Abstract
Background:
This meta-analysis explored the correlation between the C-reactive protein to albumin ratio (CAR) and survival outcomes and clinicopathological characteristics in patients with pancreatic cancer.
Methods:
PubMed, Embase, Web of Science, and Cochrane Library databases were comprehensively searched through October 17, 2019. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were used to evaluate the association between CAR and overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS) in pancreatic cancer.
Results:
The meta-analysis included 11 studies comprising 2271 patients. The pooled results showed that a high CAR was predictive of worse OS (HR = 1.84, 95% CI = 1.65-2.06, P < .001), PFS (HR = 1.53, 95% CI = 1.27-1.85, P < .001), and DFS (HR = 1.77, 95% CI = 1.30-2.41, P < .001). An elevated CAR was also associated with male sex (OR = 1.38, 95% CI = 1.10-1.74, P = .006).
Conclusion:
Elevated pretreatment CAR effectively predicts inferior survival outcomes in patients with pancreatic cancer and may be a powerful prognostic indicator for these patients.
Keywords: C-reactive protein to albumin ratio, pancreatic cancer, meta-analysis, prognosis, systemic inflammation
Introduction
Pancreatic cancer is a highly lethal disease with a very poor prognosis.1 An estimated 458 918 new cases of pancreatic cancer were diagnosed and 432 242 patients died of this disease worldwide in 2018.2 Surgical resection is the only treatment to offer a chance of curative therapy, and adjuvant chemotherapy has been shown to improve survival outcomes.3 Immunotherapy including immune checkpoint blockade and vaccine therapy has been investigated in clinical trials and shows promising effects.4 Despite treatment advances, the prognosis of pancreatic cancer is dismal, with a 5-year survival rate as low as 6% in the United States.5 Therefore, it is imperative to identify reliable and sensitive prognostic factors to aid in individualized treatment decision-making and survival outcome prediction for patients with pancreatic cancer.
Growing evidence indicates that cancer-associated inflammation and immune cells are involved in cancer progression and can promote cancer development.6,7 Many cancer-related inflammatory biomarkers derived from peripheral blood samples have been investigated as effective prognostic factors in various cancers.8,9 These serum parameters include, but are not limited to, modified Glasgow prognostic score,10 platelet-to-lymphocyte ratio,11 systemic immune-inflammation index,12 and C-reactive protein to albumin ratio (CAR).13 The CAR is a novel inflammation-based parameter that has been evaluated as a potential prognostic factor in pancreatic cancer.14-24 However, the results of previous relevant studies 14-24 were inconsistent. To quantitatively and systematically clarify this issue, we identified relevant studies and performed a meta-analysis of the prognostic value of CAR in patients with pancreatic cancer.
Materials and Methods
Guidelines and Ethics Statement
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.25 All analyses were based on previously published studies; thus, ethics approval and informed consent were not required for this study.
Search Strategy
A comprehensive and systematic literature search was conducted using PubMed, Embase, Web of Science, and Cochrane Library databases. The search strategies were the combinations of the following keywords: (“C-reactive protein/albumin ratio” or “C-reactive protein to albumin ratio” or “C-reactive protein-to-albumin ratio” or “CRP/Alb ratio”) and (“pancreatic cancer” or “pancreatic carcinoma” or “pancreatic adenocarcinoma” or “pancreatic neoplasm” or “pancreatic tumor”). The last search was updated on October 17, 2019. In addition, the reference lists of relevant studies were carefully examined for potential inclusions.
Selection Criteria
The studies eligible for the meta-analysis met the following inclusion criteria: (1) histological or pathological confirmation of the pancreatic cancer diagnosis; (2) evaluation of CAR before treatment; (3) measurement of CAR using serum-based methods; (4) assessment of the prognostic value of CAR in survival outcomes including overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS); (5) available or calculable hazard ratio (HR) with 95% confidence interval (95% CI);26,27 (6) a CAR cutoff value; and (7) published in the English language. The exclusion criteria were (1) letters, conference abstracts, case reports, or reviews; (2) animal studies; (3) duplicate studies or studies with overlapping patients; (4) studies with insufficient information for analysis; and (5) non-English studies. Overall survival was defined as the date of the first treatment to the date of death.14 Progression-free survival was defined as the time from treatment to the first observation of progression.14 Disease-free survival was defined as the time from treatment to any recurrence or death from any cause.
