Abstract
Background
Gestational alcohol exposure can contribute to fetal alcohol spectrum disorders (FASD), an array of cognitive, behavioral, and physical developmental impairments. Mammalian Target of Rapamycin (mTOR) plays a key role in regulating protein synthesis in response to neuronal activity, thereby modulating synaptic plasticity and long term memory formation in the brain. Based on our previous quantitative mass spectrometry proteomic studies, we hypothesized that gestational chronic binge alcohol exposure alters mTOR signaling and downstream pathways in the fetal hippocampus.
Methods
Pregnant Sprague-Dawley rats were assigned to either a pair-fed control (PF-Cont) or a binge alcohol (Alcohol) treatment group. Alcohol dams were acclimatized via a once-daily orogastric gavage of 4.5 g/kg alcohol (peak BAC, 216 mg/dl) from GD 5–10 and progressed to 6 g/kg alcohol (peak BAC, 289 mg/dl) from GD 11–21. Pair-fed dams similarly received isocaloric maltose dextrin.
Results
In the Alcohol group, following this exposure paradigm, fetal body weight and crown-rump length were decreased. The phosphorylation level of mTOR (P-mTOR) in the fetal hippocampus was decreased in the Alcohol group compared with controls. Alcohol exposure resulted in dysregulation of fetal hippocampal mTORC1 signaling, as evidenced by an increase in total 4E-BP1 expression. Phosphorylation levels of 4E-BP1 and p70 S6K were also increased following alcohol exposure. P-mTOR and P-4E-BP1 were exclusively detected in the dentate gyrus and oriens layer of the fetal hippocampus, respectively. DEPTOR and RICTOR expression levels in the fetal hippocampus were increased, however RAPTOR was not altered by chronic binge alcohol exposure.
Conclusion
We conclude that chronic binge alcohol exposure during pregnancy alters mTORC1 signaling pathway in the fetal hippocampus. We conjecture that this dysregulation of mTOR protein expression, its activity, and downstream proteins may play a critical role in FASD neurobiological phenotypes.
Keywords: FASD, teratogen, alcohol, fetal, pregnancy
INTRODUCTION
Gestational alcohol exposure may lead to Fetal Alcohol Spectrum Disorders (FASD), described as an array of cognitive, behavioral, and physical developmental disabilities (Riley and McGee, 2005, Sokol et al., 2003, Riley et al., 2011). A recent survey reported that 1 in 10 pregnant women consumed alcohol in the past month and that 1 in 33 women binge drink during pregnancy (Tan et al., 2015). In the United States, 2–5% of school-age children may be affected by FASD (May et al., 2018, Roozen et al., 2016). Alcohol-induced developmental deficits can be severe and may persist for a lifetime. Currently, there are no approved therapeutic drugs for treating individuals with FASD and the mechanisms underlying alcohol-mediated developmental damage remain poorly understood (Spohr and Steinhausen, 2008).
Alcohol permeates across the placental and blood brain barriers (Burd et al., 2012), and the developing brain exhibits profound vulnerability across human and animal model FASD studies (Lebel et al., 2011, Archibald et al., 2001, Gautam et al., 2015). The hippocampus, a brain region associated with cognitive behavior, learning, and memory, has been ubiquitously described as a susceptible target to alcohol-induced teratogenesis, and its susceptibility may vary depending on exposure timing, dose, duration, and pattern (Ho et al., 1972, Gil-Mohapel et al., 2010, Dudek et al., 2014, Kodituwakku, 2007, Savage et al., 2002). Alcohol exposure during hippocampal development has been shown to alter hippocampal synaptic plasticity (Bhattacharya et al., 2015, Sutherland et al., 1997, Medina, 2011, Fontaine et al., 2016), synaptic activity (Kajimoto et al., 2016, Krawczyk et al., 2016), cellular morphology (Berman and Hannigan, 2000, Ramos et al., 2002), gene expression, and DNA methylation (Chen et al., 2013, Chater-Diehl et al., 2016). We have previously reported that gestational chronic binge alcohol exposure alters hippocampal amino acid concentrations and proteomic profile (Lunde-Young et al., 2018, Davis-Anderson et al., 2018), and from this work we have identified hippocampal mammalian target of rapamycin (mTOR) pathways as distinctly vulnerable to dysregulation following gestational alcohol exposure (Davis-Anderson et al., 2018).
