Abstract
Background
Diabetic retinopathy is a well-known sight-threatening microvascular complication of diabetes mellitus. Currently, 93 million people live with diabetic retinopathy worldwide. There are insufficient studies addressing the prevalence of diabetic retinopathy and risk factors in Ethiopia.
Objective
To assess the prevalence of diabetic retinopathy and its associated factors among diabetic patients on follow-up at Debre Markos Referral Hospital, northwest Ethiopia, 2019.
Methods
This institution- based cross-sectional study was conducted among 302 patients. They were selected through systematic sampling. Explanatory data were extracted from medical records and interviews. Blood pressure, weight, height, and visual acuity tests were assessed. Retinal examination was performed with a Topcon TRC-NW7SF fundus camera. Data were entered in EpiData 3.1 and exported in to SPSS 20 for analyses. Binary logistic regression with 95% CIs was used for analyses. Simple binary logistic regression followed by multiple binary logistic regression analysis was conducted to identify associated factors.
Results
There were 302 patients in this study, of which 57 (18.9%) had diabetic retinopathy. Among the diabetic retinopathy patients, 75.4% had the preproliferative type. Four in ten (37.7%) of the patients had visual acuity problems. Poor glycemic control (AOR 4.58, 95% CI 1.86–11.31), > 10 years’ diabetes duration (AOR 3.91, 95% CI 1.86–8.23), body-mass index >25 kg/m2 (AOR 3.74, 95% CI 1.83–7.66), and hypertension (AOR 3.39, 95% CI 1.64–7.02) were factors significantly associated with diabetic retinopathy.
Conclusion
About a-fifth of diabetic patients had diabetic retinopathy. Diabetic retinopathy was significantly associated with glycemic control, hypertension, body-mass index, and duration of illness. Routine assessment and early control of those associated factors may be important in reducing both the prevalence and impact of diabetic retinopathy, as evidenced in the current study.
Keywords: diabetes mellitus, diabetic retinopathy, associated factor, Ethiopia
Background
Diabetic retinopathy (DR) is a well-known sight-threatening microvascular complication of diabetes mellitus (DM).1–3 It is characterized by varying degrees of microaneurysm, hemorrhage, hard exudates, cotton-wool spots, venous changes, and new vessel formation involved in the peripheral retina, macula, or both.4–7 Globally, approximately 95 million (35.4%)diabetic patients have DR, of which a third have vision-threatening DR and 7.6% macular edema.8,9 Global annual incidence of DR is 2.2%–12.7% and progression 3.4%–12.3-%. Progression to proliferative DR is higher in individuals with mild disease than those with no disease at baseline.10
The global prevalence of blindness is estimated to be 1.5 billion, of which 0.4 million is due to DR. Even though blindness and visual impairment has reduced globally, blindness due to DR increased from 0.2 million to 0.4 million and moderate–severe visual impairment from 1.4 million to 2.6 million from 1990 to 2015.11 Though the combination of social, nutritional, and medical support has prevented or slowed the progression of DR, it is still a global issue, because of the epidemic rise of DM, for which the risk of visual loss is 25 times higher. Screening and treatment of DR is more challenging in developing countries, due to lack of finances and skills.6,8,12 The cost of screening and treatment of DR is >US$3,190 per quality-adjusted life year. But financial loss due to social and blindness were not estimated.13
DR is the leading cause of new cases of blindness in middle-aged and elderly populations in the Asia–Pacific region. It has been estimated that it is the source of 51% of blindness and 56% of visual impairment cases globally, but awareness of DR among DM patients is 28%–84%.14 In Africa, DR rangs 7%– 62.4%, of which 15% have severe DR. Ethiopia is among the top four countries with the highest (3.8%) adult diabetic populations in sub-Saharan Africa, but without sufficient studies, screening guidelines, standard referral criteria, or retinal photocoagulation.15,16 Therefore, the aim of this study was to identify the prevalence and determinants of DR among DM patients in this area.
Methods
Study Area and Period
This was an institution-based, cross-sectional study. A total of 302 DM patients were recruited from the diabetes clinic at Debre Markos Referral Hospital through a systematic sampling method. Debre Markos Referral Hospital, in the town of Debre Markos, is 300 km from Addis Ababa, the capital of Ethiopia, and a major referral center for DM treatment in northwest Ethiopia, providing services Monday to Friday every week. Data collection was conducted from April 1 to May 30, 2019 during routine working days.
