Abstract
Addiction to psychostimulants is a major public health crisis that leads to significant morbidity and mortality, for which there are currently no FDA-approved pharmacotherapies. Female subjects have increased propensity to develop pathological substance use disorders after initial use, suggesting the possibility of different pathophysiological mechanisms between males and females. Recently, we identified the neuroactive cytokine granulocyte-colony stimulating factor (G-CSF) as a key mediator of neuronal and behavioral plasticity in response to cocaine in male mice. Here, we found that G-CSF potentiated the rewarding effects of cocaine in female mice as well; however, the dopaminergic mechanism linked to these effects was highly dependent on the ovarian hormone cycle. G-CSF treatment enhanced the ability of cocaine to inhibit dopamine clearance; however, this effect was observed specifically during pro/estrus, when circulating ovarian hormone levels were high. These findings demonstrate important sex differences in the synaptic effects of this translationally relevant neuroimmune modulator.
Keywords: Cytokine, cocaine, dopamine, voltammetry, hormone cycle, reward
Graphical Abstract
In several psychiatric disorders, such as anxiety, depression, and addiction, sex is a critical biological variable and women represent a particularly vulnerable population.1–3 In substance use disorders, females show increased propensity to transition to pathological use from first use,3 have greater problems achieving abstinence, and have higher rates of relapse than males.4 This suggests that there are fundamental biological differences in females that make them particularly vulnerable to the pharmacological and environmental factors that precipitate psychiatric diseases such as addiction. At the core of substance use disorders is a dysregulation of reward learning and motivation, suggesting that differences in the reward circuitry and its regulation may underlie sex differences in substance use disorders. The lack of data describing the neural circuitry underlying these sexual dimorphisms highlight the critical need for preclinical investigation of reward learning and motivation specifically in female subjects.
At the hub of reward and its dysregulation in disease is the mesolimbic dopamine system.5,6 Dopamine projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) are critical for reinforcement learning and mediate the attribution of salience to relevant environmental stimuli. Importantly, nearly all drugs of abuse alter the function of this pathway to increase dopamine levels and drive drug taking, seeking, and the development of substance use disorder. For stimulant drugs, such as cocaine, the ability to increase dopamine, via actions directly at dopamine terminals, is critical for their reinforcing and addictive properties. Stimulants both enhance dopamine release from terminals and reduce the rate of dopamine clearance through the dopamine transporter (DAT), thus increasing the level and duration of synaptic dopamine signaling. The mesolimbic dopamine system, and associated responses to stimulants, is also ideally situated to be regulated by a variety of peripheral factors that act to alter its encoding of information based on internal state.7 One potent regulator of reward-related behavior and the dopamine system in females is the estrous cycle, where ovarian hormones have been shown to exert dynamic control over its function. For example, when ovarian hormone levels are high (during proestrus and estrus) females have enhanced VTA dopamine cell firing, enhanced evoked dopamine release in the NAc, and enhanced cocaine effects at the DAT and show increased conditioned place preference (CPP) for cocaine.8
In addition to hormonal regulation of dopaminergic function, peripheral regulation of motivational systems by immune factors has emerged as a key regulator in the development of substance use disorder.9,10 Recently, we found that granulocyte-colony stimulating factor (G-CSF), a cytokine, is increased in the blood and brain by cocaine treatment in male mice, and it serves as a potent modulator of behavioral plasticity induced by repeated cocaine exposure.11 Interestingly, G-CSF alters responses to both natural and drug rewards by directly modulating release of dopamine at terminals in the NAc.12 While most of these studies have focused only on male subjects, sex differences in immune function are widely reported in the literature,13,14 necessitating studies that outline how these processes occur in females. The interaction of peripheral factors such as gonadal hormones and immune modulators represents an interesting avenue by which neural activity can be modulated in order to alter the function of reward systems and behavior. Here, we aimed to understand how hormonal cycles interact with the immune factor G-CSF to alter the effects of cocaine on the dopamine system and cocaine reward in female mice. Understanding the interaction between hormonal cycles and the effects of other peripheral immune factors will be critical to our understanding of the female-specific factors that underlie substance use disorder vulnerability.
RESULTS AND DISCUSSION
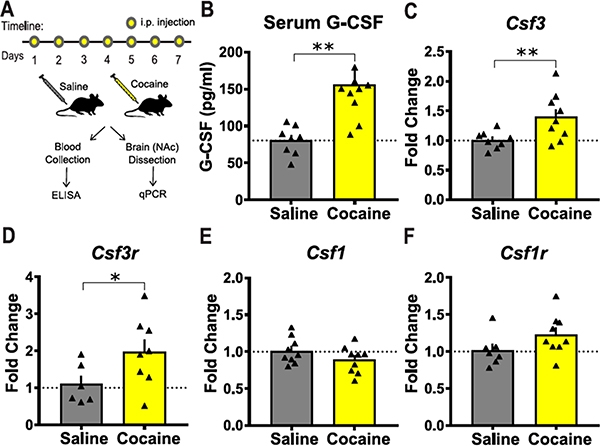
We previously showed that, in male mice, repeated cocaine injections increase levels of G-CSF in both the serum and the NAc.11 Here we performed similar cocaine treatments (20 mg/kg/day × 7 days, Figure 1A) on female mice and analyzed changes in G-CSF signaling in serum and the NAc. We found that G-CSF is increased in the serum of female mice as measured by ELISA (Figure 1B; t(16) = 3.55, p = 0.0013). Quantitative PCR demonstrated that cocaine increased levels of G-CSF transcript (Csf 3; Figure 1C; t(15) = 2.6, p = 0.01) and G-CSF receptor transcript (Csf 3r; Figure 1D; t(12) = 2.07, p = 0.03) in NAc. No significant changes were found on transcript levels of macrophage-colony stimulating factor (M-CSF; Csf1; Figure 1E) or its receptor (Csf1r; Figure 1F) highlighting that these effects are specific to G-CSF.
