Abstract
High-sodium diet may modulate the gut microbiome. Given the circulating short-chain fatty acids (SCFAs) are microbial in origin, we tested the hypothesis that the modest sodium reduction would alter circulating SCFA concentrations among untreated hypertensives, and the changes would be associated with reduced blood pressure (BP) and improved cardiovascular phenotypes. A total of 145 participants (42% blacks, 19% Asian, 34% females) were included from a randomized, double-blind, placebo-controlled crossover trial of sodium reduction with slow sodium or placebo tablets, each for 6 weeks. Targeted circulating SCFA profiling was performed in paired serum samples, which were collected at the end of each period, so as all outcome measures. Sodium reduction increased all 8 SCFAs, among which the increases in 2-methylbutyrate, butyrate, hexanoate, isobutyrate, and valerate were statistically significant (ps<0.05). Also, increased SCFAs were associated with decreased BP and improved arterial compliance. There were significant sex differences of SCFAs in response to sodium reduction (ps<0.05). When stratified by sex, the increases in butyrate, hexanoate, isobutyrate, isovalerate, and valerate were significant in females only (ps<0.05), not in males (ps>0.05). In females, changes in isobutyrate, isovalerate, and 2-methylbutyrate were inversely associated with reduced BPs (ps<0.05). Increased valerate was associated with decreased carotid-femoral pulse wave velocity (cf-PWV) (p=0.040). Our results show that dietary sodium reduction increases circulating SCFAs, supporting that dietary sodium may influence the gut microbiome in humans. There is a sex difference in SCFA response to sodium reduction. Moreover, increased SCFAs are associated with decreased BPs and improved arterial compliance.
Keywords: sodium intake, short-chain fatty acid, microbiome, hypertension, blood pressure
Summary
Moderate dietary sodium reduction increases the circulating levels of SCFAs, which may mediate the beneficial effects of sodium reduction on cardiovascular health such as reducing BP and arterial stiffness.
Graphical Abstract
Introduction
Hypertension is a serious health challenge and imposes a huge burden. Elevated blood pressure (BP) increases the risk of stroke, heart attack, and heart failure.1 Excess sodium intake is recognized as one of the most important risk factors for hypertension.2,3 However, the underlying etiologies are not well understood.
Recent research supports the concept that gut microbiota is involved in BP control and hypertension.4 In particular, animal studies suggest that microbiome is a key linkage in the causal relationship between sodium intake and elevated BP.5–7 A study found that high-sodium diet altered the gut microbiome in mice, and the treatment of L. murinus prevented salt-induced hypertension.8 High-sodium diet modulates the gut microbial composition and alters fecal short-chain fatty acids (SCFAs) production in salt-sensitive animal models.8–10 SCFAs are also involved in BP regulation.11 SCFAs are products from the fermentative activity of gut bacteria, subsequently absorbed into the bloodstream of the host, which can bind to and activate host receptors, thereby acting as a messenger between gut microbial metabolism and host physiology.11 Thus, the relationships among sodium intake, SCFAs, and BP would provide important information on the etiology of hypertension. However, evidence in humans is scarce. Given the fact that virtually all SCFAs in the circulation are microbial in origin, we tested the hypotheses that (1) modest reduction in dietary sodium intake would alter circulating SCFA levels among the untreated hypertensive participants; (2) whether there was race or sex interaction in response to sodium reduction; (3) the altered SCFAs would be associated with reduced BP and improved large artery compliance.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
The present study utilized stored serum samples from a previously conducted randomized, double-blind, placebo-controlled crossover trial (RCT) of modest dietary sodium reduction in untreated hypertensives as described before.12,13 The inclusion criteria were population aged 30 to 75 years, with sitting systolic blood pressure (SBP) 140 to 170 mmHg or diastolic blood pressure (DBP) 90 to 105 mmHg, and with no previous treatment for raised BP.12 A total of 145 participants (39% whites, 42% blacks, 19% Asian, 34% females) with serum samples available at both time points (at the end of slow sodium and placebo) were included in this study. The study was approved by the Wandsworth Local Research Ethics Committee and the Institutional Review Board of Augusta University, and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Sodium Reduction Protocol
The RCT of modest sodium reduction was carried out (Figure S1), the study protocol and compliance were described in detail elsewhere.12–15 Briefly, in the first two weeks run-in period, participants were given detailed advice by specially trained nurses on how to reduce their sodium intake, with an aim of achieving an intake of 2000 mg sodium/day (85 mmol/day). They were advised not to add salt at the table or during cooking, and avoid foods that contained a large amount of sodium. Nurses went through with the participants on what foods they usually ate, identified items with high sodium content and advised them to use lower sodium alternatives. In appropriate cases, the spouse or whoever cooked in the household was also seen. The advice was reinforced at each visit for the whole duration of the study. Sodium-free bread was provided for those who had no easy access to it. While continuing on the reduced-sodium diet, the participants were given, in a random order, either nine slow sodium tablets (10 mmol sodium per tablet) or placebos daily for six weeks. They then crossed over to receive the other tablets for another six weeks.12 Reduced-sodium diet plus slow sodium tablets represented usual sodium intake, while a reduced-sodium diet plus placebo represented a reduced-sodium intake.
