Abstract
Background:
A precise and consistent definition of return to sport (RTS) after anterior cruciate ligament (ACL) injury is lacking, and there is controversy surrounding the process of returning patients to sport and their previous activity level.
Purpose:
The aim of the Panther Symposium ACL Injury Return to Sport Consensus Group was to provide a clear definition of RTS after ACL injury and a description of the RTS continuum as well as provide clinical guidance on RTS testing and decision-making.
Study Design:
Consensus statement.
Methods:
An international, multidisciplinary group of ACL experts convened as part of a consensus meeting. Consensus statements were developed using a modified Delphi method. Literature review was performed to report the supporting evidence.
Results:
Key points include that RTS is characterized by achievement of the preinjury level of sport and involves a criteria-based progression from return to participation to RTS and, ultimately, return to performance. Purely time-based RTS decision-making should be abandoned. Progression occurs along an RTS continuum, with decision-making by a multidisciplinary group that incorporates objective physical examination data and validated and peer-reviewed RTS tests, which should involve functional assessment as well as psychological readiness. Consideration should be given to biological healing, contextual factors, and concomitant injuries.
Conclusion:
The resultant consensus statements and scientific rationale aim to inform the reader of the complex process of RTS after ACL injury that occurs along a dynamic continuum. Research is needed to determine the ideal RTS test battery, the best implementation of psychological readiness testing, and methods for the biological assessment of healing and recovery.
Keywords: anterior cruciate ligament, return to sport, rehabilitation, sports medicine
Anterior cruciate ligament (ACL) injury and subsequent treatment have been the subject of thousands of scientific investigations over the last 50 years. Among the controversies that persist in ACL treatment is the process of return to sport (RTS).24,45,57,60 The rehabilitation, as well as the RTS process, begins immediately after ACL injury, and high-quality rehabilitation is an important element in both operative and nonoperative ACL injury treatment.4,24,74 There is, however, a lack of standardization in ACL rehabilitation programs.19,43 There is also a lack of consensus on the preparation of patients for a successful RTS.5,29,41 Moreover, there has been wide variability in the criteria used in RTS decision-making.10 Although time-based decision-making is frequently used, appropriate RTS timing is uncertain, especially given the variability in the individual patient’s recovery and biological healing of the graft. Objective, criteria-based RTS programs are increasingly used, but a lack of consistency in these testing protocols still remains.9
Controversy also remains in terms of the definition of RTS after ACL injury treatment and a successful outcome. In 2016, a consensus group from the First World Congress in Sports Physical Therapy defined an RTS continuum in general for all sports, but this has not been applied to ACL injury.5 The RTS continuum emphasized a criteria-based progression from “return to participation” to “return to sport” to “return to performance.” “Return to participation” was defined as return to training or participation in sport at a lower level but not yet ready to return to full sporting activity at the previous level. “Return to sport” was defined as return to the previous level of sport but not performance at the desired or preinjury level. “Return to performance” was defined as patients’ return to performance at the preinjury level of sport. These terms are used as the patient progresses back from injury and can describe the successful RTS process. This model of a continuum is appropriate for the complex process of RTS after ACL injury because of the multiple decisions made as the patient progresses through the rehabilitation process, resumes activities, and ultimately, returns to the preinjury level of performance.
An international, multidisciplinary group of ACL clinical and research experts was convened with the task of development of evidence-based and expert opinion consensus statements on RTS after ACL injury. This applies to both operative and nonoperative treatment of ACL injury, as the RTS principles remain the same. The aim of the group was to provide a clear definition of RTS after ACL injury and a description of the RTS continuum as well as to provide guidance on RTS for patients undergoing ACL treatment. The purpose of this paper is to report the consensus statements on RTS after ACL injury and the evidence to support the statements.
Methods
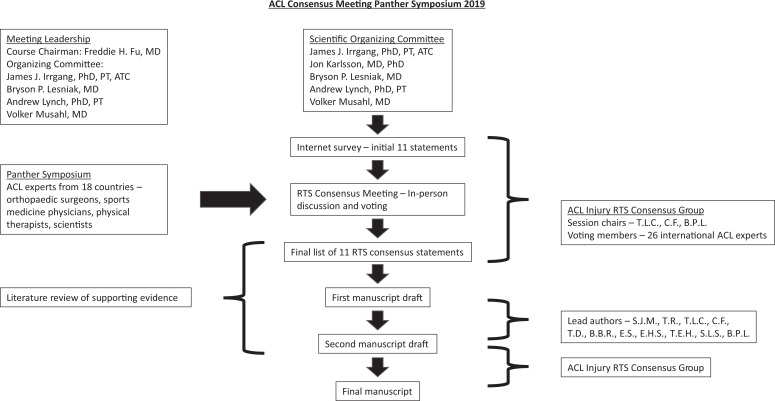
An international, multidisciplinary group of ACL clinical and research experts collaborated in a consensus building effort that culminated in the ACL Consensus Meeting–Panther Symposium 2019 on June 5 to 7, 2019, at the University of Pittsburgh Medical Center in Pittsburgh, Pennsylvania, USA (Figure 1). This global symposium included experts from 18 countries joining together to form consensus groups on current areas of ACL injury controversy, including treatment, clinical outcomes, and RTS. A total of 26 international ACL experts including orthopaedic surgeons, sports medicine physicians, physical therapists, and scientists were convened to form the Panther Symposium ACL Injury Return to Sport Consensus Group. A modified Delphi method was used to develop the consensus statements on RTS after ACL injury.26,34 This consisted of 3 rounds: internet survey with consensus group member feedback, in-person discussion facilitated by the 3 RTS session chairs (T.L.C., C.F., B.P.L.), and final vote.
Figure 1.
International anterior cruciate ligament (ACL) experts convened as part of a consensus building effort in June 2019. Through a stepwise process, the ACL Injury Return to Sport Consensus Group developed the final consensus statements and paper. RTS, return to sport.