Population and Eligibility Criteria
Source populations for this study were all patients with type 1 and type 2 DM according to World Health Organization criteria17 and on stable anti-DM medication. Patients who were critically ill, did not complete the questionnaire, physical examination, or blood tests, pregnant, or had cataracts, glaucoma, or any other eye disease, and could not completed fundus examinations for any reason were excluded from the study.
Sample-Size Determination and Sampling Procedures
The required sample size was computed using a single-population proportion formula based on the assumption of 95% CI, 5% margin of error, and 41.4% proportion (P) of DR.18 An added 10% estimated nonresponse rate made a final sample size of 302:
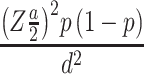 = nf
= nf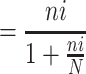 =
=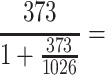 275 + 10% = 302
275 + 10% = 302
Every third patient was selected through systematic random sampling to get a sample size of 302. The patient registry was used as a sampling frame. Data were collected from each study participant from the second visit and continued with intervals of three based on visiting-card order until the desired sample size had been obtained.
Operational Definition
The definition of DR was taken as the presence of microaneurysm, hemorrhage, exudates, cotton-wool spots, intraretinal microvascular abnormalities, vein beading, and/or new blood vessels in at least one eye on retinal camera examination.
Data-Collection Procedure and Study-Variable Measurement
Explanatory variables were sociodemographic (age, sex, level of education, marital status, occupation, residence, religion, and family history of DM), behavioral (history of smoking, history of alcohol consumption), clinical (duration of DM, chronic cardiac illness, and chronic kidney disease), DM care (treatment modality, follow-up frequency, and routine DR eye screening). Data were collected through a semistructured questionnaire. Blood pressure was measured using an Android digital sphygmomanometer, keeping the respondent in a seating position. Systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg for two consecutive measurements apart from four hours apart and ongoing treatment with antihypertensive drugs defined hypertension. Those with known chronic kidney disease had been diagnosed by a physician and recorded on their patient registry book. Glycemic level was coded as poor or good. Poor glycemic control was operationally defined as mean fasting blood glucose was >130 mg/dL for at least 6 months.19 Height was measured with a movable headboard (stadiometer) and recorded to the nearest 0.1 cm. Weight was measured with a digital scale and recorded to the nearest 0.1 kg.20 Visual acuity was tested at 6 m in a well-illuminated area using a Snellen chart. If the participant’s vision were too poor to read any letters on the chart at 6 m, then counting finger, hand movement, and light perception were assessed.21
Fundus Examination and Diagnostic Criteria
Eye examinations were conducted by experienced ophthalmologists. Mydriasis of both eyes was assessed with 1% tropicamide and retinal examination, and a TRC-NW7SF fundus camera (Topcon, Tokyo, Japan) used to capture 45°C color digital images of the fundus of both eyes. Double-blind diagnoses were performed by two ophthalmologists from Debre Markose Referral Hospital. In cases of disagreement, a third ophthalmologist was consulted. According to the Early Treatment Diabetic Retinopathy Study, those with microaneurysms, hemorrhage, hard exudates, cotton-wool spots; retinal vein beading changes, microvascular abnormalities in the retina, and/or neovascularization lesions on fundus images were diagnosed with DR. On fundus examination, the presence of microaneurysms, cotton-wool spots, hemorrhage, vein beading, exudates, and/or intraretinal microvascular abnormalities were diagnosed as preproliferative DR, whereas the presence of new vessels on the dis or elsewhere and/or vitreous hemorrhage were diagnosed as as proliferative DR.5
Data-Quality Management
The questionnaire was translated into the local language (Amharic) from English, then back to English. One day’s training was provided for the data collectors on how data were to be collected and recorded. Pretesting was done on 16 DM patients at Fenoteslam General Hospital to assess the validity of the instrument, estimate time needed for data collection, and modify the questionnaire accordingly. Data were checked daily for completeness and consistency.
Data Analysis
All data were entered in EpiData 3.1 and exported toSPSS 20. Data were checked, cleaned, coded, merged, categorized, and analyzed. Frequency distributions were computed for socio-demographic, behavioral, clinical and DM-care variables. Continuous variables are expressed as means ± SD and categorical variables as proportions. ORs and CIs were used to determine the strength of association between independent and dependent variables. All independent variables were cross-tabulated with dichotomized outcomes of DR (yes/no). Simple binary logistic regression followed by multiple binary logistic regression analysis was conducted to identifyfactors associated with DR in the study population. Variables were entered into the multiple logistic regression model if P<0.25 on simple binary logistic regression analysis.22 P<0.05 wa considered a significant association between independent variables and DR. Finally, results were summarized and presented in text, tables, charts, and graphs.