Anthropometric and Laboratory Measurements
Measurements were performed at the end of each 6-week study period. Height and body weight were measured with light clothing and without shoes. Body mass index (BMI) was calculated as weight (kg) per square of height (m2). BP was measured by a validated automatic digital BP monitor (Omron HEM-705CP) in the sitting position after 5 to 10 minutes rest. Three readings were taken and the average of the last two readings was used. Twenty-four-hour ambulatory blood pressure monitoring (ABPM) was performed using SpaceLabs 90207 devices (SpaceLabs, Inc, Washington, DC) as previously described.16 Briefly, the monitor was set to take BP measurements at half-hourly intervals during the daytime and hourly intervals during the nighttime. Recordings were analyzed with the ABPM report manager system software package.16 Pulse pressure (PP) was calculated as the difference between SBP and DBP. Carotid-femoral pulse wave velocity (cf-PWV) was measured noninvasively using an automatic device Complior.12 Blood samples were collected at the end of each 6-week period for the measurements of biochemistry.
Targeted Metabolomics Analysis of SCFAs in Serum
Serum samples were collected at the end of 6-week slow sodium tablets and at the end of 6-week placebo tablets periods from each of the 145 participants. Targeted SCFA panel profiling was performed by Metabolon in paired serum samples from 145 participants. Eight SCFAs: acetic acid (C2), propionic acid (C3), isobutyric acid (C4), butyric acid (C4), 2-methyl-butyric acid (C5), isovaleric acid (C5), valeric acid (C5) and caproic acid (hexanoic acid, C6) were quantified by liquid chromatography with tandem mass spectrometry (LC-MS/MS) [Metabolon Method TAM148: “LC-MS/MS Method for the Quantitation of Short-Chain Fatty Acid (C2 to C6) in Human Plasma and Serum”]. The serum samples were spiked with stable labeled internal standards and were homogenized and subjected to protein precipitation with an organic solvent. After centrifugation, an aliquot of the supernatant was derivatized. The reaction mixture was injected onto an Agilent 1290/AB Sciex QTrap 5500 LC-MS/MS system equipped with a C18 reversed phase ultrahigh performance liquid chromatography (UHPLC) column. The mass spectrometer was operated in negative mode using electrospray ionization (ESI). The peak area of the individual analyte productions was measured against the peak area of the productions of the corresponding internal standards. Quantitation was performed using a weighted linear least squares regression analysis generated from fortified calibration standards prepared immediately prior to each run. LC-MS/MS raw data were collected and processed using AB SCIEX software Analyst 1.6.2. Data reduction was performed using Microsoft Excel 2016.
Three levels of QC were prepared in serum by diluting with PBS and/or spiking with stock solutions to obtain the appropriate concentrations for each level. Sample analysis was carried out in a 96-well plate format containing two calibration curves and six QC samples (per plate) to monitor assay performance. Precision was evaluated using the corresponding QC replicates in the sample runs. Targeted acceptance criteria are: at least 50% of QC samples at each concentration level per analyte should be within ±20.0% of the running mean, and at least 2/3 of all QC samples per analyte should fall within ±20.0% of the corresponding running mean. QCs met acceptance criteria at all levels for all analytes. Detailed results and coefficients of variations are presented in Table S1.
Statistical Analysis
The changes in cardiovascular risks and SCFAs from slow sodium to placebo in all participants are presented as mean ± standard deviation (SD). Two-tailed paired t-test was conducted to examine the differences in variables between placebo and sodium tablets. In multiple regression models, circulating SCFA levels were square-root-transformed and standardized to unit variance and zero mean. Based on the intent-to-treat principle,17 two-level mixed-effects linear regression models were used to assess the differences in SCFAs between sodium and placebo tablets while incorporating repeated measured data and controlling for age, sex, race, and BMI as confounding variables. We further tested whether the changes in the SCFAs were associated with the changes in BPs, PPs, and cf-PWV using mixed-effects models. A p < 0.05 was considered statistically significant. All analyses were performed using Stata version 12.0 (StataCorp., College Station, Texas, USA).
Results
General Characteristics of the Participants
A total of 145 participants with serum samples available were included, with a mean age of 50.7 ± 10.7 years, mean BMI 29.1 ± 5.1 kg/m2, 34% females, 42% blacks and 19% Asians. Overall, sodium reduction was associated with lowered BPs and cf-PWV (ps<0.05), as previously reported (Table S2).12 Table 1 presents the changes in cardiovascular disease (CVD) phenotypes by sex. The results remained the same, except that the decreases in nighttime DBP and office PP were not significant in the female, and the decrease in cf-PWV was not significant in the male.
Table 1.