An initial list of 11 statements was drafted by the scientific organizing committee and session chairs to address areas of current controversy and provide guidance for clinicians to address the challenges of RTS. The initial list was created as a starting point, and then the modified Delphi process commenced. For the first round, consensus group members completed an internet-based survey to indicate level of agreement or disagreement and to provide feedback on the statements. After 2 days of evidence-based presentations by symposium delegates at the ACL Consensus Meeting, the second round of the modified Delphi process was held with a structured session where each statement generated from the results of the internet-based survey was discussed and revised. The discussion was moderated by the 3 RTS session chairs (T.L.C., C.F., B.P.L.). After the discussion, a vote was taken, and 80% agreement was determined a priori to represent consensus. Statements that did not reach 80% agreement were reported as such. Then 2 assigned liaisons (S.J.M., T.R.) documented the discussion, revised each statement at the requests of the consensus group, and completed a literature review of MEDLINE to be included in support of the finalized statements. MEDLINE was searched in June 2019 using the terms anterior cruciate ligament, return to sport, and return to play with a focus on publications in the previous 5 to 10 years. To reduce potential bias, the liaisons did not submit answers to the premeeting survey, nor did they vote in the consensus process.
Consensus Statements and Discussion
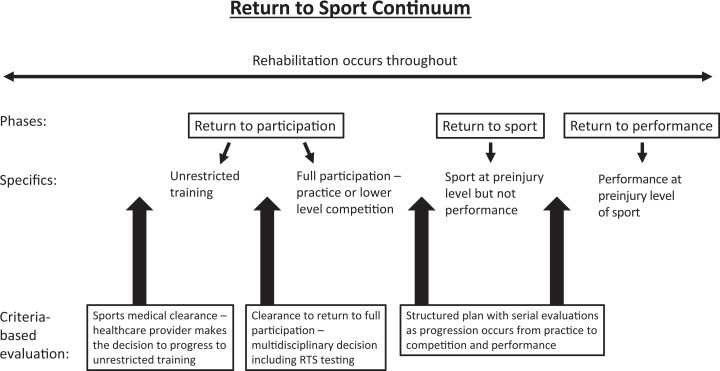
After discussion by the consensus group, 11 statements achieved consensus and are presented below (Table 1). These are accompanied by a summary of the pertinent evidence and rationale that support each statement. The previously published RTS terminology5 was used to maintain consistency in the literature and expanded upon to provide further detail (Figure 2).
TABLE 1.
ACL Injury RTS Consensus Statementsa
| Consensus Statement | Agreement, n (%) |
|---|---|
| RTS is characterized by achieving the preinjury level of sports participation as defined by the same type, frequency, intensity, and quality of performance as before injury. | 24/26 (92) |
| Sports medical clearance should be made before progressing the patient to unrestricted training and competition. | 25/26 (96) |
| Clearance to full participation (practice followed by competition) should be a multidisciplinary decision involving the patient, parent if the patient is under 18 years of age, surgeon, team physician, and physical therapist/athletic trainer. | 26/26 (100) |
| Clearance to return to full participation should be followed by a carefully structured plan to return to practice before progressive return to competition. | 26/26 (100) |
| Purely time-based RTS decision-making should be abandoned in clinical practice. | 26/26 (100) |
| RTS decision-making must include objective physical examination data (eg, clinical tests and measures). | 26/26 (100) |
| Patients should pass a standardized, validated, and peer-reviewed RTS test, with respect to the healing tissues, before returning to full participation after ACL injury with or without ACL reconstruction. | 23/26 (88) |
| RTS testing should involve assessment of specific functional skills that demonstrate appropriate quality of movement, strength, range of motion, balance, and neuromuscular control of the lower extremity and body. | 26/26 (100) |
| RTS decision-making includes psychological readiness as measured by a validated scale. | 22/26 (85) |
| The decision to release an athlete to RTS should consider contextual factors (type of sport, time of season, position, level of competition, etc). | 26/26 (100) |
| Consideration should be given to the nature and severity of concomitant injuries of the knee (eg, cartilage and menisci) when making RTS decisions. | 25/26 (96) |
aACL, anterior cruciate ligament; RTS, return to sport.
Figure 2.
The return-to-sport continuum is a criteria-based progression through the phases of return to participation, return to sport, and return to performance, with structured, serial evaluations throughout the process.
RTS is characterized by achieving the preinjury level of sports participation as defined by the same type, frequency, intensity, and quality of performance as before injury (agree 24/26; 92%)
RTS is one of the main goals of nonoperative or operative treatment for ACL injury. Anatomic ACL reconstruction is the gold-standard treatment for ACL injury in patients who wish to return to cutting or pivoting sports, have physically demanding occupations, or have persistent instability.9,24,46 Some patients are able to obtain a functionally stable knee with nonoperative management and to RTS.31,70 Previous research indicates that there is discrepancy between the reality of RTS rates after ACL injury and patients’ expectations.6,9,24,62 While approximately 90% of the patients report normal or near normal knee function on the International Knee Documentation Committee (IKDC) Subjective Knee Form, a large systematic review reported pooled rates of 74% to 87% returning to some sports activity, 59% to 72% returning to their preinjury sport, and 46% to 63% returning to competitive sports.7 The difference between the varied reports of RTS rates and patients’ subjective evaluation may be because a precise and consistent definition of RTS is lacking.6,9,24,29,62 Terms like “return to play,” “return to sport,” “return to participation,” and “return to unrestricted physical activity” are used interchangeably and cause confusion in the literature.5,6,24,29
Moreover, the definition of a successful RTS remains unclear.66 Multiple factors must be taken into consideration for determination of a successful RTS because of the differences in competition and reinjury risk. For some patients, their level of sport requires greater frequency and intensity as well as greater training to reach the desired level of performance. For other patients, the goal is not to return to the same level of sport and may actually be to return at a lower level. Successful RTS, therefore, represents different things to different patients. In addition, the aspects of the sport that include pivoting or nonpivoting and contact or noncontact can have dramatic differences on the risk of reinjury. Therefore, the consensus group determined that RTS must take into account the type of sport (pivoting or nonpivoting, contact or noncontact, and same as preinjury or a different sport), frequency (daily, weekly, monthly, etc), intensity (competitive, recreational, professional), and the performance level.39,50,66 It is important to recognize that RTS is an outcome measure that must include these specific components, but RTS is also a continuous process to reach the end goal.
