Abstract
To investigate a potential contribution of systemic physiology to recently reported BOLD fMRI signals in white matter, we compared photo-plethysmography (PPG) and whole-brain fMRI signals recorded simultaneously during long resting-state scans from an overnight sleep study. We found that intermittent drops in the amplitude of the PPG signal exhibited strong and widespread correlations with the fMRI signal, both in white matter (WM) and in gray matter (GM). The WM signal pattern resembled that seen in previous resting-state fMRI studies and closely tracked the location of medullary veins. Its temporal cross-correlation with the PPG amplitude was bipolar, with an early negative value. In GM, the correlation was consistently positive. Consistent with previous studies comparing physiological signals with fMRI, these findings point to a systemic vascular contribution to WM fMRI signals. The PPG drops are interpreted as systemic vasoconstrictive events, possibly related to intermittent increases in sympathetic tone related to fluctuations in arousal state. The counter-intuitive polarity of the WM signal is explained by long blood transit times in the medullary vasculature of WM, which cause blood oxygenation loss and a substantial timing mismatch between blood volume and blood oxygenation effects. A similar mechanism may explain previous findings of negative WM signals around large draining veins during both task- and resting-state fMRI.
Introduction
Blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) is widely used to study the brain’s activity and functional connectivity. Since the BOLD fMRI signal results from a local blood flow response to changes in synaptic activity, it is localized primarily to gray matter (GM) or to nearby downstream venous vasculature (Turner, 2002; Logothetis and Wandell, 2004), while in the less vascularized white matter (WM), BOLD signal is generally negligible. Therefore, inference of functional connectivity with fMRI has been based on temporal correlations between the activity profiles of GM regions. Yet, this indirect interpretation of functional connectivity does not consider the actual signal conduction pathways through WM fibers that enable the temporal correlation and as a result may be incomplete or inaccurate. Sensitive detection of WM BOLD signal, therefore, would be of great utility resolving ambiguities when GM signal correlation may be mediated by multiple functional pathways.
A number of recent fMRI studies have reported the detection of BOLD signal in WM, both in response to behavioral tasks (Fraser et al., 2012; Tettamanti et al., 2002; Gawryluk, et al., 2011a,b; Gawryluk et al., 2014a; Mazerolle et al., 2010; Mazerolle et al., 2013; McWhinney et al., 2012), as well as during rest (Ding et al., 2013, 2016; Marussich et al., 2017) (for review see (Gawryluk et al., 2014b)). While these results appear promising, WM signals are generally small, their occurrence is sporadic, and their origin is poorly understood. In this regard, given the low synaptic and vascular densities of WM, and the low metabolic demands of axonal signaling (Harris and Attwell, 2012; Logothetis and Wandell, 2004), it is unclear how robust a WM signal one would expect in response to brain activity changes.
One particular concern is that a portion of WM signal may result from variations in systemic physiology. Several resting-state studies have reported a WM correlation with venous blood oxygenation and total hemoglobin, either measured from the fingertip with photoplethysmography (PPG) or near-infrared spectroscopy (NIRS), or from the sagittal sinus with fMRI (see e.g. (van Houdt et al., 2010; Tong et al., 2013; Tong et al., 2017)). The observed correlation patterns strikingly resemble patterns previously attributed to WM activity observed during rest (Ding et al., 2013, 2016; Marussich et al., 2017), in which highest correlations were seen in deep white matter bordering the ventricles. In these same regions, WM signals anti-correlating with GM signals have been found under respiratory challenges (Bright et al., 2014), further indicating a potential systemic contribution. Periventricular WM is also known to contain the highest density of medullary veins that drain the deep WM, and therefore would be highly susceptible to systemic changes in vascular tone and blood oxygenation.
Despite this potential confound to WM activity, the mechanistic understanding of how hemodynamic effects explain the observed systemic correlations remains incomplete. For example, during the resting state, signals in WM were shown to have a delayed positive correlation with GM (Erdogan et al., 2016), an observation that has been attributed to a longer vascular transit time in WM (Tong et al., 2017). Delayed effects in WM have also been observed during respiratory CO2 challenges (Champagne et al., 2017; Thomas et al., 2014). At the same time, WM and GM have also been found to show opposing correlations with a peripherally measured (fingertip) PPG and NIRS signals (van Houdt et al., 2010; Tong et al., 2013), as well as with respiratory challenges (Bright et al., 2014). However, the link between these findings, while important for a mechanistic understanding and interpretation of the WM signal, has not yet been made.
To address this, we performed a detailed analysis of concurrently acquired PPG and fMRI data from an overnight sleep study. Included in the analysis was a small portion of data containing segments with a mixture of relaxed rest, drowsiness, and light sleep, conditions known to be associated with frequent and intermittent systemic variations in vascular tone. These characteristics allowed a precise analysis of the spatiotemporal characteristics of WM and GM signals, facilitating a mechanistic interpretation of the potential systemic contributions to WM fMRI activity.
Methods
Data from eight subjects participating in an overnight fMRI sleep study were analyzed. In this study, subjects were scanned for 2 consecutive nights under an IRB-approved protocol after having given written informed consent. During the fMRI scans, we concurrently acquired EEG, PPG and respiration (chest belt) data. Using EEG based sleep scoring, we excluded epochs of deep sleep and REM sleep from the analysis. After further data selection (outlined below), we then investigated the relationship between the amplitude of the PPG signal and the fMRI signal in GM and WM using lag-dependent correlation analysis.
Volunteer selection
Since the MRI scanner environment is not conducive to sleep, rigorous screening procedures were implemented to select volunteers with high probability of completing the study, and procedures were devised to help them reproduce their typical, healthy sleep patterns in the scanner.
We performed at-home screening using psychometrically validated questionnaires to increase the probability of sleeping in the scanner. Risk of airway obstruction, apnea, insomnia, claustrophobia, and back discomfort were among the measurements included in the assessments. Each volunteer maintained about 8 h of sleep (ca. 23:00–07:00) for 14 days prior to the visit. In order to acclimate subjects to the noise in the scanner environment, they were also asked to listen to a recording of the MRI acoustic noise for three consecutive days prior to the visit. Further details of the methods and experimental design have been previously presented as a conference proceeding (Moehlman et al., 2017).
BOLD fMRI and EEG data acquisition
The fMRI data were acquired on a 3 T MRI system (Skyra, Siemens, Erlangen, Germany) with a Siemens 20-channel receive array. For data acquisition, we used a slightly modified Siemens product EPI pulse sequence (ep2d_bold) that included generation of a single transistor-transistor-logic (TTL) trigger pulse per acquired volume. This trigger pulse was recorded with the EEG/ECG and PPG data for synchronization purposes. EPI scan parameters were: flip angle = 90°, TR 3 s, TE = 36 ms, in-plane resolution = 2.5 mm, slice thickness = 2 mm, slice gap = 0.5 mm, matrix size = 96 × 70 × 50, 2-fold GRAPPA undersampling.
The subjects were asked to remain still in a supine position and instructed to keep eyes open while awake (before they fell asleep), and were allowed to have brief breaks to get up, stretch, and use the bathroom between scans. Noise-cancelling headphones were used (OptoActive, OptoAcoustics, Mazor, Israel), and the scanner room was kept dark. In addition to fMRI and PPG, various other signals were acquired, including respiratory effort (measured with a chest belt), electroencephalography (EEG), electrocardiography (ECG), and video of the eyes and body.
EEG and ECG were acquired concurrently with the fMRI with a 64-channel EEG system (BrainAmp, Brain Products GmbH, Germany) featuring 61 EEG scalp electrodes, 2 electro-oculography electrodes, and 1 ECG electrode. The system was synchronized to the MRI system clock. EEG channels were referenced to the frontal-central midline (FCz) electrode and sampled at 5 kHz.