Changes in CVD variables from slow sodium to placebo in all participants
| Variable | Female (N=49) |
Male (N=96) |
||||
|---|---|---|---|---|---|---|
| Slow Sodium | Placebo | p | Slow Sodium | Placebo | p | |
| Urinary sodium (mmol/24 hours) | 152.3±52.4 | 95.8±40.1 | <0.001 | 177.2±60.8 | 120.3±51.2 | <0.001 |
| Office BP and pulse rate | ||||||
| SBP (mmHg) | 148.4±13.7 | 144.9±14.0 | 0.036 | 145.7±13.0 | 139.6±11.7 | <0.001 |
| DBP (mmHg) | 89.7±7.9 | 87.9±8.4 | 0.014 | 90.9±8.4 | 88.1±9.1 | <0.001 |
| MAP (mmHg) | 109.3±8.8 | 106.9±9.3 | 0.014 | 109.2±8.9 | 105.3±8.9 | <0.001 |
| Ambulatory BP | ||||||
| 24-hour SBP (mmHg) | 143.6±9.2 | 138.2±10.6 | <0.001 | 139.8±10.4 | 136.5±10.9 | <0.001 |
| 24-hour DBP (mmHg) | 84.8±8.4 | 82.7±8.7 | 0.006 | 86.9±8.7 | 85.3±8.4 | 0.002 |
| Day SBP (mmHg) | 149.1±9.7 | 143.1±11.4 | <0.001 | 146.9±10.6 | 142.8±11.3 | <0.001 |
| Day DBP (mmHg) | 89.7±9.1 | 87.3±9.3 | 0.003 | 92.7±9.6 | 90.6±9.4 | 0.001 |
| Night SBP (mmHg) | 137.0±9.7 | 132.3±11.7 | 0.001 | 131.9±11.4 | 129.3±12.3 | 0.006 |
| Night DBP (mmHg) | 79.4±8.4 | 77.7±8.9 | 0.115 | 80.3±9.1 | 79.0±9.4 | 0.047 |
| cf-PWV (m/s) | 11.7±1.9 | 11.1±1.6 | 0.002 | 11.7±2.5 | 11.3±2.0 | 0.062 |
| Pulse pressure | ||||||
| Office PP (mmHg) | 58.7±11.0 | 57.1±10.9 | 0.201 | 54.8±10.4 | 51.5±9.9 | <0.001 |
| 24-hour PP (mmHg) | 58.8±6.8 | 55.5±6.8 | <0.001 | 52.9±7.6 | 51.3±7.2 | <0.001 |
| Day PP (mmHg) | 59.4±7.9 | 55.8±7.9 | <0.001 | 54.2±8.2 | 52.3±7.7 | <0.001 |
| Night PP (mmHg) | 57.7±6.9 | 54.6±7.2 | <0.001 | 51.5±7.7 | 50.3±7.6 | 0.049 |
Effects of Sodium Reduction on the Circulating Levels of SCFAs
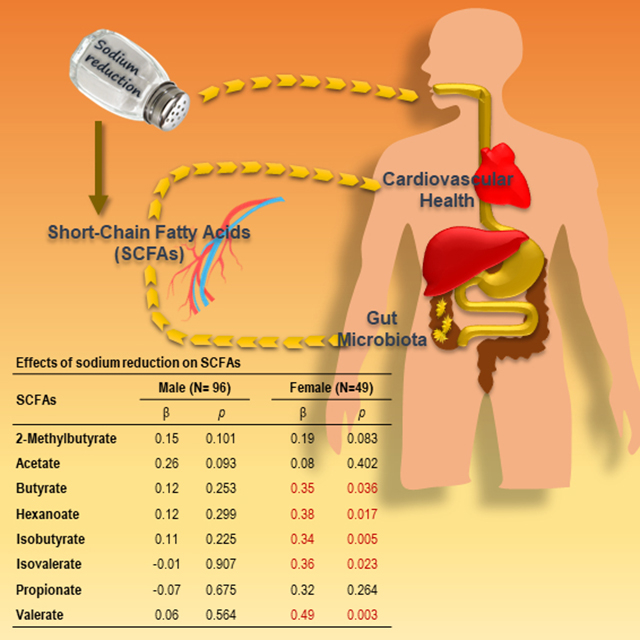
As shown in Table 2, modest sodium reduction increased the circulating levels of all the eight SCFAs, among which the increases in 2-methylbutyrate, butyrate, hexanoate, and isobutyrate were statistically significant (raw ps<0.05). Moreover, as shown in Table 3, these SCFAs remained significant after adjustment for age, sex, race, and BMI, the increase in acetate became borderline significant (p=0.065), and the increase in valerate became significant (p=0.027). Then we tested whether there was a race/ethnicity, sex or BMI interaction. Indeed, there were significant sex interactions on isovalerate and valerate (ps=0.028 for both), but not race/ethnicity nor BMI interaction (ps>0.05). The increases in butyrate, hexanoate, isobutyrate, isovalerate, and valerate induced by sodium reduction were significant in females only (ps<0.05), not in males (ps>0.05) (Table 3). Changes in SCFAs from slow sodium to placebo were presented separately by sex in Table S3 and S4.
