Abstract
Background
The use of electronic cigarettes has increased over the past decade. To determine how the abuse liability of electronic cigarette liquids (e-liquids) differs from nicotine alone, and to determine the impact of flavor, we compared nicotine-containing fruit- and tobacco-flavored e-liquids, and their nicotine-free versions, to nicotine alone in mouse models of oral consumption, reward and aversion.
Methods
Adult male C57BL/6J mice voluntarily consumed oral nicotine, equivalent nicotine concentrations of fruit- and tobacco-flavored e-liquid, and equivalent dilutions of the nicotine-free versions in 2-bottle choice tests. Conditioned place preference and place aversion were assessed with peripherally administered e-liquids or nicotine. Serum nicotine and cotinine levels were measured after subcutaneous injections of e-liquid or nicotine.
Results
Mice showed higher consumption and preference for the fruit-flavored e-liquid compared with nicotine alone. This increase was not due to the flavor itself as consumption of the nicotine-free fruit-flavored e-liquid was not elevated until the highest concentration tested. The increased consumption and preference were not observed with the tobacco-flavored e-liquid. The conditioned place preference, place aversion and nicotine pharmacokinetics of the fruit-flavored e-liquid were not significantly different from nicotine alone.
Conclusions
Our data suggest that fruit, but not tobacco flavor, increased the oral consumption of e-liquid compared with nicotine alone. Moreover, this enhancement was not due to increased consumption of the flavor itself, altered rewarding or aversive properties after peripheral administration, or altered pharmacokinetics. This flavor-specific enhancement suggests that some flavors may lead to higher nicotine intake and increased use of e-liquids compared with nicotine alone.
Keywords: Electronic cigarette, nicotine, mice, preference, aversion, consumption
1. Introduction
Electronic cigarettes (e-cigarettes) have steadily increased in popularity over the last decade (Chou et al., 2017). Over 2 million middle and high school students have used e-cigarettes, prompting the FDA to declare e-cigarette use a youth epidemic (Gottlieb, 2018; Wang et al., 2018). Alarmingly, 33% of e-cigarette users have never used combustible cigarettes, indicating that these products are appealing to and capturing a new population that may progress to nicotine dependence (McMillen et al., 2015). Indeed, e-cigarette use is significantly associated with nicotine use disorder and nicotine addiction (Chou et al., 2017), and youth who use e-cigarettes are more likely to become combustible cigarette smokers later in life (Leventhal et al., 2015; Loukas et al., 2018).
E-cigarettes vaporize a liquid (e-liquid) that contains nicotine and flavors in a mixture of propylene glycol and glycerin. The levels of tobacco-related chemicals in e-liquids are very low due to the lack of tobacco (Han et al., 2016; Beauval et al., 2017). However, e-liquids contain other unknown chemicals and e-cigarettes can deliver as much nicotine as a combustible cigarette (Wagener et al., 2017). How the abuse liability of e-liquids differs from nicotine alone has not been extensively studied, as the majority of pre-clinical studies on e-liquids have focused on toxicity in peripheral organ systems (El Golli et al., 2016; Garcia-Arcos et al., 2016; Golli et al., 2016b; Vivarelli et al., 2019). The neurocognitive effects and addiction-relevant properties of e-liquids are beginning to be examined in rodent models (Golli et al., 2016a; LeSage et al., 2016a; LeSage et al., 2016b; Harris et al., 2017; Harris et al., 2018b; Smethells et al., 2018). Intriguingly, two of these studies suggest that high concentrations of e-liquids are less aversive compared with equivalent concentrations of nicotine alone in a model of intra-cranial self-stimulation (ICSS) in male rats (LeSage et al., 2016b; Harris et al., 2018b). In addition, e-liquids are available in many different flavors, and some of the most popular flavors among youth are mint, mango and fruit (Leventhal et al., 2019). The impact of different flavors is only beginning to be determined in preclinical models, with the majority of studies focusing on menthol (Alsharari et al., 2015; Wickham, 2015; Henderson et al., 2019).
In this study, we compared the voluntary consumption and preference of fruit- and tobacco-flavored e-liquids to nicotine alone in a two-bottle choice model in mice. Two-bottle choice is a high-throughput, technically simple assay that is commonly used to measure the voluntary oral consumption and preference of nicotine in mice (Klein et al., 2004; Glatt et al., 2009; Lee and Messing, 2011; Cao et al., 2012; Locklear et al., 2012; O’Rourke et al., 2016). Although the pharmacokinetics of oral consumption are slower compared with intravenous nicotine delivery, voluntary nicotine consumption in mice can lead to physical dependence and is regulated by the same genetic and molecular factors that modulate nicotine intake in humans, such as enzymatic regulation of nicotine metabolism and expression of nicotinic acetylcholine receptors (nAChRs) (Siu et al., 2006; Locklear et al., 2012; Renda et al., 2016; Bagdas et al., 2019).
We found that mice showed greater consumption and preference for a nicotine-containing fruit-flavored e-liquid, but not for a nicotine-containing tobacco-flavored e-liquid, compared with equivalent concentrations of nicotine alone. This increase was not due to the flavor itself, as consumption and preference of a nicotine-free fruit-flavored e-liquid was not elevated until the highest concentration tested. We then assessed whether oral or peripheral routes of administration were important by examining whether peripheral administration of the fruit-flavored e-liquid had altered rewarding or aversive properties compared with nicotine alone in the conditioned place preference (CPP) and conditioned place aversion (CPA) assays, and found no significant differences compared with nicotine alone. Our data suggest that fruit, but not tobacco flavor, acts to enhance oral nicotine consumption and preference in mice. This suggests that some flavors may lead to higher nicotine intake and result in altered abuse liability of e-liquids compared with nicotine alone.
2. Methods
2.1. Animals and reagents
Eight-week old male C57BL/6J mice from The Jackson Laboratory (Sacramento, CA) acclimated to our facility for at least one week before behavioral experiments. Mice were group housed in standard cages under a 12-h light/dark cycle until the start of experiments, after which they were individually housed. All animal procedures were in accordance with the Institutional Animal Care and Use Committee at the University of Minnesota, and conformed to NIH guidelines.