PPG and chest belt signals
PPG and chest belt signals were recorded with a Biopac system (Biopac, Goleta, CA, USA, using TSD200-MRI and TSD221-MRI transducers, an MP 150 digitizer sampling at 1000 Hz, and AcqKnowledge software). For synchronization purposes, MRI scanner triggers were co-recorded with the physiological signals.
The transducer recording the PPG signal was placed on the left index finger. As this transducer is sensitive to wavelengths above 800 nm, it predominantly measures total hemoglobin content in the vasculature of the skin; thus, the amplitude of the PPG signal reflects blood volume and its pulsatile variations with the cardiac cycle (Shelley, 2007). In this regard, it is similar to the total hemoglobin measurement provided with NIRS.
Data selection and analysis
A total of sixteen healthy volunteers (9 females, age-range 20–32) were scanned with the above protocol. A subset of data was selected for analysis based on the following criteria: time segments without large subject motion (translation less than 3 mm, rotation less than 3°), no REM-sleep and a minimal amount (less than 1 min) of slow-wave (deep) sleep (see section EEG-based sleep scoring), and high quality PPG data with a clearly visible heartbeat. Around 200 resting state scans were acquired from the 16 subjects during the overnight sleep experiments, and 20% of those passed the selection criteria. We further limited data selection based on the cleanest PPG data, and accordingly, segments (>100 TRs) from 8 fMRI scans, each from a different subject, were analyzed in the current study. Exploratory analysis of the PPG data showed substantial variation in amplitude, consistent with previous reports of variations in NIRS amplitude (Tong et al., 2013, 2015). In addition, and also reported earlier (Delessert et al., 2010; Adler et al., 2013), segments with intermittent drops were observed against an otherwise stable background (see example in Fig. 1 (top)).
Fig. 1.
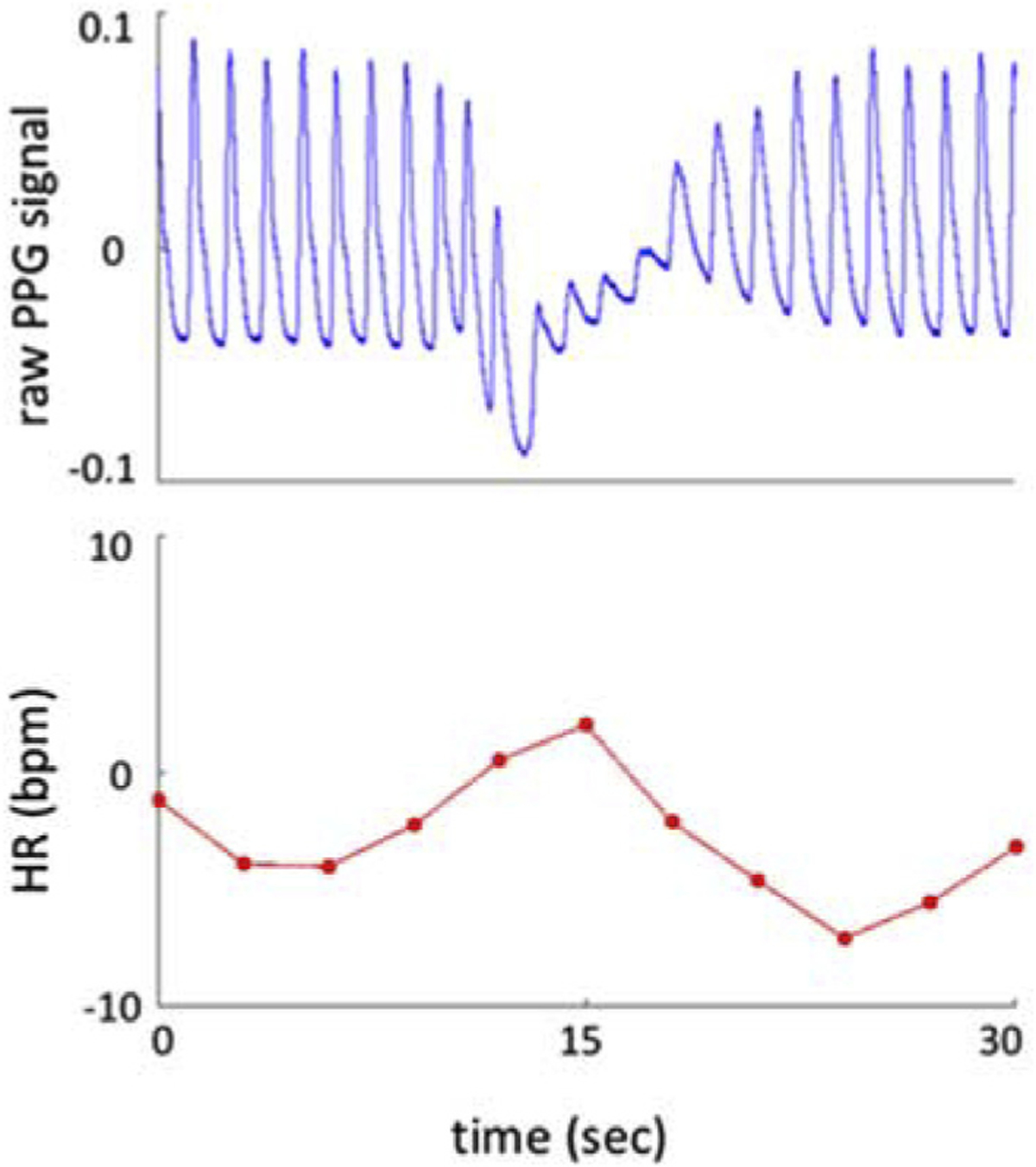
Prototypical amplitude drop in PPG signal (blue, top) and associated heart rate change (deviation around the mean heart rate; red, bottom).
fMRI preprocessing
Preprocessing of fMRI data was performed with the Analysis of Functional NeuroImages (AFNI) software (https://afni.nimh.nih.gov/afni) as follows (routine names indicated in parentheses). Motion correction (six-parameter rigid body correction with 3dvolreg) was followed by masking out voxels outside the brain (3dautomask). Correction of residual motion (3dDetrend) was performed by regressing out the motion parameters from the first registration step together with their first derivatives. Low-frequency polynomials were regressed out to remove slow artefactual drifts, where the order of the highest-order polynomial was proportional to the length L of the run (in seconds) and equal to L/150. Finally, slice-timing correction was applied to time series data (3dTshift).
EEG-based sleep scoring
As the relationship between physiology and neural activity is expected to depend on arousal state (Catcheside et al., 2002; Monstad and Guilleminault, 1999), we chose here to focus on non-REM and light sleep stages. For this purpose, sleep-scoring of the EEG data was performed after correction of MRI gradient artifacts and cardio-ballistic artifacts. Gradient artifact correction was performed with the average artifact subtraction technique (Allen et al., 2000), and the EEG data were down sampled to 250 Hz. Cardio-ballistic artifacts were removed with a two-step procedure: first, a template subtraction method was applied, and secondly, components related to artifacts were further eliminated from the EEG data via an inverse Independent Component Analysis (ICA) approach (Liu et al., 2012). The EEG signals were then band-pass filtered from 0.3 to 35 Hz. Sleep stages were manually scored with Analyzer in 30 s epochs based on the signal of the C3 electrode referenced to A2 and according to standard criteria (Iber et al., 2007). Percentages of sleep stages corresponding to time segments used in the current study are given in Table 1.
Table 1.