mTOR is a serine/threonine kinase that controls many physiological functions, including cell growth, proliferation, survival, and homeostasis through multi-protein signaling complexes by integrating extracellular and intracellular signals (Sarbassov et al., 2005, Hoeffer and Klann, 2010). mTOR resides in 2 complexes: mTORC1 [composed of mTOR, regulatory-associated protein of mTOR (RAPTOR), DEP-domain-containing mTOR-interacting protein (DEPTOR), mammalian lethal with Sec13 protein 8 (mLST8), and proline-rich AKT substrate 40kDa (PRAS40)] and mTORC2 [composed of mTOR, rapamycin-insensitive companion of mTOR (RICTOR), DEPTOR, mLST8, mammalian stress-activated MAPK-interacting protein 1 (mSin1), and Proctor] (Loewith et al., 2002, Peterson et al., 2009). mTORC1 phosphorylates eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and p70 ribosomal S6 Kinase (p70 S6K) to initiate protein synthesis, whereas mTORC2 phosphorylates AKT to promote cell survival (Qin et al., 2016, Sarbassov et al., 2005). During brain development, mTOR signaling pathways play crucial roles in mediating neuronal differentiation, growth, and proliferation, as well as in maintaining stem and progenitor cell populations (Easley et al., 2010, Cloetta et al., 2013, Romine et al., 2015, Meng et al., 2018, Lee, 2015). Dysregulation of mTOR and its downstream signaling molecules have been shown to disrupt critical brain developmental processes and have been associated with many learning disabilities (Ehninger et al., 2009, Lee, 2015). Collectively, these studies implicate plausible role(s) for the mTOR system in the developing brain. Recent studies revealed that acute administration of alcohol in non-pregnant adult mice altered hippocampal mTOR signaling pathways (Xing and Zou, 2018), and that binge alcohol exposure led to mTOR inhibition in the cerebellum and cerebral cortex of non-pregnant adult mice (Wang et al., 2018). Although mTOR has been recognized as a regulatory molecule and its signaling pathways have been mapped in brain development, the effect of alcohol on hippocampal mTOR signaling in the context of FASD remains largely unknown. The purpose of the study herein was to discern how alcohol may affect fetal hippocampal mTOR signaling in order to further elucidate FASD hippocampal effects. We hypothesized that gestational chronic binge alcohol exposure will dysregulate mTOR level/activity and disrupt the activity of proteins within its associated systems, mTORC1 and mTORC2, as well as its related downstream pathways in the fetal hippocampus.
MATERIALS & METHODS
Animals
All experimental procedures were in accordance with National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996) and approved by the Institutional Animal Care and Use Committee at Texas A&M University. Timed-pregnant Sprague Dawley rats purchased from Charles River (Wilmington, MA) were housed in a temperature-controlled room (23°C) with a 12:12-hour light–dark cycle. Animals were assigned to either a pair-fed control (PF-Cont) group (n=5 dams) or a binge alcohol treatment (Alcohol) group (n=5 dams) on gestational day (GD) 4. Alcohol group animals were acclimatized via a once-daily orogastric gavage of 4.5 g/kg (22.5% wt/v) ethanol [peak blood alcohol concentration (BAC), 216 mg/dl] from GD 5–10, and progressed to 6.0 g/kg (28.5% wt/v) ethanol (peak BAC, 289 mg/dl) from GD 11–21(Davis-Anderson et al., 2018, Subramanian et al., 2014). The exposure paradigm utilized in this study is modeled after alcohol consumption patterns in pregnant women and is commonly utilized to study FASD phenotypes in animal models (Caetano et al., 2006, Church and Gerkin, 1988, Cudd et al., 2002, Thomas et al., 2008, Thomas et al., 2010). All animals were weighed prior to the start of the study, and each Alcohol group animal was yoked with a PF-Cont group animal of similar weight for the duration of the study. The PF-Cont group animals received isocalorically matched (50% wt/v) maltose dextrin once-daily on each exposure day to account for calories that the Alcohol group received from ethanol. The Alcohol group animal received ad libitum diet and the daily feed intake was measured. The PF-Cont group animal was fed the same amount of diet consumed by the Alcohol group animal.
Fetal brain and hippocampal isolation
On GD 21, four hours after the last alcohol exposure, animals were sacrificed and fetuses were carefully separated. Each fetus was weighed and crown-rump length was measured. Fetal whole brains were extracted under a dissection microscope via craniotomy and serially washed in cold phosphate buffered saline (PBS). For immunoblotting, one male and one female pups from each dam were randomly selected from a similar position in the uterine horn, brains were dissected, meninges were removed, and hippocampi were micro-dissected in ice-cold HEPES buffer. Individual samples were then flash frozen and stored at −80°C until immunoblot analyses. For immunohistochemistry, fetal whole brain embedding was performed in optimal cutting temperature compound (OCT) (Sakura Finetek, Torrance, CA) and cryopreserved for immunofluorescence.
Immunoblotting
Hippocampal tissues were lysed in ice-cold RIPA buffer, pH 7.5 (Cell Signaling Technologies, Danvers, MA) supplemented with a protease inhibitor cocktail (cOmplete Mini, Roche Applied Biosciences). The lysates were centrifuged at 10,000 ×g for 10 min at 4°C and the supernatants were collected. Total protein concentrations were determined using the Bradford method and a BCA assay kit (Thermo Scientific, Rockford, IL). The protein samples (20 μg) were separated on 4~20% Tris–HEPES polyacrylamide gels (Bio-Rad, Hercules, CA) by electrophoresis. The separated proteins were transferred onto PVDF membranes (Immobilon-P; Millipore, Bellerica, MA). The membranes were blocked with 5% BSA in 0.01 M PBS (pH 7.4) and 0.05% Tween-20 (TPBS) for 1 hour at room temperature. Subsequently, the membranes were probed with one of the following primary antibodies (Cell signaling, Danvers, MA) overnight at 4°C: Phospho-mTOR (Ser2448) (1:1,000; #2971), mTOR (1:1,000; #4517), Phospho-4E-BP1 (Thr37/46) (1:1,000; #2855), 4E-BP1 (1:1,000; #9644), Phospho-p70S6K (Thr389) (1:1,000; #9234), P70S6K (1:1,000; #9202), DEPTOR (1:1,000; #11816), RAPTOR (1:1,000; #2280), RICTOR (1:1,000; #2114), and β-actin (1:5,000; #4967). We used one replicate for each protein of interest. The membranes were then incubated with a secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. The immune complexes were detected by chemiluminescent substrate (Thermo Scientific, Rockford, IL). Densitometry analysis was performed with AzureSpot software (Azure Biosystems, Dublin, CA).