Result
Sociodemographic Characteristics of Respondents
A total of 302 DM patients were included in the current study, with a 99.6% response rate. Respondents’ mean age was 41.20±14.20 years. Two-thirds (67.5%) of the respondents were males, half (54%) > 40 years old, two-thirds (65.6%) married, and three-quarters (76.2%) urban dwellers. Though a third (37.4%) of respondents had a family history of DM, only 5.3% of them had developed DR. Half (45.0%) the respondents had a college education, and a third (35.1%) were governmental or nongovernmental employews (Table 1).
Table 1.
Sociodemographic characteristics of patients (n=302) on follow-up
| Diabetic retinopathy | Total (%) | ||
|---|---|---|---|
| Yes | No | ||
| n (%) | n (%) | ||
| Sex | |||
| Female | 23 (7.62) | 75 (24.83) | 98 (32.45) |
| Male | 34 (11.26) | 170 (56.29) | 204 (67.55) |
| Age | |||
| ≤40 years | 9 (2.98) | 130 (43.05) | 139 (46.03) |
| >40 years | 48 (15.89) | 115 (38.08) | 163 (53.97) |
| Religion | |||
| Orthodox | 53 (17.55) | 226 (74.83) | 279 (92.38) |
| Muslim | 4 (1.32) | 19 (6.29) | 23 (7.62) |
| Residence | |||
| Rural | 8 (2.65) | 64 (21.19) | 72 (23.80) |
| Urban | 49 (16.23) | 181 (59.93) | 230 (76.20) |
| Marital status | |||
| Single | 1 (0.33) | 48 (15.890 | 49 (16.22) |
| Married | 31 (10.26) | 167 (55.30) | 198 (65.56) |
| Divorced | 5 (1.66) | 11 (3.64) | 16 (5.30) |
| Widowed | 20 (6.62) | 19 (6.30) | 39 (12.91) |
| Education | |||
| None | 9 (2.98) | 46 (15.23) | 55 (18.21) |
| Primary | 11 (3.64) | 60 (19.86) | 71 (23.51) |
| Secondary | 2 (0.66) | 38 (12.58) | 40 (13.25) |
| College and above | 35 (11.60) | 101 (33.41) | 136 (45.03) |
| Occupation | |||
| Merchant | 25 (8.28) | 83 (27.48) | 108 (35.76) |
| Employee | 24 (7.95) | 82 (27.15) | 106 (35.10) |
| Farmer | 8 (2.65) | 49 (16.23) | 57 (18.87) |
| Student | 0 | 31 (10.26) | 31 (10.26) |
| Family history of DM | |||
| No | 41 (13.58) | 148 (49.01) | 189 (62.59) |
| Yes | 16 (5.30) | 97 (32.12) | 113 (37.42) |
Behavioral, Clinical, and Diabetes-Care Characteristics of Respondents
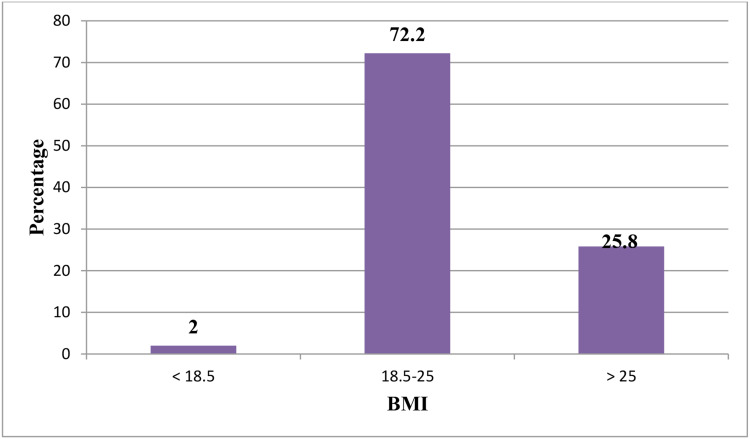
Regarding prevalence of alcohol intake, 53 (17.5%) consumed alcohol once a month or less frequently, of whom 6.2% consumed alcohol once a week or more frequently. More than 94.3% of those who used alcohol once a month or less frequently had started before diagnoses of DM. Regarding prevalence of smoking, one (0.3.%) reported smoking regularly, but no respondents had stopped smoking. Four of ten (37.1%) had had DM >10 years. Mean body-mass index was 23.79±2.6 kg/m2. Three-quarters (72.2%) of respondents body-mass index was normal and a quarter (25.8%) were overweight/obese. A quarter (23.18%) had a history of hypertension. Mean fasting blood glucose was 131.06±26.79 mg/dL, and half (50.3%) had good glycemic control. More than half (55.6%) used oral antiglycemic agents, while 43.4% respondents took insulin for treatment. A total of 184 (60.92%) were visiting a health institution every month, and one in four (39.08%) were visiting a health institution every 2 months for DM follow-up (Table 2 and Figure 1).
