = .59; EEAA β: 0.18, p = .11). [r = .73, .76] | Various. |
| (38) | LBC1936 (United Kingdom) | 248: age 79 (0.45), 47.1% ♀ | Sedentary behaviour, 7-day step count | Blood; 450K | IEAA, EEAA | ↓ Steps, EEAA (β: −0.10, p = .03), NS IEAA (β: −0.01, p = .27). ↑ Sit-to-Stand transitions, IEAA (β: 0.006, p = .05), NS EEAA (β: −0.001, p = .67). NS Sendentary Time (IEAA β: −0.11, p = .38, EEAA β: 0.18, p = .09). |
Age, sex |
| (37) | LBC1936 (United Kingdom) | 791: age 70 (0.84), 50.3% ♀. 7.8% frail and 46% pre-frail | Physical Frailty | Whole blood; 450K | IEAA, EEAA | Frailty, EEAA (RR:1.05, p = .02), NS IEAA (RR 1.02, p = .52). | Age, sex, smoking, alcohol, chronic disease |
Note: AA = epigenetic age acceleration; BMI = body mass index; EEAA = extrinsic epigenetic age acceleration; FACHS = Family and Community Health study; FEV = forced expiratory volume; FU = follow-up; IEAA = intrinsic epigenetic age acceleration; LBC1936 = Lothian Birth Cohort 1936; MCC = Melbourne Collaborative Cohort Study; MRC-NSHD = Medical Research Council National Survey of Health & Development; NS = not significant; SD = standard deviation; WHI = Women’s Health Initiative study.
Stress, Posttraumatic Stress Disorder
Ten studies included in this review investigated stress or trauma (Table 3). Nine studies included a total of 3,465 participants. The tenth study was a meta-analysis that brought together nine cohorts (33), some of which already featured in other studies shown in the table, for example, PRISMO (69) and TRACTS (70). The Grady Trauma Project (GTP) cohort was used in two of the studies (49,50), although it is unclear if the sample participants were included in both. Childhood trauma was examined in four studies, but only one of these reported a significant association with childhood sexual abuse (40), that was supported by the findings of the meta-analysis of three studies (33). Six studies investigated lifetime posttraumatic stress disorder in predominantly war veterans (33,49,61,69–71). Four of these reported no association overall. However, specific posttraumatic stress disorder symptoms (61,70) or symptom severity, were associated with increased DNAm age (33,70). Individual studies also reported increased DNAm age with increased life stress (50) and exposure to violence (55).
Table 3.
Studies Investigating the Association Between Stress and Trauma, and DNAm Age
| Ref | Study (Country) | n: Mean Age (SD) or Range, Sex, Other Information | Exposure | Sample; Platform | Epigenetic Clock | ↑ DNAm age [r With Chronological Age] | Adjustments |
|---|---|---|---|---|---|---|---|
| (50) | GTP cohort (United States) | 393: age 41.33 (12.85), 70.7% ♀, urban African-Americans | Childhood trauma, stressful life events, current stress, PTSD | Blood; 450k | AA-Horvath | Life stress (β = 0.28, p = .027), particularly personal stress (β = 0.26 p = .0087) individuals without/mild sexual/physical childhood abuse (β: 0.34, p = .003). NS others (no effect size provided, p > .10). [r = .90] | Age, sex, BMI, cell %, alcohol smoking, genetic depression. |
| (69) | PRISMO (Netherlands) | 67 high trauma (32 PTSD): age 27.4 (9.3); 32 low exposure, age 25.1 (8.1). 0% ♀. | Combat and childhood trauma, PTSD symptoms | Blood (x2); 450K | AA-Horvath |
Combat trauma (β: 1.97, p = .03) ↓ PTSD symptoms (β: −0.10, p = .04). NS childhood trauma (β: 0.13, p = .43), baseline trauma (β: −0.25, p = .90) & NS PTSD (β: −2.19, p = .41). [r = .99] |
Alcohol, smoking, medications |
| (51) | FACHS (United States) | 100: age 48.5 (9.2), 100 ♀. 94% African-American. | Childhood trauma | Blood; 450K | AA-Hannum | NS (β: −0.086, p > .05) [r = .82] | Education, BMI, smoking, alcohol, diet, exercise, married |
| (71) | War veteran cohort (United States) | 281: age 31.9 (8.4), 12.1% ♀. 56% with current & 74% lifetime PTSD | PTSD | Blood; 450K | AA-Horvath, Hannum | Lifetime PTSD severity, Hannum (β: 0.13, p = .03), NS Horvath (β: 0.02, p = .82) [r = .88] | Age, sex, cell %, ancestry |
| (55) | Case–control study of violence exposure (United States) | 101: 9.73 (1.67), 54.5% ♀. African-Americans | Neighborhood violence, stress heart rate | Saliva; 450K | AA-Horvath |
Violence exposure (r = .25, p = .02), especially direct (r = .30, p = .004). NS witnessed (r = .19, p = .06). ↓ Heart rate (β: 6.59, p = .002), especially older individuals. [r = .46] |
Age, sex, cell %, income, education |
| (40) | ALSPAC & MRC- NSHD cohorts (United Kingdom) | 989: 28.7 (5.5), FU ~10 years; 773, age 53.4 (0.2). 100% ♀. |
11 childhood psychosocial adversities | Blood & Buccal; 450K | IEAA | Sexual abuse, ALSPAC (3.4 years, p < .001), but NS NSHD (no data). [r = .45 & r = .12] | Various |
| (49) | War veteran’s (Australian) & GTP cohort (United States) | 96 males: age 68.7 (0.45), 48 with PTSD; 115 exposed males: age 44 (1.14), 70 with PTSD. | PTSD & resilience | Blood; 850K & 450K | AA-Horvath |
Resilience in veterans with PTSD (r = .32, p = .02) but NS in non-PTSD (r = −.19, p = .40) Resilience in trauma-exposed civilians (r = .23, p = .02). NS PTSD & symptom severity (no effect size stated, p > .23). [r = .50] |