Conclusion
To be precise and consistent, the RTS definition must include achieving the factors of preinjury sports type, frequency, intensity, and quality of performance.
Sports medical clearance should be made before progressing the patient to unrestricted training and competition (agree 25/26; 96%)
The decision of clearance to unrestricted training is multifactorial and should consider the time since injury, treatment, clinical examination, RTS testing, psychological readiness, and sport-specific conditions.4,5,44 Competing interests and expectations of those involved in the RTS process (eg, patient, family, coach, surgeon, team physician, physical therapist/athletic trainer) should be recognized.5,16,20 Ultimately, the decision to provide clearance to begin progressing the patient’s training is to be made by the health care provider, including physician or physical therapist/athletic trainer. This is an important distinction determining that the health care provider alone should make this initial decision to progress to unrestricted training. With any conflict of interest, the health care provider’s ethical obligation is to the patient’s health.21 Although the team physician may experience conflicting pressures, they must be transparent and inform the patient about any concerns so that the patient is adequately informed.16 These contextual factors make the clearance decision demanding and emphasize the importance of understanding the RTS process as a continuum with a criteria-based stepwise approach.74
Conclusion
It is vital that the health care provider make the sports medical clearance decision before progressing the patient to unrestricted training.
Clearance to return to full participation should be followed by a carefully structured plan to return to practice before progressive return to competition (agree 26/26; 100%)
The RTS process should be considered as a progressive course throughout the patient’s rehabilitation, taking into account the restoration of biological knee health according to the chosen treatment option, the targeted sport, and the desired level of performance as well as concomitant knee injuries and psychological readiness.¶¶ The process should be divided into phases, including specific clinical and functional milestones that are required to be met before progression to the next phase.4,5,69 As such, RTS should not be understood as an isolated decision at the end of the rehabilitation process.5 The RTS continuum as defined by Ardern et al5 emphasizes the stepwise progression through the 3 elements of the RTS process. According to the progression of activity, the 3 required elements are return to participation, RTS, and return to performance. During the return to participation phase, the athlete is physically active and may train but is medically, physically, and/or psychologically not yet ready to RTS. During the RTS phase, the athlete has returned to the defined sport, but the desired performance level is not yet reached. During the return to performance phase, the athlete returns to the defined sport and performs at the preinjury level. This model of an RTS continuum focuses on the athlete advancing through a progression of activity.
Consistent with the previous RTS continuum terminology, this consensus group used the terminology of return to participation, RTS, and return to performance but expanded this further (Figure 2). Return to participation was divided into unrestricted training, followed by full participation, to emphasize the progression of activity from training to sporting practice. RTS and then return to performance follow in stepwise progression. An athlete should be cleared to start with the next activity phase only if specific goals of the previous phase are achieved and confirmed by sport-specific clinical and functional tests.69 Serial evaluations should occur as the athlete progresses through the structured plan.
Others have similarly reported on RTS as a stepwise progression. One such group subdivided the RTS process, using the terms of graded progression from physical therapy (rehabilitation) to sport-specific training, followed by training for competition, and then actual competition.11 Another report defined the key steps of the RTS progression as on-field rehabilitation, return to training, return to competitive match play, and return to performance.13 For consistency, this consensus group limited the terminology as seen in Figure 2 to capture the RTS continuum with clear and precise terminology.
A 3-step, decision-based RTS model was reported in 2010 to synthesize and categorize different aspects of the RTS process and may also be a useful framework for providers to consider.16 Step 1 deals with medical factors to evaluate the patient’s health status, such as demographics, medical history, and physical and psychological examinations. Step 2 involves the sport-specific risk modifiers to evaluate participation risk, such as type of sport, competition level, limb dominance, and protective capabilities. Step 3 deals with decision modifiers, such as timing of season, conflict of interest, and internal and external pressure. In 2015, the Strategic Assessment of Risk and Risk Tolerance (StARRT) framework modified this 3-step model to group risk assessment by casual biological constructs and compare the risk assessment to the assessment of risk tolerance.63 This framework can be useful to the health care provider because if the risk assessment is greater than the risk tolerance, then there is reason to not allow RTS.
Conclusion
The RTS continuum emphasizes a carefully structured stepwise progression of return to practice first and then return to competition as summarized in Figure 2.
Clearance to full participation (practice followed by competition) should be a multidisciplinary decision involving the patient, parent if the patient is under 18 years of age, surgeon, team physician, and physical therapist/athletic trainer (agree 26/26; 100%)
RTS occurs along a continuum, and there is a shared decision-making process that occurs over time and with multiple contributors. There are different medical and technical competencies between the different contributors (surgeon, team physician, physical therapist/athletic trainer) in this process. The principles of shared decision-making apply, and the patient is actively involved.25,64 A multidisciplinary decision must be made with reasonable compromise from all groups if dissent exists. This multidisciplinary approach requires well-defined roles, communication among all parties, and a system to protect the athlete from disparate risk tolerances.3,5,64,69
Inclusion of the coach as a decision-maker in this consensus statement did not reach consensus (7/26; 27% agreement). There was concern that inclusion of the coach in the medical decision would create a conflict of interest, given the coach’s obligation or commitment to the team. The primary obligation of the health care provider is the patient’s health, whereas the coach remains focused on the success of the team.27 Nevertheless, the coach, as a key person in the sport development of the athlete, needs to be informed and involved in information sharing as the athlete progresses toward sports participation. The coach has the ability to evaluate the performance of the patient as he or she returns to practice and can provide an assessment of the patient’s progress to the health care providers.
Conclusion
Given that the clearance to return to full participation occurs along the RTS continuum, the decision must be multidisciplinary, including the patient, physicians, and physical therapist/athletic trainer, but the coach is not included in the decision-making.
