Abstract
The Merkel disc is a main type of tactile end organs formed by Merkel cells and Aβ-afferent endings as first tactile sensory synapses. They are highly abundant in fingertips, touch domes, and whisker hair follicles of mammals and are essential for sensory tasks including social interaction, environmental exploration, and tactile discrimination. We have recently shown that Merkel discs use serotonin to transmit tactile signals from Merkel cells to Aβ-afferent endings to drive slowly adapting type 1 impulses on the Aβ-afferent nerves. This raises a question as whether the serotoninergic transmission at Merkel discs may be regulated by serotonin transporters and whether serotonin transporter inhibitors may affect the tactile transmission. Here, we made recordings from whisker afferent nerves of mouse whisker hair follicles and tested the effects of monoamine transporter inhibitors on slowly adapting type 1 impulses. We show that methamphetamine, a monoamine releasing facilitator and reuptake inhibitor, elicited spontaneous impulses as well as increased the numbers of slowly adapting type 1 impulses elicited by whisker hair deflections. S-duloxetine, a potent inhibitor of transporters of serotonin and norepinephrine, and fluoxetine, a selective inhibitor of serotonin transporters, both also increased the numbers of slowly adapting type 1 impulses. Prolonged treatment of whisker hair follicles with methamphetamine abolished most of slowly adapting type 1 impulses. Furthermore, the treatment of whisker hair follicles with methamphetamine resulted in serotonin release from whisker hair follicles. Taken together, our results suggest that serotonin transporters play a role in regulating tactile transmission at Merkel discs.
Keywords: Merkel disc, tactile, touch, serotonin, methamphetamine, monoamine transporters, whisker hair follicles
Introduction
Tactile end organs including Merkel discs, Pacinian corpuscles, Meissner’s corpuscles, and Ruffini endings1,2 are crucial for sophisticated sensory tasks such as environmental explorations, social interactions, and tactile discrimination in mammals.2 Merkel discs, also known as Merkel cell–neurite complexes, are highly abundant in human fingertips, touch domes of the skin, whisker hair follicles, and other tactile-sensitive spots throughout the body of mammals.3,4 They are formed by Merkel cells and their associated Aβ-afferent nerve endings in a synaptic-like structure.3,5 Merkel discs are highly sensitive to skin indentation, pressure, and hair movement. Tactile stimulation of Merkel discs induces slowly adapting type 1 (SA1) impulses on Aβ-afferent fibers, which are featured electrophysiological signals essential for sensory tasks such as tactile discrimination of an object’s texture, shape, and other physical properties.2,5
Cellular and molecular mechanisms underlying tactile transduction at Merkel discs have recently been uncovered.6–8 We and others have demonstrated that Piezo2 channels are mechanoreceptors located on Merkel cells.6–8 Tactile stimuli activate Piezo2 channels to transduce mechanical stimuli into electrical signals, which leads to the generation of SA1 impulses on Aβ-afferent fibers.6–8 In addition, Piezo2 channels are also shown to be expressed at Aβ-afferent nerve endings at Merkel discs to contribute to the initiation of impulses in the dynamic phase of SA1 responses.6,7 More recently, we have further shown that tactile transduction via Piezo2 at Merkel cells results in the release of serotonin from Merkel cells, which subsequently excites Aβ-afferent endings in Merkel discs to induce SA1 impulses.9,10 We have also shown that serotonin synthases are expressed in Merkel cells. These findings indicate that serotonin is a transmitter at Merkel discs to transmit tactile signals from Merkel cells to Aβ-afferent fibers.9,10 Interestingly, a recent study on the touch domes of the skin of mice suggested that norepinephrine may be a transmitter mediating tactile signaling at Merkel discs.11 However, with the use of mouse whisker hair follicle preparations, our recent pharmacological evidence did not support this adrenergic hypothesis of tactile transmission at Merkel disc.12 On the other hand, we have shown that serotonin satisfies most of criteria for being a transmitter at Merkel discs.9 One question remains to be addressed is whether serotoninergic transmission at Merkel discs may be regulated by serotonin transporters. In the central nervous system, serotoninergic transmission is highly regulated by serotonin transporters.13 Drugs such as methamphetamine and cocaine are powerful nerve stimulants whose effects are through their inhibitory actions at transporters for monoamines including serotonin.14 The potential presence of serotonin transporters on Merkel cells and their associated afferent nerve endings have been suggested in a previous study using an immunohistochemical method.15 It is currently unknown whether serotonin transporters may play a role in regulating serotoninergic transmission at Merkel discs. In the present study, we set out to address this question by determining whether pharmacological reagents know to affect serotonin release and reuptake may affect tactile transmission at Merkel discs of mouse whisker hair follicles.