Data Extraction and Quality Assessment
Data from all candidate articles were evaluated and extracted by 2 independent investigators (Q.X. and L.W.) and any discrepancies were resolved by discussion with a third investigator (S.Z.). The following information was extracted: first author, year of publication, country, sample size, age, sex distribution, ethnicity, tumor node metastasis stage, treatment, study design, CAR cutoff value, survival outcomes and HRs with 95% CIs, and study period. The quality of the eligible studies was assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS).28 The NOS comprises 3 major aspects: study cohort selection (0-4 stars), study cohort comparability (0-2 stars), and outcome ascertainment (0-3 stars). The maximum NOS score was 9, and studies with NOS scores ≥6 were considered to be of high quality.
Statistical Analysis
The association between CAR and prognosis was evaluated by pooling HRs with 95% CIs. An HR > 1 without overlapping 95% CI suggested that an elevated CAR was correlated with poorer prognosis. The heterogeneity among studies was assessed using Cochran’s Q29 and Higgins I2 test.30 When significant heterogeneity (I2 >50% and/or P < .10) was observed, a random effect model was adopted; otherwise, a fixed-effect model was applied. Subgroup analyses were conducted to further investigate and detect the source of heterogeneity. Pooled odds ratios (ORs) with 95%CIs were calculated to assess the relationship between CAR and clinicopathological features. Sensitivity analysis was conducted to test the stability of the results by omitting each study in turn. Publication bias was examined using Begg’s funnel plots and Egger’s tests. All statistical analyses were performed using Stata SE 12.0 (Stata Corporation). A P < .05 was considered statistically significant.
Results
Study Selection and Characteristics
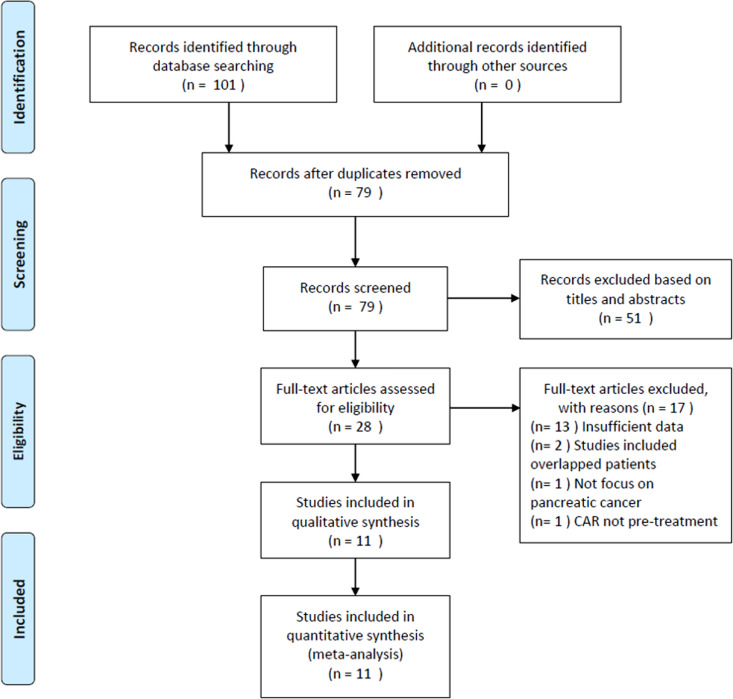
The literature selection process is summarized in Figure 1. The initial literature retrieval yielded 101 records, with 79 studies remaining after removing duplicate records. Subsequently, 51 studies were discarded following title and abstract screening, and the full texts of 28 studies were further examined. Seventeen studies were excluded after full-text review for insufficient data (n = 13), recruiting overlapping patients (n = 2), not focusing on pancreatic cancer (n = 1), and lack of pretreatment CAR measurement. Finally, the meta-analysis included a total of 11 studies14-24 comprising 2271 patients. The baseline characteristics of the included studies are shown in Table 1. The studies were published from 2016 to 2019 from 5 countries, including Japan (n = 4),17,19,20,23 China (n = 3),15,16,22 Korea (n = 2),14,21 Italy (n = 1),18 and Austria (n = 1).24 The sample sizes ranged from 43 to 595, with a median of 188. All included studies reported the association between CAR and OS,14-24 with 314,19,21 and 217,20 studies reporting the relationship between CAR and PFS and DFS, respectively. The CAR cutoff values ranged from 0.0003 to 3.85, with a median value of 0.18. Therefore, we used 0.20 for the subgroup analyses. The NOS scores ranged from 6 to 8, with a mean value of 7, indicating that all included studies were of high quality.