Details of fMRI data used for the primary analysis. For each subject, shown here are: (1) the total length of the selected scan (in units of TR = 3 s); (2) the length of the segment, within the associated scan, that was used for correlation analysis; and (3) and the distribution of sleep stages within the selected segments, expressed as a percentage of the total segment length. W: Wake, S1: Stage-1, S2: Stage-2, S3/4: deep sleep.
| Subject | Total scan length (TR) | Length of segment selected for correlation analysis (TR) | Distribution of sleep stages within segments (%) | |||
|---|---|---|---|---|---|---|
| W | S1 | S2 | S3/S4 | |||
| S1 | 1230 | 151 | 9 | 4.5 | 82 | 4.5 |
| S2 | 846 | 151 | 6 | 11 | 83 | - |
| S3 | 499 | 151 | - | - | 100 | - |
| S4 | 275 | 151 | 15 | 10 | 70 | 5 |
| S5 | 476 | 111 | - | 100 | - | - |
| S6 | 876 | 151 | - | 67 | 33 | - |
| S7 | 1880 | 101 | 21 | - | 79 | - |
| S8 | 486 | 151 | - | 6 | 94 | - |
Analysis of PPG and chest belt signals
Heart rate (HR), PPG amplitude (AMP), and respiratory volume (RV) were derived from the PPG and chest belt signals. PPG signals were aligned with fMRI data according to Biopac-recorded MR triggers and bandpass-filtered (between 0.5 and 2 Hz) to facilitate peak detection. A minimum inter-beat distance of 600 ms was used to prevent the spurious detection of smaller peaks that may occur in close proximity to a larger local peak as a result of premature ventricular contractions (Namtvedt et al., 2011). Following linear interpolation of the PPG signal, PPG amplitudes at each MR trigger time were extracted. The HR time series was computed by averaging the differences between pairs of adjacent heartbeats contained in the 6 s window around each MR trigger pulse and dividing the result by 60 to convert it to units of beats-per-minute (bpm) (for further details, please refer to (Chang et al., 2009)). Note that the mean HR was then subtracted in the display of HR time series throughout the paper. As a measure of respiration volume we calculated the standard deviation of the respiratory waveform using a sliding window of 6 s centered at each MR trigger (RV, (Chang et al., 2009)).
Lag dependent correlation analysis
Lag dependent correlation analysis between PPG amplitude and fMRI signals was performed on a voxel-by-voxel and region-of-interest (ROI) basis. For this purpose, PPG data with at least 100 fMRI volumes (100 TRs, or 300 s) and containing isolated drops in PPG amplitude were selected for each subject (see Table 1), as this would facilitate the investigation of temporal dynamics of the relationship with WM and GM fMRI signals. Correlations at several time shifts (lags) were examined, with correlations at positive lags (rightward shift of the physiological data before correlation) indicating a delayed effect of a change in physiology on the fMRI signal. For the ROI-based analysis, we averaged the fMRI signals within a spatial mask generated per subject from thresholding the maximum and minimum level of correlation at each voxel over a lag range of −9 s (−3 TR) to 9 s (3 TR) (see Fig. 5 for illustration).
Fig. 5.

a) Correlation (corr) maps between each voxel and the PPG amplitude across a lag range of −9 to 9 s (−3 to 3 TR), shown for a single subject (subject 1). Upper panel shows the maximum positive correlation value at each voxel (thresholded with r ≥ 0.1 to define the GM ROI used in panel b), and the lower panel shows the minimum negative correlation values (thresholded with r<=−0.1 to define the WM ROI used in panel b). b) Group average lag-dependent correlation values over a longer period (−30 to 30 s), based on GM and WM ROIs. Lines with x-symbols give mean over subjects, while shaded areas illustrate standard deviations (n = 8). Correlation values at extrema (arrows) are indicated together with the lag value.
As a complementary approach for ROI analysis, and in analogy to previous work (see e.g. (Tong et al., 2013)), we also subjected a segment of exemplary fMRI data (Subject 1, length of 251 TRs), to Independent Component Analysis (ICA) with 20 components (data spatially smoothed with Gaussian kernel of 3mm full width at half maximum (FWHM), MELODIC (Beckmann and Smith, 2004), part of FMRIB’s Software Library (FSL), www.fmrib.ox.ac.uk/fsl). The temporal ICA components where subsequently compared to the PPG amplitude signal by correlation analysis.
Results
A prototypical instance of an amplitude drop in the raw PPG signal is shown in Fig. 1. Over the course of about 10 s, a temporary drop in PPG amplitude is observed, that typically co-occurs with a biphasic heart rate change of up to 15 beats per minute. Following each drop, a return to a relatively stable oscillation was observed at approximately the pre-event amplitude. The occurrence rate of these drops was highly variable, reaching a maximum of about 5 per minute. To have a rough estimate of the rate, we calculated thresholds for each subject’s PPG segment by subtracting one standard deviation from the mean of the detected peak amplitudes, and counted the number of dips lower than the segment’s threshold. On average, we observed 18 ± 4 dips per 10 min, with a 54% ± 7% of PPG AMP drop from the mean within the selected segments of data. Combined, these physiological changes strongly resemble those previously attributed to sympathetic modulation of heart rate and vascular tone during arousal transitions (see e.g. (Catcheside et al., 2002; Ackner and Pampiglione, 1957)), consistent with the behavioral state of the subject during our experiments.
Examples of the relationship between physiological signals is given in Fig. 2 for three subjects. Subject average (n = 8) maximum positive correlation between PPG AMP and the whole brain fMRI signal was 0.54 ± 0.19 at lag of two TRs (6 s). Correlating AMP and with HR and RV showed negative extrema at 0 lag of −0.28 ± 0.19 and −0.37 ± 0.21, respectively. Correlation of HR and RV with fMRI signal were 0.34 ± 0.15 at 0 lag, and −0.36 ± 0.22 at lag of 3 TRs, respectively. Subject average peak-to-peak BOLD signal changes were 2.17% ± 0.39%.
Fig. 2.
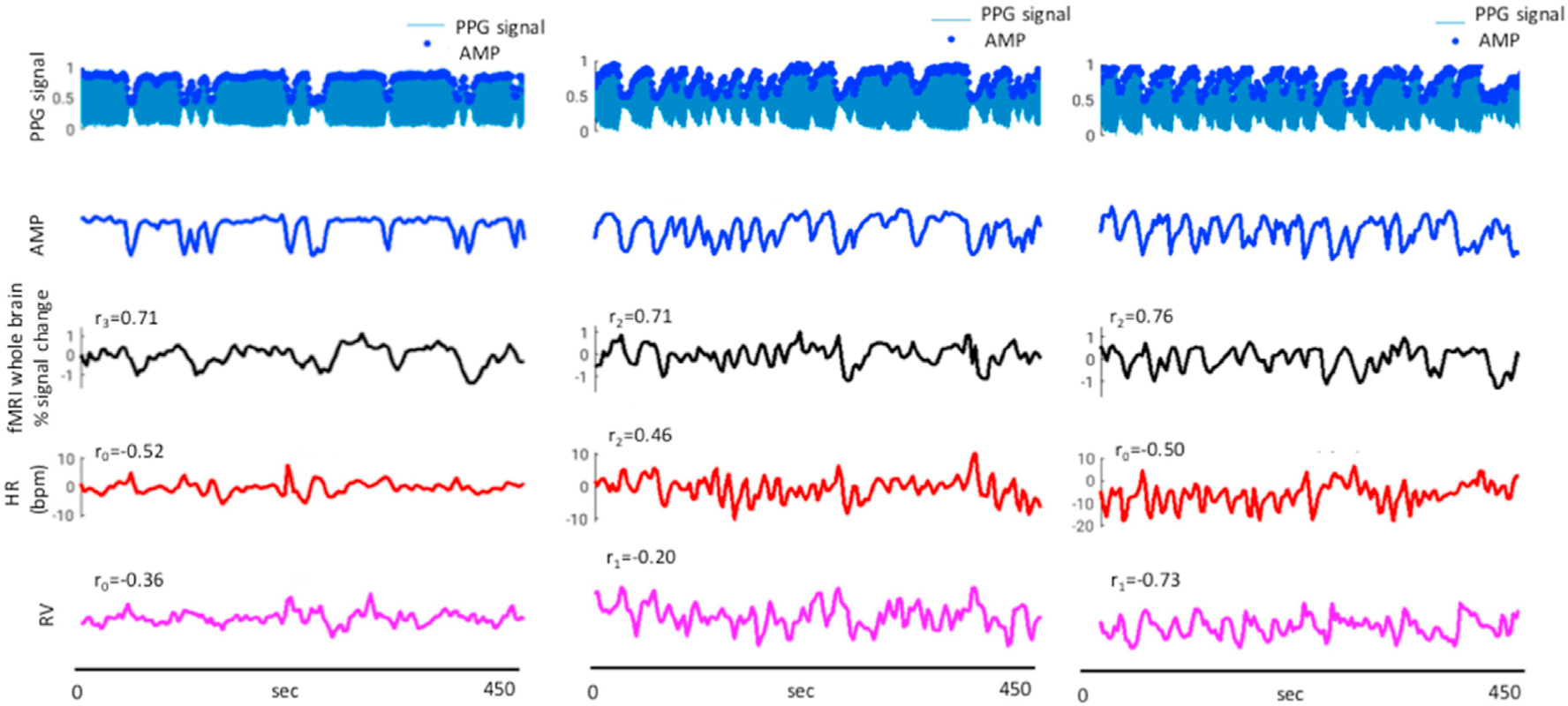
Physiological and whole brain fMRI signal fluctuations from 3 subjects. Top to bottom: normalized PPG signal (blue) with blue dots showing extracted amplitudes (AMP), mean % signal change in whole brain, HR (bpm, zero mean, red line) and RV (pink line). Correlations between PPG AMP and % fMRI signal change, HR and RV are given for each subject’s segment. The indicated correlation values correspond to the maximum absolute value among −3 TR to +3 TR time-lags (−9 to +9 s).