Ethnicity, cell %, BMI, smoking, medications |
| (61) | Veteran Affairs study: TRACTS (United States) | 179: age 32.8 (9.3), 11.7% ♀. 59% current & 76.5% lifetime PTS. 2-year FU | PTSD & symptoms | Blood; 850K | AA-Horvath, Hannum | PTSD avoidance & numbing symptoms (Horavth β: 0.43, p = .02). NS other symptoms (βs −0.04 to −0.21, p > .10), PTSD (β: 0.01, p = .92). NS all Hannum (β: −0.09 to 0.15, p > .10). [r = .90, .88] | None |
| (70) | Department of Veterans Affairs cohort (United States) | 339: age 52.6 (10.7), 13% ♀. 69% lifetime PTSD (of 241). White non-Hispanic | Trauma, PTSD symptoms | Blood; 450K | AA-Hannum | PTSD hyperarousal symptoms (β: 0.20, p = .009). NS trauma (β: 0.08, p = .16); NS PTSD severity (β: 0.03, p = .62). [r = .90] | Sex, cell % |
| (33) | Meta-analysis of nine cohorts (eight United States, one Netherlands). | Studies from 62 to 465: mean age from 22.1 to 53.9, from 0% to 72% ♀ | Childhood and lifetime trauma, current and lifetime PTSD and severity | Blood; 450K | AA-Horvath, Hannum |
Childhood trauma (CTQ) in 3 studies, Hannum (β: 0.46, p = .03), NS Horvath (β: 0.46, p = .62), NS all childhood trauma (βs: −0.03 to 0.06, p > .52) Lifetime PTSD severity, Hannum (β: 0.01, p = .02), NS Horvath (β: −0.05, p = .12); NS PTSD diagnosis, Hannum (β: 0.53, p = .06), Horvath (β: 0.12, p = .76). NS Lifetime trauma, Hannum (β: 0.02, p = .63) and Horvath (β: −0.05, p = .12) [r = .87, .87] |
Age, sex, cell %, ancestry random effects model |
Note: AA = epigenetic age acceleration; ALSPAC = Avon Longitudinal Study of Parents and Children; BMI = body mass index; EEAA = extrinsic epigenetic age acceleration; CTQ = Childhood Trauma Questionnaire; EPIC = European Prospective Investigation into Cancer and Nutrition; FACHS = Family & Community Health Studies; FU = follow-up; GTP = Grady Trauma Project; IEAA = intrinsic epigenetic age acceleration; MRC-NSHD = Medical Research Council National Survey of Health & Development; PRISMO = Dutch military personnel deployed to Afghanistan; PTSD = posttraumatic stress disorder; SD = standard deviation; TRACTS = Translational Research Centre for Traumatic brain injury and Stress disorders.
Mental Health (Excluding Posttraumatic Stress Disorder)
Five studies involving 2,176 participants investigated psychiatric symptoms or disorders, and their association with DNAm age (Supplementary Table S4). There were no consistent exposures examined across studies, and comparison of study findings is thus not possible.
Cognitive Function
Table 4 summarizes the 11 studies that looked at cognitive function or related indices. There were two studies of fluid intelligence (39,60), but they involved participants at very different stages of the lifespan. Another four studies of 1,160 investigated memory, overall cognition or cognitive decline (67,71–73). Two of these reported significant negative associations with cognition and DNAm age, but inconsistent findings regarding cognitive change (67,72). Five studies of 1,715 participants analyzed brain magnetic resonance imaging (MRI) measures (54,71,74–76). Smaller volumes (54,75) or increased white matter hyperintensities (76), were associated with older DNAm age. However, there was no finding that was replicated across two or more studies.
Table 4.
Studies Investigating the Association Between Cognitive Function or Related Indices and DNAm Age
| Ref | Study (Country) | n: Mean Age (SD) or Range, Sex, Other Information | Exposure | Sample; Platform | Epigenetic Clock | ↑ DNAm Age [r With Chronological Age] | Adjustments |
|---|---|---|---|---|---|---|---|
| (77) | PEG (United States) | 289 cases: aged 37–91, 43% ♀; 219 controls: aged 35–92, 47% ♀, Caucasians 46 cases: aged 37–83, 30% ♀; 38 controls: aged 35–92, 53% ♀, Hispanics | Parkinson’s Disease (PD) | Blood; 450k | IEAA, EEAA | PD (IEAA, p = .019; EEAA, p < .013 effect sizes not stated. [r = .55 to .90] | Age, sex, cell %, smoking, ethnicity |
| (39) | LBC1936 (United Kingdom) | Year 1: 920, age 69.5 (0.83), 49.8% ♀. Year 6: 273, age 76.2 (0.68), 48.4% ♀ | General fluid intelligence | Blood; 450K | AA-Horvath | Lower cognition (β: −0.07, p = .02). NS change in Horvath with cognition (β: −0.00044, p = .92). | Age, sex, cell %, height, smoking |
| (71) | War veteran cohort (United States) | 281: age 31.9 (8.4), 12.1% ♀. 56% with current & 74% lifetime PTSD | Working memory; Brain MRI | Blood; 450K | AA-Hannum | ↓ Working Memory (β: −0.05, p = .029) & Corpus Callosum neural integrity (β: −0.17, p = .009). [r = .88] | Age, sex, cell %, ancestry |
| (54) | Case-control, risk of familial depression (United States) | 24 high risk: age 12.87 (1.31); 22 low risk: age 12.14 (1.36); 100% ♀, 61% Caucasian. | Hippocampal & amygdala volume | Saliva; 850K | AA-Horvath | ↓ Hippocampal volume, left (β = −23.25, p = .03), NS right (β = −0.16, p = .27) NS Amygdala (effect size not stated, p > .67). [r = .34] | None |
| (72) | Betula cohort, 15 year FU (Sweden) | 16 good memory: age 57.8 (3.6), 50% ♀. 20 average memory: age 58.0 (3.5), 50% ♀. 16 decline: age 57.9 (3.6), 50%♀ | Memory (over 15 years) | Blood; 450K | AA-Horvath | ↓ Memory, baseline & decline group vs good (5.1 years, p < .001) and average (2.6 years, p = .02). [r = .69] | Age, sex |