Figure 1.
Cocaine injected female mice display elevated levels of G-CSF and G-CSF receptor. (A) Timeline of cocaine or saline injections followed by tissue collection and molecular analysis. (B) Cocaine treatment increased serum levels of G-CSF in female mice (saline, n = 8; cocaine, n = 10). (C,D) Cocaine treatment increased transcript levels of G-CSF (Csf 3: saline, n = 8; cocaine, n = 9) and receptor (Csf 3r: saline, n = 6; cocaine: n = 8) in the NAc. (E,F) Transcript levels of M-CSF (Csf1: saline, n = 9; cocaine, n = 9) and receptor (Csf1r: saline, n = 7; cocaine, n = 9) are unaffected by cocaine in the NAc. *p < 0.05, **p ≤ 0.01. All data are presented as mean ± SEM.
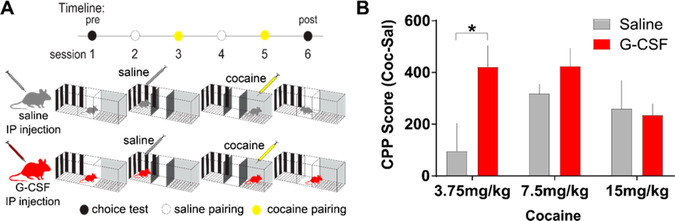
To address whether systemic G-CSF alters the ability of female mice to associate contextual cues with the rewarding properties of cocaine, we conducted conditioned place preference (CPP) for three different doses of cocaine (3.75 mg/kg, 7.5 mg/kg, and 15 mg/kg). The high dose of cocaine (15 mg/kg) typically leads to the formation of preference for cocaine in control animals, while the low dose of cocaine (3.75 mg/kg) does not.11 As was done previously,11 G-CSF (50 μg/kg) was administered during the morning of each day more than 1 h prior to the start of conditioning to raise the serum levels of G-CSF, but so as to not create an association between G-CSF injection and a chamber (Figure 2A). G-CSF treated female mice exhibited higher levels of place preference for the lowest dose of cocaine (3.75 mg/kg) compared to saline treated female mice (Figure 2B; two-way ANOVA, main effect of G-CSF: F(1,27) = 4.37, p = 0.046; cocaine dose: F(2,27) = 1.49, p = 0.24; interaction: F(2,27) = 2.5, p = 0.101; at 3.75 mg/kg saline vs G-CSF effect p = 0.007 Fisher’s post hoc test) indicating a leftward shift in the dose–response curve.
Figure 2.
G-CSF treated female mice exhibit enhanced conditioned place preference (CPP). (A) Timeline of cocaine CPP experiments in saline and G-CSF treated female mice. (B) G-CSF treatment shifted the CPP dose response curve to the left in female mice (saline, n = 5; G-CSF, n = 6). *p < 0.05 for Holm-Sidak post hoc test. All data presented as mean ± SEM.
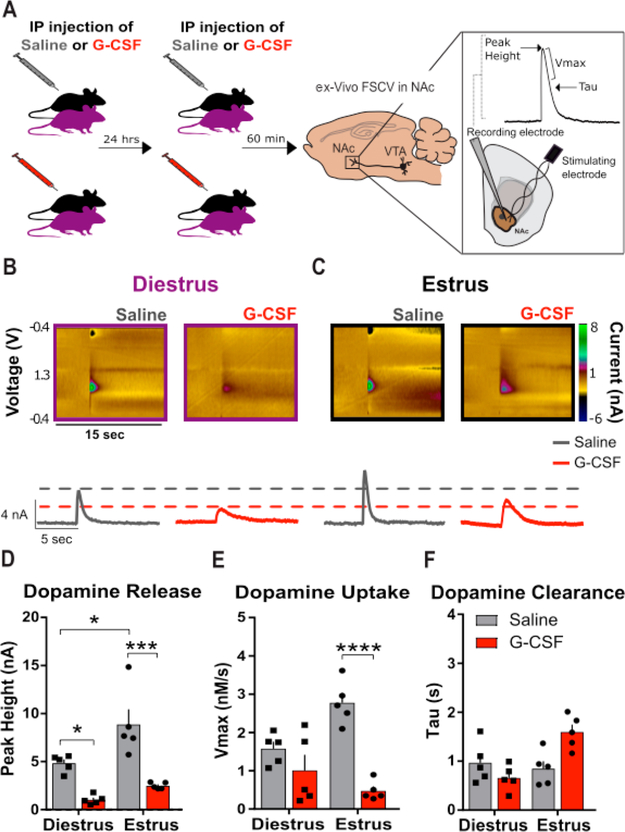
Dopamine signaling is necessary for the formation of context-reward associations;15 thus, we aimed to understand if changes in basal dopamine function or cocaine effects on this system could underlie the observed behavioral effects. First, to determine whether G-CSF treatment affected local dopaminergic circuits, we examined the effects of systemic G-CSF treatment on presynaptic dopaminergic function in the NAc. To maintain steady-state levels of systemic G-CSF, a 24 h and 60 min injection paradigm was used as in previous studies.11,12 Vaginal cytology was conducted on the day of the 24 h and 60 min G-CSF or saline i.p. injection (Figure 3A) to determine the estrous cycle stage of naturally cycling female mice. Experimental mice were chosen for fast scan cyclic voltammetry (FSCV) recordings when they were in either proestrus or estrus (termed estrus for clarity) on both days of injection or metestrus and diestrus (termed diestrus for clarity). Using FSCV, we recorded dopamine release and clearance kinetics from evoked dopamine signals in the NAc. Release and uptake kinetics were defined using modeling approaches as described: Dopamine release is reported as the peak height of the evoked dopamine signal. Vmax is a Michaelis–Menten based parameter used to assess the maximal dopamine uptake rate. Tau is a rate constant that is a measurement of the time that it takes for the signal to reach 2/3 peak height.16,30
Figure 3.