Immunofluorescence
Fetal brains were sectioned at 8 μm in a coronal plane using a Leica CM1860 cryostat (Leica Biosystems, Buffalo Grove, IL). Sections were fixed with ice-cold methanol (30 min, −20°C) and rinsed in PBS, incubated in 10% normal serum (60 min), followed by incubation with Phospho-mTOR (Ser2448) (1:250; #2971), or Phospho-4E-BP1 (Thr37/46) (1:250; #2855) primary antibody overnight at 4°C in a humidified chamber. The sections were further incubated with goat anti-rabbit IgG secondary antibody (Alexa Fluor 488, #A11008; Invitrogen, Carlsbad, CA) for 60 min at room temperature. Nuclei were stained with DAPI (ProLong Gold antifade, #P36931; Invitrogen, Carlsbad, CA). Digital images were captured using an Olympus BX63 stereomicroscope equipped with U-HGLGPS fluorescent light source, ORCA-Flash 4.0 LT digital camera (HAMAMATSU, Japan), and Olympus cellSens Dimension 1.16 software (Olympus, Japan).
Statistics
The effects of alcohol exposure on maternal and fetal body weight and crown-rump length were analyzed by two-tailed Student’s t-test. Since a sex effect was not observed for mTOR (example, P-mTOR, sex effect, P = 0.4116; total mTOR, sex effect, P = 0.4481) utilizing two-way ANOVA with Bonferroni’s multiple comparisons test, the two columns were collapsed. This finding is supported by our previous study, where a sex effect was not observed for hippocampal mTOR signaling utilizing mass spectrometry-based proteomic approaches following chronic binge alcohol exposure (Davis-Anderson et al., 2018). Immunoblotting data for the expression levels of different proteins were analyzed utilizing two-tailed Student’s t-tests between PF-Cont and Alcohol groups. All data are presented as mean ± SEM. Significance was established a priori at P < 0.05.
RESULTS
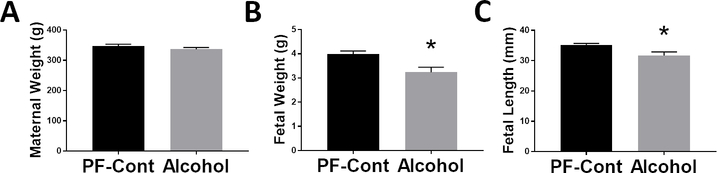
Maternal body weight in the Alcohol group was not different from that in the PF-Cont group (Fig. 1A). Fetal growth was significantly restricted in the Alcohol group. The fetal body weight was significantly lower (P = 0.0155) in the Alcohol group compared to the PF-Cont group, an approximately 19% reduction in weight (Fig. 1B). The fetal crown-rump length was also significantly lower (P = 0.0324) in the Alcohol group compared to the PF-Cont group (Fig. 1C).
Fig. 1. Effect of gestational chronic binge alcohol exposure on fetal development.
Maternal body weight, fetal body weight, and fetal crown-rump length were measured on GD 21. (A) Maternal body weight was not different between the pair-fed control (PF-Cont) and Alcohol groups (P = 0.2886). (B) Fetal body weight (P = 0.0155) and (C) fetal crown-rump length (P = 0.0324) were significantly decreased following gestational alcohol exposure. Data are shown as mean ± SEM. *, Significance was established a priori at P < 0.05.
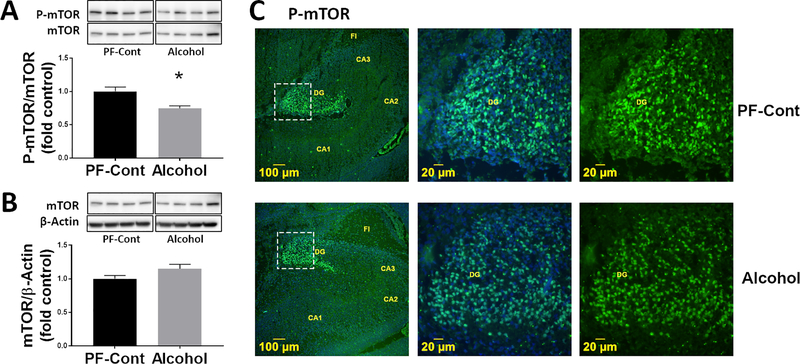
We analyzed the effect of gestational chronic binge alcohol exposure on mTOR protein expression in the fetal hippocampus. Immunoblot analysis showed that the phosphorylation level of mTOR (P-mTOR) was significantly lower (↓ 25.2%; P = 0.0038) in fetal hippocampi from the Alcohol group compared to those from the PF-Cont group (Fig. 2A). There was no difference in total mTOR expression between the PF-Cont and Alcohol groups (Fig. 2B). We then imaged for the presence of activated mTOR (P-mTOR) using immunofluorescence microscopy. Immunofluorescence imaging (Fig. 2C) demonstrated that P-mTOR was detected exclusively in the granule cells of the dentate gyrus of the fetal hippocampus in both PF-Cont and Alcohol groups. Collectively, these findings suggest that mTOR phosphorylation in the fetal hippocampus decreases following gestational chronic binge alcohol exposure.
Fig. 2. Effect of gestational chronic binge alcohol exposure on fetal hippocampal mTOR.
(A) Immunoblot analysis showed that gestational chronic binge alcohol exposure significantly decreased (↓ 25.2%; P = 0.0038) P-mTOR levels in the fetal hippocampi. (B) Total mTOR expression was not different between groups. Data are shown as mean ± SEM.*, Significance was established a priori at P < 0.05. (C) Representative immunofluorescence staining shows P-mTOR was expressed in the fetal dentate gyrus (DG) region. FI, fimbria.