Table 2.
Changes in SCFAs from slow sodium to placebo in all participants
| Metabolites | Slow sodium | Placebo | Changes | p-value |
|---|---|---|---|---|
| 2-Methylbutyrate (ng/mL) | 76.5 ± 35.5 | 81.5 ± 34.3 | 5.0 ± 27.8 | 0.032 |
| Acetate (ng/mL) | 1704.7 ± 1077.5 | 2320.8 ± 4862.7 | 616.1 ± 4948.1 | 0.136 |
| Butyrate (ng/mL) | 28.3 ± 15.8 | 32.8 ± 25.1 | 4.5 ± 24.6 | 0.031 |
| Hexanoate (ng/mL) | 33.5 ± 11.6 | 36.0 ± 12.3 | 2.5 ± 13.6 | 0.031 |
| Isobutyrate (ng/mL) | 50.2 ± 19.4 | 53.3 ± 18.1 | 3.1 ± 16.1 | 0.021 |
| Isovalerate (ng/mL) | 66.5 ± 31.6 | 69.7 ± 31.0 | 3.2 ± 31.1 | 0.217 |
| Propionate (ng/mL) | 39.7 ± 29.5 | 49.9 ± 54.9 | 9.2 ± 52.3 | 0.266 |
| Valerate (ng/mL) | 6.0 ± 3.5 | 6.6 ± 3.7 | 0.6 ± 4.1 | 0.065 |
Note: p-values were calculated from two-tailed paired t-test by comparing the differences in SCFAs between placebo and sodium tablets.
Table 3.
Effects of sodium reduction on serum SCFAs
| Metabolites | All |
Male (N= 96) |
Female (N=49) |
|||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| 2-Methylbutyrate | 0.16 | 0.020 | 0.15 | 0.101 | 0.19 | 0.083 |
| Acetate | 0.20 | 0.065 | 0.26 | 0.093 | 0.08 | 0.402 |
| Butyrate | 0.20 | 0.029 | 0.12 | 0.253 | 0.35 | 0.036 |
| Hexanoate | 0.21 | 0.027 | 0.12 | 0.299 | 0.38 | 0.017 |
| Isobutyrate | 0.19 | 0.009 | 0.11 | 0.225 | 0.34 | 0.005 |
| Isovalerate | 0.11 | 0.156 | −0.01 | 0.907 | 0.36 | 0.023 |
| Propionate | 0.09 | 0.563 | −0.07 | 0.675 | 0.32 | 0.264 |
| Valerate | 0.21 | 0.027 | 0.06 | 0.564 | 0.49 | 0.003 |
Note: Regression coefficients (β) and significance levels (p) of intervention were estimated using the mixed-effects model adjusted for age, sex, race, and BMI, and reflect changes in SCFAs from sodium tablets to placebo tablets.
Associations of Changes in SCFAs with Changes in Cardiovascular Phenotypes
Table 4 presents the associations between the changes in SCFAs and the changes in cardiovascular phenotypes. Increased circulating isovalerate was associated with reduced 24-hour DBP and night DBP (ps<0.05). Hexanoate was positively associated with daytime DBP (p=0.044).
Table 4.
Association between changes in serum SCFAs and changes in cardiovascular phenotypes in all participants
| Phenotypes | 2-Methyl butyrate | Acetate | Butyrate | Hexanoate | Isobutyrate | Isovalerate | Propionate | Valerate |
|---|---|---|---|---|---|---|---|---|
| Office BP | ||||||||
| SBP | 0.60 | −0.25 | 1.28 | 0.59 | 0.24 | −0.66 | 1.90 | −0.07 |
| DBP | 0.59 | 0.15 | 0.45 | 0.42 | 0.22 | −0.02 | 1.12 | −0.17 |
| MAP | 0.58 | 0.03 | 0.74 | 0.48 | 0.22 | −0.24 | 1.35* | −0.14 |
| Ambulatory BP | ||||||||
| 24-hour SBP | −0.02 | 0.33 | 0.66 | 0.42 | −0.12 | −0.74 | 0.73 | 0.28 |
| 24-hour DBP | −0.25 | −0.10 | 0.16 | 0.31 | −0.37 | −0.78* | 0.54 | 0.05 |
| Day SBP | 0.31 | 0.57 | 0.70 | 0.88 | 0.15 | −0.19 | 1.15 | 0.15 |
| Day DBP | 0.20 | 0.43 | 0.12 | 0.81* | 0.16 | −0.23 | 0.96 | 0.15 |
| Night SBP | −0.27 | 0.37 | 0.89 | 0.11 | −0.18 | −0.98 | 0.98 | 0.51 |
| Night DBP | −0.54 | −0.57 | 0.34 | 0.19 | −0.65 | −1.15* | 0.93 | 0.17 |
| cf-PWV | 0.09 | 0.01 | 0.05 | 0.03 | 0.01 | 0.08 | −0.14 | −0.13 |
| Pulse pressure | ||||||||
| Office PP | −0.33 | −0.52 | 0.85 | 0.21 | −0.28 | −0.74 | 0.78 | 0.19 |
| 24-hour PP | 0.08 | 0.32 | 0.36 | 0.06 | 0.05 | −0.08 | −0.26 | 0.15 |