| (75) | San Antonio Family Study (United States) | 628 from 38 families: age 45.45 (13.3), % ♀ not stated, Mexican Americans | Brain MRI, white matter integrity | Blood; 450K | AA-Horvath | ↓ White Matter, global (−0.119, p = .028) and 7 of 16 individually tracts (p < .05). [r = .93] | Cell %, diabetes, hypertension |
| (76) | ARIC study (United States) | 713: age 61.8 (4.5), African- Americans | Brain MRI, WMH | Blood; 450K | AA-Horvath, Hannum | ↑ WMH burden (Horvath β: 3.51%, Hannum β: 4.72%, both p < .0001) & Severity (11%, p < .04). [r = .51–.60] | Age, sex, cell %, BMI, health |
| (73) | The Middle-Aged Danish Twin Study (Denmark) | 243 pairs of MZ twins, age 55–79, mean 65.9 (6.1), 45.7% ♀ | Cognition: composite score | Blood; 450K | AA-Horvath, Hannum | NS Cognition, paired twin (Horvath: β: 0.02; Hannum: −0.007, p > .10) or unpaired (Horvath: β: 0.04; Hannum β 0.004, p > .10). [r = .69, .78] | Age |
| (67) | The Dunedin Study (New Zealand) | 818: aged 38 years, 48% ♀ (n = 743 with longitudinal data) | Cognitive decline | Blood; 450K | AA-Horvath, Hannum | ↓ Cognition & Decline, Horvath (r = −.16, p < .001 & r = −.09, p = .02); NS Horvath (r = −.02 & −.04, p > .31) |
Sex |
| (74) | Whitehall II imaging cohort (United Kingdom) | 47: 23 cognitively intact age 73(6), 17.4% ♀. 24 impaired, age 73(6), 16.7% ♀ | Cognitive impairment, Brain MRI | Blood; 450K | IEAA, EEAA | ↓ Diffusivity Anisotrophy & ↑ global fractional anisotrophy (p < .001). NS Cognitive Impairment (Horvath: β: 0.002; p = .98, Hannum: β = 0.11; p = .29). | Age, sex, cell %, smoking, alcohol, IQ. |
| (60) | Glycyrrhizin in Licorice study (Finland). | 239: age 12.3 (11–13.2), 51.5% ♀ | Cognition (Intelligence) | Blood; 850K | IEAA | NS Intelligence: general (β: 0.003, p = .79), verbal (β: −0.003, p = .75), performance (β: 0.006, p = .40). [r = .13] | Sex, genetics |
Note: AA = epigenetic age acceleration; ARIC = atherosclerosis risk in communities; BMI = body mass index; EEAA = extrinsic epigenetic age acceleration; FU = follow-up; IEAA = intrinsic epigenetic age acceleration; LBC1936 = Lothian Birth Cohort 1936; MCC = Melbourne Collaborative Cohort Study; MRI = magnetic resonance imaging; NS = not significant; PEG = The Parkinson’s disease, Environment and Genes; PTSD = posttraumatic stress disorder; SD = standard deviation; V90+ = Vitality 90+ (V90+); YFS = The Young Finns Study; WMH = white matter hyperintensities.
Health-Related Exposures
Eighteen studies investigated the association between other health conditions and DNAm age (Supplementary Table S5), but some exposures were only investigated in individual studies (28,32,34,67,78,79). Two studies of Down’s syndrome found increased DNAm age (56,80). Four studies involving 11,179 participants investigated the association between diabetes and hypertension and DNAm age (27,59,63,65). Diabetes was associated with increased DNAm age (specifically IEAA) in two studies (63,65), although not the other studies (27,59). In a similar manner, hypertension was associated with increased EEAA in three of the studies, but not consistently (27,59,65).
A number of studies measured biological markers associated with, health conditions. Consistent null findings were reported with respect to cytokines, including tumor necrosis factor (TNF)-alpha, and interleukin 6 (IL-6) (65,81), and two studies reported a positive association between C-reactive protein (CRP) and EEAA (36,65). Diurnal cortisol or levels at awakening were significantly associated with DNAm age (AA and IEAA respectively), in younger cohorts (54,60). Findings were mixed with regards to fasting glucose (27,35,36,65) and cholesterol (27,36,59,65).
Infections
Four studies of 234 HIV infected individuals and 200 controls examined HIV (Supplementary Table S6). HIV+ infection was associated with a significant increase in DNAm age (42,82–84). The only limitation here is that given the nature of these studies, they did have a higher risk of bias (Supplementary Table 2). Individual studies only looked at Cytomegalovirus infection and Helicobacter pylori infection (85,86).
Environmental Pollution
Two studies using data from the Veterans Affairs Normative Aging Study (Supplementary Table S7) examined air pollution. One found a positive association between a particular air pollutant (PM2.5) and AA-Horvath (43), but the second reported a negative association between PM2.5 and IEAA (44). There was only one study of pesticide exposure and they reported a positive association with some pesticide compounds (87).
Other Factors
Three additional studies were identified that did not fit into any of the exposure categories described above (Supplementary Table S8). A small case-control study found that an increasing number of years of meditation practice was associated with decreased DNAm age in mediators but not controls (88). Two studies of disadvantaged African-American youth (45,46) found that this was associated with DNAm age in stratified analysis only.