Purely time-based RTS decision-making should be abandoned in clinical practice (agree 26/26; 100%)
Based on the individual differences in biological healing, impairment resolution, neuromuscular control, functional skills, and psychological readiness, the period of time before RTS is variable.5,69 Achievement of normalized joint homeostasis (eg, absence of effusion, resolution of pain), neuromuscular control, and sufficient proprioception and strength after ACL injury may require up to 2 years and varies based on individual progress through the RTS process.41,51 Purely time based is thus insufficient, as individual patients can vary significantly. There is, however, an important role for time-based consideration respecting the healing process of the graft. Recent data showed that for every month unrestricted return to competition was delayed up to 9 months postoperatively, the reinjury incidence was reduced by 51%.30
The biology of graft healing and maturation is important, and without current biological means of graft healing assessment, time is one factor to consider. There is likely a minimum time necessary to allow graft maturation, and RTS before 6 months likely represents unacceptably high risk. Ultimately, RTS decision-making should ensure that objective criteria are met before progressing to the next stage of rehabilitation. This structure of objective measures rather than purely time-based decision-making is mirrored in the recent literature, which has shown a transition from mainly time-based rehabilitation recommendations68 to multitiered, criteria-based, sport-specific, and patient-tailored rehabilitation and RTS programs.##
Conclusion
As graft maturation and achievement of joint homeostasis are multifactorial and individual healing conditions are variable, purely time-based RTS decision-making is not sufficient.
RTS decision-making must include objective physical examination data (eg, clinical tests and measures) (agree 26/26; 100%)
The factors to consider in decision-making during the RTS continuum must be clearly defined. One major factor that must be included is objective physical examination data. Although there are limited data to guide the decision of which measures should be included, it is important to have a consistent set of objective measurements.9,10,42 Therefore, the consensus group concluded that the physical examination must include range of motion, presence of effusion, laxity testing including Lachman and pivot-shift tests, and quadriceps and hamstring muscle strength. These objective measures document that necessary knee recovery from major knee injury has occurred and therefore are key to the RTS decision-making.
A systematic review reported that greater quadriceps strength and less effusion were the physical examination findings associated with successful RTS.17 It has also been reported that hamstring-to-quadriceps strength ratio deficits and failing to pass a clinical test, involving quadriceps strength and single-leg jump testing, were associated with higher ACL graft rupture rates.37 Additionally, for every 1% increase in quadriceps limb symmetry index, there was a 3% reduction in subsequent knee injury risk.30 The objective physical examination should be conducted with the understanding of the patient’s individual sport, where some measures may be more relevant. Although the physical examination may be considered the baseline assessment for monitoring knee injury recovery, multiple other criteria, such as RTS functional testing and psychological assessment, should also be met before RTS.
Conclusion
Objective physical examination data are a minimum to establish necessary knee recovery after ACL injury or reconstruction and are widely accepted in RTS decision-making.
Patients should pass a standardized, validated, and peer-reviewed RTS test, with respect to the healing tissues, before returning to full participation after ACL injury with or without ACL reconstruction (agree 23/26; 88%)
RTS testing is an area of interest for enhancement of successful RTS. Although a systematic review in 2012 reported only 13% of RTS studies over the previous 10 years utilized objective criteria, more recent studies have increased the focus on objective and criteria-based progression of RTS.2,24,40 Resolution of knee impairments, including range of motion and effusion, and strength and hop testing are supported by the literature, and newer studies of movement symmetry are actively being studied. A positive correlation has been reported between isokinetic knee extension peak torque and subjective knee scores as well as 3 hop tests.75 Also, a good positive correlation was reported between knee extension acceleration rate and deceleration range for a timed hop test and triple crossover hop. Quadriceps strength deficits may be associated with increased risk of reinjury. One study reported that 33% of patients with quadriceps strength <90% of the contralateral extremity suffered reinjury as compared with 13% of those with >90% quadriceps strength symmetry.30 Furthermore, quadriceps strength testing has been used in assessment of ACL-deficient knees.23 In this regard, isokinetic quadriceps strength testing throughout the range of motion showed most notable deficits at less than 40° of knee flexion, and potential copers had a different strength testing profile than noncopers.
One consensus group suggested an RTS test battery should include strength testing, jump tests, and a measurement of the quality of movement.69 The Delaware-Oslo ACL cohort study has utilized an RTS test battery including isometric quadriceps strength, 4 single-leg jump tests, and 2 patient-reported outcome measures, with a 90% threshold on all criteria set as a passing score.53 Patients passing this criteria-based RTS test were more likely to report normal knee function and have more symmetric limb movement at 1 and 2 years postoperatively and were more than 6 times less likely to have a subsequent knee injury after RTS as compared with those who failed the RTS test. Passing the RTS test was also associated with higher rates of return to previous level of play. In another report from the same Delaware-Oslo ACL cohort, passing the same RTS criteria accurately predicted return to previous level of play at 1 and 2 years postoperatively with good sensitivity and specificity.30,52 Of those patients passing the RTS test at 6 months, 81% and 84% returned to the previous level of play at 1 and 2 years postoperatively, respectively, while 44% and 46% of patients who failed at 6 months returned to the previous level at 1 and 2 years postoperatively after passing subsequent RTS testing, respectively. Although the evidence is mounting for objective RTS testing, further research is needed to validate these results and clearly define the best methods of testing. There also remains the future possibility for a biological measure of the healing tissues. Advanced imaging or a biological assessment of tissue healing would be a potential useful addition to the RTS testing.
Conclusion
A standardized RTS testing battery may decrease the risk of reinjury, but further research is needed to define the exact components of the ideal test battery and which tests should take priority or be weighed more heavily.
RTS testing should involve assessment of specific functional skills that demonstrate appropriate quality of movement, strength, range of motion, balance, and neuromuscular control of the lower extremity and body (agree 26/26; 100%)
As part of the RTS testing, specific functional skills play an important role in safe RTS. Studies have shown that quadriceps strength deficits and neuromuscular control deficits are risk factors for reinjury.30,58 Therefore, of the many groups that have proposed RTS testing protocols, most routinely involve functional assessments.1,2,28,33 The most commonly reported functional tests are jump tests, including single-leg jump, crossover jump, triple jump, and timed jump tests typically comparing with the contralateral limb.1 Quadriceps and hamstring strength testing have also been extensively reported, and agility testing and motion analysis are reported commonly as well. Star excursion balance testing has been shown to be a noncontact lower extremity injury predictor, and ACL reconstruction patients have been reported to have residual deficits on these tests when returning to play.14,15 In addition, drop vertical jump testing and postural stability tests were reported to predict higher reinjury risk after ACL reconstruction in young athletes.58 There remains much variability in the functional tests included and the time points at which these occur. Regardless, functional testing remains an important consideration, and multiple measures should be included. The functional assessment should include both quantitative and qualitative measures of a range of specific skills. Further research is needed to correlate the functional tests with RTS rates and reinjury.