Nicotine tartrate salt (Acros Organics, Thermo Fisher Scientific, Chicago, IL) was mixed with tap water to the concentrations reported for each experiment. The e-liquids Retro Fruit Twist and Drops Classic American Tobacco were purchased from NicVape.com, and consisted of a 50/50 propylene glycol and glycerin mix. All e-liquid solutions were verified for their nicotine content by a standard gas chromatography assay with nitrogen phosphorus detection, based on the method of Jacob and colleagues (Jacob et al., 1981; Hieda et al., 1999; LeSage et al., 2003; Harris et al., 2008). The actual nicotine concentrations in the fruit- and tobacco-flavored e-liquids were between 16.1 to 17.7 mg/mL, and the nicotine content of the nicotine-free e-liquids were between 0.000123 to 0.000655 mg/mL (labelled nicotine concentrations=18 and 0 mg/mL). All concentrations were reported as free base. The nicotine and e-liquid solutions for voluntary consumption experiments were diluted in tap water, and the solutions for peripheral injections were diluted in 0.9% saline and pH adjusted to 7.4.
2.2. Voluntary oral drug consumption (2-bottle choice tests)
Two-bottle choice consumption was performed in a similar manner as our prior work (O’Rourke et al., 2016; Touchette et al., 2018; DeBaker et al., 2019). The mice were singly housed and presented with one bottle of tap water and one bottle of drug formulation diluted in tap water. Each experimental group in the 2-bottle choice procedure consisted of separate drug naïve mice, with a total of 5 separate groups in the 2-bottle choice experiment (nicotine alone versus water, nicotine-containing fruit-flavored e-liquid versus water, nicotine-containing tobacco-flavored e-liquid versus water, nicotine-free fruit-flavored e-liquid versus water, and nicotine-free tobacco-flavored e-liquid versus water). The nicotine group was assessed for 5 concentrations (30, 50, 75, 100 and 200 μg/mL nicotine), with each concentration presented for one week in sequential order. The nicotine-containing e-liquids were diluted to the same nicotine concentrations. As each bottle had a maximum capacity of 50 mL, the amount of 18 mg/mL nicotine-containing e-liquid required for a 50 mL concentration of 30, 50, 75, 100 and 200 μg/mL was 0.083, 0.139, 0.208, 0.278, 0.556 mL, respectively, and represents 0.17, 0.28, 0.42, 0.56 and 1.11% (v/v) of the fluid composition of the bottle. The nicotine-free e-liquids were diluted to match the same fluid amounts as the nicotine-containing e-liquids. All e-liquids mixed with water in a uniform manner without any signs of separation. The bottles were weighed every 2–3 days and the positions of the bottles were alternated each weighing to control for side preferences. All solutions were refreshed every 3–4 days. The mice were weighed once a week, and food was freely available at all times.
2.3. Place conditioning
The purpose of the place conditioning assays was to determine whether compounds within the e-liquid, other than nicotine, had rewarding or aversive effects when administered peripherally that may confound the interpretation of the oral consumption results. We used 0.5 and 2.0 mg/kg nicotine, or equivalent concentrations of nicotine-containing fruit-flavored e-liquid, for CPP and CPA, respectively. A ratio of 0.01 mL/g was used for all i.p. injections, and thus the volume of e-liquid in the injection was 0.28% for 0.5 mg/kg, and 1.1% for 2.0 mg/kg. Nicotine was dissolved in saline only. To determine whether nicotine-free fruit-flavored e-liquid had any effects alone, we compared it to saline. The chamber apparatus consisted of a two-compartment place preference insert in an open field chamber with different floor textures (Med Associates, St. Albans, VT). We used an unbiased nicotine place conditioning procedure as previously reported (Grabus et al., 2006; Lee and Messing, 2011), which consisted of one habituation session on Day 1, twice daily conditioning sessions on Days 2–4, and one test session on Day 5. For the habituation session, mice were i.p. injected with saline and placed in the apparatus with access to both chambers for 15 minutes. For the conditioning sessions, mice were i.p. injected with the drug formulation and were immediately confined to one chamber for 30 minutes. Four to five hours later, mice received an injection of saline paired with the alternate chamber, and this was repeated for 3 days for a total of 6 conditioning sessions (3 drug formulation and 3 saline). On test day, mice received an injection of saline and access to both chambers for 15 minutes. The order of the injections and the drug formulation-paired floor was counterbalanced across groups. Saline control mice received saline paired with both floors. The experiments for each formulation and concentration were performed in multiple cohorts over several months.
2.4. Nicotine and cotinine pharmacokinetics
Mice were subcutaneously injected with 2.5 mg/kg nicotine or equivalent nicotine concentrations of e-liquid and sacrificed at 10, 20, 30 or 50 minutes after injection. The volume of e-liquid in the injection was 1.4% to achieve a 2.5 mg/kg injection. Trunk blood was collected for assessment of serum nicotine and cotinine concentrations as described previously (Jacob et al., 1981; Hieda et al., 1999; LeSage et al., 2003; Harris et al., 2008).
2.5. Statistical analysis
For the oral consumption experiments, we calculated nicotine consumption (mg/kg) and preference for the drug formulation bottle. The consumption of nicotine or equivalent nicotine concentrations (mg/kg) was calculated based on of the weight of the fluid consumed and mouse weights. For the nicotine-free e-liquids, the consumption is calculated as a hypothetical mg/kg to compare with the e-liquid and nicotine groups. The average total fluid consumption (g) was calculated as the weight of all fluid consumed per week. The preference was calculated as the weight of fluid consumed from the drug formulation bottle divided by the total fluid consumed multiplied by 100. For the place conditioning experiments, we calculated a conditioning index, which was the time spent in the drug-paired chamber during test day minus time spent in that same chamber on habituation day. All analyses were calculated using Prism 8 (GraphPad, La Jolla, CA). For the place conditioning data, outliers were identified using the Grubb’s test or if the data point was outside 2X the standard deviation from the mean. The number of outliers per group were: 2 for saline, 1 each for the nicotine-free e-liquid, 0.5 mg/kg nicotine, 0.5 mg/kg e-liquid, and 2.0 mg/kg nicotine groups. The determination of whether place conditioning produced preference or aversion was established by analyzing data using one-sample t-tests against a hypothetical conditioning index of zero for each group. Drug formulation groups were compared using Student’s t-tests or one-way ANOVA followed by Tukey’s multiple comparisons tests. Comparison of data across time used two-way repeated measures ANOVA followed by Tukey’s multiple comparisons tests.