Table 2.
Behavioral, clinical, and diabetes care–related characteristics of respondents (n=302)
| Diabetic retinopathy | Total (%) | ||
|---|---|---|---|
| Yes | No | ||
| n (%) | n (%) | ||
| Alcohol consumption | |||
| Never after diagnosis | 43 (14.24) | 206 (68.21) | 249 (82.45) |
| Drinker | 14 (4.64) | 39 (12.91) | 53 (17.55) |
| Duration of DM illness | |||
| ≤10 years | 14 (4.64) | 176 (58.28) | 190 (62.92) |
| >10 years | 43 (14.24) | 69 (22.28) | 112 (37.09) |
| Glycemic control | |||
| Good | 7 (2.32) | 145 (48.01) | 153 (50.33) |
| Poor | 50 (16.56) | 100 (33.11) | 149 (49.34) |
| Hypertension | |||
| No | 25 (8.28) | 207 (68.54) | 232 (76.82) |
| Yes | 32 (10.60) | 38 (12.58) | 68 (23.18) |
| Chronic cardiac illness | |||
| No | 49 (16.22) | 243 (80.46) | 292 (96.70) |
| Yes | 8 (2.65) | 2 (0.66) | 10 (3.30) |
| Chronic kidney disease | |||
| No | 55 (18.21) | 242 (80.13) | 297 (98.35) |
| Yes | 3 (0.99) | 2 (0.66) | 5 (1.65) |
| Type of diabetes | |||
| 2 | 41 (13.57) | 127 (42.05) | 168 (55.63) |
| 1 | 14 (4.64) | 117 (38.74) | 131 (43.38) |
| Both | 2 (0.66) | 1 (0.33) | 3 (0.99) |
| Follow-up frequency | |||
| Every month | 29 (9.60) | 155 (51.32) | 184 (60.92) |
| Every 2 months | 28 (9.26) | 90 (29.80) | 118 (39.07) |
| Routine DR eye screening | |||
| No | 31 (10.26) | 220 (72.85) | 251 (83.11) |
| Yes | 26 (8.61) | 25 (8.28) | 51 (16.88) |
| DR health education | |||
| No | 49 (16.22) | 201 (66.56) | 250 (82.87) |
| Yes | 6 (2) | 46 (15.23) | 52 (17.23) |
Figure 1.
Percentages of body mass–index values among DM patients on follow-up.
Prevalence of Diabetic Retinopathy
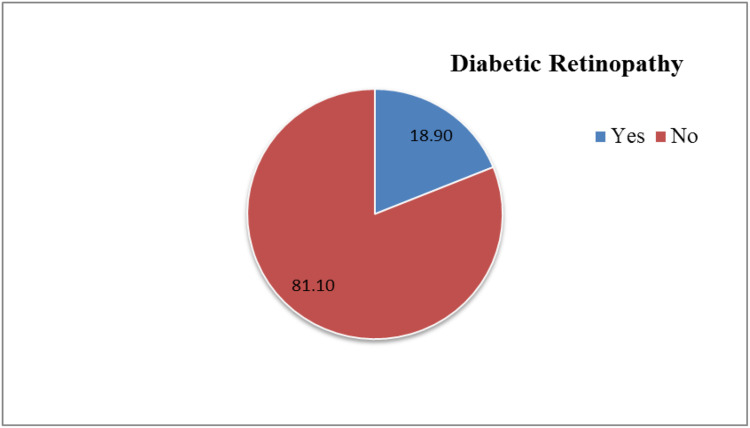
Among the 302 participants, 57 (18.9%) had DR. Three-quarters (75.4%) of DR patients had nonproliferative DR, while a quarter (24.6%) had proliferative DR. There are no fundus picture is nongradable, and 6.4% of respondents had macular edema. Four in ten (37.7%)respondents had visual acuity problems and three-quarters (86%) of DR respondents had visual acuity problems (Figure 2).