Conclusion
Functional testing with both quantitative and qualitative assessments is increasingly accepted as a standard component of RTS testing, but research is necessary to determine which assessments should be included and how they correlate with RTS and reinjury.
RTS decision-making includes psychological readiness as measured by a validated scale (agree 22/26; 85%)
Mental health among athletes is an important consideration that has recently gained more attention. The 2019 International Olympic Committee (IOC) consensus statement on mental health in athletes reported on the high prevalence rate of mental health symptoms in athletes and the relationship of mental health with physical injury and subsequent recovery.59 The IOC urged that mental health is a vital component of athlete well-being and cannot be separated from physical health. Assessment of mental health and subsequent management should be a routine part of the medical care of athletes. The IOC also concluded that cognitive, emotional, and behavioral responses are important factors in injury outcomes, and mental health disorders can complicate recovery. A systematic review of 28 studies reported 65% of those patients not returning to play cited a psychological reason for not returning.54 Fear of reinjury, lack of confidence in the knee, and depression were the most commonly cited psychological reasons.
The ACL–Return to Sport after Injury (ACL-RSI) scale has been proposed to measure the psychological impact of RTS after ACL reconstruction with the hope of being able to identify readiness to return.72 A prospective cohort study reported that patients returning to their preinjury level of sport scored significantly higher on the ACL-RSI scale preoperatively and at 4 months postoperatively as compared with those not returning to sport, indicating psychological readiness to RTS.8 This scale was validated by a large cohort study of 681 patients, which reported that an ACL-RSI threshold score at 6 months postoperatively was independently associated with return to preinjury sport at 2-year follow-up.61 In 2019, a cohort study of 329 patients who returned to sport reported that patients 20 years of age or younger with a second ACL injury had lower psychological readiness scores on the ACL-RSI scale than those without second injury.47 Early confidence may, however, be deleterious, as higher knee confidence at a younger age has been associated with a higher reinjury rate.56 Thus, it should be emphasized that the interaction of confidence, age, and time to return to play is complex and needs to be further studied. Sound research will be necessary to understand these interactions and how the testing can be implemented to improve outcomes. Given the early promising literature, the ACL-RSI scale may be a good option for assessing patients’ psychological readiness during the RTS continuum.
Further validation studies are necessary to confirm that this scale is applicable to all patient groups, to assess the risks of early low and high scores on outcomes, and to determine the effect that RTS has on patients’ reporting on the ACL-RSI scale. Advanced rehabilitation has been used to improve functional readiness, but more recently, a 5-week group training program was shown to additionally improve psychological readiness as measured with the ACL-RSI scale.48 Greater patient-reported subjective knee scores and male sex have been associated with psychological readiness for sport, and therefore, targeting specific groups may be the most beneficial for RTS.73
Conclusion
Psychological factors clearly play a role in RTS, and psychological readiness should be assessed, but currently, it remains unclear how psychological scales can be used to improve the RTS process.
The decision to release an athlete to RTS should consider contextual factors (type of sport, time of season, position, level of competition, etc) (agree 26/26; 100%)
The first priority in the RTS decision should be the patient’s health and safety, but contextual factors may also influence the timing of RTS. Multiple studies have reported that the level of competition affects the RTS rate, with professional athletes returning at greater rates.7,38 Collegiate American football and soccer athletes on scholarship also return at higher rates than nonscholarship athletes.18,35 Professional athletes and scholarship collegiate athletes have a financial interest in their RTS that may provide unique motivation. These patients may be willing to accept increased risk of returning to competition before meeting RTS criteria, and thus, the risk-benefit analysis must be considered. Furthermore, the type of sport and position played can affect RTS rates. In professional American football, quarterbacks return at higher rates than running backs and wide receivers, possibly pointing to different physical demands by position.22 Earlier National Football League draft selection, which typically represents greater potential or performance level, is also associated with greater RTS rates. These contextual factors should be considered in the decision to release an athlete to RTS, and modifications to optimize successful return should be employed.
Conclusion
RTS decision-making occurs in a dynamic continuum, and contextual factors play a role and should be considered to optimize outcomes.
Consideration should be given to the nature and severity of concomitant injuries of the knee (eg, cartilage and menisci) when making RTS decisions (agree 25/26; 96%)
Concomitant injuries are common with ACL injury, with meniscal injuries reported in 23% to 42% and cartilage lesions in 19% to 27%.12,36,65 These injuries may have additional healing considerations that could delay the RTS. There is a lack of literature to guide this decision as evidenced by a recent systematic review that failed to find a consensus on postoperative rehabilitation and RTS for concomitant ACL reconstruction and articular cartilage lesions.67 However, meniscal and cartilage injuries were reported to be associated with lower rates of RTS.32 In addition, after revision ACL reconstruction, significant chondral damage was associated with lower RTS rates.71 It is clearly important that the biological healing of the tissues is respected, but literature on RTS decision-making is lacking. Future research is needed to assess how concomitant injuries affect the RTS decision-making and how the RTS process can be optimized.
Conclusion
Concomitant injuries are common and can affect the RTS, but there is a lack of literature to guide modifications to the RTS process and decision-making.