Materials and methods
Animals
C57BL/6 mice (Harlan Laboratories, Indianapolis, IN) at the age of 4 to 8 weeks were used for making whisker hair follicle preparations to record tactile impulses from whisker afferent nerve fibers. Animal care and use conformed to NIH guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Whisker hair follicle preparations and whisker afferent fiber recordings
Whisker hair follicle preparations and whisker afferent fiber recordings were performed using our previously described method.8,9 In brief, whisker hair follicles with attached afferent fiber bundles were dissected out from whisker pads and anchored in a recording chamber. The whisker hair follicles were submerged and perfused in oxygenated Krebs solution that contained (in mM): 117 NaCl, 3.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose, bubbled with 95% O2 and 5% CO2, had a pH of 7.3 and osmolarity of 325 mOsm, and was maintained at 24°C room temperature. Unless otherwise indicated, the end of each hair follicle capsule was cut open to facilitate drug diffusion to Merkel cells in the whisker hair follicle. To record whisker afferent nerve impulses elicited by whisker deflections (SA1 responses), action potentials conducted on whisker afferent nerve fibers were recorded using a suction electrode, signals were amplified using a Multiclamp 700 A amplifier and sampled at 10 kHz with low pass filter set at 1 kHz.
Mechanical stimulation
Hair deflection was used as a tactile stimulus to elicit whisker afferent SA1 impulses as described in our previous study.8,9 In brief, we anchored whisker hair follicles in a recording chamber by affixing whisker hair shaft onto the bottom of the recording chamber and perfused them with Krebs solution. A blunted glass probe controlled by a piezo device was used for delivering mechanical stimuli. The probe was positioned at the center of the whisker hair follicle. When the mechanical probe displaced the whisker hair follicle, it generated a whisker hair shaft deflection. Unless otherwise indicated, hair deflection was induced by a 38-µm forward step to push the hair follicle for the duration of 2.612 s; the step had a 56-ms ramp at the speed of 0.68 µm/ms (dynamic phase) before reaching the 38-µm step (static phase, 2.5 s) and a revered ramp to its original position at the end of the step.
Pharmacology
Effects of monoamine transporter inhibitors on spontaneous and evoked SA1 impulses were determined. To test whether methamphetamine could induce whisker afferent impulses, methamphetamine (100 µM) was continuously bath-applied, while impulses were recorded for up to 45 min. To test the effects of methamphetamine, S-duloxetine, and fluoxetine on evoked SA1 responses, SA1 responses evoked by mechanical deflection of whisker hairs were determined before (control) and 30 min following the bath application of 100 µM methamphetamine, 10 µM S-duloxetine, or 10 µM fluoxetine. In a different set of experiments, SA1 responses were determined in whisker hair follicles treated with 100 µM methamphetamine for a prolonged period of 8 h at temperature of 24°C. Unless otherwise indicated, mechanical displacement in each set of experiments was 38 µm.
Detection of serotonin release induced by methamphetamine
Whisker hair follicles were dissected out from mice and anchored in a recording chamber that contained 120 µl Krebs solution at the room temperature of 24°C. The end of each capsule was cut open to facilitate serotonin diffusion out. In each set of experiments, five whisker hairs were used and they were incubated with the Krebs solution in the absence (control) or presence of 100 µM methamphetamine. After the treatment for different time up to 8 h, the Krebs solution was collected and centrifuged at 1000 × g for 15 min. Serotonin in the supernatant (100 µl) was detected using ELISA assay kits based on the company’s instruction (Enzo Life Sciences, Farmingdale, NY) with optical density being measured at 405 nm by a Titertrek ELISA plate reader (Flow Laboratories, Rockville, MD).