Figure 1.
Flow diagram of the study selection process.
Table 1.
Basic Characteristics of Studies Included in Meta-Analysis.
| Author | Year | Country | Sample size | Sex (M/F) | Ethnicity | TNM stage | Treatment | Age (years) mean/median (range) | Study design | Cutoff value | Survival analysis | Study duration | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee | 2016 | Korea | 82 | 49/33 | Asian | III-IV | Chemotherapy + targeted therapy | 63.5 | Retrospective | 0.5 | OS, PFS | 2011-2014 | 8 |
| Wu | 2016 | China | 233 | 156/77 | Asian | III-IV | Chemotherapy | 62 (26-85) | Retrospective | 0.54 | OS | 2011-2014 | 7 |
| Hang | 2017 | China | 142 | 92/50 | Asian | III-IV | Chemotherapy | 61 (34-86) | Retrospective | 0.156 | OS | 2009-2014 | 7 |
| Ikeguchi | 2017 | Japan | 43 | 30/13 | Asian | I-III | Surgery | 71.6 (37-88) | Retrospective | 0.04 | OS, DFS | 2006-2015 | 8 |
| Piciucchi | 2017 | Italy | 206 | 112/84 | Caucasian | I-IV | Mixed | 69.9 | Retrospective | 1 | OS | NR | 6 |
| Arima | 2018 | Japan | 142 | 74/68 | Asian | NR | Surgery | 69 (37-90) | Retrospective | 0.34 | OS, PFS | 2004-2014 | 6 |
| Fujiwara | 2018 | Japan | 188 | 115/73 | Asian | I-IV | Surgery | 67 (27-84) | Retrospective | 0.004 | OS, DFS | 2000-2015 | 7 |
| Kim | 2018 | Korea | 302 | 189/113 | Asian | III-IV | Chemotherapy + targeted therapy | 64 (38-83) | Retrospective | 3.85 | OS, PFS | 2004-2016 | 7 |
| Fan | 2019 | China | 595 | 380/215 | Asian | III-IV | Chemotherapy | 62 (33-84) | Retrospective | 0.18 | OS | 2011-2016 | 8 |
| Ikuta | 2019 | Japan | 136 | 76/60 | Asian | I-IV | Surgery | 68 (33-86) | Retrospective | 0.09 | OS | 2005-2017 | 7 |
| Vujic | 2019 | Austria | 202 | NR | Caucasian | NR | Surgery | NR | Retrospective | 0.0003 | OS | 2000-2016 | 6 |
Abbreviations: DFS, disease-free survival; F, female; M, male; NR, not reported; NOS, Newcastle Ottawa scale; OS, overall survival; PFS, progression-free survival.
Association Between CAR and OS in Pancreatic Cancer
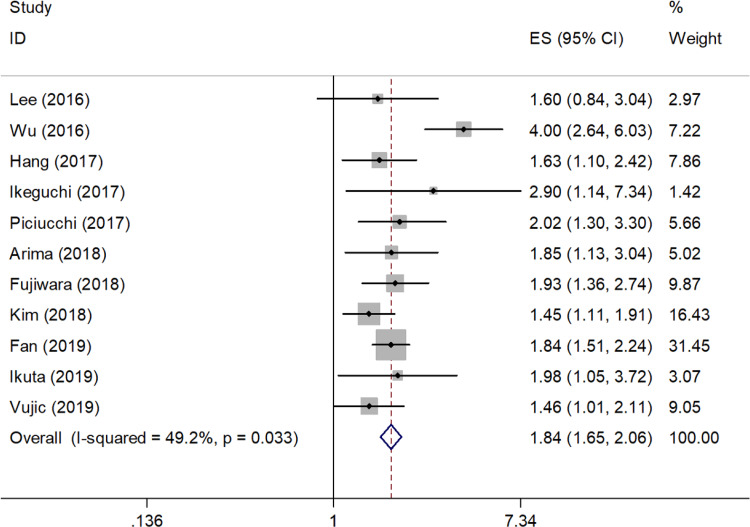
All 11 included studies (2271 cases) showed the prognostic value of CAR in OS. The pooled results were as follows: HR = 1.84, 95% CI = 1.65 to 2.06, P < .001 (Figure 2; Table 2). Subgroup analysis stratified by ethnicity, sample size, treatment, and CAR cutoff values indicated that CAR remained a significant prognostic factor in different subgroups (Table 2).