| Day PP | −0.06 | 0.08 | 0.36 | 0.08 | −0.17 | −0.05 | −0.23 | −0.07 |
| Night PP | 0.22 | 0.82 | 0.46 | −0.11 | 0.36 | 0.05 | −0.10 | 0.28 |
p<0.05
Associations of Changes in SCFAs with Changes in Cardiovascular Phenotypes in Women
Since the increases in SCFAs induced by sodium reduction were significant only in females, we further examined the associations between changes in circulating SCFAs and improved CVD measurements in this gender group (Table 5). Increased isobutyrate was associated with reductions of daytime PP, and all the ambulatory BP measurements except daytime DBP (ps<0.05). Increased isovalerate was also associated with reductions of all the ambulatory BP measurements (ps<0.05) as well as office SBP and mean arterial pressure (MAP) (ps<0.05). Moreover, increased valerate was associated with decreased cf-PWV (p=0.040). Table S5 presented the R-squared values of the full models (SCFAs, age, race, and BMI) and the reduced models (age, race, and BMI) among females. Differences in R-squared values between full models and reduced models are estimations of the proportions of variances in CVD phenotypes explained by the changes in SCFAs. Increase in isobutyrate explained 4.44% of the reduction in daytime PP. Increases in isovalerate explained 7.03%, 4.19%, and 6.90% of the reduction in office SBP, DBP, and MAP, and increase in valerate explained 6.21% of the reduction in cf-PWV.
Table 5.
Association between changes in serum SCFAs and changes in cardiovascular phenotypes among the females
| Phenotypes | 2-Methyl butyrate | Acetate | Butyrate | Hexanoate | Isobutyrate | Isovalerate | Propionate | Valerate |
|---|---|---|---|---|---|---|---|---|
| Office BP | ||||||||
| SBP | −2.45 | −0.81 | 0.68 | 0.27 | −2.36 | −2.84* | 1.51 | −0.20 |
| DBP | −0.30 | −0.34 | −0.10 | −0.58 | −0.79 | −1.00 | 0.61 | −0.80 |
| MAP | −1.01 | −0.45 | 0.15 | −0.33 | −1.31 | −1.65* | 0.86 | −0.61 |
| Ambulatory BP | ||||||||
| 24-hour SBP | −2.26 | −1.97 | −0.37 | −1.02 | −2.89† | −2.50† | 0.58 | −1.38 |
| 24-hour DBP | −1.81* | −0.25 | −0.51 | 0.26 | −2.03† | −1.97‡ | 0.36 | −0.32 |
| Day SBP | −1.62 | −2.23 | −0.59 | −0.24 | −2.59* | −2.07* | 0.40 | −1.58 |
| Day DBP | −0.91 | 0.13 | −0.84 | 0.55 | −1.08 | −1.56* | 0.69 | −0.53 |
| Night SBP | −2.35 | −1.10 | 0.42 | −1.82 | −2.65* | −2.70† | 1.09 | −0.96 |
| Night DBP | −1.70 | 0.23 | 0.28 | 0.11 | −2.11* | −2.34‡ | 0.78 | 0.05 |
| cf-PWV | −0.09 | 0.22 | 0.04 | 0.07 | −0.12 | −0.04 | 0.02 | −0.28* |
| Pulse pressure | ||||||||
| Office PP | −2.44* | −0.54 | 0.78 | 0.86 | −1.78 | −1.62 | 0.90 | 0.61 |
| 24-hour PP | −0.87 | −1.84 | −0.15 | −1.24* | −1.20 | −0.50 | −0.70 | −1.07 |
| Day PP | −0.96 | −1.92 | −0.13 | −0.75 | −1.65* | −0.38 | −1.01 | −1.05 |
| Night PP | −0.81 | −1.44 | 0.02 | −1.89† | −0.77 | −0.47 | 0.09 | −1.02 |
p<0.05
p<0.01
p<0.001.
Discussion
The present study, for the first time, shows that modest dietary sodium reduction increases circulating SCFA levels in untreated hypertensive patients, supporting that dietary sodium may influence the gut microbiome in humans. There were sex differences in SCFAs’ response to sodium reduction. Moreover, the increased circulating SCFAs were associated with reduced BPs and cf-PWV.