Meta-analysis
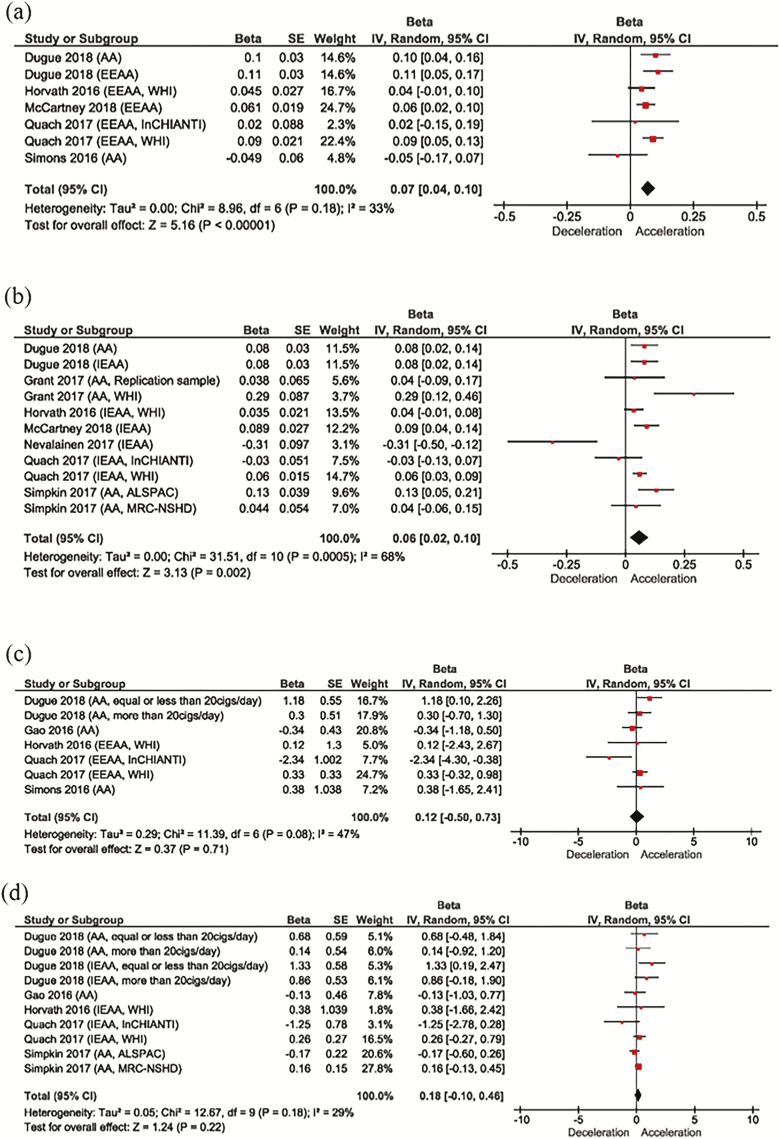
Most of the studies that measured similar exposures, were considered too heterogenous to pool together in a meta-analysis. Meta-analyses were performed, however, for both BMI and smoking, that both have standard definitions and were assessed in multiple studies. We obtained the beta coefficient and standard error (or calculated the standard error from the published 95% CI) for each cohort in the included studies. For BMI, seven cohorts (five studies) used a DNAm estimate based on the Hannum epigenetic clock, and 11 cohorts (seven studies) used one based on the Horvath epigenetic clock. Two studies could not be included because they did not provide an estimate of the variability (SE or 95% CI). BMI was significantly associated with higher DNAm (Figure 1), with a slightly greater effect size with the Hannum epigenetic clock (per one-unit increase in BMI). Heterogeneity between studies was moderate and greater for the Horvath epigenetic clock (68% vs 33%).
Figure 1.
Random-effects meta-analysis for the association between BMI or smoking status and DNAm age. (a) Forest plot of the association between BMI and DNAm age estimated using the Hannum clock; (b) Forest plot of the association between BMI and DNAm age estimated using the Horvath clock; (c) Forest plot of the association between smoking (current vs never/noncurrent) and DNAm age estimated using the Hannum clock; (d) Forest plot of the association between smoking (current vs never/noncurrent) and DNAm age estimated using the Hannum clock.
A meta-analysis was also performed for eight of the nine studies that examined smoking (Figure 1). Six cohorts from five studies used a Hannum epigenetic clock estimate (AA or EEAA), and eight cohorts (five studies) used a Horvath epigenetic clock estimate (AA or IEAA). No significant association was found between current smoking (vs never or noncurrent smoking) and DNAm age. Heterogeneity was moderate for both studies (47% and 29%). Funnel plots for all measures were approximately symmetrical, suggesting no strong evidence of publication bias (Supplementary Figure S1).
Discussion
Researchers have had a longstanding interest in understanding more about the biology of aging and the differences between individuals in the ways in which they age. A lot of effort has gone into identifying biological markers of aging that are strong predictors of an individual’s functional capacity and their risk of disease (see for review (1,4)). Since the identification of DNAm-based biomarkers of aging, “Epigenetic Clocks,” a growing number of studies have begun to investigate the extent to which external factors might influence an individual’s DNAm age. In particular, the focus has been on the discrepancy between an individual’s predicted DNAm age and actual chronological age, that is thought to reflect the speed at which an individual is (currently) aging. Positive values reflect a faster aging of cells (AA), while negative values indicate slower aging (age deceleration).
We identified 61 studies with a total of over 50,000 participants who investigated the association between environmental, lifestyle and health-related factors and DNAm age (predominantly in blood) using Horvath’s (14) and/or Hannum’s (13) epigenetic clock calculators. Despite the heterogeneity in the exact exposures that were examined, these could be grouped into common categories. Overall, 56 of the studies reported at least one significant association, and these were predominantly in the expected direction, with known risk factors for disease being associated with increased DNAm age, although the reverse direction of association was also reported (eg, (41,44,66,69)). However, overall there was a clear lack of consistent evidence for most exposures. This is likely to be driven by a number of factors such as heterogeneity between the studies (design, participant characteristics), the lack of statistical power for several of the studies (12 had a sample size less than 100), and the known positive publication bias (89). The most frequently reported null findings were with regards to educational level and the Horvath clock DNAm age (four studies totaling 5,280 participants) finding no association (27,36,41,62), and with regards to smoking (five studies of 10,574 participants) (27,36,51,53,62). However in the case of the latter, two of the largest studies found that smoking was associated with DNAm age, but in opposing directions (41,63). Education was also found to be negatively associated with DNAm age estimated using the Hannum clock specifically in four studies (27,36,59,63), including the largest (59). This finding is also supported by another study that was not identified in our systematic review (90).
The strongest evidence for an association with DNAm age was with regards to BMI. Five of nine studies found that BMI was positively associated with DNAm age even when adjusting for various confounding factors (31,35,36,59,63). The studies reporting this finding were three of the largest studies included in this systematic review (36,59,63) and involved adult and older aged participants. Interestingly, one of the studies reported an even stronger association between BMI and DNAm age in the liver (31). Of the three studies that reported no significant association between BMI and DNAm age, two had small samples sizes (n = 100 and 94 respectively) (51,62). Interestingly, the third null study had also reported no correlation (r = .02, p = .8) between chronological age and DNAm age, suggesting this was not a good marker of aging in their sample (41). However, it should also be noted that two of the five significant studies also used their own replication samples, and in both cases were not able to replicate their original finding (31,35). One study reported an association between BMI and DNAm age in the reverse direction, such that a lower BMI was associated with advanced age in their sample of 183 participants with a mean age of 44 years (66). The reason for this discrepancy is unclear, but Cachexia is a symptom of many chronic health conditions (91). Further, while BMI is not directly related to nutrient intake, it is interesting to note that the relationship between calorie intake and longevity follows a U-shaped curve, with both dietary excess and malnutrition negatively impacting survival.