3. Results
3.1. The consumption and preference of nicotine-containing fruit-flavored e-liquid compared with nicotine alone
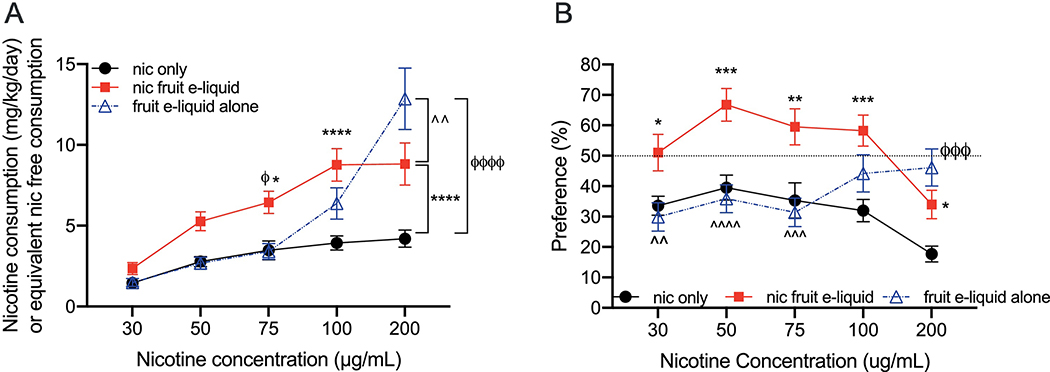
We compared the average daily consumption of the nicotine-containing fruit-flavored e-liquid and equivalent dilutions of the nicotine-free fruit-flavored e-liquid to nicotine alone. We found a significant interaction between drug formulation and concentration (Finteraction(8, 148)=14.20, P<0.0001; Fconcentration(4, 148)=68.39, P<0.0001; Fdrug(2, 37)=7.785, P=0.002; Fig. 1A). Tukey’s multiple comparisons showed that mice consumed more nicotine-containing fruit-flavored e-liquid compared with nicotine alone at the 75, 100 and 200 μg/mL concentrations. The consumption of nicotine-containing fruit-flavored e-liquid was also significantly higher than the nicotine-free version at the 75 μg/mL concentration. The consumption of the nicotine-free fruit-flavored e-liquid was greater than both the nicotine-containing fruit-flavored e-liquid and nicotine alone at only the 200 μg/mL concentration.
Fig. 1. Consumption and preference of nicotine-containing fruit-flavored e-liquid, nicotine-free fruit-flavored e-liquid and nicotine alone in 2-bottle choice tests.
(A) The average consumption and (B) preference for nicotine alone, nicotine-containing fruit-flavored e-liquid at equivalent nicotine concentrations, and nicotine-free fruit-flavored e-liquid at equivalent dilutions. The consumption for nicotine and nicotine-containing e-liquids are in mg/kg/day, and in hypothetical mg/kg/day for the nicotine-free e-liquid. *P<0.05, **P<0.01, ***P<0.001 and ***P<0.0001 for all comparisons. *indicates comparisons between nicotine-containing fruit-flavored e-liquid and nicotine alone, ϕindicates comparisons between nicotine-free fruit-flavored e-liquid and nicotine alone, and îndicates comparisons between nicotine-containing fruit-flavored e-liquid and the nicotine-free version. Mean ± SEM, n=15 for nicotine alone, n=12 for nicotine-containing fruit-flavored e-liquid, n=13 for the nicotine-free fruit-flavored e-liquid.
Similar results were observed for the bottle preference, where we found a significant interaction between drug formulation and concentration (Finteraction(8,148)=9.820, P<0.0001; Fconcentration(4, 148)=11.50, P<0.0001; Fdrug(2, 37)=7.811, P=0.002; Fig. 1B). Tukey’s multiple comparisons showed that mice had greater preference for nicotine-containing fruit-flavored e-liquid compared with nicotine alone at all concentrations. There was greater preference for the nicotine-containing fruit-flavored e-liquid compared with the nicotine-free version at the 30, 50 and 75 μg/mL concentrations. The preference for the nicotine-free fruit-flavored e-liquid exceeded that of nicotine alone at the 200 μg/mL concentration. Together, these data indicate that mice increased the consumption and preference of a nicotine-containing fruit-flavored e-liquid compared with nicotine alone, and this increase is not due to increased preference for the flavor itself since consumption of the nicotine-free fruit-flavored e-liquid was not elevated until the highest concentration tested.
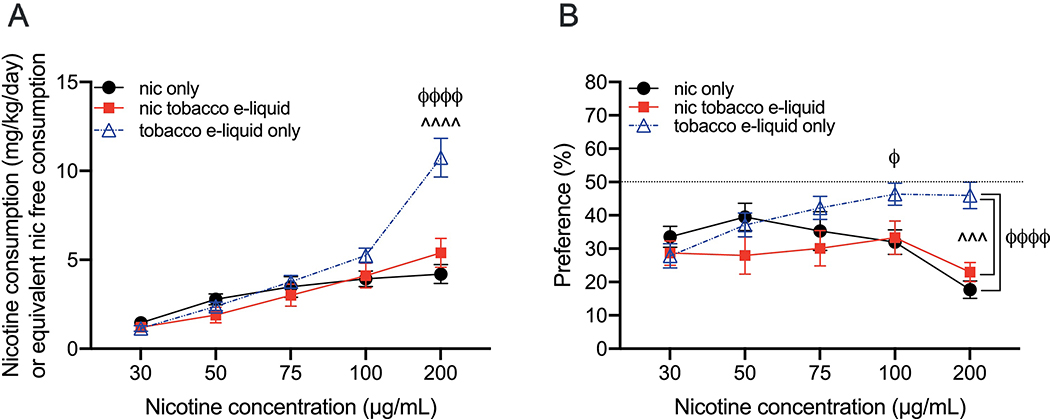
3.2. The consumption and preference of nicotine-containing tobacco-flavored e-liquid compared with nicotine alone
The increased consumption and preference that we observed for the nicotine-containing fruit-flavored e-liquid did not occur with the nicotine-containing tobacco-flavored e-liquid compared with nicotine alone. For the average daily consumption, we found a significant interaction between drug formulation and concentration (Finteraction(8, 160)=16.22, P<0.0001; Fconcentration(4, 160)=93.81, P<0.0001; Fdrug(2, 40)=4.591, P=0.02; Fig. 2A). Tukey’s multiple comparisons showed that the consumption of the nicotine-free tobacco-flavored e-liquid was significantly higher than both the nicotine-containing tobacco-flavored e-liquid and nicotine alone only at the 200 μg/mL concentration. No other significant differences between drug formulation were observed at any concentration. For bottle preference, we also found a significant interaction between drug formulation and concentration (Finteraction(8, 160)=6.933, P<0.0001; Fconcentration(4, 160)=5.572, P=0.0003; Fdrug(2, 40)=3.103, P=0.06; Fig. 2B). Tukey’s multiple comparisons showed that the preference for the nicotine-free tobacco-flavored e-liquid was greater than nicotine alone at 100 μg/mL, and greater than both the nicotine-containing tobacco-flavored e-liquid and nicotine alone at the 200 μg/mL concentration. No significant differences were observed between nicotine alone and the nicotine-containing tobacco-flavored e-liquid at any concentration.