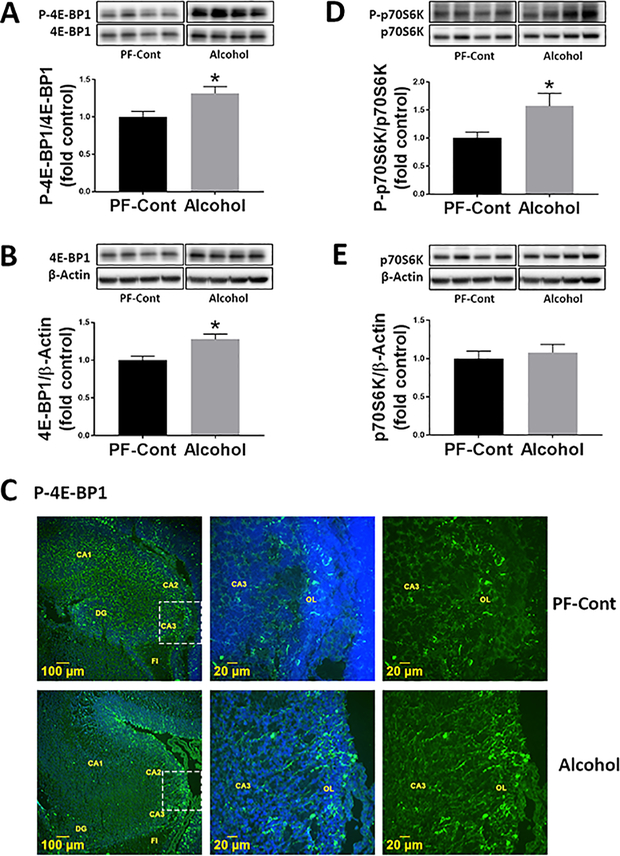
To determine if gestational chronic binge alcohol dysregulates mTOR signaling, we analyzed the abundance levels of downstream molecules within this signaling pathway. The phosphorylation level of 4E-BP1 (P-4E-BP1) was significantly higher (↑ 41%; P = 0.0156) in the fetal hippocampi from the Alcohol group compared to that of the PF-Cont group (Fig. 3A). Hippocampal total 4E-BP1 expression was also significantly elevated (↑ 20%; P = 0.0251) in the Alcohol group compared to that of the PF-Cont group (Fig. 3B). Immunofluorescence imaging of the fetal hippocampus (Fig. 3C) demonstrated that P-4E-BP1 was detected in the oriens layer of the cornu ammonis (CA) field in both groups. The hippocampal phosphorylation of p70 S6K (P-p70 S6K) was significantly higher (↑ 57%; P = 0.0383) in the Alcohol group compared to that of the PF-Cont group (Fig. 3D). There was no difference in total p70 S6K levels between the PF-Cont and Alcohol groups (Fig. 3E).
Fig. 3. Effect of gestational chronic binge alcohol exposure on mTORC1 signaling in the fetal hippocampus.
Immunoblot analysis showed that gestational chronic binge alcohol exposure significantly increased (A) P-4E-BP1 level (↑ 41%; P = 0.0156) and (B) total 4E-BP1 expression (↑ 20%; P = 0.0251) in the fetal hippocampi. (C) Immunofluorescence staining shows that P-4E-BP1 was expressed in the oriens layer (OL) of the fetal hippocampus and the representative image shows CA3 field near fimbria (FI). (D) Gestational alcohol exposure significantly increased (↑ 57%; P = 0.0383) the level of P-p70 S6K, whereas (E) total p70 S6K expression was not different between groups. Data are shown as mean ± SEM and as fold of control. *, Significance was established a priori at P < 0.05.
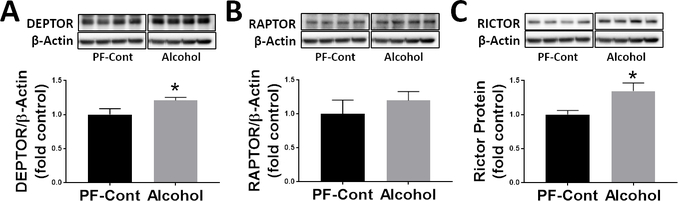
To investigate the effect of gestational chronic binge alcohol exposure on mTOR complexes, we analyzed hippocampal levels of DEPTOR, RAPTOR, and RICTOR (Fig. 4). Quantitative analysis showed that the expression levels of DEPTOR and RICTOR were higher in the Alcohol group compared to those of PF-Cont group (DEPTOR, ↑ 19.9%; P = 0.0349 and RICTOR, ↑ 34.6%; P = 0.0192). The RAPTOR expression level was not different between the PF-Cont and Alcohol groups.
Fig. 4. Effect of gestational chronic binge alcohol exposure on the proteins that complex with hippocampal mTOR.
Immunoblot analysis showed that gestational chronic binge alcohol exposure significantly increased (A) DEPTOR expression (↑ 19.9%; P = 0.0349), but (B) had no effect on RAPTOR expression. (C) RICTOR expression was significantly increased (↑ 34.6%; P = 0.0192) by alcohol exposure. DEPTOR, RAPTOR, and RICTOR proteins were normalized to β-actin and were expressed relative to PF-Cont. Data are shown as mean ± SEM. *, Significance was established a priori at P < 0.05.