Figure 2.
Meta-analysis of the associations between the CAR and overall survival in pancreatic cancer.
Table 2.
Effect of CAR on Pancreatic Cancer in Different Subgroups.
| Subgroup | No. of studies | No. of patients | Effects model | HR (95%CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I 2 (%) | P h | ||||||
| Overall survival | 11 | 2271 | Fixed | 1.84 (1.65-2.06) | <.001 | 49.2 | .033 |
| Ethnicity | |||||||
| Asian | 9 | 1863 | Random | 1.95 (1.59-2.39) | <.001 | 55.3 | .022 |
| Caucasian | 2 | 408 | Fixed | 1.65 (1.24-2.21) | .001 | 13.3 | .283 |
| Sample size | |||||||
| <200 | 6 | 733 | Fixed | 1.84 (1.50-2.25) | <.001 | 0 | .904 |
| ≥200 | 5 | 1538 | Random | 1.95 (1.44-2.65) | <.001 | 77.9 | .001 |
| Treatment | |||||||
| Surgery | 5 | 711 | Fixed | 1.79 (1.46-2.21) | <.001 | 0 | .646 |
| Chemotherapy | 3 | 970 | Random | 2.25 (1.40-3.62) | .001 | 84 | .002 |
| Chemotherapy + targeted therapy | 2 | 384 | Fixed | 1.48 (1.15-1.90) | .002 | 0 | .788 |
| Mixed | 1 | 206 | – | 2.02 (1.27-3.22) | .003 | – | – |
| Cutoff value of CAR | |||||||
| <0.20 | 6 | 1306 | Fixed | 1.79 (1.56-2.06) | <.001 | 0 | .736 |
| ≥0.20 | 5 | 965 | Random | 2.04 (1.38-3.03) | <.001 | 75.7 | .002 |
| Progression-free survival | 3 | 526 | Fixed | 1.53 (1.27-1.85) | <.001 | 0 | .892 |
| Disease-free survival | 2 | 231 | Fixed | 1.77 (1.30-2.41) | <.001 | 28.9 | .236 |
Abbreviations: CAR, C-reactive protein to albumin ratio; HR, hazard ratio.
Relationship Between CAR and PFS and DFS in Pancreatic Cancer
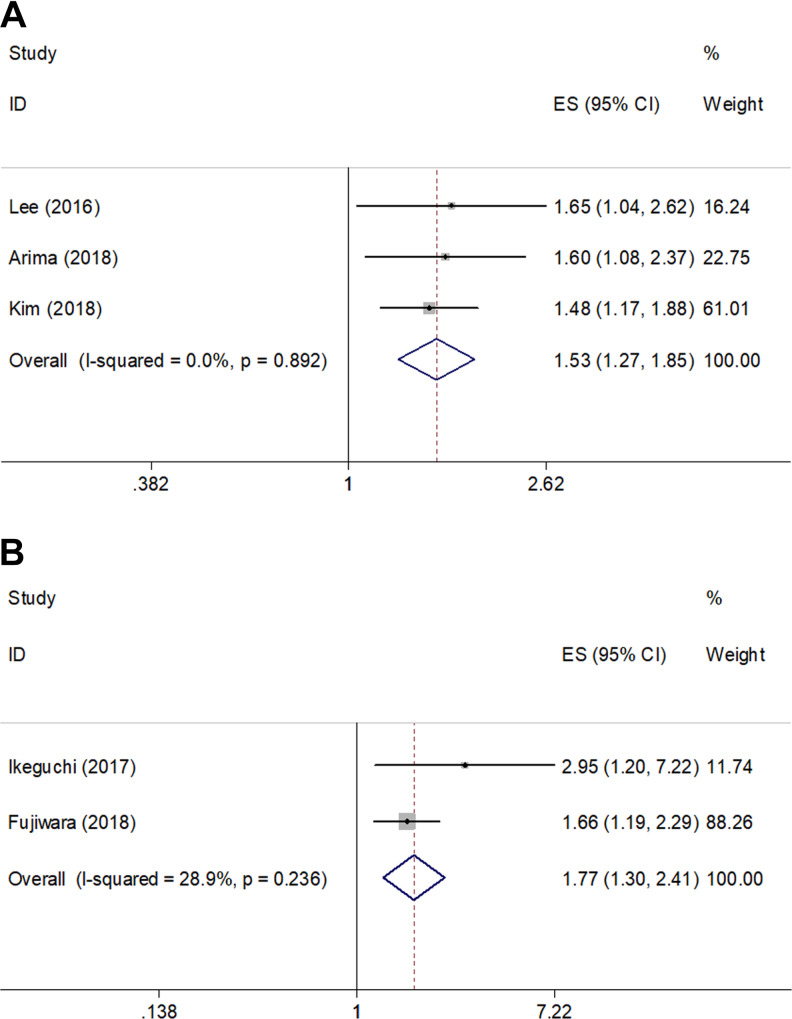