Fig. 2. Consumption and preference of nicotine-containing tobacco-flavored e-liquid, nicotine-free tobacco-flavored e-liquid and nicotine alone in 2-bottle choice tests.
(A) The average consumption and (B) preference for nicotine alone, nicotine-containing tobacco-flavored e-liquid at equivalent nicotine concentrations, and nicotine-free tobacco-flavored e-liquid at equivalent dilutions. The consumption for nicotine and nicotine-containing e-liquids are in mg/kg/day, and in hypothetical mg/kg/day for the nicotine-free e-liquid. *P<0.05, **P<0.01, ***P<0.001 and ***P<0.0001 for all comparisons. ϕindicates comparisons between nicotine-free tobacco-flavored e-liquid and nicotine alone, and îndicates comparisons between nicotine-containing tobacco-flavored e-liquid and the nicotine-free version. Mean ± SEM, n=15 for nicotine alone, n=14 for nicotine-containing tobacco-flavored e-liquid, n=14 for the nicotine-free tobacco-flavored e-liquid.
3.3. The consumption and preference of nicotine-free fruit- versus nicotine-free tobacco-flavored e-liquid, and total fluid consumption
We then evaluated the consumption and preference of the nicotine-free fruit-flavored e-liquid compared with the nicotine-free tobacco-flavored e-liquid to determine whether the flavors showed similar consumption and preference. For both consumption and preference, we found a main effect of concentration with no main effect of e-liquid or an interaction between e-liquid and dilution, indicating that mice consumed the nicotine-free versions of both flavored e-liquids similarly (mg/kg/day consumption: Finteraction(4, 100)=1.153, P=0.34; Fdilution(4, 100)=88.47, P<0.0001; Fe-liquid(1, 25)=0.779, P=0.39; bottle preference: Finteraction(4, 100)=1.616, P=0.18; Fdilution(4, 100)=13.18, P<0.0001; Fe-liquid(1, 25)=0.214, P=0.65).
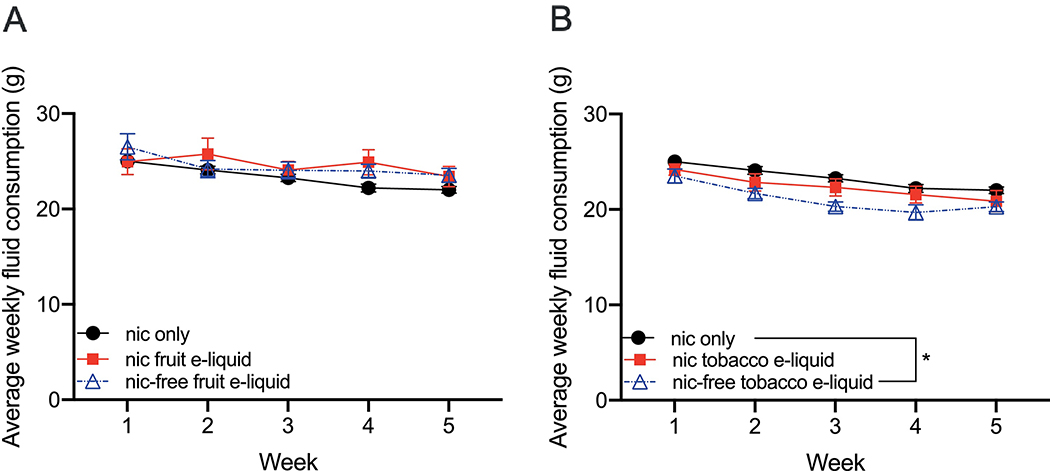
To determine whether total fluid intake differed between groups, we calculated the average total fluid consumption per week. For the nicotine-containing fruit-flavored e-liquid, nicotine-free fruit-flavored e-liquid and nicotine alone groups, we found a significant interaction between group and time (Finteraction(8, 148)=2.148, P=0.03; Fgroup(2, 37)=0.8623, P=0.43; Ftime(4, 148)=12.81, P<0.0001). However, Tukey’s multiple comparisons did not show any significant differences in total fluid intake between groups at any week (Fig. 3A). For the nicotine-containing tobacco-flavored e-liquid, nicotine-free tobacco-flavored e-liquid and nicotine groups, we found main effects of group and time, with no interaction (Finteraction(8, 160)=0.962, P=0.47; Fgroup(2, 40)=3.891, P=0.03; Ftime(4, 160)=36.16, P<0.0001). Tukey’s multiple comparisons showed that the nicotine-free tobacco-flavored e-liquid group was significantly different from nicotine, and consumed less total fluid compared with the nicotine alone group (Fig. 3B). There was no difference in total fluid intake between nicotine alone and the nicotine-containing tobacco-flavored e-liquid groups, nor was there a difference between the nicotine-containing tobacco-flavored e-liquid and the nicotine-free tobacco-flavored e-liquid groups.
Fig. 3. Total fluid consumption.
(A) The total fluid consumption in the 2-bottle choice over the 5-week experiment did not differ between the nicotine-containing fruit-flavored e-liquid, nicotine-free fruit-flavored e-liquid and nicotine alone groups at any week. (B) The total fluid consumption of the nicotine-free tobacco-flavored e-liquid group was significantly different from the nicotine group. Mean ± SEM, n=15 for nicotine alone, n=12 for nicotine-containing fruit-flavored e-liquid, n=13 for the nicotine-free fruit-flavored e-liquid, n=14 for nicotine-containing tobacco-flavored e-liquid, n=14 for the nicotine-free tobacco-flavored e-liquid.