Figure 2.
Prevalence of diabetic retinopathy among diabetic patients on follow-up.
Associated Factors for Diabetic Retinopathy among DM Patients
On bivariate analysis, DR had statistically significant associations with age, glycemic control, hypertension, body-mass index, type of DM, and duration of illness. On bivariate logistic regression, P<0.25 results were included in multivariate logistic regression. Glycemic control, hypertension, body-mass index, and duration of illness had statistically significant associations with DR. The odds of developing DR among those with poor glycemic control were about five times (AOR 4.58, 95% CI 1.86–11.31) those of patients with good glycemic control. The odds of developing DR in hypertensive patients were triple (AOR 3.39, 95% CI 1.64–7.02) those of nonhypertensive patients. The odds of developing DR among overweight/obese respondents were about four times more likely (AOR 3.74, 95% CI1.83–7.66) those of patients with normal body weight. The odds of developing DR among patients who had had DM >10 years were quadruple (AOR (95% CI 3.91 (1.86,8.23) those of their counterparts (Table 3).
Table 3.
Multivariate analysis of sociodemographic, clinical and diabetes care, and treatment modality–related characteristics of patients (n=302)
| Retinopathy | COR (95% CI) | AOR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Age | |||||
| ≤40 years | 9 | 130 | 1 | 1 | |
| >40 years | 48 | 115 | 6.03 (2.83–12.83) | 1.39 (0.532–3.65) | 0.500 |
| Glycemic control | |||||
| Good | 7 | 145 | 1 | 1 | |
| Poor | 50 | 100 | 10.35 (4.512–23.77) | 4.58 (1.86–11.31) | 0.001** |
| Hypertension | |||||
| No | 25 | 207 | 1 | 1 | |
| Yes | 32 | 38 | 6.97 (3.72–13.1) | 3.39 (1.64–7.02) | 0.001** |
| Body-mass index | |||||
| 18.5–25 kg/m2 | 23 | 195 | 1 | 1 | |
| >25 kg/m2 | 34 | 44 | 6.55 (3.52–12.204) | 3.74 (1.83–7.66) | 0.001** |
| <18.5 kg/m2 | 0 | 6 | 0 | 0 | 0.999 |
| Type of diabetes | |||||
| 2 | 41 | 127 | 1 | 1 | |
| 1 | 14 | 117 | 0.36 (0.19–0.69) | 1.306 (0.51–3.35) | 0.578 |
| Both | 2 | 1 | 1.5 (0.13–16.97) | 1.52 (0.10–22.56) | 0.762 |
| Duration of DM | |||||
| ≤10 years | 14 | 176 | 1 | 1 | |
| >10 years | 43 | 69 | 7.83 (4.03–15.2) | 3.91 (1.86–8.23) | 0.001** |
Note: **P<0.05.
Discussion
The findings of the current study showed that one in five (18.9%, 95% CI 14.5%–23.3%) DM patients had DR. This is consistent with studies conducted in Brazil (15%)23 and India (21.2%)24 and meta-analyses in China (18.45%).25 It is higher than studies conducted in Beijing (8.1%)26 and Arbamnech General Hospital (13%),27 but lower than studies conducted in Armenia (36.2%),28 Zimbabwe (28.4%),29 Khartoum (82.6%),30 and Jimma, Ethiopia (41.4%).31 This discrepancy among studies might be due to variations in genetics, methodology, setting, DR-risk comorbidities, diagnostic method, quality of care, and health-seeking behavior among study participants. In our study, among DR patients, three-quarters (75.4%) had nonproliferative DR. This is lower than study results in India (85.3%)30 and Armenia (90.2%),28 but higher than studies in southern Iran (56.9%)32 and Khartoum (51.7%).30 This variation might be due to quality of care for DM patients and diagnostic methods. Four of ten (37.7%) respondents had visual acuity problems, and more than three-quarters (86%) of DR patients had visual acuity problems. This was higher than a study conducted at Nobel Medical College in Biratnagar (24.6%).33 This variation might be due to quality of care for diabetic patients and lifestyle. In the multivariate logistic regression model, glycemic control, hypertension, body-mass index, and duration of illness were significantly associated with DR. In this study, the odds of developing DR among those with poor glycemic control were about five times (AOR 4.58, 95% CI 1.86–11.31) those of patients with good glycemic control. This result is in line with systematic reviews in China,25 southern Iran,34 Tanzania,35 and Jimma University Hospital.31 The possible mechanism might be that poor glycemic control causes vascular cell apoptosis by abnormal glucose metabolism, activation of protein kinase C, formation of advanced glycosylation end product, and increased production of reactive oxygen species.36–38 The odds of developing DR among hypertensive patients were three times (AOR 3.39 95% CI 1.64–7.02) those of nonhypertensive patients. This is consistent with studies conducted in Beijing,26 Tanzania,35 Kenya,39 Khartoum,30 Arbamnech General Hospital,27 and Jimma University Hospital,31 but inconsistent with a study conducted in Iran.34 This discrepancy might be due to methodology, confounding effects, variations in self-care practices, and variations in hypertensive prevalence among studies. There also existed an association between duration of DM and DR in our findings. The odds of developing DR among patients who had had DM >10 years (AOR 3.91, 95% CI 1.86_.23) were quadruple() those of their counterparts. This finding is in line with studies conducted in Armenia,28 Beijing,26 Iran,32 Kenya,39 Tanzania,35 Zimbabwe,29 Khartoum,30 Arbamnech General Hospital,27 and Jimma University Hospital.31