We further calculated voxel-wise correlations of PPG AMP and fMRI signals. The 0-lag correlation clearly shows the antipolar characteristics of the correlation between GM and WM (Fig. 3 displays the mean map over 8 subjects for a selected set of slices). This individual subjects’ maps are provided in Fig. SI1. As can be seen from Fig. 3, negative values dominate in the white matter, whereas positive values dominate in the gray matter. The strongest negative correlation values are seen in periventricular WM.
Fig. 3.

Exemplary EPI magnitude images from Subject 1, and subject-averaged (n = 8) correlation between the PPG amplitude (AMP) and fMRI at 0-lag in 6 axial slices. Positive r indicates decrease of fMRI signal during PPG AMP dip.
For comparison with previous studies of WM signals during rest, we also compared ICA-derived fMRI temporal components with PPG AMP for one subject. The components having the three strongest positive and negative temporal correlations with AMP are given in Fig. SI2, again showing that the deep WM had the strongest negative correlation.
In the absence of PPG AMP drops, the correlation between AMP and fMRI was generally low. This is exemplified in Fig. SI3, where a time segment with relatively stable AMP was examined. Antipolar correlation patterns, as well as the distinction between GM and WM effects, are virtually absent in this example.
To further investigate the temporal characteristics of the fMRI-AMP relationship, the lag dependence of the correlation was examined, both at voxel (Fig. 4) and ROI level (Fig. 5). Consistent patterns were observed across subjects, with a striking negative correlation observed in white matter and vein/ventricular regions at 0-lag, and a delayed positive correlation in the same regions (Fig. 4). Plots of the ROI-averaged temporal behavior of the cross-correlations in WM (Fig. 5) further accentuate this: in contrast to the exclusively positive correlation seen in GM, WM has a clearly biphasic correlate, whose initial negative peak precedes the positive peak seen in GM.
Fig. 4.
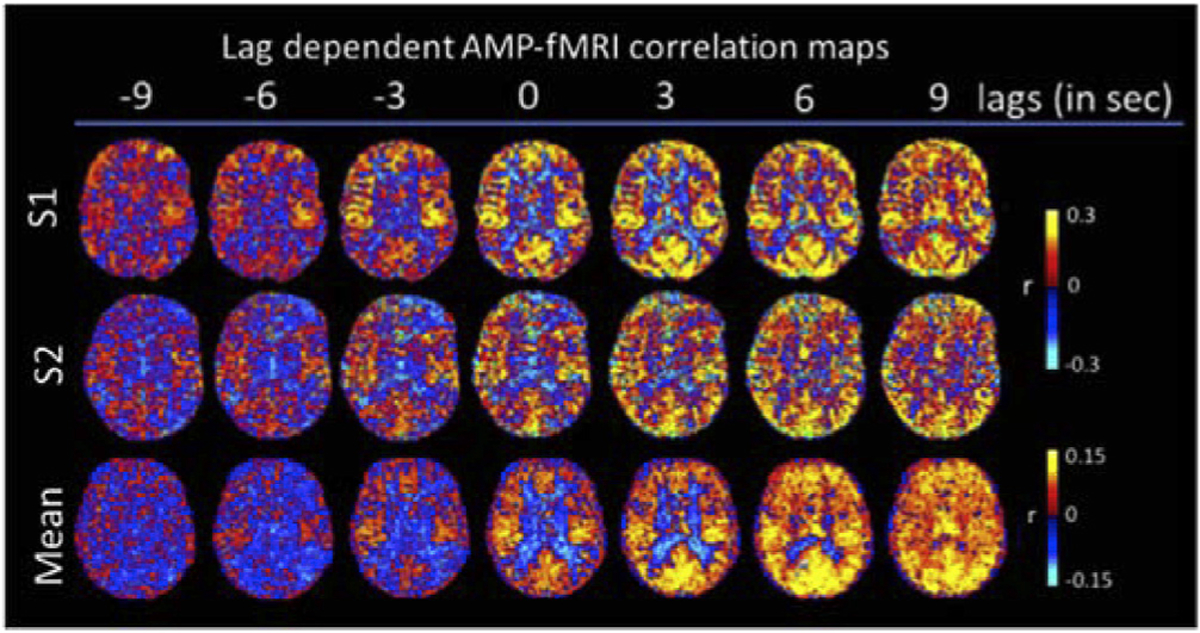
Subject level and averaged (n = 8) lag-dependent correlation, with lag varying from −3 TR (−9 s) to 9 TR (+9 s). Positive lags indicate a delayed fMRI effect relative to PPG AMP dip. Positive r indicates decrease of fMRI signal during PPG AMP dip.
Discussion
General comments
In order to investigate the contribution of systemic physiological variations to BOLD fMRI signal in WM and to clarify their nature and potential origin, we studied the relationship between PPG amplitude, a peripheral measure of vascular tone, and fMRI acquired during drowsy resting conditions and light sleep. For this purpose, we analyzed data from an overnight sleep study, acquired during drowsiness and light sleep, conditions conducive to strong, spontaneous and intermittent fluctuations in systemic physiology and arousal state. This intermittency facilitates interpretation of the temporal relationship between PPG AMP and the fMRI signal.
We observed a consistent relationship between systemic variations and the BOLD fMRI signal. Specifically, the PPG signal, an indicator of peripheral vascular tone, showed strong amplitude variations that were generally aperiodic and brief (~5–10 s). The covariation of fMRI signal and PPG AMP resulted in BOLD effects reaching about 2% of the baseline amplitude, which is in the range of GM activity levels evoked during typical fMRI experiments (Kruger et al., 2001). In our data, the level of covariation with PPG AMP often exceeded correlations with more conventional physiological measures such as heart rate and respiratory volume (Shmueli et al., 2007; Birn et al., 2006). Notably, the WM and GM correlates of PPG AMP drops had distinctly different lag dependencies, and at short lags had opposing polarities: In WM, PPG AMP drops were associated with a rapid negative and delayed positive fMRI correlation, corresponding to a positive and a negative BOLD signal change, respectively. Interestingly, the (negative) correlation in WM appeared to precede the PPG AMP signal (Fig. 5b) which may be related to the relatively slow conduction velocity (Guyton and Hall. 1996) of the (non-myelinated) sympathetic nervous innervation of the skin vasculature and the long pathway of this innervation to the finger.