Data analysis
Data were analyzed using Clampfit software. Data are presented as mean ± SEM. Statistical significance was evaluated using Student’s t test; one-way analysis of variance with Bonferroni post hoc tests for multiple groups, *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
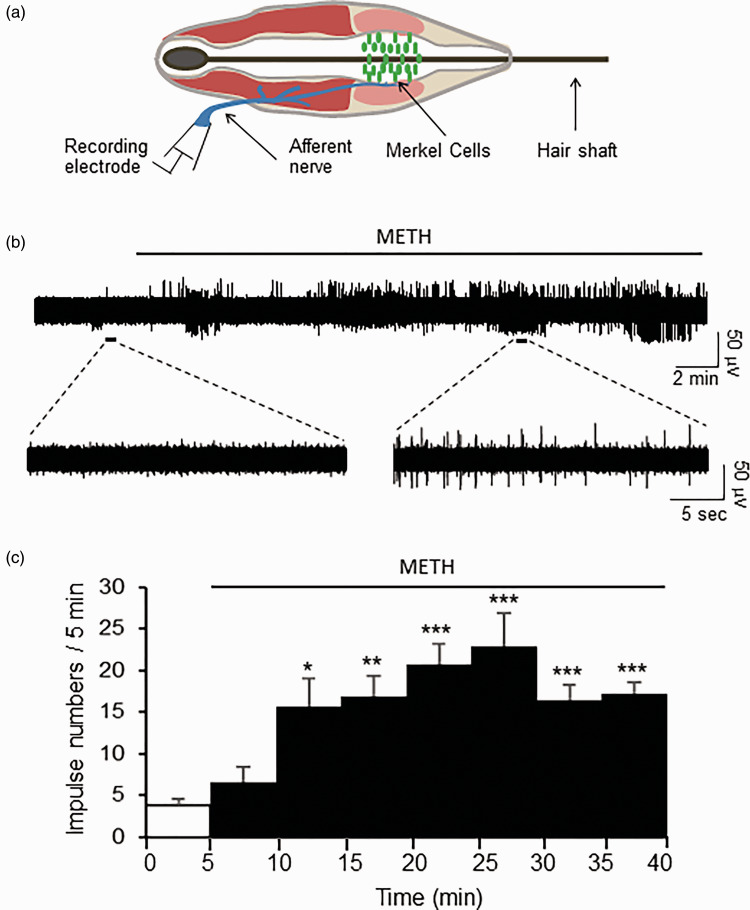
Spontaneous whisker afferent impulses were rarely observed in normal bath solution but we found that the treatment of whisker hair follicles with methamphetamine induced spontaneous whisker afferent impulses. In this set of experiments, whisker afferent impulses were recorded (Figure 1(a)) before and following the bath application of 100 µM methamphetamine for up to 45 min. Spontaneous nerve impulses were as few as 3.7 ± 0.9 (n = 7) during the first 5 min before the bath application of methamphetamine (Control, Figure 1(b) and (c)). However, spontaneous nerve impulses were significantly increased during 10 to 15 min of the bath application of 100 µM methamphetamine, and the impulse numbers during this period of time were 15.7 ± 4.3 (n = 7), over four times of the impulse numbers in control (P < 0.05, Figure 1(c)). The increases in the impulse numbers induced by methamphetamine lasted for 45 min, the longest time of the recordings made in this set of experiments (Figure 1(c)).
Figure 1.
METH increases spontaneous impulses recorded from afferent nerves of mouse whisker hair follicles. (a) Schematic diagram illustrates essential structures of a whisker hair follicle and electrophysiological recording of nerve impulses from the whisker afferent nerve. (b) Sample trace on the top panel shows impulses before and following the continuous bath application of 100 µM METH. On the bottom panels are two traces at expanded time scales to show few spontaneous impulses before METH (left) and many impulses following the application of METH. (c) Summary data of recordings (n = 7) of impulse numbers before (0–5 min) and following the bath applications of 100 µM METH from 5 to 40 min. In both (b) and (c), the line on the top of each graph indicates the duration of METH application. Data represent the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance with Bonferroni post hoc tests. METH: methamphetamine.