G-CSF decreases presynaptic dopamine release in the nucleus accumbens of female mice. (A) Timeline of G-CSF injections. Animals were injected with either saline or G-CSF 24 h and then 60 min before ex vivo voltammetry (left; n = 5 per group) to ensure that G-CSF blood levels were elevated for a comparable time as compared to the CPP experiments. FSCV was used to record dopamine release and uptake in the NAc (right). (Inset) Peak height, Vmax, and tau measurements were used to assess the signal. (B,C) Color plots (top) and current versus time plots (bottom) showing the presence of dopamine after electrical stimulation in diestrus (B) and estrus (C) animals. (D) Group data showing enhanced dopamine release in the saline treated animals in estrus compared to diestrus, and decreased dopamine release in the G-CSF treated animals in diestrus and estrus. (E) G-CSF treatment decreased maximal rates of dopamine uptake (Vmax) only in estrus. (F) There was no difference in dopamine clearance as measured by tau. *p < 0.05, **p < 0.01, ***p < 0.0001. Data presented as mean ± SEM.
First, we assessed the interaction between G-CSF treatment and estrous cycle stage on dopamine release and clearance. We found a significant main effect of cycle stage (Figure 3B–D; two-way ANOVA, main effect of estrous cycle: F(1, 16) = 11.22, p = 0.0041), consistent with previous studies.8 Pairwise Holm–Sidak analysis revealed that animals in estrus exhibited enhanced dopamine release compared to animals in diestrus (Figure 3B–D; Holm–Sidak multiple comparisons test: diestrus-saline vs estrus-saline: p = 0.0124). We also found a significant main effect of G-CSF treatment (Figure 3B–D, two-way ANOVA, main effect of G-CSF: F(1, 16) = 38.72, p < 0.0001). Planned posthoc analysis revealed significant decreases in dopamine release in both groups (Figure 3B–D; diestrus-saline vs diestrus-G-CSF, p = 0.0137; estrus-saline vs estrus-G-CSF, p = 0.0002). Analysis of dopamine uptake showed a significant main effect of G-CSF (Figure 3E, two-way ANOVA, main effect of G-CSF: F(1, 16) = 30.16, p < 0.0001), but not cycle stage (F(1, 16) = 1.629, p = 0.22) on Vmax. There was also a significant G-CSF × estrous cycle interaction (F(1, 16) = 10.99, p = 0.004). Posthoc analysis of this interaction revealed that G-CSF treatment attenuated the maximal rate of dopamine uptake in estrus, but not diestrus (Figure 3E; estrus-saline vs estrus-G-CSF, p < 0.0001; diestrus-saline vs diestrus-G-CSF, p = 0.439). However, there were no significant effects of dopamine clearance as measured by tau (Figure 3F).
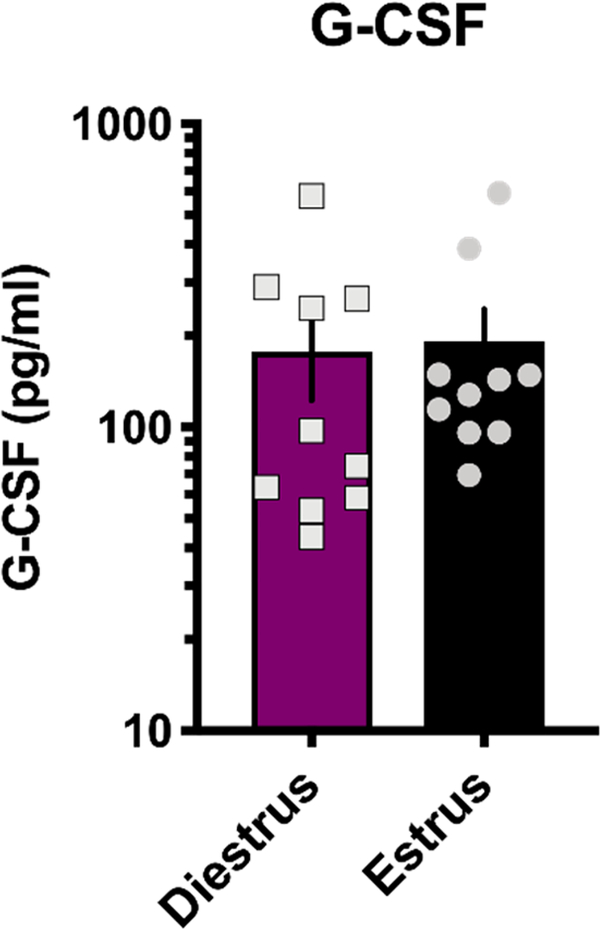
Given that G-CSF differentially affected dopamine uptake depending on estrous cycle stage (Figure 3), we next examined levels of circulating G-CSF over the estrous cycle to see if this could be contributing to the effect. G-CSF levels were assessed in freely cycling mice during estrus and diestrus (Figure 4, two-tailed t test p = 0.84, t = 0.2, df = 18). These data confirm that there are no significant alterations in circulating G-CSF depending on cycle stage and suggest that the differential effects of G-CSF during estrus are due to altered responsivity to injections of G-CSF rather than pre-existing difference in levels.