Typically, in fMRI, only the pulsatile component of the PPG signal is analyzed and used to correct for pulsation artifacts and variations in heart rate (Glover et al., 2000; Chang et al., 2009). On the other hand, the (low-pass filtered) amplitude of the PPG signal and the variation in pulse-height, which are both indicators of vascular tone, are generally not considered in fMRI. Nevertheless, a previous study has shown that a PPG amplitude based regressor can explain unique variations in the fMRI signal beyond what is explained with cardiac rate and respiratory volume regressors (van Houdt et al., 2010).
The correlation between fMRI and PPG AMP is consistent with previous studies comparing peripheral indicators of vascular tone using PPG and NIRS with fMRI during rest (van Houdt et al., 2010; Tong et al., 2013, 2017; Erdogan et al., 2016). In particular, the correlation pattern observed in WM between these previous studies and the current studies is highly similar, with the strongest (negative) effects seen in the deep (periventricular) WM. The bipolar characteristics of the correlate in WM found in the current study explains the apparent dichotomy between the findings in (Tong et al., 2013) and (Tong et al., 2017), where either (delayed) positive or negative correlations between NIRS and the fMRI signal in WM were observed. Nevertheless, while these findings indicate a potential systemic contribution to WM fMRI signal, the origin of the distinctly different temporal characteristics of the GM and WM correlates of systemic variations remains unexplained. In the following, we address this by examining differences in vascular architecture between these tissues.
White matter vasculature
To interpret the WM fMRI signal, it is important to consider its blood supply. The strongest anticorrelation between PPG and fMRI was found in deep WM locations, which have a blood supply that is distinctly different from that of GM. Much of the deep WM blood supply originates from centimeters long, but thin medullary arteries that branch off of pial arteries (Fig. 6a). Much of the blood exits deep WM through a network of long, thin medullary veins, which in turn drain into large medullary veins that surround the ventricles (Fig. 6b). As in GM, arteries in WM possess intrinsic (local) innervation that allows for local changes in vascular tone in response to neural activity or blood CO2 concentration. The vascular tone of the larger arteries in the brain, including those on the pial surface, is also under central control, mediated by the sympathetic innervation originating from the superior cervical ganglion (Hamel, 2006). Medullary arteries originating from the pial surface, however, lack this extrinsic sympathetic innervation that serves to effectuate global changes in vascular tone elsewhere in the CNS and in other organs (including the skin). Putative effects of these vascular features on the WM hemodynamic response and BOLD effect will be discussed in the explanatory model outlined below.
Fig. 6.

Geometry of WM blood supply. a) Contrast-enhanced X-ray of adult human arterial supply in a coronal brain slice including periventricular WM, approximately covering the area outlined by the yellow box in (b). Notice the long, thin arteries supplying the deep WM. Figure reproduced with permission from (Okudera et al., 1999). b) Schematic of deep medullary drainage, coronal view. Notice the long thin veins, and their fan-shaped pattern of increasing density towards the large draining veins surrounding the ventricles. The yellow box indicates the field of view of the image shown in a). Figure reproduced with permission from (Ruiz et al., 2009).
Model for BOLD fMRI response in WM
Based on the particular blood supply of the deep WM, we propose a parsimonious model to explain observations in the current and in previous work of the distinctly different systemic effects in GM and WM. In a typical scenario of local vasodilation in GM in response to neural activity (Fig. 7a), the BOLD response results from a combination of an increase in both blood volume (BV) and flow, leading to an elevated blood oxygenation (BO) (Buxton et al., 1998). These processes have opposing effects on the BOLD signal, with the positive effects of BO increases (and concomitant deoxyhemoglobin concentration decreases) generally overwhelming the BV effects. In GM, BO and BV effects to large extent overlap in time, although the BO effects maybe slightly delayed as they are secondary to negative BV changes and rely on the arterio-venous transit time. Since the BV changes are generated locally, this transit time is short, and as a result the BOLD response is generally positive over its entire time course.
Fig. 7.
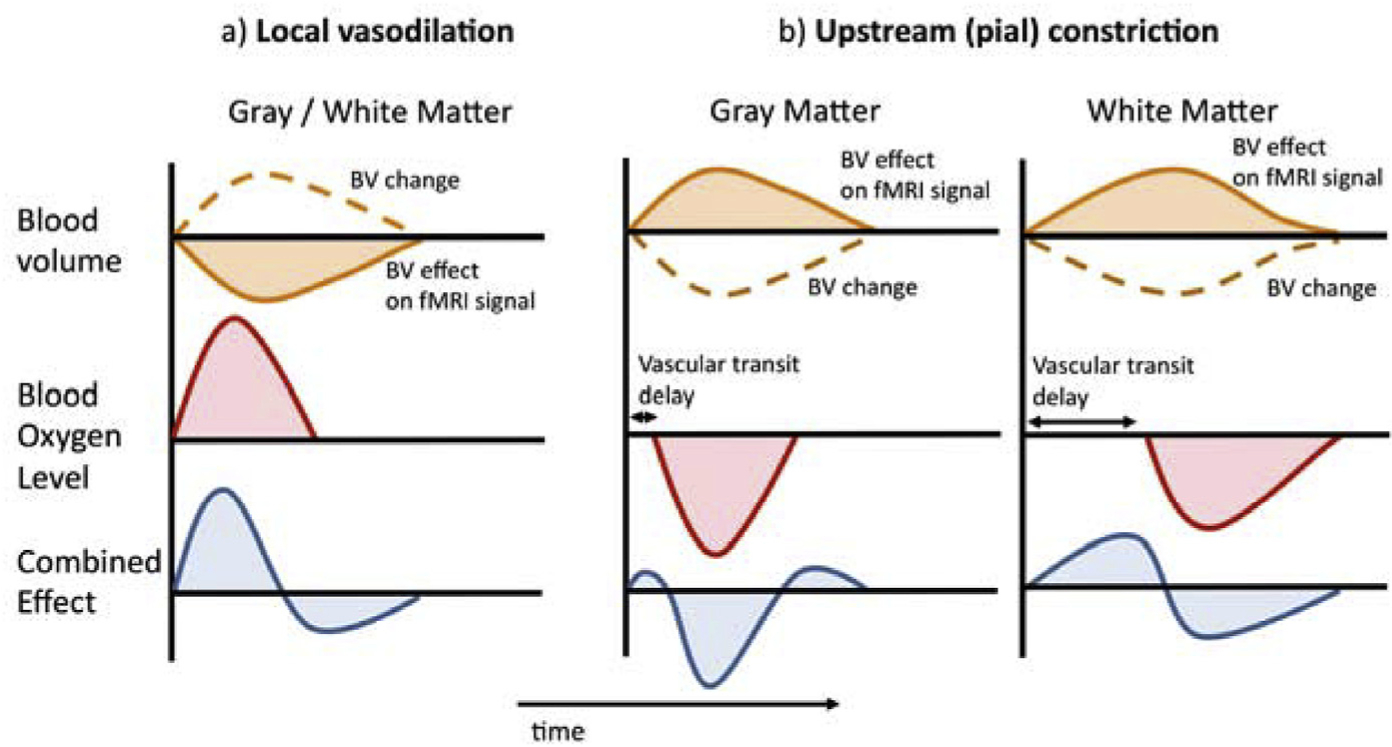
Mechanistic interpretation of WM signal based on a balloon model and delayed vascular transit. a) Typical fMRI response to a brief, local (neuro) vascular dilatory event results from the combination of a (positive) blood oxygenation (Oxygenation) effect and a (negative) cerebral blood volume (CBV) effect. b) The fMRI response to an upstream arterial event, in this example brief vasoconstriction, is affected by vascular transit delay, which is strongly different for GM and deep WM. The substantial temporal shift between BO and BV effects in WM leads to a strongly bipolar response that is temporally antipolar to the GM response.