Three studies14,19,21 (526 patients) reported a correlation between CAR and PFS. As shown in Figure 3A and Table 2, the pooled HR and 95% CI were 1.53 and 1.27 to 1.85, respectively (P < .001). Moreover, data extracted from 2 studies17,20 (231 subjects) demonstrated that elevated CAR was associated with poor DFS (HR = 1.77, 95% CI = 1.30-2.41, P < .001; Figure 3B; Table 2). Subgroup analysis of PFS and DFS was not performed because of the limited number of studies.
Figure 3.
Meta-analysis of the pooled hazard ratios for patients with high CAR with different survival outcomes. (A) Progression-free survival (PFS) and (B) disease-free survival (DFS).
Correlation Between CAR and Clinicopathological Features in Pancreatic Cancer
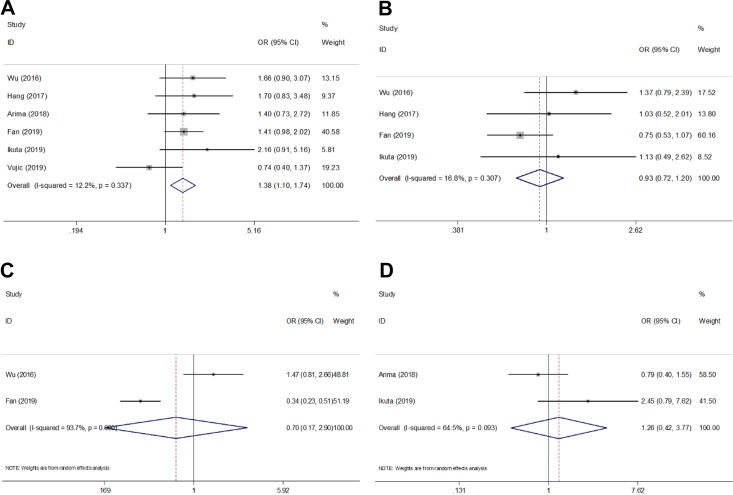
Six studies15,16,19,22-24 reported the relevance of CAR and clinicopathological characteristics including sex (male vs female), tumor location (head vs body or tail), disease stage (metastasis vs locally advanced), and pN stage (N1 vs N0). As shown in Figure 4 and Table 3, the pooled data indicated that high pretreatment CAR was associated with male sex (OR = 1.38, 95% CI = 1.10-1.74, P = .006) but not tumor location (OR = 0.93, 95% CI = 0.72-1.20, P = .582), disease stage (OR = 0.70, 95% CI = 0.17-2.90, P = .623), or pN stage (OR = 1.26, 95% CI = 0.42-3.77, P = .675).
Figure 4.
Meta-analysis of the association between CAR and clinicopathological features of pancreatic cancer. (A) Gender (male vs female), (B) tumor location (head vs body or tail), (C) disease stage (metastasis vs locally advanced), and (D) pN stage (N1 vs N0).
Table 3.