Conclusion
RTS after ACL injury is ultimately characterized by achievement of the preinjury level of sport. The RTS process occurs along a continuum from return to participation, which includes unrestricted training, followed by full participation, to RTS, and ultimately, return to performance. This consensus paper helps define the stages of the RTS continuum after ACL injury as summarized in Figure 2. Additionally, purely time-based RTS decision-making should be abandoned, and a criteria-based progression involving a multidisciplinary team that includes the surgeon, sports medicine physician, physical therapist, and athletic trainer should be utilized. The patient should progress through a structured plan as specific clinical and functional milestones are met. RTS decision-making should include objective physical examination data; validated and peer-reviewed RTS testing that involves functional assessment and psychological readiness; and consideration for biological healing, contextual factors, and concomitant injuries. Further research is needed in determining the ideal RTS testing battery, the best implementation and use of psychological readiness testing, and the biological assessment of healing and recovery.
Footnotes
Final revision submitted April 6, 2020; accepted April 20, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: C.F. has received research support from Zimmer Biomet, consulting fees from Karl Storz and Medacta, speaking fees from Medacta, and royalties from Karl Storz. S.L.S. has received research support from Zimmer Biomet and consulting fees from Arthrex, Conmed, Flexion Therapeutics, JRF Ortho, Olympus, Smith & Nephew, and Vericel. B.P.L. has received royalties from Wolters Kluwer Health–Lippincott Williams & Wilkins.
Contributing authors have the following disclosures: L.E. has received grants from Smith & Nephew. C.C.K. has received grant support from DJO, educational support from CDC Medical, consulting fees from Zimmer Biomet, and nonconsulting fees from Arthrex. R.K. has received grants from Smith & Nephew, Zimmer Biomet, Stryker, and Johnson & Johnson; consulting fees from Medacta International, Arthrex, Japan Tissue Engineering, and Hirosaki Life Science Innovation; and speaking fees from Arthrex, Smith & Nephew, Zimmer Biomet, Johnson & Johnson, and Japan Tissue Engineering. V.M. has received educational support from Arthrex and Smith & Nephew. S.J.R. has received educational support from Mid-Atlantic Surgical. R.S. has received personal fees from Medacta International. C.v.E. has received educational support from Arthrex, Mid-Atlantic Surgical, and Smith & Nephew and grant support from DJO and Zimmer Biomet. D.V. has received educational support from Mid-Atlantic Surgical and hospitality payments from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abrams GD, Harris JD, Gupta AK, et al. Functional performance testing after anterior cruciate ligament reconstruction: a systematic review. Orthop J Sports Med. 2014;2(1):2325967113518305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther. 2012;42(7):601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ardern CL, Bizzini M, Bahr R. It is time for consensus on return to play after injury: five key questions. Br J Sports Med. 2016;50(9):506–508. [DOI] [PubMed] [Google Scholar]
- 4. Ardern CL, Ekas G, Grindem H, et al. 2018. International Olympic Committee consensus statement on prevention, diagnosis and management of paediatric anterior cruciate ligament (ACL) injuries. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):989–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ardern CL, Glasgow P, Schneiders A, et al. 2016. consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016;50(14):853–864. [DOI] [PubMed] [Google Scholar]
- 6. Ardern CL, Taylor NF, Feller JA, Webster KE. Return-to-sport outcomes at 2 to 7 years after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2012;40(1):41–48. [DOI] [PubMed] [Google Scholar]
- 7. Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–1552. [DOI] [PubMed] [Google Scholar]
- 8. Ardern CL, Taylor NF, Feller JA, Whitehead TS, Webster KE. Psychological responses matter in returning to preinjury level of sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2013;41(7):1549–1558. [DOI] [PubMed] [Google Scholar]
- 9. Ardern CL, Webster KE, Taylor NF, Feller JA. Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011;45(7):596–606. [DOI] [PubMed] [Google Scholar]
- 10. Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. [DOI] [PubMed] [Google Scholar]
- 11. Biedert RM, Hintermann B, Hörterer H, et al. WISSENSCHAFTLICHER BEITRAG: 8. GOTS-Schweiz Tagung Universität Basel, 2. Februar 2006: Sportfähigkeit nach Verletzungen und Operationen. Sports Orthopaedics and Traumatology Sport-Orthopädie—Sport-Traumatologie. 2006;22(4):249–254. [Google Scholar]
- 12. Borchers JR, Kaeding CC, Pedroza AD, Huston LJ, Spindler KP, Wright RW. Intra-articular findings in primary and revision anterior cruciate ligament reconstruction surgery: a comparison of the MOON and MARS study groups. Am J Sports Med. 2011;39(9):1889–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckthorpe M, Frizziero A, Roi GS. Update on functional recovery process for the injured athlete: return to sport continuum redefined. Br J Sports Med. 2019;53(5):265–267. [DOI] [PubMed] [Google Scholar]
- 14. Butler RJ, Lehr ME, Fink ML, Kiesel KB, Plisky PJ. Dynamic balance performance and noncontact lower extremity injury in college football players: an initial study. Sports Health. 2013;5(5):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clagg S, Paterno MV, Hewett TE, Schmitt LC. Performance on the modified star excursion balance test at the time of return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2015;45(6):444–452. [DOI] [PubMed] [Google Scholar]
- 16. Creighton DW, Shrier I, Shultz R, Meeuwisse WH, Matheson GO. Return-to-play in sport: a decision-based model. Clin J Sport Med. 2010;20(5):379–385. [DOI] [PubMed] [Google Scholar]
- 17. Czuppon S, Racette BA, Klein SE, Harris-Hayes M. Variables associated with return to sport following anterior cruciate ligament reconstruction: a systematic review. Br J Sports Med. 2014;48(5):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daruwalla JH, Greis PE, Hancock R, Xerogeanes JW. Rates and determinants of return to play after anterior cruciate ligament reconstruction in NCAA Division 1 college football athletes: a study of the ACC, SEC, and PAC-12 Conferences. Orthop J Sports Med. 2014;2(8):2325967114543901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Mille P, Osmak J. Performance: bridging the gap after ACL surgery. Curr Rev Musculoskelet Med. 2017;10(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dingenen B, Gokeler A. Optimization of the return-to-sport paradigm after anterior cruciate ligament reconstruction: a critical step back to move forward. Sports Med. 2017;47(8):1487–1500. [DOI] [PubMed] [Google Scholar]