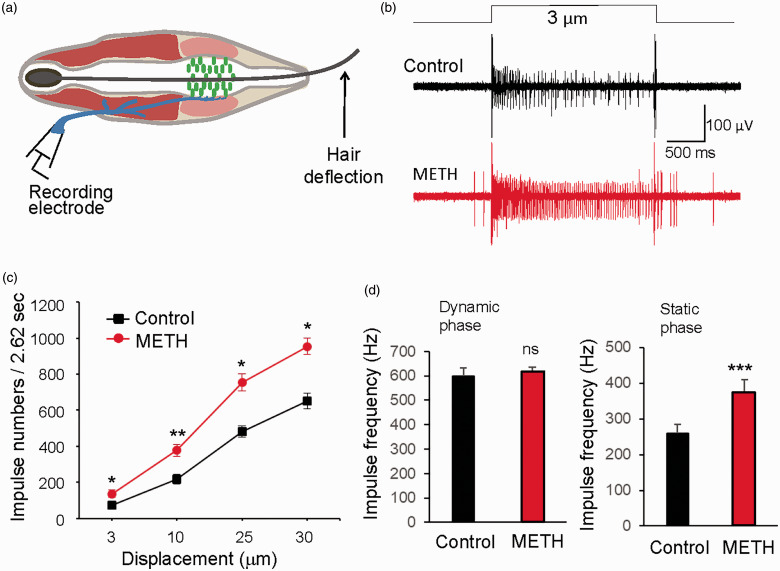
We next determined whether methamphetamine might affect SA1 impulses evoked by whisker hair deflections. In this set of experiments, SA1 impulses were elicited by mechanical displacements to deflect whisker hairs in a series of ramp-and-hold steps using a piezo probe (Figure 2(a)). Figure 2(b) are the sample traces of SA1 responses evoked by a 3 µm mechanical displacement before (control) and following the bath application of 100 µM methamphetamine for 30 min, which shows increases of the numbers of SA1 impulses by methamphetamine. At all displacement steps tested including 3, 10, 25, and 30 µm, SA1 impulse numbers were significantly increased by 100 µM methamphetamine in comparison with those before the application of methamphetamine. For example, with a 30-µm displacement step for the duration of 2.612 s, the total impulse numbers were 481.3 ± 15.2 (n = 6) in control and were significantly increased to 753 ± 23.8 (n = 6) following the bath application of 100 µM methamphetamine (Figure 2(c)). We analyzed impulse frequencies during ramp phase (dynamic phase) and holding phase (static phase) of displacements at the step of 10 µm, and we found that impulse frequencies in static phase but not dynamic phase were significantly enhanced by methamphetamine (n = 6, Figure 2(d)).
Figure 2.
METH increases SA1 impulses in the static phase in mouse whisker hair follicles. (a) Graph illustrates mechanical displacement to deflect whisker hair. (b) Two sample traces show SA1 impulses elicited by a 3-µm displacement step before (top) and following the application of 100 µM METH for 30 min (bottom). (c) Summary data of SA1 impulse numbers evoked by displacement at steps of 3, 10, 25, and 30 µm in recordings before (control, n = 6) and following the application of 100 µM METH. The impulses during both dynamic phase (112 ms) and static phases (2.5 s) were counted together. (d) Summary data (n = 6) of impulses frequencies during dynamic phase (left panel) and static phase (right) in control (black bars) and following the application of METH (red bars). Data represent the mean ± SEM, ns, no significant difference, *P < 0.05, **P < 0.01, ***P < 0.001, two-way analysis of variance with Bonferroni post hoc tests or paired Student’s t test. METH: methamphetamine.