Meta-Analysis of Correlation Between CAR and Clinicopathological Features in Patients With Pancreatic Cancer.
| Clinicopathological features | No. of studies | No. of patients | Effects model | OR (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| I 2 (%) | P h | ||||||
| Gender (male vs female) | 6 | 1450 | Fixed | 1.38 (1.10-1.74) | .006 | 12.2 | .337 |
| Tumor location (head vs body or tail) | 4 | 1106 | Fixed | 0.93 (0.72-1.20) | .582 | 16.8 | .307 |
| Disease stage (metastasis vs locally advanced) | 2 | 828 | Random | 0.70 (0.17-2.90) | .623 | 93.7 | <.001 |
| pN stage (N1 vs N0) | 2 | 278 | Random | 1.26 (0.42-3.77) | .675 | 64.7 | .093 |
Abbreviations: CAR, C-reactive protein to albumin ratio; OR, Odds ratio.
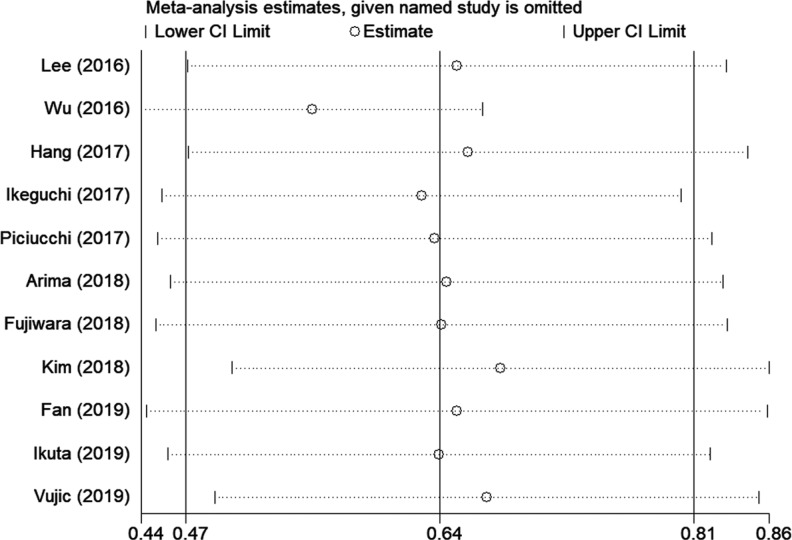
Sensitivity Analysis and Publication Bias
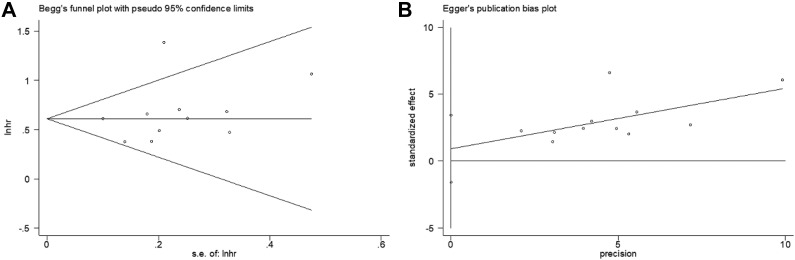
Sensitivity analysis to test the stability and credibility of the results, by sequentially omitting each study showed that the results were stable (Figure 5). Both Begg’s funnel plot and Egger’s linear regression test were applied to detect potential publication bias. The results suggested that there was no significant publication bias in this meta-analysis (Begg’s P = .213, Egger’s P = .431; Figure 6).
Figure 5.
Sensitivity analysis. The plot showed the pooled hazard ratios with 95% CIs after omitting any of the studies. The elimination of any studies did not alter the statistical significance.
Figure 6.
Publication bias test. (A) Begg’s funnel plot for publication bias test of overall survival (P = .213) and (B) Egger’s linear regression for publication bias test of overall survival (P = .431).
Discussion
Pancreatic cancer has aggressive biological behaviors, and the disease prognosis is dismal. Sensitive and effective prognostic biomarkers are helpful for the clinical management of pancreatic cancer.1 The current meta-analysis pooled data from 11 studies and found that elevated CAR was predictive of poor OS, PFS, and DFS. Furthermore, a high CAR was also associated with male patients. Thus, CAR could be a reliable and easily accessible prognostic factor for patients with pancreatic cancer.