3.4. Place conditioning of fruit-flavored e-liquid compared with nicotine alone
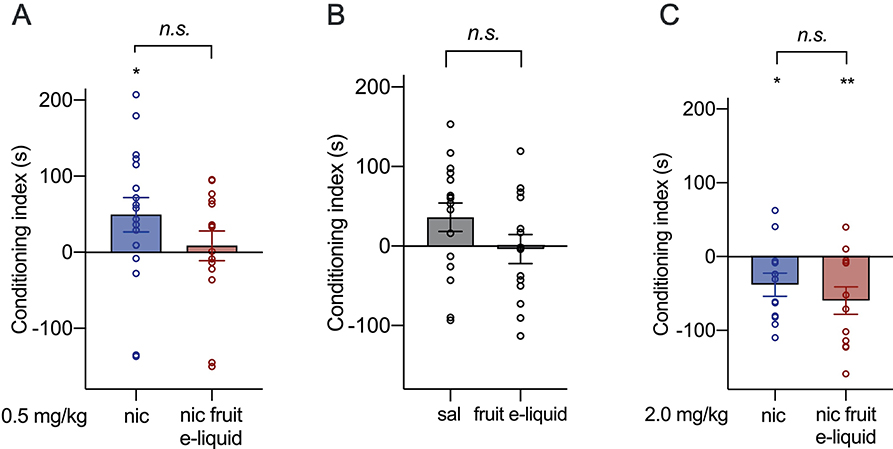
To determine whether compounds within the fruit-flavored e-liquid, other than nicotine, had rewarding or aversive properties that may confound the oral consumption results, we evaluated the nicotine-containing fruit-flavored e-liquid compared with nicotine alone in peripheral place conditioning tests. We first assessed CPP using 0.5 mg/kg nicotine and equivalent concentrations of nicotine-containing fruit-flavored e-liquid, and found no significant difference in the conditioning index (t=1.342, df=30, P=0.19, Fig. 4A). However, when assessing whether each group had significant place conditioning, we found that conditioning with 0.5 mg/kg nicotine produced a conditioning index that was significantly greater than zero, indicating a significant place preference (one-sample t-test: t=2.172, df=16, P=0.045). The conditioning index of the nicotine-containing fruit-flavored e-liquid was not significantly different from zero, indicating no preference or aversion was produced (one-sample t-test: t=0.432, df=14, P=0.67).
Fig. 4. Nicotine-containing fruit-flavored e-liquid does not differ from nicotine alone in conditioned place preference or conditioned place aversion assays.
(A) The conditioning index after CPP with 0.5 mg/kg nicotine or nicotine-containing fruit-flavored e-liquid at an equivalent nicotine concentration. n=17 for nicotine, n=15 for e-liquid groups. (B) The conditioning index after saline or nicotine-free fruit-flavored e-liquid at an equivalent dilution to 0.5 mg/kg nicotine. n=16 for saline, n=14 for nicotine-free e-liquid groups. (C) The conditioning index after CPA with 2.0 mg/kg nicotine or nicotine-containing fruit-flavored e-liquid at an equivalent nicotine concentration. n=12 for nicotine and e-liquid groups. *P<0.05, **P<0.01 for a one-sample t-test between the conditioning index and a hypothetical index of zero.
We also assessed whether the nicotine-free fruit-flavored e-liquid at an equivalent 0.5 mg/kg nicotine dilution produced any preference or aversion in the place conditioning assay compared with saline alone. We found no significant difference between place conditioning with saline compared with the nicotine-free fruit-flavored e-liquid (t=1.553, df=28, P=0.13, Fig. 4B). Neither saline nor the nicotine-free fruit-flavored e-liquid produced a conditioning index that was significantly different from zero, indicating no preference or aversion was produced with either substance (one-sample t-tests saline: t=2.014, df=15, P=0.06; nicotine-free e-liquid: t=0.204, df=13, P=0.84).
We next assessed place aversion using CPA at a concentration of 2.0 mg/kg nicotine and equivalent nicotine concentrations of fruit-flavored e-liquid. There was no significant difference in the conditioning index between substances (t=0.896, df=22, P=0.38, Fig. 4C). Both nicotine alone and the nicotine-containing fruit-flavored e-liquid produced a conditioning index that was significantly below zero, indicating that both substances produced place aversion (one-sample t-tests nicotine: t=2.454, df=11, P=0.03; fruit-flavored e-liquid: t=3.196, df=11, P=0.009). Overall, these data show that i.p. administered nicotine-containing fruit-flavored e-liquid produced similar effects compared with nicotine alone, although the nicotine-containing fruit-flavored e-liquid did not produce significant CPP when assessed using a one-sampled t-test. These data suggest that the oral route of administration is important in mediating the enhancement in nicotine-containing fruit-flavored e-liquid consumption and preference, and suggests that no additional compounds, other than nicotine, within the nicotine-containing fruit-flavored e-liquid have rewarding or aversive properties when administered peripherally.
3.5. Nicotine and cotinine pharmacokinetics
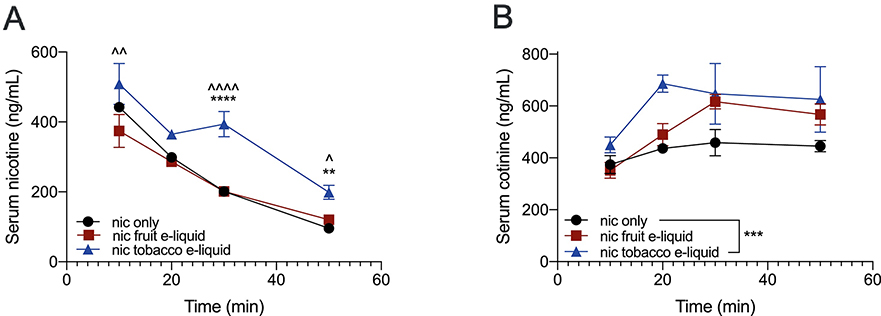
We then determined whether the nicotine and cotinine pharmacokinetics differed between peripheral inejctions of nicotine alone compared with nicotine-containing flavored e-liquids. To ensure that we could measure plasma nicotine and cotinine levels from individual mice, we used a 2.5 mg/kg s.c. injection of nicotine alone and equivalent nicotine concentrations of fruit-flavored and tobacco-flavored e-liquids. For the average serum nicotine levels (ng/mL), we found a significant interaction between drug formulation and time (Finteraction(6, 20)=2.925, P=0.03; Ftime(3, 20)=95.03, P<0.0001; Fdrug(2, 20)=35.56, P<0.0001; Fig. 5A). Tukey’s multiple comparisons showed that serum nicotine levels after the injection of nicotine-containing tobacco-flavored e-liquid was significantly higher than nicotine alone at the 30 and 50 minute timepoints, and significantly higher than the nicotine-containing fruit-flavored e-liquid at the 10, 30 and 50 minute timepoints. There was no difference between the nicotine-containing fruit-flavored e-liquid and nicotine alone at any timepoint.
Fig. 5. Nicotine and cotinine clearance.