- 21. Dunn WR, George MS, Churchill L, Spindler KP. Ethics in sports medicine. Am J Sports Med. 2007;35(5):840–844. [DOI] [PubMed] [Google Scholar]
- 22. Eisenstein ED, Rawicki NL, Rensing NJ, Kusnezov NA, Lanzi JT. Variables affecting return to play after anterior cruciate ligament injury in the National Football League. Orthop J Sports Med. 2016;4(10):2325967116670117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eitzen I, Eitzen TJ, Holm I, Snyder-Mackler L, Risberg MA. Anterior cruciate ligament-deficient potential copers and noncopers reveal different isokinetic quadriceps strength profiles in the early stage after injury. Am J Sports Med. 2010;38(3):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellman MB, Sherman SL, Forsythe B, LaPrade RF, Cole BJ, Bach BR., Jr Return to play following anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2015;23(5):283–296. [DOI] [PubMed] [Google Scholar]
- 25. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eubank BH, Mohtadi NG, Lafave MR, et al. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flint FA, Weiss MR. Returning injured athletes to competition: a role and ethical dilemma. Can J Sport Sci. 1992;17(1):34–40. [PubMed] [Google Scholar]
- 28. Gokeler A, Welling W, Zaffagnini S, Seil R, Padua D. Development of a test battery to enhance safe return to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grassi A, Vascellari A, Combi A, Tomaello L, Canata GL, Zaffagnini S. Return to sport after ACL reconstruction: a survey between the Italian Society of Knee, Arthroscopy, Sport, Cartilage and Orthopaedic Technologies (SIGASCOT) members. Eur J Orthop Surg Traumatol. 2016;26(5):509–516. [DOI] [PubMed] [Google Scholar]
- 30. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grindem H, Wellsandt E, Failla M, Snyder-Mackler L, Risberg MA. Anterior cruciate ligament injury: who succeeds without reconstructive surgery? The Delaware-Oslo ACL cohort study. Orthop J Sports Med. 2018;6(5):23259 67118774255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamrin Senorski E, Svantesson E, Beischer S, et al. Low 1-year return-to-sport rate after anterior cruciate ligament reconstruction regardless of patient and surgical factors: a prospective cohort study of 272 patients. Am J Sports Med. 2018;46(7):1551–1558. [DOI] [PubMed] [Google Scholar]
- 33. Hildebrandt C, Muller L, Zisch B, Huber R, Fink C, Raschner C. Functional assessments for decision-making regarding return to sports following ACL reconstruction, part I: development of a new test battery. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hohmann E, Cote MP, Brand JC. Research pearls: expert consensus based evidence using the Delphi method. Arthroscopy. 2018;34(12):3278–3282. [DOI] [PubMed] [Google Scholar]
- 35. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in National Collegiate Athletic Association Division I soccer athletes: a study of the Southeastern Conference. Am J Sports Med. 2016;44(2):433–439. [DOI] [PubMed] [Google Scholar]
- 36. Kvist J, Kartus J, Karlsson J, Forssblad M. Results from the Swedish national anterior cruciate ligament register. Arthroscopy. 2014;30(7):803–810. [DOI] [PubMed] [Google Scholar]
- 37. Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E. Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med. 2016;50(15):946–951. [DOI] [PubMed] [Google Scholar]
- 38. Lai CCH, Ardern CL, Feller JA, Webster KE. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med. 2018;52(2):128–138. [DOI] [PubMed] [Google Scholar]
- 39. Lee DY, Karim SA, Chang HC. Return to sports after anterior cruciate ligament reconstruction: a review of patients with minimum 5-year follow-up. Ann Acad Med Singapore. 2008;37(4):273–278. [PubMed] [Google Scholar]
- 40. Logerstedt D, Lynch A, Axe MJ, Snyder-Mackler L. Symmetry restoration and functional recovery before and after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Losciale JM, Zdeb RM, Ledbetter L, Reiman MP, Sell TC. The association between passing return-to-sport criteria and second anterior cruciate ligament injury risk: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2019;49(2):43–54. [DOI] [PubMed] [Google Scholar]
- 42. Lynch AD, Logerstedt DS, Grindem H, et al. Consensus criteria for defining “successful outcome” after ACL injury and reconstruction: a Delaware-Oslo ACL cohort investigation. Br J Sports Med. 2015;49(5):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Makhni EC, Crump EK, Steinhaus ME, et al. Quality and variability of online available physical therapy protocols from academic orthopaedic surgery programs for anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(8):1612–1621. [DOI] [PubMed] [Google Scholar]
- 44. Marshall NE, Keller RA, Dines J, Bush-Joseph C, Limpisvasti O. Current practice: postoperative and return to play trends after ACL reconstruction by fellowship-trained sports surgeons. Musculoskelet Surg. 2019;103(1):55–61. [DOI] [PubMed] [Google Scholar]
- 45. Marshall S, Padua D, McGrath M. Incidence of ACL injury. In: Hewett TE, Shultz SJ, Griffin LY, eds. Understanding and Preventing Noncontact ACL Injuries. Human Kinetics; 2007:5–29. [Google Scholar]
- 46. Marx RG, Jones EC, Angel M, Wickiewicz TL, Warren RF. Beliefs and attitudes of members of the American Academy of Orthopaedic Surgeons regarding the treatment of anterior cruciate ligament injury. Arthroscopy. 2003;19(7):762–770. [DOI] [PubMed] [Google Scholar]
- 47. McPherson AL, Feller JA, Hewett TE, Webster KE. Psychological readiness to return to sport is associated with second anterior cruciate ligament injuries. Am J Sports Med. 2019;47(4):857–862. [DOI] [PubMed] [Google Scholar]
- 48. Meierbachtol A, Yungtum W, Paur E, Bottoms J, Chmielewski TL. Psychological and functional readiness for sport following advanced group training in patients with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(11):864–872. [DOI] [PubMed] [Google Scholar]
- 49. Mohtadi NG, Chan DS. Return to sport-specific performance after primary anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2018;46(13):3307–3316. [DOI] [PubMed] [Google Scholar]