In support of the finding that BMI is positively associated with DNAm age, increased BMI and obesity have also been associated with accelerated telomere erosion, another biomarker of aging (92). Obesity is an important risk factor for many age-related diseases (93). It is associated with heightened oxidative stress and pro-inflammatory state, that enhances white blood cell turnover and thus is considered pro-aging (94). Although not a new concept, recent evidence also indicates that caloric restriction may be beneficial in delaying aging and the mechanisms appear to involve metabolic regulators that sense nutrient and energy (95).
The other finding with some consistent evidence is frailty, with all three studies (n = 3,092 participants) reporting a positive association with DNAm age. Frailty is a clinical syndrome that is characterized by a loss of physical and cognitive reserve and a decline in function (96). It has been shown to be strongly associated with age-related phenotypes and reduced longevity (97) and thus, has itself been used as a measure of biological aging (98). Frailty is commonly defined using the Fried criteria, where an individual is required to meet three of five criteria that includes low grip strength, low energy, slow walking speed, low physical activity, and unintentional weight loss. Two other studies included in this review examined grip strength and reported that decreased strength was associated with increased DNAm age (39,41), lending further support to the frailty association.
Horvath Versus Hannum Epigenetic Clocks
In this systematic review, we focused on studies that had measured DNAm age using either of the two most widely studied epigenetic clock calculators developed by Horvath (14) and Hannum and colleagues (13). DNAm age is calculated based on methylation levels at 353 and 71 CpGs sites, respectively. The residual after regressing DNAm age on known chronological age provides an estimate of AA (AA-Horvath and AA-Hannum). AA-Horvath was used by the majority of studies (39 of 61), but more often studies used a slightly modified version that takes into account blood cell counts (IEAA).
The majority of studies examined more than one epigenetic clock estimates but seldom reported consistent associations. This suggests that these different clock estimates, while highly related, are both measuring a slightly different aspect of biological aging. Horvath’s epigenetic clock is a multi-tissue age predictor, designed using data gathered from 7,844 non-cancer samples of all ages across 51 different tissue and cell types of all ages. It is particularly accurate in children and adolescents (14) and has been strongly correlated with gestational age and early-life development (58). On the other hand, Hannum’s calculator was derived from blood samples using only adult participants aged 19 to 101 years, although it strongly correlates with age in most tissues/cells (13). The IEAA measures exhibit greater consistency across cell types and tissues, and although not relevant to the current review (as most studies focused on blood), this indicates that IEAA may be under stronger genetic control. However, given the changes in cell-type composition of blood with aging, the EEAA estimator that incorporates a weighted average of cell counts may be better at predicting age-related decline (18) and mortality (16). Indeed, IEAA is said to provide a measure of cell-intrinsic aging, while EEAA is a measure of immune system aging. From our systematic review, we found no consistent evidence that certain environmental, lifestyle or health factors were more likely to be associated with one clock measure over another, with the exception of education. BMI was significantly associated with AA estimated using with AA-Horvath (31,35), IEAA (36,59), and EEAA (36,59,63). Likewise, frailty was associated with increased AA-Horvath (52), IEAA (68), and EEAA measures (37).
Most studies reported a high correlation (median r = .82) between chronological age and DNAm in their sample, which is expected and in line with these calculators functioning as an “epigenetic clock”. The samples on which these algorithms were originally developed demonstrated very high correlation with chronological age (r = .96 for both Horvath’s and Hannum’s clocks) (13,14). However, some studies reported a much lower correlation (overall range r = .13 to .99). This is likely due to the inclusion of select samples of participants with specific conditions or a very narrow age range. Both clocks were originally developed based on a wide range of ages and in generally healthy individuals. Further, in small samples, individual outliers would exert a stronger influence on the coefficient, particularly if a parametric test was used.
Strengths and Limitations of the Review
This systematic review was conducted in line with PRISM guidelines (http://www.prisma-statement.org) and used a systematic search strategy with defined inclusion and exclusion criteria. Multiple authors independently assessed studies for inclusion and independently completed the quality of appraisal of the studies. These steps have helped minimize bias in the selection of studies to be included in this review. We have provided a comprehensive summary of the studies investigating the association between environmental, lifestyle and health-related factors and DNAm age, and have assessed the strength of evidence. A meta-analysis was performed for BMI and smoking, separately for the Hannum clock and the Horvath clock, as these studies were considered sufficiently homogenous to enable pooling of the results. The forest plot indicated, however, a moderate degree of heterogeneity between studies.
In this systematic review, we did not consider a number of “fixed” exposures, including genetic factors, sex, ethnicity or race. A number of studies have previously reported that males have an older blood DNAm age than females (27), which would support their on average longer lifespan and ethnic differences have also been reported previously (27). Most studies included in this review controlled for sex, although ethnicity was reported by only a few studies. A recent study using data from a number of cohorts identified a couple of genetic loci that were associated with IEAA and EEAA and these variants suggest a causal role of menarche, menopause, and lipoproteins in influencing IEAA (99).
In terms of the external validity, the studies included in this systematic review investigated a range of exposures, but in predominantly adult participants and mostly recruited in the United States. While not always specifically mentioned, it is likely that the studies included a large number of white Caucasians with less representation from other ethnic groups. This will limit the generalizability of the findings. Furthermore, many of the studies examined multiple exposures and in some cases, numerous epigenetic clocks, but there was very little mention of the issue of multiple testing. For example, one study examined more than 18 exposures and five DNAm age measures but did not control for multiple testing in their analysis, increasing the risk of a Type I error and raising the possibility of reporting false positive results (63). Another factor that needs to be considered is the influence of cell composition. Blood is composed of different cell types, including lymphocytes, monocytes, and granulocytes (that can be broken down further) and each is likely to have a unique epigenetic profile (and thus, different DNAm age). While a number of studies controlled for cell type composition in their analysis, this was often estimated based on cell-specific DNAm marks, rather than directly measured. This could influence or drive some of the associations reported. These factors, as well as the likelihood of positive publication bias, need to be taken into account when interpreting the findings from this review.