DISCUSSION
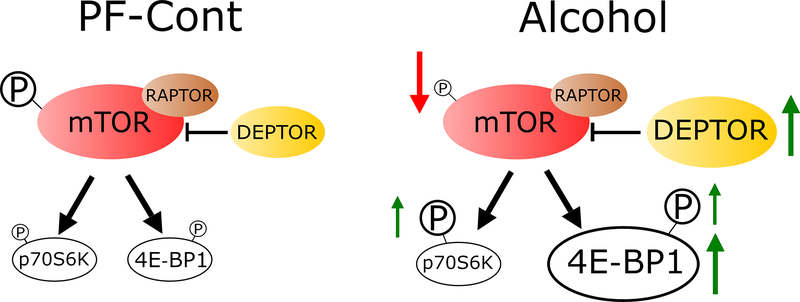
To our knowledge, this is the first investigation of alcohol’s effects on fetal hippocampal mTOR signaling following a gestational chronic binge exposure paradigm. Three findings can be gleaned from this study: gestational chronic binge alcohol exposure 1) dysregulates the fetal hippocampal mTOR system, 2) has an impact on mTORC1 signaling, which plays essential roles in brain development, and 3) alters the proteins that complex with hippocampal mTOR, whose functions have also been implicated in brain developmental processes (Fig. 5). Collectively, these findings suggest that gestational chronic binge alcohol exposure alters mTORC1 signaling, activity indices, and related downstream pathways in the fetal hippocampus, and these alcohol-induced alterations may contribute to hippocampal FASD pathogenesis.
Fig. 5. Schematic representation: Chronic binge alcohol exposure during pregnancy alters mTOR system in rat fetal hippocampus.
The phosphorylation level of mTOR (P-mTOR) in the fetal hippocampus was decreased in the Alcohol group compared with controls. Alcohol exposure resulted in dysregulation of fetal hippocampal mTORC1 signaling, as evidenced by an increase in total 4E-BP1 expression. Phosphorylation levels of 4E-BP1 and p70 S6K were also increased following alcohol exposure. DEPTOR and RICTOR (not shown) expression levels in the fetal hippocampus were increased, however RAPTOR was not altered by chronic binge alcohol exposure.
Alcohol dysregulates fetal hippocampal mTOR
It is widely accepted that mTOR is a critical regulator of cell growth, proliferation, and homeostasis via the modulation of protein synthesis (Sarbassov et al., 2005, Hoeffer and Klann, 2010) and that mTOR is activated during memory formation through several brain areas, including the hippocampus proper and the dentate gyrus (Bekinschtein et al., 2007, Macias et al., 2013, Giovannini et al., 2015). Therefore, the regulation of hippocampal protein synthesis is a major control step in synaptic plasticity and memory formation (Graber et al., 2013, Bekinschtein et al., 2007). Recent studies demonstrated that alcohol administration in juvenile rats and adult mice induced memory deficits, and also showed that alcohol administration resulted in decreased mTOR phosphorylation and apoptosis in hippocampal neuronal cells (Yang et al., 2017, Xing and Zou, 2018, Wang et al., 2018). In alignment with these reports, our data also showed that fetal hippocampal mTOR phosphorylation is decreased following a gestational chronic binge alcohol exposure paradigm. Interestingly, we observed that phosphorylated mTOR was expressed in the fetal hippocampal dentate gyrus.
Alcohol has an impact on mTORC1 signaling pathway
mTOR signals through two distinct multi-protein complexes known as mTORC1 and mTORC2. mTORC1 plays a pivotal role in mediating synthesis of synaptic proteins and is involved in normal neurocognitive development associated with learning and memory. The dysregulation of mTORC1 has been reported in various neuronal disorders (Neasta et al., 2014, Hoeffer and Klann, 2010). mTORC2 plays integral roles in iron transport, cell survival and growth, and cytoskeletal remodeling (Loewith et al., 2002, Hoeffer and Klann, 2010). mTORC1 regulates two critical components of translation initiation machinery: 4E-BP1and p70 S6K (Sarbassov et al., 2005). Activation of mTORC1 induces phosphorylation of 4E-BP1 and p70 S6K, which subsequently promotes an initiation of protein synthesis (Klann and Dever, 2004, Ma and Blenis, 2009). We found that gestational chronic binge alcohol exposure increased phosphorylation of 4E-BP1 and p70 S6K in the fetal hippocampus. This aligns with previous work reporting that binge alcohol exposure increased the levels of 4E-BP1 and p70 S6K phosphorylation in adult mouse and rat brains (Neasta et al., 2010). Interestingly, we observed an increase in total 4E-BP1 expression in response to our alcohol exposure paradigm. 4E-BP1 functions as a protein synthesis repressor by binding to eIF4E and its activity is regulated by mTORC1 signaling (Graber et al., 2013, Showkat et al., 2014). Overall, increased activity of 4E-BP1 is associated with decreased protein translation levels (Hay and Sonenberg, 2004). The expression of phosphorylated 4E-BP1 protein was also observed in the fetal hippocampal dentate gyrus. To date, the mechanism by which alcohol impairs mTOR activity is poorly defined. Alteration of the mTORC1 pathway via an increase in total 4E-BP1 expression and phosphorylation of 4E-BP1 and p70 S6K may be implicated in alcohol-induced hippocampal neuropathogenesis.