Figure 4.
Circulating G-CSF levels are not affected by estrous cycle stage. ELISA analysis of serum from animals in diestrus and estrus reveals no significant fluctuations in levels of G-CSF at different stages of the estrous cycle. Data are mean ± SEM.
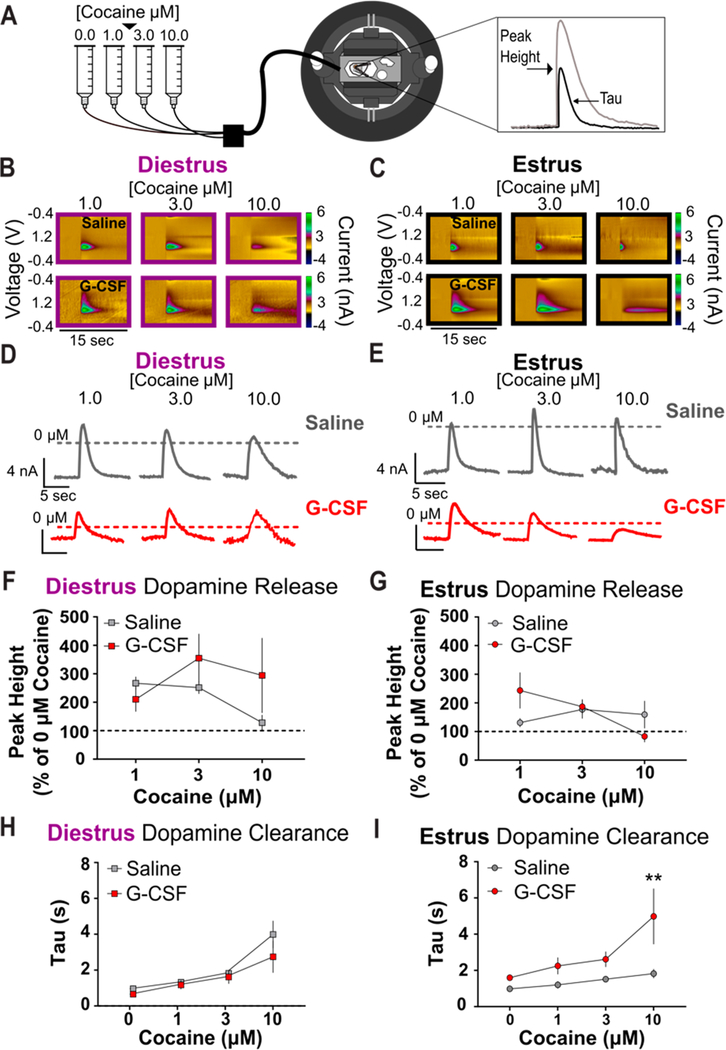
Next, we aimed to assess how the effect of systemic G-CSF altered the pharmacodynamic properties of cocaine, considering that G-CSF treatment enhances the rewarding and reinforcing properties of cocaine in male mice,11 and we observed similar effects in the present study with females (Figure 2). A range of cocaine doses was bath applied to acute NAc slices obtained from naturally cycling female mice in either diestrus or estrus that had been treated with either saline or G-CSF 24 h and 60 min prior to FSCV recordings (Figure 5A). Cocaine has been shown to have two separate mechanisms of action at dopamine terminals: (1) it inhibits the DAT, thus preventing dopamine clearance and increasing the half-life of dopamine in the synaptic cleft,17 and (2) it mobilizes vesicles from the releasable pool to actively enhance dopamine release.18,20 These mechanisms are important for the cumulative effects of cocaine on the dopamine system and can be altered independently of one another. Here we aimed to understand how G-CSF could alter these mechanisms and if there was an interaction between the hormonal cycle stage and how G-CSF acted to enhance the effects of cocaine on the dopamine system.
Figure 5.
G-CSF treatment alters cocaine effects in a cycle-dependent fashion. (A) Schematic of experiment. (B) Diestrus and (C) estrus color plots showing the presence of dopamine after electrical stimulation and bath application of 1, 3, and 10 μM cocaine (n = 5 per group) in animals treated with saline (top) and G-CSF (bottom). (D) Diestrus and (E) estrus current versus time plots showing cocaine effects on electrically evoked dopamine release in animals treated with saline (top) or G-CSF (bottom). (F) Group data showing electrically evoked dopamine release was not changed by G-CSF treatment in animals in diestrus and (G) estrus. (H) There was no difference in dopamine clearance (tau) between diestrus animals treated with saline or G-CSF. I, G-CSF treatment in estrus females enhances the effects of cocaine on dopamine clearance. **p < 0.01 from saline treated. All data are presented as mean ± SEM.