(A) The average serum nicotine and (B) serum cotinine levels after injection of 2.5 mg/kg s.c. of nicotine alone, nicotine-containing fruit-flavored e-liquid and nicotine-containing tobacco-flavored e-liquid. *P<0.05, **P<0.01, ***P<0.001 and ***P<0.0001 for all comparisons. *indicates comparison between nicotine-containing tobacco-flavored e-liquid and nicotine alone, and îndicates comparison between nicotine-containing tobacco-flavored e-liquid and nicotine-containing fruit-flavored e-liquid.
For the average serum cotinine levels (ng/mL), we found main effects of time and drug formulation without a significant interaction (Finteraction(6, 24)=0.8097, P=0.57; Ftime(3, 24)=5.769, P=0.004; Fdrug(2, 24)=8.713, P=0.001; Fig. 5B). We examined the main effects of drug formulation using a Tukey’s multiple comparisons and found an overall significant difference between the nicotine-containing tobacco-flavored e-liquid and nicotine alone.
4. Discussion
The prevalence of e-cigarette use has steadily increased over the past decade, and the numerous flavors available contributes to the popularity of these products among adolescents and young adults (McMillen et al., 2015; Leventhal et al., 2019). How the abuse liability of flavored e-liquid differs from nicotine alone has not been extensively studied in pre-clinical models, and the studies that have been published have mainly used peripheral administration routes. In this study, we used oral and peripheral routes of administration, and evaluated voluntary oral consumption, CPP, CPA, and nicotine pharmacokinetics of nicotine-containing fruit- and/or tobacco-flavored e-liquids compared with nicotine alone. We found that mice had greater oral consumption and preference for the nicotine-containing fruit-flavored e-liquid compared with nicotine alone. Interestingly, this enhancement of consumption and preference was not due to the flavor or to non-nicotine ingredients in the e-liquid alone, since the consumption and preference of the nicotine-free fruit-flavored e-liquid was not elevated until the highest concentration tested. Thus, the enhancement of consumption and preference observed at the lower concentration range was not driven by the consumption or preference for the flavor and non-nicotine ingredients in the e-liquid. One possible mechanism underlying the enhancement may be that fruit flavoring acts as an orosensory cue to enhance the reinforcing effects of moderate nicotine concentrations, similar to how light and tone cues enhance the responding to i.v. nicotine self-administration in rats (Chaudhri et al., 2005; Chaudhri et al., 2006). Human data shows that young adult smokers rate green apple and chocolate flavored e-cigarettes as more rewarding compared with unflavored e-cigarettes, and are willing to work harder for the flavored e-cigarettes compared with unflavored e-cigarettes (Audrain-McGovern et al., 2016). Interestingly, we did not observe an increase in consumption and preference with the nicotine-containing tobacco-flavored e-liquids, which suggests that some flavors, but not others, can enhance intake. We believe the mice perceived both the fruit and tobacco flavoring equally, as there was no difference in the consumption or preference of the nicotine-free versions of both flavors of e-liquids. Alternatively, fruit flavor, but not tobacco flavor, may mask the aversive orosensory effects of nicotine, thus promoting greater consumption. Further research on individual flavors and flavor categories will be important in identifying the scope and nature of this enhancement of nicotine consumption and preference.
We then compared the nicotine-containing fruit-flavored e-liquid to nicotine alone in place conditioning assays to determine whether compounds within the e-liquid, other than nicotine, had rewarding or aversive effects that may confound the interpretation of the oral consumption results. We used peripheral administration of the fruit-flavored e-liquid in CPP and CPA tests, which eliminated the taste of the flavor. We found no significant difference in the CPP generated by 0.5 mg/kg nicotine compared with equivalent nicotine concentrations of the fruit-flavored e-liquid. However, when examining each group separately, nicotine, but not the nicotine-containing fruit-flavored e-liquid, produced a CPP index that was significantly different compared with 0 indicating conditioned preference was achieved. We only tested 0.5 mg/kg nicotine, which is a concentration that we and others have used to successfully produce CPP in mice (Grabus et al., 2006; Lee and Messing, 2011). It is possible that the nicotine-containing fruit-flavored e-liquid may show differences in conditioned reward compared with nicotine alone at alternative concentrations. We found no significant differences in the CPA produced by nicotine-containing fruit-flavored e-liquid and nicotine alone at 2.0 mg/kg, suggesting that the aversive properties of both drug formulations was similar. Additionally, the nicotine-free fruit-flavored e-liquid was not rewarding or aversive. Together, these data indicate that the nicotine-containing fruit-flavored e-liquid acts similarly compared with nicotine alone when administered peripherally, that the non-nicotine compounds in the e-liquid had no rewarding or aversive effects alone, and strongly suggest that an oral route of administration is important in the enhancement of oral consumption and preference observed with the nicotine-containing fruit-flavored e-liquid.
We also did not observe any significant differences in nicotine or cotinine pharmacokinetics between the nicotine-containing fruit-flavored e-liquid and nicotine alone after a 2.5 mg/kg s.c. injection. Thus, it is unlikely that any pharmacokinetic differences exist between the nicotine-containing fruit-flavored e-liquid and nicotine alone at the lower 0.5 and 2.0 mg/kg concentrations used in the place conditioning experiments. Our data suggest that the fruit-flavored enhancement of oral consumption and preference was not due to altered drug clearance, which is known to influence nicotine intake in humans and animals (Rao et al., 2000; Siu et al., 2006). Nicotine metabolism pathways in mice and humans are similar, as nicotine is converted to cotinine by cytochrome P450 2A5 in mice and 2A6 in humans (Matta and Elberger, 2007; Raunio et al., 2008). Interestingly, we found that the nicotine-containing tobacco-flavored e-liquid resulted in higher serum nicotine and cotinine levels compared with the nicotine-containing fruit-flavored e-liquid and nicotine alone. However, the increases in serum nicotine and cotinine levels in the nicotine-containing tobacco-flavored e-liquid group were not associated with altered oral consumption and preference compared with nicotine alone. The mechanism underlying the higher serum concentrations is unclear, and one possibility is that the fruit- and tobacco-flavored e-liquids differ in beta-nicotyrine levels, which is formed by the oxidation of nicotine and can inhibit cytochrome P450 2A enzymes, thus inhibiting nicotine pharmacokinetics (Abramovitz et al., 2015).
Together, our data suggest that the increase in consumption and preference observed with the nicotine-containing fruit-flavored e-liquid is primarily due to the orosensory properties of the flavor, and not to an interaction with nicotine when administered peripherally. This has important implications for behavioral assays that require peripheral administration, such as intravenous self-administration, which are unable to detect the orosensory effects and interactions between nicotine and flavor.