SCFAs are a class of gut microbial metabolites and virtually all the SCFAs in the circulation are microbial in origin.18 It is well recognized that dietary intakes influence the gut microbiome.19 However, the effects of sodium intake on SCFAs, which mediate the effects of the microbiome on host physiology, have been rarely studied in humans and there are contradictory findings in animal studies. To the best of our knowledge, our study is the first to address the question that whether changes in dietary sodium intake alter circulating SCFAs in hypertensive patients. By leveraging a completed RCT, we were able to establish a causal relationship, that is modest dietary sodium reduction increased circulating SCFAs (in particular, butyrate, isobutyrate, 2-methylbutyrate, hexanoate, and valerate) in untreated hypertensives. Our findings were supported by several recent animal studies. Miranda et al. reported that 4 weeks of high-salt diet treatment altered gut microbiota composition by decreasing Lactobacillus levels and decreased luminal butyrate production in mice.9 Decreased Lactobacillus levels in the gut microbiota by high-salt diet were also observed in other studies.8,20 On the other hand, fecal SCFAs (acetate, propionate, and isobutyrate, but not butyrate) were found to be significantly elevated by high-salt diet in male Dahl salt-sensitive rats.9 Moreover, Wilck et al. reported that a high-salt challenge conducted in 12 European adults reduced intestinal survival of Lactobacillus spp., increased Th 17 cells and increased BP.8
Recent work generated from SCFA receptor null mice suggests that SCFAs play a role in BP regulation.18,21. SCFAs have an anti-inflammatory effect on both colonic epithelial and immune cells.21 SCFAs are products from the fermentative activity of gut bacteria, subsequently absorbed into the bloodstream of the host, which can bind to and activate host receptors such as olfactory receptor 78 (Olfr78) and G-protein coupled receptor 41 (Gpr41), thereby acting as a messenger between gut microbial metabolism and host physiology.11,18 Olfr78 is localized in the renal afferent arteriole, the site of renin storage and secretion. Olfr78 null mice have lowered plasma renin levels and lowered baseline BP.22 Whereas Gpr41 is localized in the vascular endothelium, the Gpr41 null mice have isolated systolic hypertension with stiffer vessels.23
There is evidence that gut microbiota and serum metabolites are different between African American and white American hypertensive patients.24 However, there was no race/ethnicity interaction with sodium reduction on circulating SCFAs in our study. We did identify a sex difference in the present study. Our results showed that the effects of sodium reduction on SCFAs were significant in females only, not in males. Modest sodium reduction significantly increased serum levels of butyrate, isobutyrate, hexanoate, isovalerate and valerate in untreated female hypertensives. Moreover, increased SCFAs by sodium reduction were associated with reduced BPs. Among the aforementioned animal studies, only male animals were used. To the best of our knowledge, our study is the only study to examine the effect of dietary sodium on SCFAs in humans. Further replications are needed. It is well recognized that there are sex differences in hypertension and CVD.25 In line with our findings, some studies found larger BP elevation by sodium loading in women than in men.26–28 A randomized clinical trial (RCT) found that there were no differences in hemodynamics after 1-week salt loading (200 mmol Na+/day) in men while women had higher 24-hour SBP, daytime SBP, nighttime SBP, nighttime DBP, and mean arterial pressure.26 The reason that women have greater sensitivity to dietary sodium intake is unclear. Several underlying mechanisms have been identified. For example, hypertensive females may have greater anti-inflammatory immune profiles.25 Whereas endothelial nitric oxide (NO) production is sensitive to dietary sodium changes in men but not women.29 Recently, sex differences in gut microbiota composition have been observed in several human studies.30–32 Our findings suggest a novel mechanism that changes in gut microbiota composition leading to changes in circulating SCFAs may underlie the BP responses to dietary sodium reduction in females, but not in males. Other mechanisms, e.g. endothelial NO pathway may play a role in salt-sensitive hypertension in males. Sex differences in SCFA response to sodium reduction warrant further investigation.
We found that increased circulating levels of isobutyrate and isovalerate by sodium reduction were associated with decreased BPs in untreated female hypertensives. Gomez-Arango et al. observed that in overweight and obese pregnant women at 16 weeks gestation, the abundance of butyrate-producing bacteria and butyrate production in the gut microbiota is significantly negatively associated with BPs.33 In animal studies, SCFAs are also found to be negatively associated with BP. In rats, the infusion of acetate into the cecum prevented obstructive sleep apnea-induced hypertension.34 Propionate significantly attenuated cardiac hypertrophy, fibrosis, vascular dysfunction, and hypertension in atherosclerosis mice model.35 However, de la Cuesta-Zuluaga et al. found that higher fecal acetate, propionate, and butyrate were associated with higher BP in humans.36 Huart et al. also found higher stool levels of acetate, butyrate, and propionate in hypertensive versus normotensive individuals in men.37 The inconsistency of the findings between these studies and ours may be due to the difference in specimens used. Both de la Cuesta-Zuluaga and Huart used fecal SCFA excretion, which cannot be equated to SCFA production or absorption, and the association of higher fecal SCFA concentrations with obesity and cardiometabolic dysregulation may be due to less efficient absorption and utilization of these metabolites.36 A human study showed that the participants with lower fecal acetate tended to have higher acetate absorption.38 A recent study suggested that SCFAs measured in circulation might better represent SCFA absorption.36 Future studies assessing both fecal and circulating SCFAs are needed.