We further tested the effects of S-duloxetine, a potent and selective inhibitor of monoamine transporters,16 on SA1 impulses evoked by whisker hair deflections. In this set of experiments, SA1 impulses were evoked by mechanical displacement at the step of 38 µm before (control) and following the application of 10 µM S-duloxetine (Figure 3(a) to (c)). While S-duloxetine did not have a significant effect on the impulses at dynamic phase of SA1, SA1 impulses in the static phase were increased in the presence of S-duloxetine in comparison with the control before S-duloxetine application (Figure 3(a) to (c)). Overall, the frequencies of SA1 impulses in static phase were 274.5 ± 21.3 Hz (n = 6) in the absence of S-duloxetine, and increased to 322.3 ± 22.8 Hz (n = 6, P < 0.05) following the bath applications of S-duloxetine. We also tested the effects of fluoxetine, a potent and selective inhibitor of serotonin transporter,17 on SA1 impulses evoked by whisker hair deflections. Similar to S-duloxetine, 10 µM fluoxetine had no effects on SA1 impulses in dynamic phase but significantly increased the frequency of SA1 impulses in the static phase (Figure 3(d)). The frequencies of SA1 impulses in static phase were 265.3 ± 14.9 Hz (n = 6) in the absence of fluoxetine, and increased to 323.4 ± 17.2 Hz (n = 6, P < 0.01) following the applications of 10 µM fluoxetine (Figure 3(d)).
Figure 3.
S-duloxetine and fluoxetine increase SA1 impulses in the static phase in mouse whisker hair follicles. (a) Sample traces show SA1 impulses elicited by whisker hair deflection with a 38-µm displacement step before (control) and following the bath application of 10 µM S-duloxetine for 30 min. (b) Summary data (n = 6) of SA1 impulse frequencies over time in control (black circles) and following the bath application of S-duloxetine (red circles). Time bin for each data point is 100 ms. (c) Summary data (n = 6) of SA1 impulse frequencies during dynamic phase (left panel) and static phase (right) in control (n = 6) and following the applications of S-duloxetine (n = 6). (d) Summary data of SA1 impulse frequencies during dynamic phase (left panel) and static phase (right) in control (n = 6) and following the applications of 10 µM fluoxetine. Data represent the mean ± SEM, ns, not significantly different, *P < 0.05, **P < 0.01, paired Student’s t test.
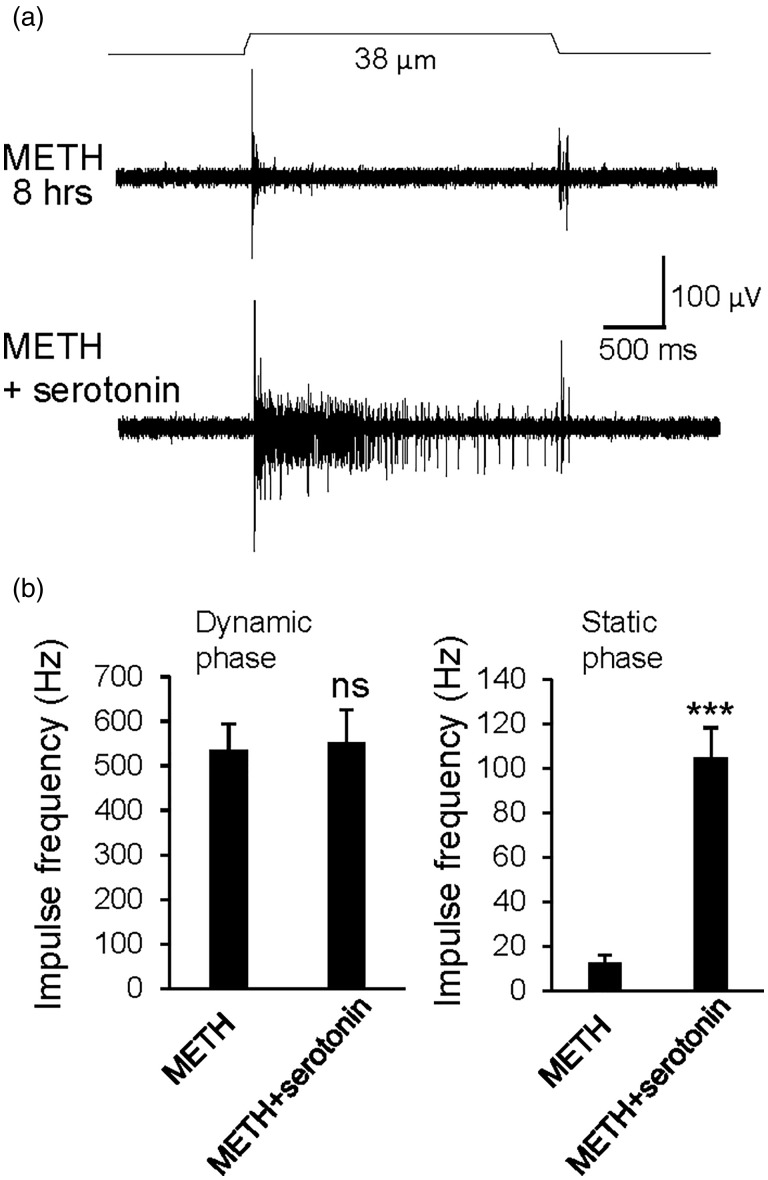
Prolonged treatment of whisker hair follicles with methamphetamine may result in the depletion of serotonin in Merkel cells to eventually disrupt tactile transmission at Merkel discs. To test this idea, we incubated whisker hair follicles with 100 µM methamphetamine for 8 h and then examined SA1 impulses evoked by mechanical displacement. We found that the prolonged treatment of whisker hair follicles with methamphetamine resulted in substantial decreases