The CAR is calculated as the C-reactive protein to albumin ratio and was initially used as a long-term marker of prognosis in patients with sepsis.31,32 In recent years, an increasing number of studies have investigated the prognostic role of CAR in various types of cancer33-35 and demonstrated that pretreatment CAR is an effective predictor of poor survival. The exact mechanisms underlying the prognostic function of CAR in cancer remain to be elucidated. The possible explanations include the following: C-reactive protein (CRP) is an acute phase protein synthesized by the liver, and its level rapidly increases in response to inflammation in patients with cancer.36 As a widely used inflammatory marker, elevated CRP is associated with poor prognosis in various cancers.37-39 Albumin (Alb) is a circulating protein in the plasma and is used as a nutritional indicator. Hypoalbuminemia, as a usual symptom of chronic malnutrition, is also common in patients with cancer. Therefore, CAR, which combines CRP and Alb, has the rationale to be a prognostic indicator for patients with cancer. High CAR levels could be a marker of inferior survival outcomes in cancer.
Many recent studies have also investigated the prognostic impact of CAR on patients with diverse cancer using meta-analytic methods. A meta-analysis including 4592 patients with tumor demonstrated significantly poorer OS in patients with a high pretreatment CAR than that in those with a low CAR (HR: 2.01, 95% CI = 1.58-2.56, P < .001).8 Another meta-analysis also indicated that elevated CAR predicted poor OS in patients with lung cancer.40 In addition, a recent meta-analysis showed that a high pretreatment CAR was associated with worse OS and DFS/PFS in patients with renal cell carcinoma (RCC), and a high CAR was also correlated with a variety of clinicopathological features.41 The pooled results in the present meta-analysis on pancreatic cancer also suggested that an elevated CAR was associated with poor OS, PFS, and DFS, consistent with the findings from other types of cancer. We also found that a high CAR was associated with male sex; that is, male patients with pancreatic cancer tend to have a higher CAR. This finding may suggest that male patients are inclined to suffer from worse prognosis. Notably, in our meta-analysis, only 2 studies were conducted in Italy and Austria; the other 9 studies were performed in Korea, China, and Japan. Considering that most included studies were performed in Asia, the results of this meta-analysis could be more applicable for Asian patients with pancreatic cancer.
Although this study is the first meta-analysis on CAR in patients with pancreatic cancer, it has several limitations. First, the CAR cutoff values differed in the included studies, which may cause selection bias. The cutoff values varied from 0.0003 to 3.85 and, thus affected the stratification of patients in different studies. Second, most of the studies were from Asia. Although subgroup analysis indicated that a high CAR also predicted poor OS in Caucasian patients, more studies on patients of various ethnicities are needed. Third, the sample size for the analysis of CAR and clinical factors was small, which may account for the negative results regarding the correlation between CAR and tumor location, disease stage, and pN stage.
Conclusions
In summary, the results of the current meta-analysis showed that an elevated CAR was predictive of poor prognosis in patients with pancreatic cancer. Moreover, we also found that male patients with pancreatic cancer tend to have a higher CAR. The CAR may be an effective prognostic marker for pancreatic cancer. However, due to several limitations, more large-scale studies are necessary to validate these results.
Footnotes
Authors’ Note: Q.X. and L.W. collected and analyzed the data, wrote the article; S.Z. analyzed the data; Q.X. and L.W. conceived and designed this study, analyzed the data, wrote the article; and all authors reviewed the article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Medical and Health Science and Technology Project of Zhejiang Province (No. 2019338599).