The strengths of our study include that we leveraged a randomized, double-blind, placebo-controlled, cross-over trial of dietary sodium reduction with well-characterized CVD phenotypes. The cross-over RCT design enables each participant to serve as his/her own control, diminishing the inter-person variations. However, our study is limited by its relatively modest sample size and the lack of an independent replication sample. Moreover, it was a post-hoc analysis of an RCT, which was not originally designed to test the effect of sodium reduction on SCFAs; therefore the causal relationships between dietary sodium-driven changes in SCFAs and BP/cf-PWV cannot be established. Future RCTs are needed.” Last not the least, raw p-values were reported without multiple testing correction due to the modest sample size. Evidence shows that gut microbiota is different between hypertensive and normotensive individuals.36 Our findings that sodium reduction increased circulating SCFAs were based on hypertensive patients. Whether circulating SCFAs have the same response to sodium reduction among normotensive population is unknown. Future replications in large scale RCTs including both hypertensive and normotensive individuals are warranted.
In conclusion, our results for the first time show that dietary sodium reduction increases the circulating levels of SCFAs among untreated hypertensive individuals, supporting the concept that dietary sodium may influence the gut microbiome in humans.8 In addition, there was a sex difference in SCFA response to sodium reduction. Moreover, increased circulating SCFAs are associated with decreased BPs and improved arterial compliance.
Perspectives
Gut microbiota is involved in BP regulation and hypertension. SCFAs are products from the fermentative activity of gut bacteria. Our data show that moderate sodium reduction increases serum SCFAs, which are also associated with reduced BP and arterial stiffness. High dietary sodium intake may play a role in gut microbiota dysbiosis.
Supplementary Material
Novelty and Significance.
What Is New?
Dietary sodium reduction increases circulating short-chain fatty acids (SCFAs), suggesting that dietary sodium may influence the gut microbiome in hypertensives given that almost all circulating SCFAs are microbial in origin.
There is a sex difference in SCFA response to sodium reduction, the increases are statistically significant only in females.
Increased circulating SCFAs by sodium reduction are further associated with decreased blood pressure (BP), and improved arterial compliance in untreated hypertensive patients.
What Is Relevant?
Animal studies suggest that microbiome is a key linkage in the causal relationship between sodium intake and elevated BP. Our study provides the evidence in hypertensives.
SCFAs, the gut microbial metabolites, play a role in BP regulation in SCFA receptor null mice. Our study shows that changes in circulating SCFAs are associated with changes in BP in hypertensives.
The sex difference of SCFA in response to sodium reduction observed in our study may contribute to the understanding of sex differences in hypertension and CVD.
Acknowledgments
Sources of Funding
The original study was in part funded by the United Kingdom Food Standards Agency (N02034). The current study was in part funded by the American Heart Association (16GRNT31250002 to HZ).
Footnotes
Disclosures
FJH is a member of Consensus Action on Salt & Health (CASH) and World Action on Salt & Health (WASH). Both CASH and WASH are non-profit charitable organizations and FJH does not receive any financial support from CASH or WASH.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00152074.
References
- 1.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS data brief. 2017(289):1–8. [PubMed] [Google Scholar]
- 2.Cook NR, Appel LJ, Whelton PK. Lower Levels of Sodium Intake and Reduced Cardiovascular Risk. Circulation. 2014;129(9):981–989. doi: 10.1161/Circulationaha.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378(9789):380–382. doi: 10.1016/s0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 4.Toral M, Robles-Vera I, de la Visitacion N, Romero M, Yang T, Sanchez M, Gomez-Guzman M, Jimenez R, Raizada MK, Duarte J. Critical Role of the Interaction Gut Microbiota - Sympathetic Nervous System in the Regulation of Blood Pressure. Front Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):403–409. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47(6):187–197. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65(6):1331–1340. doi: 10.1161/hypertensionaha.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y, Leibowitz A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018;10(9). doi: 10.3390/nu10091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, Verdu EF, Collins SM, Bercik P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6(1):57. doi: 10.1186/s40168-018-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Current hypertension reports. 2017;19(4):25–25. doi: 10.1007/s11906-017-0722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;54(3):482–488. doi: 10.1161/hypertensionaha.109.133223. [DOI] [PubMed] [Google Scholar]