To dissociate the effects on cocaine release mechanisms we analyzed both the peak height of dopamine release and tau as a measure of cocaine’s ability to inhibit dopamine clearance. During diestrus, there was a trend toward a main effect of G-CSF increasing dopamine release; however, this did not reach significance (Figure 5B, D, and F, two-way ANOVA, main effect of G-CSF: F(1,24) = 1.591, p = 0.2194). G-CSF pretreatment also did not have an effect on tau in diestrus females (Figure 5B, D, and H, two-way ANOVA, main effect of G-CSF: F(1,32) = 2.190, p = 0.1487). Conversely, G-CSF treatment in estrus animals did enhance the effects of cocaine with a main effect of G-CSF on tau (Figure 5C, E, and I, two-way ANOVA, main effect of G-CSF: F(1, 32) = 14.71, p = 0.0006), but not dopamine release (Figure 5C, E, and G, two-way ANOVA, main effect of G-CSF: F(1,24) = 0.2381, p = 0.63). Posthoc analysis revealed that G-CSF pretreatment significantly increased cocaine’s effects on dopamine clearance inhibition (tau) at 10 μM (Figure 5C, E, and I, p = 0.0095) but not at lower concentrations of cocaine. These data, along with the results from Figure 2, suggest a DAT-mediated mechanism underlying the enhanced effect of G-CSF treatment on associative learning for cocaine reward in female mice.
CONCLUSIONS
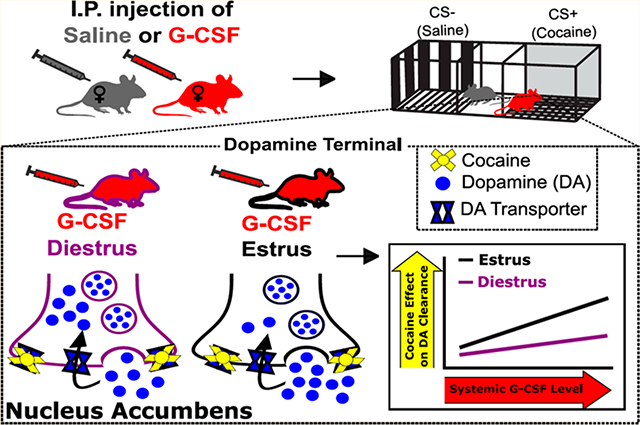
In the present study, we define a mechanism for the complex interaction between reward, cytokine signaling, and hormonal cycles and how these interacting factors alter stimulant effects in females. We find that prolonged treatment with cocaine leads to significant increases in levels of circulating G-CSF as well as increases in expression of G-CSF and its receptor in the NAc of female mice. Additionally, injections of G-CSF lead to a leftward shift in the CPP dose response curve, with G-CSF treated animals forming strong preference for lower doses of cocaine as compared to control animals. Both of these effects are similar to those we have previously reported in male mice.12 Additionally, G-CSF treatment altered the pharmacodynamic properties of cocaine in females, enhancing its ability to increase synaptic dopamine levels by regulating dopamine clearance mechanisms in the NAc - providing a potential mechanism for the observed behavioral effects. Interestingly, the effects of G-CSF on the pharmacodynamic properties of cocaine were enhanced during estrus, highlighting that this process can be regulated by ovarian hormone cycles. Given the known differences between male and female animals in immune function, hormonal cycles, and behavioral response to drugs of abuse, it is critical to understand how these sex differences manifest on the molecular, neural, and behavioral level.
There has been growing interest in recent years in the interaction between immune systems and neuronal function, and emerging evidence suggests that immune dysfunction plays a critical role in the etiology of psychiatric disease. Accordingly, immune dysregulation has been implicated in addiction,9 and only recently have studies begun to examine the mechanistic link between altered immune function and the pathology underlying these disorders.9–11,21,22 There is evidence that proinflammatory cytokines can reduce mesolimbic dopamine function by increasing dopamine clearance, decreasing dopamine receptor expression, or altering synthesis and releasable pool content,23 suggesting that cytokines could be acting through synaptic mechanisms, especially via the dopamine system, to exert their effect on mood and reward-related behaviors. Similarly, ovarian hormonal systems have also been shown to be potent regulators of reward behaviors and mood and can alter similar mechanisms. Several studies have shown the ability of estradiol to enhance release of dopamine from dopamine terminals,3,24 alter uptake mechanisms, and alter dopamine receptor function and levels, among other effects, highlighting the potential contribution of hormones in these processes. Although there have been reported inconsistencies in the ability of the hormone cycle to regulate dopamine release and uptake kinetics, sex differences have consistently been reported,25 again emphasizing the role of sex in this process. These similar effects allow for interactions between proinflammatory cytokines and hormonal factors in the regulation of synaptic processes, especially in regard to reward. Indeed, here we find different effects of G-CSF on dopaminergic function in females that are influenced by estrous cycle stage and influence cocaine’s pharmacodynamic properties and associated reward learning.