A similar sequence of processes occurs in GM during systemic vasoconstriction that affects the nearby (pial) arteries and arteries further upstream, albeit with opposite polarity (Fig. 7b, left panel). In deep WM however, the situation can be quite different. Sympathetic vasoconstriction (at innervated pial arteries or further upstream) would occur remotely, potentially delaying the BO effects relative to the BV effects. This delay, which depends on the arterio-venous transit time, can be substantial due to the particular geometry of the medullary vasculature. Both medullary veins and arteries are long (up to 5 cm) and thin (100–200 μm) and have low flow velocities of <2 cm/s (Deeg and Lode, 2005). As a result, BO changes in WM may trail those seen in GM by several seconds, supported by transit times measured by MRI-based tracking of a bolus of intravascular Gadolinium contrast agent (van Gelderen et al., 2008). BV changes on the other hand may occur more rapidly, as they do not depend on vascular transit but rather more closely follow upstream pressure changes. As a result, the temporal overlap between WM BO and BV changes is reduced, resulting in strongly bipolar signal changes (Fig. 7b, right panel). In addition, the BO change (and associated effect on the signal) may be somewhat reduced because of increased loss of oxygen to WM due to the long transit through the upstream vasculature (Vovenko, 1999). This reduction is expected to be largest for veins the furthest downstream, such as the medullary veins near the ventricles (c.f. Fig. 8c below).
Fig. 8.

Comparison of spatial characteristics of WM signal (subject 3) a), vascular transit delays b), anatomy of the deeps venous system c). A striking resemblance is seen between these different aspects, supporting the mechanistic interpretation of the WM effects based on the balloon model with delayed vascular transit. WM signal (a) was calculated for 2 MRI slices from 0-lag correlation with AMP (overlaid on EPI magnitude images, r < −0.05). Images in b) give results from another subject, showing delayed (~5 s) arrival in WM of intravenously injected bolus of Gd-DTPA T2* contrast agent (van Gelderen et al., 2008). The schematic of the medullary vascular system in c) was reproduced with permission from (Lee et al., 1996).
Further support for our mechanistic interpretation of the WM signal comes from its particular spatial distribution (Fig. 8). A striking resemblance is seen between this distribution on one hand (Fig. 8a) and the bolus arrival time map (Fig. 8b) and the medullary vasculature’s geometry (Fig. 8c) on the other.
Origin of intermittent vasoconstriction
The presence of spontaneous temporal variations in cerebral hemodynamics has been acknowledged since at least as early as the 1880’s (Mosso, 1880). These variations may have various origins, and often involve periodic changes in sympathetic vasoconstriction. Phenomena like Mayer waves and vasomotion (for reviews see (Julien, 2006; Haddock and Hill, 2005)) are oscillatory and periodic in nature, and their potential contribution to the spontaneous fMRI signal has been recognized for some time (Hudetz et al., 1998; Biswal et al., 1995). In contrast, the intermittent variations in hemodynamics described here, and their effect on the fMRI signal, have been less well studied.
The PPG amplitude dips observed here suggest the intermittent occurrence of brief sympathetic activity of the autonomic nervous system (ANS). Since sympathetic innervation by the superior cervical ganglion extends to most central nervous system (CNS) arteries all the way up to the pial surface, it can effectuate vasoconstriction and reduced blood flow in much of the brain (Busija and Heistad, 1984). Importantly, the superior cervical ganglion also exerts vasoconstrictive influence over the vasculature in the skin, consistent with the positive correlation seen between PPG AMP and GM fMRI signal.
Thus, the ANS alone could be responsible for the observed variations in the fMRI signal. This is not to say that the latter exclusively reflect vascular activity. In fact, the ANS vasoconstrictive activity may be associated with or secondary to some form of CNS neural activity as there are multiple pathways that integrate these systems (Delessert et al., 2010; Grote and Zou, 2016). One candidate phenomenon that may simultaneously affect CNS neural and sympathetic vascular activity is that of arousal (Halasz et al., 2004). It is well established that low arousal states such as sleep and relaxed wakefulness are punctuated by brief cortical micro-arousals as well a sub-cortical (autonomic) arousals. Such arousals can cause changes in electro-cortical activity (Nir et al., 2011; Cash et al., 2009) that often co-occur with sympathetic effects as evidenced by changes in peripheral arterial tone, heart rate, blood pressure, and PPG amplitude (Guilleminault et al., 2006; Bonnet and Arand, 1997; Catcheside et al., 2002; Bangash et al., 2008; Goff et al., 2008; Monstad and Guilleminault, 1999; Hord et al., 1966).
Almost unavoidably, resting-state fMRI studies typically deal with conditions of low or varying arousal, which may be susceptible to global signal changes similar to those observed in the current study. This is not to say that the current observations are specific to resting-state fMRI, as arousal changes elicited by tasks would also be expected to affect systemic physiology, including vascular tone.
Alternative explanations
While the spatio-temporal characteristics of the WM fMRI correlate of PPG amplitude drops observed here suggest the involvement of the sympathetic arousal, alternative mechanisms should be considered. For example, it is well established that blood-borne vasoactive stimuli such as such CO2 may cause vasodilation through local mechanisms, and possibly jointly change vascular tone in WM, GM and peripherally (at the fingers). While this may occur under certain conditions, by itself it does not explain the current and previous observations (van Houdt et al., 2010; Tong et al., 2013) of and early anti-correlated effect in WM. It is possible however that, similar to the effect of sympathetic vasoconstriction, early, large scale CO2 mediated changes upstream from the parenchyma (Iadecola, 2004) create an blood pressure effect that cause a rapid BV change in WM. Such blood pressure effects may also be caused by changes in respiration. The effect of the resulting BV change on BO in WM would be affected by the long vascular transit delay in the medullary vasculature (see above), and hence result in a bipolar WM signal. Similarly, strong, large scale changes in neural activity that recruit pial arteries and arteries further upstream may create this early BV effect in WM as well. Thus, the current observations may not be specific to conditions of varying arousal (such as drowsiness and light sleep) but rather be exemplary of systemic variations in hemodynamics in general. Also, we would like to emphasize that the indications of systemic contributions laid out above by no means exclude the possibility that a local vasodilatory mechanism in response to WM metabolic activity (‘neuronal activation’) contributes to the fMRI signal as well.
A previous explanation of WM activity suggested that the opposing changes in WM and GM may be explained by considering the CNS blood supply to be arranged as a 2-pool system, in which a WM pool serves as a reserve for GM pool (Bhogal et al., 2015). It was envisioned that when GM demand is high, blood is shuttled (“stolen”) from the WM pool to fulfill GM demands. While possible, there is little evidence for this mechanism, and it does not explain the temporal characteristics of the WM effects.
Implications
The data and hypotheses presented here suggest that, due to the characteristics of the vascular geometry of WM, and in particular that of medullary arteries and veins, WM fMRI signals may have significant contributions from remote hemodynamics. Because of this, care has to be taken with the interpretation of fMRI signals in WM, including those reported previously (Gawryluk et al., 2011a,b; Mazerolle et al., 2010; Ding et al., 2013; Ding et al., 2016). Interpretation of the WM fMRI signal in terms of WM connections between GM functional nodes therefore needs to fully account for potential systemic contributions, especially as the inspection of the medullary vasculature presented in Figs. 6 and 8 indicates that its geometry does not generally align with known fiber orientations. Thus, even if systemic effects are fully accounted for, inference of WM fiber orientation based on the geometry of WM functional patterns (see e.g. (Ding et al., 2013; Ding et al., 2016)), may not be straightforward.