of the frequencies of SA1 impulses in static phase. The SA1 impulses frequencies in static phase were 278.4 ± 41.2 Hz (n = 6) in control group without methamphetamine treatment and decreased to 13 ± 1.6 Hz (n = 6, P < 0.001) in methamphetamine-treated group (Figure 4(a) and (b)). On the other hand, impulses in the dynamic phase were not significantly affected following the prolonged treatment of methamphetamine (Figure 4(b)). Interestingly, following the prolonged treatment with methamphetamine to abolish most of SA1 impulses, the impulses in the static phases could be partially restored when 100 µM serotonin was bath applied to whisker hair follicles (Figure 5(a) and (b)). Overall, the impulse frequency in static phase were 13 ± 1.6 Hz (n = 6) after 8 h of methamphetamine treatment without the addition of serotonin and were significantly increased to 104.9 ± 15.2 Hz (n = 6, P < 0.001, Figure 5(b)) with the addition of 100 µM serotonin. On the other hand, impulse frequency in dynamic phase was not significantly different between the two groups (Figure 5(b)).
Figure 4.
Prolonged METH treatment abolishes impulses in static phase of SA1 responses recorded from afferent nerves of mouse whisker hair follicles. (a) Sample traces show SA1 impulses recorded from afferent nerves of mouse whisker hair follicles incubated at 24°C either in the absence (control, top panel) or the presence of 100 µM METH for 8 h. In each recording, SA1 impulses were evoked by a whisker hair deflection with a 38-µm displacement step indicated above the sample traces. (b) Summary data of impulse frequencies during dynamic phase (left panel) and static phase (right panel) in the prolonged incubation without (control, open bars, n = 6) and with 100 µM METH (closed bars, n = 6). Whisker hair follicles were incubated for 8 h at 24°C without or with 100 µM METH. Data represent the mean ± SEM, ns, not significantly different, **P < 0.001, unpaired Student’s t test. METH: methamphetamine.
Figure 5.
The abolishment of SA1 impulses by prolonged METH treatment could be partially restored by serotonin. (a) Sample traces show whisker afferent impulses evoked by whisker hair deflections under two experimental conditions. One was with a whisker hair follicle treated with 100 µM METH for 8 h (top panel). Another was with a whisker hair follicle treated with 100 µM METH for 8 h and then with 100 µM serotonin added in bath solution during recordings of SA1 impulses (bottom panel). (b) Summary data of impulse frequencies in dynamic phase (left panel) and static phase (right panel) for the recordings from whisker hair follicles treated with METH for 8 h (METH, n = 6) and with the addition of 100 µM serotonin (METH + serotonin). Data represent the mean ± SEM, ns, not significantly different, ***P < 0.001, unpaired Student’s t test. METH: methamphetamine.