Alcohol alters the proteins that complex with hippocampal mTOR
mTOR complexes form with multiple mTOR-interacting proteins, however the exact function of many of these proteins still remains elusive. DEPTOR functions as an inhibitor of both mTORC1 and mTORC2. DEPTOR and mTOR interact and regulate each other via a negative feedback loop (Peterson et al., 2009). Depletion of DEPTOR activates mTOR signaling and induces cell growth and survival, but overexpression of DEPTOR inhibits mTORC1 signaling in different cancer cell types (Catena and Fanciulli, 2017). Our data demonstrate that gestational chronic binge alcohol exposure increased the expression of DEPTOR in the fetal hippocampus. Therefore, the increased DEPTOR expression observed here could inhibit the mTORC1 signaling following our alcohol exposure paradigm (Peterson et al., 2009, Catena and Fanciulli, 2017). RAPTOR functions as a scaffold protein specific to mTORC1 and may affect mTORC1 activity by recruiting and interacting with substrates for mTOR, such as 4E-BP1 and p70 S6K (Hara et al., 2002). In the current study, RAPTOR expression in the fetal hippocampus was not altered, even though mTORC1 signaling was impacted by gestational chronic binge alcohol exposure. RICTOR is specific to mTORC2 and is required for regulating cell survival and the cell cycle (Sarbassov et al., 2005). It has been reported that alcohol may increase mTORC2 activity by elevating the level of RICTOR expression in mouse muscle cells (Hong-Brown et al., 2011). We also observed increased RICTOR expression in the fetal hippocampus in response to chronic binge alcohol exposure.
Perspectives and significance
The effect of alcohol on the hippocampal mTOR signaling in the context of FASD remains largely unknown. Although we report deficits in fetal body weight and length, we did not examine facial dysmorphology and neuroanatomic/structural deficits. We utilized an established gestational chronic binge alcohol exposure paradigm to discern how alcohol impacts the fetal hippocampal mTOR signaling system in order to further elucidate FASD hippocampal pathogenesis. Based on our results, we conclude that gestational chronic binge alcohol exposure alters mTOR signaling, its activity indices, and related downstream pathways in the fetal hippocampus. These findings are corroborated by other reports; altered activity of mTOR and its downstream molecules may impact development of brain (Lee, 2015), skeletal system (McCarthy et al., 2013, Luo et al., 2017), and muscles (Lang et al., 2009, Steiner and Lang, 2015). Future studies are warranted to determine the mechanism(s) underlying alcohol-induced dysregulation of hippocampal mTOR signaling and how this relates to fetal hippocampal neurodevelopment in the context of FASD, which in turn may facilitate development of targeted pharmacological therapeutic strategies for FASD.
Acknowledgements
This study was supported by National Institutes of Health [AA19446, AA23520, AA23035] and Texas A&M University [Tier One Program] (JR).
Footnotes
Conflict of interest: The authors have no conflicts of interest.
REFERENCES
- ARCHIBALD SL, FENNEMA-NOTESTINE C, GAMST A, RILEY EP, MATTSON SN & JERNIGAN TL 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol, 43, 148–54. [PubMed] [Google Scholar]
- BEKINSCHTEIN P, KATCHE C, SLIPCZUK LN, IGAZ LM, CAMMAROTA M, IZQUIERDO I & MEDINA JH 2007. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiology of Learning and Memory, 87, 303–307. [DOI] [PubMed] [Google Scholar]
- BERMAN RF & HANNIGAN JH 2000. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus, 10, 94–110. [DOI] [PubMed] [Google Scholar]
- BHATTACHARYA D, DUNAWAY EP, BHATTACHARYA S, BLOEMER J, BUABEID M, ESCOBAR M, SUPPIRAMANIAM V & DHANASEKARAN M 2015. Impaired ILK Function Is Associated with Deficits in Hippocampal Based Memory and Synaptic Plasticity in a FASD Rat Model. PLOS ONE, 10, e0135700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURD L, BLAIR J & DROPPS K 2012. Prenatal alcohol exposure, blood alcohol concentrations and alcohol elimination rates for the mother, fetus and newborn. J Perinatol, 32, 652–9. [DOI] [PubMed] [Google Scholar]
- CAETANO R, RAMISETTY-MIKLER S, FLOYD LR & MCGRATH C 2006. The epidemiology of drinking among women of child-bearing age. Alcohol Clin Exp Res, 30, 1023–30. [DOI] [PubMed] [Google Scholar]
- CATENA V & FANCIULLI M 2017. Deptor: not only a mTOR inhibitor. J Exp Clin Cancer Res, 36, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATER-DIEHL EJ, LAUFER BI, CASTELLANI CA, ALBERRY BL & SINGH SM 2016. Alteration of Gene Expression, DNA Methylation, and Histone Methylation in Free Radical Scavenging Networks in Adult Mouse Hippocampus following Fetal Alcohol Exposure. Plos One, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y, OZTURK NC & ZHOU FC 2013. DNA methylation program in developing hippocampus and its alteration by alcohol. PLoS One, 8, e60503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH MW & GERKIN KP 1988. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics, 82, 147–54. [PubMed] [Google Scholar]
- CLOETTA D, THOMANETZ V, BARANEK C, LUSTENBERGER RM, LIN S, OLIVERI F, ATANASOSKI S & RUEGG MA 2013. Inactivation of mTORC1 in the Developing Brain Causes Microcephaly and Affects Gliogenesis. Journal of Neuroscience, 33, 7799–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUDD TA, CHEN WJ & WEST JR 2002. Fetal and maternal thyroid hormone responses to ethanol exposure during the third trimester equivalent of gestation in sheep. Alcohol Clin Exp Res, 26, 53–8. [PubMed] [Google Scholar]