As indicated above, the WM phenomenon as seen in our study may occur more generally with large scale changes in remote, upstream arteries where vascular transit delays create timing shifts in the BV and BO effects and/or diminish the BO effect. For example, our balloon-model (Fig. 6) explanation of downstream effects of arterial dilation may also explain “negative BOLD effects” observed occasionally in task fMRI (see e.g. (Bianciardi et al., 2011; Bianciardi et al., 2014)). To the extent that fMRI tasks trigger changes in behavioral (e.g. arousal, alertness, and attention) or physiological state (heart rate, respiration and vascular tone), a systemic GM fMRI effect accompanied by a downstream WM effect is to be expected. Similarly, anti-correlation between the WM and GM effect in response to respiratory challenges (Bright et al., 2014), and the delayed WM correlation seen with CO2 breathing challenges may be at least partially explained by this proposed mechanism (Champagne et al., 2017; Thomas et al., 2014).
Fluctuations in systemic physiology are a ubiquitous problem in fMRI and may affect both GM and WM signals. Proper interpretation of fMRI experiments therefore will require a comprehensive accounting of these fluctuations (Tong et al., 2015; Erdogan et al., 2016) that will likely require the inclusion of multiple physiological measures, including heart rate (Shmueli et al., 2007; Chang et al., 2009), respiratory rate and depth (Birn et al., 2006), and peripheral measures of vascular tone. As shown here and in previous work (van Houdt et al., 2010; Tong et al., 2013, 2017), the latter can be conveniently measured with PPG or NIRS, and the former being standard equipment on many fMRI systems. Considering the widely varying conditions of fMRI studies and the complex interaction between the various components that control systemic physiology, the inter-relationship between the various physiological measures, and their relative contribution to the fMRI signal will likely vary and depend on experimental conditions. In particular in “awake” resting state studies, where subject behavior is generally poorly controlled, intermittent bouts of sleep may occur, and incomplete accounting for changes in systemic physiology may affect interpretation of the functional measures (Tagliazucchi and Laufs, 2014; Glasser et al., 2017). The results presented here also suggest that previous approaches for removing systemic effects by spectral filtering or the use of a reference signal from WM (Caballero-Gaudes and Reynolds, 2017) may lead to incorrect or incomplete removal of systemic hemodynamic variations.
Conclusions
We characterized the spatio-temporal pattern of fMRI activity in WM in response to changes in peripheral vascular tone. Our results suggest that the temporal dynamics of signal characteristics in WM often observed in the fMRI literature can be explained by a temporal mismatch between blood volume and blood oxygenation effects originating from vascular transit delays resulting from the vascular geometry. These results highlight the possible contribution of non-local systemic effects to fMRI signals in WM, along with the value of using readily available peripheral indicators of vascular tone to account for them.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.neuroimage.2018.04.045.
References
- Ackner B, Pampiglione G, 1957. Some relationships between peripheral vasomotor and E.E.G. changes. J. Neurol. Neurosurg. Psychiatry 20, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler D, Bridevaux PO, Contal O, Georges M, Dupuis-Lozeron E, Claudel E, Pepin JL, Janssens JP, 2013. Pulse wave amplitude reduction: a surrogate marker of micro-arousals associated with respiratory events occurring under non-invasive ventilation? Respir. Med 107, 2053–2060. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R, 2000. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12, 230–239. [DOI] [PubMed] [Google Scholar]
- Bangash MF, Xie A, Skatrud JB, Reichmuth KJ, Barczi SR, Morgan BJ, 2008. Cerebrovascular response to arousal from NREM and REM sleep. Sleep 31, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM, 2004. ‘Probabilistic independent component analysis for functional magnetic resonance imaging’. IEEE Trans. Med. Imaging 23, 137–152. [DOI] [PubMed] [Google Scholar]
- Bhogal AA, Philippens ME, Siero JC, Fisher JA, Petersen ET, Luijten PR, Hoogduin H, 2015. Examining the regional and cerebral depth-dependent BOLD cerebrovascular reactivity response at 7T. Neuroimage 114, 239–248. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, de Zwart JA, Duyn JH, 2011. Negative BOLD-fMRI signals in large cerebral veins. J. Cereb. Blood Flow. Metab 31, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, van Gelderen P, Duyn JH, 2014. Investigation of BOLD fMRI resonance frequency shifts and quantitative susceptibility changes at 7 T. Hum. Brain Mapp 35, 2191–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA, 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31, 1536–1548. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson Med 34, 537–541. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL, 1997. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr. Clin. Neurophysiol 102, 390–396. [DOI] [PubMed] [Google Scholar]
- Bright MG, Bianciardi M, de Zwart JA, Murphy K, Duyn JH, 2014. Early anti-correlated BOLD signal changes of physiologic origin. Neuroimage 87, 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Heistad DD, 1984. Effects of activation of sympathetic nerves on cerebral blood flow during hypercapnia in cats and rabbits. J. Physiol 347, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR, 1998. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson Med 39, 855–864. [DOI] [PubMed] [Google Scholar]
- Caballero-Gaudes C, Reynolds RC, 2017. Methods for cleaning the BOLD fMRI signal. Neuroimage 154, 128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I, 2009. The human K-complex represents an isolated cortical down-state. Science 324, 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD, 2002. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep 25, 797–804. [DOI] [PubMed] [Google Scholar]
- Champagne AA, Bhogal AA, Coverdale NS, Mark CI, Cook DJ, 2017. A novel perspective to calibrate temporal delays in cerebrovascular reactivity using hypercapnic and hyperoxic respiratory challenges. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH, 2009. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 44, 857–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg KH, Lode HM, 2005. Trans-fontanellar Doppler sonography of the intracranial veins in infants–part I–Normal values. Ultraschall Med. 26, 507–517. [DOI] [PubMed] [Google Scholar]
- Delessert A, Espa F, Rossetti A, Lavigne G, Tafti M, Heinzer R, 2010. Pulse wave amplitude drops during sleep are reliable surrogate markers of changes in cortical activity. Sleep 33, 1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Newton AT, Xu R, Anderson AW, Morgan VL, Gore JC, 2013. Spatiotemporal correlation tensors reveal functional structure in human brain. PLoS ONE 8, e82107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z, Xu R, Bailey SK, Wu TL, Morgan VL, Cutting LE, Anderson AW, Gore JC, 2016. Visualizing functional pathways in the human brain using correlation tensors and magnetic resonance imaging. Magn. Reson Imaging 34, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan SB, Tong Y, Hocke LM, Lindsey KP, de B. Frederick B, 2016. Correcting for blood arrival time in global mean regression enhances functional connectivity analysis of resting state fMRI-bold signals. Front. Hum. Neurosci 10, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser LM, Stevens MT, Beyea SD, D’Arcy RC, 2012. White versus gray matter: fMRI hemodynamic responses show similar characteristics, but differ in peak amplitude. BMC Neurosci. 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, D’Arcy RC, Mazerolle EL, Brewer KD, Beyea SD, 2011a. Functional mapping in the corpus callosum: a 4T fMRI study of white matter. Neuroimage 54, 10–15. [DOI] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, Beyea SD, D’Arcy RC, 2014a. Functional MRI activation in white matter during the symbol digit modalities test. Front. Hum. Neurosci 8, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, Brewer KD, Beyea SD, D’Arcy RC, 2011b. Investigation of fMRI activation in the internal capsule. BMC Neurosci. 12, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryluk JR, Mazerolle EL, D’Arcy RC, 2014b. Does functional MRI detect activation in white matter? A review of emerging evidence, issues, and future directions. Front. Neurosci 8, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser Matthew F., Coalson Timothy S., Bijsterbosch Janine D., Harrison Samuel J., Harms Michael P., Anticevic Alan, Van Essen David C., Smith Stephen M., 2017. Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D, 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn. Reson Med 44, 162–167. [DOI] [PubMed] [Google Scholar]