The abuse liability of e-liquids compared with nicotine alone has been understudied compared with the rapid increase in popularity of these products. Two previous studies in adult male rats using peripheral administration of a nicotine-containing fruit-flavored e-liquid showed that high concentrations of the e-liquid are less aversive compared with nicotine alone in an ICSS model of aversion, whereas no differences were observed in an ICSS model of reward, i.v. self-administration, or nicotine pharmacokinetics (LeSage et al., 2016b; Harris et al., 2018b). Further investigation found that propylene glycol, a main component of all e-liquids, is able to attenuate the aversive effect of nicotine alone in the ICSS procedure, without affecting ICSS thresholds itself (Harris et al., 2018a). In this study, peripheral administration of nicotine-containing fruit-flavored e-liquid was not different from nicotine alone in the CPA procedure, indicating equal aversive conditioning was produced. This difference in findings may be due to several factors, including a species difference, differences in the doses of e-liquid used, in the behavioral assay, or possible batch differences in the composition of the fruit-flavored e-liquids. We did not assess the effect of propylene glycol in the place conditioning assay, and there is no data on how propylene glycol affects place conditioning in mice. However, we tested the effect of the nicotine-free fruit-flavored e-liquid, which contains a 50/50 propylene glycol and glycerin mixture, and did not observe any conditioned preference or aversion.
The exact chemical composition of the compounds used as flavorings in e-liquids is unknown as manufacturers are not required to provide a list of ingredients. Recent evaluation of flavor preferences of JUUL e-liquid in US youth from 8th to 12th grade shows that mint, mango and fruit are the most preferred (Leventhal et al., 2019). Menthol, the compound primarily used in mint flavoring, has been extensively studied, as it is the only flavor allowed in combustible cigarettes (Wickham, 2015). Menthol acts through several mechanisms, such as reduction of the aversive sensory effects of smoking in humans, attenuation of the aversion to high nicotine concentrations in two-bottle choice tests in rats (Wickham, 2015; Wickham et al., 2018), and delaying the clearance of nicotine (Alsharari et al., 2015). Unlike menthol, mango flavoring in e-liquid appears to be a combination of at least 7 chemical compounds (Eddingsaas et al., 2018). The chemical composition of fruit and tobacco flavoring in the e-liquids used in the present study is unknown, but it is highly likely that they are composed of multiple chemicals, similar to mango flavoring. Identifying whether these individual chemicals are important in the abuse liability of e-liquids will be a challenge.
In this study, one limitation is that we assessed adult male mice only. Determining whether the enhancement of fruit-flavored e-liquid consumption and preference also occurs in adult female mice and adolescent mice of both sexes is critical to understanding the biological impact of these products. In addition, the technology to enable voluntary self-administration of inhaled e-liquids is still under development. Future replication of these flavor effects in an inhalation model or through the use of aerosolized e-liquid extracts would be important to further understand the impact of flavored e-liquids.
5. Conclusions
We found that mice had higher consumption and preference of a nicotine-containing fruit-flavored e-liquid compared with nicotine alone. Importantly, this was not due to the flavor itself, as the nicotine-free fruit-flavored e-liquid was not preferred until the highest concentration. Moreover, this increase in consumption and preference was not observed with the nicotine-containing tobacco-flavored e-liquid. There was no significant difference in the CPP or CPA of the fruit-flavored e-liquid compared with nicotine, suggesting that the increased consumption and preference requires oral experience and was likely not due to altered rewarding or aversive effects of the e-liquid when administered peripherally. Together, our results suggest that certain flavors may enhance nicotine consumption. Identifying which flavors produce this effect, the chemical composition of the flavors, and the mechanism underlying the enhancement will be important in determining how the abuse liability of flavored e-liquids may differ compared with nicotine alone, and which regulatory steps may be required to limit the abuse of these products.
Highlights.
Fruit flavor, but not tobacco flavor, enhances e-liquid consumption and preference
The nicotine-free flavored e-liquid is not preferred over nicotine alone
Conditioning rewarding and aversive effects are equal between nicotine and e-liquid
Acknowledgments
We thank Theresa Harmon for assistance with data collection.
Role of Funding Source
This work was supported by the National Institute of Neurological Disorders and Stroke T32NS105604 (SM Mulloy), the National Institute on Alcohol Abuse and Alcoholism R01AA026598 (AML), and the National Institute on Drug Abuse R01DA046318 (MGL, ACH). The funding sponsors were not involved in study design.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitz A, McQueen A, Martinez RE, Williams BJ, Sumner W, 2015. Electronic cigarettes: The nicotyrine hypothesis. Med Hypotheses. 85, 305–310. [DOI] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI, 2015. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PLoS One. 10, e0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP, 2016. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend. 166, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Diester CM, Riley J, Carper M, Alkhlaif Y, AlOmari D, Alayoubi H, Poklis JL, Damaj MI, 2019. Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology. 157, 107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauval N, Antherieu S, Soyez M, Gengler N, Grova N, Howsam M, Hardy EM, Fischer M, Appenzeller BMR, Goossens JF, Allorge D, Garçon G, Lo-Guidice JM, Garat A, 2017. Chemical Evaluation of Electronic Cigarettes: Multicomponent Analysis of Liquid Refills and their Corresponding Aerosols. J Anal Toxicol. 41, 670–678. [DOI] [PubMed] [Google Scholar]
- Cao J, Gautier NM, Li MD, 2012. CD-1 mice Show Individual Differences in Nicotine Preference in a Modified Two-Bottle Oral Self-Administration Model. Front Psychiatry. 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA, 2005. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl). 180, 258–266. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF, 2006. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl). 184, 353–366. [DOI] [PubMed] [Google Scholar]
- Chou SP, Saha TD, Zhang H, Ruan WJ, Huang B, Grant BF, Blanco C, Compton W, 2017. Prevalence, correlates, comorbidity and treatment of electronic nicotine delivery system use in the United States. Drug Alcohol Depend. 178, 296–301. [DOI] [PubMed] [Google Scholar]
- DeBaker MC, Robinson JM, Moen JK, Wickman K, Lee AM, 2019. Differential patterns of alcohol and nicotine intake: Combined alcohol and nicotine binge consumption behaviors in mice. Alcohol. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddingsaas N, Pagano T, Cummings C, Rahman I, Robinson R, Hensel E, 2018. Qualitative Analysis of E-Liquid Emissions as a Function of Flavor Additives Using Two Aerosol Capture Methods. Int J Environ Res Public Health. 15, 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Golli N, Jrad-Lamine A, Neffati H, Rahali D, Dallagi Y, Dkhili H, Ba N, El May MV, El Fazaa S, 2016. Impact of e-cigarette refill liquid with or without nicotine on liver function in adult rats. Toxicol Mech Methods. 26, 419–426. [DOI] [PubMed] [Google Scholar]
- Garcia-Arcos I, Geraghty P, Baumlin N, Campos M, Dabo AJ, Jundi B, Cummins N, Eden E, Grosche A, Salathe M, Foronjy R, 2016. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 71, 1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt AR, Denton K, Boughter JDJ, 2009. Variation in Nicotine Consumption in Inbred Mice Is Not Linked to Orosensory Ability. Chem Senses. 34, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golli NE, Dallagi Y, Rahali D, Rejeb I, Fazaa SE, 2016. a. Neurobehavioral assessment following e-cigarette refill liquid exposure in adult rats. Toxicol Mech Methods. 26, 435–442. [DOI] [PubMed] [Google Scholar]
- Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, El May MV, El Fazaa S, 2016b. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol. 77, 109–116. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, 2018. Statement from FDA Commissioner Scott Gottlieb, M.D., on new steps to address epidemic of youth e-cigarette use.