We determined whether serotonin was released following the treatment of whisker hair follicles with methamphetamine. In one set of experiments, whisker hair follicles were incubated at 24°C for 2 h in Krebs bath solution in the absence (control group) or presence of 100 µM amphetamine. Serotonin released into the supernatant was then detected using ELISA. Serotonin concentrations in the supernatant were measured to be 6.5 ± 2.4 nM (n = 6) in control group and were increased to 26.4 ± 2.9 nM (n = 6, P < 0.01) in amphetamine-treated group (Figure 6(a)). In a different set of experiments, we determined time course of serotonin release in the presence of 100 µM amphetamine (Figure 6(b)). We found that serotonin concentrations in the supernatant were elevated over time and peaked at 4 h time point of incubation with methamphetamine. For example, serotonin concentrations were 0.75 ± 0.06 nM (n = 6) in the first 5 min of incubation with amphetamine and the concentrations increased to 41.7 ± 9.6 nM (n = 6, P < 0.01, Figure 6(b)) following 4 h of incubation with amphetamine. Serotonin concentrations following 8 h of incubations with amphetamine were 38.4 ± 11.2 nM (n = 6) and were similar to those following 4 h of incubation with amphetamine (Figure 6(b)).
Figure 6.
METH induces serotonin release from whisker hair follicles. (a) Serotonin concentrations in the supernatant of Krebs solution in which hair follicles were incubated in the absence (control, open bar, n = 6) and presence of 100 µM METH (n = 6). In each experiment, five whisker hair follicles were pooled together and incubated at 24°C for a period of 2 h. (b) Serotonin concentrations in the supernatant of Krebs solution in which hair follicles were incubated with 100 µM METH for 5 min, 1, 2, 4, and 8 h (n = 6). In each experiment, five whisker hair follicles were pooled together and incubated at 24°C for the time indicated. Data represent the mean ± SEM. Sample sizes are numbers of experiments. *P < 0.05, **P < 0.01, ***P < 0.001, one-way analysis of variance with Bonferroni post hoc test. METH: methamphetamine.
Discussion
In the present study, we have shown that SA1 impulses recorded from afferent nerves of mouse whisker hair follicles are significantly changed by compounds that affect the release and reuptake of serotonin. Our findings provide a new line of evidence supporting our previous idea of serotonin being a transmitter at Merkel discs in whisker hair follicles.9 Our findings also indicate that the serotoninergic transmission at Merkel discs are highly regulated by serotonin transporters.
We have shown that methamphetamine induces spontaneous impulses and potentiates SA1 impulses evoked by tactile stimulation following short-time treatment of whisker hair follicles with methamphetamine. Methamphetamine is known to be a facilitator of monoamine release in the CNS synapses.14Consistently, we have shown that serotonin is released following the treatment of whisker hair follicles with methamphetamine. This effect of methamphetamine may account for methamphetamine-induced spontaneous impulses. Methamphetamine is also known to be an inhibitor of transporters for monoamines including serotonin.14 Methamphetamine-induced serotonin releases together with its inhibitory effects on serotonin reuptake would increase serotonin concentrations at synaptic clefts of Merkel discs, which may be a cause of methamphetamine-induced potentiation of SA1 impulses. Consistently, we have previously shown that SA1 impulses could be significantly potentiated following the application of exogenous serotonin to Merkel discs.10 In addition to methamphetamine, we have shown that S-duloxetine, a potent inhibitors of serotonin and norepinephrine transporters,16 and fluoxetine, a selective inhibitor of serotonin transporters, both also significantly potentiate SA1 impulses.17 These results together strengthen the idea that serotonin transporters play a role in regulating tactile transmission at the Merkel discs of whisker hair follicles.