- Goff EA, O’Driscoll DM, Simonds AK, Trinder J, Morrell MJ, 2008. The cardiovascular response to arousal from sleep decreases with age in healthy adults. Sleep 31, 1009–1017. [PMC free article] [PubMed] [Google Scholar]
- Grote L, Zou D, 2016. Pulse Wave Analysis during Sleep Principles and practice of sleep medicine, W. B. Saunders: Philadelphia. [Google Scholar]
- Guilleminault C, Abad VC, Philip P, Stoohs R, 2006. The effect of CNS activation versus EEG arousal during sleep on heart rate response and daytime tests. Clin. Neurophysiol 117, 731–739. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE, 1996. Nervous regulation of the circulation, and rapid control of arterial pressure In: Textbook of medical physiology. W.B. Saunders Co, Philadelphia. [Google Scholar]
- Haddock RE, Hill CE, 2005. Rhythmicity in arterial smooth muscle. J. Physiol 566, 645–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz P, Terzano M, Parrino L, Bodizs R, 2004. The nature of arousal in sleep. J. Sleep. Res 13, 1–23. [DOI] [PubMed] [Google Scholar]
- Hamel E, 2006. Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol 100 (1985), 1059–1064. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D, 2012. The energetics of CNS white matter. J. Neurosci 32, 356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hord DJ, Lubin A, Johnson LC, 1966. The evoked heart rate response during sleep. Psychophysiology 3, 47–54. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Biswal BB, Shen H, Lauer KK, Kampine JP, 1998. Spontaneous fluctuations in cerebral oxygen supply. An introduction. Adv. Exp. Med. Biol 454, 551–559. [DOI] [PubMed] [Google Scholar]
- Iadecola C, 2004. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci 5, 347–360. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL Jr., Quan SF, 2007. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine, Westchester. [Google Scholar]
- Julien C, 2006. The enigma of Mayer waves: facts and models. Cardiovasc Res. 70, 12–21. [DOI] [PubMed] [Google Scholar]
- Kruger G, Kastrup A, Glover GH, 2001. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn. Reson Med 45, 595–604. [DOI] [PubMed] [Google Scholar]
- Lee C, Pennington MA, Kenney CM 3rd, 1996. MR evaluation of developmental venous anomalies: medullary venous anatomy of venous angiomas. AJNR Am. J. Neuroradiol 17, 61–70. [PMC free article] [PubMed] [Google Scholar]
- Liu Z, de Zwart JA, van Gelderen P, Kuo LW, Duyn JH, 2012. Statistical feature extraction for artifact removal from concurrent fMRI-EEG recordings. Neuroimage 59, 2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA, 2004. Interpreting the BOLD signal. Annu. Rev. Physiol 66, 735–769. [DOI] [PubMed] [Google Scholar]
- Marussich L, Lu KH, Wen H, Liu Z, 2017. Mapping white-matter functional organization at rest and during naturalistic visual perception. Neuroimage 146, 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle EL, Beyea SD, Gawryluk JR, Brewer KD, Bowen CV, D’Arcy RC, 2010. Confirming white matter fMRI activation in the corpus callosum: co-localization with DTI tractography. Neuroimage 50, 616–621. [DOI] [PubMed] [Google Scholar]
- Mazerolle EL, Gawryluk JR, Dillen KN, Patterson SA, Feindel KW, Beyea SD, Stevens MT, Newman AJ, Schmidt MH, D’Arcy RC, 2013. Sensitivity to white matter FMRI activation increases with field strength. PLoS ONE 8, e58130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney SR, Mazerolle EL, Gawryluk JR, Beyea SD, D’Arcy RC, 2012. Comparing gray and white matter fMRI activation using asymmetric spin echo spiral. J. Neurosci. Methods 209, 351–356. [DOI] [PubMed] [Google Scholar]
- Moehlman TM, de Zwart JA, Liu X, McClain IB, Chang C, Mandelkow H, Bieber RE, Fernandez KA, King KA, Zalewski CK, Brewer CC, van Gelderen P, Duyn JH, Picchioni D, 2017. A method for studying neural circuits during all-night functional magnetic resonance imaging sleep studies In: Associated Professional Sleep Societies Annual Meeting (Boston, MA: ). [Google Scholar]
- Monstad P, Guilleminault C, 1999. Cardiovascular changes associated with spontaneous and evoked K-complexes. Neurosci. Lett 263, 211–213. [DOI] [PubMed] [Google Scholar]
- Mosso A, 1880. Sulla circolazione del sangue nel cervello dell’uomo. Atti della R. Acad. Lincei, Mem. Cl. Sci. Fis. Mat. Nat 3, 237–358. [Google Scholar]
- Namtvedt SK, Randby A, Einvik G, Hrubos-Strom H, Somers VK, Rosjo H, Omland T, 2011. Cardiac arrhythmias in obstructive sleep apnea (from the akershus sleep apnea project). Am. J. Cardiol 108, 1141–1146. [DOI] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G, 2011. Regional slow waves and spindles in human sleep. Neuron 70, 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudera T, Huang YP, Fukusumi A, Nakamura Y, Hatazawa J, Uemura K, 1999. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology 19, 93–111. [DOI] [PubMed] [Google Scholar]
- Ruiz DS, Yilmaz H, Gailloud P, 2009. Cerebral developmental venous anomalies: current concepts. Ann. Neurol 66, 271–283. [DOI] [PubMed] [Google Scholar]
- Shelley KH, 2007. Photoplethysmography: beyond the calculation of arterial oxygen saturation and heart rate. Anesth. Analg 105, S31–S36 tables of contents. [DOI] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, Duyn JH, 2007. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage 38, 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi sE., Laufs H, 2014. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron 82, 695–708. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Paulesu E, Scifo P, Maravita A, Fazio F, Perani D, Marzi CA, 2002. Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J. Neurophysiol 88, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Thomas BP, Liu P, Park DC, van Osch MJ, Lu H, 2014. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J. Cereb. Blood Flow. Metab 34, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Hocke LM, Fan X, Janes AC, Frederick Bd, 2015. Can apparent resting state connectivity arise from systemic fluctuations? Front. Hum. Neurosci 9, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Hocke LM, Nickerson LD, Licata SC, Lindsey KP, Frederick Bd, 2013. Evaluating the effects of systemic low frequency oscillations measured in the periphery on the independent component analysis results of resting state networks. Neuroimage 76, 202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Lindsey KP, Hocke LM, Vitaliano G, Mintzopoulos D, Frederick BD, 2017. Perfusion information extracted from resting state functional magnetic resonance imaging. J. Cereb. Blood Flow. Metab 37, 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R, 2002. How much cortex can a vein Drain? Downstream dilution of activation-related cerebral blood oxygenation changes. Neuroimage 16, 1062–1067. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Duyn JH, 2008. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn. Reson Med 59, 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houdt PJ, Ossenblok PP, Boon PA, Leijten FS, Velis DN, Stam CJ, de Munck JC, 2010. Correction for pulse height variability reduces physiological noise in functional MRI when studying spontaneous brain activity. Hum. Brain Mapp 31, 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovenko E, 1999. Distribution of oxygen tension on the surface of arterioles, capillaries and venules of brain cortex and in tissue in normoxia: an experimental study on rats. Pflugers Arch. 437, 617–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