The odds of developing DR among overweight/obese respondents were (AOR (95% CI3.74 (1.83,7.66) were about quadruple those of patients with normal body weight. This result is in line with studies conducted in the US,40 Iran,32 and Beijing,26 but inconsistent with studies in Croatia41 and Minnesota.42 Possible reasons for this discrepancy among studies might bemethodological differences, differences in study participants, lack of comprehensive anthropometric measurements, and confounding effects, but being overweight/obese causes increasing blood viscosity, oxidative stress, vascular growth factors, leptin, cytokines, and ICAM1, which leads to DR.43,44
Strengths and Limitations of the Study
There are many important strengths to the present study. This is the first study to investigate the prevalence of DR and associated factors in Debre Markose, Ethiopia. The use of a cross-sectional design also provided a sufficiently large sample and including many independent variables. Another strength is that fundus examinations were performed by experienced ophthalmologists. This study is not without limitations. The study design was cross-sectional, so we could not take account of the temporal relationship between potential risk factors and outcomes. Another limitation is its short duration. Despite these factors, we believe that this study is novel and its findings reflect the trend of rising DR frequency in developing countries. Moreover, fasting blood sugar was used to assess glycemic control, due to the lack of facilities to assess HbA1c in the study area.
Conclusion and Recommendation
The prevalence of DR in this study was 18.9% (95% CI 14.3%–23.5%). Threequarters (75.4%) of DR patients had nonproliferative DR and a quarter (24.6%) proliferative DR. Poor glycemic control, hypertension, overweight, obesity, and longer DM duration were significantly associated with DR. The results emphasize that the Ministry of Health should establish strategies and polices to control DR. We recommend that health workers also provide sustainable health information to diabetic patients on possible risk factors of DR (hypertension, overweight/obese, and poor glycemic control), as these were evidenced in the current study.
Acknowledgments
The authors would like to thank Debre Markos Referral Hospital administration, health workers, and data collectors. We are also indebted to the study participants for their kind cooperation.
Funding Statement
There was no funding or sponsoring organization for this study.
Abbreviations
AOR, adjusted OR; BMI, body-mass index; COR, crude oOR; DM, diabetes mellitus; DR, diabetic retinopathy; FBS, fasting blood sugar; IRMAs, intraretinal microvascular abnormalities; NGO, nongovernmental organization; NPDR, nonproliferative dDRr; PDR, proliferative DR; SBP, systolic blood pressure; SLB, slit-lamp biomicroscopy; WHO, World Health Organization.
Data-Sharing Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the manuscript.
Ethics Approval and Consent to Participate
This study was performed in accordance with the revised Declaration of Helsinki guidelines for biomedical research involving human subjects,45 and ethical clearance was obtained from the Institutional Review Board, College of Health Science, and Jimma University. Following endorsement from the university, Debre Markos Referral Hospital (study setting) was informed about the objectives of the study through a support letter from the Institutional Review Board. After obtaining informed consent (oral) from clients, data were collected. Study participants had the right to refuse to join, ask any question, or withdraw at any time. Privacy and confidentiality were assured. Respondents who were diagnosed with retinopathy were referred to the ophthalmic clinic for further management.
Consent for Publication
Not applicable.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All the authors declare that they have no competing interests.