- 13.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56(2):253–259. doi: 10.1161/hypertensionaha.110.155747. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium Reduction, Metabolomic Profiling, and Cardiovascular Disease Risk in Untreated Black Hypertensives. Hypertension. 2019:Hypertensionaha11912880. doi: 10.1161/hypertensionaha.119.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium Reduction, miRNA Profiling and CVD Risk in Untreated Hypertensives: a Randomized, Double-Blind, Placebo-Controlled Trial. Sci Rep-Uk. 2018;8(1):12729. doi: 10.1038/s41598-018-31139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest Salt Reduction Lowers Blood Pressure and Albumin Excretion in Impaired Glucose Tolerance and Type 2 Diabetes Mellitus: A Randomized Double-Blind Trial. Hypertension. 2016;67(6):1189–1195. doi: 10.1161/hypertensionaha.115.06637. [DOI] [PubMed] [Google Scholar]
- 17.Xi W, Pennell ML, Andridge RR, Paskett ED. Comparison of intent-to-treat analysis strategies for pre-post studies with loss to follow-up. Contemporary clinical trials communications. 2018;11:20–29. doi: 10.1016/j.conctc.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raizada MK, Joe B, Bryan NS, et al. Report of the National Heart, Lung, and Blood Institute Working Group on the Role of Microbiota in Blood Pressure Regulation: Current Status and Future Directions. Hypertension. 2017. doi: 10.1161/hypertensionaha.117.09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, Huang Z, Yu K, Ding R, Ye K, Dai C, Xu X, Zhou G, Li C. High-Salt Diet Has a Certain Impact on Protein Digestion and Gut Microbiota: A Sequencing and Proteome Combined Study. Front Microbiol. 2017;8:1838. doi: 10.3389/fmicb.2017.01838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pevsner-Fischer M, Blacher E, Tatirovsky E, Ben-Dov IZ, Elinav E. The gut microbiome and hypertension. Curr Opin Nephrol Hypertens. 2017;26(1):1–8. doi: 10.1097/mnh.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 22.Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. P Natl Acad Sci USA. 2013;110(11):4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiological Genomics. 2016;48(11):826–834. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walejko JM, Kim S, Goel R, Handberg EM, Richards EM, Pepine CJ, Raizada MK. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. Int J Cardiol. 2018;271:336–339. doi: 10.1016/j.ijcard.2018.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillis EE, Sullivan JC. Sex Differences in Hypertension: Recent Advances. Hypertension. 2016;68(6):1322–1327. doi: 10.1161/hypertensionaha.116.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvarajah V, Mäki-Petäjä KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB. Novel Mechanism for Buffering Dietary Salt in Humans: Effects of Salt Loading on Skin Sodium, Vascular Endothelial Growth Factor C, and Blood Pressure. Hypertension (Dallas, Tex : 1979). 2017;70(5):930–937. doi: 10.1161/HYPERTENSIONAHA.117.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, Chen JC, Duan X, Huang JF, Chen CS, Kelly TN, Bazzano LA, Whelton PK. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overlack A, Ruppert M, Kolloch R, Gobel B, Kraft K, Diehl J, Schmitt W, Stumpe KO. Divergent hemodynamic and hormonal responses to varying salt intake in normotensive subjects. Hypertension. 1993;22(3):331–338. doi: 10.1161/01.hyp.22.3.331. [DOI] [PubMed] [Google Scholar]
- 29.Eisenach JH, Gullixson LR, Kost SL, Joyner MJ, Turner ST, Nicholson WT. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol (1985). 2012;112(6):1049–1053. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haro C, Rangel-Zúñiga OA, Alcalá-Díaz JF, Gómez-Delgado F, Pérez-Martínez P, Delgado-Lista J, Quintana-Navarro GM, Landa BB, Navas-Cortés JA, Tena-Sempere M, Clemente JC, López-Miranda J, Pérez-Jiménez F, Camargo A. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PloS one. 2016;11(5):e0154090–e0154090. doi: 10.1371/journal.pone.0154090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Applied and environmental microbiology. 2006;72(2):1027–1033. doi: 10.1128/aem.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominianni C, Sinha R, Goedert JJ, Pei Z, Yang L, Hayes RB, Ahn J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PloS one. 2015;10(4):e0124599. doi: 10.1371/journal.pone.0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68(4):974–981. doi: 10.1161/hypertensionaha.116.07910. [DOI] [PubMed] [Google Scholar]
- 34.Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr., Durgan DJ. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension. 2018;72(5):1141–1150. doi: 10.1161/HYPERTENSIONAHA.118.11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolomaeus H, Balogh A, Yakoub M, et al. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139(11):1407–1421. doi: 10.1161/CIRCULATIONAHA.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, Carmona JA, Abad JM, Escobar JS. Higher Fecal Short-Chain Fatty Acid Levels Are Associated with Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients. 2018;11(1):51. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, Krzesinski JM, Melin P, de Tullio P, Jouret F. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension. 2019:Hypertensionaha11812588. doi: 10.1161/hypertensionaha.118.12588. [DOI] [PubMed] [Google Scholar]
- 38.Vogt JA, Wolever TM. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133(10):3145–3148. doi: 10.1093/jn/133.10.3145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