While the changes we see in G-CSF expression and behavior largely parallel our results from male mice, we see some interesting sex differences in the present experiments as compared to previous work. Our previously published results found that injection of mice with G-CSF prior to slice preparation potentiated dopamine release from the VTA to the NAc.12 In female mice, we see that G-CSF pretreatment leads to a reduction in peak dopamine release regardless of estrous cycle stage (Figure 3C), suggesting an underlying sex difference that is not driven by hormonal factors. Our previous findings in males observed an increase in dopamine release that was associated with increased reward learning and performance in operant tasks; these data suggest that these effects may not hold true in female mice. Additionally, G-CSF abolished cycle regulation of dopamine transport mechanisms by reducing Vmax on its own. It is important to note that the effects of G-CSF on transport mechanisms was only significant during estrus, suggesting the effects are occluded in diestrus and could be happening through mechanisms that are only present in estrus. While the baseline changes in dopamine release were interesting and significant, they alone could not explain the increased CPP as they were opposite of what would be predicted if basal dopamine changes were responsible for enhanced learning mechanisms.
To address other potential mechanisms by which G-CSF could alter cocaine CPP, we focused on the pharmacodynamic properties of cocaine. Cocaine exerts its actions via inhibiting the uptake function of the DAT where it functions as a transporter blocker. Cocaine’s ability to bind to and inhibit the DAT has been directly linked to its ability to cause CPP, where point mutations in the DAT prevent cocaine’s ability to induce a conditioned place preference.26 Thus, actions directly at the DAT are a critical component of the rewarding properties of cocaine. Here we find that G-CSF treatment increases the potency of cocaine at the DAT, providing a potential mechanism for how G-CSF can increase cocaine CPP; however, these effects were only observed in estrus females at the highest concentration, while G-CSF effects on CPP were most pronounced at the lowest concentration. Although there are a multitude of factors between ex vivo and behavior experiments, it is important to note the difficulties in assessing the exact amount of cocaine at the DAT compared to concentrations of cocaine in the brain. In consideration of this, these results still suggest that natural fluctuations in levels of ovarian hormones can alter the way in which potential immune-related treatments exert their effects.
With regard to translational potential, targeting of neuroimmune interactions is an area of growing interest in the neurobiology of substance use disorders, and there is considerable promise for targeting these systems to modulate the symptoms of addiction.27 G-CSF specifically is a promising target as compounds that modulate its levels or function are already FDA approved for use in clinical populations.28 However, in order to translate findings such as these into individuals with substance use disorder, it is imperative to understand the factors that influence its actions at a molecular and behavioral level. First, the findings in the present paper are in agreement with our previously published reports performed in male animals, where repeated cocaine injections increased G-CSF and G-CSF receptor expression levels and G-CSF treatment increased cocaine CPP,11 suggesting that targeting G-CSF with treatments that reduce its levels could have efficacy in both sexes. It is particularly important to note that while we saw cycle-dependent effects on neural activity, we, however, did not control for cycle stage in the CPP testing in order to look at a translationally relevant behavioral output. Thus, it is important that we observe the same left-shift in the dose–response curve in freely cycling females not prescreened for estrous stage; as human subjects abusing cocaine use throughout the menstrual cycle, and long-term drug use can disrupt normal hormonal cycles, therefore understanding the changes in freely cycling animals gives this higher relevance.
Together, these data highlight the potent control that immune cytokines have on behaviors associated with drugs of abuse. G-CSF levels are increased by cocaine exposure and are capable of enhancing CPP and cocaine’s effects at the dopamine transporter, providing a potential therapeutic target for reducing drug-induced plasticity that promotes addictive behaviors. Importantly, there were complex interactions between G-CSF effects and estrous cycle stage, with G-CSF only exerting its neurochemical effects during estrus highlighting the importance of understanding hormonal interactions with immune signaling. This work is particularly important as women are more susceptible to a number of psychiatric disorders that are characterized by deficits in reward and motivation; thus, understanding how the neural systems that control these processes are regulated is critical to both our basic understanding of the process as well as translation. Moving forward, it will be important to expand our understanding of the potential benefits of neuroimmune factors as treatment strategies for symptoms of neurological disorders.
METHODS
Animals
Female 6- to 8-week-old C57BL/6J mice were obtained from The Jackson Laboratory (SN:000664). Mice were housed three to five per cage and maintained on a 12 h reverse light/dark cycle at 22–25 °C with ad libitum access to food and water. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Vanderbilt University School of Medicine and/or Icahn School of Medicine at Mount Sinai, which approved and supervised all animal protocols. Experimenters were blind to experimental groups and order of testing was counterbalanced during behavioral experiments.
Drug Treatments
Systemic G-CSF (GenScript) was administered by intraperitoneal (i.p.) injection at a dose of 50 μg/kg at the times specified. This dose was chosen based on our previous findings that it altered behavioral response to cocaine as well as dopamine release in male mice.11,12 Cocaine hydrochloride was provided by the NIDA drug supply program and administered at specified doses via i.p. injection.