References
- 1.Lee P, WHO members. Prevention of blindness from diabetes mellitus. World Health Organ. 2006:1–48. [Google Scholar]
- 2.Ghanchi F, Bailey C, Chakravarthy U, Cohen S, Dobson P, Gibson J. The royal college of ophthalmologists diabetic retinopathy guidelines. R Coll Ophthalmol. 2013;2:1–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook L, Kanski S. Clinical Ophthalmology. Vol. 8 ELSEVER; 2016:1–887. [Google Scholar]
- 4.Canada D, Practice C, Expert G. Retinopathy diabetes canada clinical practice guidelines expert committee. Can Diabetes Assoc. 2018;42:210–216. [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study (ETDRS) Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the modified airlie house classification. ETDRS report number 10. Ophthalmology. 1991;98(5):786–806. doi: 10.1016/S0161-6420(13)38012-9 [DOI] [PubMed] [Google Scholar]
- 6.Stry M, Health OF. Ministry of health guidelines for screening and management of diabetic retinopathy in Kenya. Minist Heal Kenya. 2017;4(2):1–64. [Google Scholar]
- 7.Wong TY, Sun J, Kawasaki R, et al. Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018;125(10):1608–1622. doi: 10.1016/j.ophtha.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 8.Hancho N, Kirigia J, Claude KO. IDF Diabetes Atlas. Vol. 8 International Diabetes Fedration; 2017:1–150. [Google Scholar]
- 9.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2(1):1–25. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabanayagam C, Riswana Banu D, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet. 2018;7(2):140–149. doi: 10.1016/S2213-8587(18)30128-1 [DOI] [PubMed] [Google Scholar]
- 11.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):1221–1234. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 12.Shu D, Ting W, Ophth M, Chui G, Cheung M. Review diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. [DOI] [PubMed] [Google Scholar]
- 13.Lamoureux EL, Hassell JB, Keeffe JE. The impact of diabetic retinopathy on participation in daily living. Arch Ophthalmol. 2004;122(2):84–88. doi: 10.1001/archopht.122.1.84 [DOI] [PubMed] [Google Scholar]
- 14.Chua J, Xin C, Lim Y, Wong TY. Diabetic retinopathy in the asia-pacific. Asia Pacific J Ophthalmol. 2018;7(1):3–16. [DOI] [PubMed] [Google Scholar]
- 15.Gojka R, Cowan M. Global report on diabetes. World Health Organ. 2016;3:1–88. [Google Scholar]
- 16.Abebe N, Kebede T, Addise D. Review article diabetes in Ethiopia 2000–2016 – prevalence and related acute and chronic complications; a systematic review. African J Diabetes Med. 2017;25(2):7–12. [Google Scholar]
- 17.Heller M, Edelstein P, Mayer M. Membrane-bound enzymes. III. Protease activity in leucocytes in relation to erythrocyte membranes. BBA Biomembr. 1975;413(3):472–482. doi: 10.1016/0005-2736(75)90130-3 [DOI] [PubMed] [Google Scholar]
- 18.Dell RB, Holleran S, Ramakrishnan R. NIH public access. Dell Al. 2012;43(4):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ADA. Standards of medical care in diabetes. Diabet Care. 2013;36(1):11–66. doi: 10.2337/dc13-S011 [DOI] [Google Scholar]
- 20.American Academy of Pediatrics. Guideline # 4 Anthropometric Measurements. Vol. 8 2016:1–7. Available from: marketing@aap.org. [Google Scholar]
- 21.Long J, Grasso J. Eye education for emergency clinicians eye. State Ophthalmol Serv. 2008;16(3):1–21. [Google Scholar]
- 22.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):1–8. doi: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borges D, Mendanha DA, Martins M, Vilar C. Risk factors and incidence of diabetic retinopathy. Rev Bras Oftalmol. 2016;75(6):443–446. [Google Scholar]
- 24.Bharathi N, Kalpana S, Sujatha L, Nawab A, Kumar H. Prevalence of diabetic retinopathy in diabetics of rural population belonging to Ramanagara and Chikkaballapura districts of Karnataka. Int J Sci Res Publ. 2015;5(1):2250–3153.:. [Google Scholar]