We have shown that prolonged treatment of whisker hair follicles with methamphetamine abolishes most SA1 impulses evoked by tactile stimulation. This effect of methamphetamine may be attributed to serotonin depletion from Merkel cells since methamphetamine can facilitate serotonin release and inhibit serotonin reuptake.14 In our study, we have shown that the treatment of whisker hair follicles with methamphetamine increases the concentrations of serotonin in the supernatants for up to 4 h but no further increases in serotonin concentrations are observed from 4 to 8 h of methamphetamine treatment. These results suggest that serotonin in Merkel cells is released by methamphetamine initially and serotonin in Merkel cells is likely to be depleted following the prolonged methamphetamine treatment. Prolonged treatment of whisker hair follicles with methamphetamine abolished SA1 impulses in static phase but not dynamic phase, suggesting that SA1 impulses in static phase but not dynamic phase were mediated by serotoninergic transmission. This is consistent with the two-receptor model of SA1 impulses proposed in a previous study, which suggests that the static phase relies on Merkel cells and the dynamic phase is due to a direct mechanotransduction at Aβ-afferent terminals.6
While our previous studies9,10 and also the present one consistently indicate that serotonin is a transmitter at Merkel discs in whisker hair follicles, a recent study has suggested that norepinephrine is a transmitter of Merkel discs in the touch domes of mouse skin.11 However, the adrenergic hypothesis of tactile transmission at Merkel discs has been challenged by our recent study with whisker hair follicles.12 It is currently unclear about the causes of the discrepancy between our studies9,10 and the study by another group.11 Nevertheless, our studies including the one presented here have provided several lines of evidence indicating that serotonin satisfies the criteria of being a transmitter at Merkel discs. These criteria include (i) synthesis and storage of serotonin in Merkel cells9, (ii) release of serotonin following mechanical stimulation9, (iii) interaction of serotonin with their receptors at postsynaptic sites in β-afferent endings at Merkel discs9,10, and (iv) transporters for serotonin reuptake at Merkel discs.
We have shown in the present study that the serotoninergic synapses for tactile transmission at Merkel discs can be regulated by compounds affecting serotonin uptake and release. It is conceivable that serotonin uptake inhibitors such as cocaine, methamphetamine, and other recreational drugs in this category may act at Merkel disc serotoninergic synapses to alter tactile sensations. It will be interesting to study whether tactile hallucination in people following the use of methamphetamine and other recreational drugs may be partially due to their potential peripheral actions at Merkel discs. It will also be interesting to know in human patients whether serotonin transporter system at Merkel discs may be targeted and tactile sensitivity modified after taking other serotonin transporter inhibitors.
Author Contributions
WC performed recordings of electrophysiological tactile responses and serotonin detection. JGG designed the study and wrote the manuscript. All authors were involved in data collection and analysis, result discussion, and commented on the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants DE018661 and DE023090 (to JGG).
ORCID iD
Jianguo G Gu https://orcid.org/0000-0002-8404-9850
References
- 1.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 2014; 346: 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 2001; 11: 455–461. [DOI] [PubMed] [Google Scholar]
- 3.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol 2003; 271: 225–239. [DOI] [PubMed] [Google Scholar]
- 4.Merkel F. Tastzellen and Tastkoerperchen bei den Hausthieren und beim Menschen. Archiv f Mikrosk Anat 1875; 11: 636–652. [Google Scholar]
- 5.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol (Lond). 1969; 200: 763–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo S-H, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 2014; 509: 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo S-H, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 2014; 509: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive Abeta-afferent impulses. Cell 2014; 157: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ, Gu JG. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci USA 2016; 113: E5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang W, Kanda H, Ikeda R, Ling J, Gu JG. Serotonergic transmission at Merkel discs: modulation by exogenously applied chemical messengers and involvement of Ih currents. J Neurochem 2017; 141: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo S-H, Roybal DD, Karsenty G, Patapoutian A, Sulzer D, Lumpkin EA. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 2018; 100: 1401–1413. e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonekatsu M, Gu SL, Kanda H, Gu JG. Effects of norepinephrine and beta2 receptor antagonist ICI 118,551 on whisker hair follicle mechanoreceptors dissatisfy Merkel discs being adrenergic synapses. Mol Brain 2019; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 2007; 10: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 14.Halpin LE, Collins SA, Yamamoto BK. Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci 2014; 97: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana T, Endoh M, Fujiwara N, Nawa T. Receptors and transporter for serotonin in Merkel cell-nerve endings in the rat sinus hair follicle. An immunohistochemical study. Arch Histol Cytol 2005; 68: 19–28. [DOI] [PubMed] [Google Scholar]
- 16.Shelton RC. Serotonin and norepinephrine reuptake inhibitors. Handb Exp Pharmacol 2019; 250: 145–180. [DOI] [PubMed] [Google Scholar]
- 17.Fuller RW, Wong DT, Robertson DW. Fluoxetine, a selective inhibitor of serotonin uptake. Med Res Rev 1991; 11: 17–34. [DOI] [PubMed] [Google Scholar]