Serum Analysis
For cocaine injection experiments, mice were injected once daily for 7 days with 20 mg/kg cocaine and sacrificed by rapid decapitation 24 h after the final treatment. For estrous cycle dependent measurements, animals had their cycle stage monitored via vaginal lavage and cytology across 3 days and were then killed without any further treatment. Trunk blood was allowed to clot at RT for 1 h before being spun down at 1500g for 15 min at 4 °C. The supernatant was analyzed for G-CSF levels utilizing a quantitative ELISA kit according to manufacturer’s instructions (R&D Systems, product # MCS00).
qPCR
The same animals used for serum analysis were also utilized for qPCR analysis. After sacrifice, the nucleus accumbens was rapidly dissected and frozen on dry ice. RNA isolation, cDNA preparation and quantitative PCR were carried out as previously published.12 Primers used for these experiments were as follows:
Csf 3 F: TATAAAGGCCCCCTGGAGCTG // R: GCTGCAGGGCCATTAGCTTC
Csf 3r F: GTTTTGTGGGGAGTGGGGAT // R: TAACGCGGTGCTTGTCACTA
Csf1 F: CTCTAGCCGAGGCCATGTG // R: CCCCCAACAGTCAGCAAGAC
Csf1r F: CTCTTCCTCTGTTCCCTTTCAGG // R: AGTTCTGTGAGGACGGGAAC
Gapdh F: TTGTCAGCAATGCATCCTGCACCACC // R: CTGAGTGGCAGTGATGGCATGGAC
Conditioned Place Preference
Unbiased conditioned place preference experiments were carried out as previously described.11 Animals received injections of G-CSF (50 μg/kg) or saline at least 1 h prior to the start of any conditioning or testing. On day one of the procedure, animals were allowed to freely explore all chambers, and drug-paired chambers were assigned. On days 2 and 3, animals were injected with saline in the morning and placed in the saline-paired chamber, and in the afternoon they were injected with cocaine (3.75, 7.5, or 15 mg/kg) and placed in the cocaine-paired chamber. On day 4, the animals were again allowed to freely explore all chambers with the index of preference taken as the difference in time spent in the cocaine paired chamber.
Vaginal Cytology
To monitor the estrous cycle of female mice, vaginal cytology was monitored in experimental naturally cycling female mice for 5 days. On G-CSF injection days the lavage was performed approximately 1 h before saline or G-CSF injections for FSCV experiments. The lavage technique29 was conducted to confirm that female mice were in either diestrus (low circulating hormone) or estrus (high circulating hormone) on both days of injections.
Fast-Scan Cyclic Voltammetry
Ex vivo fast-scan cyclic voltammetry (FSCV) was used to characterize dopamine release, dopamine transporter kinetics, and the pharmacodynamic properties of cocaine in the NAc. As mentioned above, this was done after two intraperitoneal injections of G-CSF or saline, one 24 h and another 60 min before FSCV. A vibrating tissue slicer was used to prepare 300 μM thick coronal brain sections containing the NAc, which were immersed in oxygenated aCSF containing the following (in mM): 126 NaCl (126), 2.5 KCl (2.5), 1.2 NaH2PO4, 2.4 CaCl2, 1.2 MgCl2, 25 NaHCO3, 11 glucose, 0.4 L-ascorbic acid, pH adjusted to 7.4. The slice was transferred to the testing chambers containing aCSF at 32 °C with a 1 mL/min flow rate. A carbon fiber microelectrode (100–200 μM length, 7 μM radius) and bipolar stimulating electrode were placed in close proximity into the NAc. Dopamine release was evoked by a single electrical pulse (350 μA, 4 ms, monophasic) applied to the tissue every 3 min. The extracellular dopamine level was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s). Once the peak of evoked dopamine release was stabilized (3 collections with <10% variability), the amount of evoked dopamine release and maximal rate of uptake (Vmax) were assessed.
For experiments with cocaine, aCSF containing 0 μM cocaine was applied to brain slices for acquisition of a stable baseline. Following this, aCSF containing 1 μM cocaine was bath applied to the same slice. Once the peak of evoked dopamine release was stabilized (3 collections with <10% variability), aCSF containing 3 μM cocaine was washed onto the slice and allowed to stabilize. After the peak of evoked dopamine release stabilized with 3 μM cocaine-containing aCSF, 10 μM cocaine-containing aCSF was bath applied until a baseline was established.
Recording electrodes were calibrated by recording responses (in electrical current; nA) to a known concentration of dopamine (3 μM) using a flow-injection system. This was used to convert an electrical current to a dopamine concentration.
Voltammetric Data Analysis
Demon voltammetry and analysis software was used for all analysis of FSCV data.30 Data were modeled either using Michaelis–Menten kinetics to determine dopamine release and Vmax or via analyzing peak and decay kinetics to determine the peak height and tau. For Michaelis–Menten modeling, we assumed that all parameters were floating and found the best fit line for each data point as described by Calipari et al.8
Statistical Analysis
Data were analyzed using GraphPad Prism version 8.0 (La Jolla, CA). For analyses with only two groups, unpaired t tests were run. For all other experiments, two-way ANOVA was used with Holm–Sidak’s multiple comparisons tests, including planned tests for pairwise comparisons in analyses in which there was no interaction. Type I error rate (α) was set to 0.05 for all statistical tests. The n for each experiment is reported in the figure captions.
ACKNOWLEDGMENTS
The authors would like to thank NIDA Drug supply for provision of cocaine hydrochloride. The authors would like to thank Dr. Deena Walker for her assistance in performance and interpretation of vaginal cytology.