Future Evaluation of Novel Clocks
In our review, we only focused on the Horvath and Hannum clocks, and thus cannot draw any conclusions about possible associations between environmental, lifestyle and health exposures with other epigenetic clocks. In 2011, the first epigenetic age predictor was published (12) and since then, a number of other epigenetic clocks have also been developed. These include one based on blood DNAm changes at only 3 CpGs (26), a highly sensitive age estimator (called the skin and blood clock) that is accurate for buccal cells and in children (25), as well as a novel measure using MBD-seq data (24). Furthermore, a couple of very recent publications have reported the development of exciting new epigenetic biomarkers of aging, referred to as DNAm PhenoAge (20) and DNAm GrimAge (21). Initial results suggest that both of these clocks outperform current measures of biological aging. DNAm PhenoAge is strongly associated with a number of age-related health outcomes (20) and DNAm GrimAge has very high predictive accuracy for time to death, as well as time to coronary heart disease and cancer (21). Importantly, especially given the conclusions of this review, both the DNAm PhenoAge and DNAm GrimAge exhibited strong correlations with smoking. It was not possible to include these clocks in this review given they were only recently published. However, it will be interesting to see the results of subsequent studies that include these measures in their analysis. It is thus likely that the associations reported here may be quite specific for the original (and slightly modified) Horvath and Hannum epigenetic clocks, but may not be generalizable to other clocks that have been developed.
Conclusion
For the majority of exposures, there was insufficient evidence to indicate whether or not they were associated with DNAm age, largely due to the heterogeneity in terms of the exact exposure examined, the epigenetic clock measure and the unique study characteristics. Many exposures were only investigated in a small number of studies and the findings were often inconsistent. However, this systematic review found some preliminary evidence that BMI and frailty were associated with accelerated DNAm age. Further research is needed to provide more conclusive evidence about the potential influence of external factors on DNAm age.
This field of research holds much promise and has the potential to provide further insights into how to promote slower biological aging and ultimately prolong healthy life. It also has the potential that DNAm age could be used as a tool to monitor the effectiveness of lifestyle and preventative interventions. Many of the external factors have been described as substantial risk factors for chronic disease. The ability to potentially “change” many of them through modifying behaviors is why they are the primary points for prevention of age-related disease. Better knowledge of this could provide crucial insights into how an individual could reduce their biological age and thus could permit enhanced survival.
Funding
This work was supported by a National Health & Medical Research Leader Fellowship (grant number APP1135727 to J.R.).
Conflict of interest statement
None declared.
Supplementary Material
References
- 1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bao Q, Pan J, Qi H, et al. Aging and age-related diseases–from endocrine therapy to target therapy. Mol Cell Endocrinol. 2014;394:115–118. doi: 10.1016/j.mce.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 3. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561:45–56. doi: 10.1038/s41586-018-0457-8 [DOI] [PubMed] [Google Scholar]
- 4. Jylhävä J, Pedersen NL, Hägg S. Biological Age Predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- 6. Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913 [DOI] [PubMed] [Google Scholar]
- 7. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuke C, Shimabukuro M, Petronis A, et al. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68(Pt 3):196–204. doi: 10.1046/j.1529-8817.2004.00081.x [DOI] [PubMed] [Google Scholar]
- 9. Sierra MI, Fernández AF, Fraga MF. Epigenetics of Aging. Curr Genomics. 2015;16:435–440. doi: 10.2174/1389202916666150817203459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;16:593–610. doi: 10.1038/nrm4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones MJ, Goodman SJ, Kobor MS. DNA methylation and healthy human aging. Aging Cell. 2015;14:924–932. doi: 10.1111/acel.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bocklandt S, Lin W, Sehl ME, et al. Epigenetic predictor of age. PLoS One. 2011;6:e14821. doi: 10.1371/journal.pone.0014821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 16. Chen BHM, Colicino RE, Peters E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging. 2016;8(9):1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dugué PA, Bassett JK, Joo JE, et al. DNA methylation-based biological aging and cancer risk and survival: pooled analysis of seven prospective studies. Int J Cancer. 2018;142:1611–1619. doi: 10.1002/ijc.31189 [DOI] [PubMed] [Google Scholar]
- 18. Marioni RE, Sonia S, Allan FM. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin EM, Fry RC. Environmental influences on the epigenome: exposure- associated DNA methylation in human populations. Annu Rev Public Health. 2018;39:309–333. doi: 10.1146/annurev-publhealth-040617-014629 [DOI] [PubMed] [Google Scholar]
- 20. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11:303–327. doi: 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377:108–109. doi: 10.1016/S0140-6736(10)60903-8 [DOI] [PubMed] [Google Scholar]
- 24. Han LK, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. Am J Psychiatry. 2018;175(8):774–782. doi: 10.1176/appi.ajp.2018.17060595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horvath S, Oshima J, Martin GM, et al. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging (Albany NY). 2018;10:1758–1775. doi: 10.18632/aging.101508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weidner CI, Lin Q, Koch CM, et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014;15(2):R24. doi: 10.1186/gb-2014-15-2-r24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horvath S, Gurven M, Levine ME, et al. An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol. 2016;17(1):171. doi: 10.1186/s13059-016-1030-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maierhofer A, Flunkert J, Oshima J, Martin GM, Haaf T, Horvath S. Accelerated epigenetic aging in Werner syndrome. Aging. 2017;9(4):1143–1152. doi: 10.18632/aging.101217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA. DNA methylation age is not accelerated in brain or blood of subjects with schizophrenia. Schizophr Res. 2018;196:39–44. doi: 10.1016/j.schres.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beach SR, Dogan MV, Lei MK, et al. Methylomic aging as a window onto the influence of lifestyle: tobacco and alcohol use alter the rate of biological aging. J Am Geriatr Soc. 2015;63:2519–2525. doi: 10.1111/jgs.13830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horvath S, Erhart W, Brosch M, et al. Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA. 2014;111(43):15538–15543. doi: 10.1073/pnas.1412759111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levine MEL, Lu AT, Chen BH, et al. Menopause accelerates biological aging. Proc Natl Acad Sci USA. 2016;113(33):9327–9332. doi:dx.doi.org/ 10.1007/s13365-015-0406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf EJ, Maniates H, Nugent N, et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology. 2018;92:123–134. doi: 10.1016/j.psyneuen.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, Horvath S. Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative Study. Biol Psychiatry. 2017;81(2):136–144. doi:dx.doi.org/ 10.1016/j.biopsych.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grant CD, Jafari N, Hou L, et al. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience. 2017;39:475–489. doi: 10.1007/s11357-017-0001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9(2):419–446. doi:dx.doi.org/ 10.18632/aging.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gale CR, Marioni RE Harris SE, Starr JM, Deary IJ. DNA methylation and the epigenetic clock in relation to physical frailty in older people: the Lothian Birth Cohort 1936. Clin Epigenetics. 2018;10(1):101. doi: 10.1186/s13148-018-0538-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gale CR, Marioni RE, Čukić I, et al. The epigenetic clock and objectively measured sedentary and walking behavior in older adults: the Lothian Birth Cohort 1936. Clin Epigenetics. 2018;10:4. doi: 10.1186/s13148-017-0438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44(4):1388–1396. doi: 10.1093/ije/dyx233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawn RB, Anderson EL, Suderman M, et al. Psychosocial adversity and socioeconomic position during childhood and epigenetic age: analysis of two prospective cohort studies. Hum Mol Genet. 2018;27:1301–1308. doi: 10.1093/hmg/ddy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simpkin AJ, Cooper R, Howe LD, et al. Are objective measures of physical capability related to accelerated epigenetic age? Findings from a British birth cohort. BMJ Open. 2017;7:e016708. doi: 10.1136/bmjopen-2017-016708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horvath SL, Levine AJ, HIV-1 infection accelerates age according to the epigenetic clock. J Infect Dis. 2015;212(10):1563–1573. doi: 10.1093/infdis/jiv277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nwanaji-Enwerem JC, Dai L, Colicino E, et al. Associations between long-term exposure to PM2.5 component species and blood DNA methylation age in the elderly: the VA normative aging study. Environ Int. 2017;102:57–65. doi: 10.1016/j.envint.2016.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ward-Caviness CK, Nwanaji-Enwerem JC, Wolf K, et al. Long-term exposure to air pollution is associated with biological aging. Oncotarget. 2016;7(46):74510–74525. doi: 10.18632/oncotarget.12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brody GH, Miller GE, Yu T, Beach SR, Chen E. Supportive family environments amelioratethe link between racial discrimination and epigenetic aging: areplication across two longitudinal cohorts. Psychol Sci. 2016;27(4):530–541. doi: 10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller GE, Yu T, Chen E, Brody GH, Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proc Natl Acad Sci USA. 2015;112(33):10325–10330. doi: 10.1073/pnas.1505063112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brody GH, Yu T, Chen E, Beach SR, Miller GE. Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. J Child Psychol Psychiatry & Allied Disciplines. 2016;57(5):566–74. doi: 10.1111/jcpp.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen EM, Miller GE, Yu T, Brody GH, The Great Recession and health risks in African American youth. Brain Behav Immun. 2016;53:234–241. doi: 10.1016/j.bbi.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mehta DB, Bruenig D, Lawford B. Accelerated DNA methylation aging and increased resilience in veterans: the biological cost for soldiering on. Neurobiol Stress. 2018;8:112–119. doi: 10.1016/j.ynstr.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zannas AS, Arloth J, Carrillo-Roa T, Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16:266. doi: 10.1186/s13059-015-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simons RL, Lei MK, Beach SR, et al. Economic hardship and biological weathering: the epigenetics of aging in a U.S. sample of black women. Soc Sci Med. 2016;150:192–200. doi: 10.1016/j.socscimed.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Breitling LP, Saum KU, Perna L, Schöttker B, Holleczek B, Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clinical Epigenetics. 2016;8:21. doi: 10.1186/s13148-016-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao X, Zhang Y, Breitling LP, Brenner H. Relationship of tobacco smoking and smoking-related DNA methylation with epigenetic age acceleration. Oncotarget. 2016;7(30):46878–46889. doi: 10.18632/oncotarget.9795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davis EG, Humphreys KL, McEwen LM, et al. Accelerated DNA methylation age in adolescent girls: associations with elevated diurnal cortisol and reduced hippocampal volume. Transl Psychiatry Psychiatry. 2017;7(8):e1223. doi: 10.1038/tp.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jovanovic T, Vance LA, Cross D. Exposure to violence accelerates epigenetic aging in children. Sci Rep. 2017;7(1):8962. doi: 10.1038/s41598-017-09235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Horvath S, Garagnani P, Bacalini MG. Accelerated epigenetic aging. Aging Cell. 2015;14(3):491–495. doi: 10.1111/acel.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Levine AJ, Quach A, Moore DJ. Accelerated epigenetic aging in brain is associated with pre-mortem HIV-associated neurocognitive disorders. J Neurovirol. 2016;22(3):366–375. doi: 10.1007/s13365-015-0406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simpkin AJ, Howe LD, Tilling K, The epigenetic clock and physical development during childhood and adolescence: longitudinal analysis from a UK birth cohort. Int J Epidemiol. 2017;46(2):549–558. doi: 10.1093/ije/dyw307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCartney DL, Stevenson AJ, Walker RM, et al. Investigating the relationship between DNA methylation age acceleration and risk factors for Alzheimer’s disease. Alzheimers Dement (Amst). 2018;10:429–437. doi: 10.1016/j.dadm.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suarez A, Lahti J, Czamara D, et al. The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clin Epigenetics. 2018;10:96. doi: 10.1186/s13148-018-0528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wolf EJ, Logue MW, Morrison FG. Posttraumatic psychopathology and a quickening pace of the epigenetic clock. Biol Psychiatry. 2018;83: S1–S107. doi: 10.1017/S0033291718001411 [DOI] [Google Scholar]
- 62. Binder AM, Corvalan C, Mericq V. Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics. 2018;13(1):85–94. doi: 10.1080/15592294.2017.1414127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dugué PA, Bassett JK, Joo JE. Association of DNA methylation-based biological age with health risk factors and overall and cause-specific mortality. Am J Epidemiol. 2018;187(3):529–538. doi: 10.1093/aje/kwx291 [DOI] [PubMed] [Google Scholar]