- Grabus SD, Martin BR, Brown SE, Damaj MI, 2006. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl). 184, 456–463. [DOI] [PubMed] [Google Scholar]
- Han S, Chen H, Zhang X, Liu T, Fu Y, 2016. Levels of Selected Groups of Compounds in Refill Solutions for Electronic Cigarettes. Nicotine Tob Res. 18, 708–714. [DOI] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG, 2008. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 16, 86–97. [DOI] [PubMed] [Google Scholar]
- Harris AC, Muelken P, Haave Z, Swain Y, Smethells JR, LeSage MG, 2018a. Propylene glycol, a major electronic cigarette constituent, attenuates the adverse effects of high-dose nicotine as measured by intracranial self-stimulation in rats. Drug Alcohol Depend. 193, 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Muelken P, Smethells JR, Krueger M, LeSage MG, 2017. Similar precipitated withdrawal effects on intracranial self-stimulation during chronic infusion of an e-cigarette liquid or nicotine alone. Pharmacol Biochem Behav. 161, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Muelken P, Smethells JR, Yershova K, Stepanov I, Olson TT, Kellar KJ, LeSage MG, 2018b. Effects of nicotine-containing and “nicotine-free” e-cigarette refill liquids on intracranial self-stimulation in rats. Drug Alcohol Depend. 185, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Grant S, Chu BW, Shahoei R, Huard SM, Saladi SSM, Tajkhorshid E, Dougherty DA, Lester HA, 2019. Menthol Stereoisomers Exhibit Different Effects on α4β2 nAChR Upregulation and Dopamine Neuron Spontaneous Firing. eNeuro. 5, ENEURO.0465–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR, 1999. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl). 143, 150–157. [DOI] [PubMed] [Google Scholar]
- Jacob P, Wilson M, Benowitz NL, 1981. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 222, 61–70. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM, 2004. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 78, 13–25. [DOI] [PubMed] [Google Scholar]
- Lee AM, Messing RO, 2011. Protein kinase C epsilon modulates nicotine consumption and dopamine reward signals in the nucleus accumbens. Proc Natl Acad Sci U S A. 108, 16080–16085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Muelken P, Harris AC, 2016a. Self-Administration of Smokeless Tobacco Products in Rats. Tob Regul Sci. 2, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR, 2003. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl). 170, 278–286. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC, 2016b. Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend. 168, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Miech R, Barrington-Trimis J, Johnston LD, O’Malley PM, Patrick ME, 2019. Flavors of e-Cigarettes Used by Youths in the United States. JAMA. 322, 2132–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J, 2015. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. JAMA. 314, 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear LL, McDonald CG, Smith RF, Fryxell KJ, 2012. Adult mice voluntarily progress to nicotine dependence in an oral self-selection assay. Neuropharmacology. 63, 582–592. [DOI] [PubMed] [Google Scholar]
- Loukas A, Marti CN, Cooper M, Pasch KE, Perry CL, 2018. Exclusive e-cigarette use predicts cigarette initiation among college students. Addict Behav. 76, 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Elberger AJ, 2007. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology (Berl). 193, 199–213. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD, 2015. Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nicotine Tob Res. 17, 1195–1202. [DOI] [PubMed] [Google Scholar]
- O’Rourke KY, Touchette JC, Hartell EC, Bade EJ, Lee AM, 2016. Voluntary co-consumption of alcohol and nicotine: Effects of abstinence, intermittency, and withdrawal in mice. Neuropharmacology. 109, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, Tyndale RF, 2000. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 58, 747–755. [DOI] [PubMed] [Google Scholar]
- Raunio H, Pokela N, Puhakainen K, Rahnasto M, Mauriala T, Auriola S, Juvonen RO, 2008. Nicotine metabolism and urinary elimination in mouse: in vitro and in vivo. Xenobiotica. 38, 34–47. [DOI] [PubMed] [Google Scholar]
- Renda A, Penty N, Komal P, Nashmi R, 2016. Vulnerability to nicotine self-administration in adolescent mice correlates with age-specific expression of α4* nicotinic receptors. Neuropharmacology. 108, 49–59. [DOI] [PubMed] [Google Scholar]
- Siu EC, Wildenauer DB, Tyndale RF, 2006. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology (Berl). 184, 401–408. [DOI] [PubMed] [Google Scholar]
- Smethells JR, Harris AC, Burroughs D, Hursh SR, LeSage MG, 2018. Substitutability of nicotine alone and an electronic cigarette liquid using a concurrent choice assay in rats: A behavioral economic analysis. Drug Alcohol Depend. 185, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette JC, Maertens JJ, Mason MM, O’Rourke KY, Lee AM, 2018. The nicotinic receptor drug sazetidine-A reduces alcohol consumption in mice without affecting concurrent nicotine consumption. Neuropharmacology. 133, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivarelli F, Canistro D, Cirillo S, Cardenia V, Rodriguez-Estrada MT, Paolini M, 2019. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-free conditions. Life Sci. 228, 53–65. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, Leavens EL, Tackett AP, Molina N, Queimado L, 2017. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control. 26, e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A, 2018. Tobacco Product Use Among Middle and High School Students - United States, 2011–2017. MMWR Morb Mortal Wkly Rep. 67, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham RJ, 2015. How Menthol Alters Tobacco-Smoking Behavior: A Biological Perspective. Yale J Biol Med. 88, 279–287. [PMC free article] [PubMed] [Google Scholar]
- Wickham RJ, Nunes EJ, Hughley S, Silva P, Walton SN, Park J, Addy NA, 2018. Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology. 128, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]