Funding
This work is supported by NIDA Grants DA042111 (to E.S.C.) and DA044308 (to D.D.K.) as well as NARSAD Young Investigator awards to R.S.H., E.S.C., and D.D.K. This work was supported by the Academic Pathways program at Vanderbilt University (L.J.B.). Funding from the Whitehall Foundation and Edward Mallinckrodt Jr. Foundation to E.S.C. also supported this work.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).McLean CP, Asnaani A, Litz BT, and Hofmann SG (2011) Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45 (8), 1027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Altemus M, Sarvaiya N, and Epperson CN (2014) Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35 (3), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Becker JB, and Hu M (2008) Sex Differences in Drug Abuse. Front. Neuroendocrinol. 29 (1), 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).NIDA, Substance Use in Women. National Institute on Drug Abuse website, August 10, 2018. [Google Scholar]
- (5).Lambert C, Silva SD, Ceniti AK, Rizvi SJ, Foussias G, and Kennedy SH (2018) Anhedonia in depression and schizophrenia: A transdiagnostic challenge. CNS Neurosci. Ther. 24, 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hägele C, Schlagenhauf F, Rapp M, Sterzer P, Beck A, Bermpohl F, Stoy M, Ströhle A, Wittchen H-U, Dolan RJ, and Heinz A (2015) Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology 232, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kiraly DD, Walker DM, and Calipari ES (2018) Modeling drug addiction in females: how internal state and environmental context facilitate vulnerability. Current Opinion in Behavioral Sciences 23 (27–35), 27–35. [Google Scholar]
- (8).Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han MH, and Nestler EJ (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 8, 13877–13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, and Stellwagen D (2016) Microglial TNFα suppresses cocaine-induced plasticity and behavioral sensitization. Neuron 90 (3), 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lacagnina MJ, Rivera PD, and Bilbo SD (2017) Glial and Neuroimmune Mechanisms as Critical Modulators of Drug Use and Abuse. Neuropsychopharmacology 42, 156–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Calipari ES, Godino A, Peck EG, Salery M, Mervosh NL, Landry JA, Russo SJ, Hurd YL, Nestler EJ, and Kiraly DD (2018) Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat. Commun. 9 (1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kutlu MG, Brady LJ, Peck EG, Hofford RS, Yorgason JT, Siciliano CA, Kiraly DD, and Calipari ES (2018) Granulocyte Colony Stimulating Factor Enhances Reward Learning through Potentiation of Mesolimbic Dopamine System Function. J. Neurosci. 38 (41), 8845–8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Brown MA, and Su MA (2019) An Inconvenient Variable: Sex Hormones and Their Impact on T Cell Responses. J. Immunol. 202, 1927–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Selmi C, and Gershwin ME (2019) Sex and autoimmunity: proposed mechanisms of disease onset and severity. Expert Rev. Clin. Immunol. 15 (6), 607–615. [DOI] [PubMed] [Google Scholar]
- (15).Baik J-H (2013) Dopamine signaling in reward-related behaviors. Front. Neural Circuits 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Schwarz J, Storch A, Koch W, Pogarell O, Radau PE, and Tatsch K (2004) Loss of Dopamine Transporter Binding in Parkinson’s Disease Follows a Single Exponential Rather Than Linear Decline. J. Nucl. Med. 45, 1694–1697. [PubMed] [Google Scholar]
- (17).Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, and Jones SR (2013) Temporal Pattern of Cocaine Intake Determines Tolerance vs Sensitization of Cocaine Effects at the Dopamine Transporter. Neuropsychopharmacology 38, 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Venton BJ, Seipel AT, Phillips PEM, Augustine GJ, and Wightman RM (2006) Cocaine Increases Dopamine Release by Mobilization ofa Synapsin-Dependent Reserve Pool. J. Neurosci. 26 (12), 3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ferris MJ, Calipari ES, Yorgason JT, and Jones SR (2013) Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci. 4 (5), 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Siciliano CA, Calipari ES, Ferris MJ, and Jones SR (2015) Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci. 6 (1), 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Northcutt A, Hutchinson M, Wang X, Baratta M, Hiranita T, Cochran T, Pomrenze M, Galer E, Kopajtic T, Li C, Amat J, Larson G, Cooper D, Huang Y, O’Neill C, Yin H, Zahniser N, Katz J, Rice K, Maier S, Bachtell R, and Watkins L (2015) DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry 20, 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lacagnina MJ, Watkins LR, and Grace PM (2018) Toll-like receptors and their role in persistent pain. Pharmacol. Ther. 184, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Felger JC, and Treadway MT (2017) Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Walker QD, Cabassa J, Kaplan KA, Li S-T, Haroon J, Spohr HA, and Kuhn CM (2001) Sex Differences in Cocaine-Stimulated Motor Behavior: Disparate Effects of Gonadectomy. Neuropsychopharmacology 25, 118. [DOI] [PubMed] [Google Scholar]
- (25).Walker QD, Rooney MB, Wightman RM, and Kuhn CM (1999) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95, 1061–1070. [DOI] [PubMed] [Google Scholar]
- (26).O’Neill B, Tilley MR, and Gu HH (2013) Cocaine produces conditioned place aversion in mice with a cocaine insensitive dopamine transporter. Genes Brain Behav 12 (1), 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hofford RS, Russo SJ, and Kiraly DD (2018) Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur. J. Neurosci, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Singh VK, Newman VL, and Seed TM (2015) Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): A review. Cytokine+ 71, 22–37. [DOI] [PubMed] [Google Scholar]
- (29).McLean AC, Valenzuela N, Fai S, and Bennett SA (2012) Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Visualized Exp. 67 (e4389), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yorgason JT, Espana RA, and Jones SR (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 202 (2), 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]