- 64. Austin MK, Chen E, Ross KM, et al. Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology. 2018;97:131–134. doi: 10.1016/j.psyneuen.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 65. Irvin MR, Aslibekyan S, Do A, et al. Metabolic and inflammatory biomarkers are associated with epigenetic aging acceleration estimates in the GOLDN study. Clin Epigenetics. 2018;10:56. doi: 10.1186/s13148-018-0481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nevalainen T, Kananen L, Marttila S. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clinical Epigenetics. 2017;9:20. doi: 10.1186/s13148-016-0301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Belsky DW, Moffitt TE, Cohen AA, et al. , Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187(6):1220–1230. doi: 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim S, Myers L, Wyckoff J, Cherry KE, Jazwinski SM. The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. GeroScience. 2017;39(1):83–92. doi: 10.1007/s11357-017-9960-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Boks MP, van Mierlo HC, Rutten BP, et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011 [DOI] [PubMed] [Google Scholar]
- 70. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with posttraumatic stress disorder and mortality. Psychosom Med. 2018;80:42–48. doi: 10.1097/PSY.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wolf EJ, Morrison FG, Sullivan DR, Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. doi: 10.1016/j.psyneuen.2015.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Degerman S, Josefsson M, Nordin Adolfsson A, Maintained memory in aging is associated with young epigenetic age. Neurobiol Aging. 2017;55:167–171. doi: 10.1016/j.neurobiolaging.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 73. Starnawska A, Tan Q, Lenart A, Blood DNA methylation age is not associated with cognitive functioning in middle-aged monozygotic twins. Neurobiol Aging. 2017;50:60–63. doi: 10.1016/j.neurobiolaging.2016.10.025 [DOI] [PubMed] [Google Scholar]
- 74. Chouliaras L, Pishva E, Haapakoski R, et al. Peripheral DNA methylation, cognitive decline and brain aging: pilot findings from the Whitehall II imaging study. Epigenomics. 2018;10:585–595. doi: 10.2217/epi-2017-0132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hodgson K, Carless MA, Kulkarni H. Epigenetic age acceleration assessed with human white-matter images. J Neurosci. 2017;37(18):4735–4743. doi: 10.1523/JNEUROSCI.0177-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raina A, Zhao X, Grove ML, et al. Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: the atherosclerosis risk in communities study. Clinical Epigenetics. 2017;9:21. doi: 10.1186/s13148-016-0302-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Horvath S, Ritz BR. Increased epigenetic age and granulocyte counts in the blood of Parkinson’s disease patients. Aging. 2015;7(12):1130–1142. doi: 10.18632/aging.100859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ambatipudi S, Horvath S, Perrier F, et al. DNA methylome analysis identifies accelerated epigenetic ageing associated with postmenopausal breast cancer susceptibility. Eur J Cancer. 2017;75:299–307. doi: 10.1016/j.ejca.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Steinberg J, Shah KM, Gartland A, Zeggini E, Wilkinson JM. Effects of chronic cobalt and chromium exposure after metal-on-metal hip resurfacing: an epigenome-wide association pilot study. J Orthop Res. 2017;35(10):2323–2328. doi: 10.1002/jor.23525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Walker RF, Liu JS, Peters BA, Ritz BR, Wu T, Ophoff RA, Horvath S. Epigenetic age analysis of children who seem to evade aging. Aging. 2015;7(5):334–339. doi: 10.18632/aging.100744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Verschoor CP, McEwen LM, Kohli V, et al. The relation between DNA methylation patterns and serum cytokine levels in community-dwelling adults: a preliminary study. BMC Genet. 2017;18(1):57. doi: 10.1186/s12863-017-0525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gross AM, Jaeger PA, Kreisberg JF, et al. Methylome-wide analysis of chronic hiv infection reveals five-year increase in biological age and epigenetic targeting of HLA. Mol Cell. 2016;62:157–168. doi: 10.1016/j.molcel.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Leung JM, Fishbane N, Jones M, et al. Longitudinal study of surrogate aging measures during human immunodeficiency virus seroconversion. Aging (Albany NY). 2017;9:687–705. doi: 10.18632/aging.101184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nelson KN, Hui Q, Rimland D, Xu K, Freiberg MS, Justice AC, Marconi VC, Sun YV. Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV-positive, treatment-naive U.S. veterans. AIDS. 2017;31(4):571–575. doi: 10.1097/QAD.0000000000001360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gao X, Zhang Y, Brenner H. Associations of Helicobacter pylori infection and chronic atrophic gastritis with accelerated epigenetic ageing in older adults. Br J Cancer. 2017;117(8):1211–1214. doi: 10.1038/bjc.2017.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kananen L, Nevalainen T, Jylhävä J, Marttila S, Hervonen A, Jylhä M, Hurme M. Cytomegalovirus infection accelerates epigenetic aging. Exp Gerontol. 2015;72:227–229. doi: 10.1016/j.exger.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 87. Lind PM, Salihovic S, Lind L. High plasma organochlorine pesticide levels are related to increased biological age as calculated by DNA methylation analysis. Environ Int. 2018;113:109–113. doi: 10.1016/j.envint.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 88. Chaix R, Alvarez-López MJ, Fagny M, et al. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology. 2017;85:210–214. doi: 10.1016/j.psyneuen.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Joober R, Schmitz N, Annable L, Boksa P. Publication bias: what are the challenges and can they be overcome? J Psychiatry Neurosci. 2012;37:149–152. doi: 10.1503/jpn.120065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Karlsson Linnér R, Marioni RE, Rietveld CA, et al. An epigenome-wide association study meta-analysis of educational attainment. Mol Psychiatry. 2017;22:1680–1690. doi: 10.1038/mp.2017.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7:507–509. doi: 10.1002/jcsm.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5 [DOI] [PubMed] [Google Scholar]
- 93. Frasca D, Blomberg BB, Paganelli R. Aging, obesity, and inflammatory age-related diseases. Front Immunol. 2017;8:1745. doi: 10.3389/fimmu.2017.01745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 95. Balasubramanian P, Howell PR, Anderson RM. Aging and caloric restriction research: a biological perspective with translational potential. EBioMedicine. 2017;21:37–44. doi: 10.1016/j.ebiom.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. At J, Bryce R, Prina M, et al. Frailty and the prediction of dependence and mortality in low- and middle-income countries: a 10/66 population-based cohort study. BMC Med. 2015;13:138. doi: 10.1186/s12916-015-0378-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 99. Lu AT, Xue L, Salfati EL, et al. GWAS of epigenetic aging rates in blood reveals a critical role for TERT. Nat Commun. 2018;9(1):387. doi: 10.1038/s41467-017-02697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.