ORCID iD: Qinfen Xie  https://orcid.org/0000-0002-1001-4962
https://orcid.org/0000-0002-1001-4962
References
- 1. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73–85. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torphy RJ, Zhu YW, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2(4):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillen S, Schuster T, zum Buschenfelde CM, Friess H, Kleeff J.Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. [DOI] [PubMed] [Google Scholar]
- 8. Li N, Tian GW, Wang Y, Zhang H, Wang ZH, Li G. Prognostic role of the pretreatment c-reactive protein/albumin ratio in solid cancers: a meta-analysis. Sci Rep. 2017;7:41298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. [DOI] [PubMed] [Google Scholar]
- 10. Hu X, Wang Y, Yang WX, Dou WC, Shao YX, Li X. Modified Glasgow prognostic score as a prognostic factor for renal cell carcinomas: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:6163–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang LX, Chen HX. Establishing the prognostic value of platelet-to-lymphocyte ratio in cervical cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2019;29(4):683–690. [DOI] [PubMed] [Google Scholar]
- 12. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–75388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan Y, Gu X, Gao Z. Prognostic value of C-reactive protein to albumin ratio in patients with hepatocellular carcinoma: a meta-analysis. Clin Res Hepatol Gastroenterol. 2020;44(2):241–243. [DOI] [PubMed] [Google Scholar]
- 14. Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol. 2016;8(7):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu MW, Guo J, Guo LH, Zuo Q. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumor Biol. 2016;37(9):12525–12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep. 2017;7(1):2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeguchi M, Hanaki T, Endo K, et al. C-reactive protein/albumin ratio and prognostic nutritional index are strong prognostic indicators of survival in resected pancreatic ductal adenocarcinoma. J Pancreat Cancer. 2017;3(1):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piciucchi M, Stigliano S, Archibugi L, et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci. 2017;18(4):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arima K, Yamashita YI, Hashimoto D, et al. Clinical usefulness of postoperative C-reactive protein/albumin ratio in pancreatic ductal adenocarcinoma. Am J Surg. 2018;216(1):111–115. [DOI] [PubMed] [Google Scholar]
- 20. Fujiwara Y, Haruki K, Shiba H, et al. C-reactive protein-based prognostic measures are superior at predicting survival compared with peripheral blood cell count-based ones in patients after curative resection for pancreatic cancer. Anticancer Res. 2018;38(11):6491–6499. [DOI] [PubMed] [Google Scholar]
- 21. Kim HJ, Lee SY, Kim DS, et al. Inflammatory markers as prognostic indicators in pancreatic cancer patients who underwent gemcitabine-based palliative chemotherapy. Korean J Int Med. 2020;35(1):171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Z, Fan K, Gong Y, et al. The CRP/albumin ratio predicts survival and monitors chemotherapeutic effectiveness in patients with advanced pancreatic cancer. Cancer Manag Res. 2019;11:8781–8788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikuta S, Aihara T, Yamanaka N. Preoperative C-reactive protein to albumin ratio is a predictor of survival after pancreatic resection for pancreatic ductal adenocarcinoma. Asia Pac J Clin Oncol. 2019;15(5):e109–e114. [DOI] [PubMed] [Google Scholar]
- 24. Vujic J, Marsoner K, Wienerroither V, Mischinger HJ, Kornprat P. The predictive value of the CRP-to-albumin ratio for patients with pancreatic cancer after curative resection: a retrospective single center study. In Vivo. 2019;33(6):2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 26. Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. [DOI] [PubMed] [Google Scholar]
- 27. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. [DOI] [PubMed] [Google Scholar]
- 29. Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 30. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 31. Ranzani OT, Zampieri FG, Forte DN, Azevedo LCP, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. Plos One. 2013;8(3):e59321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. 2009;9(1):30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer. 2015;15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu XC, Sun XW, Liu JJ, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. [DOI] [PubMed] [Google Scholar]
- 36. Sciarra A, Gentilucci A, Salciccia S, et al. Prognostic value of inflammation in prostate cancer progression and response to therapeutic: a critical review. J Inflamm (London). 2016;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fang Y, Xu C, Wu P, et al. Prognostic role of C-reactive protein in patients with nasopharyngeal carcinoma: A meta-analysis and literature review. Medicine. 2017;96(45):e8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou L, Cai X, Liu Q, Jian Z-Y, Li H, Wang K-J. Prognostic role of C-reactive protein in urological cancers: a meta-analysis. Sci Rep. 2015;5:12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Woo HD, Kim K, Kim J. Association between preoperative C-reactive protein level and colorectal cancer survival: a meta-analysis. Cancer Causes Control. 2015;26(11):1661–1670. [DOI] [PubMed] [Google Scholar]
- 40. Deng TB, Zhang J, Zhou YZ, Li WM. The prognostic value of C-reactive protein to albumin ratio in patients with lung cancer. Medicine (Baltimore). 2018;97(50):e13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou W, Zhang GL. C-reactive protein to albumin ratio predicts the outcome in renal cell carcinoma: A meta-analysis. PLoS One. 2019;14(10):e0224266. [DOI] [PMC free article] [PubMed] [Google Scholar]