- 25.Song P, Yu J, Chan KY, Theodoratou E, Rudan I. Prevalence, risk factors and burden of diabetic retinopathy in China: a systematic review and meta-analysis. J Glob Health. 2018;8(1). doi: 10.7189/jogh.08.010803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui J, Ren JP, Chen DN, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ Open. 2017;7(8):1–6. doi: 10.1136/bmjopen-2016-015473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chisha Y, Terefe W, Assefa H, Lakew S, Reboldi G. Prevalence and factors associated with diabetic retinopathy among diabetic patients at Arbaminch General Hospital, Ethiopia: cross sectional study. PLoS One. 2017;12(3):1–9. doi: 10.1371/journal.pone.0171987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giloyan A, Harutyunyan T, Petrosyan V. The prevalence of and major risk factors associated with diabetic retinopathy in Gegharkunik province of Armenia: cross-sectional study. BMC Ophthalmol. 2015;15(46):1–7. doi: 10.1186/s12886-015-0032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okwanga PN, Mukona M, Mateveke K, Machingura PI, Macheka B, Gomo E. Prevalence and risk factors associated with retinopathy in diabetic patients at Parirenyatwa Hospital outpatients’ clinic in Harare, Zimbabwe. Arch Med Biomed Res. 2017;3(2):104. doi: 10.4314/ambr.v3i2.6 [DOI] [Google Scholar]
- 30.Elwali ES, Almobarak AO, Hassan MA, Mahmooud AA, Awadalla H, Ahmed MH. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: population based study. Int J Ophthalmol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharew G, Ilako DR, Kimani KGY, Gelaw Y. Prevalence of diabetic retinopathy in Jimma University Hospital, Southwest Ethiopia. Ethiop Med J. 2013;51(2):105–113. [PubMed] [Google Scholar]
- 32.Ghaem H, Daneshi N, Riahi S, Dianatinasab M. The prevalence and risk factors for diabetic retinopathy in Shiraz, Southern Iran. Diabetes Metab J. 2018;42(6):538. doi: 10.4093/dmj.2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhikari BN, Gautam PS, Bekoju B, Basnet S, Bhandari H. Prevalence and associated risk factors for diabetic retinopathy among in-patients diagnosed with diabetes mellitus: a retrospective study conducted in nobel medical college and teaching hospital, Biratnagar. J Nobel Med Coll. 2018;7(1):50–55. [Google Scholar]
- 34.Rasoulinejad SA, Hajian-Tilaki K, Mehdipour E. Associated factors of diabetic retinopathy in patients that referred to teaching hospitals in Babol. Caspian J Intern Med. 2015;6(4):224–228. [PMC free article] [PubMed] [Google Scholar]
- 35.Cleland CR, Burton MJ, Hall C, et al. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop Med Int Health. 2016;21(3):417–426. doi: 10.1111/tmi.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ola MS, Nawaz MI. Cellular and molecular mechanism of diabetic retinopathy. INTECH Open. 2010;1:1–29. [Google Scholar]
- 37.Rask-madsen C, King GL. Review vascular complications of diabetes: mechanisms of injury and protective factors. CMET. 2012;17(1):20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cahalan MD, Nader N, Hodeify R, Kulkarni RPE-N-N. Effects of hyperglycemia on vascular smooth muscle Ca2+ signaling and oxidative stress. Biomed Res Int. 2017;(February):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathenge W, Bastawrous A, Peto T, et al. Prevalence and correlates of diabetic retinopathy in a population-based survey of older people in Nakuru, Kenya. Ophthalmic Epidemiol. 2014;21(3):169–177. doi: 10.3109/09286586.2014.903982 [DOI] [PubMed] [Google Scholar]
- 40.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–835. doi: 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaštelan S, Tomić M, Gverović Antunica A, Ljubić S, Salopek Rabatić J, Karabatić M. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballard DJ, Melton LJ, Dwyer MS, et al. Risk factors for diabetic retinopathy: a population-based study in Rochester, Minnesota. Diabetes Care. 1986;9(4):334–342. doi: 10.2337/diacare.9.4.334 [DOI] [PubMed] [Google Scholar]
- 43.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes. 2005;29(11):1308–1314. doi: 10.1038/sj.ijo.0802987 [DOI] [PubMed] [Google Scholar]
- 44.Dorchy H, Claes C, Verougstraete C. Risk factors of developing proliferative retinopathy in type 1 diabetic patients: role of BMI. Diabetes Care. 2002;25(4):798–799. doi: 10.2337/diacare.25.4.798 [DOI] [PubMed] [Google Scholar]
- 45.Rennie D. Trial registration: a great idea switches from ignored to irresistible. J Am Med Assoc. 2004;292(11):1359–1362. doi: 10.1001/jama.292.11.1359 [DOI] [PubMed] [Google Scholar]