- DAVIS-ANDERSON KL, WESSELING H, SIEBERT LM, LUNDE-YOUNG ER, NAIK VD, STEEN H & RAMADOSS J 2018. Fetal regional brain protein signature in FASD rat model. Reprod Toxicol, 76, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEK J, SKOCIC J, SHEARD E & ROVET J 2014. Hippocampal abnormalities in youth with alcohol-related neurodevelopmental disorder. J Int Neuropsychol Soc, 20, 181–91. [DOI] [PubMed] [Google Scholar]
- EASLEY CA, BEN-YEHUDAH A, REDINGER CJ, OLIVER SL, VARUM ST, EISINGER VM, CARLISLE DL, DONOVAN PJ & SCHATTEN GP 2010. mTOR-Mediated Activation of p70 S6K Induces Differentiation of Pluripotent Human Embryonic Stem Cells. Cellular Reprogramming, 12, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHNINGER D, DE VRIES PJ & SILVA AJ 2009. From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. Journal of Intellectual Disability Research, 53, 838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONTAINE CJ, PATTEN AR, SICKMANN HM, HELFER JL & CHRISTIE BR 2016. Effects of pre-natal alcohol exposure on hippocampal synaptic plasticity: Sex, age and methodological considerations. Neurosci Biobehav Rev, 64, 12–34. [DOI] [PubMed] [Google Scholar]
- GAUTAM P, LEBEL C, NARR KL, MATTSON SN, MAY PA, ADNAMS CM, RILEY EP, JONES KL, KAN EC & SOWELL ER 2015. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum Brain Mapp, 36, 2318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIL-MOHAPEL J, BOEHME F, KAINER L & CHRISTIE BR 2010. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev, 64, 283–303. [DOI] [PubMed] [Google Scholar]
- GIOVANNINI MG, LANA D & PEPEU G 2015. The integrated role of ACh, ERK and mTOR in the mechanisms of hippocampal inhibitory avoidance memory. Neurobiol Learn Mem, 119, 18–33. [DOI] [PubMed] [Google Scholar]
- GRABER TE, MCCAMPHILL PK & SOSSIN WS 2013. A recollection of mTOR signaling in learning and memory. Learn Mem, 20, 518–30. [DOI] [PubMed] [Google Scholar]
- HARA K, MARUKI Y, LONG X, YOSHINO K, OSHIRO N, HIDAYAT S, TOKUNAGA C, AVRUCH J & YONEZAWA K 2002. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell, 110, 177–89. [DOI] [PubMed] [Google Scholar]
- HAY N & SONENBERG N 2004. Upstream and downstream of mTOR. Genes Dev, 18, 1926–45. [DOI] [PubMed] [Google Scholar]
- HO BT, FRITCHIE GE, IDANPAAN-HEIKKILA JE & MCISAAC WM 1972. Placental transfer and tissue distribution of ethanol-1– 14 C. A radioautographic study in monkeys and hamsters. Q J Stud Alcohol, 33, 485–93. [PubMed] [Google Scholar]
- HOEFFER CA & KLANN E 2010. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci, 33, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG-BROWN LQ, BROWN CR, NAVARATNARAJAH M, HUBER DS & LANG CH 2011. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res, 35, 1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAJIMOTO K, VALENZUELA CF, ALLAN AM, GE SY, GU Y & CUNNINGHAM LA 2016. Prenatal Alcohol Exposure Alters Synaptic Activity of Adult Hippocampal Dentate Granule Cells Under Conditions of Enriched Environment. Hippocampus, 26, 1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLANN E & DEVER TE 2004. Biochemical mechanisms for translational regulation in synaptic plasticity. Nat Rev Neurosci, 5, 931–42. [DOI] [PubMed] [Google Scholar]
- KODITUWAKKU PW 2007. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev, 31, 192–201. [DOI] [PubMed] [Google Scholar]
- KRAWCZYK M, RAMANI M, DIAN J, FLOREZ CM, MYLVAGANAM S, BRIEN J, REYNOLDS J, KAPUR B, ZOIDL G, POULTER MO & CARLEN PL 2016. Hippocampal hyperexcitability in fetal alcohol spectrum disorder: Pathological sharp waves and excitatory/inhibitory synaptic imbalance. Experimental Neurology, 280, 70–79. [DOI] [PubMed] [Google Scholar]
- LANG CH, PRUZNAK AM, NYSTROM GJ & VARY TC 2009. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutr Metab (Lond), 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBEL C, ROUSSOTTE F & SOWELL ER 2011. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev, 21, 102–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE DY 2015. Roles of mTOR Signaling in Brain Development. Exp Neurobiol, 24, 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWITH R, JACINTO E, WULLSCHLEGER S, LORBERG A, CRESPO JL, BONENFANT D, OPPLIGER W, JENOE P & HALL MN 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Molecular Cell, 10, 457–468. [DOI] [PubMed] [Google Scholar]
- LUNDE-YOUNG R, DAVIS-ANDERSON K, NAIK V, NEMEC M, WU G & RAMADOSS J 2018. Regional dysregulation of taurine and related amino acids in the fetal rat brain following gestational alcohol exposure. Alcohol, 66, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO Z, LIU Y, LIU Y, CHEN H, SHI S & LIU Y 2017. Cellular and molecular mechanisms of alcohol-induced osteopenia. Cell Mol Life Sci, 74, 4443–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA XM & BLENIS J 2009. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol, 10, 307–18. [DOI] [PubMed] [Google Scholar]