- 50. Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39(3):127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagelli CV, Hewett TE. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nawasreh Z, Logerstedt D, Cummer K, Axe M, Risberg MA, Snyder-Mackler L. Functional performance 6 months after ACL reconstruction can predict return to participation in the same preinjury activity level 12 and 24 months after surgery. Br J Sports Med. 2018;52(6):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nawasreh Z, Logerstedt D, Cummer K, Axe MJ, Risberg MA, Snyder-Mackler L. Do patients failing return-to-activity criteria at 6 months after anterior cruciate ligament reconstruction continue demonstrating deficits at 2 years? Am J Sports Med. 2017;45(5):1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nwachukwu BU, Adjei J, Rauck RC, et al. How much do psychological factors affect lack of return to play after anterior cruciate ligament reconstruction? A systematic review. Orthop J Sports Med. 2019;7(5):23259 67119845313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nyland J, Brand E, Fisher B. Update on rehabilitation following ACL reconstruction. Open Access J Sports Med. 2010;1:151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paterno MV, Huang B, Thomas S, Hewett TE, Schmitt LC. Clinical factors that predict a second ACL injury after ACL reconstruction and return to sport: preliminary development of a clinical decision algorithm. Orthop J Sports Med. 2017;5(12):2325967117745279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reardon CL, Hainline B, Aron CM, et al. Mental health in elite athletes: International Olympic Committee consensus statement (2019). Br J Sports Med. 2019;53(11):667–699. [DOI] [PubMed] [Google Scholar]
- 60. Rishiraj N, Taunton JE, Lloyd-Smith R, Woollard R, Regan W, Clement DB. The potential role of prophylactic/functional knee bracing in preventing knee ligament injury. Sports Med. 2009;39(11):937–960. [DOI] [PubMed] [Google Scholar]
- 61. Sadeqi M, Klouche S, Bohu Y, Herman S, Lefevre N, Gerometta A. Progression of the psychological ACL-RSI score and return to sport after anterior cruciate ligament reconstruction: a prospective 2-year follow-up study from the French Prospective Anterior Cruciate Ligament Reconstruction Cohort Study (FAST). Orthop J Sports Med. 2018;6(12):2325967118812819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shah VM, Andrews JR, Fleisig GS, McMichael CS, Lemak LJ. Return to play after anterior cruciate ligament reconstruction in National Football League athletes. Am J Sports Med. 2010;38(11):2233–2239. [DOI] [PubMed] [Google Scholar]
- 63. Shrier I. Strategic Assessment of Risk and Risk Tolerance (StARRT) framework for return-to-play decision-making. Br J Sports Med. 2015;49(20):1311–1315. [DOI] [PubMed] [Google Scholar]
- 64. Shrier I, Safai P, Charland L. Return to play following injury: whose decision should it be? Br J Sports Med. 2014;48(5):394–401. [DOI] [PubMed] [Google Scholar]
- 65. Tandogan RN, Taser O, Kayaalp A, et al. Analysis of meniscal and chondral lesions accompanying anterior cruciate ligament tears: relationship with age, time from injury, and level of sport. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):262–270. [DOI] [PubMed] [Google Scholar]
- 66. Thomeé R, Kaplan Y, Kvist J, et al. Muscle strength and hop performance criteria prior to return to sports after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1798–1805. [DOI] [PubMed] [Google Scholar]
- 67. Thrush C, Porter TJ, Devitt BM. No evidence for the most appropriate postoperative rehabilitation protocol following anterior cruciate ligament reconstruction with concomitant articular cartilage lesions: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1065–1073. [DOI] [PubMed] [Google Scholar]
- 68. van Grinsven S, van Cingel RE, Holla CJ, van Loon CJ. Evidence-based rehabilitation following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1128–1144. [DOI] [PubMed] [Google Scholar]
- 69. van Melick N, van Cingel RE, Brooijmans F, et al. Evidence-based clinical practice update: practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br J Sports Med. 2016;50(24):1506–1515. [DOI] [PubMed] [Google Scholar]
- 70. van Yperen DT, Reijman M, van Es EM, Bierma-Zeinstra SMA, Meuffels DE. Twenty-year follow-up study comparing operative versus nonoperative treatment of anterior cruciate ligament ruptures in high-level athletes. Am J Sports Med. 2018;46(5):1129–1136. [DOI] [PubMed] [Google Scholar]
- 71. Webster KE, Feller JA, Kimp A, Devitt BM. Medial meniscal and chondral pathology at the time of revision anterior cruciate ligament reconstruction results in inferior mid-term patient-reported outcomes. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1059–1064. [DOI] [PubMed] [Google Scholar]
- 72. Webster KE, Feller JA, Lambros C. Development and preliminary validation of a scale to measure the psychological impact of returning to sport following anterior cruciate ligament reconstruction surgery. Phys Ther Sport. 2008;9(1):9–15. [DOI] [PubMed] [Google Scholar]
- 73. Webster KE, Nagelli CV, Hewett TE, Feller JA. Factors associated with psychological readiness to return to sport after anterior cruciate ligament reconstruction surgery. Am J Sports Med. 2018;46(7):1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wilk KE, Arrigo CA. Rehabilitation principles of the anterior cruciate ligament reconstructed knee: twelve steps for successful progression and return to play. Clin Sports Med. 2017;36(1):189–232. [DOI] [PubMed] [Google Scholar]
- 75. Wilk KE, Romaniello WT, Soscia SM, Arrigo CA, Andrews JR. The relationship between subjective knee scores, isokinetic testing, and functional testing in the ACL-reconstructed knee. J Orthop Sports Phys Ther. 1994;20(2):60–73. [DOI] [PubMed] [Google Scholar]
- 76. Wilke C, Grimm L, Hoffmann B, Frobose I. [Functional testing as guideline criteria for return to competition after ACL rupture in game sports]. Sportverletz Sportschaden. 2018;32(3):171–186. [DOI] [PubMed] [Google Scholar]
- 77. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731–735. [DOI] [PubMed] [Google Scholar]
- 78. Zarzycki R, Failla M, Capin JJ, Snyder-Mackler L. Psychological readiness to return to sport is associated with knee kinematic asymmetry during gait following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2018;48(12):968–973. [DOI] [PMC free article] [PubMed] [Google Scholar]