- MACIAS M, BLAZEJCZYK M, KAZMIERSKA P, CABAN B, SKALECKA A, TARKOWSKI B, RODO A, KONOPACKI J & JAWORSKI J 2013. Spatiotemporal characterization of mTOR kinase activity following kainic acid induced status epilepticus and analysis of rat brain response to chronic rapamycin treatment. PLoS One, 8, e64455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY PA, CHAMBERS CD, KALBERG WO, ZELLNER J, FELDMAN H, BUCKLEY D, KOPALD D, HASKEN JM, XU R & HONERKAMP-SMITH G 2018. Prevalence of fetal alcohol spectrum disorders in 4 US communities. JAMA, 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTHY N, WETHERILL L, LOVELY CB, SWARTZ ME, FOROUD TM & EBERHART JK 2013. Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD. Development, 140, 3254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDINA AE 2011. Fetal alcohol spectrum disorders and abnormal neuronal plasticity. Neuroscientist, 17, 274–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENG DL, FRANK AR & JEWELL JL 2018. mTOR signaling in stem and progenitor cells. Development, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEASTA J, BARAK S, HAMIDA SB & RON D 2014. mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem, 130, 172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEASTA J, BEN HAMIDA S, YOWELL Q, CARNICELLA S & RON D 2010. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proceedings of the National Academy of Sciences of the United States of America, 107, 20093–20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON TR, LAPLANTE M, THOREEN CC, SANCAK Y, KANG SA, KUEHL WM, GRAY NS & SABATINI DM 2009. DEPTOR Is an mTOR Inhibitor Frequently Overexpressed in Multiple Myeloma Cells and Required for Their Survival. Cell, 137, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIN X, JIANG B & ZHANG Y 2016. 4E-BP1, a multifactor regulated multifunctional protein. Cell cycle (Georgetown, Tex.), 15, 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMOS AJ, EVRARD SG, TAGLIAFERRO P, TRICARICO MV & BRUSCO A 2002. Effects of chronic maternal ethanol exposure on hippocampal and striatal morphology in offspring. Cellular and Molecular Mechanisms of Drugs of Abuse Ii: Cocaine, Substituted Amphetamines, Ghb, and Opiates, 965, 343–353. [DOI] [PubMed] [Google Scholar]
- RILEY EP, INFANTE MA & WARREN KR 2011. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev, 21, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY EP & MCGEE CL 2005. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood), 230, 357–65. [DOI] [PubMed] [Google Scholar]
- ROMINE J, GAO X, XU XM, SO KF & CHEN JH 2015. The proliferation of amplifying neural progenitor cells is impaired in the aging brain and restored by the mTOR pathway activation. Neurobiology of Aging, 36, 1716–1726. [DOI] [PubMed] [Google Scholar]
- ROOZEN S, PETERS G-JY, KOK G, TOWNEND D, NIJHUIS J & CURFS L 2016. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcoholism: Clinical and Experimental Research, 40, 18–32. [DOI] [PubMed] [Google Scholar]
- SARBASSOV DD, ALI SM & SABATINI DM 2005. Growing roles for the mTOR pathway. Curr Opin Cell Biol, 17, 596–603. [DOI] [PubMed] [Google Scholar]
- SAVAGE DD, BECHER M, TORRE AJ & SUTHERLAND RJ 2002. Dose‐dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcoholism: Clinical and Experimental Research, 26, 1752–1758. [DOI] [PubMed] [Google Scholar]
- SHOWKAT M, BEIGH MA & ANDRABI KI 2014. mTOR Signaling in Protein Translation Regulation: Implications in Cancer Genesis and Therapeutic Interventions. Mol Biol Int, 2014, 686984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOL RJ, DELANEY-BLACK V & NORDSTROM B 2003. Fetal alcohol spectrum disorder. JAMA, 290, 2996–9. [DOI] [PubMed] [Google Scholar]
- SPOHR HL & STEINHAUSEN HC 2008. Fetal alcohol spectrum disorders and their persisting sequelae in adult life. Dtsch Arztebl Int, 105, 693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINER JL & LANG CH 2015. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab, 308, E699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBRAMANIAN K, NAIK VD, SATHISHKUMAR K, YALLAMPALLI C, SAADE GR, HANKINS GD & RAMADOSS J 2014. Chronic binge alcohol exposure during pregnancy impairs rat maternal uterine vascular function. Alcohol Clin Exp Res, 38, 1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTHERLAND RJ, MCDONALD RJ & SAVAGE DD 1997. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus, 7, 232–8. [DOI] [PubMed] [Google Scholar]
- TAN CH, DENNY CH, CHEAL NE, SNIEZEK JE & KANNY D 2015. Alcohol use and binge drinking among women of childbearing age - United States, 2011–2013. MMWR Morb Mortal Wkly Rep, 64, 1042–6. [DOI] [PubMed] [Google Scholar]
- THOMAS JD, IDRUS NM, MONK BR & DOMINGUEZ HD 2010. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol, 88, 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS JD, SATHER TM & WHINERY LA 2008. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci, 122, 1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG YC, WANG X, LI H, XU M, FRANK J & LUO J 2018. Binge ethanol exposure induces endoplasmic reticulum stress in the brain of adult mice. Toxicology and Applied Pharmacology, 356, 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XING Y & ZOU D 2018. Ethanol-induced cognitive dysfunction is associated with alterations in the mammalian target of rapamycin signalling pathway in the hippocampus of male mice. Neuroreport, 29, 1230–1237. [DOI] [PubMed] [Google Scholar]
- YANG Y, FENG J, XU F & WANG J 2017. Piracetam inhibits ethanol (EtOH)-induced memory deficit by mediating multiple pathways. Brain Res, 1676, 83–90. [DOI] [PubMed] [Google Scholar]